Abstract
VirB5 is a minor component of the extracellular T pilus determined by the Agrobacterium tumefaciens type IV secretion system. To identify proteins that interact with VirB5 during the pilus assembly process, we purified VirB5 as a recombinant fusion protein and, by using a gel overlay assay, we detected a 26-kDa interacting protein in Agrobacterium cell lysates. The VirB5-binding protein was purified from A. tumefaciens and identified as the cytokinin biosynthetic enzyme Tzs. The VirB5-Tzs interaction was confirmed using pulldown assays with purified proteins and the yeast two-hybrid system. An analysis of the subcellular localization in A. tumefaciens showed that Tzs was present in the soluble as well as the membrane fraction. Tzs was extracted from the membranes with the mild detergent dodecyl-β-d-maltoside in complexes of different molecular masses, and this association was strongly reduced in the absence of VirB5. Using immunoelectron microscopy, we also detected Tzs on the Agrobacterium cell surface. A functional type IV secretion system was required for efficient translocation to the surface, but Tzs was not secreted into the cell supernatant. The fact that Tzs localizes on the cell surface suggests that it may contribute to the interaction of Agrobacterium with plants.
Agrobacterium tumefaciens is a gram-negative plant pathogen that incites crown gall tumors after infecting plant wounds (34, 54, 55). Tumor formation is a consequence of the transfer of a piece of single-stranded DNA, the T-DNA (transferred DNA), and of its integration into the plant genome. The T-DNA encodes proteins that direct the synthesis of plant hormones, such as cytokinin (Tmr) and indole acetic acid (Tms), and the subsequent deregulation of the plant phytohormone balance leads to tumor formation (1, 2). The Tmr protein exerts its function after the uptake into the chloroplasts, where it diverts an intermediate of the methyl-erythritol phosphate (MEP) pathway of isoprenoid biosynthesis for the production of hydroxylated trans-zeatin ribotides (40). Cytokinin biosynthesis in plants predominantly uses isoprenoid metabolites from the MEP pathway as well, but the primary products are isopentenyl ribotides and those are subsequently hydroxylated to trans-zeatin ribotides (36, 39). Alternatively, isopentenyl ribotides are synthesized from metabolites of the cytoplasmic mevalonate pathway or they are produced as products of the degradation of modified tRNAs resulting in cis-zeatin ribotides.
A subgroup of A. tumefaciens, the nopaline strains, encodes the Tmr homologous trans-zeatin synthesis (Tzs) protein on the tumor-inducing (Ti) plasmid (9, 20, 51). In contrast to the T-DNA gene tmr, the tzs gene is not translocated into plant cells and its gene product Tzs catalyzes the last step of the biosynthesis of the trans-zeatin ribotides inside A. tumefaciens (24, 27, 37). The biological significance of Tzs action is believed to be that the produced cytokinins stimulate plant cell growth in the wound callus. This stimulation may increase the efficacy of T-DNA transformation, and the fact that Tzs is coregulated with the T-DNA translocation machinery is in accord with this notion (24, 37). However, so far there is no direct evidence for this role of Tzs during the Agrobacterium-plant interaction. As Tzs is not produced in all agrobacteria, it is not considered to be essential for virulence and it may be a host range factor that contributes to the infection of certain plants. In addition to the proteins that impact plant hormone homeostasis, the T-DNA encodes proteins that mediate the production of opines, a special family of conjugates between organic acids and amino acids (50, 54). These compounds serve as nutrients for A. tumefaciens, which, unlike most other bacteria, has the ability to metabolize them. The unique strategy of A. tumefaciens to exploit the resources of plants was named genetic colonization, and it relies on the ability to transfer genes from the bacteria into the host cell (45).
A type IV secretion system (T4SS) mediates the translocation of the single-stranded T-DNA covalently linked to the VirD2 protein into plant cells (6, 14, 34). The T4SS required for this translocation process consists of 12 components, the 11 VirB proteins (VirB1-VirB11) and VirD4. Three of the components of the T4SS, VirB4, VirB11, and VirD4, contain Walker nucleotide binding and hydrolysis motifs; they interact and energize T4SS functions (5). VirB1, VirB3, VirB6, VirB7, VirB8, VirB9, and VirB10 assemble into a complex that spans the inner and the outer membrane and may form the substrate translocation channel (13, 25, 28, 49). The proteins VirB2 and VirB5 are components of the T4SS-determined T pilus, an extracellular structure that is believed to initiate cell-cell contact with plant cells prior to the initiation of T-complex transfer (16). VirB2 is the major T-pilus component that forms the main body of this extracellular structure (31). A yeast two-hybrid screen identified interaction partners in Arabidopsis thaliana, suggesting that VirB2 directly contacts the host cell during the substrate translocation process (23). VirB5 and its homologs TraC and TrbF were identified as minor components of T4SS-determined surface structures (42-44). VirB5 localizes at the T-pilus tip, and C-terminal variants were differentially affected in DNA transfer to different hosts, suggesting that VirB5 may also be involved in host-cell contact (3).
Details on the mechanism of T-pilus assembly are beginning to emerge, and based on work with purified components and analyses of membrane-bound T4SS complexes, a VirB4-VirB8-VirB2-VirB5 pilus assembly sequence was proposed (53). As an independent approach to gain insights into the mechanism of T-pilus incorporation of VirB5, we pursued a gel overlay approach to isolate interaction partners from A. tumefaciens. We detected a 26-kDa VirB5-binding protein from A. tumefaciens, and this protein was subsequently purified and identified as the trans-zeatin biosynthetic protein Tzs. The VirB5-Tzs interaction was confirmed, and an analysis of its subcellular localization showed that Tzs associates with discrete membrane protein complexes and that it is also exposed at the cell surface. A functional T4SS was required for efficient incorporation of Tzs into membrane complexes and for efficient translocation to the cell surface, suggesting that the secretion system and VirB5 may enable these processes by directly binding to Tzs.
MATERIALS AND METHODS
Cultivation of microorganisms.
Cultures of Escherichia coli for cloning and protein overproduction were grown in LB medium (1% tryptone, 0.5% yeast extract, 0.5% NaCl) or in LBON medium (LB medium without NaCl) following standard procedures (12, 32). A. tumefaciens virulence genes were induced with acetosyringone (AS) in liquid or on solid AB minimal medium (1% glucose, 0.39% morpholineethanesulfonic acid [MES], 1 mM KNa-phosphate, 1× AB salts [20 g NH4Cl, 6 g MgSO4·7H2O, 3 g KCl, 0.2 g CaCl2, 0.05 g FeSO4·7H2O per liter, pH 5.5], pH 5.5, 20× AB salts) as described previously (53). Yeast strains (Saccharomyces cerevisiae) for two-hybrid analysis were propagated in YEPD medium (2% tryptone, 1% yeast extract, 2% glucose, pH 7) for routine culture or in SD medium (6.7 g yeast nitrogen base without amino acid, 20 g glucose, and 0.87 g dropout mix [0.8 g adenine, 0.8 g Arg, 4 g Asp, 0.8 g His, 2.4 g Leu, 1.2 g Lys, 0.8 g Met, 2 g Phe, 8 g Thr, 0.8 g Trp, 1.2 g Tyr, 0.8 g uracil per liter] per liter, pH 7) for the analysis of interactions as described previously (15).
Plasmid and strain constructions.
Standard molecular biology procedures were followed for the construction of strains (Table 1) and plasmids (Table 2) (32), and the oligonucleotides used are listed in Table 3. All PCR-amplified gene sequences were verified after cloning by DNA sequencing.
TABLE 1.
Bacteria and yeast strains used in this study
| Strain | Genotype and characteristics | Source/reference |
|---|---|---|
| C58 | A. tumefaciens A136 pTiC58, virulent nopaline wild-type strain | 46 |
| CB1002 | A. tumefaciens A136 pTiC58ΔvirB2 | This work |
| CB1005 | A. tumefaciens A136 pTiC58ΔvirB5 | 43 |
| CB1008 | A. tumefaciens A136 pTiC58ΔvirB8 | This work |
| A348 | A. tumefaciens A136 pTiA6NC, virulent octopine wild-type strain | 19 |
| Ach5 | A. tumefaciens, Achillea millefolium isolate, octopine-type Ti-plasmid | 26 |
| Chry 5 | A. tumefaciens, Chrysanthemum morifolium isolate, succinamopine-type Ti-plasmid | 26 |
| A208 | A. tumefaciens A136 pTiT37, nopaline-type Ti-plasmid | 26 |
| JM109 | E. coli, [F′ traD36 proAB+lacIqlacZΔM15] recA1 endA1 gyrA96 thi-1 hsdR17 relA1 supE44 Δ(lac-proAB) λ− | 52 |
| GJ1158 | E. coli, proU promoter-controlled chromosomal RNA polymerase gene | 12 |
| Saccharomyces cerevisiae Y153 | MATaleu2-3,112 ura3-52 trp1-901 his3-Δ200 ade2-101 LYS2::GAL-HIS3 gal4Δgal80ΔURA3::GAL-lacZ | 15 |
TABLE 2.
