Abstract
The infectivity of flock house virus (FHV) requires autocatalytic maturation cleavage of the capsid protein at residue 363, liberating the C-terminal 44-residue γ peptides, which remain associated with the particle. In vitro studies previously demonstrated that the amphipathic, helical portion (amino acids 364 to 385) of γ is membrane active, suggesting a role for γ in RNA membrane translocation during infection. Here we show that the infectivity of a maturation-defective mutant of FHV can be restored by viruslike particles that lack the genome but undergo maturation cleavage. We propose that the colocalization of the two defective particle types in an entry compartment allows the restoration of infectivity by γ.
The process of membrane fusion, orchestrated by enveloped viruses, is now moderately well understood (12, 17-19), but understanding of the mechanism for membrane translocation of the genomes of nonenveloped viruses is still being developed. A common theme in nonenveloped virus entry has, however, emerged—the involvement of small, amphipathic or hydrophobic viral polypeptides (1). One or more such polypeptides have been discovered in Poliovirus (VP4 and the N terminus of VP1) (10), Reovirus (μ1N) (5, 6), and Adenovirus (protein VI) (21). Flock house virus (FHV), a nonenveloped insect virus of the family Nodaviridae, contains a similar peptide, γ, that is postulated to permeabilize membranes during virus entry (7). A synthetic polypeptide corresponding to the N-terminal amphipathic helix of γ is partitioned into lipid bilayers and releases dye from liposomes (2, 3, 11). FHV infectivity requires the autocatalytic cleavage of the 43-kDa capsid protein α at residue 363 (α → β + γ) (16), making the γ peptide (amino acids 364 to 407) covalently independent but still associated with the particle. The γ peptides are located predominantly in the interior of the FHV capsid but can be transiently exposed on the capsid surface (4). The capacity for transient exposure is likely to be essential for the process of membrane penetration during viral entry. Interestingly, entry intermediates of FHV, called eluted particles, have lost 25% of their γ peptides (20) while retaining overall capsid architecture, underscoring the role of γ in the early stages of entry. In spite of the obvious importance of this peptide in promoting FHV infectivity, little is known about the behavior or localization of γ in host cells.
To understand the role of γ in vivo, we developed an approach for supplying the γ peptide in trans during FHV entry. Two different FHV constructs were generated in separate cell culture systems. The first construct was a virus containing two point mutations in capsid protein α: a threonine replacing asparagine at position 363 (N363T) and an asparagine replacing aspartate at position 75 (D75N). It was shown previously that either mutation separately blocks the maturation cleavage of α (16, 22), and both substitutions were included in this construct, designated N363T/D75N FHV, to reduce the possibility of reversion to the wild-type FHV sequence. N363T/D75N FHV, although it packages the FHV genome, is noninfectious due to the lack of cleaved γ. It was generated by the transfection of Drosophila sp. cells (Schneider's line 1) with mutated viral genomic RNA (13), leading to defective virus production in transfected cells. Although N363T/D75N FHV particles cannot propagate through normal infection, sufficient quantities were purified from transfected cells for the experiments described herein.
The second construct utilized was a viruslike particle (VLP), generated by expressing the wild-type FHV coat protein α from a baculovirus vector in Spodoptera frugiperda cells (line IPLB-Sf21), as described previously (15). The VLPs undergo autocatalytic maturation cleavage to produce γ and were indistinguishable from authentic FHV by X-ray crystallography (unpublished result). They package insect cellular RNA instead of the FHV genome and are therefore replication incompetent and noninfectious.
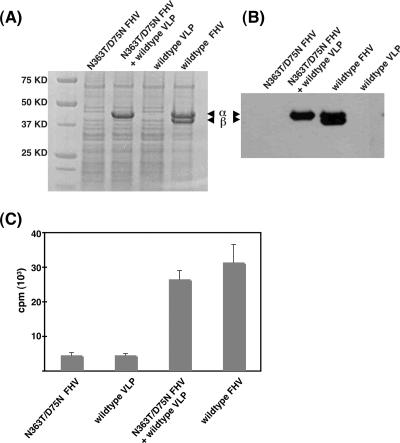
We confirmed that both FHV constructs were noninfectious in a control experiment. Drosophila DL-1 cells were infected with N363T/D75N FHV or wild-type VLP for 15 h and lysed with 0.5% NP-40, virus particles were pelleted through a 30% sucrose cushion in 50 mM HEPES (pH 7.0), and the pellets were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by Coomassie blue staining (Fig. 1A) or immunoblotting with an anti-FHV antibody (Fig. 1B). No progeny corresponding to that produced by wild-type FHV could be detected in the virus pellets from cells infected with N363T/D75N FHV or wild-type VLP. In contrast, when DL-1 cells were coinfected with N363T/D75N FHV and wild-type VLP for 15 h, FHV capsid protein was detected in the pellets by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting (Fig. 1A and B). Gel electrophoretic analysis revealed that virus produced during this coinfection was the maturation-defective N363T/D75N FHV mutant. Thus, coinfection with wild-type VLPs launched the genome packaged in the N363T/D75N FHV particles, and the mutant genome gave rise to maturation-defective progeny virions.
