Abstract
The B-lymphotropic Epstein-Barr virus (EBV) encodes two isoforms of latent membrane protein 2 (LMP2), LMP2A and LMP2B, which are expressed during latency in B cells. The function of LMP2B is largely unknown, whereas LMP2A blocks B-cell receptor (BCR) signaling transduction and induction of lytic EBV infection, thereby promoting B-cell survival. Transfection experiments on LMP2B in EBV-negative B cells and the silencing of LMP2B in EBV-harboring Burkitt's lymphoma-derived Akata cells suggest that LMP2B interferes with the function of LMP2A, but the role of LMP2B in the presence of functional EBV has not been established. Here, LMP2B, LMP2A, or both were overexpressed in EBV-harboring Akata cells to study the function of LMP2B. The overexpression of LMP2B increased the magnitude of EBV switching from its latent to its lytic form upon BCR cross-linking, as indicated by a more-enhanced upregulation and expression of EBV lytic genes and significantly increased production of transforming EBV compared to Akata vector control cells or LMP2A-overexpressing cells. Moreover, LMP2B lowered the degree of BCR cross-linking required to induce lytic EBV infection. Finally, LMP2B colocalized with LMP2A as demonstrated by immunoprecipitation and immunofluorescence and restored calcium mobilization upon BCR cross-linking, a signaling process inhibited by LMP2A. Thus, our findings suggest that LMP2B negatively regulates the function of LMP2A in preventing the switch from latent to lytic EBV replication.
Epstein-Barr virus (EBV) is a ubiquitous B-lymphotropic gammaherpesvirus which persists after primary infection latently in the host for life and may switch periodically to its lytic form (28). In vitro, EBV undergoes very efficient growth transformation and immortalizes infected B cells by latent infection, resulting in lymphoblastoid cell lines (LCLs) expressing a limited number of viral genes, including six viral nuclear antigens (EBNAs) and latent membrane protein 1 (LMP1) and LMP2 (30). The ability to transform B cells implicates EBV as the culprit for a variety of malignancies, including Burkitt's lymphoma, Hodgkin's disease, and posttransplant lymphoproliferative disease (8, 24, 38). In vivo, EBV persists in latently infected memory B cells circulating in the peripheral blood (30). These latently infected cells do not express EBNAs or LMP1, but may express LMP2 (1, 2). Since LMP2 has no transformation capacity (12), this may suggest a pivotal role of LMP2 in the regulation of the balance between latent and lytic EBV.
Transcription of LMP2 is controlled by two promoters separated in the viral DNA by 3 kb (31). Two mRNAs that have different 5′ exons followed by eight common exons encode two distinct proteins, LMP2A and LMP2B, respectively. LMP2A contains an N-terminal cytoplasmic domain of 119 amino acids with eight tyrosines that are phosphorylated in LCLs, 12 transmembrane domains, and a C-terminal domain of 12 amino acids. LMP2A blocks B-cell receptor (BCR) signal transduction through specific phosphotyrosine motifs in its N-terminal domain and promotes B-cell survival. This function is dependent on the expression level of LMP2A (1, 2, 5, 6, 15, 21, 35). LMP2B lacks the entire N-terminal cytoplasmic domain. A recent work using transfection of LMP2 into EBV-negative cells has suggested possible roles for LMP2B. LMP2B colocalized with LMP2A in the membrane where the C terminus of both splice variants can interact and regulate the activity of each other (17). Furthermore, LMP2B was shown to negatively regulate LMP2A activity by interfering with its aggregation (29). Another study revealed protein domains of LMP2B which are required for intra- and extracellular localization and self-aggregation (37), which raised the question of whether the function of LMP2B in EBV is bound to its localization independently of LMP2A. Nevertheless, whether and how LMP2B is involved in the regulation of latent and lytic EBV infection in B cells harboring the functional virus remains a largely unresolved question.
Burkitt's lymphoma-derived Akata cells provide an optimal model to study the balance between latent and lytic EBV. Specifically, lytic EBV infection can be initiated in Akata cells by cross-linking their BCR using anti-immunoglobulin G (anti-IgG) (36). Importantly, upon induction of lytic EBV infection, the majority of viral genes are expressed (3, 39). Since these EBV genes could have an impact on the function of LMP2B, we used Akata cells to investigate the function of LMP2B in cells harboring functional EBV. Recently, we found that the silencing of LMP2B reduces susceptibility to induction of lytic EBV infection upon BCR cross-linking (27). This result indicated a role of LMP2B distinct from that of LMP2A in the regulation of EBV lytic activation. In this work, we further pursue the hypothesis that LMP2B exhibits a negative-regulatory effect on LMP2A maintenance of EBV latency. Thus, we compared the effects of overexpression of LMP2B and LMP2A on the susceptibility to induction of lytic EBV infection and on cellular signaling pathways in Akata cells.
MATERIALS AND METHODS
Cell lines and primary cells.
The Burkitt's lymphoma cell line Akata (36) was grown in RPMI 1640 supplemented with 10% fetal calf serum, l-glutamine (2 mM), penicillin (100 U/ml), and streptomycin (100 μg/ml). Akata cells were a kind gift of A. Bell (Birmingham, United Kingdom). Akata cells transfected with the plasmids pEneo (33), pEneo-LMP2A (27), and pEneo-FLAG-LMP2B, named Akata-vector (27), Akata-LMP2A (27), and Akata-LMP2B pools 1 to 3, respectively, were cultured in the same medium supplemented with 0.4 mg/ml G418 (Promega, Mannheim, Germany). Akata-cre cells that stably overexpress the regulatable creERT2 recombinase (9) integrated into the vector pcDNA3.1 were cultured in the same medium supplemented with 0.4 mg/ml G418 (Promega, Mannheim, Germany). Cord blood mononuclear cells (CBMC) were obtained from heparinized blood by Ficoll-Hypaque gradient centrifugation (Amersham Biosciences Europe GmbH, Otelfingen, Switzerland) and washed with phosphate-buffered saline (Gibco, Invitrogen Life Sciences, Basel, Switzerland). Informed consent was obtained from parturient women. The Zurich institutional ethics committee approved the collection and use of clinical material. B95.8 cells were cultured in Dulbecco's minimal essential medium supplemented with 10% fetal calf serum, l-glutamine (2 mM), penicillin (100 U/ml), and streptomycin (100 μg/ml) (23).
Plasmids.
The sequence coding for LMP2B was PCR amplified from the vector pSG5-LMP2 (15, 31) with following primers, including cloning adapters and 3× FLAG for LMP2B tagging: FLAG-LMP2B-F (5′-CGCGTTTAAACATGGACTACAAAGACCATGACGGTGATTATAAAGATCATGATATCGATTACAAGGATGACGATGACAAGAATCCAGTATGCCTGCCTG-3′) and LMP2-Rev (5′-GCGCTCGAGTTATACAGTGTTGCGATATGGGGTC-3′). The PCR product was cloned via PmeI/XhoI into the Moloney murine leukemia virus-derived pEneo bicistronic expression vector containing an internal ribosome entry site with a neomycin selection marker (33), resulting in the vectors pEneo-FLAG-LMP2B and pEneo-LMP2A (27). Positive clones were verified by sequencing. The parental pEneo plasmid was used as the control vector.