Plasmids used in this study
| Plasmid | Genotype and characteristics | Source/reference |
|---|---|---|
| pPZP300 | Strr Spcr; carries extra copies of the gene encoding the transcription factor VirG for increased virulence gene induction | 28 |
| pTrcB2 | Strr Spcr; pTrc200 derivative for lacIq/trc promoter-controlled expression of virB2 | 42 |
| pTrcB5 | Strr Spcr; pTrc200 derivative for lacIq/trc promoter-controlled expression of virB5 | 43 |
| pK*mobsacB | KanrsacB; mobilizable vector for the construction of in-frame deletion variants | 41 |
| pT7-H6TrxFus | Carbr; T7 promoter-controlled expression of hexahistidyl-thioredoxin (TrxA) fusion proteins | 29 |
| pT7-H6TrxVirB5 | Carbr; for expression of hexahistidyl-thioredoxin-VirB5 fusion protein | This work |
| pT7-StrepII | Carbr; T7 promoter-controlled expression of StrepII fusion proteins | 53 |
| pT7-7StrepIIVirB5 | Carbr; for expression of StrepII-VirB5 fusion protein | This work |
| pT7-7tzs | Carbr; T7 promoter-controlled expression of Tzs | 27 |
| pAS2 | Carbr; for expression of fusions with the GAL4 DNA binding domain; selection in yeast in the absence of Trp | 15 |
| pACTII | Carbr; for expression of fusions with the GAL4 activation domain; selection in yeast in the absence of Leu | 15 |
| pAS2-VirB5 | Carbr; virB5 in pAS2 | This work |
| pAS2-VirE2 | Carbr; virE2 in pAS2 | 8 |
| pAS2-Tzs | Carbr; tzs in pAS2 | This work |
| pACTII-VirB5 | Carbr; virB5 in pACTII | This work |
| pACTII-VirE2 | Carbr; virE2 in pACTII | 8 |
| pACTII-Tzs | Carbr; tzs in pACTII | This work |
TABLE 3.
Oligonucleotides used in this study
| Application | Name | Sequence and restriction sitea |
|---|---|---|
| Construction of pT7-7 StrepIIVirB5 | VirB5-5′ | 5′-CAGGGTACCCAGTTCGTTGTCAGCGATCCGGCG-3′ |
| and pT7-7H6TrxA-VirB5 | VirB5-3′ | 5′-GAGCTGCAGTCAGGGGACGGCCCCAAAGATG-3′ |
| Construction of pAS2-Tzs and | AS2-Tzs-5′ | 5′-GGAGGCTCATGATACTCCATCTCATCTACGGACC-3′ |
| pACTII-Tzs | AS2-Tzs-3′ | 5′-GACGGGGATCCTCACCGAATTCGCGTCAGCGTG-3′ |
| Cloning of virB2 | B2-5 | 5′-CCACACGAATTCCAAGTCGTGATGGACCGTCTCGA-3′ |
| B2-3 | 5′-CCACACGAATTCGACGGCAACGTGCATTGCGCATTT-3′ | |
| Deletion of virB2 | ΔB2-5 | 5′-AGGAGGTCCGCAATAATGAATGATCGTCTGGAAGCAACCCTT-3′ |
| ΔB2-3 | 5′-TTATTGCGGACCTCCTTGATTTAAGTCGAACAAGAGTTGATCGTC-3′ | |
| Cloning of virB8 | B8-5 | 5′-CGCAGTCTAGAGCAAAGTGGATCGGGCAACTTAT-3′ |
| B8-3 | 5′-CGCAGTCTAGACCTCTGCTCTCTGTTGATATTGCGCTT-3′ | |
| Deletion of virB8 | ΔB8-5 | 5′-CCGTGCTCGAGTTATTCAGACCCCTTCATGGCGACCACCT-3′ |
| ΔB8-3 | 5′-CCGTGCTCGAGATGACGAAAAAAGCATTTCTCA-3′ |
Restriction enzyme cleavage sites are underlined.
For the overexpression of fusions of VirB5 without the N-terminal signal peptide, the gene was PCR amplified with the oligonucleotides VirB5-5′ and VirB5-3′, followed by cleavage with Acc65I and PstI and ligation into similarly cut pT7-H6TrxFus and pT7-7StrepII. To analyze the interactions of Tzs with the yeast two-hybrid system, the gene was PCR amplified with the oligonucleotides AS2-Tzs-5′ and AS2-Tzs-3′ and cleaved with BsphI and BamHI, followed by ligation into NcoI and BamHI-cleaved pAS2 and pACTII.
Strains CB1002 and CB1008, carrying in-frame deletions of virB2 and virB8, respectively, on the Ti plasmid of strain C58, were constructed essentially as described previously (10, 43) using the oligonucleotides shown in Table 3. The virB2 gene with 500 bp of upstream and downstream sequence was PCR amplified from intact A. tumefaciens cells by using oligonucleotides B2-5 and B2-3, and the fragment was cloned into the precleaved plasmid pTZ57R/T (InsTAclone kit; Fermentas), followed by deletion of the gene by inverse PCR with the oligonucleotides ΔB2-5 and ΔB2-3. The 1-kb fragment with the flanking regions was then excised with EcoRI and cloned into vector pK*mobsacB (41), and the gene deletion was introduced into the chromosome by double recombination (10, 43). A similar procedure was followed for the construction of CB1008 using oligonucleotides B8-5 and B8-3 for PCR amplification of virB8 and of its flanking regions, followed by cloning and inverse PCR with the oligonucleotides ΔB8-5 and ΔB8-3. The fragment containing the flanking regions was then excised with XbaI and cloned into pK*mobsacB, and the gene deletion was introduced into the chromosome by double recombination as described above.
Overproduction and purification of VirB5 and of Tzs.
Soluble fusion proteins (hexahistidyl- and StrepII-tagged VirB5) were overproduced in the NaCl-inducible T7 promoter expression strain GJ1158 as described previously (53) after expression for 90 min at 37°C (His6TrxAVirB5) and for 48 h at 20°C (StrepIIVirB5). They were subsequently purified by StrepTactin-Sepharose or immobilized metal affinity chromatography (IMAC) and gel filtration chromatography as described previously (53). Native Tzs protein was overproduced and purified as described previously (27), and 500 μg was used for the generation of a specific antiserum after injection into rabbits (BioGenes, Germany).
Protein analytical methods, subcellular fractionation, and gel overlay assay.
Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (30), followed by Western blotting with specific antisera following standard procedures (21). Membrane protein complexes were isolated from A. tumefaciens by extraction with the mild detergent dodecyl-β-d-maltoside (DDM), followed by blue native electrophoresis as described previously (53). Proteins from other subcellular fractions were isolated after shearing and ultracentrifugation (T pili) and after precipitation of cell-free supernatants with acetone as described previously (7, 42).
A gel overlay assay based on the method described previously by Homann et al. (22) was applied for the detection of interaction partners of VirB5. Samples were separated by SDS-PAGE, followed by Western blotting onto polyvinylidene difluoride (PVDF) membranes and incubation for 12 h at 4°C in renaturation buffer (10 mM HEPES, 10 mM MgCl2, 50 mM NaCl, 0.1 mM EDTA, 1 mM dithiothreitol, 10% glycerol, pH 7.5) to remove the SDS and to enable refolding. The membranes were then washed three times with Tris-buffered saline containing Tween 20 (TBS-T) (20 mM Tris-HCl, 137 mM NaCl, 0.1% Tween 20, pH 8), and protein binding sites were blocked with 5% dry milk powder in TBS-T, followed by incubation with purified VirB5 fusion protein (1.5 μg/ml) in TBS-T with 5% dry milk powder for 5 h at 4°C. The membranes were then washed three times with TBS-T, and the bound VirB5 fusion protein was detected by Western blotting with a specific antiserum.
Interaction studies using affinity matrices (pulldown assays).
