Abstract
Streptomyces davawensis synthesizes the antibiotic roseoflavin, one of the few known natural riboflavin analogs, and is roseoflavin resistant. It is thought that the endogenous flavokinase (EC 2.7.1.26)/flavin adenine dinucleotide (FAD) synthetase (EC 2.7.7.2) activities of roseoflavin-sensitive organisms are responsible for the antibiotic effect of roseoflavin, producing the inactive cofactors roseoflavin-5′-monophosphate (RoFMN) and roseoflavin adenine dinucleotide (RoFAD) from roseoflavin. To confirm this, the FAD-dependent Sus scrofa d-amino acid oxidase (EC 1.4.3.3) was tested with RoFAD as a cofactor and found to be inactive. It was hypothesized that a flavokinase/FAD synthetase (RibC) highly specific for riboflavin may be present in S. davawensis, which would not allow the formation of toxic RoFMN/RoFAD. The gene ribC from S. davawensis was cloned. RibC from S. davawensis was overproduced in Escherichia coli and purified. Analysis of the flavokinase activity of RibC revealed that the S. davawensis enzyme is not riboflavin specific (roseoflavin, kcat/Km = 1.7 10−2 μM−1 s−1; riboflavin, kcat/Km = 7.5 10−3 μM−1 s−1). Similar results were obtained for RibC from the roseoflavin-sensitive bacterium Bacillus subtilis (roseoflavin, kcat/Km = 1.3 10−2 μM−1 s−1; riboflavin, kcat/Km = 1.3 10−2 μM−1 s−1). Both RibC enzymes synthesized RoFAD and RoFMN. The functional expression of S. davawensis ribC did not confer roseoflavin resistance to a ribC-defective B. subtilis strain.
Riboflavin (vitamin B2) analogs have the potential to serve as basic structures for the development of novel anti-infectives (3). Consequently, both the mode of action of riboflavin analogs and the mechanism of resistance to these compounds are of substantial interest. Only very few natural riboflavin analogs are known (24). Moreover, the only known organism to produce a riboflavin analog with antibiotic activity is the gram-positive bacterium Streptomyces davawensis (32).
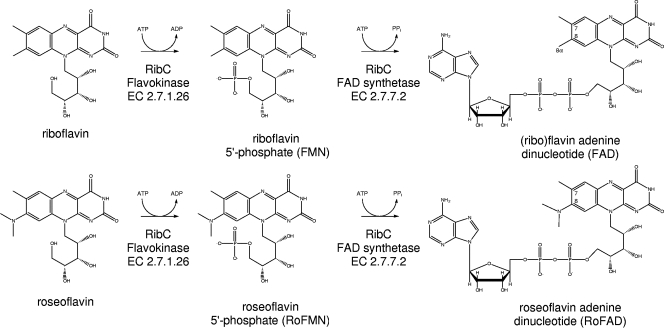
S. davawensis synthesizes the anti-vitamin 8-dimethyl-amino-8-demethyl-d-riboflavin, or roseoflavin (Fig. 1), from riboflavin (27) via 8-amino- and 8-methylamino-8-demethyl-d-riboflavin (22). Roseoflavin is toxic to gram-positive but also to gram-negative bacteria if the compound is able to enter the cell (16). The molecular basis for roseoflavin toxicity is not clear. In all organisms, riboflavin serves as the direct precursor for the cofactors (ribo)flavin-5′-monophosphate (FMN) and flavin adenine dinucleotide (FAD) (12). FAD and (to a lesser extent) FMN are active components of flavoproteins, being involved in a wide range of redox and other reactions (15). FMN is synthesized from riboflavin by flavokinase (EC 2.7.1.26), and FAD is produced from FMN by FAD synthetase (EC 2.7.7.2) (2). In microorganisms, both enzymatic activities seem to be present in sufficient amounts, since only traces of free riboflavin are detectable in the cytoplasm (11). Accordingly, cytoplasmic roseoflavin may quickly be converted to the corresponding FMN/FAD analogs roseoflavin-5′-monophosphate (RoFMN) and roseoflavin adenine dinucleotide (RoFAD). The synthesis of these inactive cofactors may explain the antibiotic activity of roseoflavin (Fig. 1) (31, 36).
FIG. 1.
Enzymatic conversion of riboflavin (top) into FMN/FAD and of roseoflavin (bottom) into RoFMN and RoFAD.
S. davawensis is roseoflavin resistant, and the mechanism of self-resistance could involve a flavokinase/FAD synthetase with a high substrate specificity for riboflavin. Such an enzyme would not accept roseoflavin as a substrate and thus would not produce toxic RoFMN and RoFAD. In order to test this hypothesis, the S. davawensis flavokinase/FAD synthetase was identified, purified, kinetically characterized, and compared to the enzyme of the roseoflavin-sensitive organism Bacillus subtilis. The present study demonstrates that flavokinases/FAD synthetases in general are responsible for the production of inactive/inhibitory RoFMN and RoFAD and that the S. davawensis flavokinase/FAD synthetase RibC is not involved in roseoflavin resistance.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
S. davawensis (Streptomyces strain 768) was aerobically grown at 37°C in a nutrient broth (YS) containing yeast extract (2 g/liter) and soluble potato starch (10 g/liter). For growth, a 250-ml Erlenmeyer flask containing 50 ml YS medium and a coiled stainless steel spring for good aeration and cell dispersion was used. The cultures were agitated at 260 rpm in an orbital shaker. For susceptibility testing, roseoflavin (50 μg/ml) was added to YS agar plates. Escherichia coli and B. subtilis were cultivated on LB agar (33). The plasmids used in this study are described in Table 1.
TABLE 1.
