Abstract
A number of novel infectious bronchitis viruses (IBVs) were previously identified in commercial poultry in Australia, where they caused significant economic losses. Since there has been only limited characterization of these viruses, we investigated the genomic and phenotypic differences between these novel IBVs and other, classical IBVs. The 3′ 7.5 kb of the genomes of 17 Australian IBV strains were sequenced, and growth properties of 6 of the strains were compared. Comparison of sequences of the genes coding for structural and nonstructural proteins revealed the existence of two IBV genotypes: classical and novel. The genomic organization of the classical IBVs was typical of those of other group III coronaviruses: 5′-Pol-S-3a-3b-E-M-5a-5b-N-untranslated region (UTR)-3′. However, the novel IBV genotype lacked either all or most of the genes coding for nonstructural proteins at the 3′ end of the genome and had a unique open reading frame, X1. The gene order was either 5′-Pol-S-X1-E-M-N-UTR-3′ or 5′-Pol-S-X1-E-M-5b-N-UTR-3′. Phenotypically, novel and classical IBVs also differed; novel IBVs grew at a slower rate and reached lower titers in vitro and in vivo and were markedly less immunogenic in chicks. Although the novel IBVs induced histopathological lesions in the tracheas of infected chicks that were comparable to those induced by classical strains, they did not induce lesions in the kidneys. This study has demonstrated for the first time the existence of a naturally occurring IBV genotype devoid of some of the genes coding for nonstructural proteins and has also indicated that all of the accessory genes are dispensable for the growth of IBV and that such viruses are able to cause clinical disease and economic loss. The phylogenic differences between these novel IBVs and other avian coronaviruses suggest a reservoir host distinct from domestic poultry.
Infectious bronchitis virus (IBV) is a pathogen of domestic chickens that causes acute, highly contagious respiratory disease (8). IBV is a member of the genus Coronavirus (order Nidovirales, family Coronaviridae). Coronaviruses have been divided into three groups based on antigenic cross-reactivity and nucleotide sequence analysis. Group 1 and group 2 coronaviruses contain the mammalian coronaviruses, while group 3 contains coronaviruses from birds and includes IBV (18). IBV replicates in ciliated epithelial cells of the respiratory tract (and the oviduct), causing deciliation, edema, desquamation, hyperplasia, and mononuclear cell infiltration of the submucosa between 3 and 9 days after infection. In the kidney, cytopathic changes occur in the tubular epithelium, with degradation, necrosis, and an interstitial inflammatory response (4, 9, 13).
All coronaviruses maintain a set of essential genes, including those that encode the polymerase (Pol), spike (S), small membrane (E), membrane (M), and nucleocapsid (N) proteins, in the invariable order 5′-Pol-S-E-M-N-3′ (24). In addition to these essential genes, the genomes of all coronaviruses contain group-specific, or accessory, genes, which encode small proteins of unknown function (24, 35). The 27.6-kb IBV genome contains nine functional genes, four of which encode structural proteins. The polymerase gene, gene 1, occupying approximately two-thirds of the genome at the 5′ end, encodes two partially overlapping open reading frames (ORFs), 1a and 1b. Gene 2 consists of one ORF that encodes the spike glycoprotein (S), which is posttranslationally cleaved into the amino-terminal S1 (92-kDa) and the carboxyl-terminal S2 (84-kDa) subunits (24). In the mature virion, S2 associates with S1, anchoring the S1 protein to the membrane to form the multimeric coiled-coil S protein (3). The S1 subunit is involved in virus entry and also contains epitopes for virus-neutralizing and hemagglutination-inhibiting antibodies (5, 22, 29). The S1 sequences from different strains vary significantly, usually by between 2 and 25% at the amino level, whereas the S2 subunit is conserved (24).
Gene 3 contains three ORFs, 3a (174 nucleotides), 3b (195 nucleotides), and 3c (321 nucleotides). ORF 3c encodes the E protein, which is a structural protein required for virion assembly, while 3a and 3b encode nonstructural proteins of unknown function (24). ORFs 3a and 3b have been found in all IBV isolates discovered thus far (26) and also in other group 3 coronaviruses (turkey and pheasant coronaviruses) (6, 7). Recently, it was shown using reverse genetics that neither the 3a nor the 3b protein is essential for IBV replication in vitro and that these proteins can thus be considered to be accessory proteins (17). In a study using plasmid expression, Liu and Inglis (27) suggested that the translation of ORF 3c, which encodes the E protein, is dependent on the upstream sequence elements (3a and 3b), which together may serve as an internal ribosomal entry site. However, the suggestion that these genes were essential for initiating the translation of the E protein has now been challenged by the generation of mutant IBVs lacking 3a and 3b genes (17). Gene 4 contains one ORF encoding the M glycoprotein, which is essential for the production of coronavirus-like particles (12, 24). IBV is unique among members of genus Coronavirus in that it has two ORFs in gene 5 (5a and 5b). Gene 5 is present in all group 3 coronaviruses characterized to date, but it was shown to be dispensable for replication in vitro using reverse genetics (1). Gene 6 has one ORF, encoding the N protein, which, together with the genomic RNA, forms the helical nucleocapsid (12, 24). This protein is highly conserved, differing between different IBV isolates by only 2 to 6% at the amino acid level (37, 38). There is an untranslated region (UTR) located immediately downstream of gene 6, which is thought to be important for the initiation of negative-strand RNA synthesis. This region has been found to be highly conserved among different strains of the same coronavirus (10, 36). In porcine, canine, and feline coronaviruses, the 3′ UTR contains at least one ORF, but functional ORFs have not been detected in the 3′ UTR of IBV (30).