Pulldown assays to detect the interaction of purified Tzs with hexahistidyl- or StrepII-tagged VirB5 fusion proteins bound to affinity matrices were conducted as described previously (53).
Purification of a VirB5-binding protein from A. tumefaciens.
The VirB5-binding protein was purified from A. tumefaciens, and the gel overlay assay described above was applied to monitor the progress of individual purification steps. A. tumefaciens strain C58 carrying plasmid pPZP300 was cultivated under virulence gene-inducing conditions on eight AB minimal medium agar plates (15 cm diameter) for 4 days at 20°C in the presence of 200 μM AS for virulence gene induction. The bacteria were washed from the plates with 10 ml 50-mM Na-K-phosphate buffer per plate, sedimented, and resuspended in 20 ml 50-mM Na-K-phosphate buffer. Next, the bacteria were lysed by passage through a French press at 20,000 lb/in2, followed by low-speed centrifugation (40 min at 12,000 rpm in an SS-34 rotor, Sorvall RC5B centrifuge) to remove cell debris and ultracentrifugation (2 h at 40,000 rpm in a Ti50.2 rotor, Sorvall OTD-50B ultracentrifuge) to remove membrane proteins. The VirB5-binding protein in the soluble fraction was further enriched by differential (NH4)2SO4 precipitation (30 to 70% in steps of 10%), and the highest amount was detected in the 40% fraction. The precipitate was suspended in 1 ml 50 mM HEPES (pH 7), dialyzed in 2 liters of this buffer at 4°C for 12 h and then applied onto a Mono Q-Sepharose anion exchange column (Amersham Biosciences). The column was washed with 50 mM HEPES (pH 7), followed by elution with a linear gradient (50 mM to 1 M NaCl in 50 mM HEPES-puffer, pH 7) over 10 column volumes, and the fractions were precipitated by the addition of acetone. The samples were separated by electrophoresis on a 12% acrylamide gel and blotted onto a PVDF membrane, and the VirB5-binding protein localized by gel overlay assay was identified by N-terminal sequencing using standard protocols.
Yeast two-hybrid system analysis.
Analyses of protein-protein interactions using the yeast two-hybrid system were conducted as described previously following standard procedures for the Matchmaker two-hybrid system (Clontech) (8, 15). The genes were cloned into pAS2 and pACTII, and plasmid-containing yeast cells were selected on SD medium in the absence of Leu and Trp. Six transformants from each plasmid combination were streaked on SD agar plates and lysed in liquid nitrogen, followed by soaking in Z buffer (16.1 g Na2HPO4, 5.5 g NaH2PO4, 0.75 g KCl, 0.25 g MgCl2, 2.7 ml β-mercaptoethanol/liter) containing X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) (1 mg/ml) to identify β-galactosidase production. The strength of the interaction was assessed based on the number of blue colonies among six transformants each from six independent transformation experiments.
Immunoelectron microscopy.
Immunoelectron microscopy to detect cell-bound Tzs was conducted as described previously (3) using 1:250 diluted Tzs-specific primary antiserum and 1:10-diluted anti-rabbit 10-nm gold conjugates (Sigma-Aldrich).
RESULTS
Overlay assays detect a VirB5-binding virulence-induced protein in A. tumefaciens.
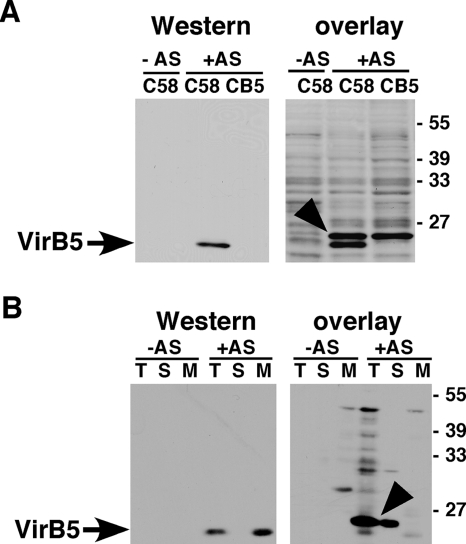
In an attempt to identify A. tumefaciens proteins that contribute to the incorporation of the minor T-pilus component VirB5 into these extracellular structures, we overexpressed VirB5 as a fusion to hexahistidyl-thioredoxin. His6TrxAVirB5 was purified by immobilized metal affinity chromatography, and a gel-overlay assay was conducted to identify binding partners in A. tumefaciens cell lysates. To this end, the lysates were separated by SDS-PAGE, followed by blotting onto a PVDF membrane and removal of the SDS to enable renaturation. The membranes were then incubated with purified His6TrxAVirB5, and the bound fusion protein was detected after incubation with a VirB5-specific antiserum and a horseradish peroxidase-coupled secondary antiserum by chemiluminescence detection. This approach identified a 26-kDa protein only in lysates of virulence gene-induced strain C58, not in lysates from noninduced cells (Fig. 1A). This protein was slightly larger than the 25-kDa VirB5, which was detected by standard Western blotting as well as during the overlay assay procedure. The VirB5-binding protein was also present in lysates from the virB5 gene deletion strain CB1005 (CB5) (Fig. 1A), excluding the possibility that it represents a modified VirB5 with a higher apparent molecular mass. In order to characterize its subcellular localization, we analyzed subcellular fractions and detected the VirB5-binding protein in the total cell lysate and in the soluble fraction (Fig. 1B). In contrast, VirB5 was detected in the total cell lysate and in the membrane fraction. Since all T4SS components localize either exclusively or primarily in the membranes, we concluded that the VirB5-binding protein is not likely a component of the transmembrane complex but that it may be an AS-induced soluble factor that aids in VirB5 assembly. To assess this possibility, the protein was purified and identified next.
FIG. 1.
Detection of a VirB5-binding protein using overlay assays. Wild-type C58 and the virB5 deletion mutant CB1005 carrying pPZP300 were grown under virulence gene-inducing (+AS) or noninducing conditions (−AS). For subcellular fractionation, the cells were lysed in a French press, followed by separation of the total cell lysate (T) into soluble (S) and membrane fractions (M). (A) Cell lysates were separated by SDS-PAGE, followed by Western blotting with VirB5-specific antiserum (left panel). The overlay assay was conducted by incubating the PVDF membrane after electrotransfer of the proteins with purified His6TrxAVirB5, followed by washing and Western blotting with VirB5-specific antiserum (right panel). (B) To analyze the subcellular localization of VirB5 and the interacting protein, samples of the T, S, and M fractions were separated by SDS-PAGE, followed by Western blotting (left panel) or the overlay assay was conducted, followed by Western blotting as described above (right panel). Arrows indicate VirB5, and arrowheads indicate the His6TrxAVirB5-binding protein detected in the overlay assay; numbers on the right indicate the molecular masses of reference proteins.
Purification and identification of the VirB5-binding protein as Tzs.
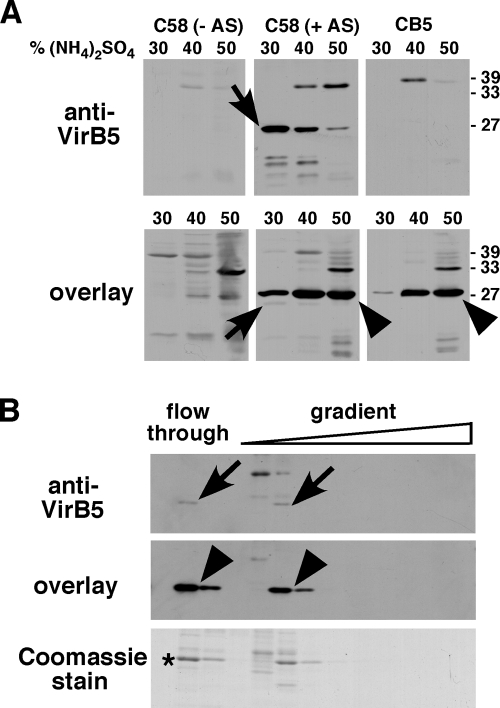
To purify the His6TrxAVirB5-binding protein, strain C58 carrying the plasmid pPZP300 (for increased virulence gene expression) was grown in the presence of the virulence gene inducer AS and as controls, we also cultivated the negative controls CB1005 (with AS) and C58 (without AS). Cell lysis was conducted in a French press, the cell debris was removed by low-speed centrifugation, and the membranes were then separated from the soluble proteins by ultracentrifugation. To enrich the His6TrxAVirB5-binding protein, we subjected the soluble fraction to stepwise fractionation with (NH4)2SO4 and the highest amounts were detected in the 40% and 50% fractions in the case of strain C58 as well as that of CB1005 (Fig. 2A). VirB5 was also detected in the soluble fraction here, which is likely due to the more highly concentrated sample. This result is in accord with previous reports showing that a small portion of VirB5 is present in the soluble fraction (42, 53). The highest amount of VirB5 was precipitated with 30% (NH4)2SO4, but the protein was also detected in the 40% and 50% fractions. These data are in accord with the possibility that VirB5 and the interacting protein form a complex in vivo.