Plasmids used in this study
| Plasmid | Relevant feature(s)a | Reference or source |
|---|---|---|
| pTZ18cm− | High-copy-no. cloning vector, cm | Trenzyme |
| pCR2.1 | High-copy-no. cloning vector, amp | Invitrogen |
| pNCO113 | High-copy-no. expression vector, amp | 40 |
| pTrcHisA | High-copy-no. expression vector, amp | Invitrogen |
| pXI12 | pBR322 sacB5′ ermAM cryT RBS PvegI sacB3′ amp, B. subtilis integration/expression vector | 18 |
| pBEST501 | B. subtilis integration vector, neo amp | 21 |
| pBluescriptII SK− | High-copy-no. cloning vector, amp | Stratagene |
| pSG01 | pTrcHisA containing a 0.960-kbp BamHI/PstI fragment carrying S. davawensis ribCopt | This study |
| pSG02 | pNCO113 containing a 0.960-kbp NcoI/HindIII fragment carrying S. davawensis ribC | This study |
| pSG03 | pTrcHisA containing a 0.948-kbp BamHI/PstI fragment carrying B. subtilis ribC | This study |
| pSG05 | pCR2.1 containing an internal 0.5-kbp S. coelicolor ribC (SCO5711) fragment | This study |
| pSG06 | pTZ18cm− containing a 5,837-bp S. davawensis genomic fragment (ribC locus) | This study |
| pSG10 | pIXI12 containing a 0.960-kbp NdeI/XmaI fragment carrying S. davawensis ribCopt | This study |
| pSG60 | pBluescript SK− containing a 2.609-kbp XhoI/SpeI fragment carrying the B. subtilis ribC locus; ribC of this locus was replaced by neo from pBEST501 | This study |
Abbreviations: cm, chloramphenicol resistance cassette; amp, ampicillin resistance cassette; sacB, integration of genes by homologous double-crossover recombination at B. subtilis sacB; ermAM, erythromycin resistance cassette; neo, neomycin resistance cassette; PvegI, medium-strength vegI promoter; ribC, flavokinase/FAD synthetase gene.
Isolation of total DNA and other molecular biology techniques.
For the isolation of total DNA from S. davawensis, the Kirby mix procedure was used (23). Other molecular biology techniques were carried out according to standard procedures (33).
Library construction and preparation of a DNA colony array.
Total DNA (44 μg) of S. davawensis was partially digested with Sau3A1 to fragments with approximate sizes of 5 kb. The resulting DNA fragments were separated by agarose gel electrophoresis. The 5-kb fragments were extracted from the agarose gel and ligated to 1.5 μg BamHI-treated, dephosphorylated plasmid vector pTZ18 cm− (Trenzyme, Konstanz, Germany). The ligation reaction was used to transform E. coli TZ102α (Trenzyme). For the preparation of the DNA colony array, 9,216 single, white colonies were transferred into 24 384-well plates, with each well containing 30 μl 2× YT-glycerol broth (0.5% yeast extract, 1.6% tryptone, 1% NaCl, and 15% glycerol). Cells were grown at 37°C for 72 h, and aliquots of the cultures were spotted onto a nylon membrane as described previously (41). The colonies were grown on the nylon membrane by incubation on LB agar. The colonies were lysed with alkali, and the DNA was fixed to the nylon membrane by UV cross-linking.
Screening of an S. davawensis DNA colony array with a heterologous probe.
An internal 500-bp fragment of the hypothetical gene ribC of Streptomyces coelicolor (SCO5711) was amplified by PCR using the modifying oligonucleotides SCO5711 fw (5′-AAAAGCTTAGGAGGTGTCACAGTGCAGCGCTGGCGTG-3′) and SCO5711 rev (5′-ATGAATTCGCGCGCTAGAAGGCAGCCGGCCGCCCTTT-3′) (sites for restriction endonucleases are underlined). Chromosomal DNA of S. coelicolor was used as a template and prepared using the Kirby mix procedure (see above). The PCR product was ligated to pCR2.1 (Invitrogen GmbH, Karlsruhe, Germany) and cloned using E. coli TOP10F′ (Invitrogen). The resulting plasmid, pSG05, was isolated, 2 μg was digested with HindIII/EcoRI, and the ribC insert was purified from contaminating DNA molecules using agarose gel electrophoresis. The insert DNA of pSG05 (200 ng) was used as a template for random hexanucleotide-primed labeling with [α-33P]dCTP (50 μCi; 3,000 Ci/mmol) and Klenow enzyme. The probe was diluted to 0.5 × 106 cpm/ml in hybridization buffer (250 mM sodium phosphate, pH 7.2; 1 mM EDTA; 7% sodium dodecyl sulfate [SDS]) and hybridized to the DNA array for 20 h at 65°C. Washing was performed twice for 15 min each at 30°C in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-0.1% SDS and once for 30 min at 65°C in 0.5 × SSC-0.1% SDS (33).
Heterologous expression of S. davawensis ribC in E. coli.
For integration of S. davawensis ribC into NcoI/HindIII-treated pNCO113 (40), the modifying oligonucleotides ribCfw (5′-AACCATGGAGGAGGTGTCACAGTGCAGCGCTGGCGTG-3′) and ribCrev (5′-ATAAGCTTGCGCGCTAGAAGGCAGCCGGCCGCCCTTT-3′) were used. E. coli DH5α was transformed with pNCO113ribC (pSG02). For ribC expression, the resulting strain was aerobically cultivated at 37°C on LB medium. Expression of ribC was induced by adding 1 mM isopropylthiogalactopyranoside (IPTG) after the culture had reached an optical density at 600 nm of 0.6. After 1 h of further aerobic incubation, cells were harvested by centrifugation. The ribC gene from S. davawensis has a relatively high G+C content (72%) and was optimized with respect to the codon usage of E. coli. The optimized ribC gene (ribCopt) was synthesized using overlapping oligonucleotides and ligase and PCR techniques. The produced PCR fragment was ligated to the vector pTrcHisA (Invitrogen). The resulting plasmid (pSG01) was introduced into E. coli DH5α, and gene expression was carried out as described above.
Heterologous expression of B. subtilis ribC in E. coli.
