Abstract
The extracellular signal-regulated kinase (ERK) mitogen-activated protein kinase (MAPK) pathway is essential for infection by a variety of viruses. The p90 ribosomal S6 kinases (RSKs) are direct substrates of ERK and functional mediators of ERK MAPK signaling, but their roles in viral infection have never been examined. We demonstrate that ORF45 of Kaposi's sarcoma-associated herpesvirus (KSHV) interacts with RSK1 and RSK2 and strongly stimulates their kinase activities. The activation of RSK by ORF45 is correlated with ERK activation but does not require MEK. We further demonstrate that RSK1/RSK2 is activated during KSHV primary infection and reactivation from latency; a subset of RSK1/RSK2 is present in the viral replication compartment in the nucleus. Depletion of RSK1/RSK2 by small interfering RNA or the specific inhibitor BI-D1870 suppresses KSHV lytic gene expression and progeny virion production, suggesting an essential role of RSK1/RSK2 in KSHV lytic replication.
Kaposi's sarcoma-associated herpesvirus (KSHV), also known as human herpesvirus 8, is a human DNA tumor virus etiologically linked to Kaposi's sarcoma (KS), primary effusion lymphoma (PEL), and multicentric Castleman disease (MCD) (6, 14, 17, 28). Like all herpesviruses, KSHV has two alternative life cycles: latent and lytic. KSHV establishes latent infection in the majority of infected cells in cases of KS, PEL, and MCD, but lytic replications occur only in a small fraction. During latent infection, the viral genome is maintained as an episome, and only a few viral genes are expressed. Under appropriate conditions, latent genomes can be reactivated to express the full panel of viral genes in a cascade fashion, beginning with immediate-early genes, followed by early genes and then late genes (14, 28). Successful completion of this lytic replication leads to release of progeny viruses and ultimately cell death. Despite its destruction of cells, lytic replication is believed to play a critical role in KSHV tumorigenesis (14, 17, 39).
For successful infection and propagation, viruses rely on and modulate cellular signaling machineries, including the mitogen-activated protein kinases (MAPKs), which respond to various extracellular stimuli, ranging from growth factors and cytokines to cellular stress (7, 35). The MAPK signal-transduction cascade is activated by sequential phosphorylation of a three-component module: MAPK, MAPK kinase (MAPKK), and MAPK kinase kinase (MAPKKK). MAPKKKs are often activated by extracellular stimuli through a small GTP-binding protein of the Ras/Rho family and phosphorylate MAPKK, which in turn activates MAPK. The best-characterized groups of MAPKs in mammalian cells are extracellular signal-regulated kinases 1 and 2 (ERK1/ERK2), p38 MAPK (p38α, p38β, p38γ, and p38δ), and stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK1/JNK2/JNK3). Activated MAPK phosphorylates its specific substrate to exert its diverse biological functions. MAPKs also directly phosphorylate several protein kinases, including a family of 90-kDa ribosomal S6 kinases (RSKs), which represents an additional signaling amplification step in the MAPK cascade (18, 35).
The RSKs are serine-threonine kinases and direct substrates of ERK1/ERK2. The four isoforms in humans share 75 to 80% amino acid (aa) identity. All RSK isoforms have two distinct kinase domains: the N-terminal (NTKD) and the C-terminal (CTKD). The NTKD phosphorylates downstream targets and is activated through a sequential phosphorylation cascade involving ERK1/ERK2, the CTKD, and 3-phosphoinositide-dependent protein kinase 1. The RSKs are involved in the regulation of multiple processes in the cell, including gene expression, protein synthesis, the cell cycle, and cell growth, survival, proliferation, and differentiation (18, 35).
The RAF (as MAPKKK)-MEK (as MAPKK)-ERK (as MAPK) signaling cascade is activated during infection by a variety of DNA and RNA viruses, including cytomegalovirus, human immunodeficiency virus 1 (HIV-1), influenza virus, respiratory syncytial virus, hepatitis B virus, coronavirus, vaccinia virus, and coxsackievirus (2, 5, 22, 23, 26, 27, 30, 32, 34, 45). The MEK/ERK pathway is activated with biphasic kinetics by KSHV during de novo infection to modulate initial cellular and viral gene expression (29, 37, 40, 44). The activation of ERK1/ERK2 is important for efficient KSHV infection because the MEK inhibitor U0126 inhibits key viral gene expression (40). Consistently, overexpression of RAF or ERK increases KSHV infectivity at the postattachment stage (1, 31). RAF-MEK-ERK signaling has also been shown to be essential for 12-O-tetradecanoylphorbol-13-acetate (TPA)-mediated reactivation of KSHV (11, 16). Recently, a comprehensive expression screening of a mammalian cDNA library also determined RAS-RAF-MEK-ERK signaling to be involved in reactivation of KSHV (47). Although, RAF-MEK-ERK signaling is essential for KSHV infection, the role of RSKs is unknown.
ORF45 is a KSHV gene product that plays a critical role in the KSHV lytic replication cycle. It is an immediate-early, highly phosphorylated, mostly cytoplasmic protein with expression beginning immediately after the virus enters the lytic cycle. Its expression level rises as lytic replication progresses and remains high through the late stage. In the end, a significant amount of ORF45 is encapsidated in the virion (49, 50, 53). Its unique temporal and spatial expression put it in the forefront of the process of coping with the host cellular environment. Previously, we demonstrated that KSHV ORF45 interacts with interferon regulatory factor 7 (IRF-7) and suppresses its activation, suggesting a role of ORF45 in defeating the host antiviral response (51). Recently, with bacterial artificial chromosome (BAC)-based mutagenesis, we demonstrated that disruption of ORF45 causes a lower yield of progeny viruses, even though it has no significant effect on viral gene expression or DNA replication in a reconstituted 293T cell system (52). The mutant virus also showed lower infectivity and diminished viral gene expression upon primary infection of 293T cells. ORF45 therefore has multiple roles at both early and late stages of the viral lytic life cycle (52). We reasoned that ORF45 may also interact with other cellular pathways, and in the research reported here, we demonstrate that KSHV ORF45 interacts with RSK1 and RSK2 and activates their kinase activities. We also showed that the sustained activation of RSKs is essential for KSHV lytic replication.
MATERIALS AND METHODS
Cells, virus, antibodies, and chemicals.
BCBL-1 cells were cultured in RPMI 1640 medium with 10% heat-inactivated fetal bovine serum (FBS) and antibiotics. HEK293T cells and 293 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% FBS, 2 mM l-glutamine, and antibiotics. BAC36 and BAC-stop45, (which carries the entire KSHV genome or the ORF45-null recombinant virus genome, respectively, have been described previously (52). Rabbit antibodies detecting MAPKK ERK1/ERK2, MEK1/MEK2, p38 MAPK, and ERK5; the phosphorylated forms of ERK1/ERK2 (Thr202/Tyr204), MEK1/MEK2 (Ser217/Ser221), p38 MAPK (Thr180/Tyr182), ERK5 (Thr218/Tyr220), and RSK1 (Thr359/Ser363, Ser380, and Thr573) antibodies; and U0126 (MEK1 inhibitor) were from Cell Signaling Technology, Beverly, MA. Rat anti-LANA antibody was from Advanced Biotechnologies, Inc. Rabbit anti-RSK1 and -RSK2 antibodies were purchased from Upstate (Charlottesville, VA). Mouse monoclonal anti-RTA, anti-ORF65, and rabbit anti-PF8/ORF59 antibodies were gifts from K. Ueda, S.-J. Gao, and R. Ricciardi, respectively. Anti-ORF45 and anti-K8 antibodies have been described previously (49, 51). TPA, sodium butyrate, and Polybrene; anti-actin, anti-green fluorescent protein (GFP), and anti-hemagglutinin (HA) antibodies; EZview red anti-Flag M2 and anti-HA affinity gel beads; and 3× Flag and HA peptides were purchased from Sigma (St. Louis, MO). RSK inhibitor BI-D1870 was purchased from the MRC Protein Phosphorylation Unit, University of Dundee, Dundee, Scotland.
Plasmid constructs.
Plasmid pCR3.1-ORF45 has been described previously (51). Flag-tagged ORF45 constructs of aa 1 to 407 (full length), 1 to 332, 1 to 237, 1 to 115, 19 to 407, 77 to 407, 90 to 407, and 115 to 407 and deletion mutants Δ(19-77) and Δ(90-115) were cloned by PCR into pCMV-TAG2 (Stratagene). Plasmids encoding glutathione S-transferase (GST) fusion proteins of ORF45 aa 1 to 115, 115 to 237, 237 to 332, and 332 to 407 were cloned by PCR into pGEX-5X (Pharmacia). pGEX-S6 was constructed by cloning of the corresponding coding sequence of S6 peptide (KEAKEKRQEQIAKRRRLSSLRASTSKSESSQK) into pGEX-5X. Plasmids pKH3, pKH3-RSK2, and pKH3-RSK2-Y707A (constitutive active) and pKH3-RSK2-K100A/Y707A (kinase-dead mutant) were kindly provided by J. Smith and D. Lannigan at the University of Virginia (10). Human RSK1 coding sequence was amplified from a full-length cDNA clone obtained from ATCC and cloned into pKH3.
Immunoprecipitation (IP).