Coronaviruses replicate via the synthesis of a 5′-coterminal nested set of subgenomic mRNAs. Preceding the coding sequence of each subgenomic mRNA are transcription regulation sequences (TRSs) that lie 5′ of the first ORF. The TRSs are conserved in each coronavirus group, and the distance between the TRS and the first ORF is different in each subgenomic mRNA of different coronaviruses.
Antigenic analyses of S, N, and M proteins (20) and sequence analyses of S1 and N genes (30, 31) of Australian IBV strains have identified two genotypically and antigenically distinct groups, tentatively classified as “classical” and “novel” strains. The classical group shared 80.7 to 98.3% identity in the deduced amino acid sequences of their S1 regions, whereas novel strains shared only 53.8 to 61.7% identity with classical strains at the amino acid level in this region (31). Analysis of the N gene confirmed the observations made with S1, that classical and novel strains belonged to phylogenetically distinct groups of viruses. The 3′ UTR immediately downstream from the N gene was also distinct, with extensive deletions apparent in the novel strains (30). A longitudinal study of IBV strains in commercial poultry in Australia indicated that these novel IBV strains emerged suddenly at three geographically distant commercial sites. The novel strains persisted at the sites at which they were initially isolated for a period of 3 to 4 years, causing respiratory disease and mortalities and necessitating the introduction of vaccines to control the problem (21).
In this study, the 3′ 7.5 kb of the genomes of 17 different Australian IBV strains, including four novel strains, was sequenced and analyzed to compare the genes encoding their structural (S, E, M, and N) and nonstructural (3a, 3b, 5a, and 5b) proteins in order to better understand the evolution of IBV in its natural host. Some phenotypic characteristics of the novel and classical strains were also compared in embryos and in chickens.
MATERIALS AND METHODS
IBV strains.
The IBV vaccine strains Vic S, S, and Armidale were obtained from the manufacturers (Fort Dodge Australia Pty. Ltd., Intervet Australia Pty. Ltd., and Fort Dodge Australia Pty. Ltd., respectively). Strains Vic S and S belong to the same serotype (serotype B) by virus neutralization and also to the same antigenic type as determined by monoclonal antibody typing. The Armidale strain belongs to a different serotype (serotype C) (20). The 11 other strains used in this study, N1/62, V1/71, V2/71, Q1/73, N2/75, Q1/76, N1/88, Q3/88, V5/90, V18/91, and V6/92, were described previously (20). In addition, three recently discovered field isolates, Q1/99, V2/02, and V3/02, were used. All viruses were isolated from either tracheal or kidney tissue collected from field cases and propagated in specific-pathogen-free (SPF) embryonated chicken eggs for three to five passages.
Isolation of IBV from field samples and propagation in embryonated eggs.
Samples of kidney and trachea were pooled, and 10% (wt/vol) tissue suspensions were made in 0.1% phosphate-buffered saline (PBS) containing 100 U penicillin and 100 μg streptomycin/ml. After 12 h of incubation at 4°C, 200 μl of supernatant was inoculated into the allantoic cavity of 9- to 11-day-old embryos of SPF chickens (SPAFAS; Woodend, Victoria, Australia). Five eggs were used for each sample. The inoculated eggs were incubated at 37°C and candled daily. After 72 h of incubation, the eggs were chilled at 4°C, and allantoic fluids were harvested, pooled, clarified by centrifugation at 3,000 × g, and stored at −70°C.
Virus titration and virus neutralization tests.
Virus titers were determined in tracheal organ cultures (TOCs) made from SPF chicken embryos at 19 days of incubation and expressed in median ciliostatic doses (CD50). For each dilution, five rings with 100% of the cilia beating were used. Complete deciliation at 5 days after inoculation in at least three rings indicated that virus replication had taken place. Virus neutralization tests were performed with 100 CD50 of virus and twofold dilution series of sera (starting dilution, 1:20).
Growth of IBV in ovo.
Approximately 100 CD50 of IBV strains Q1/76, N1/62, N1/88, Q3/88, V18/91, and V6/92 as allantoic fluid at egg passage level 4 or 5 were inoculated into the allantoic cavities of five 10- to 11-day-old SPF chicken embryos. After incubation for 48 or 72 h at 37°C, eggs were chilled overnight at 4°C, and allantoic fluid was collected, pooled, centrifuged at 3,000 × g for 30 min, and stored at −70°C.
Assessment of IBV growth in TOCs.
Medium was removed from five TOCs with 100% ciliary activity, 100 μl allantoic fluid, diluted with PBS to contain 10 CD50 of virus, was added, and the TOCs were incubated at room temperature to allow virus adsorption. After 1 h, 1 ml of PBS was added, the TOCs were incubated for 5 min, the inoculum was removed, and 1 ml of incubation medium was added (medium 199, containing 100 IU penicillin/ml, 100 μg streptomycin/ml, and 20 mM α-methyl-d-glycoside). After 3 and 5 days of incubation, medium from the five rings was collected and pooled, fetal calf serum was added to a concentration of 10%, and the medium was stored at −70°C. The titer of virus in the medium was subsequently determined in TOCs as described above.
Assessment of growth and immunogenicity of IBV strains in chickens.
SPF chicks (n = 20) housed in positive-pressure isolation units were inoculated intraocularly at 2 weeks of age with approximately 103 CD50 of each strain of IBV. At 3, 5, and 7 days postinoculation (p.i.), four chicks were euthanized, and their tracheas were collected aseptically. Tracheal scrapings were collected in 2 ml of medium 199 supplemented with 5% fetal calf serum, penicillin (100 IU/ml), streptomycin (100 μg/ml), and neomycin (50 μg/ml) and stored at −70°C until testing. Viral titers were determined for individual tracheal scrapings using TOCs as described above. At 6 weeks of age, serum was collected from the remaining chicks (n = 8), the chicks were then reinoculated intraocularly with 2 × 103 CD50 of the same virus, and serum was collected when the chicks were 10 weeks of age.