The bifunctional flavokinase/FAD synthetase RibC from B. subtilis (25) served as a positive control throughout this study and was compared to the corresponding enzyme (RibC) from S. davawensis. For integration of B. subtilis ribC into BamHI/PstI-treated pTrcHisA, the modifying oligonucleotides ribBSCfw (5′-AAGGATCCATGAAGACGATACATATTACA-3′) and ribBSCrev (5′-AACTGCAGTTATTTCCGCAAATTGCTCAA-3′) were used. E. coli DH5α was transformed with the resulting plasmid (pSG03). As a template for PCR amplification of wild-type B. subtilis ribC, the plasmid pMM01 (25) was used. For purification of the plasmid DNA, a standard protocol (33) was employed. For expression of B. subtilis ribC, the recombinant strain was aerobically cultivated at 37°C on LB medium. Gene expression was carried out as described above.
Purification of overproduced His-tagged S. davawensis RibC and His-tagged B. subtilis RibC.
All procedures were carried out at 0 to 4°C. Frozen cell paste (5 g) of E. coli DH5α overproducing RibC was resuspended in 25 ml of lysis buffer (50 mM NaH2PO4, pH 8; 300 mM NaCl; 10 mM imidazole). Cells were disrupted in a French press at 108 Pa. All subsequent centrifugation steps were performed at 10,000 × g and 4°C. Centrifugation for 45 min removed cell debris and unbroken cells. The clear lysate was then incubated with 2 ml Ni-nitrilotriacetic acid-agarose for 16 h. This suspension was applied to a 10-ml column and washed five times with 10 ml washing solution (50 mM NaH2PO4, pH 8; 300 mM NaCl; 20 mM imidazole). Elution of protein bound to Ni-nitrilotriacetic acid was performed by adding five 1-ml portions of elution buffer (50 mM NaH2PO4, pH 8; 300 mM NaCl; 250 mM imidazole). Aliquots of the fractions were analyzed by SDS-polyacrylamide gel electrophoresis and staining with Coomassie brilliant blue R-250. The apparently homogeneous fractions were tested directly for flavokinase activity and stored at −20°C. The enzyme was stable for at least 2 weeks under these conditions.
Protein concentration determination and sequence analysis.
The protein concentration was estimated by the method of Bradford (7). N-terminal sequencing of RibC was done by the method of Hunkapiller et al. (19).
Flavokinase assay and HPLC analysis of flavins.
Flavokinase activity was measured in a final volume of 1 ml of 100 mM potassium phosphate (pH 7.5) containing 50 μM riboflavin, 3 mM ATP, 15 mM MgCl2, and 10 mM Na2SO3. The mixture was preincubated at 37°C for 5 min, and the reaction was started by addition of the enzyme. After appropriate time intervals, an aliquot was removed and applied directly to a high-pressure liquid chromatography (HPLC) column (Nucleosil 10 C18; 4.6 by 250 mm; Macherey & Nagel). The following solvent system was used at a flow rate of 2.5 ml/min: 25% (vol/vol) methanol-100 mM formic acid-100 mM ammonium formate (pH 3.7). Detection of riboflavin, FMN, and FAD was carried out with a fluorescence detector (excitation, 470 nm; emission, 530 nm) (Waters Associates, Inc.). Flavokinase activity is expressed as nanomoles of FMN formed from riboflavin and ATP. For testing roseoflavin in the flavokinase/FAD synthetase assay, a modified procedure was used. Since roseoflavin is not fluorescent, roseoflavin, RoFMN, and RoFAD had to be detected photometrically at 470 nm in the described HPLC method. The flavokinase assay is not continuous. The reaction velocity v was determined separately for each substrate concentration by linear regression using multiple data points. Three (but not all) substrate concentrations were tested in triplicate, and the data were found to be highly reproducible. The kinetic constants Km and Vmax were evaluated with the Michaelis-Menten equation and Lineweaver-Burk plots using the Microsoft Excel program. The turnover numbers, kcat, were calculated with the subunit molecular masses of 36 kDa for B. subtilis RibC and of 35 kDa for S. davawensis RibC.
Integration of S. davawensis ribCopt in B. subtilis and subsequent deletion of the ribC gene from the chromosome of B. subtilis.
The ribC gene from B. subtilis is essential and cannot be deleted from the genome without compensation (25). Thus, prior to deletion of this gene, ribCopt from S. davawensis was introduced into the genome of B. subtilis (at the sacB locus) under control of the constitutive PvegI promoter present in the integration/expression vector pXI12 (18). Subsequently, endogenous B. subtilis ribC could be inactivated by insertional mutagenesis using a neomycin cassette. For gene integration of ribCopt into the sacB locus of B. subtilis, pSG10 was constructed. The gene ribCopt was amplified by PCR using the modifying oligonucleotides NdeISG10 (5′-CATATGCAACGTTGGCGTGGCC-3′) and XmaISG11 (5′-TTCCCGGGTCAGCGATCCCCAGCTTC-3′) and pSG01 (containing ribCopt) as a template. The oligonucleotides contained heterologous NdeI/XmaI restriction sites to allow ligation of the digested PCR fragment to the expression/integration vector pXI12 (erythromycin resistant), producing pSG10. B. subtilis 168 (a wild-type strain) was transformed with PstI-linearized pSG10 (5 μg), and erythromycin-resistant transformant B. subtilis strains were selected. In the resulting B. subtilis strain (B. subtilis 168 PvegI ribCopt ermAM@ sacB) having the additional ribCopt gene, endogenous ribC was replaced by a neomycin cassette. The neomycin cassette was introduced by homologous recombination as well. The plasmid pSG60 was constructed for this purpose. Adjacent regions homologous to ribC within the ribC locus of the B. subtilis chromosome were truB (5′) and rpsO (3′). The gene fragment truB was amplified by PCR from B. subtilis 168 chromosomal DNA using the modifying oligonucleotides XhoISG20 (5′-CCGCTCGAGCTCAAAAGAAGGAGTG-3′) and EcoRI/SpeISG21 (5′-ACTAGTACGACGCGGAATTCCACAGAACGGTCACC-3′). The gene rpsO was amplified by PCR from B. subtilis 168 chromosomal DNA using the modifying oligonucleotides BcuISG22 (5′-AATCTGCAGCAGGAAGCCATCCGTTATTTCAG-3′) and PstISG23 (5′-AATACTAGTTTCTTGTCCCATACAATTACGAACTCCTC-3′). The neomycin cassette was amplified by PCR using pBEST501 (21) as a template and the modifying oligonucleotides EcoRISG24 (5′-CGGAATTCGCTTGGGCAGCAGGTCG-3′) and PstI/SpeISG25 (5′-ACTAGTACGACGAAACTGCAGTTCAAAATGGTATGCG-3′). The plasmid pSG60 was assembled by ligation of endonuclease-treated PCR fragments of truB, neo, and rpsO (in that order) into XhoI/SpeI-digested pBluescript II SK−. B. subtilis 168 ΩribCopt was transformed with PstI-linearized pSG60 (5 μg), and erythromycin/neomycin-resistant transformant B. subtilis strains (B. subtilis 168 ΔribCΩneo PvegI ribCopt ermAM@sacB) obtained by homologous double-crossover recombination into the B. subtilis ribC locus were selected.