FIG. 2.
Purification of the VirB5-binding protein from the soluble fraction of C58 cell lysates. Wild-type C58 and the virB5 deletion variant CB1005 were grown under virulence gene-inducing (+AS) or noninducing conditions (−AS). (A) Cells were lysed in a French press and the soluble fractions were separated from the membranes by ultracentrifugation, followed by precipitation of proteins with increasing concentrations of (NH4)2SO4 as indicated. The precipitates were analyzed by SDS-PAGE and Western blotting with VirB5-specific antiserum (upper panel) or by overlay assay with His6TrxAVirB5, followed by the detection of bound VirB5 (lower panel). (B) Proteins precipitated with 40% (NH4)2SO4 from C58 extracts were dialyzed and applied to a MonoQ anion exchange column, and the fractions eluted from the column (flowthrough and NaCl gradient) were analyzed by SDS-PAGE and Western blotting, overlay assay and detection of VirB5, or Coomassie staining. The protein indicated by the asterisk was electrotransferred to a PVDF membrane and subjected to Edman sequencing. Arrows show VirB5, and arrowheads indicate the His6TrxAVirB5-binding protein detected using the overlay assay; numbers on the right indicate the molecular masses of reference proteins.
We used Coomassie staining of SDS gels to analyze the differential salt solubility. This analysis revealed that the 40% (NH4)2SO4 fraction contained a 26-kDa protein and the smallest amount of other proteins (data not shown), and it was therefore chosen for further purification by anion exchange chromatography. Only a portion of the VirB5-binding protein (revealed by overlay assay) and of soluble VirB5 (revealed by Western blotting) from the 40% (NH4)2SO4 fraction bound to the anion exchange column, whereas most of both proteins eluted in the wash fractions (Fig. 2B). Interestingly, VirB5 and its interaction partner eluted in identical fractions during this procedure and this result is also consistent with the notion that they may form a complex. An analysis of the fractions eluted from the column by SDS-PAGE and Coomassie staining revealed that this procedure constituted an enrichment of the VirB5-binding protein, and we concluded that the purity was high enough for its identification. To this end, we blotted the flowthrough fraction with the highest amount of the VirB5-binding protein onto a PVDF membrane and its identity was analyzed by N-terminal (Edman) sequencing. This analysis identified 11 of the 12 N-terminal amino acids counted from the N terminus as MLLHLIYGPTXS (X indicates an amino acid that could not be identified), which matches Tzs, the trans-zeatin biosynthetic protein from A. tumefaciens strain C58. The finding of Tzs as the VirB5 binding protein was unexpected, as there is no obvious connection between a protein involved in phytohormone biosynthesis in the cytoplasm and a T4SS component. However, the biological role of Tzs has not been firmly established and, in the following work, we verified the interaction with VirB5 and assessed its biological significance.
Pulldown and yeast two-hybrid analysis confirm the interaction between VirB5 and Tzs.
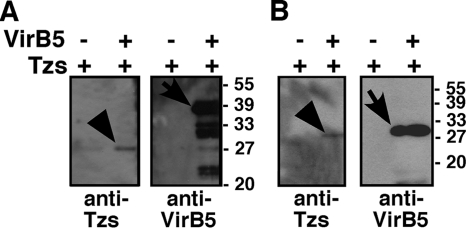
To further characterize the VirB5-Tzs interaction, we cloned, overproduced, and purified Tzs as described previously (27) and we used the purified protein for the generation of a specific antiserum. We also overproduced and purified N-terminally StrepII-affinity-tagged VirB5 and used this fusion protein as well as His6TrxAVirB5 to analyze the interaction with purified Tzs in pulldown assays. N-terminally affinity-tagged His6TrxAVirB5 protein was attached to the IMAC affinity matrix and incubated with Tzs, followed by sedimentation of the matrix by centrifugation, washing, detection of the bound proteins by SDS-PAGE, and detection with specific antisera. This approach demonstrated that a VirB5 fusion protein bound Tzs to the IMAC matrix, and the use of a negative control (matrix without His6TrxAVirB5) showed that this was not due to unspecific binding of Tzs (Fig. 3A). Similar results were obtained when the pulldown assays were conducted with StrepII affinity-tagged VirB5 and StrepTactin-Sepharose affinity matrix (Fig. 3B). These results also lead to the conclusion that Tzs binds to the VirB5 portion of the fusion proteins and not to the His6TrxA domain.
FIG. 3.
Analysis of the VirB5-Tzs interaction by pulldown assays. Purified Tzs was incubated with affinity bead-bound StrepIIVirB5 and His6TrxAVirB5 fusion proteins or with affinity beads alone, followed by sedimentation of the beads, washing, elution, and analysis of the bead-bound material by SDS-PAGE and Western blotting with specific antisera. (A) Analysis of proteins bound to Ni-nitrilotriacetic acid Sepharose after elution with imidazole. (B) Analysis of proteins bound to StrepTactin magnetic beads after elution with biotin. Arrows indicate VirB5 fusion proteins, and arrowheads indicate Tzs eluted from the affinity matrices. Molecular masses of reference proteins are shown on the right. −, absence of; +, presence of.
As an independent assay to assess the VirB5-Tzs interaction, we used the yeast two-hybrid system. The genes encoding VirB5, Tzs, and (as a positive control) the VirE2 protein were fused to the DNA and the activation domains of the yeast GAL4 transcription factor. Interaction between two fusion partners resulted in the activation of the lacZ promoter and was monitored by the blue color of the yeast colonies. As in previous work, VirE2 was found to interact with itself (8) and we also detected a VirB5-VirB5 interaction, but this assay did not provide evidence for self-interaction of Tzs (Table 4). When pairwise combinations of the three proteins were tested, we noticed that VirB5 bound to the DNA binding domain led to lacZ gene activation when both Tzs and VirE2 were coexpressed as fusions to the activation domain, but the reciprocal experiments did not lead to gene activation. As the expression of VirB5 fused to the DNA binding domain alone did not activate the lacZ gene (data not shown), we conclude that the results of this assay lend additional support for the notion that Tzs and VirB5 interact. The evidence for a VirB5-VirE2 interaction presented here is novel, but we have not analyzed this question further in the context of this work. After confirming the VirB5-Tzs interaction with independent in vitro methods, we next analyzed whether Tzs associates with the T4SS complex in vivo.
TABLE 4.
Analysis of VirB protein and Tzs interactions using the yeast two-hybrid system
| Combination of vectors | Strength of interaction assessed by the no. of blue coloniesa |
|---|---|
| pAS2-VirB5/pACTII-VirB5 | ++ |
| pAS2-VirE2/pACTII-VirE2 | +++ |
| pAS2-Tzs/pACTII-Tzs | − |
| pAS2-VirB5/pACTII-Tzs | ++ |
| pAS2-Tzs/pACTII-VirB5 | − |
| pAS2-VirB5/pACTII-E2 | +++ |
| pAS2-Tzs/pACTII-VirE2 | − |
Relative numbers of β-galactosidase-producing colonies; six transformants each were tested from six independent transformation experiments.
Analysis of Tzs production and membrane association.
Using the Tzs-specific antiserum, we first studied the conditions for production of Tzs in different agrobacteria. Tzs was detected exclusively in virulence gene-induced cells of A. tumefaciens strains C58 and A208 but not in strains A348, A281, Ach5, and Chry5 (data not shown). These results are in accord with its occurrence only on nopaline-type Ti-plasmids, such as in strains C58 and A208.
We next used the antiserum to determine the subcellular localization of Tzs in A. tumefaciens strain C58, and the T-pilus components VirB2 and VirB5 were used as controls. As described above, the cells were fractionated into total cell lysate (T), soluble (S), and membrane proteins (M) and Tzs was detected in all three fractions (data not shown). This result differs from that of the overlay assay, which detected the VirB5-binding protein exclusively in the soluble fraction (Fig. 1B). The fact that we analyzed more concentrated fractions of the membranes here and that detection with the Tzs-specific antiserum was more sensitive than the overlay assay likely explains this discrepancy.