Assessment of pathogenicity of IBV strains.
For each IBV strain, groups of 20 2-week-old chicks were inoculated intraocularly with approximately 103 CD50 of virus. At 3, 5, 7, and 10 days p.i., four chicks were euthanized, and their tracheas and the cranial right kidney lobes were removed, placed into 10% buffered formalin, sectioned, stained with hematoxylin and eosin, and examined histologically for lesions. The tracheal lesions scored included cellular infiltration, loss of cilia, squamous metaplasia of surface epithelium, and loss of glands. Renal lesions scored included cellular infiltration, edema, epithelial degeneration, and tubular dysplasia. Lesions were scored as follows: 0 for no lesions, 1+ for mild lesions, 2+ for moderate lesions, 3+ for severe lesions, and 4+ for extensive lesions.
Extraction of RNA.
After 72 h of incubation, the eggs were chilled at 4°C, and allantoic fluids were harvested and tested for the presence of IBV using reverse transcription-PCR. Allantoic fluids containing IBV were clarified by centrifugation at 1,100 × g at 4°C for 10 min, and virus was pelleted by centrifugation at 34,000 × g at 4°C for 1 h. The virus pellet was resuspended in sterile PBS at a 1:100 dilution of its original volume and stored at −72°C. Kidney and lung tissues from diseased birds were prepared as 10% to 15% suspensions in Tris-buffered saline by repeated passage through a three-way tap or by grinding with a mortar and pestle. Tracheas were scraped with a scalpel blade, and the tracheal mucosa was then homogenized by passage through a three-way tap in Tris-buffered saline. Purification of RNA was performed using RNeasy kits (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Approximately 50 μl of virus from allantoic fluid or 100 μl of homogenized tissue was used for each extraction, and RNA was eluted in 30 μl of buffer. The extracted RNA was used as a template in a reverse transcription reaction using a method described previously (28).
Synthesis of cDNA.
For the synthesis of cDNA, 5 μl of extracted RNA was denatured at 100°C for 1 min, cooled by placing it on ice for 5 min, and then mixed with 20 μl of premix containing 10 μl diethyl-pyrocarbonate-treated water, 0.5 μM oligo(dT), 0.65 U RNAguard, 50 μM each of dATP, dTTP, dGTP, and dCTP, 5 μl of 5× reaction buffer, and 50 U of Moloney murine leukemia virus reverse transcriptase. The reaction mixture was incubated at 42°C for 1 h and subsequently incubated at 100°C for 5 min to inactivate the reverse transcriptase. The resultant cDNA was immediately used for PCR or stored at −70°C for later use.
PCR.
For the amplification reaction, two primers, POLY-F1 (5′-GATTGTGCATGGTGGACAATG-3′) and UTR-R1 (5′-CTGTACCCTCGATCGTACTC-3′), binding to the 3′ end of the polymerase gene (nucleotides 20,070 to 20,090; Beaudette strain; GenBank accession number NC_001451) and the 3′ UTR (nucleotides 27,489 to 27,508; Beaudette strain) of the IBV genome, respectively, were used to amplify a 7.5-kb fragment of the IBV genome that contains all the genes for the structural and nonstructural proteins. The PCRs were carried out in 50-μl volumes containing 50 μM each of dATP, dTTP, dGTP, and dCTP, 0.5 μM of each primer, 5 μl of 10× High Fidelity PCR buffer, 2 mM magnesium sulfate, 1.5 U platinum Taq high-fidelity DNA polymerase, and 5 μl cDNA as a template. Amplification was performed using 35 cycles of incubation at 94°C for 30 s, 57°C for 30 s, and 68°C for 8 min, with a final extension step at 68°C for 10 min. The resultant PCR products were separated in a 0.8% agarose gel.
Purification of PCR products.
PCR products were purified using a PCR purification kit (Ultra Clean PCR Clean-Up; Mo Bio Laboratories) according to the manufacturer's instructions.
DNA sequencing.
The 3′ 7.5-kb PCR products from all the Australian IBV strains and field isolates were cloned in pGEM-T using the pGEM-T vector system cloning kit (Promega, Madison, WI). Different primers (13 to 15 in total) were designed and used for sequencing of the 3′ 7.5 kb of the genome of the IBV strains. The cloned PCR products were sequenced using the Big Dye Terminator v3.1 cycle sequencing kit (Applied Biosystems), and the reaction products were sent to the Australian Genomic Research Facility, Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia, for analysis on an ABI Prism 3100 genetic analyzer.
Sequence analysis.
The sequences of the different genes of the IBV strains were aligned using ClustalW (34). The nucleotide and amino acid sequence identities were calculated using the OldDistance program in the GCG package. Phylogenic trees were inferred from nucleotide sequences using the DNAml program in the PHYLIP package (14). To obtain the most accurate phylogenetic analysis, different transition/transversion rates were assessed, and the transition/transversion rate (1.35) that gave the tree with the greatest maximum likelihood was then used for inferences of all subsequent trees.
Molecular evolutionary genetic analysis.
The relative abundances of synonymous and nonsynonymous substitutions in individual genes were determined using the Molecular Evolutionary Genetics Analysis (MEGA) program (23).
Nucleotide sequence accession numbers.