BCBL-1 and control BJAB cells (0.5 × 106/ml) were induced with 20 ng TPA for 48 h. Of the induced cells, 5 × 107 were collected and washed with cold phosphate-buffered saline (PBS) and lysed with 0.5 ml of whole-cell lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% NP-40, 1 mM sodium orthovanadate [Na3VO4], 40 mM β-glycerophosphate, 1 mM sodium fluoride, 10% glycerol, 5 mM EDTA, 5 μg/ml of aprotinin, 5 μg/ml of leupeptin, 5 mM benzamidine, and 1 mM phenylmethylsulfonyl fluoride). The cell lysates were centrifuged at 10,000 × g for 5 min at 4°C. Supernatants were incubated with 5 μg anti-ORF45 monoclonal antibody 2D4A5 with gentle agitation at 4°C for 2 h. Protein G-coated paramagnetic beads (Invitrogen) were added, and the lysates were incubated with gentle agitation for an additional 2 h at 4°C. After three washes with lysis buffer and three with TBS buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl), the precipitates were boiled in loading buffer and resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
For IP with anti-Flag or anti-HA antibodies, the cell lysates were incubated with EZview red anti-Flag M2 or anti-HA affinity beads for 4 h or overnight at 4°C. After washing with lysis buffer and TBS, proteins were eluted by incubation with 150 μg/ml 3× Flag or HA peptide in TBS for 1 h at 4°C.
Mass spectrometry analysis.
Bands excised from colloidal blue-stained gels were subjected to liquid chromatography-tandem mass spectrometry by the mass spectrometry facility at The Wistar Institute as previously described (49).
Expression and preparation of GST proteins.
Escherichia coli BL21 cultures transformed with plasmids encoding GST or GST fusion proteins were induced with 100 μM isopropyl-β-d-thiogalactopyranoside (IPTG) for 3 h at room temperature. Cells were pelleted, washed once with PBS, and resuspended in PBS plus lysozyme and protease inhibitors. The cell suspension was sonicated, and Triton X-100 was added to a final concentration of 1%. After 30 min of incubation at 4°C with gentle agitation, cell debris was removed by centrifugation at 10,000 × g for 10 min. The supernatant was incubated with glutathione agarose beads at 4°C overnight. After five washes with PBS, GST proteins were eluted with 10 mM glutathione in 50 mM Tris HCl, pH 8.5. The eluates were dialyzed in buffer A150 containing 25 mM Tris, pH 7.5, 1 mM EDTA, 150 mM NaCl, 0.1% NP-40, and 10% glycerol. The protein concentration was determined with a bicinchoninic acid protein assay kit (Pierce). The purity was assessed by Coomassie brilliant blue staining of proteins separated by SDS-PAGE. The purified GST proteins were divided into aliquots and stored at −80°C until use.
In vitro kinase assay.
293 cells seeded into 100-mm dishes were transfected with 10 μg of HA-tagged RSK expression vector. After serum starvation for 24 h, the transfected cells were induced with 20% FBS for 10 min. Whole-cell lysates were made, and HA-RSK1 or HA-RSK2 proteins were immunoprecipitated with 50 μl of EZview red anti-HA affinity beads. After two washes with lysis buffer and three with TBS buffer, the immunoprecipitated beads were resuspended in 100 μl of TBS plus 1 mM phenylmethylsulfonyl fluoride, 1 mM Na3VO4, and 1× protease inhibitor cocktail (Roche). The kinase reaction was performed by incubation of 5 μl of IP complexes with 2.5 μg GST substrates in 25 μl of 1× kinase assay buffer (25 mM HEPES, pH 7.5, 50 mM NaCl, 20 mM β-glycerolphosphate, 1 mM dithiothreitol, 20 mM MgCl2, 1 mM Na3VO4, 1 μg/ml bovine serum albumin, 20 μM ATP, and 5 μCi [γ-32P]ATP). The reactions were kept at 30°C for 30 min and stopped by addition of PAGE loading buffer. After fractionation of samples by 10% SDS-PAGE, the gel was dried and exposed to X-ray film or analyzed with a PhosphorImager. The immunoprecipitated HA-RSK proteins were processed for Western analysis with anti-HA antibody.
Stable cell lines in which both RSK1 and RSK2 are knocked down by siRNA.
Hairpin-forming oligonucleotides were designed and cloned into RNAi-Ready pSIREN-RetroQ vector (Clontech) according to the manufacturer's instructions. Four small interfering RNAs (siRNAs) for each gene were initially designed, and the two most-effective ones were chosen for further studies. The two for human RSK1 (accession no. NM_002953) are siRNA-RSK1-2 (CCATGACACTGATTCTGAA) and siRNA-RSK1-4 (TGAGGAGGGCCACATCAAA). The two for human RSK2 (accession no. NM_004586) are siRNA-RSK2-1 (GAAGAAGGCCACACTGAAA) and siRNA-RSK2-3 (GCCTGAAGATACATTCTAT). Oligonucleotides encoding missense siRNA and luciferase siRNA were also cloned into the vector as controls. The pSIREN-RetroQ plasmid includes a puromycin-resistance gene, so transduced cells can be selected by puromycin exposure. To select cells that were transduced by both RSK1 and RSK2 siRNAs, we modified the pSIREN-RetroQ-RSK1 vectors that express RSK1 siRNAs. We replaced the puromycin-resistance gene in the pSIREN-RetroQ backbone with a hygromycin-resistance gene from pTRE2hyg (Clontech). Retroviruses were packaged in GP2-293 cells and used to infect 293 or BCBL-1 cells. The cells infected with both siRNA-RSK1-expressing retroviruses and siRNA-RSK2-expressing retroviruses were selected with 2 μg/ml puromycin (Clontech) and 200 μg/ml hygromycin (Invitrogen). The pooled cells were examined for RSK1 and RSK2 levels by Western blotting.
Virion purification and infection.
BCBL-1 cells were induced with 20 ng/ml TPA, and BAC3636 or BAC-stop45 stable 293T cells were induced with 20 ng/ml TPA and 0.3 mM sodium butyrate for 4 to 5 days. The extracellular viruses were purified from the culture supernatant as described previously (49, 52). Briefly, media from the induced cultures were collected and passed through 0.45-μm filters. The virions were then pelleted at 27,000 rpm for 1 h on a 25% sucrose cushion with a Beckman SW28 rotor. The pellets were dissolved in 1% original volume of 1× PBS or Dulbecco's modified Eagle's medium and stored at −80°C. Viral genomes were determined by real-time PCR with primers amplifying the KSHV ORF73 gene. The infectivity of virus stocks was determined by latent nuclear antigen immunofluorescence assay (LANA IFA) for BCBL-1-derived virus and by GFP expression for BAC36-derived virus.
IFA.
The primary antibodies used in the immunohistochemistry studies included anti-ORF45 2D4A5 (1:500), anti-RSK1 (1:200), anti-RSK2 (1:200), anti-K8 monoclonal immunoglobulin G (IgG) (1:1,000), and sheep anti-bromodeoxyuridine (anti-BrdU) antibody (1:200 dilution; purchased from Capralogics, Inc., Hardwick, MA).
BCBL-1 cells were treated with TPA (20 ng/ml) for 48 h. The cells were washed with PBS and fixed with 2% paraformaldehyde in PBS for 10 min at room temperature. The fixed cells were spun onto Shandon double cytoslides at 1,000 rpm for 5 min. The slides were immersed in PBS for 5 min, permeabilized in 0.2% Triton X-100 in PBS for 20 min on ice, and washed once with PBS. For the double-label experiment, the primary mouse monoclonal and rabbit polyclonal antibodies were diluted together and incubated with cells on the slides at room temperature for 1 h. After washing, the cells were incubated with fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG and Texas Red-conjugated anti-rabbit antibodies (Vector Laboratories, Inc., Burlingame, CA) at room temperature for 1 h.
For BrdU staining, TPA-treated BCBL-1 cells were incubated in medium containing 10 μM BrdU (Sigma) for 60 min before fixation. After immunostaining with primary antibodies and secondary antibodies tagged with Cy5 or FITC according to the procedure described above, the pulse-labeled cells were fixed again with 2% paraformaldehyde in PBS for 20 min at room temperature and then incubated with 2 N HCl for 30 min at room temperature so that incorporated BrdU residues were exposed. Sheep anti-BrdU antibody was added and incubated with the cells for 45 min. The cells were then incubated with Texas Red-tagged secondary antibody. All secondary antibodies were diluted at 1:250.
The slides were examined with a Leica TCS SPII confocal laser scanning system. Two or three channels were recorded simultaneously or sequentially. Data acquisition was controlled for possible breakthrough between the green and red channels and between the blue and red channels.
Nucleotide sequence accession numbers.
The sequences for siRNA-RSK1-2 and siRNA-RSK1-4 and for siRNA-RSK2-1 and siRNA-RSK2-3 were submitted to GenBank under accession no. NM_002953 and NM_004586, respectively.
RESULTS
Association of RSK1/RSK2 with KSHV ORF45.
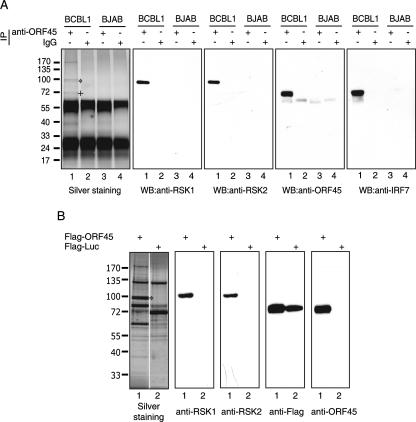
To identify potential ORF45-associated cellular and viral proteins, we immunoprecipitated ORF45 and associated molecules from lysate of TPA-induced BCBL-1 cells using monoclonal anti-ORF45 2D4A5. As controls, we included KSHV-free BJAB cell lysate in the IP experiment. The IP protein complexes were separated by SDS-PAGE, and resolved proteins were visualized by silver staining (Fig. 1A). Several specific protein bands were present in the samples derived from the induced BCBL-1 cells (Fig. 1A, lane 1) but not in those from BJAB cells (Fig. 1A, lane 3) or in the sample precipitated with regular IgGs (Fig. 1A, lanes 2 and 4). These proteins were excised from the gel and subjected to trypsin digestion followed by liquid chromatography-tandem mass spectrometry analysis as described previously (49). The doublet bands around 90 kDa were found to be p90 RSK1 and p90 RSK2. Western blotting with specific anti-RSK1 and anti-RSK2 antibodies confirmed their identities (Fig. 1A). A previously identified ORF45 binding partner, IRF-7, that comigrated with ORF45 was also confirmed by Western blotting (Fig. 1A). To exclude the possibility that RSKs are directly recognized by the antibody 2D4A5 because of cross-reactivity, we used anti-Flag affinity beads to immunoprecipitate Flag-ORF45 from lysates of 293 cells that were transiently transfected with Flag-tagged ORF45 expression vector. The immunoprecipitated ORF45 and associated proteins were eluted with 3× Flag peptides and analyzed by SDS-PAGE followed by silver staining. The doublet bands of RSK1 and RSK2 were present in the immunocomplex of Flag-tagged ORF45 but not in that of Flag-tagged luciferase (Fig. 1B). Their identities were confirmed by Western blotting (Fig. 1B). Collectively, these data demonstrated that RSK1/RSK2 interacted with ORF45 in the cell. Other bands that appear to be specific were identified as cellular proteins related to the cytoskeleton, implying that ORF45 may be involved in intracellular viral trafficking. However, the specificity and functional role of the interaction between these cytoskeleton proteins and ORF45/RSKs await further study.
