Abstract
The mechanisms mediating protective immunity to hepatitis C virus (HCV) infection are incompletely understood because early infection in humans is rarely identified, particularly in those individuals who subsequently demonstrate spontaneous virus eradication. We have established a large national network of patients with acute HCV infection. Here, we comprehensively examined total HCV-specific CD4+ and CD8+ T-cell responses and identified functional T-cell thresholds that predict recovery. Interestingly, we found that the presence of HCV-specific cytotoxic T lymphocytes (CTLs) that can proliferate, exhibit cytotoxicity, and produce gamma interferon does not ensure recovery, but whether these CTLs were primed in the presence or absence of CD4+ T-cell help (HCV-specific interleukin-2 production) is a critical determinant. These results have important implications for early prediction of the virologic outcome following acute HCV and for the development of novel immunotherapeutic approaches.
Hepatitis C virus (HCV) is the major causative agent of chronic hepatitis, with an estimated global prevalence of 3% (31). Patients who develop chronic infection are at risk of developing hepatic fibrosis, liver failure, and hepatocellular carcinoma. The mechanisms by which HCV establishes viral persistence in the vast majority of exposed individuals remains incompletely understood. Considerable evidence indicates that the outcome of acute HCV infection is determined by the magnitude, diversity, and quality of the adaptive immune response (3). In particular, a vigorous, multispecific CD4+ T-cell response that is maintained long term appears to be a prerequisite for spontaneous recovery from HCV infection (6, 7, 11, 16, 17, 23). By contrast, persistence is predicted by a failure to generate or sustain HCV antigen-driven proliferation and production of Th1 cytokines by CD4+ T cells (3, 16, 22, 27). Moreover, this outcome can be recapitulated by anti-CD4 antibody treatment of immune chimpanzees, indicating that loss of HCV-specific T-cell help is a central event contributing to immune evasion (3, 10). HCV-specific CD8+ T cells or cytotoxic T lymphocytes (CTLs) that target multiple epitopes, with frequencies often exceeding 3 to 4%, characterize successful responses (3). However, as shown elegantly by Cox and colleagues (5), the majority of acutely infected patients demonstrate HCV-specific CTLs irrespective of the subsequent virologic outcome (although CD4+ T cells were not examined in the study). Emerging data indicate that the phenotypic characteristics of CTLs are also important, as shown by the recent demonstration that the loss of CD127 expression on HCV-specific CTLs predates the development of persistence in patients with acute HCV infection (8, 28).
In the present study, we comprehensively assessed HCV genome-wide CD4+ and CD8+ T cells in patients with acute infection followed prospectively who developed well-defined virologic outcomes, i.e., recovery versus chronicity. For the first time, we identified a quantitative threshold of gamma interferon (IFN-γ)-producing T cells that was correlated with spontaneous recovery from acute HCV infection. We found highly variable immune response patterns in the acute phase of HCV infection, including the presence of vigorous HCV-specific CTLs with or without IFN-γ-producing CD4+ T cells. Notably, the presence of HCV-specific CTLs that produced IFN-γ and displayed cytotoxicity did not ensure spontaneous recovery from acute HCV infection; however, adequate help (i.e., HCV-specific interleukin-2 [IL-2] production by CD4+ T cells) during the primary exposure to HCV was essential to promoting CTL effectors into long-lived effector memory cells conferring immune protection. These results have important implications for early prediction of the virologic outcome following acute HCV and for the development of novel immunotherapeutic approaches.
MATERIALS AND METHODS
Study population and HCV testing.
The study group was comprised of acutely HCV-infected patients recruited from multiple sites (Portland, Seattle, Memphis, and Pittsburgh). The study protocol was approved by all appropriate institutional review boards. Acute HCV was diagnosed based on HCV antibody seroconversion in a subject with previously negative HCV test results, an HCV antibody-positive test in a subject with new-onset risk factors and an alanine aminotransferase (ALT) level greater than 10 times normal, or HCV RNA positivity with HCV antibody negativity. Thirty-one treatment-naïve patients (including 16 men) were recruited for the present study; the mean and median ages were 36 and 33, respectively. The majority of patients were Caucasian (84%), and injection drug use was the primary risk factor in 42% of the patients. Spontaneous virus resolution and chronicity were defined as the absence or presence of HCV RNA at 6 months postenrollment with at least two (range, two to five) virus determinations.
The duration of infection describes the time from the estimated date of HCV acquisition to enrollment. For subjects with known iatrogenic exposures, the acquisition date was defined as the date of exposure. For subjects who lacked known iatrogenic exposures but who reported symptoms, the acquisition date was defined as 6 weeks before the onset of symptoms. Among the remaining participants, the midpoint between the last negative antibody test and the earlier of the first positive antibody test or positive HCV RNA test was used as the date of acquisition.
Plasma preparation tubes (Becton Dickinson) were used to isolate plasma from whole blood at months 0, 2, 4, 6, 9, and 12, which was frozen and later thawed for viral-load and genotype or serotype (Abbott Murex HCV Serotyping 1-6 assay; HC03) testing. HCV genotyping (line probe assay) and viral-level determination (HCV RNA 3.0 branched chain DNA; lower limit, 615 copies/ml) were performed by the Bayer Reference Testing Laboratory (Berkeley, CA).
Synthetic peptides for T-cell analysis.
Overlapping peptides (n = 750) were synthesized to span the complete amino acid sequence of the HCV polyprotein derived from HCV type 1 (genotype 1a; accession number M62321) and divided evenly into subgenomic peptide pools (Fig. 1) (25, 26). These pentadecamers (15-mer peptides) overlapping by 11 amino acids were resuspended at 20 mg/ml in dimethyl sulfoxide (DMSO) and then concentrated so that the final volume of DMSO in the assay would not exceed 0.5%.
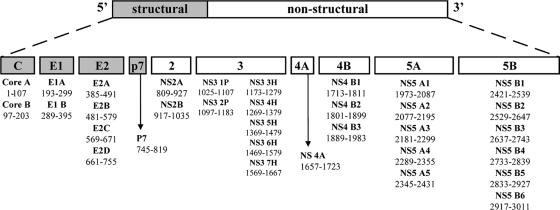
FIG. 1.
Peptide pools used for stimulation in ELISPOT assays. The hepatitis C virus polyprotein (genotype 1a; 3,010 amino acids; 750 peptides in total) is composed of four structural proteins (Core to p7) and six nonstructural proteins (NS2 to NS5B). This polyprotein was divided into 33 peptide pools (with 18 to 25 overlapping peptides in each pool), as described in Materials and Methods.
Cell preparation.
Peripheral blood mononuclear cells (PBMCs) from a unit blood draw or following leukophoresis were separated on Ficoll-Histopaque density gradients (Amersham Pharmacia Biotech, Sweden) and then cryopreserved in medium containing 80% fetal calf serum, 10% DMSO, and 10% RPMI 1640 (Gibco) with penicillin-streptomycin according to the manufacturer's recommendation. Generation of monocyte-derived dendritic cells (DCs) was based upon the method described by Romani and colleagues (21) and by us (25).