Transfection.
Cells (1 × 106) were electroporated with 2 μg of the pEneo, pEneo-LMP2A, or pEneo-FLAG-LMP2B plasmid with Nucleofector II (Amaxa GmbH, Cologne, Germany) with Buffer T and the program A-23. Electroporated cells were allowed to recover for 2 days and were then selected with 0.8 mg/ml G418 (Promega) for 2 to 3 weeks until resistant cells arose. These cells were named Akata-vector, Akata-LMP2A, and Akata-LMP2B pools 1 to 3, respectively. One month after selection, cells were supplemented with 0.4 mg/ml G418 and used for experiments. To generate double-transfected Akata cells, either stable Akata-LMP2B cells were electroporated transiently (t) with the vector pEneo-LMP2A (named 2B + At) or Akata-LMP2A cells were electroporated transiently with the vector pEneo-FLAG-LMP2B (named 2A + Bt).
Immunoblot analysis.
Total cellular protein extracts were prepared by disruption of cells in radioimmunoprecipitation assay lysis buffer (150 mM NaCl, 1% NP-40, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate [SDS], and 50 mM Tris, pH 7.5). After denaturation for 10 min in 4× loading LDS buffer (Invitrogen, Basel, Switzerland), samples were separated on a 10% Bis-Tris gel (Invitrogen) and transferred to a nitrocellulose membrane (Schweitzer & Schell Bioscience GmbH, Dassel, Germany). Membranes were blocked with 5% low-fat milk in 1× phosphate-buffered saline (PBS)-0.1% Tween 20 and incubated with either mouse anti-FLAG M2 (Sigma, St. Louis, MO), rat anti-LMP2A (clone 14B7) (5), or anti-c-myc (A-14; Santa Cruz Biotech, Inc., Santa Cruz, CA) antibody overnight at 4°C. Immunoreactive proteins were detected by the secondary antibodies against mouse and rabbit, respectively (Cell Signaling Technology, Danvers, MA) and an enhanced chemiluminescence detection kit (SuperSignal West Femto; Perbio Science Switzerland S.A., Lausanne, Switzerland).
IP.
Total cellular protein extracts were prepared by disruption of 5 × 106 cells in Tris nondenaturing lysis buffer (150 mM NaCl, 1% Triton X-100, 1 mM EDTA, and 50 mM Tris, pH 7.5), 2 mM NaF, and 2 mM Orthovanadate (all from Sigma). Isolated proteins were immunoprecipitated with a Sigma immunoprecipitation (IP) kit according to the manufacturer's instructions. IPs were performed with the same antibodies used for immunoblotting or without an antibody.
Immunostaining.
Cells (5 × 104) were centrifuged by Cytospin on double Cytofunnel glass slides (Thermo, Waltham, MA) and fixed with methanol for 10 min at −20°C. Cells were permeabilized with 1% Triton X-100 in 1× PBS for 20 min at room temperature. Immunoreactive proteins were detected with a rat anti-LMP2A antibody, 14B7 (1:100), and a secondary Alexa 488-labeled goat anti-rat IgG antibody diluted 1:200 (Molecular Probes, Invitrogen). FLAG-tagged LMP2B was detected with anti-FLAG M2 from Sigma (1:500) and a secondary Alexa 594-labeled goat anti-mouse IgG antibody diluted 1:200 (Molecular Probes, Invitrogen). BZLF1 was detected with anti-BZLF1 (Argene, North Massapequa, NY) diluted 1:100 and probed with the same secondary antibody used for FLAG detection.
Flow cytometry.
To evaluate the surface IgG (sIgG) content of transfected cells for later stimulation experiments with BCR cross-linking, cells were fixed in 4% paraformaldehyde (Sigma) and stained with a phycoerythrin-labeled anti-human IgG1,κ antibody (BD Biosciences, Basel, Switzerland). A phycoerythrin-labeled anti-mouse IgG1,κ antibody (BD Biosciences) was used as an isotype control. To determine the percentage of cells in which EBV was activated by BCR cross-linking, 0.5 × 106 cross-linked and non-cross-linked cells were stained with a fluorescein isothiocyanate (FITC)-labeled antiviral gp350/220 antibody diluted 1:10 (Biodesign International, Saco, ME) and analyzed by flow cytometry (FC-500; Beckmann-Coulter, Krefeld, Germany).
BCR cross-linking of Akata cells.
Akata cells were split to 0.5 × 106 cells/ml 24 h before BCR cross-linking. Cells (0.5 × 106/ml) were then BCR cross-linked with 0.1 μg/μl polyclonal rabbit anti-human-IgG (Dako, Zug, Switzerland) for 3 h and suspended afterwards in fresh RPMI 1640. Cross-linked and non-cross-linked cells were collected for subsequent analyses.
qRT-PCR.
Total RNA was extracted from 0.5 × 106 cells with an RNeasy kit from Qiagen (Hombrechtikon, Switzerland). DNase [DNA-free; Ambion (Europe), Huntington, Cambridgeshire, United Kingdom] treatment was performed before cDNA synthesis with an Omniscript RT kit (Qiagen). Quantitative real-time PCR (qRT-PCR) was done with validated TaqMan systems for the housekeeping gene HMBS and the lytic EBV genes BZLF1, BXLF1, and LMP2 (14) on an ABI 7200 (Applied Biosystems). TaqMan data were analyzed using SDS 2.2 (Applied Biosystems), and mRNA expression was normalized to HMBS mRNA, resulting in threshold cycle (ΔCT) values. ΔCT values were further normalized by dividing “cross-linked” by “not cross-linked” values, resulting in ΔΔCT values. qRT-PCR data for control vector ΔΔCT were set to 100% and LMP2B- or LMP2A-overexpressing cells were compared to it.
Transformation assay.
Fifty thousand freshly isolated CBMC per well were seeded in a 96-well plate. Culture supernatants were filtered through a 0.45-μm polyvinylidene difluoride Millex-HV filter (Millipore Corporation, MA). Fifty microliters of filtered culture supernatant of cells 24 h after anti-IgG cross-linking or no cross-linking was added to a final volume of 100 μl per well. A total of 10 wells for each filtered culture supernatant was plated and inoculated. Filtered supernatant from B95.8 served as the positive control, whereas the negative control was CBMC cultured with medium only (23). The transformation capacity was monitored by counting the wells after 6 weeks, when growth and clustering of cells could be observed (34). The percentage of transformation was calculated by setting 10 transformed wells to 100% and by normalization to the transformation capacity of the supernatant of cells that were not cross-linked, representing the spontaneous activation of EBV in Akata cells. Statistics were done with Prism 4 (GraphPad Software, Inc.).