FIG. 1.
Association of RSK1 and RSK2 with KSHV ORF45. (A) Lysates of TPA-induced BCBL-1 (lanes 1 and 2) and BJAB (lanes 3 and 4) were immunoprecipitated with anti-ORF45 monoclonal antibody 2D4A5 (lanes 1 and 3) or control mouse IgG (lanes 2 and 4). The immunoprecipitated complexes were resolved by 4 to 12% NuPAGE and visualized by silver staining. The doublet bands (marked by an asterisk) around 90 kDa were identified as human RSK1 and RSK2. The position of comigrated ORF45 and IRF-7 is marked by a plus sign. The IP complexes were subjected to Western blotting (WB) with anti-RSK1, anti-RSK2, anti-IRF7, and anti-ORF45 antibodies as indicated. (B) Lysates of 293T cells transfected with Flag-tagged ORF45 (lane 1) or luciferase (lane 2) were immunoprecipitated with anti-Flag M2 affinity gel. The IP complexes were eluted with 3× Flag peptides, and the eluates were resolved by SDS-PAGE and visualized by silver staining. The doublet bands marked by an asterisk are RSK1 and RSK2. The IP eluates were also analyzed by Western blotting with antibodies anti-RSK1, anti-RSK2, anti-Flag, and anti-ORF45 as indicated.
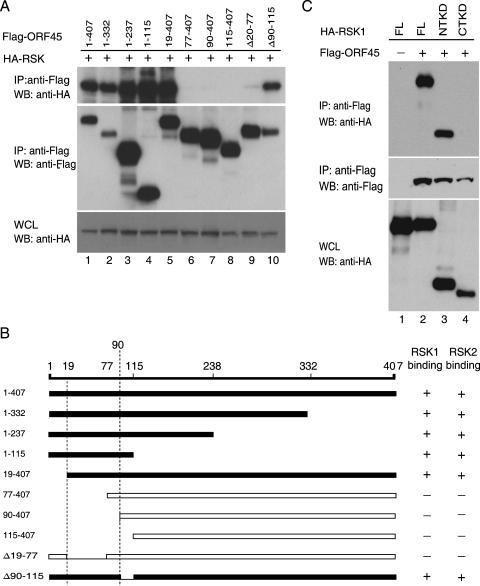
Next we determined the domains of ORF45 that bind to RSKs. A series of ORF45 truncation mutants were generated and cotransfected into 293 cells with HA-tagged RSK1 expression vector pKH3-RSK1 (Fig. 2A). The lysates were immunoprecipitated using anti-Flag M2 affinity gel, followed by Western blot analysis. Truncation from the C terminus up to aa 115 (mutants 1 to 332, 1 to 237, or 1 to 115) did not affect binding to RSK1. Mutants with deletions of the first N-terminal 19 aa retained function, but further deletions (aa 77 to 407, 90 to 407, and 115 to 407) abolished the interaction between ORF45 and RSK1. Deletion of the acidic amino acid cluster (90 to 115 aa) did not affect the interaction, while deletion of the 20- to 77-aa region abolished the interaction. The experiment was repeated with a RSK2 construct, and a similar result was obtained (data not shown). These experiments demonstrated that the region between aa 20 to 90 of ORF45 is essential for binding to RSK1/RSK2.
FIG. 2.
Mapping the binding domains of ORF45 and RSKs. (A) In vivo co-IP assay. HEK293 cells were transiently transfected with HA-RSK1 and full-length Flag-ORF45 (1 to 407 aa) or its deletion mutants aa 1 to 332, 1 to 237, 1 to 115, 19 to 407, 77 to 407, 90 to 407, 115 to 407, Δ(20-77), and Δ(90-115). Cell lysates were immunoprecipitated on an anti-Flag M2 affinity gel. The IP complexes were immunoblotted with anti-HA (top panel) and anti-Flag (middle panel). The whole-cell lysates (WCL), analyzed with anti-HA, showed similar expression of HA-RSK1 in all samples (bottom panel). (B) Diagram of ORF45 constructs used in the binding assays. Their binding abilities with RSK1 or with RSK2 are summarized on the right. (C) The NTKD binds to KSHV ORF45. HEK293 cells were transfected with Flag-ORF45 only (lane 1) or cotransfected with Flag-ORF45 and full-length HA-tagged RSK (FL, lane 2), the NTKD (lane 3), or the CTKD construct (lane 4). The lysates of transfected cells were immunoprecipitated on an anti-Flag affinity gel. The IP complexes were eluted with 3× Flag peptides, and the eluates were analyzed by immunoblotting with anti-HA (top panel) and anti-Flag (middle panel). Aliquots of whole-cell lysates (bottom panel) were analyzed by immunoblotting with anti-HA to reveal the input RSK. WB, Western blot.
We next mapped the region of RSK1/RSK2 that interacts with ORF45. RSKs are known to have two kinase domains: the NTKD and CTKD (35). Full-length and truncation mutants containing either the NTKD (1 to 418 aa) (Fig. 2C, lane 3) or the CTKD (321 to 735 aa) (Fig. 2C, lane 4) were cotransfected into 293 cells with Flag-tagged ORF45. Co-IP and Western blotting showed that NTKD interacts with ORF45 (Fig. 2C, lane 3).
Phosphorylation of ORF45 by RSKs.
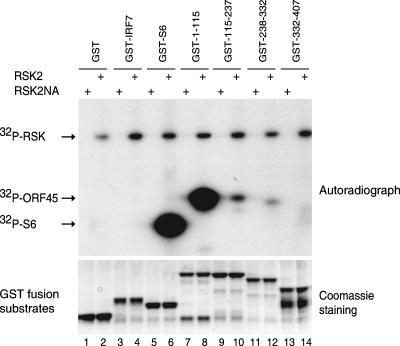
We have shown before that ORF45 is heavily phosphorylated in the cells (53). The association of ORF45 and RSKs raises the possibility that ORF45 may be a target for phosphorylation. To test whether RSKs phosphorylate ORF45 directly, we performed in vitro kinase assays using GST-ORF45 deletion mutants collectively covering the whole ORF45 molecule (Fig. 3). The immunoprecipitated HA-RSK2 was incubated with substrates in the presence of [γ-32P]ATP. As a positive control, GST-S6 fusion protein was efficiently phosphorylated by HA-RSK2 (lane 6) but not by kinase-dead mutant HA-RSK2NA (lane 5). In addition, neither GST alone nor GST-IRF-7 fusion protein were phosphorylated under the same conditions, confirming the specificity of the assay (lanes 1 to 4). Among the GST-ORF45 mutants, only one which encompasses aa 1 to 115 was strongly phosphorylated. This region overlaps the RSK binding domain (aa 20 to 90), as shown above. In all cases, autophosphorylation of active RSK2 was easily detected. The experiment was repeated with immunoprecipitated RSK1 and similar results were obtained (data not shown). These experiments demonstrate that RSK1/RSK2 directly phosphorylates ORF45 in vitro.
FIG. 3.
Phosphorylation of ORF45 by RSK2. HA-tagged pKH3-RSK2 and pKH3-RSK2NA (kinase-dead mutant) vectors were transfected into HEK293 cells. The transfected cells were starved for 24 h and then treated with 20% FBS for 10 min to activate RSK. Cell lysates were immunoprecipitated with anti-HA affinity gel. The IP HA-RSK2 (even lanes) and HA-RSK2NA (odd lanes) were used for in vitro kinase assays with GST-ORF45 fusion proteins encompassing aa 1 to 115 (lanes 7 and 8), 115 to 237 (lanes 9 and 10), 238 to 332 (lanes 11 and 12), and 332 to 407 (lanes 13 and 14) as substrates. GST-S6 (lanes 5 and 6) was included as a positive control. GST (lanes 1 and 2) and a GST-IRF7 fusion protein (lanes 3 and 4) were not phosphorylated under the assay conditions and served as negative controls.
ORF45 activates RSK1/RSK2.