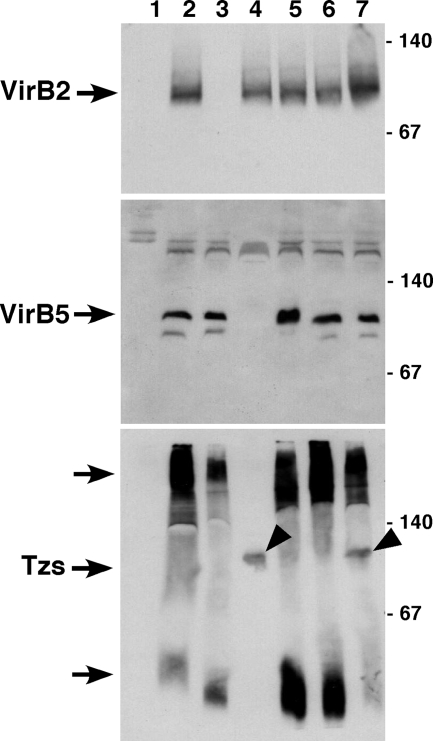
To analyze the impact of VirB5 on the membrane association of Tzs in more detail, we next extracted membrane proteins with the mild detergent DDM, followed by separation under native conditions by blue native electrophoresis. This method was initially developed to assess the interactions between T4SS components, and it was applied here to determine the association of the translocation machinery with Tzs. As in previous work, both VirB2 and VirB5 were detected in the 100-kDa molecular mass range and, as expected, VirB2 was absent in CB1002 and VirB5 was absent in CB1005 (Fig. 4). An analysis with Tzs-specific antiserum revealed that the largest portion of Tzs was present in high-molecular-mass fractions larger than 140 kDa and in low-molecular-mass fractions smaller than 67 kDa (Fig. 4C). A minor fraction was also present in the 100-kDa molecular mass range, but the signal was not as well defined as in the cases of VirB2 and VirB5. These results were qualitatively similar in extracts from CB1002 and CB1008, but the amounts of Tzs were strongly reduced in CB1005. Tzs was not detected in the high- and low-molecular-mass fractions, but a relatively well-defined signal was present in the 100-kDa molecular mass range in CB1005 as well as in CB1008. Complementation of the virB5 deletion in CB1005 pTrcB5 restored the wild-type pattern of Tzs fractionation, and these results demonstrate that VirB5 has a profound impact on the membrane association of Tzs. The fractionation of Tzs observed here is reminiscent of that of T4SS core components and of translocated substrates that were extracted in high-molecular-mass complexes with DDM. To assess whether Tzs is translocated from Agrobacterium by the T4SS, we analyzed next whether it is transferred to plant cells or to the cell exterior.
FIG. 4.
Analysis of DDM-extracted membrane protein complexes by blue native electrophoresis. Strains C58, CB1002 (ΔvirB2), CB1005 (ΔvirB5), and CB1008 (ΔvirB8) and complemented variants were cultivated on AB minimal medium in the absence (−AS, lane 1) or in the presence of AS (+AS, lanes 2 to 7) for virulence gene induction, followed by cell lysis, sedimentation of the membranes and extraction with 2% DDM. The samples were separated by blue native PAGE on a 15% gel, followed by Western blotting with VirB2-, VirB5-, and Tzs-specific antiserum. Lanes: 1, C58 without AS; 2, C58 with AS; 3, CB1002; 4, CB1005; 5, CB1002 pTrcB2; 6, CB1005 pTrcB5; 7, CB1008. Arrowheads point to Tzs in a 100-kDa complex in CB1005 and CB1008, and molecular masses of reference proteins are shown on the right (in kilodaltons). This experiment was conducted twice with qualitatively similar results.
Extracellular localization of Tzs depends on T4SS function.
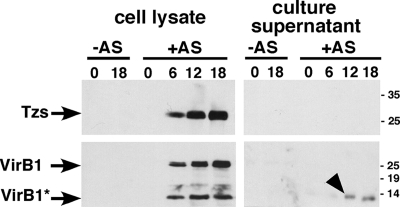
The T4SS-dependent membrane association and the presence of positively charged residues at the C terminus of Tzs, which is a translocation signal of other T4SS substrates (48), opened the possibility that it may be translocated to plant cells. To test this possibility, the gene encoding Tzs was fused in frame to that encoding the Cre recombinase and its translocation to plant cells was tested following standard protocols (47). This analysis did not provide any evidence for a translocation of the fusion protein, indicating that Tzs is not a substrate translocated to plant cells (A. den Dulk-Raas and A. Vergunst, personal communication). Next, we tested whether Tzs is secreted to the cell exterior of strain C58 grown in liquid medium. As a positive control, we monitored the secretion of the C-terminal VirB1* fragment that is proteolytically processed from VirB1 (7). Tzs was not detected in the cell supernatant, and it is therefore not a secreted protein (Fig. 5).
FIG. 5.
Analysis of proteins secreted from A. tumefaciens. Strain C58 was cultivated in liquid AB minimal medium in the absence (−) or in the presence (+) of AS for virulence gene induction. The presence of VirB1, VirB1*, and Tzs was monitored in cell lysates as well as in culture supernatants at different time points after the induction of virulence gene expression (0 h, 6 h, 12 h, and 18 h). Samples were separated by SDS-PAGE, followed by Western blotting with VirB1 and Tzs-specific antiserum. The arrowhead points to secreted VirB1*, and molecular masses of reference proteins are shown on the right (in kilodaltons).
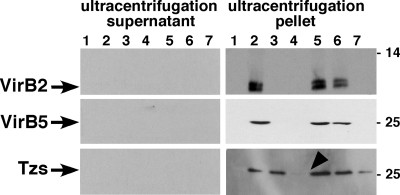
T4SS-mediated translocation may alternatively lead to the incorporation or Tzs into T pili or to its display at the cell surface. To address these possibilities, we cultivated agrobacteria on solid agar medium, followed by the shearing of the cells to remove cell-bound T pili and flagella and ultracentrifugation to separate these high-molecular-mass extracellular structures in the ultracentrifugation pellet from low-molecular-mass structures in the ultracentrifugation supernatant. The T-pilus major component VirB2 and the minor component VirB5 as well as Tzs were detected in the ultracentrifugation pellet of samples from wild-type strain C58, suggesting that Tzs is incorporated into a high-molecular-mass structure that can be removed from the cells by shearing (Fig. 6). In accord with previous work, VirB2 and VirB5 were not detected in the ultracentrifugation pellets from strains CB1002, CB1005, and CB1008, but as expected, complementation of CB1002 and CB1005 restored pilus formation. In contrast, Tzs was detected in the ultracentrifugation pellets isolated from CB1002, in reduced amounts in those from CB1008, and in strongly reduced amounts in those from CB1005 (Fig. 6). The reduced association with the ultracentrifugation pellet fraction was complemented in CB1005 pTrcB5. These results demonstrated that Tzs can be sheared in a high-molecular-mass complex from the cell exterior, but since this association was not strictly dependent on VirB2 and VirB8, it is likely not a T-pilus component.
FIG. 6.
Analysis of the composition of T-pilus fractions. Strains C58, CB1002 (ΔvirB2), CB1005 (ΔvirB5), and CB1008 (ΔvirB8) and complemented variants were cultivated on AB minimal medium in the absence (−) of AS (lane 1) or in the presence (+) of AS (lanes 2 to 7) for virulence gene induction, followed by shearing of cells and ultracentrifugation for separation of extracellular high-molecular-mass structures (ultracentrifugation pellet) and low-molecular-mass proteins released from the cells (ultracentrifugation supernatant). Samples were separated by SDS-PAGE, followed by Western blotting with VirB2-, VirB5-, and Tzs-specific antiserum. Lanes: 1, C58 without AS; 2, C58 with AS; 3, CB1002; 4, CB1005; 5, CB1002 pTrcB2; 6, CB1005 pTrcB5; 7, CB1008. The arrowhead points to the reduced amount of Tzs in the ultracentrifugation pellet from CB1005, and molecular masses of reference proteins are shown on the right (in kilodaltons).