ELISPOT assays.
IFN-γ production was detected using an established enzyme-linked immunospot (ELISPOT) protocol (25, 26). Magnetic-bead-purified CD8+ T cells (1.25 × 105) were cultured with peptide-pulsed DCs, and 2.5 × 105 “CD4+ others” (PBMCs depleted of CD8+ cells) were incubated in plates for 40 h before the plates were developed. The spots were visualized with a peroxidase substrate kit (Vector; SK-4200). After the plates were dry, the spots were quantified using AID Elispot Reader version 3.4, build 1428 (Strasberg, Germany). Responses to peptide pools were calculated following subtraction of the spots in the eight control wells (medium plus DMSO alone). The percentage of CD4+ T cells used in each ELISPOT assay was calculated by analyzing the CD4 content of the CD8-depleted cells by flow cytometry. The spot number was then adjusted for and reported as spots per 2.5 × 105 CD4+ cells. The breadth for each subject was determined by counting the pools that had a qualitative response, defined as 3 standard deviations above the mean in the control wells that contained DMSO but no peptide and at least 5 spot-forming units above background (25).
Antibodies for analysis of cell surface antigen and intracellular expression/fluorescence-activated cell sorter (FACS) analysis.
Multiparameter flow cytometry was performed using a BD FACSAria instrument (BD Biosciences) compensated with single fluorochromes and analyzed using Diva software (BD Biosciences). Fluorochrome-labeled (fluorescein isothiocyanate, phycoerythrin [PE], PE-Cy5, allophycocyanin [APC]-Cy7, Pacific Blue, and APC) monoclonal antibodies specific for CD4, CD8, CD62 ligand (CD62L), CD25, CD69, and intracellular IFN-γ were obtained from BD Biosciences. Anti-CD127 was supplied by R&D systems (Minneapolis, MN). Anti-PD-1 fluorescein isothiocyanate/APC was obtained from eBioscience (San Diego, CA). Anti-CD3 Texas Red was supplied by Immunotech (Fullerton, CA) and anti-CD8-AF405 by Caltag (Invitrogen, Carlsbad, CA). PBMCs (1 × 106 to 2 × 106) were stained for cell surface antigen expression at 4°C in the dark for 30 min and then washed twice in 2 ml phosphate-buffered saline containing 1% bovine serum albumin and 0.01% sodium azide and subsequently fixed in 200 μl of 1% paraformaldehyde (Sigma-Aldrich, St. Louis, MO). Intracellular staining for IFN-γ was carried out using Caltag Fix and Perm media and protocol. Isotype-matched control antibodies and fluorescence minus one (FMO) control stains were used to determine background levels of staining. Patients expressing the appropriate HLA class I alleles were also assessed for antigen-specific responses to HCV by pentamer staining as described previously (9). PE-labeled Pro5 pentamers were supplied by ProImmune (Springfield, VA). For flow cytometric analysis of antigen-specific cells, a minimum of 1 × 105 CD8+ events were acquired for each pentamer stain.
Analysis of antigen-specific CD8+ T-cell responses by FACS.
Overlapping HCV peptides that elicited ELISPOT responses were used to assess intracellular production of IFN-γ by FACS. In addition, 32 CEF peptides (AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, NIH) derived from human cytomegalovirus, Epstein-Barr virus, and influenza virus were used as positive controls. PBMCs were cultured at 37°C and 5% CO2 in polypropylene culture tubes set at an angle of 45° at a concentration of 5 million cells/ml in RPMI culture medium (supplemented with 10% normal human serum). The cultures were stimulated with 3 μg/ml anti-CD28 and anti-CD49d (BD Biosciences) in the presence of CEF peptides, DMSO, or specific HCV pools shown to elicit ELISPOT responses (final concentration, 10 μg/ml peptide and 0.5% DMSO). To measure degranulation, 10 μl of PE-Cy5-labeled anti-CD107a (BD Biosciences) was added to the wells at the beginning of the stimulation. Following 2 h of stimulation, 4 μl of brefeldin A (Sigma) was added to the wells to inhibit secretion of IFN-γ, and the cells were cultured for a further 4 h. The cells were then harvested, washed, and stained for flow cytometric analysis using anti-CD3-Texas Red (Immunotech), anti-CD8-AF405 (Caltag), and anti-IFN-γ-PE (BD Biosciences). CTLs were identified by initially gating them on lymphocytes and then on CD8+ CD3+ T cells within the lymphocyte population. Antigen-specific CTLs (HCV/CEF) were identified as IFN-γ-positive cells within the CTL population. Interval gates were set using FMO and isotype controls. CD107a positivity was defined as the percentage of the population staining positive for PE-CY5 above negative controls.
CTL proliferation assays.
CD4+ cells were depleted from whole PBMCs using magnetic beads (Miltenyi Biotech) according to the manufacturer's instructions. These “CD8 others” were stained with carboxyfluorescein diacetate succinimidyl ester (CFSE) (10 μmol/liter; Molecular Probes, Eugene, OR) for 10 min, washed, and resuspended at 5 million cells/ml for proliferation assays. The CFSE cultures were stimulated with 3 μg/ml anti-CD28 (BD Biosciences) and anti-CD49d (BD Biosciences) in the presence of HCV peptide (final concentration, 10 μg/ml) specific for a pentamer to which an individual patient had known reactivity. Cultures were performed at 37°C and 5% CO2 in polypropylene culture tubes set at an angle of 45° for 7 days. Blocking antibodies against PD-L1 and PD-L2 (eBiosciences) were added to the cultures at a concentration of 1 μg/ml. Cells were harvested and stained with anti-CD3, -CD8, -PD-1, -CD62L, and -CD127 and an appropriate pentamer. The phenotype of CFSE and its loss in pentamer-positive populations were analyzed by flow cytometry.
Assessment of CD4 help.
The CD4+ cell fractions of PBMCs used in the CTL proliferation assays were stimulated for 48 h in the presence of HCV proteins (10-μg/ml final concentration of each protein) and lymphoid cell lines (LCLs) derived from the same patient. In a 250-μl volume in 96-well plates, 2.5 × 105 CD4+ cells were cultured in duplicate wells with 1 × 104 autologous LCLs that had been pulsed with either HCV core and NS3 (Mikrogen), HCV NS4 and NS5 (Chiron), or relevant negative controls. After 48 h, the cell supernatants were examined by Luminex for cytokine production.
Luminex assays.
Samples were transferred to MultiScreen filter plates (Millipore, Billerica, MA) and assayed with Beadlyte technology (Upstate, Charlottesville, VA) in conjunction with a Luminex100 IS System (Luminex Corp., Austin, TX) to determine the quantities of IL-2 and IL-10 within these samples. Duplicate samples and standards were processed according to Multiple Cytokine Detection Protocol B (Upstate), opting for overnight incubation with Beadmates from Upstate's Human Multi-Cytokine Flex Kit (catalog no. 48-505; bead no. 06, 21, 34, 36, and 53). Beadlyte standards 4 and 6 (catalog no. 47-015 and 47-022) were mixed and serially diluted 1:2 in tissue culture medium for maximum detection range. The results were analyzed using five-parameter logistic curves (fluorescence intensity versus pg/ml) generated by Luminex100 IS software (version 2.3).