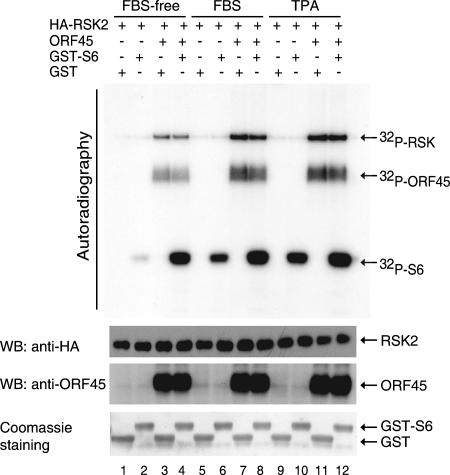
To explore the functional consequence of this interaction, we determined whether ORF45 affects RSK kinase activity. We cotransfected HA-tagged RSK2 expression vector pKH3-RSK2 with pCR3.1-ORF45 or empty vector pCR3.1 into 293 cells. Cells were serum starved for 24 h, then stimulated with serum or TPA as indicated. In vitro IP kinase assays were performed with GST-S6 as substrate and GST as control. In the absence of serum or TPA stimulation, RSK2 had low basal activity (Fig. 4, lane 2); ORF45 greatly increased RSK2 activity toward phosphorylation of GST-S6 peptide (Fig. 4, lane 4). The stimulation by ORF45 is greater than that caused by serum (compare lanes 4 and 6) or TPA treatment (compare lanes 4 and 10). In the presence of serum, ORF45 further stimulated RSK2 activity severalfold (lanes 8 and 6). Similarly, in the presence of TPA, ORF45 further stimulates RSK2 activity (lanes 12 and 10). In the cells cotransfected with ORF45, serum (lanes 8 and 4) and TPA (lanes 12 and 4) only minimally increased RSK2 activities, suggesting that ORF45 can maximally activate RSK activity. In addition, RSKs prepared from ORF45-expressing cells all showed strong autophosphorylation (lanes 3, 4, 7, 8, 11, and 12), suggesting that ORF45 increases RSK2 autophosphorylation. In control reactions, GST was not phosphorylated by the IP complex (odd lanes). Western blotting showed the levels of input RSK2 in each reaction to be comparable. All these data support that ORF45 activates RSK2 kinase activity. Furthermore, strong diffuse bands were detected on autoradiography of kinase reactions derived from ORF45-expressing cells. Western blotting confirmed that they are ORF45. This result not only confirmed the interaction of RSK2 and ORF45 but also suggested that ORF45 was phosphorylated, presumably by the associated RSK2. Experiments with the RSK1 construct gave identical results.
FIG. 4.
ORF45 stimulates RSK kinase activity. pKH3-RSK2 plasmids were cotransfected with pCR3.1-ORF45 or empty vector pCR3.1 into 293 cells. After 24 h of serum starvation, the transfected cells were left untreated (lanes 1 to 4) or were treated with 20% FBS (lanes 5 to 8) or TPA (lanes 9 to 12) for 10 min. HA-RSK2 kinases were immunoprecipitated with anti-HA and mixed with GST (odd lanes) or GST-S6 (even lanes) for in vitro kinase assays. Bands on the autoradiography panel are phosphorylated GST-S6, coimmunoprecipitated ORF45, and autophosphorylated HA-RSK2, as marked. The IP complexes in each reaction were analyzed by Western blotting (WB) to show the equal input of HA-RSK2 in each reaction and to confirm the identity of the coimmunoprecipitated ORF45. Coomassie blue staining of the GST-S6 and GST substrates in each reaction was also shown.
RSK activation by ORF45 depends on ERK1/ERK2 but not on MEK.
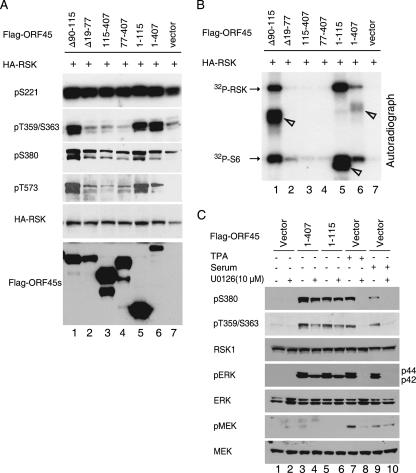
Activation of RSKs by ERK MAPK signaling involves sequential phosphorylation events at several serine and threonine residues (Ser221, Ser359/Ser363, Ser380, and Thr573; positions represent the human RSK1 aa sequence) (35). We were interested in determining whether these sites are also phosphorylated in ORF45-activated RSKs. We cotransfected pKH3-RSK1 with either the full length of ORF45 or its truncation mutants into 293 cells. After serum starvation, we prepared cell lysate for Western blotting with phosphorylation-specific antibodies to examine the phosphorylation statuses of these key residues (Fig. 5A). We also performed IP kinase assays to assess the abilities of ORF45 mutants to activate RSK (Fig. 5B). The results showed that ORF45 constructs that preserved the RSK binding domain (aa 20 to 90) including the full length of aa 1 to 407 (lane 6) and 1 to 115 (lane 5) and the internal deletion mutant Δ(90-115) (lane 1) are able to activate RSK (Fig. 5B), as shown by phosphorylated GST-S6 and autophosphorylation of RSK. The three ORF45 products all coprecipitated with HA-RSK and were phosphorylated in the assay conditions (Fig. 5B). All three ORF45s of these constructs induced RSK phosphorylation at the Ser359/Ser363, Ser380, and Thr573 sites (Fig. 5A, lanes 1, 5, and 6). Other constructs that lost the RSK binding domain were unable to activate RSK (Fig. 5B, lanes 2, 3, and 4) and also failed to induce RSK phosphorylation at these sites (Fig. 5A, lanes 2, 3, and 4). In contrast, Ser221 of RSK1 was phosphorylated at a similar level in all samples regardless of ORF45 expression (Fig. 5A), confirming that this site is constitutively phosphorylated. These results demonstrate that the increased RSK kinase activities were correlated with the phosphorylation status of the key residues.
FIG. 5.
Activation of RSK1/RSK2 by ORF45 in vivo. (A) Phosphorylation status of key residues of RSK activated by ORF45. pKH3-RSK1 plasmids were transfected with full-length Flag-tagged ORF45 or deletion mutants as indicated. After serum starvation for 24 h, cell lysates were prepared and analyzed by Western blotting with phosphorylation-specific antibodies to phospho-S221, phospho-T359/S363, phospho-S380, phospho-T573, and anti-Flag antibody. (B) Kinase activities of RSK with coexpressed ORF45 or its mutants. The cell lysates were also immunoprecipitated with anti-HA, and the IP complexes were mixed with GST-S6 for an in vitro kinase assay measuring the kinase activity of RSK1. The full-length sample (1 to 407 aa, lane 6), the mutant containing aa 1 to 115 (lane 5), and the mutant from which aa 90 to 115 are deleted are able to bind to RSK and were coprecipitated with HA-RSK. They were phosphorylated under the assay conditions, and their positions are marked with arrowheads. (C) Activation of RSK by ORF45 depends on ERK but not on MEK. pKH3-RSK1 plasmids were cotransfected into 293 cells with empty vectors (lanes 1, 2, and 7 to 10), full-length ORF45 (lanes 3 and 4), or the ORF45 deletion mutant containing aa 1 to 115 (lanes 5 and 6). The transfected cells were starved for 24 h and then treated with vehicle (lanes 1 to 6), with TPA (lanes 7 and 8), or with 20% FBS (lanes 9 and 10) in the presence (even lanes) or absence (odd lanes) of MEK inhibitor U0126. Whole-cell lysates were subjected to Western blotting with the phosphorylation-specific antibodies to RSK phospho-S380, phospho-T359/S363, pERK, and pMEK. The blots were then stripped and reprobed with anti-RSK1, ERK, and MEK as indicated.
Ser359/Ser363 and Thr573 of RSK are phosphorylated by ERK1/ERK2 in MAPK signaling. We next examined whether ERK1/ERK2 and its upstream kinase MEK are involved in RSK activation by ORF45. Human embryonic kidney (HEK293) cells were transfected with empty vector (Fig. 5C, lanes 1, 2, and 7 to 10), full-length ORF45 (lanes 3 and 4), or truncated mutant aa 1 to 115 (lanes 5 and 6). Cells were serum starved and treated with 10 μM MEK inhibitor U0126 before serum or TPA stimulation as indicated. Cell lysates were analyzed by Western blotting with phosphorylation-specific antibodies against MEK, ERK1/ERK2, and RSK (Fig. 5C). Full-length ORF45 and the truncated mutant aa 1 to 115 induced RSK phosphorylation as expected. ORF45 also induced ERK phosphorylation but, surprisingly, no MEK phosphorylation, suggesting that RSK activation by ORF45 does not require MEK. In the presence of MEK inhibitor U0126, ORF45-induced RSK1 phosphorylations at Ser380 and Thr359/Ser363 were barely affected (Fig. 5C, lanes 4 and 6), but such phosphorylations induced by TPA (lane 8) and serum (lane 10) were completely abolished. Interestingly, in the presence of U0126 and transfected ORF45, p44 ERK1 phosphorylation was completely abolished, but p42 ERK2 phosphorylation was not affected. These results suggest that ORF45-induced RSK activation is ERK dependent but MEK independent. Examination of other MAPKs such as p38 and ERK5 revealed that ORF45 specifically induced ERK/RSK activation (data not shown).
RSKs are activated during KSHV primary infection and reactivation from latency.
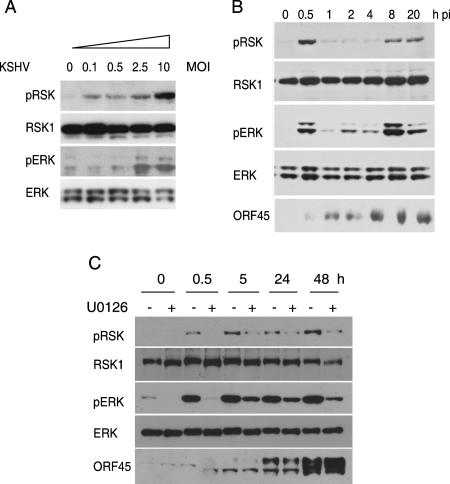
Several reports have shown that ERK MAPK signaling is activated during KSHV infection (40, 44). To determine whether RSKs are also activated, we infected serum-starved 293 cells with KSHV at different multiplicities of infection (MOI) (viral genome copies/cell) for 30 min. The lysate of the infected cells was analyzed by Western blotting with anti-phospho-RSK1/RSK2 and anti-phospho-ERK1/ERK2. KSHV infection increased RSK and ERK phosphorylation dose dependently (Fig. 6A). The maximal activation was seen at an MOI of 10, but substantial induction was also observed at lower MOI, suggesting that this phosphorylation event is physiologically relevant. Total RSK and ERK protein levels were not affected by KSHV infection, as determined by reprobing of the membrane with antibodies against total RSK1 and ERK1/ERK2 (Fig. 6A). These results demonstrated that KSHV de novo infection activates the endogenous RSK and ERK.