Experiments with DAAO.
d-Amino acid oxidase (DAAO) (EC 1.4.3.3) from porcine kidney was purchased from Fluka (Germany). The enzyme contains noncovalently bound FAD. FAD was removed from this enzyme by dialysis (24 h) against 1 M KBr in 100 mM Na2HPO4 (pH 8.5). Subsequently, KBr was removed by dialysis (24 h) against 100 mM Na2HPO4 (pH 8.5). DAAO activity was measured in a final volume of 1 ml of 65 mM Na2HPO4 (pH 8.5) containing 4 mM phenylglycine, 40 μM FAD (or 40 μM RoFAD), and 25 μg DAAO. FAD was from Sigma-Aldrich or prepared from riboflavin using S. davawensis RibC. RoFAD was prepared from roseoflavin using S. davawensis RibC. The samples were incubated at 25°C, and absorbance at 252 nm was recorded. The concentration of the product benzoylformic acid was estimated photometrically using an absorption coefficient at 252 nm of 11,810 mM−1 cm−1. Activity is expressed as nanomoles of benzoylformic acid produced per minute per milligram of protein.
Nucleotide sequence accession number.
The sequence reported here has been deposited in the GenBank database under accession number EF397307.
RESULTS
Identification of the flavokinase/FAD synthetase gene (ribC) in S. davawensis.
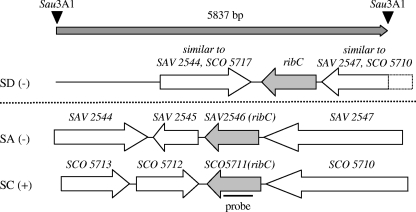
The open reading frame SCO5711, predicted to encode the bifunctional S. coelicolor flavokinase/FAD synthetase (RibC) (6), was used to produce a heterologous nucleic acid ribC probe. This probe from S. coelicolor was hybridized under stringent conditions to a DNA colony array representing the complete genome of S. davawensis in threefold coverage. Three independent colony doublets (out of 9,216) produced a strong hybridization signal. The plasmids of the corresponding E. coli strains were isolated and subjected to restriction analysis. From the restriction pattern it was concluded that the three plasmids contained identical genomic fragments. The insert of the largest plasmid (pSG06) (Table 1) was completely sequenced and analyzed by BLAST (1). Within the 5,837-bp fragment a putative ribC gene was detected, in addition to other putative genes (Fig. 2).
FIG. 2.
RibC loci in Streptomyces davawensis (SD), Streptomyces avermitilis (SA), and Streptomyces coelicolor (SC). Genes related to flavin biosynthesis are shown in gray. The gene ribC from S. coelicolor (SCO5711), coding for a putative bifunctional flavokinase/FAD synthetase, was used as a template to generate a heterologous nucleic acid probe which hybridized to a 5,837-bp Sau3AI subgenomic fragment of S. davawensis (arrow shaded in gray at the top). Upon BLAST analysis, open reading frames were identified and compared to the respective sequences of S. coelicolor and S. avermitilis (below the dotted line). The putative ribC gene products share an overall similarity of between 80% and 90% on the amino acid level. The other open reading frames are annotated as follows (6, 20): SAV2544, unknown; SAV2545, unknown; SAV2547, putative large Pro/Ala/Gly-rich protein; SCO5713/SCO5712, putative peptide transport ATP-binding proteins; SCO5710, putative large Pro/Ala/Gly-rich protein.
Overproduction and purification of recombinant RibC from S. davawensis and B. subtilis.
A sequence comparison between putative RibC from S. davawensis and bifunctional bacterial flavokinases/FAD synthetases with confirmed enzymatic activity revealed an identity of >37% on the amino acid sequence level. The newly identified open reading frame ribC from S. davawensis was introduced into E. coli using the expression vector pNCO113 (pSG02). The corresponding recombinant strain, however, did not overproduce RibC from S. davawensis. The nucleotide sequence of ribC from S. davawensis has a relatively high G+C content (72%), and the codon usage within this gene is significantly different from the preferred codon usage that was reported for E. coli (35). Therefore, an artificial ribC gene (ribCopt) with an optimized codon usage for E. coli was constructed. The artificial gene was proof sequenced, the obtained DNA sequence was translated, and, besides an N-terminal His6 tag, no amino acid alterations were detected compared to the translated wild-type ribC gene. The ribCopt gene was introduced into E. coli, and the corresponding recombinant strain overproduced a His-tagged protein with an approximate mass of 34 kDa, corresponding to RibC from S. davawensis. This protein was subsequently purified to apparent homogeneity (as judged by SDS-polyacrylamide gel electrophoresis and Coomassie blue staining). Edman degradation revealed a single sequence matching the tentative S. davawensis RibC N terminus, which was deduced from the DNA sequence data. Similarly, RibC from B. subtilis (25) was purified as a His-tagged recombinant enzyme from a cell extract of an overproducing E. coli strain.