GenBank accession numbers for the sequences obtained in this study are as follows: DQ490205 for Armidale, DQ490206 for N1/62, DQ490207 for N1/88, DQ490208 for N2/75, DQ490209 for Q1/73, DQ490210 for Q1/76, DQ490211 for Q1/99, DQ490212 for Q3/88, DQ490213 for S, DQ490214 for V1/71, DQ490215 for V2/02, DQ490216 for V2/71, DQ490217 for V3/02, DQ490218 for V5/90, DQ490219 for V6/92, DQ490220 for V18/91, and DQ490221 for Vic S.
RESULTS
Nucleotide and amino acid sequences of the 3′ 7.5 kb of the genomes of classical and novel IBVs. (i) Structural genes.
The overall nucleotide and amino acid sequence identities of individual genes within the classical (Armidale, V2/02, N1/62, V3/02, S, Vic S, Q1/76, V5/90, Q1/99, Q1/73, N2/75, V1/71, and V2/71) and novel (V18/91, V6/92, N1/88, and Q3/88) strains are summarized in Table 1. Comparison of the sequences of the structural genes of the 13 classical strains and the four novel IBV strains revealed that the S1 genes had the lowest nucleotide identities among all the structural genes in both the classical (80.1 to 96.8%) and the novel (77.3 to 96.4%) groups. The highest nucleotide identities were found between the S2 genes of the classical strains (91.8 to 99.6%) and the M genes of the novel strains (96.5 to 99.9%). With the exception of the S2 gene, the nucleotide sequences of all of the structural genes of the novel strains differed markedly from those of the classical strains (60.0 to 66.0% identity). However, within each of the groups of viruses, both the structural and nonstructural genes shared greater degrees of identity. Nucleotide identities between the classical viruses were between 80.1 and 100%, and those between the novel viruses were between 76.2 and 99.9%.
TABLE 1.
Percent nucleotide and amino acid sequence identities of individual genes of the Australian classical and novel IBV strainsa
| Strain type | % Sequence identity (range) for:
|
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1
|
S2
|
3a
|
3b
|
E
|
M
|
5a
|
5b
|
N
|
3′ 7.5 kb
|
|||||||||||
| nt | aa | nt | aa | nt | aa | nt | aa | nt | aa | nt | aa | nt | aa | nt | aa | nt | aa | nt | aa | |
| Classical | 80.1-96.8 | 82.3-96.6 | 91.8-99.6 | 91.4-99.5 | 92.5-00.0 | 93.1-100.0 | 86.7-100.0 | 86.2-100.0 | 87.0-97.3 | 84.6-97.3 | 84.7-98.7 | 88.1-98.7 | 86.9-100.0 | 86.4-100.0 | 85.1-92.9 | 84.9-96.5 | 87.1-96.5 | 93.1-97.4 | 86.5-94.9 | 91.1-99.4 |
| Novel | 77.3-96.4 | 76.2-96.2 | 89.9-99.7 | 93.3-99.7 | NA | NA | NA | NA | 92.1-94.6 | 90.9-94.6 | 96.5-99.9 | 97.8-99.1 | NA | NA | 88.1 | 89.5 | 81.7-96.1 | 86.9-97.1 | 77.2-88.3 | 89.0-99.5 |
| Classical vs novel | 61.8-64.9 | 60.8-67.2 | 73.8-76.3 | 78.0-83.9 | NA | NA | NA | NA | 60.0-64.6 | 62.7-69.1 | 66.1-63.6 | 68.7-72.3 | NA | NA | 48.9-50.8 | 32.6-34.9 | 64.1-66.0 | 67.1-69.9 | 58.9-60.1 | 69.8-73.0 |
nt, nucleotide sequence; aa, amino acid sequence; NA, not applicable.
(ii) Nonstructural genes.
Comparison of the entire gene 3 and gene 5 nucleotide sequences revealed major differences in these genes, both in length and in the number of ORFs, between the classical and novel strains.
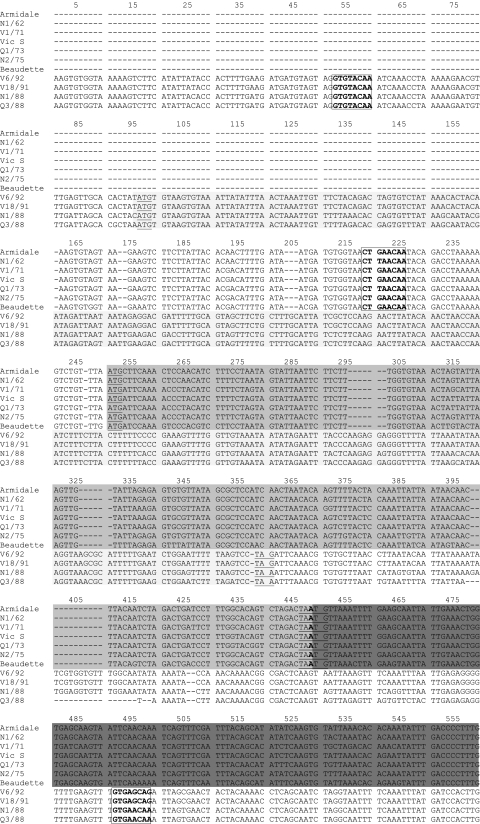
(a) Gene 3. Gene 3 of the classical strains encoded three ORFs, 3a, 3b, and 3c, of 174, 195, and 321 nucleotides, respectively. The gene 3 region of the novel strains encoded only two ORFs (Fig. 1). The first ORF was 264 nucleotides in length and was present in all four novel strains. No significant similarity between this ORF and other coronavirus accessory genes or any other protein in the GenBank database was found. Therefore, this ORF in the novel IBVs was named X1 because the significance of the putative gene product was uncertain. The second ORF in the novel strains was similar to the 3c (E gene) ORFs of classical IBVs, although it differed in length between the novel strains: 318 nucleotides in N1/88 and Q3/88 and 312 nucleotides in V18/91 and V6/92 (Fig. 1). In the novel IBVs, there was a region between ORFs X1 and 3c (nucleotides 362 to 623) that corresponded in position to ORF 3b of classical strains but did not contain an ORF. No similarity between this region and any sequence in the GenBank database was found.