FIG. 6.
KSHV infection induces RSK phosphorylation. (A) Dose-dependent RSK phosphorylation upon KSHV infection. HEK293 cells were serum starved for 24 h and then were either mock infected or infected with KSHV at different MOIs (genome copies/cell) for 30 min at 37°C. The lysates was subjected to Western blotting and reacted with anti-phospho-RSK and anti-phospho-ERK1/ERK2 antibodies. The blots were stripped and reprobed with anti-RSK1 and anti-ERK. (B) Kinetics of RSK activation by KSHV infection. After 24 h of serum starvation, the HEK293 cells were either mock infected or infected with KSHV (MOI of 10) for the times indicated. Cell lysates were analyzed by Western blotting with anti-phospho-RSK, anti-phospho-ERK1/ERK2, and anti-ORF45 antibodies. The membranes were stripped and reprobed to detect total RSK1 and ERK. (C) RSKs are activated during KSHV reactivation. BCBL-1 cells were induced with TPA in the absence and presence of U0126 for the times indicated. Cell lysates were analyzed by Western blotting with anti-ORF45 and phosphorylation-specific antibodies of RSK and ERK. The membranes were stripped and reprobed with antibodies to total RSK1 and ERK.
To determine the kinetics of RSK phosphorylation induced by KSHV infection, we analyzed lysate of serum-starved cells infected with KSHV (MOI of 10) for different lengths of time. A strong increase in RSK phosphorylation was seen at 30 min postinfection (pi), and the phosphorylation level decreased at 1, 2, and 4 h pi (Fig. 6B). The RSK phosphorylation increased again at 8 h pi and remained at that level. Similarly, ERK phosphorylation peaked at 30 min pi, decreased at later time points, and then increased again (Fig. 6B). The early RSK activation by KSHV requires virus binding to the target cells because prevention of virus binding with heparin blocks RSK activation (data not shown).
Next we examined whether RSKs are activated during reactivation. BCBL-1 cells carrying latent KSHV infection were treated with TPA. RSK and ERK phosphorylation increased after TPA induction. Beginning 5 h after induction, ERK and RSK phosphorylation became partially resistant to U0126 treatment (Fig. 6C). Since the ORF45-induced ERK/RSK activation is MEK independent, it implies that ORF45 may play a role in ERK/RSK activation during KSHV lytic replication.
Role of ORF45 in RSK activation during KSHV infection.
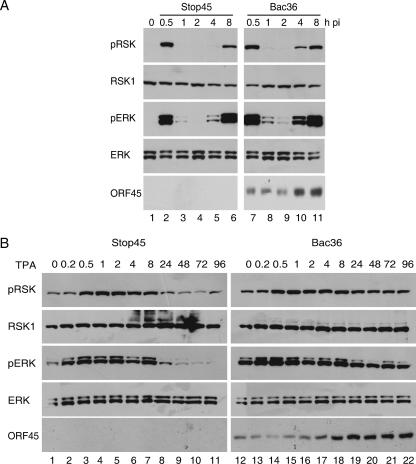
To investigate the role of ORF45 in RSK activation during KSHV infection, we compared the levels of RSK phosphorylation in cells infected with recombinant KSHV viruses BAC36 (wild type) with levels in cells infected with BAC-stop45 (ORF45 null) (52). Serum-starved 293 cells were infected with BAC36 and BAC-stop45 at 10 genome copies per cell. Lysates were prepared at different intervals after infection and analyzed for RSK phosphorylation. ORF45-null virus infection induced about the same levels of activation of RSK (Fig. 7A, top row, lanes 2 and 7) and ERK1/ERK2 (third row, lanes 2 and 7) at 0.5 h pi, indicating that ORF45 does not seem to play a major role in the immediate-early transient activation of RSK and ERK. This result is not surprising because the KSHV virion envelope proteins have been shown to trigger ERK activation (40). But beginning at 4 h pi, the second cycle of RSK (Fig. 7A, top row, lanes 5 and 10, lanes 6 and 11) and ERK (third row, lanes 5 and 10, lanes 6 and 11) phosphorylation was noticeably lower in ORF45-null-infected cells. This result suggests that ORF45 played a more significant role in the second phase of RSK and ERK activation during KSHV infection.
FIG. 7.
Role of ORF45 in RSK activation during KSHV infection. (A) RSK activation by ORF45 wild-type and ORF45-null recombinant KSHV viruses. HEK293 cells were infected with recombinant KSHV BAC36 wild-type or ORF45-null BAC-stop45 viruses for the times indicated. Cell lysates were analyzed by Western blotting with anti-phospho-RSK, anti-phospho-ERK, or anti-ORF45 antibodies. Membranes were stripped and reprobed with anti-ERK or anti-RSK antibodies. (B) RSK activation by ORF45 wild-type and ORF45-null viruses during TPA-induced reactivation. Stable 293T cells carrying BAC36 wild-type or ORF45-null BAC-stop45 viral genomes were treated with TPA for the times indicated. Cell lysates were analyzed by Western blotting with anti-phospho RSK, anti-phospho-ERK1/ERK2, or anti-ORF45 antibodies. Membranes were stripped and reprobed with anti-ERK or anti-RSK antibodies.
To determine whether ORF45 plays any role in RSK and ERK activation during reactivation, we examined the phosphorylation of RSK and ERK in TPA-induced BAC36 and BAC-stop45 stable cells at the different time points. The levels of RSK and ERK phosphorylation were comparable at the early stage up to 8 h after TPA treatment (Fig. 7B), suggesting that the role of ORF45 is minimal in the early phase of RSK and ERK activation. The levels of RSK and ERK phosphorylation decreased in ORF45-null cells after TPA induction for 24 h; however, the levels in ORF45 wild-type cells were sustained. These results support that ORF45 contributes to sustained RSK and ERK phosphorylation in KSHV reactivation.
Role of RSKs in KSHV primary infection.
The MAPK signal pathways have been shown to be involved in different aspects of the virus life cycle. To determine whether RSKs play any role in the KSHV life cycle, we generated stable 293 cell lines in which both RSK1 and RSK2 were knocked down by retrovirus-based siRNAs. Western blotting showed that expressions of both RSK1 and RSK2 were reduced by >90%, to undetectable levels. These cells show no obvious difference in morphology or growth rate from their parental cells. The cells do not trigger nonspecific antiviral response because both herpes simplex virus type 1 and vesicular stomatitis virus replicate equally well in the siRNA-transduced cells and the parental cells (data not shown).
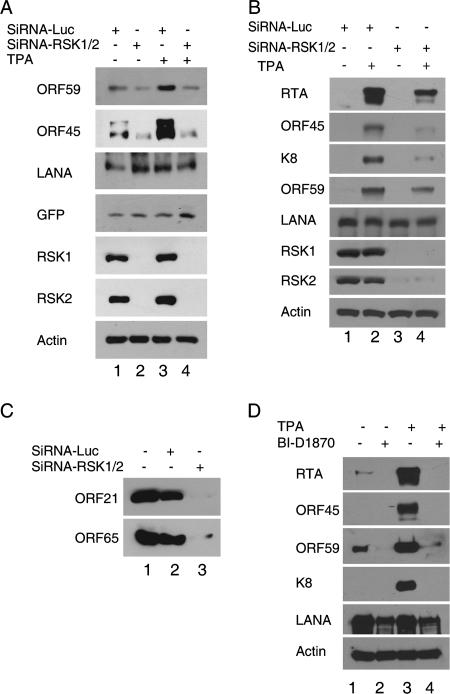
To determine the effect of RSK1/RSK2 knockdown on KSHV infection, we infected siRNA-RSK1/RSK2-transduced cells and control cells with BAC36 recombinant viruses. BAC36 contains a GFP-expressing cassette, so KSHV infection can be monitored by GFP expression. Three days after infection, examination of the infected cells with fluorescence microscopy revealed similar infection rates in the RSK1/RSK2 siRNA-transduced and control cells (data not shown). Western blot analysis confirmed similar expression levels of GFP and KSHV LANA in RSK1/RSK2 knockdown and control cells (Fig. 8A), suggesting that RSK1 and RSK2 do not affect KSHV primary infection which automatically results in latency. However, the expressions of lytic genes such as PF8/ORF59 and ORF45 were noticeably lower in siRNA-RSK1/RSK2-transduced cells than in control cells (Fig. 8A, lanes 1 and 2), indicating that RSK1 and RSK2 affect KSHV reactivation from latency. Indeed, when the infected cells were incubated in the presence of TPA, expressions of PF8/ORF59 and ORF45 were significantly lower in RSK1/RSK2 siRNA-transduced cells than in control cells while levels of LANA and GFP expression were the same (Fig. 8A, lanes 3 and 4). These results suggest that RSK1 and RSK2 play an essential role in lytic replication.
FIG. 8.