Calcium mobilization.
Cells (5 × 106) were stained with Fluo-3 (6 μM final concentration) and Fura-Red (15 μM final concentration; both from Invitrogen) for 45 min in RPMI 1640 at 37°C. After a washing step with 1× PBS, cells were stored at room temperature in the dark. Before measurement, cells were incubated for 5 min at 37°C. Calcium mobilization was measured by adding ionomycin (2 μg/ml final concentration; Invitrogen) as the control and anti-IgG at the same concentration as in stimulation experiments (100 μg/ml) by using a FC-500 (Beckmann-Coulter) with an argon laser at 488 nm. Fluorescent emission was recorded at 520 nm (Fluo-3) and 670 nm (Fura-Red), and the Fluo-3/Fura-Red ratio was plotted against time. The baseline was recorded prior to anti-IgG addition for 30 s and for 5 min after BCR cross-linking. The increase over the baseline level was calculated for the time of peak of calcium mobilization (tp) by using FlowJo 5.7.2 software. The percentage of responding cells was calculated for the time slice from tp to tp + 2 min. As an additional control, Akata-cre cells were stimulated and the calcium mobilization was measured.
RESULTS
Construction of LMP2B-overexpressing Akata cells.
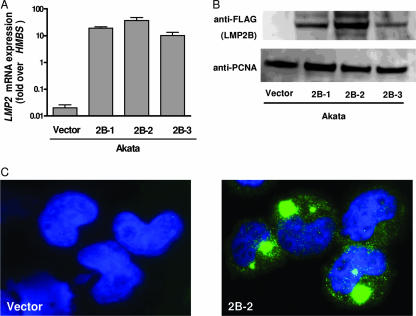
In order to investigate the effects of LMP2B on LMP2A and the switch from latent to lytic EBV replication, we first constructed EBV-harboring Akata cells overexpressing either LMP2B or LMP2A (27) and Akata cells with the vector control (27). Given that no specific antibody against LMP2B exists, we chose to tag LMP2B with a 3× FLAG sequence at the N terminus. The tag was placed at the N terminus, since it has been suggested that clustering of LMP2A and LMP2B occurs over the common C termini and that LMP2B influences the activity of its LMP2A isoform only when they colocalize (18, 29). As the transfection efficiency of B cells is low with common protocols, we decided to establish Akata cell pools stably overexpressing LMP2B, LMP2A, or a control vector. Thus, Akata cells were transfected independently with pEneo-FLAG-LMP2B, pEneo-LMP2A, or the control vector pEneo alone, as described in Materials and Methods. After neomycin selection, stable overexpression of LMP2B was verified for all three independently transfected Akata cell pools at the RNA level by qRT-PCR and at the protein level by immunoblotting and immunostaining. The three LMP2B-overexpressing cell pools (Akata-LMP2B pools 1, 2, and 3) showed different levels of overexpression of LMP2B after transfection (Fig. 1A). The mRNA expression levels correlated with protein levels, whereby Akata-LMP2B pool 1 showed a medium level, pool 2 a high level, and pool 3 the lowest level of LMP2B mRNA and protein, respectively (Fig. 1B). Immunostaining was done with Akata-LMP2B pool 2. Most overexpressed LMP2B localized to cytosolic compartments, whereas smaller amounts were detected in the plasma membrane (Fig. 1C). Furthermore, all cell lines were stained for sIgG and were found to express sIgG in similar percentages (Table 1). Thus, similar prerequisites for susceptibility to stimulation by BCR cross-linking with anti-IgG were ensured in the distinct cell lines.
FIG. 1.
Generation of LMP2B-overexpressing Akata cells. (A) Overexpression of LMP2B pools 1 to 3 (2B-1, 2B-2, 2B-3) by qRT-PCR using specific TaqMan systems targeting HMBS and LMP2 mRNA, respectively. (B) Immunoblot of FLAG-LMP2B pools 1 to 3 (2B-1, 2B-2, 2B-3). (C) Immunostaining of FLAG-LMP2B in Akata-vector control cells and Akata-LMP2B pool 2 (2B-2).
TABLE 1.
Characteristics of Akata cells useda
| Cell line | Vector construct | % sIgG+ cells | % Cells activated by gp350/220 staining
|
||
|---|---|---|---|---|---|
| 0 h | 24 h
|
||||
| Cross-linked | Not cross-linked | ||||
| Akatab | None | 96 ± 1 | ND | ND | ND |
| Akata-vectorb | pEneo | 92 ± 2 | 0.30 ± 0.11 | 4.62 ± 0.06 | 0.58 ± 0.09 |
| Akata-LMP2Bc | pEneo-FLAG-LMP2B | 88 ± 4 | 0.36 ± 0.20 | 5.42 ± 0.94 | 0.69 ± 0.23 |
| Akata-LMP2Ab | pEneo-LMP2A | 92 ± 1 | 0.24 ± 0.05 | 1.00 ± 0.81 | 0.34 ± 0.13 |
ND, not detected.
Values are presented as means ± SD from three independent experiments.
Values are presented as means ± SD from pools 1 to 3.
LMP2B overexpression increases the magnitude of EBV lytic activation after BCR cross-linking.
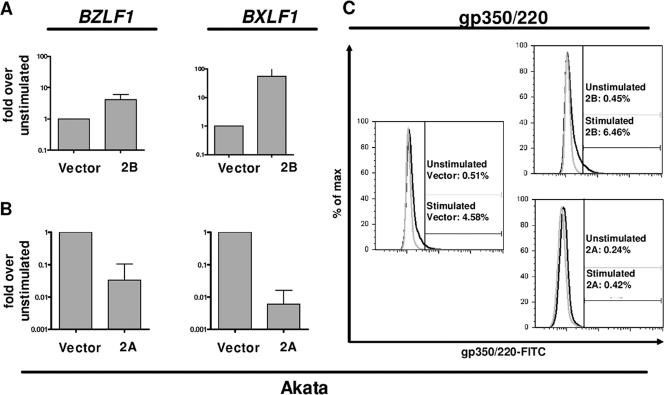
To assess the distinct effects of LMP2B and LMP2A on lytic activation of EBV in Akata cells, we stimulated Akata-LMP2B cells or Akata-LMP2A cells and the corresponding Akata-vector control cells by BCR cross-linking. After 24 h, cross-linked and non-cross-linked cells were collected and examined by qRT-PCR for expression of the immediate-early lytic gene BZLF1 and the early lytic gene BXLF1 encoding the viral thymidine kinase. The data were normalized to HMBS mRNA expression and are presented as ratios of cross-linked to non-cross-linked cells (Fig. 2). Akata-LMP2B cells showed mRNA expression levels of BZLF1 and BXLF1 that increased 4-fold and 55-fold, respectively (Fig. 2A). By contrast and as expected, transcription levels of lytic EBV genes were reduced in Akata-LMP2A cells, where expression of BZLF1 and BXLF1 mRNAs was reduced by 97% and 99%, respectively (Fig. 2B). To confirm these results at the protein level, we stained cells before and 24 h after BCR cross-linking with a FITC-labeled antiviral gp350/220 antibody in three independent stimulations. Indeed, as determined by flow cytometry, Akata-LMP2B cell pools expressed up to 5.4-fold- and 4.6-fold-higher gp350/220 levels than Akata-LMP2A cells and Akata-vector control cells, respectively (Fig. 2C; Table 1).