Kinetic characterization of the flavokinase activity of S. davawensis RibC in comparison to B. subtilis RibC.
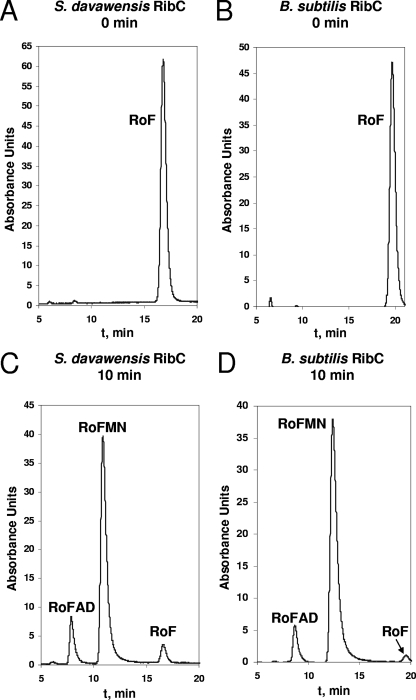
The pure RibC proteins of S. davawensis and B. subtilis were kinetically characterized using the substrates riboflavin and roseoflavin. Both enzymes produced the toxic FMN analog RoFMN and also the toxic FAD analog RoFAD (Fig. 3). In Table 2 the kinetic parameters for the flavokinase activity of the enzymes are summarized. For the S. davawensis enzyme, roseoflavin (kcat/Km = 1.7 10−2 μM−1 s−1) apparently is a somewhat better substrate (by a factor of 2.3) than riboflavin (kcat/Km = 7.5 10−3 μM−1 s−1). The B. subtilis enzyme accepts both substrates equally well (roseoflavin, kcat/Km = 1.3 10−2 μM−1 s−1; riboflavin, kcat/Km = 1.3 10−2 μM−1 s−1).
FIG. 3.
Flavokinase/FAD synthetase assays. Assay mixtures containing 50 μM roseoflavin, 3 mM ATP, 15 mM MgCl2, and 10 mM sodium sulfite (Na2SO3) were preincubated at 37°C for 5 min. Purified RibC from Streptomyces davawensis (A and C) or Bacillus subtilis (B and D) (3 μg of each) was added, and the mixtures were incubated for 0 (A and B) or 10 (C and D) min. An aliquot was removed, and the compounds were separated on an HPLC column (Nucleosil 10 C18; 4.6 by 250 mm; Macherey & Nagel). The following solvent system was used at a flow rate of 2.5 ml/min: 25% (vol/vol) methanol, 100 mM formic acid, and 100 mM ammonium formate (pH 3.7). Peak intensity is given in arbitrary absorbance units. The chromatograms show three resolved peaks of roseoflavin, RoFMN, and/or RoFAD. The retention times in panels A and C are shifted toward shorter times (compared to those in B and D) because of use of a new column for separation.
TABLE 2.
Kinetic constants for the flavokinase activities of RibC from Streptomyces davawensis and Bacillus subtilis
| RibC | Substrate | Km (μM) | Vmax (U mg−1)a | kcat (s−1) | kcat/Km (μM−1 s−1) |
|---|---|---|---|---|---|
| S. davawensis | Riboflavin | 40 | 500 | 0.3 | 7.5 × 10−3 |
| Roseoflavin | 30 | 900 | 0.5 | 1.7 × 10−2 | |
| B. subtilis | Riboflavin | 55 | 1,200 | 0.7 | 1.3 × 10−2 |
| Roseoflavin | 30 | 600 | 0.4 | 1.3 × 10−2 |
Specific activities (U mg−1) are in nmol min−1 mg−1 protein.
RoFAD is not an active cofactor with DAAO.
The FAD-containing pig kidney DAAO (EC 1.4.3.3) catalyzes the oxidative deamination of d-amino acids, giving the corresponding α-ketoacids, ammonia, and hydrogen peroxide. The ease of removal of FAD from DAAO and the stability of the resulting apoenzyme have encouraged many researchers to use this enzyme to study flavin-protein interactions (13, 14). Extensive dialysis of a DAAO solution removed FAD and produced inactive DAAO. RibC from S. davawensis was used to synthesize FAD and also RoFAD. Subsequently, these cofactors were tested for reactivation of DAAO (Table 3). It was possible to reactivate DAAO using FAD. Incubation of DAAO with RoFAD, however, did not produce an active enzyme, indicating that roseoflavin-derived cofactors are not functional.
TABLE 3.
Reactivation experiments with DAAO (EC 1.4.3.3)
| Cofactora | DAAO activityb |
|---|---|
| FAD (Sigma-Aldrich) | 4.62 |
| FAD (S. davawensis RibC) | 4.55 |
| RoFAD (S. davawensis RibC) | 0 |
| FAD, without enzyme | 0 |
| None, without enzyme | 0 |
| Nondialyzed enzyme | 45.5 |
FAD and RoFAD were used at 40 μM.
Activity is expressed as nanomoles of benzoylformic acid produced per minute per milligram of protein.
The ribC gene from S. davawensis is able to complement a ribC-defective B. subtilis strain and does not confer roseoflavin resistance to B. subtilis.
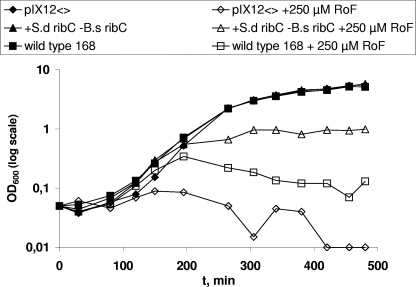
The successful deletion of B. subtilis ribC was confirmed by Southern blotting experiments using a ribC-specific probe. The presence of S. davawensis ribCopt under control of the constitutive PvegI promoter was confirmed in a similar experiment using a ribCopt-specific probe. The strain B. subtilis 168 ΔribCΩneo PvegI ribCopt ermAM@sacB was able to grow in a minimal medium with a growth rate comparable to that of the wild-type strain, which showed that the S. davawensis ribC gene is capable of complementing the flavokinase/FAD synthetase function of the B. subtilis ribC gene (Fig. 4). In an additional experiment, the B. subtilis ribC deletion strain was grown in a minimal medium containing roseoflavin (Fig. 4). The strain did not grow in this medium, indicating that the presence of S. davawensis RibC was not able to reduce the antibiotic effect of roseoflavin. Similar results were obtained in an agar diffusion assay (data not shown).