Essential role of RSK1 and RSK2 in KSHV lytic replication. (A) Silencing of RSK1/RSK2 by siRNA suppresses KSHV lytic infection. HEK293 cells were transduced with retrovirus-based control siRNA-Luc or were transduced with both siRNA-RSK1 and siRNA-RSK2. Stable siRNA-RSK1/RSK2-transduced and control cells were infected with KSHV in the presence of 2 μg/ml Polybrene for 4 h and then washed with PBS and placed in fresh medium. Forty-eight hours after infection, the cells were left untreated or were treated with TPA for 24 h. Cells were then lysed and assayed by Western blotting. The experiments were repeated with different siRNA construct combinations; only a representative result is shown here. (B) Silencing of RSK1/RSK2 by siRNA suppresses KSHV lytic replication in BCBL-1 cells. BCBL-1 cells stably transduced with control siRNA-Luc or with siRNA-RSK1/RSK2 were left untreated or were treated with TPA for 72 h. Cell lysates were then prepared and assayed by Western blotting with the antibodies indicated. (C) Silencing of RSK1/RSK2 by siRNA suppresses KSHV progeny virion production. The virions secreted into the medium 4 days after TPA treatment were collected, purified, and analyzed by Western blotting for the presence of ORF21 (tegument) and ORF65 (capsid). (D) RSK inhibitor blocks KSHV lytic replication. BCBL-1 cells were induced with TPA for 72 h in the absence and presence of RSK inhibitor BI-D1870. Cell lysates were then prepared and assayed by Western blotting with the antibodies indicated.
RSK1 and RSK2 are required for KSHV reactivation and virion production.
To confirm that RSK1/RSK2 play a role in KSHV reactivation, we tested the effect of RSK1/RSK2 knockdown on KSHV reactivation in BCBL-1 cells. The level of RSK1/RSK2 expression was reduced by >90% in stable siRNA-RSK1/RSK2-treated cells (Fig. 8B). We induced both siRNA-RSK1/RSK2-transduced and control cells with TPA for 72 h to trigger KSHV lytic replication. The lysates of the induced cells were examined with Western blotting. Expressions of lytic genes RTA/ORF50, ORF45, K8, and ORF59 were all greatly inhibited at 72 h in siRNA-RSK1/RSK2-transduced cells, whereas expression of latent gene LANA was not affected (Fig. 8B, lanes 3 and 4). In the absence of TPA induction, depletion of RSK1/RSK2 by siRNA also inhibited KSHV spontaneous lytic gene expression.
Lytic replication leads to production of infectious virus progeny. To determine whether knockdown of RSK1/RSK2 interferes with progeny virus production, we determined the yields of viruses purified from the TPA-induced medium of siRNA-RSK1/RSK2-treated BCBL-1 and control cells by Western blotting with antibodies against capsid protein ORF65 and tegument protein ORF21. In contrast to that from control cells, the release of virus from RSK1/RSK2 knockdown BCBL-1 cells was inhibited to undetectable levels (Fig. 8C), so we conclude that RSK1 and RSK2 are required for KSHV lytic replication.
A specific RSK NTDK inhibitor, BI-D1870, has recently been developed (38). To determine whether inhibition of RSK activity affects KSHV lytic replication, we induced BCBL-1 cells with TPA in the presence of 10 μM BI-D1870 (38), a concentration that has been tested to have no cytotoxicity. Cells were collected 48 h after induction and analyzed for lytic gene expression by Western blotting. The data suggested that inhibition of RSK activity by BI-D1870 greatly suppressed KSHV lytic gene expression induced by TPA and also the spontaneous lytic replication, but it had little effect on latent gene LANA expression (Fig. 8D).
RSK1 and RSK2 are colocalized with ORF45 in both the cytoplasm and the nucleus.
To explore the functional role of the interaction of ORF45 and RSK and the mechanism of RSK1 and RSK2 involvement in KSHV lytic replication, we examined their subcellular localizations during KSHV lytic replication, induced by treatment of BCBL-1 cells with TPA for 48 h. The cells were fixed, permeabilized, and examined with a double-staining IFA. ORF45 is localized predominantly in the cytoplasm, but a portion accumulates in the intranuclear structures that resembles viral replication compartments (VRCs) (Fig. 9A). VRCs have been described as globular intranuclear structures where viral DNA synthesis, initial DNA packaging, and virion assembly take place (13). The distributions of RSK1 and RSK2 differ in ORF45-positive and -negative cells. In ORF45-negative cells, RSK1 and RSK2 were distributed in both the cytoplasm and nucleus with a slight preference for the cytoplasm (Fig. 9A). In the ORF45-positive cells, a subset of RSK1 and RSK2 was recruited into the VRC-like structures that are colocalized with ORF45. (We attempted to determine whether the intranuclear RSK1 and RSK2 are active forms by IFA with various phosphorylation-specific antibodies from different sources, but we failed to obtain a clear result because of low sensitivities of the antibodies.) To confirm that the intranuclear bodies are indeed functional VRC where viral DNA synthesis indeed takes place, we pulse-labeled the induced BCBL-1 cells with BrdU and performed triple staining to detect RSK2, K8, and freshly synthesized DNA. K8 has been shown to be involved in KSHV lytic DNA replication and to colocalize with other viral replication proteins in the VRC (43). Figure 9B shows that RSK2 (green) colocalizes with both BrdU (red) and K8 (blue), confirming that RSKs are present in functional VRCs. The presence of RSK1 and RSK2 in the VRCs further supports that RSK1 and RSK2 play an essential role in KSHV lytic replication.
FIG. 9.
A portion of RSK1/RSK2 accumulates in the VRC during KSHV lytic replication. (A) BCBL-1 cells were treated with TPA for 48 h, fixed, permeabilized, and double stained for ORF45 (green) and RSK1 (red) or RSK2 (red) antibodies. (B) BCBL-1 cells were treated with TPA for 48 h and pulse-labeled with BrdU for 60 min. The cells were subjected to triple-label IFA with mouse monoclonal anti-K8 (blue), rabbit polyclonal anti-RSK2 (green), and sheep anti-BrdU (red) antibodies.
DISCUSSION
The research reported here demonstrates that a KSHV immediate-early and tegument protein, ORF45, interacts with RSK1 and RSK2 and significantly stimulates their kinase activities. We found that these kinases are activated during KSHV primary infection. ERK/RSK signaling are also activated and sustained during KSHV reactivation. Depletion of RSK1/RSK2 gene expression by siRNA or inhibition of their activities by a specific inhibitor, BI-D1870, blocks KSHV lytic gene expression and abolishes progeny virion production, demonstrating an essential role of RSK1/RSK2 in KSHV lytic replication.
Activation of RSKs during KSHV primary infection and reactivation.
Even though MEK/ERK activation has been shown to be important for KSHV primary infection and reactivation (11, 16, 31, 40, 47), whether the signaling relays to RSKs, direct substrates and functional mediators of ERK1/ERK2, remains unknown. Our studies suggest that RSK1 and RSK2 are activated with biphasic kinetics similar to those of ERK1/ERK2 during KSHV primary infection. The first wave of activation by KSHV infection relies on binding and/or entry and is independent of viral gene expression, because UV-irradiated KSHV also activates ERK1/ERK2 (40). The second wave of ERK/RSK activation is sustained and coincides with viral gene expression. Although other viral factors could also be involved, we demonstrated that ORF45 contributes significantly to the late sustained ERK/RSK activation. During primary infection, the ORF45-null recombinant KSHV virus BAC-stop45 triggered weaker activation of ERK/RSK than did wild-type BAC36 in the second phase. During reactivation, although the activation of ERK and RSK remains at high levels in BAC36-293T cells 24 h after infection, the level is significantly lower in BAC-stop45 cells after that time. In TPA-induced BCBL-1 cells, a U0126-resistant ERK/RSK phosphorylation occurred 5 h after induction. Because ORF45-mediated ERK/RSK activation is independent of MEK, ORF45 could play a significant role at this stage.
Activation of RSKs by KSHV ORF45.
ORF45 is tightly associated with RSK1/RSK2 and activates its activity. Analysis of the phosphorylation status of key regulatory residues of RSK, ERK, and MEK revealed that ERK is activated but MEK is not. Indeed, MEK-specific inhibitor U0126 has little effect on RSK activation by ORF45. The independence of MEK suggests that the activation of RSK by ORF45 represents a novel mechanism of MAPK activation. How does ORF45 activate RSK1/RSK2? ORF45 lacks a typical kinase domain and seems not to have intrinsic kinase activity. Activation of RSK by ORF45 depends on its ability to bind to RSK. The N-terminal third of the ORF45 coding region (aa 1 to 115) is able to bind to and stimulate RSK1/RSK2 as efficiently as full-length ORF45. How does the binding of ORF45 trigger RSK1/RSK2 activation? One possibility is that binding of RSKs to ORF45 could increase the association of ERK to RSKs. ERK has previously been shown to be associated with RSKs in mammalian cells through the docking site near the carboxyl end in quiescent cells and to dissociate transiently upon mitogen stimulation. Increased association of ERK with RSK extends the duration of RSK activation (36). We observed that ORF45 increases the interaction of RSK/ERK even though it does not bind to ERK directly (E. Kuang and F. Zhu, unpublished data). A second possibility is that ORF45 could alter the conformation of RSK by binding to the RSK NTKD and subsequently triggering phosphorylation activity of the CTKD and possibly ERK1/ERK2. Finally, bound ORF45 might also protect activated RSK1/RSK2 or the ERK1/ERK2 from cellular phosphatases or other cellular negative regulators.
RSKs are essential for KSHV lytic replication.