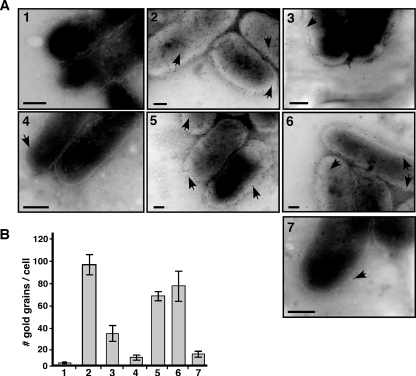
The results of the shearing experiments suggested that Tzs may be displayed on the cell surface, and we directly tested this possibility by immunoelectron microscopy. This approach has identified VirB5 on the cell surface and on the tips of T pili, whereas VirB2 was detected along the entire length of isolated T pili (3). When we conducted the immunoelectron microscopic analysis with Tzs-specific antiserum, the protein was detected evenly distributed across the entire surface of the wild-type strain C58, but it was not associated with T pili (Fig. 7). The number of gold grains indicating the presence of Tzs on the cell surface was modestly reduced on CB1002 and strongly reduced on CB1008, and the lowest amount was detected on CB1005. These reductions were complemented in CB1002 pTrcB2 and CB1005 pTrcB5 (Fig. 7). The results of the immunoelectron microscopic analysis correlated well with the detection of Tzs in pellets after shearing of the cells and ultracentrifugation (Fig. 6), showing that both assays detected a surface-exposed form of Tzs. Taken together, the work presented here demonstrates that Tzs is translocated to the cell surface of A. tumefaciens strain C58 and that the high efficiency of this process depends on VirB5 in the context of a functional T4SS.
FIG. 7.
Immunoelectron microscopy detects Tzs on the A. tumefaciens cell surface. Strains C58, CB1002 (ΔvirB2), CB1005 (ΔvirB5), and CB1008 (ΔvirB8) and complemented variants were cultivated on AB minimal medium in the absence or in the presence of AS for virulence gene induction, followed by immunoelectron microscopy with Tzs-specific primary antibody and 10 nm gold-labeled secondary antibody. (A) Representative images of transmission electron micrographs; arrowheads point to gold grains on the cell surfaces of samples as follows: 1, C58 without AS; 2, C58 with AS; 3, CB1002; 4, CB1005; 5, CB1002 pTrcB2; 6, CB1005 pTrcB5; 7, CB1008. The contrast was increased to visualize the outline of cells for the purpose of presentation, but counting was conducted with reduced contrast settings that allowed the visualization of grains in even more heavily stained regions of the cells. Bars, 100 nm. (B) Quantification of results of the transmission electron microscopy analysis of Tzs on the cell surface; numbering of bars as for panel A. We counted 10 cells each from three independent induction experiments for each strain (total of 30 cells), and error bars show the standard deviations.
DISCUSSION
The original goal of the work described here was to isolate proteins from A. tumefaciens that mediate the incorporation of VirB5 into T pili. To this end, we pursued an unbiased biochemical approach to isolate proteins from cell lysates that bind to purified VirB5 fusion protein and we envisaged that we may identify VirB proteins, other virulence-induced proteins, and possibly, non-virulence-induced proteins. The identification of Tzs, a protein that is believed to synthesize trans-zeatin ribotide phytohormones in the A. tumefaciens cytoplasm, came as a surprise, as there was no a priori reason to believe that such a protein would interact with the T4SS. In the course of this study, we have provided several lines of evidence supporting the notion that the Tzs-VirB5 interaction occurs in vivo and we gained insights into the mechanism of Tzs translocation across the cell envelope.
The first line of evidence suggesting a specific interaction was the finding that the VirB5 fusion protein bound only to one protein from A. tumefaciens cell lysate on PVDF membranes, the Tzs protein. Thus, VirB5 did not show features of a “sticky” protein that interacts with many partners. Under the conditions used, this interaction was apparently stronger than that with other known interaction partners of VirB5, such as VirB8 and VirB10 (53), that were not detected by overlay assay. This difference may be due to the fact that VirB8 and VirB10 do not refold on the membranes, but in any case, the limited number of interaction partners is consistent with a specific interaction. The second line of evidence stems from the apparent copurification of Tzs with a portion of soluble VirB5, which was observed during the initial purification of the VirB5-binding protein. Whereas this does not constitute a strict proof for an in vivo interaction, the results are consistent with the interaction detected with the overlay assay. The interaction between VirB5 and Tzs was subsequently confirmed using pulldown assays with purified proteins and the yeast two-hybrid system. The third line of evidence is based on the observation that a significant portion of Tzs associated with the membrane fraction. In the absence of VirB5, Tzs did not cofractionate with detergent-extracted high-molecular-mass complexes (larger than 140 kDa) and low-molecular-mass complexes (smaller than 67 kDa) and only small amounts of Tzs fractionated in the 100-kDa molecular mass range, similar to that of the VirB2-VirB5 pilus assembly complex. It is tempting to speculate that the detection of Tzs in the 100-kDa molecular mass range may reflect an interaction with VirB5 and VirB2, whereas high-molecular-mass Tzs may reflect its interaction with the core T4SS that was shown to fractionate in this molecular mass range (27, 53). The fourth line of evidence is based on the finding that the integrity of the T4SS is necessary for the efficient translocation of Tzs to the cell-surface that was monitored by the analysis of extracellular high-molecular-mass structures as well as by immunoelectron microscopy. Taken together, our data suggest a model for the contribution of the VirB5-Tzs interaction to the translocation of Tzs to the cell surface.
Surface-exposed Tzs was not released as a soluble protein into the supernatant, but it was removed from the cells by shearing and fractionated in a high-molecular-mass complex, together with T pili. This observation is reminiscent of findings made in the case of VirB7, which is a small lipoprotein and T4SS core complex component that translocates to the cell surface and is removed from the cells in a high-molecular-mass complex by shearing (38). The extracellular localization of VirB7 does not depend on T-pilus assembly, indicating that it is not an integral pilus component, but VirB7 may contribute to the assembly of this extracellular structure (38). Similar to VirB7, Tzs was detected on the surface of a virB2 deletion strain, albeit at reduced levels. In contrast to VirB7, we observed that the amount of surface-exposed Tzs was strongly reduced in the absence of VirB8 and even more so in the absence of VirB5. Thus, an efficient translocation of Tzs to the surface depends on the integrity of the T4SS, and VirB5 is especially critical for this process. These observations differ from those obtained in the case of VirB7, which translocates to the surface independently of individual T4SS components (38). The fact that VirB7 and Tzs are surface exposed in the absence of VirB2 and in immunoelectron microscopic analyses conducted here and elsewhere suggests that these proteins are not T-pilus components (3). The nature of the extracellular high-molecular-mass structure isolated by shearing remains elusive. One possibility is that shearing removes outer membrane vesicles (blebs) from the cells. Such structures are implicated in the translocation of virulence factors from bacteria (33, 35), and it may be interesting to assess in the future whether membrane vesicles contribute to the Agrobacterium-plant interaction.
The presented data suggest a mechanism for the translocation of Tzs to the cell surface. Tzs may bind to VirB5 in the inner membrane and it may subsequently interact with T4SS core complex components, followed by its translocation to the cell surface. This model is supported by the observation that a major portion of detergent-extracted Tzs fractionated in the molecular mass range of the T4SS core components. The absence of VirB5, but not of VirB2 and VirB8, reduced the amount of Tzs in these complexes, indicating that VirB5 is likely required to enable an early step of the interaction with the T4SS. Both VirB2 and VirB8 apparently facilitate a later step of the translocation, as the amount of surface-exposed Tzs was reduced in CB1002 and even more so in CB1008. The interaction partners among the T4SS core components are not known, but the data presented here suggest that in contrast to the case for VirB7, a functional T4SS and especially VirB5 are required for efficient translocation of Tzs to the cell surface.