FIG. 2.
LMP2B overexpression increases the magnitude of EBV lytic activation after BCR cross-linking, whereas LMP2A overexpression results decreased magnitude. qRT-PCR with specific systems for HMBS, BZLF1, and BXLF1 mRNA, respectively, for Akata-vector control, Akata-LMP2B (A), and Akata-LMP2A (B) 24 h after BCR cross-linking. Means and standard deviations (SD) of qRT-PCR results are from three independent stimulation experiments on one representative polyclonal population. (C) Flow cytometry for gp350/220-FITC-labeled unstimulated (gray line) or stimulated (black line) cells 24 h after BCR cross-linking. One representative measurement is shown for Akata-vector control, Akata-LMP2B, and Akata-LMP2A cells with gp350/220-FITC-positive cells gated and indicated as percentages. Means and SD for three independent experiments are summarized in Table 1.
LMP2B-overexpressing Akata cells produce more infectious EBV than control cells upon BCR cross-linking.
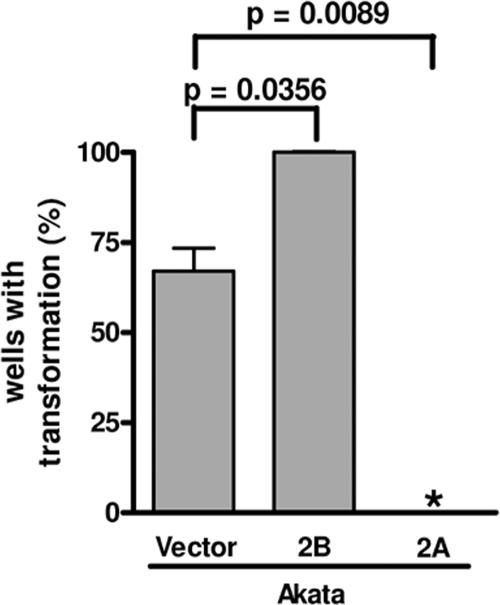
To verify the complete activation of the EBV lytic cycle following BCR cross-linking, the production of infectious EBV was monitored by the transformation of primary human B cells (34). Following BCR cross-linking of Akata-LMP2B, Akata-LMP2A, and Akata-vector control cells, supernatants were prepared from three independent experiments and added to freshly isolated CBMC to determine the transformation capacity of the infectious EBV produced. After normalization to non-cross-linked cells, the transformation capacities of supernatants from Akata-vector control cells were 67%, but those from Akata-LMP2B-cell pools were up to 100% (P = 0.0356), in contrast to the 0% transformation capacity of supernatants from Akata-LMP2A cells (P = 0.0089; Fig. 3).
FIG. 3.
More infectious EBV is produced in LMP2B-overexpressing Akata cells than in the control. Isolated CBMC were infected with supernatant from Akata-vector control, Akata-LMP2A, or Akata-LMP2B pools 1 to 3 collected 24 h after stimulation by BCR cross-linking. The transformation capacity was monitored by counting the wells after 6 weeks, at which time growth and clustering of cells indicated transformation. The percentage of transformation was calculated by setting 10 wells showing signs of transformation to 100% and by normalization to the transformation capacity of the supernatant of the corresponding BCR non-cross-linked control cells, representing the spontaneous EBV lytic activation in Akata cells. Means and SD are from three independent stimulation experiments with subsequent collection of supernatant and infection of CBMC. t tests were performed with a 95% confidence interval. *, no wells showed signs of transformation.
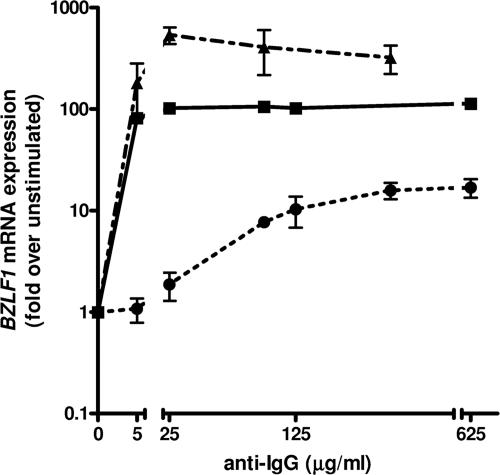
Overexpression of LMP2B decreases the degree of BCR stimulation required to induce lytic EBV infection, in contrast to overexpression of LMP2A.
LMP2A blocks BCR signaling, thereby impeding EBV lytic activation (6). It is not clear how the expression level of LMP2A influences the degree of BCR cross-linking (BCR activation) required to induce lytic EBV infection and how this is affected by LMP2B overexpression. Therefore, we assessed the magnitude of EBV lytic activation as a function of the anti-IgG dose to engage BCR by cross-linking Akata-vector, Akata-LMP2B, or Akata-LMP2A cells with increasing concentrations of anti-IgG. To quantify the activation of EBV lytic infection, mRNA expression of BZLF1 at 24 h after BCR cross-linking was measured (Fig. 4). Cross-linked Akata-vector control cells compared to non-cross-linked control cells showed an almost 100-fold increase of BZLF1 mRNA expression with the lowest anti-IgG concentration of 5 μg/ml and around 100-fold-higher peak BZLF1 mRNA expression levels with anti-IgG concentrations of 25 μg/ml or higher. Akata-LMP2B cells showed a similar but greater increase in BZLF1 mRNA expression and around fivefold-higher peak levels than Akata-vector cells with anti-IgG concentrations of 25 μg/ml or higher. By contrast, Akata-LMP2A cells required anti-IgG concentrations of at least 25 μg/ml to show an increase in BZLF1 mRNA expression and an anti-IgG concentration of 625 μg/ml to show maximal BZLF1 mRNA expression levels, which were around 10-fold lower than peak expression levels in Akata-vector cells. Thus, cells with higher expression levels of LMP2A required higher doses of anti-IgG to induce an EBV lytic activation, which was still of a considerably lower magnitude than that for Akata-vector control cells. These results suggest that the expression level of LMP2A has an impact on the amount of BCR cross-linking required to induce lytic EBV infection and that higher expression levels of LMP2A can be overridden, though only partially, with a higher degree of BCR cross-linking. On the other hand, higher LMP2B expression levels seemed to lower the degree of BCR cross-linking required to induce EBV lytic activation and to increase the magnitude of inducible EBV lytic activation.