Evidence presented here suggests that RSK1 and RSK2 play critical roles in KSHV lytic replication. (i) Depletion of the expression of both RSK1 and RSK2 by siRNA dramatically inhibits TPA-induced KSHV lytic gene expression and virion production. These results suggest that RSK1 and RSK2 affect an upstream signaling pathway that controls reactivation of KSHV. (ii) Inhibition of KSHV lytic gene expression by RSK inhibitor BI-D1870 suggests that the kinase activities of RSK1 and RSK2 are needed for the KSHV lytic cycle. (iii) A subset of RSK1 and RSK2 accumulates in VRCs in the nuclei of cells that are undergoing lytic replication. The VRC consists of viral and cellular proteins and is the location where viral DNA replication and subsequent capsid assembly take place (13). The functional role of RSK1/RSK2 in VRCs is not clear, but this localization strongly supports that RSK1 and RSK2 play an indispensable role in KSHV lytic replication. They could modulate cellular factors that reside in the VRCs and regulate viral DNA replication. Viral gene transcription also takes place on the freshly synthesized DNA, and RSK may also be needed for regulation of viral gene expression. RSK1 and RSK2 have been shown to phosphorylate many cellular nuclear and cytoplasmic targets such as CREB, c-Fos, ETS, CBP, ATF4, Bad, GSK3β, IκBα, p27KIP1, histone H3, filamin A, and others to regulate many cellular processes, including transcription, the cell cycle, survival, and proliferation (18, 35). It remains to be determined which factors and substrates are directly involved in KSHV lytic replication. It is possible that some of these factors may collectively determine the fate of KSHV replication. Besides cellular targets, RSK1 and RSK2 could phosphorylate viral proteins and modulate their functions. ERK1/ERK2 have been shown to phosphorylate viral proteins such as influenza virus NEP and M1 and HIV p6Gag (19, 32) and to participate in various aspects of the viral life cycle.
Interestingly, loss of ORF45 does not significantly affect overall viral gene expression during reactivation, whereas knockdown of RSK1/RSK2 severely suppresses expression of many viral lytic genes (52). One possible explanation is that other viral factors can substitute for ORF45 to activate the ERK/RSK MAPK pathway partially. Indeed several KSHV gene products have been shown to activate ERK MAPK signaling, including K15, vGPCR, kaposin, and others (4, 25, 42).
Implication for KS pathogenesis.
Sustained activation of ERK/RSK signaling by KSHV has important implications for KSHV-related diseases. The activated ERK/RSK signaling during the KSHV lytic cycle could activate a number of transcription factors that reprogram host gene expression. For example, ERK1/ERK2 and other MAPKs p38 and JNK are known to activate AP-1 and Ets, which were recently shown to activate angiogenesis factor angiopoietin 2 (46). The ERK/RSK pathway is also known as an important regulator for cell survival. Extended cell survival during lytic replication allows spontaneously reactivated cells to keep producing paracrine signaling to the neighboring cells. In addition, aberrant RAS/RAF/ERK activation may be directly linked to carcinogenesis, and development of pharmacological inhibitors to each kinase has been a focus of intensive research (33). Misregulation of RSK2 has recently been implicated in cell transformation and human prostate cancers (8, 9, 24, 41). Thus, sustained activation of ERK/RSK signaling probably plays an important role in KSHV pathogenesis.
Also of interest is that RSK2 may be a key factor mediating the reciprocal interaction between KSHV and HIV-1. HIV-1 infection is known to increase KS incidence significantly in individuals infected with KSHV. Direct interplay between KSHV and HIV involving the HIV-encoded protein Tat has been suggested. Huang et al. found that ORF45 is the only KSHV immediate-early protein that interacts synergistically with Tat in activating expression of the HIV-1 long terminal repeat (21). Intracellular expression of Tat also increased human herpesvirus 8 lytic gene expression (21, 48). Extracellular Tat has also been shown to act as a growth factor promoting KS cell growth and increasing KSHV infectivity (3, 15). Recently, Tat was demonstrated to recruit and activate RSK2, which is required for Tat transcriptional activation (20). Thus, RSK could be the mediator of the synergistic effect between HIV-1 and KSHV.
The essential role of RSKs in KSHV lytic replication suggests that RSKs could be a potential target for treatment of KSHV-related diseases. Several recently developed RSK inhibitors (SL0101, BI-D1870, and fmk) (12, 38, 41) not only provide powerful new tools for dissection of the functions of RSKs in normal and pathological situations but also provide the potential for therapies directed at the misregulated ERK/RSK pathway. Inhibition of RSK not only would disrupt KSHV replication but could also block the proliferation of KS-associated tumor cells. Therefore, development of specific RSK inhibitors should be a very attractive approach for therapy of KSHV-associated diseases.
Acknowledgments
We are grateful to Yan Yuan for his support at the initial stage of this project. We thank Dario Alessi, Melanie Cobb, Shou-Jiang Gao, Deborah Lannigan, Jeffrey Smith, Robert Ricciardi, and Keiji Ueda for kindly providing reagents. We also thank Anne B. Thistle at the Florida State University for excellent editorial assistance.
This work was supported by National Institutes of Health grant R01DE016680 and by FSU setup funds to F.Z.
Footnotes
Published ahead of print on 5 December 2007.
REFERENCES
- 1.Akula, S. M., P. W. Ford, A. G. Whitman, K. E. Hamden, J. G. Shelton, and J. A. McCubrey. 2004. Raf promotes human herpesvirus-8 (HHV-8/KSHV) infection. Oncogene 235227-5241. [DOI] [PubMed] [Google Scholar]
- 2.Andrade, A. A., P. N. Silva, A. C. Pereira, L. P. De Sousa, P. C. Ferreira, R. T. Gazzinelli, E. G. Kroon, C. Ropert, and C. A. Bonjardim. 2004. The vaccinia virus-stimulated mitogen-activated protein kinase (MAPK) pathway is required for virus multiplication. Biochem. J. 381437-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aoki, Y., and G. Tosato. 2004. HIV-1 Tat enhances Kaposi sarcoma-associated herpesvirus (KSHV) infectivity. Blood 104810-814. [DOI] [PubMed] [Google Scholar]
- 4.Brinkmann, M. M., M. Glenn, L. Rainbow, A. Kieser, C. Henke-Gendo, and T. F. Schulz. 2003. Activation of mitogen-activated protein kinase and NF-κB pathways by a Kaposi's sarcoma-associated herpesvirus K15 membrane protein. J. Virol. 779346-9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai, Y., Y. Liu, and X. Zhang. 2007. Suppression of coronavirus replication by inhibition of the MEK signaling pathway. J. Virol. 81446-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 2661865-1869. [DOI] [PubMed] [Google Scholar]
- 7.Chen, Z., T. B. Gibson, F. Robinson, L. Silvestro, G. Pearson, B. Xu, A. Wright, C. Vanderbilt, and M. H. Cobb. 2001. MAP kinases. Chem. Rev. 1012449-2476. [DOI] [PubMed] [Google Scholar]
- 8.Cho, Y. Y., K. Yao, A. M. Bode, H. R. Bergen III, B. J. Madden, S. M. Oh, S. Ermakova, B. S. Kang, H. S. Choi, J. H. Shim, and Z. Dong. 2007. RSK2 mediates muscle cell differentiation through regulation of NFAT3. J. Biol. Chem. 2828380-8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark, D. E., T. M. Errington, J. A. Smith, H. F. Frierson, Jr., M. J. Weber, and D. A. Lannigan. 2005. The serine/threonine protein kinase, p90 ribosomal S6 kinase, is an important regulator of prostate cancer cell proliferation. Cancer Res. 653108-3116. [DOI] [PubMed] [Google Scholar]
- 10.Clark, D. E., C. E. Poteet-Smith, J. A. Smith, and D. A. Lannigan. 2001. Rsk2 allosterically activates estrogen receptor alpha by docking to the hormone-binding domain. EMBO J. 203484-3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen, A., C. Brodie, and R. Sarid. 2006. An essential role of ERK signalling in TPA-induced reactivation of Kaposi's sarcoma-associated herpesvirus. J. Gen. Virol. 87795-802. [DOI] [PubMed] [Google Scholar]
- 12.Cohen, M. S., C. Zhang, K. M. Shokat, and J. Taunton. 2005. Structural bioinformatics-based design of selective, irreversible kinase inhibitors. Science 3081318-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Bruyn Kops, A., and D. M. Knipe. 1988. Formation of DNA replication structures in herpes virus-infected cells requires a viral DNA binding protein. Cell 55857-868. [DOI] [PubMed] [Google Scholar]
- 14.Dourmishev, L. A., A. L. Dourmishev, D. Palmeri, R. A. Schwartz, and D. M. Lukac. 2003. Molecular genetics of Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8) epidemiology and pathogenesis. Microbiol. Mol. Biol. Rev. 67175-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ensoli, B., and M. Sturzl. 1998. Kaposi's sarcoma: a result of the interplay among inflammatory cytokines, angiogenic factors and viral agents. Cytokine Growth Factor Rev. 963-83. [DOI] [PubMed] [Google Scholar]
- 16.Ford, P. W., B. A. Bryan, O. F. Dyson, D. A. Weidner, V. Chintalgattu, and S. M. Akula. 2006. Raf/MEK/ERK signalling triggers reactivation of Kaposi's sarcoma-associated herpesvirus latency. J. Gen. Virol. 871139-1144. [DOI] [PubMed] [Google Scholar]
- 17.Ganem, D. 2006. KSHV infection and the pathogenesis of Kaposi's sarcoma. Annu. Rev. Pathol. 1273-296. [DOI] [PubMed] [Google Scholar]
- 18.Hauge, C., and M. Frodin. 2006. RSK and MSK in MAP kinase signalling. J. Cell Sci. 1193021-3023. [DOI] [PubMed] [Google Scholar]