FIG. 1.
Analysis of progeny virus synthesized in singly or doubly infected Drosophila cells. (A and B) Drosophila DL-1 cells were infected with wild-type FHV at 3 ×103 particles/cell (corresponding to a MOI of 10), N363T/D75N FHV at 3 ×103 particles/cell (corresponding to a MOI of 10), or wild-type VLP at 1.8 ×104 particles/cell (corresponding to a MOI of 60) or coinfected with N363T/D75N FHV at 3 ×103 particles/cell and wild-type VLP at 1.8 ×104 particles/cell. At 15 h postinfection, virus was partially purified by pelleting through a 30% sucrose cushion, and the pellets were subjected to Coomassie blue staining (A) and immunoblotting with a polyclonal anti-FHV antibody (B). The positions of FHV coat protein α and cleavage product β are indicated. The γ peptide (4 kDa) ran off the gel under these conditions and is not visible. (C) At 15 h postinfection, progeny virus was labeled with [35S]methionine-cysteine for 2 h, purified on a 10 to 40% sucrose gradient, and quantified using liquid scintillation counting.
We wanted to ensure that virus being detected during coinfection was progeny and not input virus. To this end, we metabolically labeled newly synthesized protein in virus-infected cells with [35S]methionine-cysteine (Fig. 1C). Radiolabel was added 15 h postinfection, since it was previously demonstrated that the rate of FHV coat protein production in infected cells reaches its peak around this time (9). Drosophila DL-1 cells (108) were infected separately with wild-type FHV, N363T/D75N FHV, or wild-type VLP or coinfected with N363T/D75N FHV and wild-type VLP. After 15 h, cells were washed twice with methionine- and cysteine-free Sf-900 II serum-free medium (GIBCO) and incubated in the same medium with 0.6 mCi EasyTag express protein labeling mix (PerkinElmer) at 27°C for 2 h. Cells were harvested and lysed with 0.5% NP-40, total virus was pelleted through a 30% sucrose cushion, and virus in the resultant pellet was further purified on a 10 to 40% sucrose gradient in 50 mM HEPES, pH 7.0. The gradient was fractionated on an Isco fractionator, and the labeled progeny virus was quantified by measuring the amount of 35S in the peak fractions (Fig. 1C). While cells infected with wild-type FHV and those coinfected with N363T/D75N FHV and wild-type VLP produced similar amounts of labeled progeny virus, the quantities of progeny obtained from cells infected separately with either N363T/D75N FHV or wild-type VLPs were far lower (∼14% of the amount of progeny pelleted from FHV-infected cells) (Fig. 1C).
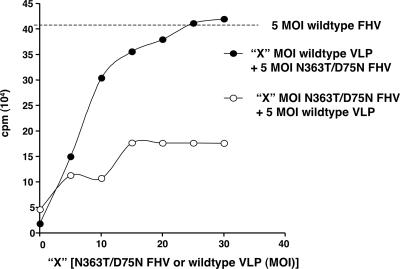
In order to quantitatively determine the efficiency of coinfection, we compared the amount of 35S-labeled progeny produced by cells infected with wild-type FHV at a multiplicity of infection (MOI) of 5 (corresponding to 1.5 ×103 particles/cell) with that produced during coinfection with different quantities of N363T/D75N FHV and wild-type VLP (Fig. 2). Since both N363T/D75N FHV and wild-type VLP are noninfectious, their concentrations described in terms of MOI reflect a virtual value, calculated as the number of wild-type FHV particles needed to achieve that desired MOI. Coinfecting cells with wild-type VLP at a MOI of 5 (1.5 ×103 particles/cell) and increasing amounts of N363T/D75N FHV restored some infectivity, as the amount of 35S-labeled progeny produced increased linearly at first, but the amount then plateaued quickly, presumably due to the limited amount of γ available from VLP (Fig. 2). In contrast, when N363T/D75N FHV at a MOI of 5 was added to cells along with increasing amounts of wild-type VLP, infectivity increased linearly with the amount of VLP added. When wild-type VLP was added at a MOI of 30 (corresponding to 9 ×103 particles/cell) during this coinfection, the quantity of radiolabled progeny virus produced was equivalent to that produced by infecting cells with wild-type FHV at a MOI of 5 (Fig. 2). These data suggest that given enough γ peptide, the noninfectious mutant N363T/D75N FHV can be just as infectious as wild-type FHV.