FIG. 4.
Overexpression of LMP2B decreases the degree of BCR stimulation required to induce lytic EBV infection, in contrast to overexpression of LMP2A. BZLF1 mRNA expression in Akata-vector control (▪), Akata-LMP2A (•), or Akata-LMP2B (▴) 24 h after BCR cross-linking using increasing doses of anti-IgG. Means and SD are from three independent stimulation experiments on one representative polyclonal population.
LMP2B physically interacts with LMP2A before and after BCR cross-linking in Akata cells.
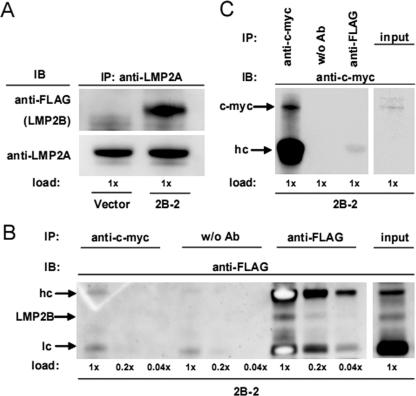
To investigate whether overexpressed LMP2B physically interacts with endogenous LMP2A, we isolated whole-cell protein extracts from Akata-LMP2B pool 2 (2B-2) and Akata-vector control cells with a subsequent pull-down IP of LMP2A. Subsequently, we performed immunoblotting against FLAG tags and LMP2A (Fig. 5A). Indeed, coimmunoprecipitated LMP2B was detected by anti-FLAG in Akata-LMP2B cells but not in Akata-vector control cells, whereas endogenous LMP2A was detected in both. Control IPs, with an anti-c-myc antibody or without antibody and subsequent immunoblotting against c-myc or FLAG (Fig. 5B and C) showed no unspecific pull-down, confirming the specificity of the IPs, despite the overexpression of FLAG-LMP2B.
FIG. 5.
LMP2B coimmunoprecipitates with LMP2A. Whole-cell protein lysates of Akata-LMP2B pool 2 (2B-2) and the Akata-vector control were immunoprecipitated (IP) with anti-LMP2A (A). The IPs were separated on a SDS gel and immunoblotted (IB) against FLAG and LMP2A. The heavy band in the LMP2A immunoblot represents the heavy Ig chain of the mouse antibody used for pull-down, whereas the lower, narrow band represents the equal amount of LMP2A pulled down in 2B-2 and vector input lysates. (B) The input lysate of 2B-2 was split into IPs without antibody, against c-myc, and FLAG and immunoblotted (three dilutions; 1×, 0.2×, 0.04×) against FLAG. No unspecific pull-down with anti-c-myc was observed due to the overexpression of FLAG-LMP2B. (C) The same IPs as those used in panel B were loaded on a SDS gel and immunoblotted against c-myc to verify that the IP was working. The upper band (65 kDa) and the lower band (63 kDa) in the input lysate of 2B-2 represent two forms of c-myc.
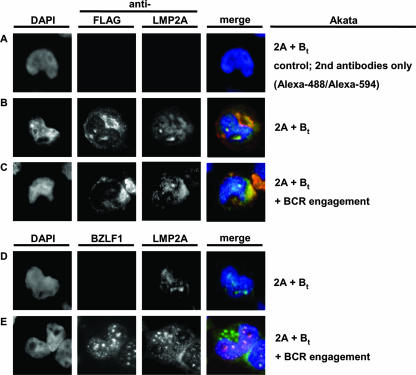
Next, to elucidate in which compartment LMP2B and LMP2A localize, we transfected Akata-LMP2A (2A) transiently with FLAG-LMP2B (Bt) (indicated as 2A + Bt) and immunostained LMP2A or FLAG-LMP2B for fluorescent microscopy. The images shown in Fig. 6B suggest partial colocalization of both LMP2 isoforms in the same cellular compartments (Fig. 6B). Additionally, we found an accumulation of LMP2B in the cytosolic region, as seen in stable Akata-LMP2B and described above (Fig. 1C). To determine if there is a relocalization of LMP2A or LMP2B upon BCR cross-linking, we double-stained Akata-LMP2A cells transiently transfected with FLAG-LMP2B (2A + Bt) for FLAG-LMP2B and for LMP2A (Fig. 6C), and for BZLF1 and LMP2A (Fig. 6D) after BCR cross-linking. An immunofluorescence analysis indicated a rather modest shift of LMP2A and LMP2B into the cytosol after BCR cross-linking, but still-adequate amounts of both LMP2s were located in the same compartments as before BCR cross-linking.
FIG. 6.
Overexpressed LMP2B and LMP2A colocalize before and after BCR cross-linking. (A) Negative control for secondary antibodies. (B) To investigate where LMP2B and LMP2A localize, we transiently transfected Akata-LMP2A cells with FLAG-LMP2B (2A + Bt) and stained for LMP2A and FLAG-LMP2B. To determine if there occurs a relocalization of LMP2A or LMP2B upon BCR cross-linking, we stained the double-transfected Akata cells (2A + Bt) for FLAG-LMP2B and for LMP2A (C) and for BZLF1 and LMP2A (D) 3 h after BCR cross-linking (E). DAPI, 4′,6′-diamidino-2-phenylindole.
LMP2B restores calcium mobilization in LMP2A-overexpressing Akata cells.
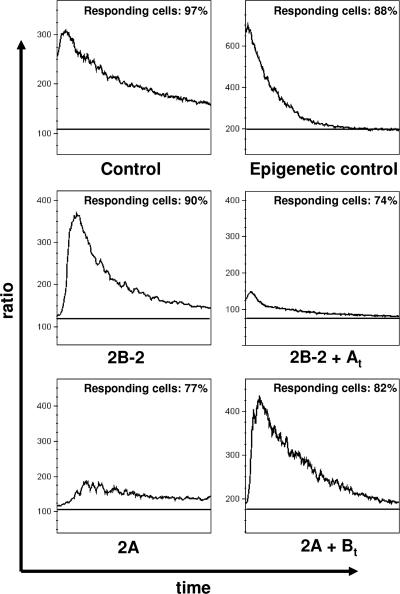
It has been previously established that LMP2A blocks calcium mobilization induced by BCR cross-linking (20-22). To investigate the impact of LMP2B overexpression on calcium mobilization, we determined the calcium levels before (baseline) and after BCR cross-linking, monitoring the kinetics for 5 min in Akata-vector control, Akata-LMP2B pool 2 (2B-2), and Akata-LMP2A (2A) cells, respectively. Additionally, Akata-cre cells overexpressing creERT2 recombinase in the cytosol were stimulated and monitored in parallel to exclude any epiphenomena due merely to overexpression which could influence calcium mobilization. Calcium mobilization reached up to 3-fold, 3.3-fold, and 3.5-fold peaks compared to baseline levels after BCR cross-linking in Akata-vector control cells, Akata-cre cells, and Akata-LMP2B cells, respectively (Fig. 7). Next, we generated double transfectants by electroporation of Akata-LMP2B cells transiently with the vector pEneo-LMP2A (2B + At) as described in Materials and Methods and measured the calcium mobilization after BCR cross-linking. We observed not only a decrease of calcium mobilization from 3.5-fold to 1.9-fold in Akata-LMP2B cells but also a decrease of responding cells from 90% to 74%. These results are in agreement with previous studies (20-22). The calcium mobilization in Akata-LMP2A cells revealed the same low calcium mobilization and percentage of responding cells as observed in the double-transfected 2B + At cells (1.8-fold and 77%, respectively). To address the question of whether it is possible to rescue the phenotype of Akata-vector control cells, we transiently transfected Akata-LMP2A cells with the vector pEneo-FLAG-LMP2B (2A + Bt). Interestingly, the calcium mobilization was restored to Akata-vector control cell levels from 1.8- to 2.9-fold after BCR cross-linking. Additionally, responding cells increased from 77% to 82% in double-transfected 2A + Bt cells (Fig. 7).