FIG. 1.
Comparison of the nucleotide sequence of the region coding for gene 3 (3a, 3b, and E) of classical and novel IBV strains with that of the reference strain Beaudette (GenBank accession number AJ311317). Underlined bases are the putative start and stop codons. The putative TRSs, which are located upstream of the start codon, are boxed. Missing bases or deletions are indicated with dashes (-). Unshaded sequences upstream and downstream of ORF X1 in the novel IBVs are the 3′ end of the S gene and noncoding region, respectively.  , ORF X1;
, ORF X1;  , 3a gene;
, 3a gene;  , 3b gene;
, 3b gene;  , 3c gene.
, 3c gene.
Sequence analysis of the 5′ terminal end of the S2 gene and of the intergenic region between the S2 gene and the putative gene 3 regions of the classical and novel IBVs revealed that the X1 gene was preceded by a TRS, which was located 36 nucleotides upstream of its predicted initiation codon. The 3a TRS in the classical strains was 23 bp upstream of the translational initiation codon. The X1 TRS sequence was GTGTACAA, whereas the TRS for the 3a gene in the classical strains was CT(T/G)AACAA. The X1 ORF was predicted to code for a protein of 88 amino acids, and the amino acid sequence identity between novel IBV strains in ORF X1 was 89 to 100%.
The 3a and 3b genes were absent from the novel IBVs (Fig. 1). Sequence analysis of gene 3 (Fig. 1) in the novel IBVs revealed a potential TRS for the 3c gene upstream of its initiation codon. This putative TRS sequence was GTGAACAA in N1/88 and Q3/88 and GTGAGCAG in V18/91 and V6/92 and was located about 124 nucleotides upstream from the start codon (Fig. 1).
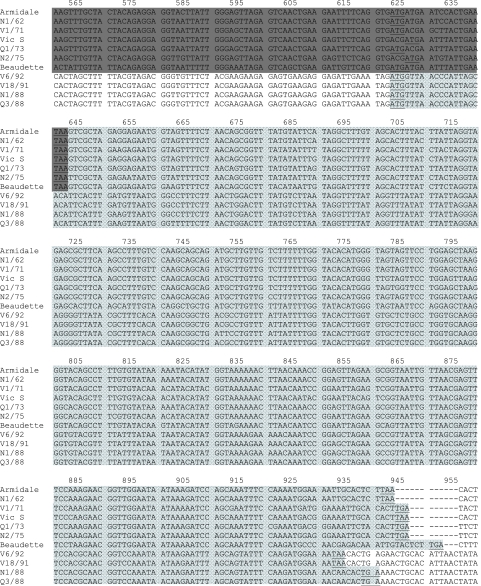
(b) Gene 5. The nucleotide and deduced amino acid sequences of gene 5 from 17 Australian IBV strains and the Beaudette strain were compared (Fig. 2). In the classical strains and the Beaudette strain, this gene encoded two ORFs, 5a and 5b, of 198 and 249 nucleotides, respectively. However, the sequences in the gene 5 region in V18/91 and V6/92 (nucleotides 280 to 545) (Fig. 2) did not contain an ORF of any significant length. The novel strains N1/88 and Q3/88, on the other hand, had a single ORF of 246 nucleotides in this region. This ORF was similar, but not identical, to ORF 5b in the classical IBV strains. Thus, the novel strains N1/88 and Q3/88 lacked ORF 5a but had ORF 5b. The predicted TRS for the 5b gene in N1/88 and Q3/88 was AGAAACAA and was located within the M gene sequence 124 nucleotides upstream from the start codon for the 5b protein (Fig. 2), the same distance between the predicted TRS for gene 3c in the novel IBVs and its start codon.
FIG. 2.
Comparison of the nucleotide sequence of the region coding for gene 5 (5a and 5b) of classical and novel IBV strains with that of the reference strain Beaudette (GenBank accession number AJ311317). Underlined bases are the putative start and stop codons. The TRSs, which are located upstream from the start codons, are boxed. Missing bases or deletions are indicated with dashes (-).  , 5a gene;
, 5a gene;  , 5b gene.
, 5b gene.
Genomic organization of the 3′ 7.5 kb of Australian IBV strains.
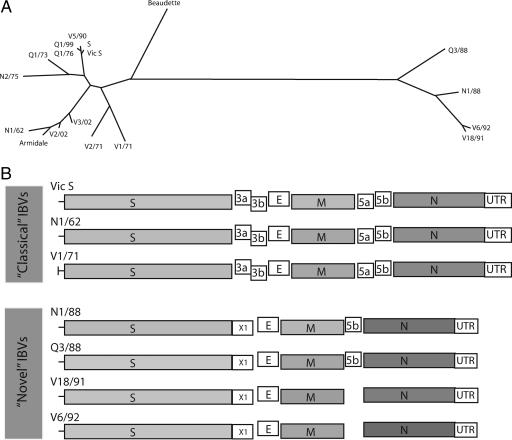
All the classical strains had the predicted gene order of 5′-Pol-S-3a-3b-E-M-5a-5b-N-UTR-3′ (Fig. 3B). This contrasted with the genomic organization of the novel IBVs: strains N1/88 and Q3/88 lacked the nonstructural genes 3a, 3b, and 5a and had an additional gene in the gene 3 region, resulting in the genomic organization 5′-Pol-S-X1-E-M-5b-N-UTR-3′, while strains V18/91 and V6/92 had the gene order 5′-Pol-S-X1-E-M-N-UTR-3′.