Whereas this work demonstrates the surface localization of Tzs and suggests a key role of the VirB5 interaction for the translocation, it does not reveal the biological significance of this localization. The production of trans-zeatin ribotides in the cytoplasm is believed to be the primary function of Tzs (24, 37). However, the surface localization reported here opens up the possibility that Tzs may provide an additional or even an entirely different contribution to the A. tumefaciens-plant interaction. Different functions of surface-localized Tzs could be imagined, but further experimentation is required to assess these possibilities. First, the translocation of Tzs to the cell surface may down-regulate the cytoplasmic production of phytohormones. This metabolic pathway may be costly for the cells, and it may not contribute to the host cell interaction once the T-complex has been assembled and the tmr gene has been transferred. Thus, it may be advantageous to remove Tzs from the cytoplasm at this stage of the interaction. Second, surface-localized Tzs may convert metabolites from wounded and destroyed plant cells at the infection site to active phytohormones and thereby stimulate plant cell growth in the wound callus. Third, Tzs may be translocated to plant cells as a component of membrane vesicles and, in a manner similar to that of the Tmr protein, it may translocate to the chloroplasts and divert metabolites of the MEP pathway for the production of hydroxylated trans-zeatin ribotides (40). So far, there is no evidence for the formation of membrane blebs by agrobacteria, but blebs were detected in the closely related genus Brucella and it is therefore possible that they are also produced by Agrobacterium species (4, 17). The finding that Tzs as well as VirB7 associates with high-molecular-mass extracellular structures after shearing of cells is consistent with the possibility that membrane vesicles may be produced at the natural pathogen-host interface. Fourth, surface-localized Tzs may directly stimulate host-cell contact by binding to plant surface structures. The discovery of surface-localized GroEL, a protein that exerts an essential function in the bacterial cytoplasm, but contributes on the cell surface to pathogen-host adhesion in some bacteria, constitutes a precedent for this possibility (11, 18). The above-mentioned possibilities are speculative at this point, but the discovery of Tzs on the cell surface opens interesting avenues for the analysis of the Agrobacterium-plant interaction in the future.
Acknowledgments
We are indebted to August Böck (Munich, Germany) and Meinhard Zenk (St. Louis, MO) for continued support and discussions and to Natalie Domke (Munich, Germany) for technical assistance. We are grateful to Annette Vergunst (Nîmes, France) and Amke den Dulk-Raas (Leiden, The Netherlands) for performing Cre recombinase reporter assays to determine whether Tzs is translocated into plant nuclei and to John Morala (McMaster) for help during the construction of strain CB1008.
This work was supported by grants from the Natural Sciences and Engineering Research Council of Canada (NSERC grant 262104), the Canada Foundation for Innovation (CFI), the Ontario Innovation Trust (OIT), and the Deutsche Forschungsgemeinschaft (via SFB 369).
Footnotes
Published ahead of print on 28 December 2007.
REFERENCES
- 1.Akiyoshi, D. E., H. Klee, R. M. Amasino, E. W. Nester, and M. P. Gordon. 1984. T-DNA of Agrobacterium tumefaciens encodes an enzyme of cytokinin biosynthesis. Proc. Natl. Acad. Sci. USA 815994-5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akiyoshi, D. E., R. O. Morris, R. Hinz, B. S. Mischke, T. Kosuge, D. J. Garfinkel, M. P. Gordon, and E. W. Nester. 1983. Cytokinin/auxin balance in crown gall tumors is regulated by specific loci in the T-DNA. Proc. Natl. Acad. Sci. USA 80407-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aly, K. A., and C. Baron. 2007. The VirB5 protein localizes to the T-pilus tips in Agrobacterium tumefaciens. Microbiology 1533766-3775. [DOI] [PubMed] [Google Scholar]
- 4.Aragon, V., R. Diaz, E. Moreno, and I. Moriyon. 1996. Characterization of Brucella abortus and Brucella melitensis native haptens as outer membrane O-type polysaccharides independent from the smooth lipopolysaccharide. J. Bacteriol. 1781070-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atmakuri, K., E. Cascales, and P. J. Christie. 2004. Energetic components VirD4, VirB11 and VirB4 mediate early DNA transfer reactions required for bacterial type IV secretion. Mol. Microbiol. 541199-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baron, C. 2005. From bioremediation to biowarfare: on the impact and mechanism of type IV secretion systems. FEMS Microbiol. Lett. 253163-170. [DOI] [PubMed] [Google Scholar]
- 7.Baron, C., M. Llosa, S. Zhou, and P. C. Zambryski. 1997. VirB1, a component of the T-complex transfer machinery of Agrobacterium tumefaciens, is processed to a C-terminal secreted product, VirB1. J. Bacteriol. 1791203-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baron, C., Y. R. Thorstenson, and P. C. Zambryski. 1997. The lipoprotein VirB7 interacts with VirB9 in the membranes of Agrobacterium tumefaciens. J. Bacteriol. 1791211-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beaty, J. S., G. K. Powell, L. Lica, D. A. Regier, E. M. S. MacDonald, N. G. Hommes, and R. O. Morris. 1986. Tzs, a nopaline Ti plasmid gene from Agrobacterium tumefaciens associated with trans-zeatin biosynthesis. Mol. Genet. Genomics 203274-280. [Google Scholar]
- 10.Berger, B. R., and P. J. Christie. 1994. Genetic complementation analysis of the Agrobacterium tumefaciens virB operon: virB2 through virB11 are essential virulence genes. J. Bacteriol. 1763646-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergonzelli, G. E., D. Granato, R. D. Pridmore, L. F. Marvin-Guy, D. Donnicola, and I. E. Corthesy-Theulaz. 2006. GroEL of Lactobacillus johnsonii La1 (NCC 533) is cell surface associated: potential role in interactions with the host and the gastric pathogen Helicobacter pylori. Infect. Immun. 74425-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhandari, P., and J. Gowrishankar. 1997. An Escherichia coli host strain useful for efficient overproduction of cloned gene products with NaCl as the inducer. J. Bacteriol. 1794403-4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cascales, E., and P. J. Christie. 2004. Definition of a bacterial type IV secretion pathway for a DNA substrate. Science 3041170-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christie, P. J., K. Atmakuri, V. Krishnamoorthy, S. Jakubowski, and E. Cascales. 2005. Biogenesis, architecture, and function of bacterial type IV secretion systems. Annu. Rev. Microbiol. 59451-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durfee, T., K. Becherer, P.-L. Chen, S.-H. Yeh, Y. Yang, A. E. Kilburn, W.-H. Lee, and S. J. Elledge. 1993. The retinoblastoma protein associates with the protein phosphatase type 1 catalytic subunit. Genes Dev. 7555-569. [DOI] [PubMed] [Google Scholar]
- 16.Fullner, K. J., J. L. Lara, and E. W. Nester. 1996. Pilus assembly by Agrobacterium T-DNA transfer genes. Science 2731107-1109. [DOI] [PubMed] [Google Scholar]
- 17.Gamazo, C., and I. Moriyon. 1987. Release of outer membrane fragments by exponentially growing Brucella melitensis cells. Infect. Immun. 55609-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garduno, R. A., E. Garduno, and P. S. Hoffman. 1998. Surface-associated Hsp60 chaperonin of Legionella pneumophila mediates invasion in a HeLa cell model. Infect. Immun. 664602-4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garfinkel, D. J., R. B. Simpson, L. W. Ream, F. F. White, M. P. Gordon, and E. W. Nester. 1981. Genetic analysis of crown gall: fine structure map of the T-DNA by site-directed mutagenesis. Cell 27143-153. [DOI] [PubMed] [Google Scholar]
- 20.Goodner, B., G. Hinkle, S. Gattung, N. Miller, M. Blanchard, B. Qurollo, B. S. Goldman, Y. Cao, M. Askenazi, C. Halling, L. Mullin, K. Houmiel, J. Gordon, M. Vaudin, O. Iartchouk, A. Epp, F. Liu, C. Wollam, M. Allinger, D. Doughty, C. Scott, C. Lappas, B. Markelz, C. Flanagan, C. Crowell, J. Gurson, C. Lomo, C. Sear, G. Strub, C. Cielo, and S. Slater. 2001. Genome sequence of the plant pathogen and biotechnology agent Agrobacterium tumefaciens C58. Science 2942323-2328. [DOI] [PubMed] [Google Scholar]