- 19.Hemonnot, B., C. Cartier, B. Gay, S. Rebuffat, M. Bardy, C. Devaux, V. Boyer, and L. Briant. 2004. The host cell MAP kinase ERK-2 regulates viral assembly and release by phosphorylating the p6gag protein of HIV-1. J. Biol. Chem. 27932426-32434. [DOI] [PubMed] [Google Scholar]
- 20.Hetzer, C., D. Bisgrove, M. S. Cohen, A. Pedal, K. Kaehlcke, A. Speyerer, K. Bartscherer, J. Taunton, and M. Ott. 2007. Recruitment and activation of RSK2 by HIV-1 Tat. PLoS ONE 2e151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang, L. M., M. F. Chao, M. Y. Chen, H. Shih, Y. P. Chiang, C. Y. Chuang, and C. Y. Lee. 2001. Reciprocal regulatory interaction between human herpesvirus 8 and human immunodeficiency virus type 1. J. Biol. Chem. 27613427-13432. [DOI] [PubMed] [Google Scholar]
- 22.Jacque, J. M., A. Mann, H. Enslen, N. Sharova, B. Brichacek, R. J. Davis, and M. Stevenson. 1998. Modulation of HIV-1 infectivity by MAPK, a virion-associated kinase. EMBO J. 172607-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson, R. A., X. L. Ma, A. D. Yurochko, and E. S. Huang. 2001. The role of MKK1/2 kinase activity in human cytomegalovirus infection. J. Gen. Virol. 82493-497. [DOI] [PubMed] [Google Scholar]
- 24.Kang, S., S. Dong, T. L. Gu, A. Guo, M. S. Cohen, S. Lonial, H. J. Khoury, D. Fabbro, D. G. Gilliland, P. L. Bergsagel, J. Taunton, R. D. Polakiewicz, and J. Chen. 2007. FGFR3 Activates RSK2 to mediate hematopoietic transformation through tyrosine phosphorylation of RSK2 and activation of the MEK/ERK pathway. Cancer Cell 12201-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kliche, S., W. Nagel, E. Kremmer, C. Atzler, A. Ege, T. Knorr, U. Koszinowski, W. Kolanus, and J. Haas. 2001. Signaling by human herpesvirus 8 kaposin A through direct membrane recruitment of cytohesin-1. Mol. Cell 7833-843. [DOI] [PubMed] [Google Scholar]
- 26.Kong, X., H. San Juan, A. Behera, M. E. Peeples, J. Wu, R. F. Lockey, and S. S. Mohapatra. 2004. ERK-1/2 activity is required for efficient RSV infection. FEBS Lett. 55933-38. [DOI] [PubMed] [Google Scholar]
- 27.Luo, H., B. Yanagawa, J. Zhang, Z. Luo, M. Zhang, M. Esfandiarei, C. Carthy, J. E. Wilson, D. Yang, and B. M. McManus. 2002. Coxsackievirus B3 replication is reduced by inhibition of the extracellular signal-regulated kinase (ERK) signaling pathway. J. Virol. 763365-3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore, P. S., and Y. Chang. 2001. Kaposi's sarcoma-associated herpesvirus, p. 2803-2833. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, and S. E. Straus (ed.), Fields virology, 4th ed., vol. 2. Lippincott, Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 29.Naranatt, P. P., S. M. Akula, C. A. Zien, H. H. Krishnan, and B. Chandran. 2003. Kaposi's sarcoma-associated herpesvirus induces the phosphatidylinositol 3-kinase-PKC-ζ-MEK-ERK signaling pathway in target cells early during infection: implications for infectivity. J. Virol. 771524-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nijhara, R., S. S. Jana, S. K. Goswami, A. Rana, S. S. Majumdar, V. Kumar, and D. P. Sarkar. 2001. Sustained activation of mitogen-activated protein kinases and activator protein 1 by the hepatitis B virus X protein in mouse hepatocytes in vivo. J. Virol. 7510348-10358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan, H., J. Xie, F. Ye, and S. J. Gao. 2006. Modulation of Kaposi's sarcoma-associated herpesvirus infection and replication by MEK/ERK, JNK, and p38 multiple mitogen-activated protein kinase pathways during primary infection. J. Virol. 805371-5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pleschka, S., T. Wolff, C. Ehrhardt, G. Hobom, O. Planz, U. R. Rapp, and S. Ludwig. 2001. Influenza virus propagation is impaired by inhibition of the Raf/MEK/ERK signalling cascade. Nat. Cell Biol. 3301-305. [DOI] [PubMed] [Google Scholar]
- 33.Roberts, P. J., and C. J. Der. 2007. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 263291-3310. [DOI] [PubMed] [Google Scholar]
- 34.Rodems, S. M., and D. H. Spector. 1998. Extracellular signal-regulated kinase activity is sustained early during human cytomegalovirus infection. J. Virol. 729173-9180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roux, P. P., and J. Blenis. 2004. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol. Mol. Biol. Rev. 68320-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roux, P. P., S. A. Richards, and J. Blenis. 2003. Phosphorylation of p90 ribosomal S6 kinase (RSK) regulates extracellular signal-regulated kinase docking and RSK activity. Mol. Cell. Biol. 234796-4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sadagopan, S., N. Sharma-Walia, M. V. Veettil, H. Raghu, R. Sivakumar, V. Bottero, and B. Chandran. 2007. Kaposi's sarcoma-associated herpesvirus induces sustained NF-κB activation during de novo infection of primary human dermal microvascular endothelial cells that is essential for viral gene expression. J. Virol. 813949-3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sapkota, G. P., L. Cummings, F. S. Newell, C. Armstrong, J. Bain, M. Frodin, M. Grauert, M. Hoffmann, G. Schnapp, M. Steegmaier, P. Cohen, and D. R. Alessi. 2007. BI-D1870 is a specific inhibitor of the p90 RSK (ribosomal S6 kinase) isoforms in vitro and in vivo. Biochem. J. 40129-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schulz, T. F. 2006. The pleiotropic effects of Kaposi's sarcoma herpesvirus. J. Pathol. 208187-198. [DOI] [PubMed] [Google Scholar]
- 40.Sharma-Walia, N., H. H. Krishnan, P. P. Naranatt, L. Zeng, M. S. Smith, and B. Chandran. 2005. ERK1/2 and MEK1/2 induced by Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) early during infection of target cells are essential for expression of viral genes and for establishment of infection. J. Virol. 7910308-10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith, J. A., C. E. Poteet-Smith, Y. Xu, T. M. Errington, S. M. Hecht, and D. A. Lannigan. 2005. Identification of the first specific inhibitor of p90 ribosomal S6 kinase (RSK) reveals an unexpected role for RSK in cancer cell proliferation. Cancer Res. 651027-1034. [PubMed] [Google Scholar]
- 42.Sodhi, A., S. Montaner, V. Patel, M. Zohar, C. Bais, E. A. Mesri, and J. S. Gutkind. 2000. The Kaposi's sarcoma-associated herpes virus G protein-coupled receptor up-regulates vascular endothelial growth factor expression and secretion through mitogen-activated protein kinase and p38 pathways acting on hypoxia-inducible factor 1alpha. Cancer Res. 604873-4880. [PubMed] [Google Scholar]
- 43.Wang, Y., Q. Tang, G. G. Maul, and Y. Yuan. 2006. Kaposi's sarcoma-associated herpesvirus ori-Lyt-dependent DNA replication: dual role of replication and transcription activator. J. Virol. 8012171-12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xie, J., H. Pan, S. Yoo, and S. J. Gao. 2005. Kaposi's sarcoma-associated herpesvirus induction of AP-1 and interleukin 6 during primary infection mediated by multiple mitogen-activated protein kinase pathways. J. Virol. 7915027-15037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang, X., and D. Gabuzda. 1999. Regulation of human immunodeficiency virus type 1 infectivity by the ERK mitogen-activated protein kinase signaling pathway. J. Virol. 733460-3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ye, F. C., D. J. Blackbourn, M. Mengel, J. P. Xie, L. W. Qian, W. Greene, I. T. Yeh, D. Graham, and S. J. Gao. 2007. Kaposi's sarcoma-associated herpesvirus promotes angiogenesis by inducing angiopoietin-2 expression via AP-1 and Ets1. J. Virol. 813980-3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu, F., J. N. Harada, H. J. Brown, H. Deng, M. J. Song, T. T. Wu, J. Kato-Stankiewicz, C. G. Nelson, J. Vieira, F. Tamanoi, S. K. Chanda, and R. Sun. 2007. Systematic identification of cellular signals reactivating Kaposi sarcoma-associated herpesvirus. PLoS Pathog. 3e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeng, Y., X. Zhang, Z. Huang, L. Cheng, S. Yao, D. Qin, X. Chen, Q. Tang, Z. Lv, L. Zhang, and C. Lu. 2007. Intracellular Tat of human immunodeficiency virus type 1 activates lytic cycle replication of Kaposi's sarcoma-associated herpesvirus: role of JAK/STAT signaling. J. Virol. 812401-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu, F. X., J. M. Chong, L. Wu, and Y. Yuan. 2005. Virion proteins of Kaposi's sarcoma-associated herpesvirus. J. Virol. 79800-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu, F. X., T. Cusano, and Y. Yuan. 1999. Identification of the immediate-early transcripts of Kaposi's sarcoma-associated herpesvirus. J. Virol. 735556-5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu, F. X., S. M. King, E. J. Smith, D. E. Levy, and Y. Yuan. 2002. A Kaposi's sarcoma-associated herpesviral protein inhibits virus-mediated induction of type I interferon by blocking IRF-7 phosphorylation and nuclear accumulation. Proc. Natl. Acad. Sci. USA 995573-5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu, F. X., X. Li, F. Zhou, S. J. Gao, and Y. Yuan. 2006. Functional characterization of Kaposi's sarcoma-associated herpesvirus ORF45 by bacterial artificial chromosome-based mutagenesis. J. Virol. 8012187-12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu, F. X., and Y. Yuan. 2003. The ORF45 protein of Kaposi's sarcoma-associated herpesvirus is associated with purified virions. J. Virol. 774221-4230. [DOI] [PMC free article] [PubMed] [Google Scholar]