FIG. 3.
(A) Phylogenic tree inferred from the 3′ 7.5-kb nucleotide sequences of 17 Australian IBV strains and the Beaudette strain using the DNAml program in the PHYLIP package. (B) Comparison of the structures of the 3′ 7.5 kb of the genome of the “classical” and “novel” Australian IBVs strains.
Comparison of the 3′ 7.5 kb of Australian IBVs.
The classical strains shared 86.5 to 94.9% nucleotide sequence identity with each other but only 58.9 to 60.1% nucleotide sequence identity with strains in the novel group. The novel strains had 77.2 to 88.3% nucleotide sequence similarity with each other (Table 2). Amino acid sequences of all structural and nonstructural proteins were aligned and compared with each other. The classical strains shared more than 91.0% amino acid identity with each other, and five strains, Vic S, S, Q1/76, V5/90, and Q1/99, shared more than 98.5% amino acid sequence identity with each other. The novel strains shared 69.8 to 73.0% amino acid sequence identity with the classical strains and 89.0 to 99.5% amino acid sequence identity with each other (Table 2). The genomes of the novel strains, excluding the polymerase gene, which was assumed to be the same size in all strains, were 468 to 509 bases shorter than the genomes of classical IBV strains. These missing bases in the novel IBVs corresponded mostly to the 3a, 3b, 5a, and 5b genes and the 3′ UTR regions. Overall, the genomes of the classical strains were predicted to range between 27,608 and 27,674 bases, and those of the novel strains were predicted to range between 27,140 and 27,165 bases.
TABLE 2.
Nucleotide and amino acid sequence identities of the 3′ 7.5 kb of the genomes of 17 Australian IBV strains and strain Beaudettea
| Strain | % Identity with:
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Armidale | V2/02 | N1/62 | V3/02 | S | Vic S | Q1/76 | V5/90 | Q1/99 | Q1/73 | N2/75 | V1/71 | V2/71 | Beaudette | V18/91 | V6/92 | N1/88 | Q3/88 | |
| Armidale | 100.0 | 98.3 | 97.0 | 97.0 | 92.6 | 92.7 | 92.7 | 92.9 | 92.5 | 92.8 | 92.0 | 91.6 | 92.1 | 90.2 | 72.5 | 72.6 | 70.7 | 71.6 |
| V2/02 | 94.1 | 100.0 | 97.0 | 97.5 | 93.5 | 93.6 | 93.7 | 93.7 | 93.4 | 93.4 | 92.0 | 92.0 | 92.0 | 90.4 | 72.8 | 73.0 | 71.0 | 71.8 |
| N1/62 | 93.3 | 93.1 | 100.0 | 96.1 | 93.0 | 93.1 | 93.1 | 93.2 | 93.0 | 92.9 | 92.3 | 91.6 | 91.8 | 90.3 | 72.6 | 72.6 | 70.9 | 71.7 |
| V3/02 | 92.6 | 93.5 | 91.6 | 100.0 | 94.8 | 94.9 | 94.8 | 95.0 | 94.6 | 94.0 | 92.6 | 92.4 | 92.5 | 90.1 | 72.6 | 72.8 | 70.9 | 71.7 |
| S | 86.9 | 88.5 | 87.1 | 90.0 | 100.0 | 99.1 | 99.0 | 99.2 | 99.2 | 94.8 | 93.9 | 92.8 | 92.5 | 90.2 | 72.4 | 72.6 | 70.5 | 71.1 |
| Vic S | 86.9 | 88.5 | 87.1 | 89.9 | 94.9 | 100.0 | 99.2 | 99.4 | 98.9 | 95.0 | 94.0 | 92.9 | 92.7 | 90.1 | 72.6 | 72.8 | 70.6 | 71.4 |
| Q1/76 | 86.6 | 88.2 | 86.8 | 89.6 | 94.6 | 94.6 | 100.0 | 99.4 | 98.8 | 94.9 | 94.1 | 92.8 | 92.7 | 90.1 | 72.5 | 72.7 | 70.6 | 71.3 |
| V5/90 | 86.9 | 88.4 | 87.1 | 89.9 | 94.9 | 94.9 | 94.6 | 100.0 | 99.0 | 95.1 | 94.2 | 93.0 | 92.8 | 90.2 | 72.5 | 72.7 | 70.6 | 71.4 |
| Q1/99 | 86.7 | 88.2 | 86.9 | 89.7 | 94.8 | 94.6 | 94.3 | 94.6 | 100.0 | 94.6 | 93.9 | 92.8 | 92.5 | 90.1 | 72.4 | 72.5 | 70.4 | 71.2 |
| Q1/73 | 87.9 | 89.1 | 88.0 | 89.2 | 90.6 | 90.6 | 90.2 | 90.5 | 90.3 | 100.0 | 92.9 | 91.7 | 91.5 | 90.2 | 72.5 | 72.7 | 70.5 | 71.4 |
| N2/75 | 87.2 | 87.2 | 87.4 | 87.9 | 89.0 | 88.9 | 88.6 | 88.9 | 88.7 | 89.3 | 100.0 | 91.1 | 91.8 | 88.8 | 72.1 | 72.4 | 70.5 | 71.8 |
| V1/71 | 86.5 | 86.9 | 87.0 | 87.5 | 88.0 | 87.9 | 87.6 | 87.9 | 87.8 | 87.5 | 86.9 | 100.0 | 94.4 | 88.5 | 71.6 | 71.7 | 69.9 | 70.5 |
| V2/71 | 87.7 | 87.7 | 87.7 | 88.2 | 87.9 | 87.9 | 87.6 | 87.9 | 87.7 | 87.7 | 87.5 | 89.3 | 100.0 | 88.5 | 71.4 | 71.5 | 69.8 | 70.6 |
| Beaudette | 82.7 | 82.6 | 82.8 | 82.9 | 82.5 | 82.5 | 82.2 | 82.6 | 82.4 | 82.8 | 82.4 | 82.3 | 82.8 | 100.0 | 72.2 | 72.2 | 70.3 | 70.3 |
| V18/91 | 59.4 | 59.6 | 59.6 | 59.6 | 59.5 | 59.5 | 59.4 | 59.5 | 59.4 | 59.1 | 59.2 | 59.5 | 59.9 | 58.6 | 100.0 | 99.5 | 94.6 | 89.0 |