FIG. 4.
Growth of a flavokinase/FAD synthetase ribC-defective Bacillus subtilis strain containing Streptomyces davawensis ribCopt at sacB under control of the constitutive PvegI promoter (+S.d ribC −B.s ribC) (B. subtilis 168 ΔribCΩneo PvegI ribCopt ermAM@sacB). This strain grows at a rate similar to that of the wild-type B. subtilis 168 strain. It does not grow in the presence of 250 μM roseoflavin (+S.d ribC −B.s ribC + 250 μM RoF), indicating that ribC does not confer roseoflavin resistance. The wild-type B. subtilis strain 168 grows in the absence (wild-type 168) but not in the presence (wild-type 168 + 250 μM RoF) of roseoflavin. The control strains (pXI12 and pXI12 + 250 μM RoF) contain the empty integration/expression plasmid pXI12 at sacB.
DISCUSSION
The ribC gene from S. davawensis encodes a bifunctional flavokinase/FAD synthetase. This was shown in vitro by analyzing the enzymatic activity of the purified gene product RibC and also in vivo by complementation experiments using a ribC-defective B. subtilis strain. Flavokinases/FAD synthetases have been purified from several sources (2, 26, 30, 37). In Corynebacterium ammoniagenes (RibF), B. subtilis (RibC), and E. coli (RibF), the flavokinase/FAD synthetase activities are provided by a single, bifunctional polypeptide chain (25, 26; K. Kitatsuji, S. Ishino, S. Teshiba, and M. Arimoto, Process for producing flavine mononucleotides. European patent application 0 542 240 A2, 1993). In contrast, both enzymatic activities were purified separately from eukaryotic organisms (2, 34). B. subtilis is the only known organism containing a second cryptic monofunctional flavokinase (RibR) (17, 38, 39). There is evidence that RibR may be involved in regulation of the rib biosynthetic operon (17).
Our data show that RibC from S. davawensis and RibC from B. subtilis both accept the antibiotic roseoflavin as a substrate and produce the toxic FMN/FAD derivatives RoFMN and RoFAD (Fig. 3). Riboflavin analogs with electron-donating substituents at position 8 (e.g., roseoflavin) were described earlier to be inert to several biological reductants and consequently were thought not to function as redox-active components (roseoflavin E0′ = −222 mV; riboflavin E0′ = −208 mV). It was discussed that these analogs may be good steric replacements for riboflavin but not catalytic substitutes (42). Our experiments with RoFAD-reconstituted porcine DAAO apoenzyme (10) showed that RoFAD indeed is not functional. In the case of RoFAD-DAAO, we speculate that it is not the altered redox potential of RoFAD that is primarily responsible for inactive DAAO but rather the fact that RoFAD is not properly positioned in the enzyme. The exact position of FAD in the active site of DAAO is known (29). In the case of FAD-DAAO (Fig. 1), the flavin 8α-methyl group is in close contact to the atoms of various amino acids of the polypeptide chain. It seems that the dimethyl-amino group of RoFAD (Fig. 1) cannot be easily accommodated unless conformational changes are assumed. These changes probably alter the geometry of the whole substrate binding site and consequently may reduce enzyme activity. The addition of roseoflavin does not inactivate FAD-containing DAAO (data not shown). Thus, at least to some extent, unspecific flavokinases/FAD synthetases are responsible for the antibiotic action of flavin analogs in vivo.
The mechanism of resistance to roseoflavin and riboflavin analogs in general is less clear. Roseoflavin was reported to exhibit antibiotic activity against gram-positive bacteria only (32). E. coli and other members of the Enterobacteriaceae were not affected by this compound. However, these cells are devoid of a transport system for riboflavin, and their resistance is due to a limited uptake of roseoflavin (4, 16). S. davawensis is able to grow in the presence of 250 μM roseoflavin, a concentration which is toxic to many gram-positive organisms tested and also to S. coelicolor and Streptomyces avermitilis (data not shown). Therefore, the presence of a highly riboflavin-specific S. davawensis RibC not producing toxic RoFMN and RoFAD was anticipated. The kinetic parameters, however, now show that S. davawensis RibC is even less discriminative in respect to roseoflavin phosphorylation than RibC of the roseoflavin-sensitive bacterium B. subtilis. Also, the functional introduction of S. davawensis RibC did not confer roseoflavin resistance to a ribC-defective B. subtilis strain. From these results, it is concluded that RibC from S. davawensis is not involved in roseoflavin resistance. Interestingly, it was reported that the macrolide resistance gene mreA of Streptococcus agalactiae encodes a monofunctional flavokinase (8, 9). The mechanism by which the enzyme conferred macrolide resistance, however, remained unclear.
In B. subtilis some roseoflavin-resistant strains were found to overproduce riboflavin (28). In these strains, accumulating riboflavin dilutes roseoflavin and prevents the formation of toxic levels of RoFMN and RoFAD. However, when grown under laboratory conditions, S. davawensis clearly was found not to be a natural riboflavin overproducer (data not shown), such as Ashbya gossypii (11).
We suggest that most FMN/FAD-dependent flavoproteins are not active with RoFMN or RoFAD. For E. coli, the BRENDA database lists 11 different FMN-dependent proteins and 43 FAD-dependent proteins (5). S. davawensis certainly contains a similar (high) number of flavoproteins. It is not likely that in S. davawensis this many proteins all evolved not to bind RoFMN or RoFAD.