Statistical analyses.
Results were expressed as medians and means ± standard deviations and analyzed using paired and unpaired Student's t, χ2, nonparametric Mann-Whitney U, or Wilcoxon rank sum tests where appropriate. Correlation between different parameters was performed using Spearman's rank test. Logistic regression methodology (12) was used for prediction models of the binary response variable “resolved or persistent” following acute HCV infection. A P value of less than 0.05 was considered significant. The JMP 6.0 (SAS Institute, Inc., Cary NC) statistical package and Prizm 5.01 (GraphPad Software, San Diego CA) were used.
RESULTS
Breadth and vigor of CD4+ and CD8+ T-cell responses in patients with acute HCV infection: definition of a quantitative threshold associated with recovery.
PBMCs from the earliest time point after HCV infection were studied in 17 patients with acute infections who subsequently developed viral persistence and 14 patients with acute-resolving infections. In order to characterize the total HCV-specific immune response, overlapping peptides (n = 750) derived from genotype 1a were synthesized and pooled into 33 subgenomic regions to stimulate T cells in an ELISPOT assay using CD4+ and purified CD8+ T cells as previously described (25, 26) (Fig. 1).
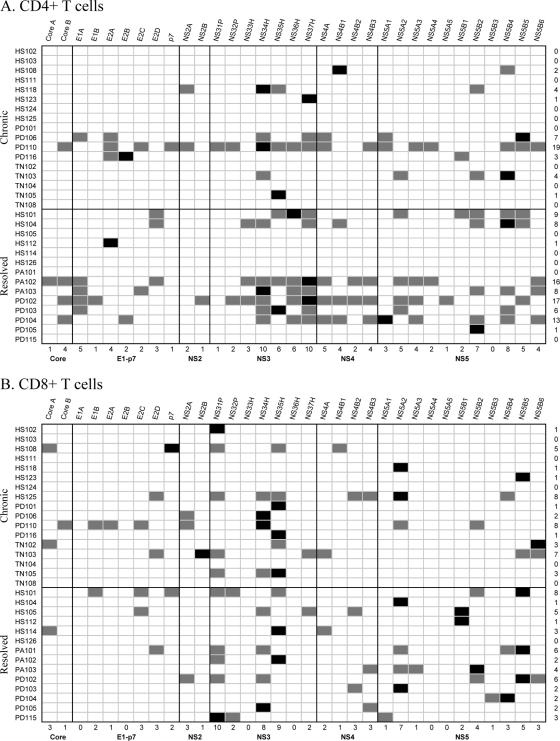
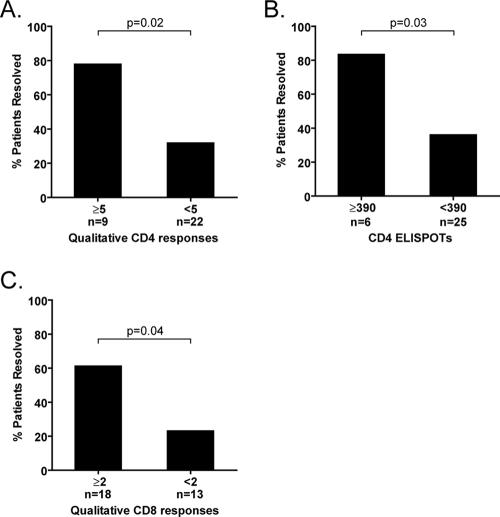
The T-cell response in acute HCV infection demonstrates striking heterogeneity; indeed, essentially any peptide pool can be targeted. Figure 2 shows HCV pools that elicited CD4+ and CD8+ T-cell responses and their relative immunodominance. Irrespective of the ultimate virologic outcome, the strongest CD4+ or CD8+ T-cell response was most frequently noted within the nonstructural 3 (NS3) or NS5 region. Among patients who demonstrated CD4+ T-cell responses to five or more pools, 78% (7 of 9) later spontaneously resolved HCV infection, whereas only 32% (7/22) of the patients who targeted less than five peptide pools with CD4+ T cells subsequently cleared HCV (Fig. 3A) (odds ratio, 7.5; confidence interval, 1.4 to 60; P = 0.03; logistic regression analysis). As shown in Fig. 2, 7 of the 14 (50%) patients who resolved infection demonstrated responses to five or more pools, whereas only 2 out of the 17 (12%) patients who developed chronicity did so. Moreover, patients displaying more than 390 ELISPOTs per 2.5 × 105 CD4+ T cells (∼0.15% total CD4+ T cells) had eight times greater odds of spontaneously eradicating HCV than patients who did not reach this threshold (83.3 versus 36%; 5 of 6 versus 9 of 25; odds ratio, 8.8; P = 0.03) (Fig. 3B).
FIG. 2.
Summary of the HCV peptide pool specificities recognized by each individual with acute-chronic and acute-resolving HCV infections. All 31 study patients are shown. The HCV pools are grouped according to HCV protein. The grey-shaded boxes indicate qualitatively positive CD4+ T-cell responses (A) or CD8+ T-cell responses (B). The black boxes represent the strongest CD4+ or CD8+ T-cell responses in an individual subject. The first column indicates the patient identifier, and the far-right column shows the total number of pools recognized for each individual patient. The bottom row represents the number of patients in the cohort who demonstrated qualitative responses to individual peptide pools. Among the 14 patients with acute-resolving infections, 29 of the 33 distinct HCV pools elicited responses from CD4+ T cells and 23 distinct pools elicited responses from CD8+ T cells.
FIG. 3.
T-cell thresholds associated with spontaneous recovery following acute HCV infection. Using 33 subgenomic pools spanning the entire HCV polyprotein, total T-cell responses were assessed by IFN-γ ELISPOT assays. (A) For CD4+ T cells, qualitative responses to five or more pools at the earliest time point (enrollment) were associated with a statistically higher rate of recovery (P = 0.02 by logistic regression analysis). (B) More than 80% of patients demonstrating more than 390 CD4+ T-cell ELISPOTs per 2.5 × 105 CD4+ T cells spontaneously recovered from HCV infection, whereas less than 40% not meeting this quantitative threshold did so. The total sum of ELISPOTs for 33 peptide pools was added for each individual subject. (C) For CD8+ T cells, qualitative responses to two or more HCV peptide pools was associated with a statistically higher rate of recovery (P = 0.04; logistic regression analysis).