FIG. 7.
LMP2B restores calcium mobilization in LMP2A-overexpressing Akata cells. Calcium levels were determined before and after BCR cross-linking, and the kinetics were measured for 5 min. Upper panels, calcium mobilization after BCR cross-linking in Akata control cells and, as an additional control, in Akata cells with epigenetic overexpression of an unspecific protein in the cytosol (see Materials and Methods). Middle panels, calcium mobilization after BCR cross-linking in Akata-LMP2B cells without (2B) and with (2B + At) transiently transfected LMP2A. Lower panels, calcium mobilization after BCR cross-linking in Akata-LMP2A cells without (2A) and with (2A + Bt) transiently transfected FLAG-LMP2B. Baselines were measured just before BCR cross-linking. The percentage of responding cells was calculated as described in Materials and Methods.
DISCUSSION
In this work, we investigated the impact of LMP2B on the potential of LMP2A to maintain EBV in its latent state. We demonstrate that LMP2B increases the magnitude of EBV switching from its latent to its lytic form upon BCR cross-linking, lowers the degree of BCR cross-linking required to provoke this switching, and is involved in augmenting signaling via calcium mobilization upon BCR cross-linking in Akata cells harboring functional EBV. These observations suggest a negative regulatory effect of LMP2B on the ability of LMP2A to block BCR signaling, thereby preventing EBV from switching from latent to lytic infection in B cells.
Although LMP2A and LMP2B are similar in their structure, the lack of the signaling amino-terminal domain in LMP2B indicates distinct functions for these two proteins. Indeed, the overexpression of LMP2B in Akata cells resulted in higher mRNA expression of immediate-early and early lytic EBV genes upon BCR cross-linking, whereas the overexpression of LMP2A results in lower mRNA expression in these genes. Moreover, LMP2B overexpression leads as well to production of more EBV envelope protein gp350/220 and functional virus than do LMP2A-overexpressing or control Akata cells upon BCR cross-linking. These findings are compatible with our previous observation that silencing of LMP2B in Akata cells reduces the susceptibility of these cells to undergo EBV lytic activation induced by BCR cross-linking (27). Thus, our current and previous data provide evidence that LMP2B is involved in the regulation of EBV switching from latent to lytic infection EBV in B cells harboring the whole virus in its latent form.
The overexpression of LMP2B did not result in spontaneous switching of latent to lytic EBV. Nevertheless, the higher magnitude of EBV lytic activation in LMP2B-overexpressing Akata cells than that in control Akata cells upon BCR cross-linking with similar doses of anti-IgG suggested that LMP2B exerts its mode of action through lowering the required degree of BCR cross-linking and thus BCR signaling needed. Since LMP2A blocks BCR signaling (5, 6, 12, 15, 21, 29, 30, 35), we addressed the fundamental question of whether the magnitude of EBV lytic activation at given expression levels of the LMP2s depends on the dose of anti-IgG required to cross-link BCR, i.e., the degree of BCR cross-linking. Indeed, although increasing doses of anti-IgG elevated the levels of activation of lytic EBV in LMP2A-overexpressing Akata cells until they reached a plateau, peak levels of induced lytic EBV in these cells were around at least 10-fold lower than in control Akata cells or around 30- to 50-fold lower than in LMP2B-overexpressing Akata cells. This result suggests that both endogenous and overexpressed LMP2A is able to reduce BCR signaling very effectively and cannot be completely counteracted, even by saturated levels of anti-IgG added for cross-linking. Thus, the amount of overexpressed LMP2B was able to decrease the activity of endogenous LMP2A on BCR signaling but could not abolish it completely in whole-EBV-containing cells.
An important question to be addressed was if there is an interaction of LMP2A and LMP2B in Akata cells harboring EBV and, if so, where the two isoforms of LMP2 colocalize. The physical interaction between LMP2A and LMP2B was verified by pulling down FLAG-LMP2B with endogenous LMP2A. Our immunostaining results suggest an accumulation of LMP2B in intracellular compartments and to a lesser extent on the plasma membranes of Akata cells harboring whole EBV. Lynch et al. (17) reported that transiently expressed LMP2B localized to perinuclear regions and colocalized with transiently or constitutively expressed LMP2A in EBV-negative BJAB cells or LCL B95-8CR, respectively. Studies using HEK 293 cells overexpressing full-length or deletion mutants of LMP2A revealed a clustering signal of LMP2A at the C terminus leading to homodimerization (18). As LMP2A and LMP2B share eight exons and the C terminus, Rovedo and Longnecker (29) hypothesized that LMP2B colocalizes with LMP2A, forming heterodimers, and subsequently negatively regulates LMP2A activity, leading to decreased degradation of the tyrosine kinase Lyn. A more-recent study showed that when tagged LMP2B was overexpressed in BJAB cells or HEK 293T cells, LMP2B was found exclusively in intracellular perinuclear compartments (29, 37), a result which is in apparent contrast with the results of the aforementioned study. When LMP2B was truncated at any domain, it resulted in localization to the cell surface. Based on these results taken together, one can hypothesize that the major impact of LMP2B on LMP2A takes place in endosomes, where it interferes either with the activity of LMP2A and subsequent ubiquitination and degradation of Lyn or with the trafficking of LMP2A back to the plasma membrane.