Another flavokinase/FAD synthetase which does not show sequence similarity to known enzymes and thus escaped our screening approach may be present in S. davawensis. Alternatively, a membrane-bound roseoflavin-exporting protein may account for the roseoflavin resistance of S. davawensis. Since the flavokinase/FAD synthetase activity of S. davawensis RibC accepts both flavins as a substrate, the transporter would have to eliminate roseoflavin efficiently and specifically from the cytoplasm.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (DFG) (Research Training Group 886).
We thank A. Mattevi for helpful discussions.
This work is dedicated to Antonie and Erich Mack.
Footnotes
Published ahead of print on 21 December 2007.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 253389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bacher, A. 1991. Riboflavin kinase and FAD synthetase, p. 349-370. In F. Muller (ed.), Chemistry and biochemistry of flavoenzymes, vol. 1. CRC Press, Boca Raton, FL. [Google Scholar]
- 3.Bacher, A., S. Eberhardt, W. Eisenreich, M. Fischer, S. Herz, B. Illarionov, K. Kis, and G. Richter. 2001. Biosynthesis of riboflavin. Vitam. Horm. 611-49. [DOI] [PubMed] [Google Scholar]
- 4.Bandrin, S. V., M. Beburov, P. M. Rabinovich, and A. I. Stepanov. 1979. Riboflavin auxotrophs of Escherichia coli. Genetika 152063-2065. [PubMed] [Google Scholar]
- 5.Barthelmes, J., C. Ebeling, A. Chang, I. Schomburg, and D. Schomburg. 2007. BRENDA, AMENDA and FRENDA: the enzyme information system in 2007. Nucleic Acids Res. 35511-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bentley, S. D., K. F. Chater, A. M. Cerdeno-Tarraga, G. L. Challis, N. R. Thomson, K. D. James, D. E. Harris, M. A. Quail, H. Kieser, D. Harper, A. Bateman, S. Brown, G. Chandra, C. W. Chen, M. Collins, A. Cronin, A. Fraser, A. Goble, J. Hidalgo, T. Hornsby, S. Howarth, C. H. Huang, T. Kieser, L. Larke, L. Murphy, K. Oliver, S. O'Neil, E. Rabbinowitsch, M. A. Rajandream, K. Rutherford, S. Rutter, K. Seeger, D. Saunders, S. Sharp, R. Squares, S. Squares, K. Taylor, T. Warren, A. Wietzorrek, J. Woodward, B. G. Barrell, J. Parkhill, and D. A. Hopwood. 2002. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417141-147. [DOI] [PubMed] [Google Scholar]
- 7.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72248-254. [DOI] [PubMed] [Google Scholar]
- 8.Clancy, J., F. Dib-Hajj, J. W. Petitpas, and W. Yuan. 1997. Cloning and characterization of a novel macrolide efflux gene, mreA, from Streptococcus agalactiae. Antimicrob. Agents Chemother. 412719-2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarebout, G., C. Villers, and R. Leclercq. 2001. Macrolide resistance gene mreA of Streptococcus agalactiae encodes a flavokinase. Antimicrob. Agents Chemother. 452280-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curti, B., S. Ronchi, U. Branzoli, G. Ferri, and C. H. Williams, Jr. 1973. Improved purification, amino acid analysis and molecular weight of homogenous d-amino acid oxidase from pig kidney. Biochim. Biophys. Acta 327266-273. [DOI] [PubMed] [Google Scholar]
- 11.Demain, A. L. 1972. Riboflavin oversynthesis. Annu. Rev. Microbiol. 26369-388. [DOI] [PubMed] [Google Scholar]
- 12.Fischer, M., and A. Bacher. 2005. Biosynthesis of flavocoenzymes. Nat. Prod. Rep. 22324-350. [DOI] [PubMed] [Google Scholar]
- 13.Fonda, M. L., and B. M. Anderson. 1968. d-Amino acid oxidase. 3. Studies of flavin adenine dinucleotide binding. J. Biol. Chem. 2435635-5643. [PubMed] [Google Scholar]
- 14.Fonda, M. L., and B. M. Anderson. 1967. d-Amino acid oxidase. I. Spectrophotometric studies. J. Biol. Chem. 2423957-3962. [PubMed] [Google Scholar]
- 15.Fraaije, M. W., and A. Mattevi. 2000. Flavoenzymes: diverse catalysts with recurrent features. Trends Biochem. Sci. 25126-132. [DOI] [PubMed] [Google Scholar]
- 16.Grill, S., H. Yamaguchi, H. Wagner, L. Zwahlen, U. Kusch, and M. Mack. 2007. Identification and characterization of two Streptomyces davawensis riboflavin biosynthesis gene clusters. Arch. Microbiol. 188377-387. [DOI] [PubMed] [Google Scholar]
- 17.Higashitsuji, Y., A. Angerer, S. Berghaus, B. Hobl, and M. Mack. 2007. RibR, a possible regulator of the Bacillus subtilis riboflavin biosynthetic operon, in vivo interacts with the 5′-untranslated leader of rib mRNA. FEMS Microbiol. Lett. 27448-54. [DOI] [PubMed] [Google Scholar]
- 18.Hümbelin, M., V. Griesser, T. Keller, W. Schurter, M. Haiker, H. P. Hohmann, H. Ritz, G. Richter, A. Bacher, and A. P. G. M. van Loon. 1999. GTP cyclohydrolase II and 3,4-dihydroxy-2-butanone 4-phosphate synthase are rate-limiting enzymes in riboflavin synthesis of an industrial Bacillus subtilis strain used for riboflavin production. J. Ind. Microbiol. Biotechnol. 221-7. [Google Scholar]
- 19.Hunkapiller, M. W., R. M. Hewick, W. J. Dreyer, and L. E. Hood. 1983. High-sensitivity sequencing with a gas-phase sequenator. Methods Enzymol. 91399-413. [DOI] [PubMed] [Google Scholar]
- 20.Ikeda, H., J. Ishikawa, A. Hanamoto, M. Shinose, H. Kikuchi, T. Shiba, Y. Sakaki, M. Hattori, and S. Omura. 2003. Complete genome sequence and comparative analysis of the industrial microorganism Streptomyces avermitilis. Nat. Biotechnol. 21526-531. [DOI] [PubMed] [Google Scholar]
- 21.Itaya, M., K. Kondo, and T. Tanaka. 1989. A neomycin resistance gene cassette selectable in a single copy state in the Bacillus subtilis chromosome. Nucleic Acids Res. 174410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juri, N., Y. Kubo, S. Kasai, S. Otani, M. Kusunose, and K. Matsui. 1987. Formation of roseoflavin from 8-amino- and 8-methylamino-8-demethyl-d-riboflavin. J. Biochem. (Tokyo) 101705-711. [DOI] [PubMed] [Google Scholar]
- 23.Kieser, T., M. J. Bibb, M. J. Buttner, K. F. Chater, and D. A. Hopwood. 2000. Practical Streptomyces genetics. The John Innes Foundation, Norwich, United Kingdom.