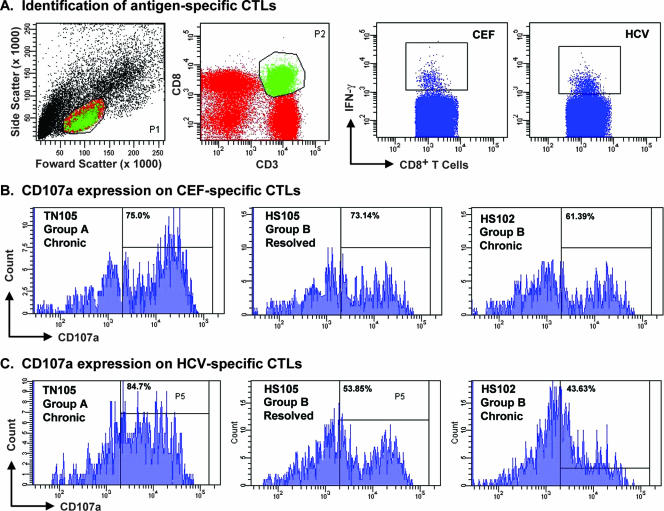
FIG. 7.
Antigen-specific IFN-γ production and degranulation. CD4-depleted PBMCs were stimulated with CEF peptides or specific HCV pools as described in Materials and Methods in 10 patients (shown are 3 representative patients). (A) Gating strategy used to identify antigen-specific CTLs and CEF- and HCV-specific responses for one patient (TN105). CTLs were identified by initially gating on lymphocytes (P1) and then on CD8+ CD3+ T cells within the lymphocyte population (P2). Antigen-specific CTLs (HCV/CEF) were identified as IFN-γ-positive cells within the CTL population. (B and C) CD107a expression on CEF-specific responses for three representative patients (B) and HCV-specific responses for the same patients (C). Interval gates were set using FMO and isotype controls. CD107a positivity was defined as the percentage of the population staining positive for PE-CY5 above negative controls. Upregulation of CD107a did not correlate with the outcome of infection. The HCV peptide pools used were as follows: for TN105, NS3-1P, NS3-4H, and NS3-5H; for HS105, E2C, NS3-4H, NS3-7H, NS4-B2, and NS5-B1; for HS102, NS3-1P.
The breadth of CD8+ T cells also predicted the virologic outcome; specifically, patients demonstrating HCV-specific IFN-γ-producing CTL responses to at least two HCV peptide pools were statistically more likely to contain HCV infection (61% [11 of 18 patients]) than patients demonstrating responses to one or no HCV peptide pool (23% [3 of 13 patients]; odds ratio,5.24; P = 0.04) (Fig. 3C). As shown in Fig. 2, 11 of the 14 (79%) patients who resolved HCV spontaneously demonstrated CTL responses to two or more peptide pools, whereas 7 of the 17 (41%) patients with chronic infection did so.
The median sums of IFN-γ-producing HCV-specific CTLs were equivalent in patients with acute-resolving (183 ELISPOTs per 2.5 × 105 CD8+ T cells) and acute-chronic (175 ELISPOTs per 2.5 × 105 CD8+ T cells) infection. Unlike what we described for CD4+ T cells, there was no quantitative cutoff for CD8+ T cells predictive of outcome. The fact that the breadth of the CTL response appears to be more important than its overall strength is congruent with the recent recognition in humans and chimpanzees that vigorous but narrowly focused CTLs (particularly in the absence of CD4 help) can lead to mutational escape and viral persistence (10, 26).
“Helped” and “helpless” HCV-specific CTLs in acute HCV infection.
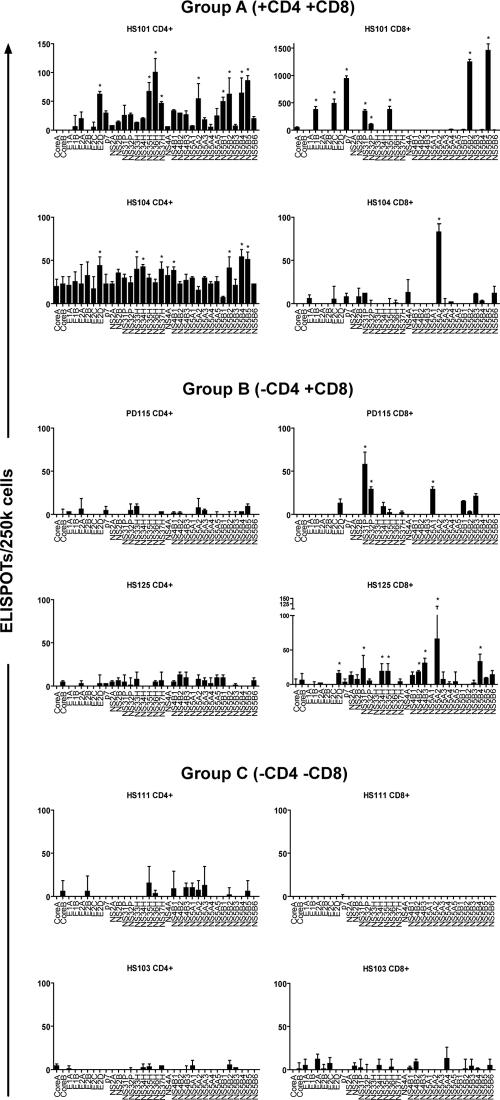
Our HCV genome-wide ELISPOT analyses of CD4 and CD8 T-cell IFN-γ responses revealed three distinct immunologic patterns (Fig. 4): pattern A (n = 17) was characterized by the presence of HCV-specific CD4+ and CD8+ T cells (“helped” CTLs) and pattern B (n = 8) displayed HCV-specific CD8+ T cells but no IFN-γ-producing CD4+ T cells, while six patients failed to demonstrated either CD4+ or CD8+ T cells that specifically targeted HCV peptides (pattern C). The demographic, biochemical, and clinical features of the three immunologic groups were similar (Table 1 and Fig. 5). With regard to the ultimate virologic outcome, only 1 of the 6 patients in group C (16.7%) spontaneously resolved HCV versus 13 of 25 (52%) in the groups that demonstrated T-cell responses; although these different proportions were not statistically significant, this was likely related to sample size.
FIG. 4.
Representative examples of different immunologic patterns according to whole HCV genome analyses of CD4+ and CD8+ IFN-γ ELISPOTs (the asterisks indicate significant responses compared to negative control wells). Group A demonstrated both CD4+ and CD8+ T-cell responses. Group B demonstrated CD8+ T-cell responses but no CD4+ T-cell responses, and group C demonstrated no significant CD4+ or CD8+ T-cell responses. Both representative patients for group A resolved HCV infection. In group B, HS125 developed chronic infection and PD115 resolved. The majority of patients (five of six) in group C developed chronic infection, including both patients depicted here. Table 1 shows the demographic and clinical features of the three immunologic groups. The error bars indicate standard deviations.
TABLE 1.