FIG. 2.
Yield of N363T/D75N FHV progeny as a function of the amount of γ in trans. DL-1 cells were coinfected with a constant amount of wild-type VLP and increasing amounts (X) of N363T/D75N FHV or with a constant amount of N363T/D75N FHV and increasing amounts of wild-type VLP. The amount of gradient-purified, radiolabeled progeny virus was quantified by liquid scintillation counting. The amount of 35S-labeled progeny produced by infecting cells with wild-type FHV at a MOI of 5 (1.5 ×103 particles/cell) is represented by a dashed line. Three hundred FHV particles corresponds to a MOI of 1.
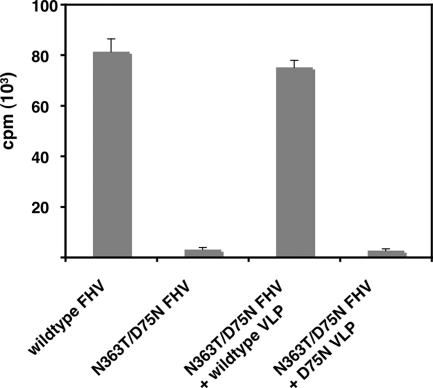
To determine if the transactivation of N363T/D75N FHV infectivity was indeed due to the supply of γ from wild-type VLP, DL-1 cells were coinfected with N363T/D75N FHV and a mutated, cleavage-defective VLP (D75N VLP). This coinfection produced ∼4% of the quantity of 35S-labeled progeny produced by coinfection with N363T/D75N FHV and wild-type VLP (Fig. 3), indicating that the presence of cleaved γ in VLP was of primary importance for restoring infectivity to N363T/D75N FHV.
FIG. 3.
Transactivation requires γ. The amounts of 35S-labeled progeny produced by coinfecting Drosophila DL-1 cells with N363T/D75N FHV at a MOI of 5 (1.5 ×103 particles/cell) and either wild-type or D75N VLP at a MOI of 30 (9 ×103 particles/cell) were quantified and compared. The amounts of radiolabeled progeny virus produced by separate infections with wild-type FHV and N363T/D75N FHV, both at a MOI of 5 (1.5 ×103 particles/cell), are also presented for comparison.
Our data show that providing γ peptide from a replication-incompetent VLP can restore the infectivity of a maturation-defective mutant of FHV. This remarkable result resolves several points about the role of γ in FHV entry. First, the restoration of infectivity by membrane-active (2, 3) γ supports the hypothesis that γ is the permeabilization protein of FHV and is required for membrane disruption during entry. Second, conformational changes necessary for genome escape still occur in the N363T/D75N capsid. That the mutant particles can provide their genome for replication indicates that genome release from FHV is independent of capsid architectural changes triggered by the release of γ. Third, the rupture of cellular membrane vesicles elicited by γ is general enough to facilitate genome entry from other virions and not only from the particle from which γ came. The escape of replication-competent genomes from N363T/D75N FHV particles probably requires that these particles be incarcerated in the same membrane-bound compartment as the VLPs that provide γ for the disruption of that particular compartment.
There is a precedent for nonenveloped virus permeabilization proteins functioning in trans. The reovirus membrane-interacting protein μ1 undergoes autocatalytic cleavage to produce an N-terminal, amphipathic peptide, μ1N, analogous to FHV γ. Virions with an N42A mutation in μ1, which cannot undergo this cleavage, are not competent for endosomal escape. Cleaved μ1N from a noninfectious empty wild-type reovirus can function in trans to facilitate the cytoplasmic entry of μ1 N42A mutant virions (14). Similarly, the entry of a mutant parvovirus deficient in membrane disruption ability is facilitated by coinfection with adenovirus (8). It is tempting to think that permeabilization proteins of other nonenveloped viruses may function in a similar, general way, rather than in a particle-specific manner, to trigger the disruption of host plasma or endosomal membranes. The striking parallels observed among the cell entry mechanisms of these diverse pathogens suggest convergent evolution of a protein-lipid permeabilization system among nonenveloped viruses.
Acknowledgments
We acknowledge support by NIH grants GM034220 (J.E.J.) and GM053491 (A.S.).
Footnotes
Published ahead of print on 12 December 2007.