An immunofluorescence analysis of LMP2A and LMP2B suggested a colocalization of LMP2A and LMP2B in Akata cells overexpressing both proteins. We detected a rather modest shift of LMP2B and LMP2A into the cytosol, which suggests their internalization after BCR cross-linking. Nevertheless, a large amount of both LMP2s remains detectable on the plasma membrane, demonstrating an intact turnover process. Moreover, this experiment allowed us to exclude the possibility of misfolded and degraded protein in the endoplasmic reticulum due to overexpression, as has been reported in earlier studies (10, 19). It is known that LMP2A aggregates in lipid rafts, assembling as a signalosome which enables a transient interaction with the tyrosine kinases Syk and Lyn with a subsequent block of the BCR signal (5-7, 15, 16, 21, 25, 26, 35). One can hypothesize that after BCR cross-linking, the anti-IgG/BCR complex is internalized together with closely located lipid rafts and LMP2A signalosomes. If there is an additional function of LMP2A in preventing the whole complex from being transported again to the cell membrane, in this way blocking continuous stimulation, one can hypothesize that there is a loss of LMP2A at the cell surface and an accumulation in endosomes located in the cytosol after BCR cross-linking. LMP2B, which is found in cytosolic compartments, may intervene in this step, disrupting homodimerized LMP2A and restoring the turnover.
As demonstrated here for the first time, calcium mobilization upon BCR cross-linking is dependent on the expression level of LMP2B in EBV-harboring Akata cells. As expected from previous reports (20-22), we observed virtually no calcium mobilization in LMP2A-overexpressing cells upon BCR cross-linking. By contrast, after transient transfection of LMP2B into LMP2A-overexpressing Akata cells, calcium mobilization after BCR cross-linking is increased to levels comparable to those observed for Akata cells overexpressing LMP2B. Conversely, we measured a reduced calcium mobilization in LMP2B-overexpressing Akata cells transiently transfected with LMP2A. As transiently transfected vectors expressing the gene of interest lead to high levels of protein, the dominant effect on the stably transfected Akata cells was predictable. Three different signaling pathways which are activated upon BCR cross-linking in Akata cells have been analyzed: (i) calcium mobilization through phosphatidylinositol 3-kinase, (ii) c-Jun N-terminal kinase activation through Syk and Lyn signaling, and (iii) ERK1/2 phosphorylation through the RAS protein (4). Nevertheless, whether LMP2B is involved partially or throughout all these signaling cascades, taking a key regulatory function upstream, remains unknown. Rovedo and Longnecker showed recently that in the EBV-negative B-cell line BJAB, ectopically expressed LMP2B decreases the activity of LMP2A by alteration of the phosphorylation status (29). Thus, LMP2B would function at an initial step of BCR signaling to restore BCR signal transduction which was blocked by LMP2A. As a more downstream read-out, they chose to measure the calcium mobilization in BJAB cells upon BCR cross-linking. As has previously been shown, the overexpression of LMP2A nearly abolished calcium mobilization (20-22). According to the hypothesis of BCR signal restoration by LMP2B, ectopic overexpression of both splice variants of LMP2 together resulted in the phenotype of BJAB transfected with vector control only.
LMP2B may have important functions not only in the modulation of latent and lytic EBV infection in tumor cells or memory B cells in the periphery. As was reported previously, LMP2A is expressed in newly infected naïve B cells before latency is established and serves as a tonic signal for survival. This scenario might be true for B cells with crippled or a total loss of BCR expression, leading in the worst case to Hodgkin's lymphoma (11, 13). In contrast, if a naïve B cell which has a functional BCR is newly infected and receives the survival signal, the additional signal of LMP2A might resemble in total an activated BCR, forcing EBV to lytic infection. In the presence of LMP2B, the signal of LMP2A would be downregulated, not leading to activated lytic EBV as was suggested previously for high levels of LMP2A (32).
In conclusion, the data presented here provide evidence that LMP2B is involved in the regulation of switching from latent to lytic EBV in B cells harboring functional EBV. Based on our present and previous findings for Akata cells (27) together with observations made for the EBV-negative cell line BJAB (29), we suggest that LMP2B has an impact on the activity of LMP2A, resulting in increased susceptibility to induction of lytic EBV infection through modulation of BCR and downstream signaling.
Acknowledgments
This work was supported by the Cancer League of the Canton of Zurich, the Edoardo, R. Giuseppe and Christina Sassella Foundation, and the Novartis Foundation for Research in Medical Biology.
Footnotes
Published ahead of print on 5 December 2007.
REFERENCES
- 1.Babcock, G. J., D. Hochberg, and A. D. Thorley-Lawson. 2000. The expression pattern of Epstein-Barr virus latent genes in vivo is dependent upon the differentiation stage of the infected B cell. Immunity 13497-506. [DOI] [PubMed] [Google Scholar]
- 2.Babcock, G. J., and D. A. Thorley-Lawson. 2000. Tonsillar memory B cells, latently infected with Epstein-Barr virus, express the restricted pattern of latent genes previously found only in Epstein-Barr virus-associated tumors. Proc. Natl. Acad. Sci. USA 9712250-12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernasconi, M., C. Berger, J. A. Sigrist, A. Bonanomi, J. Sobek, F. K. Niggli, and D. Nadal. 2006. Quantitative profiling of housekeeping and Epstein-Barr virus gene transcription in Burkitt lymphoma cell lines using an oligonucleotide microarray. Virol. J. 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bryant, H., and P. J. Farrell. 2002. Signal transduction and transcription factor modification during reactivation of Epstein-Barr virus from latency. J. Virol. 7610290-10298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fruehling, S., S. K. Lee, R. Herrold, B. Frech, G. Laux, E. Kremmer, F. A. Grasser, and R. Longnecker. 1996. Identification of latent membrane protein 2A (LMP2A) domains essential for the LMP2A dominant-negative effect on B-lymphocyte surface immunoglobulin signal transduction. J. Virol. 706216-6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fruehling, S., and R. Longnecker. 1997. The immunoreceptor tyrosine-based activation motif of Epstein-Barr virus LMP2A is essential for blocking BCR-mediated signal transduction. Virology 235241-251. [DOI] [PubMed] [Google Scholar]
- 7.Fukuda, M., and R. Longnecker. 2005. Epstein-Barr virus (EBV) latent membrane protein 2A regulates B-cell receptor-induced apoptosis and EBV reactivation through tyrosine phosphorylation. J. Virol. 798655-8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gottschalk, S., C. M. Rooney, and H. E. Heslop. 2005. Post-transplant lymphoproliferative disorders. Annu. Rev. Med. 5629-44. [DOI] [PubMed] [Google Scholar]