Clinical features of the three immunologic groups
| Parameter | Value for group:
|
P value | ||
|---|---|---|---|---|
| A (+CD4 +CD8) (n = 17) | B (−CD4 +CD8) (n = 8) | C (−CD4 −CD8) (n = 6) | ||
| Clinical features | ||||
| Estimated duration of infection at blood collection (days) | 133 | 136 | 171 | NSa |
| No. (%) of spontaneous resolutions | 9 (52.9) | 4 (50) | 1 (16.7) | NS |
| ALT (IU/liter) | 441 | 144 | 231 | NS |
| AST (IU/liter) | 247 | 77 | 114 | NS |
| T-bilirubin (mg/dl) | 1.68 | 0.84 | 0.77 | NS |
| Alkaline phosphatase (IU/liter) | 95.9 | 112.4 | 61.0 | NS |
| White blood cell count (million/ml) | 6.7 | 5.8 | 9.0 | NS |
| HCV RNA level (log10 IU/ml) | ||||
| Mean (SD) | 4.32 (0.38) | 3.55 (0.56) | 5.14 (0.64) | NS |
| Median (25th, 75th) | 3.53 (2.79, 5.68) | 2.79 (2.79, 5.04) | 5.25 (4.24, 6.25) | |
| Age (yr) | ||||
| Mean (SD) | 36 (3.6) | 39 (5.2) | 33 (6.0) | NS |
| Median (25th, 75th) | 35 (27, 46) | 33 (23, 48) | 31 (20, 47) | |
| Gender | ||||
| No. (%) male | 8 (47) | 3 (38) | 5 (83) | NS |
| No. (%) female | 9 (53) | 5 (62) | 1 (17) | |
| Genotype | ||||
| No. (%) 1a | 10 (58.8) | 2 (25) | 2 (33) | NSb |
| No. (%) 1b | 2 (11.8) | 1 (12.5) | 3 (50) | |
| No. (%) 2 | 0 | 1 (12.5) | 0 | |
| No. (%) 3 | 0 | 1 (12.5) | 1 (17) | |
| No. (%) Untypeable | 0 | 1 (12.5) | 0 | |
| No. (%) other | 5 (29.4) | 2 (25) | 0 | |
| 1 | 1 | 0 | 0 | |
| 1 and 2 | 1 | 1 | 0 | |
| Serotype 1 | 3 | 1 | 0 | |
| ELISPOT results | ||||
| Avg CD4 ELISPOTs (qualitative responses/33 pools) | 7.1 | 0 | 0 | 0.0008 |
| No. (%) with >5 qualitative CD4 ELISPOT responses | 9 (52.9) | 0 | 0 | 0.0032 |
| Avg CD8 ELISPOTs (qualitative responses/33 pools) | 3.2 | 3.8 | 0 | 0.0103 |
| No. (%) with >2 qualitative CD8 ELISPOT responses | 7 (41.2) | 6 (75) | 0 | 0.0160 |
| Avg CD4 + CD8 ELISPOTs (qualitative responses/2 × 33 pools) | 10.3 | 3.8 | 0 | 0.0014 |
NS, not significant.
Comparisons were made for genotype 1a versus all others.
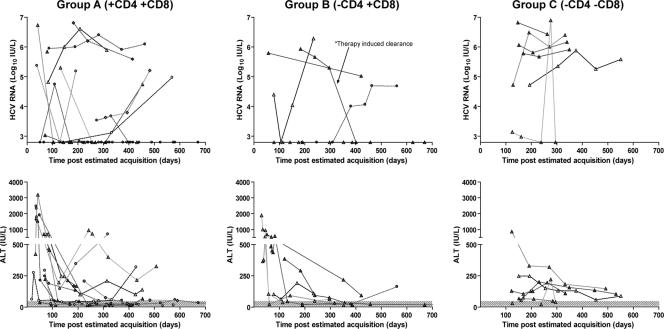
FIG. 5.
The HCV RNA and ALT kinetics in the three immunologic pattern groups. Data points for each individual subject are represented by different symbols at the indicated time points. The shaded area represents the threshold of sensitivity of the HCV RNA assay and the normal range of values for ALT. Of note, only one patient (the previously described [26] PD101) received antiviral therapy.
As shown in Table 1, the median number of HCV peptide pools (7.1) targeted by CD4+ T cells and the proportion responding to more than five pools (53%) in the pattern A group were statistically higher than in the other groups (P = 0.0008 and P = 0.0032, respectively). Interestingly, the proportions of patients within pattern group A and pattern group B who spontaneously cleared HCV and who developed persistence were equivalent. In addition, among the patients demonstrating HCV-specific CTLs but lacking IFN-γ CD4+ T-cell responses (pattern B), the average numbers of pools targeted by CTLs were equivalent irrespective of the outcome (3.25 in the acute-chronic versus 4.25 in the acute-resolved).
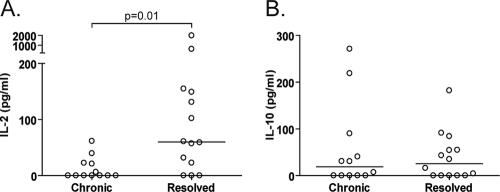
These divergent virologic outcomes in the face of seemingly indistinguishable in vitro IFN-γ ELISPOT results raise a number of important questions. Is the nature of CD4 help different in those patients who resolve spontaneously than in those with persistence? Do additional functional or phenotypic features of CTLs (e.g., cytotoxicity, proliferation, and central versus effector memory) explain why some patients without IFN-γ CD4 ELISPOTs are able to spontaneously contain HCV infection whereas others develop viral persistence? To this end, we cultured bead-purified CD4+ T cells with LCLs pulsed with recombinant HCV proteins (or negative controls) and measured the secretion of IL-2 and IL-10 using multiplex technology. Because of limiting cell numbers, we combined HCV core/NS3 proteins and NS4/NS5 proteins in duplicate wells. As shown in Fig. 6A, HCV-specific, CD4-derived IL-2 secretion was significantly higher in those patients with HCV-specific CTLs who spontaneously eradicated HCV than in those who failed to do so. Notably, patients assigned to the “helpless” CTL category (based on IFN-γ ELISPOTs, pattern B) demonstrated IL-2 secretion to at least one of the HCV protein combinations, albeit in some cases at very low levels (range, 6.4 pg/ml to 155 pg/ml). Patients who went on to develop persistence did not demonstrate higher levels of HCV-specific, CD4-derived IL-10 production (Fig. 6B) than whose who resolved spontaneously.
FIG. 6.
Secretion of IL-2 and IL-10 by HCV-specific CD4+ T cells in patients who demonstrated HCV-specific CTLs by IFN-γ ELISPOT. Briefly, bead-purified CD4+ cells (2.5 ×105) were cultured in duplicate wells with 1 × 104 autologous LCLs that had been pulsed with either HCV core and NS3 (Mikrogen), HCV NS4 and NS5 (Chiron), or relevant negative controls. After 48 h, the cell supernatants were examined by Luminex for cytokine production. Each point represents the amount of cytokine produced in response to HCV proteins after the subtraction of negative controls. Shown are the results for 6 patients in the pattern A group (+CD4+CD8 ELISPOTs) and 7 patients in the pattern B group (no CD4 ELISPOTs, +CD8 ELISPOTs); each point represents cytokine values following stimulation with either the core/NS3 or NS4/NS5 combination of HCV antigens (thus, 26 values for 13 patients). Patients with HCV-specific CTLs who spontaneously resolved HCV infection demonstrated significantly greater HCV-specific, CD4-derived IL-2 secretion (A) but no difference in the secretion of IL-10 (B).