| V6/92 | 59.2 | 59.4 | 59.4 | 59.4 | 59.3 | 59.3 | 59.2 | 59.3 | 59.2 | 58.9 | 58.9 | 59.3 | 59.7 | 58.4 | 88.3 | 100.0 | 95.0 | 89.4 |
| N1/88 | 59.8 | 59.9 | 59.9 | 60.0 | 59.7 | 59.7 | 59.6 | 59.7 | 59.6 | 59.3 | 59.4 | 59.5 | 60.1 | 58.8 | 82.9 | 82.6 | 100.0 | 90.0 |
| Q3/88 | 59.9 | 60.0 | 60.1 | 60.0 | 59.6 | 59.6 | 59.5 | 59.6 | 59.5 | 59.4 | 59.7 | 59.5 | 60.1 | 58.9 | 77.5 | 77.2 | 79.5 | 100.0 |
Boldface type indicates nucleotide identity, whereas lightface type indicates amino acid identity.
Phylogenetic analysis.
Phylogenetic analysis of the 3′ 7.5 kb of the genome divided IBV strains into two distinct groups, classical and novel strains. In the classical group, there were three subclusters, with Vic S, S, V5/90, Q1/99, and Q1/76 grouped with Q1/73 and N2/75 into one subcluster; N1/62, Armidale, V2/02, and V3/02 grouped into a second subcluster; and V1/71 and V2/71 grouped into a third subcluster. The Beaudette strain clustered separately from all Australian IBV strains, between the classical and novel IBV clusters but closer to the classical strain cluster (Fig. 3A). Phylogenetic analysis of each individual gene revealed similar phylogenetic clusterings (results not shown).
Molecular evolutionary genetic analysis.
To investigate evolutionary pressures on different IBV genes, tests for neutral evolution, positive selection, and purifying selection were performed. Purifying selection results in selection against nonsynonymous substitutions at the DNA level, while positive selection results in selection in favor of nonsynonymous substitutions. For all tests, the null hypothesis was that synonymous substitution rates (dS) were equal to nonsynonymous substitution rates (dN), while the alternative hypotheses were as follows: dS ≠ dN (for neutral evolution), dN > dS (for positive selection), and dN < dS (for purifying selection). Tables 3 and 4 show the P values for these tests for all the structural genes for five classical and four novel IBVs. The five classical strains that clustered together phylogenetically were chosen for comparison. This group of strains included two of the three most commonly used vaccine strains in Australia and three field isolates. The tests were not performed for the small nonstructural genes because the sequences of these genes were very similar.
TABLE 3.
P values for tests of neutral evolution, positive selection, and purifying selection in genes of vaccine strains and closely related field isolatesa
| Gene and strain |
P value for:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutral evolution
|
Positive selection
|
Purifying selection
|
||||||||||
| S | Q1/76 | Q1/99 | V5/90 | S | Q1/76 | Q1/99 | V5/90 | S | Q1/76 | Q1/99 | V5/90 | |
| S1 | ||||||||||||
| Q1/76 | <0.01 | <0.01 | 1.00 | |||||||||
| Q1/99 | 0.94 | 0.82 | 0.47 | 0.41 | 1.00 | 1.00 | ||||||
| V5/90 | <0.01 | <0.01 | 0.81 | <0.01 | <0.01 | 0.41 | 1.00 | 1.00 | 1.00 | |||
| Vic S | 0.26 | 0.24 | 0.71 | 0.19 | 0.11 | 0.11 | 1.00 | 0.08 | 1.00 | 1.00 | 0.34 | 1.00 |
| S2 | ||||||||||||
| Q1/76 | 0.92 | 1.00 | 0.46 | |||||||||
| Q1/99 | 0.12 | 0.23 | 1.00 | 1.00 | 0.06 | 0.12 | ||||||
| V5/90 | 0.13 | 0.48 | 0.31 | 0.06 | 1.00 | 1.00 | 1.00 | 0.25 | 0.16 | |||
| Vic S | 0.06 | 0.70 | 0.36 | 0.27 | 0.03 | 1.00 | 1.00 | 0.14 | 1.00 | 0.35 | 0.19 | 1.00 |
| M | ||||||||||||
| Q1/76 | 0.32 | 1.00 | 0.17 | |||||||||
| Q1/99 | <0.01 | 0.32 | <0.01 | 1.00 | <0.01 | 0.17 | ||||||
| V5/90 | 0.32 | <0.01 | 0.32 | 1.00 | <0.01 | 1.00 | 0.17 | <0.01 | 0.17 | |||
| Vic S | 0.50 | 0.31 | 0.50 | 0.31 | 1.00 | 0.17 | 1.00 | 0.17 | 0.26 | 1.00 | 0.26 | 1.00 |
| N | ||||||||||||
| Q1/76 | 0.95 | 0.47 | 1.00 | |||||||||
| Q1/99 | 0.14 | 0.74 | 0.07 | 1.00 | 1.00 | 0.37 | ||||||
| V5/90 | 0.43 | 0.28 | 0.26 | 1.00 | 1.00 | 1.00 | 0.21 | 0.31 | 0.12 | |||
| Vic S | 0.86 | 0.62 | 0.70 | 0.25 | 0.43 | 1.00 | 1.00 | 1.00 | 1.00 | 0.31 | 0.34 | 0.11 |
Boldface type indicates that the alternative hypothesis has been accepted.