- 24.Mack, M., and S. Grill. 2006. Riboflavin analogs and inhibitors of riboflavin biosynthesis. Appl. Microbiol. Biotechnol. 71265-275. [DOI] [PubMed] [Google Scholar]
- 25.Mack, M., A. P. van Loon, and H. P. Hohmann. 1998. Regulation of riboflavin biosynthesis in Bacillus subtilis is affected by the activity of the flavokinase/flavin adenine dinucleotide synthetase encoded by ribC. J. Bacteriol. 180950-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manstein, D. J., and E. F. Pai. 1986. Purification and characterization of FAD synthetase from Brevibacterium ammoniagenes. J. Biol. Chem. 26116169-16173. [PubMed] [Google Scholar]
- 27.Matsui, K., N. Juri, Y. Kubo, and S. Kasai. 1979. Formation of roseoflavin from guanine through riboflavin. J. Biochem. (Tokyo) 86167-175. [PubMed] [Google Scholar]
- 28.Matsui, K., H. Wang, T. Hirota, H. Matsukawa, S. Kasai, K. Shinagawa, and S. Otani. 1982. Riboflavin production by roseoflavin-resistant strains of some bacteria. Agric. Biol. Chem. 462003-2008. [Google Scholar]
- 29.Mattevi, A., M. A. Vanoni, F. Todone, M. Rizzi, A. Teplyakov, A. Coda, M. Bolognesi, and B. Curti. 1996. Crystal structure of d-amino acid oxidase: a case of active site mirror-image convergent evolution with flavocytochrome b2. Proc. Natl. Acad. Sci. USA 937496-7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merrill, A. H., Jr., and D. B. McCormick. 1979. Preparation and properties of immobilized flavokinase. Biotechnol. Bioeng. 211629-1638. [DOI] [PubMed] [Google Scholar]
- 31.Otani, S., Y. Note, Y. Nishina, and Y. Matsumura. 1980. Interactions between roseoflavin and flavoproteins, p. 593-597. In K. Yagi and T. Yamano (ed.), Flavins and flavoproteins. Japan Scientific Societies Press, Tokyo, Japan.
- 32.Otani, S., M. Takatsu, M. Nakano, S. Kasai, and R. Miura. 1974. Roseoflavin, a new antimicrobial pigment from Streptomyces. J. Antibiot. (Tokyo) 2786-87. [PubMed] [Google Scholar]
- 33.Sambrook, J., E. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 34.Santos, M. A., A. Jimenez, and J. L. Revuelta. 2000. Molecular characterization of FMN1, the structural gene for the monofunctional flavokinase of Saccharomyces cerevisiae. J. Biol. Chem. 27528618-28624. [DOI] [PubMed] [Google Scholar]
- 35.Sharp, P. M., E. Cowe, D. G. Higgins, D. C. Shields, K. H. Wolfe, and F. Wright. 1988. Codon usage patterns in Escherichia coli, Bacillus subtilis, Saccharomyces cerevisiae, Schizosaccharomyces pombe, Drosophila melanogaster and Homo sapiens; a review of the considerable within-species diversity. Nucleic Acids Res. 168207-8211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shinkai, S., K. Kameoka, N. Honda, K. Ueda, O. Manabe, and J. Lindsey. 1986. Spectral and reactivity studies of roseoflavin analogs: correlation between reactivity and spectral parameters. Bioorg. Chem. 14119-133. [Google Scholar]
- 37.Sobhanaditya, J., and N. A. Rao. 1981. Plant flavokinase. Affinity-chromatographic procedure for the purification of the enzyme from mung-bean (Phaseolus aureus) seeds and conformational changes on its interaction with orthophosphate. Biochem. J. 197227-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Solovieva, I. M., R. A. Kreneva, L. Errais Lopes, and D. A. Perumov. 2005. The riboflavin kinase encoding gene ribR of Bacillus subtilis is a part of a 10 kb operon, which is negatively regulated by the yrzC gene product. FEMS Microbiol. Lett. 24351-58. [DOI] [PubMed] [Google Scholar]
- 39.Solovieva, I. M., R. A. Kreneva, D. J. Leak, and D. A. Perumov. 1999. The ribR gene encodes a monofunctional riboflavin kinase which is involved in regulation of the Bacillus subtilis riboflavin operon. Microbiology 14567-73. [DOI] [PubMed] [Google Scholar]
- 40.Stüber, D., H. Matile, and G. Garotta. 1990. System for high-level production in Escherichia coli and rapid purification of recombinant proteins: application to epitope mapping, preparation of antibodies, and structure-function analysis. Immunol. Methods 4121-152. [Google Scholar]
- 41.Vente, A., B. Korn, G. Zehetner, A. Poustka, and H. Lehrach. 1999. Distribution and early development of microarray technology in Europe. Nat. Genet. 2222. [DOI] [PubMed] [Google Scholar]
- 42.Walsh, C., J. Fisher, R. Spencer, D. W. Graham, W. T. Ashton, J. E. Brown, R. D. Brown, and E. F. Rogers. 1978. Chemical and enzymatic properties of riboflavin analogues. Biochemistry 171942-1951. [DOI] [PubMed] [Google Scholar]