In order to examine additional aspects of HCV-specific CTL function or phenotype that might not be revealed by the ELISPOT assay, we used a multiparameter approach. First, we examined intracellular cytokine production following stimulation with specific HCV pools that had elicited ELISPOT responses in individual patients. PBMCs depleted of CD4+ cells were cultured in the presence of the relevant HCV peptide pools, CEF, or DMSO control for 6 h. HCV-specific CTLs from patients who subsequently developed persistence demonstrated intracellular production of IFN-γ that was comparable to what we observed in patients who spontaneously eradicated HCV. In addition, the cytolytic potential of HCV-specific CTLs was examined by assessing the upregulation of CD107a on HCV peptide-stimulated, CEF-stimulated, and unstimulated CD8+ T cells after 6 h of incubation (Fig. 7). As described recently (19), CD107a is a lysosome-associated membrane glycoprotein expressed on the cell surface following release of the cytotoxic granule contents, and thus, its ex vivo expression correlates with the degranulation capacity of virus-specific CTLs. Gating on IFN-γ-producing HCV-specific CTLs in 10 patients, we found that although CD107a expression tended to be lower following HCV peptide versus CEF peptide stimulation (median, 31% versus 51.2%), the HCV-induced expression levels were comparable among those acute patients who resolved versus those who became chronically infected.
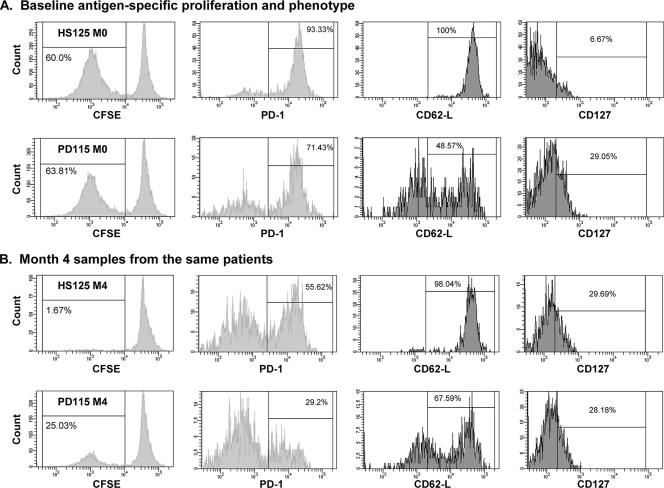
Next, we assessed the proliferative capacities and memory phenotypes of HCV-specific CTLs in early HCV infection. Patients HS125 (acute-chronic) and PD115 (acute-recovered) are shown as illustrative examples in Fig. 8. IL-2 production following stimulation with core/NS3 or NS4/NS5 were 0 and 6.4 pg/ml, respectively, for patient HS125 and 61 and 131.2 pg/ml, respectively, for PD115. Both patients demonstrated HLA A2-restricted responses to the NS31073 epitope. CFSE-labeled PBMCs (10 × 106/ml) depleted of CD4+ cells were incubated for 7 days in the presence of cognate HCV-specific peptides (10 μg/ml) and blocking antibodies against PD-L1 and PD-L2, as described recently (9). The two patients demonstrated comparable levels of HCV-specific proliferation (∼60%), as represented by the CFSElow population. Interestingly, however, the level of surface expression of PD-1, recently shown to be the primary determinant of apoptosis sensitivity of virus-specific CTLs (20), was higher on pentamer-specific CTLs in HS125 than in PD115 (Fig. 8A). More than four times the number of pentamer-specific CTLs expressed CD127 in the patient who resolved infection (PD115) than in the acute-chronic patient (HS125). As recently described by our group (8), CD127 (IL-7Rα) serves as a “signature” for cellular fitness, demarcating effector cells that survive long term and retain functional responsiveness (15). The phenotypes associated with the trafficking pattern also appeared to be different in the two patients; the lymph node homing receptor CD62L is downregulated when T cells differentiate into effector cells (8). In HS125, all of the HCV-specific CTLs expressed CD62L, consistent with a central memory instead of an effector memory phenotype; in contrast, less than half of the HCV-specific CTLs in PD115 expressed CD62L. Four months later, the percentage of CTLs that proliferated in response to cognate peptide decreased significantly to 1.67% in patient HS125 and 25% in PD110. An additional pair of patients demonstrated similar patterns in phenotypes and virologic outcomes (data not shown). Taken together, these results demonstrate that the majority of patients in the early “priming” phase of HCV infection demonstrate HCV-specific CTLs that produce IFN-γ and proliferate; however, phenotypic differences between CTLs primed in the presence or absence of CD4+ T-cell help may be ultimately associated with whether HCV is cleared or becomes persistent.
FIG. 8.
Proliferative capacities and phenotypes of HCV1073-specific CTLs in two representative patients: HS125, who developed chronicity, and PD115, who spontaneously cleared HCV. CFSE-stained PBMCs depleted of CD4 T cells were stimulated with NS31073 as described in Materials and Methods. The cells were gated on CD8 and A2-1073 pentamer double positivity and analyzed for loss of CFSE, as well as expression of PD-1, CD62L, and CD127. (A) The levels of CTL proliferation were comparable in the two patients at the earliest time point (enrollment). A higher percentage of total pentamer-positive CTLs in HS125 expressed PD-1 and CD62L and fewer expressed CD127 than in PD115. (B) Four months later, the proliferative capacity of HCV1073-reactive CTLs was decreased, markedly so for patient HS125, compared to baseline responses. PD-1 and CD62L expression on pentamer-positive CTLs remained higher for patient HS125 at that time.
DISCUSSION
Most patients with acute HCV infection are asymptomatic, and therefore, the disease is rarely identified in the earliest stages; furthermore, because the majority of individuals develop persistence, the precise correlates of spontaneous recovery remain unelucidated. As a result of establishing a large prospective study to screen and identify patients with acute HCV infection at multiple sites around the United States, we were able to compare the immunologic features of approximately equivalent numbers of patients with spontaneously resolved and chronic infections. Moreover, because of the large sample, we were able to limit the analysis to mostly patients who did not receive antiviral therapy, thus allowing the identification of host immune features that determine the natural history of acute HCV infection. For our larger cohort of 67 patients with acute HCV infection from whom the current study patients were selected, the spontaneous viral clearance rate after 6 months of infection was 18% (95% confidence interval, 11%, 31%) (29).