REFERENCES
- 1.Banerjee, M., and J. E. Johnson. Activation, exposure and penetration of virally encoded, membrane-active polypeptides during nonenveloped virus entry. Curr. Protein Peptide Sci., in press. [DOI] [PubMed]
- 2.Bong, D. T., A. Janshoff, C. Steinem, and M. R. Ghadiri. 2000. Membrane partitioning of the cleavage peptide in flock house virus. Biophys. J. 78839-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bong, D. T., C. Steinem, A. Janshoff, J. E. Johnson, and M. Reza Ghadiri. 1999. A highly membrane-active peptide in Flock House virus: implications for the mechanism of nodavirus infection. Chem. Biol. 6473-481. [DOI] [PubMed] [Google Scholar]
- 4.Bothner, B., X. F. Dong, L. Bibbs, J. E. Johnson, and G. Siuzdak. 1998. Evidence of viral capsid dynamics using limited proteolysis and mass spectrometry. J. Biol. Chem. 273673-676. [DOI] [PubMed] [Google Scholar]
- 5.Chandran, K., D. L. Farsetta, and M. L. Nibert. 2002. Strategy for nonenveloped virus entry: a hydrophobic conformer of the reovirus membrane penetration protein μ1 mediates membrane disruption. J. Virol. 769920-9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandran, K., and M. L. Nibert. 2003. Animal cell invasion by a large nonenveloped virus: reovirus delivers the goods. Trends Microbiol. 11374-382. [DOI] [PubMed] [Google Scholar]
- 7.Cheng, R. H., V. S. Reddy, N. H. Olson, A. J. Fisher, T. S. Baker, and J. E. Johnson. 1994. Functional implications of quasi-equivalence in a T = 3 icosahedral animal virus established by cryo-electron microscopy and X-ray crystallography. Structure 2271-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farr, G. A., L. G. Zhang, and P. Tattersall. 2005. Parvoviral virions deploy a capsid-tethered lipolytic enzyme to breach the endosomal membrane during cell entry. Proc. Natl. Acad. Sci. USA 10217148-17153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallagher, T. M., and R. R. Rueckert. 1988. Assembly-dependent maturation cleavage in provirions of a small icosahedral insect ribovirus. J. Virol. 623399-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hogle, J. M. 2002. Poliovirus cell entry: common structural themes in viral cell entry pathways. Annu. Rev. Microbiol. 56677-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janshoff, A., D. T. Bong, C. Steinem, J. E. Johnson, and M. R. Ghadiri. 1999. An animal virus-derived peptide switches membrane morphology: possible relevance to nodaviral transfection processes. Biochemistry 385328-5336. [DOI] [PubMed] [Google Scholar]
- 12.Kielian, M. 2006. Class II virus membrane fusion proteins. Virology 34438-47. [DOI] [PubMed] [Google Scholar]
- 13.Marshall, D., and A. Schneemann. 2001. Specific packaging of nodaviral RNA2 requires the N-terminus of the capsid protein. Virology 285165-175. [DOI] [PubMed] [Google Scholar]
- 14.Odegard, A. L., K. Chandran, X. Zhang, J. S. Parker, T. S. Baker, and M. L. Nibert. 2004. Putative autocleavage of outer capsid protein μ1, allowing release of myristoylated peptide μ1N during particle uncoating, is critical for cell entry by reovirus. J. Virol. 788732-8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneemann, A., R. Dasgupta, J. E. Johnson, and R. R. Rueckert. 1993. Use of recombinant baculoviruses in synthesis of morphologically distinct viruslike particles of flock house virus, a nodavirus. J. Virol. 672756-2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneemann, A., W. Zhong, T. M. Gallagher, and R. R. Rueckert. 1992. Maturation cleavage required for infectivity of a nodavirus. J. Virol. 666728-6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sieczkarski, S. B., and G. R. Whittaker. 2005. Viral entry. Curr. Top. Microbiol. Immunol. 2851-23. [DOI] [PubMed] [Google Scholar]
- 18.Skehel, J. J., and D. C. Wiley. 2000. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu. Rev. Biochem. 69531-569. [DOI] [PubMed] [Google Scholar]
- 19.Tamm, L. K., and X. Han. 2000. Viral fusion peptides: a tool set to disrupt and connect biological membranes. Biosci. Rep. 20501-518. [DOI] [PubMed] [Google Scholar]
- 20.Walukiewicz, H. E., J. E. Johnson, and A. Schneemann. 2006. Morphological changes in the T=3 capsid of Flock House virus during cell entry. J. Virol. 80615-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiethoff, C. M., H. Wodrich, L. Gerace, and G. R. Nemerow. 2005. Adenovirus protein VI mediates membrane disruption following capsid disassembly. J. Virol. 791992-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zlotnick, A., V. S. Reddy, R. Dasgupta, A. Schneemann, W. J. Ray, Jr., R. R. Rueckert, and J. E. Johnson. 1994. Capsid assembly in a family of animal viruses primes an autoproteolytic maturation that depends on a single aspartic acid residue. J. Biol. Chem. 26913680-13684. [PubMed] [Google Scholar]