- 21.Harlow, E., and D. Lane (ed.). 1988. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 22.Homann, H. E., W. Willenbrink, C. J. Buchholz, and W. J. Neubert. 1991. Sendai virus protein-protein interactions studied by a protein-blotting protein-overlay technique: mapping of domains on NP protein required for binding to P protein. J. Virol. 651304-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hwang, H. H., and S. B. Gelvin. 2004. Plant proteins that interact with VirB2, the Agrobacterium tumefaciens pilin protein, mediate plant transformation. Plant Cell 163148-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.John, M. C., and R. M. Amasino. 1988. Expression of an Agrobacterium Ti plasmid gene involved in cytokinin biosynthesis is regulated by virulence loci and induced by plant phenolic compounds. J. Bacteriol. 170790-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Judd, P. K., R. B. Kumar, and A. Das. 2005. Spatial location and requirements for the assembly of the Agrobacterium tumefaciens type IV secretion apparatus. Proc. Natl. Acad. Sci. USA 10211498-11503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kovacs, L. G., and S. G. Pueppke. 1994. Mapping and genetic organization of pTiChry5, a novel Ti plasmid from a highly virulent Agrobacterium tumefaciens strain. Mol. Gen. Genet. 242327-336. [DOI] [PubMed] [Google Scholar]
- 27.Krall, L., M. Raschke, M. H. Zenk, and C. Baron. 2002. The Tzs protein from Agrobacterium tumefaciens C58 produces zeatin riboside 5′-phosphate from 4-hydroxy-3-methyl-2-(E)-butenyl diphosphate and AMP. FEBS Lett. 517315-318. [DOI] [PubMed] [Google Scholar]
- 28.Krall, L., U. Wiedemann, G. Unsin, S. Weiss, N. Domke, and C. Baron. 2002. Detergent extraction identifies different VirB protein subassemblies of the type IV secretion machinery in the membranes of Agrobacterium tumefaciens. Proc. Natl. Acad. Sci. USA 9911405-11410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kromayer, M., R. Wilting, P. Tormay, and A. Böck. 1996. Domain structure of the prokaryotic selenocysteine-specific elongation factor SelB. J. Mol. Biol. 262413-420. [DOI] [PubMed] [Google Scholar]
- 30.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227680-685. [DOI] [PubMed] [Google Scholar]
- 31.Lai, E.-M., and C. I. Kado. 1998. Processed VirB2 is the major subunit of the promiscuous pilus of Agrobacterium tumefaciens. J. Bacteriol. 1802711-2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maniatis, T. A., J. Sambrook, and E. F. Fritsch. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 33.Mashburn-Warren, L. M., and M. Whiteley. 2006. Special delivery: vesicle trafficking in prokaryotes. Mol. Microbiol. 61839-846. [DOI] [PubMed] [Google Scholar]
- 34.McCullen, C. A., and A. N. Binns. 2006. Agrobacterium tumefaciens and plant cell interactions and activities required for interkingdom macromolecular transfer. Annu. Rev. Cell Dev. Biol. 22101-127. [DOI] [PubMed] [Google Scholar]
- 35.Miller, S. I., M. Bader, and T. Guina. 2003. Bacterial vesicle formation as a mechanism of protein transfer to animals. Cell 1152-3. [DOI] [PubMed] [Google Scholar]
- 36.Miyawaki, K., P. Tarkowski, M. Matsumoto-Kitano, T. Kato, S. Sato, D. Tarkowska, S. Tabata, G. Sandberg, and T. Kakimoto. 2006. Roles of Arabidopsis ATP/ADP isopentenyltransferases and tRNA isopentenyltransferases in cytokinin biosynthesis. Proc. Natl. Acad. Sci. USA 10316598-16603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Powell, G. K., N. G. Hommes, J. Kuo, L. A. Castle, and R. O. Morris. 1988. Inducible expression of cytokinin biosynthesis in Agrobacterium tumefaciens by plant phenolics. Mol. Plant-Microbe Interact. 1235-242. [DOI] [PubMed] [Google Scholar]
- 38.Sagulenko, V., E. Sagulenko, S. Jakubowski, E. Spudich, and P. J. Christie. 2001. VirB7 lipoprotein is exocellular and associates with the Agrobacterium tumefaciens T pilus. J. Bacteriol. 1833642-3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakakibara, H. 2006. Cytokinins: activity, biosynthesis, and translocation. Annu. Rev. Plant Biol. 57431-449. [DOI] [PubMed] [Google Scholar]
- 40.Sakakibara, H., H. Kasahara, N. Ueda, M. Kojima, K. Takei, S. Hishiyama, T. Asami, K. Okada, Y. Kamiya, T. Yamaya, and S. Yamaguchi. 2005. Agrobacterium tumefaciens increases cytokinin production in plastids by modifying the biosynthetic pathway in the host plant. Proc. Natl. Acad. Sci. USA 1029972-9977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schafer, A., A. Tauch, W. Jager, J. Kalinowski, G. Thierbach, and A. Puhler. 1994. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 14569-73. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt-Eisenlohr, H., N. Domke, C. Angerer, G. Wanner, P. C. Zambryski, and C. Baron. 1999. Vir proteins stabilize VirB5 and mediate its association with the T pilus of Agrobacterium tumefaciens. J. Bacteriol. 1817485-7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmidt-Eisenlohr, H., N. Domke, and C. Baron. 1999. TraC of IncN plasmid pKM101 associates with membranes and extracellular high molecular weight structures in Escherichia coli. J. Bacteriol. 1815563-5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmidt-Eisenlohr, H., M. Rittig, S. Preithner, and C. Baron. 2001. Biomonitoring of pJP4-carrying Pseudomonas chlororaphis with Trb protein-specific antisera. Environ. Microbiol. 3720-730. [DOI] [PubMed] [Google Scholar]
- 45.Stachel, S. E., and P. C. Zambryski. 1986. Agrobacterium tumefaciens and the susceptible plant cell: a novel adaptation of extracellular recognition and DNA conjugation. Cell 47155-157. [DOI] [PubMed] [Google Scholar]
- 46.van Larebeke, N., G. Engler, M. Holsters, S. van den Elsacker, I. Zaenen, R. A. Schilperoort, and J. Schell. 1974. Large plasmids in Agrobacterium tumefaciens essential for crown gall-inducing ability. Nature 252169-170. [DOI] [PubMed] [Google Scholar]
- 47.Vergunst, A. C., B. Schrammeijer, A. den Dulk-Ras, C. M. de Vlaam, T. J. Regensburg-Tuink, and P. J. Hooykaas. 2000. VirB/D4-dependent protein translocation from Agrobacterium into plant cells. Science 290979-982. [DOI] [PubMed] [Google Scholar]
- 48.Vergunst, A. C., M. C. van Lier, A. den Dulk-Ras, T. A. Stuve, A. Ouwehand, and P. J. Hooykaas. 2005. Positive charge is an important feature of the C-terminal transport signal of the VirB/D4-translocated proteins of Agrobacterium. Proc. Natl. Acad. Sci. USA 102832-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ward, D. V., O. Draper, J. R. Zupan, and P. C. Zambryski. 2002. Peptide linkage mapping of the A. tumefaciens vir-encoded type IV secretion system reveals novel protein subassemblies. Proc. Natl. Acad. Sci. USA 9911493-11500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Winans, S. C. 1992. Two-way chemical signalling in Agrobacterium-plant interactions. Microbiol. Rev. 5612-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wood, D. W., J. C. Setubal, R. Kaul, D. Monks, L. Chen, G. E. Wood, Y. Chen, L. Woo, J. P. Kitajima, V. K. Okura, N. F. Almeida Jr., Y. Zhou, D. Bovee Sr., P. Chapman, J. Clendenning, G. Deatherage, W. Gillet, C. Grant, D. Guenthner, T. Kutyavin, R. Levy, M. Li, E. McClelland, A. Palmieri, C. Raymond, G. Rouse, C. Saenphimmachak, Z. Wu, D. Gordon, J. A. Eisen, I. Paulsen, P. Karp, P. Romero, S. Zhang, H. Yoo, Y. Tao, P. Biddle, M. Jung, W. Krespan, M. Perry, B. Gordon-Kamm, L. Liao, S. Kim, C. Hendrick, Z. Zhao, M. Dolan, S. V. Tingey, J. Tomb, M. P. Gordon, M. V. Olson, and E. W. Nester. 2001. The genome of the natural genetic engineer Agrobacterium tumefaciens C58. Science 2942317-2323. [DOI] [PubMed] [Google Scholar]
- 52.Yanisch-Perron, C., J. Viera, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequence of the M13mp18 and pUC18 vectors. Gene 33103-119. [DOI] [PubMed] [Google Scholar]
- 53.Yuan, Q., A. Carle, C. Gao, D. Sivanesan, K. Aly, C. Höppner, L. Krall, N. Domke, and C. Baron. 2005. Identification of the VirB4-VirB8-VirB5-VirB2 pilus assembly sequence of type IV secretion systems. J. Biol. Chem. 28026349-26359. [DOI] [PubMed] [Google Scholar]
- 54.Zhu, J., P. M. Oger, B. Schrammeijer, P. J. Hooykaas, S. K. Farrand, and S. C. Winans. 2000. The bases of crown gall tumorigenesis. J. Bacteriol. 1823885-3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zupan, J., T. R. Muth, O. Draper, and P. C. Zambryski. 2000. The transfer of DNA from Agrobacterium tumefaciens into plants: a feast of fundamental insights. Plant J. 2311-28. [DOI] [PubMed] [Google Scholar]