A number of previous studies showed that the robustness of the T-cell response during the acute phase of HCV is associated with the outcome of infection (5-8, 16, 17, 28), but this study provides the first clear evidence that quantitative T-cell thresholds exist above which spontaneous recovery occurs following acute HCV infection. The likelihood of recovery is considerably greater in an individual subject exceeding these thresholds; for example, if five or more HCV peptide pools (or ∼15% of the HCV genome) are targeted by CD4+ T cells early after infection, the chance of recovery is more than seven times higher than if this threshold is not achieved. Similarly, by logistic regression analysis, we found that patients demonstrating HCV-specific IFN-γ-producing CTL responses to at least two HCV peptide pools were statistically more likely to contain HCV infection than patients demonstrating responses to one or no HCV peptide pools. The fact that five of six patients lacking any HCV-specific CD4+ and CD8+ T-cell responses by ELISPOT developed persistence has therapeutic implications: these patients should be considered candidates for early antiviral treatment. Further, by defining in quantitative terms the breadth of T-cell responses that need to be induced for containment of HCV, our findings have important implications for vaccine design.
Generation of effective CTL memory depends on the provision of CD4+ T-cell help via production of cytokines (15) or by assisting professional antigen-presenting cells via CD40/CD40L-mediated activation (1, 18). From previous models, it is understood that if CD4+ T-cell help is lacking, the primary infection may be cleared but the resulting “helpless” CTLs are impaired in the ability to generate a secondary response upon rechallenge (13, 14). Our comprehensive IFN-γ ELISPOT analyses, incorporating all potential HCV epitopes in the earliest stages of infection, revealed distinct immunologic patterns, including the absence of HCV-specific IFN-γ-producing CD4+ T cells in the presence of vigorous CTLs (pattern B); equal proportions of patients in this category resolved infection or became persistently infected. The experiments aimed at elucidating the mechanistic basis for divergent virologic outcomes despite seemingly indistinguishable immunologic patterns revealed the critically important role of IL-2 produced by HCV-specific CD4+ T cells. Levels of IL-2 were significantly higher in the patients with HCV-specific CTLs who successfully eradicated the virus than in those who became persistently viremic. Thus, CD4-derived IL-2 may be one of the factors required during the primary immunization with HCV to program the differentiation of fully functional CTL memory (30). These results are in keeping with the recent demonstration that T lymphocytes lacking IL-7Rα (CD127) demonstrate impaired IL-2 secretion and predate the development of viral persistence (8, 28). Further work is needed to elucidate the mechanisms underlying the primary failure of HCV-specific CD4+ T cells to program or imprint the complete differentiation of CTLs.
Our results also underscore the limitations of restricting immune analyses to one functional read-out (e.g., IFN-γ ELISPOT) and the need to use complementary techniques. Using highly purified CD8+ T cells that were cocultured with autologous DCs, previously shown by us to significantly enhance the ability to detect HCV-specific responses (25), we found that the majority of patients had functional HCV-specific CTLs. In fact, as shown in Fig. 8, the functional CTL responses to HCV, when assayed at the peak of the primary response early after infection, were remarkably similar in patients with and without adequate CD4 help. However, phenotypic defects (increased PD-1 and CD62L expression) were observed that predated the crippling of the CTL effector functions and their demise in persistent infection (2). Accordingly, 4 months later, the “unhelped” memory CTLs divided less and were unable to provide complete protection against HCV.
The facts that hepatitis C viremia may be sustained in the face of multispecific CD8+ T-cell responses and that the number of CTLs synchronously functional in early infection may not be entirely predictive of an individual's ability to control HCV replication suggest that the processes that mediate recovery are more complex than initially appreciated (4). Indeed, one of the limitations of HCV vaccine studies performed to date is their incomplete characterization of the T cells induced (24). Our results provide additional evidence that the effector/memory phenotype of CTLs and the contribution of CD4 T-cell help will be essential to understanding how to maintain long-lived protective responses to HCV.
In summary, we have confirmed that a multispecific IFN-γ-producing T-cell response in the earliest stages of acute HCV infection is critical for governing spontaneous recovery or viral persistence. We defined quantitative T-cell thresholds associated with higher likelihood of effective virus containment, suggesting that recapitulation of these T-cell responses by vaccination might provide an integral component of future strategies. Our data, however, indicate that HCV-specific responses as assessed by IFN-γ ELISPOT assays may not represent adequate surrogates for protective T-cell immunity (3), since a significant proportion of patients with robust CTLs develop persistence. Further work is required to elucidate precisely how HCV undermines CD4+ Th cells and their instructive signals in the earliest stages of infection.
Acknowledgments
We thank Lana McBride for coordinating clinical enrollment and follow-up and the patients for their willingness to participate in this study.
We do not have a commercial or other association that might pose a conflict of interest (e.g., pharmaceutical stock ownership, consultancy, advisory board membership, relevant patents, or research funding).
This research was supported by RO1 DK060590 and a VA Merit Review Grant to H.R.R. The study was also supported by the GCRC at UCHSC (5M01 RR000051).
Footnotes
Published ahead of print on 28 November 2007.
REFERENCES
- 1.Bennett, S. R., F. R. Carbone, F. Karamalis, R. A. Flavell, J. F. Miller, and W. R. Heath. 1998. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature 393478. [DOI] [PubMed] [Google Scholar]
- 2.Bevan, M. J. 2004. Helping the CD8+ T-cell response. Nat. Rev. Immunol. 4595-602. [DOI] [PubMed] [Google Scholar]
- 3.Bowen, D. G., and C. M. Walker. 2005. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature 436946-952. [DOI] [PubMed] [Google Scholar]
- 4.Cooper, S., A. L. Erickson, E. J. Adams, J. Kansopon, A. J. Weiner, D. Y. Chien, M. Houghton, P. Parham, and C. M. Walker. 1999. Analysis of a successful immune response against hepatitis C virus. Immunity 10439-449. [DOI] [PubMed] [Google Scholar]
- 5.Cox, A. L., T. Mosbruger, G. M. Lauer, D. Pardoll, D. L. Thomas, and S. C. Ray. 2005. Comprehensive analyses of CD8+ T cell responses during longitudinal study of acute human hepatitis C. Hepatology 42104-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Folgori, A., E. Spada, M. Pezzanera, L. Ruggeri, A. Mele, A. R. Garbuglia, M. P. Perrone, P. Del Porto, E. Piccolella, R. Cortese, A. Nicosia, and A. Vitelli. 2006. Early impairment of hepatitis C virus specific T cell proliferation during acute infection leads to failure of viral clearance. Gut 551012-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerlach, J. T., H. M. Diepolder, M. C. Jung, N. H. Gruener, W. W. Schraut, R. Zachoval, R. Hoffman, C. A. Schirren, A. Santantonio, and G. R. Pape. 1999. Recurrence of hepatitis C virus after loss of virus-specific CD4+ T-cell response in acute hepatitis C. Gastroenterology 117933-941. [DOI] [PubMed] [Google Scholar]
- 8.Golden-Mason, L., J. R. Burton, Jr., N. Castelblanco, J. Klarquist, S. Benlloch, C. Wang, and H. R. Rosen. 2006. Loss of IL-7 receptor alpha-chain (CD127) expression in acute HCV infection associated with viral persistence. Hepatology 441098-1099. [DOI] [PubMed] [Google Scholar]
- 9.Golden-Mason, L., B. Palmer, J. Klarquist, J. A. Mengshol, N. Castelblanco, and H. R. Rosen. 2007. Upregulation Of PD-1 expression on circulating and intrahepatic hepatitic C virus-specific CD8+ T cells associated with reversible immune dysfunction. J. Virol. 819249-9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grakoui, A., N. H. Shoukry, D. J. Woollard, J.-H. Han, and J. Ghrayeb. K. K. Murthy, C. M. Rice, and C. M. Walker. 2003. HCV persistence and immune evasion in the absence of memory T cell help. Science 302659-662. [DOI] [PubMed] [Google Scholar]
- 11.Grant, M. 2002. Secondary persistent infection with hepatitis C virus: a challenge for adaptive immunity. Lancet 3591452. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton, L. C. 1992. Regression with graphics: a second course in applied statistics, p. 183-216. Duxbury Press, Belmont, CA.