- 9.Indra, A. K., X. Warot, J. Brocard, J. M. Bornert, J. H. Xiao, P. Chambon, and D. Metzger. 1999. Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: comparison of the recombinase activity of the tamoxifen-inducible Cre-ER(T) and Cre-ER(T2) recombinases. Nucleic Acids Res. 274324-4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim, P. S., and P. Arvan. 1998. Endocrinopathies in the family of endoplasmic reticulum (ER) storage diseases: disorders of protein trafficking and the role of ER molecular chaperones. Endocr. Rev. 19173-202. [DOI] [PubMed] [Google Scholar]
- 11.Kluiver, J., K. Kok, I. Pfeil, D. de Jong, T. Blokzijl, G. Harms, P. van der Vlies, A. Diepstra, C. Atayar, S. Poppema, R. Kuppers, and A. van den Berg. 2007. Global correlation of genome and transcriptome changes in classical Hodgkin lymphoma. Hematol. Oncol. 2521-29. [DOI] [PubMed] [Google Scholar]
- 12.Konishi, K., S. Maruo, H. Kato, and K. Takada. 2001. Role of Epstein-Barr virus-encoded latent membrane protein 2A on virus-induced immortalization and virus activation. J. Gen. Virol. 821451-1456. [DOI] [PubMed] [Google Scholar]
- 13.Küppers, R., I. Schwering, A. Brauninger, K. Rajewsky, and M. L. Hansmann. 2002. Biology of Hodgkin's lymphoma. Ann. Oncol. 13(Suppl. 1)11-18. [DOI] [PubMed] [Google Scholar]
- 14.Ladell, K., M. Dorner, L. Zauner, C. Berger, F. Zucol, M. Bernasconi, F. K. Niggli, R. F. Speck, and D. Nadal. 2007. Immune activation suppresses initiation of lytic Epstein-Barr virus infection. Cell. Microbiol. 92055-2069. [DOI] [PubMed] [Google Scholar]
- 15.Longnecker, R., B. Druker, T. M. Roberts, and E. Kieff. 1991. An Epstein-Barr virus protein associated with cell growth transformation interacts with a tyrosine kinase. J. Virol. 653681-3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Longnecker, R., and E. Kieff. 1990. A second Epstein-Barr virus membrane protein (LMP2) is expressed in latent infection and colocalizes with LMP1. J. Virol. 642319-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lynch, D. T., J. S. Zimmerman, and D. T. Rowe. 2002. Epstein-Barr virus latent membrane protein 2B (LMP2B) co-localizes with LMP2A in perinuclear regions in transiently transfected cells. J. Gen. Virol. 831025-1035. [DOI] [PubMed] [Google Scholar]
- 18.Matskova, L., I. Ernberg, T. Pawson, and G. Winberg. 2001. C-terminal domain of the Epstein-Barr virus LMP2A membrane protein contains a clustering signal. J. Virol. 7510941-10949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meusser, B., C. Hirsch, E. Jarosch, and T. Sommer. 2005. ERAD: the long road to destruction. Nat. Cell Biol. 7766-772. [DOI] [PubMed] [Google Scholar]
- 20.Miller, C. L., A. L. Burkhardt, J. H. Lee, B. Stealey, R. Longnecker, J. B. Bolen, and E. Kieff. 1995. Integral membrane protein 2 of Epstein-Barr virus regulates reactivation from latency through dominant negative effects on protein-tyrosine kinases. Immunity 2155-166. [DOI] [PubMed] [Google Scholar]
- 21.Miller, C. L., J. H. Lee, E. Kieff, and R. Longnecker. 1994. An integral membrane protein (LMP2) blocks reactivation of Epstein-Barr virus from latency following surface immunoglobulin crosslinking. Proc. Natl. Acad. Sci. USA 91772-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller, C. L., R. Longnecker, and E. Kieff. 1993. Epstein-Barr virus latent membrane protein 2A blocks calcium mobilization in B lymphocytes. J. Virol. 673087-3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller, G., and M. Lipman. 1973. Release of infectious Epstein-Barr virus by transformed marmoset leukocytes. Proc. Natl. Acad. Sci. USA 70190-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murray, P. G., and L. S. Young. 2002. The Role of the Epstein-Barr virus in human disease. Front. Biosci. 7d519-d540. [DOI] [PubMed] [Google Scholar]
- 25.Portis, T., and R. Longnecker. 2004. Epstein-Barr virus (EBV) LMP2A alters normal transcriptional regulation following B-cell receptor activation. Virology 318524-533. [DOI] [PubMed] [Google Scholar]
- 26.Portis, T., and R. Longnecker. 2004. Epstein-Barr virus (EBV) LMP2A mediates B-lymphocyte survival through constitutive activation of the Ras/PI3K/Akt pathway. Oncogene 238619-8628. [DOI] [PubMed] [Google Scholar]
- 27.Rechsteiner, M. P., C. Berger, M. Weber, J. A. Sigrist, D. Nadal, and M. Bernasconi. 2007. Silencing of latent membrane protein 2B reduces susceptibility to activation of lytic Epstein-Barr virus in Burkitt's lymphoma Akata cells. J. Gen. Virol. 881454-1459. [DOI] [PubMed] [Google Scholar]
- 28.Rickinson, A., and E. Kieff. 2001. Epstein-Barr virus, p. 2575-2627. In Knipe, D. M., P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 29.Rovedo, M., and R. Longnecker. 2007. Epstein-Barr virus latent membrane protein 2B (LMP2B) modulates LMP2A activity. J. Virol. 8184-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rowe, D. T. 1999. Epstein-Barr virus immortalization and latency. Front. Biosci. 4D346-D371. [DOI] [PubMed] [Google Scholar]
- 31.Sample, J., D. Liebowitz, and E. Kieff. 1989. Two related Epstein-Barr virus membrane proteins are encoded by separate genes. J. Virol. 63933-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaadt, E., B. Baier, J. Mautner, G. W. Bornkamm, and B. Adler. 2005. Epstein-Barr virus latent membrane protein 2A mimics B-cell receptor-dependent virus reactivation. J. Gen. Virol. 86551-559. [DOI] [PubMed] [Google Scholar]
- 33.Schaefer, B. C., T. C. Mitchell, J. W. Kappler, and P. Marrack. 2001. A novel family of retroviral vectors for the rapid production of complex stable cell lines. Anal. Biochem. 29786-93. [DOI] [PubMed] [Google Scholar]
- 34.Sugden, B., and W. Mark. 1977. Clonal transformation of adult human leukocytes by Epstein-Barr virus. J. Virol. 23503-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swart, R., I. K. Ruf, J. Sample, and R. Longnecker. 2000. Latent membrane protein 2A-mediated effects on the phosphatidylinositol 3-kinase/Akt pathway. J. Virol. 7410838-10845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takada, K. 1984. Cross-linking of cell surface immunoglobulins induces Epstein-Barr virus in Burkitt lymphoma lines. Int. J. Cancer 3327-32. [DOI] [PubMed] [Google Scholar]
- 37.Tomaszewski-Flick, M. J., and D. T. Rowe. 2007. Minimal protein domain requirements for the intracellular localization and self-aggregation of Epstein-Barr virus latent membrane protein 2. Virus Genes 35225-234. [DOI] [PubMed] [Google Scholar]
- 38.Young, L. S., and P. G. Murray. 2003. Epstein-Barr virus and oncogenesis: from latent genes to tumours. Oncogene 225108-5121. [DOI] [PubMed] [Google Scholar]
- 39.Yuan, J., E. Cahir-McFarland, B. Zhao, and E. Kieff. 2006. Virus and cell RNAs expressed during Epstein-Barr virus replication. J. Virol. 802548-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]