- 13.Hamilton, S. E., M. Prlic, and S. C. Jameson. 2004. Environmental conservation: bystander CD4 T cells keep CD8 memories fresh. Nat. Immunol. 5873-874. [DOI] [PubMed] [Google Scholar]
- 14.Hamilton, S. E., M. C. Wolkers, S. P. Schoenberger, and S. C. Jameson. 2006. The generation of protective memory-like CD8+ T cells during homeostatic proliferation requires CD4+ T cells. Nat. Immunol. 7475-481. [DOI] [PubMed] [Google Scholar]
- 15.Kaech, S. M., E. J. Whery, and R. Ahmed. 2002. Effector and memory T-cell differentiation: implications for vaccine development. Nat. Rev. Immunol. 2251-262. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan, D. E., K. Sugimoto, K. Newton, M. E. Valiga, F. Ikeda, A. Aytaman, F. A. Nunes, M. R. Lucey, B. A. Vance, R. H. Vonderheide, K. R. Reddy, J. A. McKeating, and K. M. Chang. 2007. Discordant role of CD4 T-cell response relative to neutralizing antibody and CD8 T-cell responses in acute hepatitis C. Gastroenterology 132654-666. [DOI] [PubMed] [Google Scholar]
- 17.Lechner, F., D. K. H. Wong, P. R. Dunbar, R. Chapman, R. T. Chung, P. Dohrenwend, G. Robbins, R. Philliops, P. Klenerman, and B. D. Walker. 2000. Analysis of successful immune responses in persons with hepatitis C virus. J. Exp. Med. 91499-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu, Z., L. Yuan, X. Zhou, E. Sotomayor, H. I. Levitsky, and D. M. Pardoll. 2000. CD40-independent pathways of T cell help for priming of CD8+ cytotoxic T lymphocytes. J. Exp. Med. 191541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Penna, A., M. Pilli, A. Zerbini, A. Orlandini, S. Mezzadri, L. Sacchelli, G. Missale, and C. Ferrari. 2007. Dysfunction and functional restoration of HCV-specific CD8 responses in chronic hepatitis C virus infection. Hepatology 45588-601. [DOI] [PubMed] [Google Scholar]
- 20.Petrovas, C., J. P. Casazza, J. M. Brenchley, D. A. Price, E. Gostick, W. C. Adams, M. L. Precopio, T. Schacker, M. Roederer, D. C. Douek, and R. A. Koup. 2006. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J. Exp. Med. 2032281-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romani, N., S. Gruner, D. Brang, E. Kampgen, A. Lenz, B. Trockenbacker, G. Konwalinka, P. O. Fritsch, R. M. Steinman, and G. Schuler. 1994. Proliferating dendritic cell progenitors in human blood. J. Exp. Med. 18083-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosen, H. R., C. Miner, A. W. Sasaki, D. M. Lewinsohn, A. J. Conrad, A. Bakke, H. G. Bouwer, and D. J. Hinrichs. 2002. Frequencies of HCV-specific effector CD4+ T cells by flow cytometry: correlation with clinical disease stages. Hepatology 35190-198. [DOI] [PubMed] [Google Scholar]
- 23.Schulze zur Wiesch, J., G. M. Lauer, C. L. Day, A. Y. Kim, K. Ouchi, J. E. Duncan, A. G. Wurcel, J. Timm, A. M. Jones, B. Mothe, T. M. Allen, B. McGovern, L. Lewis-Ximenex, J. Sidney, A. Sette, R. T. Chung, and B. D. Walker. 2005. Broad repertoire of the CD4+ Th cell response in spontaneously controlled hepatitis C virus infection includes dominant and highly promiscuous epitopes. J. Immunol. 1753603-3613. [DOI] [PubMed] [Google Scholar]
- 24.Shiina, M., and B. Rehermann. 2006. Hepatitis C vaccines: inducing and challenging memory T cells. Hepatology 431395-1398. [DOI] [PubMed] [Google Scholar]
- 25.Smyk-Pearson, S., I. A. Tester, D. Lezotte, A. W. Sasaki, D. M. Lewinsohn, and H. R. Rosen. 2006. Differential antigenic hierarchy associated with spontaneous recovery from hepatitis C virus infection: implications for vaccine design. J. Infect. Dis. 194454-463. [DOI] [PubMed] [Google Scholar]
- 26.Tester, I., S. Smyk-Pearson, P. Wang, A. Wertheimer, E. Yao, D. M. Lewinsohn, J. E. Tavis, and H. R. Rosen. 2005. Immune evasion versus recovery after acute hepatitis C virus infection from a shared source. J. Exp. Med. 2011725-1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thimme, R., D. Oldach, K. M. Chang, C. Steiger, S. C. Ray, and F. V. Chisari. 2001. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J. Exp. Med. 1941395-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urbani, S., B. Amadei, P. Fisicaro, D. Tola, A. Orlandini, L. Sacchelli, C. Mori, G. Missale, and C. Ferrari. 2006. Outcome of acute hepatitis C is related to virus-specific CD4 function and maturation of antiviral memory CD8 responses. Hepatology. 44126-139. [DOI] [PubMed] [Google Scholar]
- 29.Wang, C. C., E. Krantz, J. Klarquist, M. Krows, L. McBride, E. P. Scott, T. Shaw-Stiffel, S. J. Weston, H. Thiede, A. Wald, and H. R. Rosen. 2007. Acute hepatitis C in a contemporary US cohort: modes of acquisition and factors influencing viral clearance. J. Infect. Dis. 1961474-1482. [DOI] [PubMed] [Google Scholar]
- 30.Williams, M. A., and M. J. Bevan. 2007. Effector and memory CTL differentiation. Annu. Rev. Immunol. 25171-192. [DOI] [PubMed] [Google Scholar]
- 31.World Health Organization. 1999. Global surveillance and control of hepatitis C. Report of a WHO consultation organized in collaboration with the Viral Hepatitis Prevention Board, Antwerp, Belgium. J. Viral Hepat. 635-47. [PubMed] [Google Scholar]