TABLE 4.
P values for tests of neutral evolution, positive selection, and purifying selection in genes of novel IBV strainsa
| Gene and strain |
P value for:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Neutral evolution
|
Positive selection
|
Purifying selection
|
|||||||
| N1/88 | Q3/88 | V6/92 | N1/88 | Q3/88 | V6/92 | N1/88 | Q3/88 | V6/92 | |
| S1 | |||||||||
| Q3/88 | <0.01 | 1.00 | <0.01 | ||||||
| V6/92 | <0.01 | <0.01 | 1.00 | 1.00 | <0.01 | <0.01 | |||
| V18/91 | 0.01 | <0.01 | <0.01 | 1.00 | 1.00 | 1.00 | <0.01 | <0.01 | 0.14 |
| S2 | |||||||||
| Q3/88 | <0.01 | 1.00 | <0.01 | ||||||
| V6/92 | <0.01 | <0.01 | 1.00 | 1.00 | <0.01 | <0.01 | |||
| V18/91 | <0.01 | <0.01 | <0.01 | 1.00 | 1.00 | <0.01 | <0.01 | <0.01 | <0.01 |
| M | |||||||||
| Q3/88 | <0.01 | 1.00 | <0.01 | ||||||
| V6/92 | <0.01 | <0.01 | 1.00 | 1.00 | <0.01 | <0.01 | |||
| V18/91 | <0.01 | <0.01 | <0.01 | 1.00 | 1.00 | 1.00 | <0.01 | <0.01 | 0.16 |
| N | |||||||||
| Q3/88 | <0.01 | 1.00 | <0.01 | ||||||
| V6/92 | <0.01 | <0.01 | 1.00 | 1.00 | <0.01 | <0.01 | |||
| V18/91 | <0.01 | <0.01 | 0.30 | 1.00 | 1.00 | 1.00 | <0.01 | <0.01 | 0.15 |
Boldface type indicates that the alternative hypothesis has been accepted.
The analysis indicated that there had been either neutral evolution or positive selection of the S1 gene within the classical strains, suggesting that the S1 gene may have been under immune pressure. No clear evidence for selection was detected in the S2, M, or N genes (Table 3). In contrast, positive selection was not detected in any of the genes of the novel strains, but there was evidence for neutral evolution or purifying selection within genes of the novel strains (Table 4).
Comparisons of growth in embryos and growth and immunogenicity in chicks.
As strains N1/88 and Q3/88 were found to lack the 3a, 3b, and 5a genes and strainsV18/91 and V6/92 were found to lack these genes as well as gene 5b, we assessed the growth rates of these strains in embryos and in chickens. The classical strains Q1/76 and N1/62, which possess all four of these genes, were included for comparison. In embryonated eggs, TOCs, and infected chicks, all four novel strains had slower growth rates and produced less virus than the two classical strains (Table 5). The novel strains were also poorly immunogenic (Table 5). The virus-neutralizing antibody titers against homologous virus after a single inoculation and after a second inoculation were markedly lower in chicks infected with N1/88, Q3/88, V18/91, or V6/92 (titers of 80 to 160 after the second inoculation) than in chicks inoculated with Q1/76 or N1/62 (titers of 2,560 after the second inoculation).
TABLE 5.
Growth rates of different IBV strains in ovo, in vitro, in tracheal explants, and in the trachea of infected chickens and their immunogenicities in chickensf
| IBV strain | Virus titer ina:
|
Neutralizing antibody titer in chickense
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Allantoic fluidb
|
Tracheal organ culturec
|
Trachea of infected chicksd
|
4 wk | 8 wk | |||||||
| 48 h
|
72 h
|
Day 3 | Day 5 | Day 3 | Day 5 | Day 7 | |||||
| Expt 1 | Expt 2 | Expt 1 | Expt 2 | ||||||||
| Q1/76 | 105 | 106 | 105 | 105 | 103 | 103 | 103 | 104 | 102 | 320 | 2,560 |
| N1/62 | 106 | 106 | 106 | 105 | 103 | 103 | 104 | 105 | 103 | ND | 2,560 |
| N1/88 | 102 | 103 | 103 | 104 | 101 | 102 | 10 | 103 | 10 | 20 | 80 |
| Q3/88 | 102 | 102 | 102 | 104 | 101 | 102 | 10 | 102 | 10 | 40 | 160 |
| V18/91 | 102 | 101 | 104 | 103 | 102 | 102 | 102 | 10 | 80 | 160 | |
| V6/92 | 103 | 103 | 104 | 104 | NT | NT | 10 | 102 | 20 | 80 | |
Virus titers determined in TOCs.
Embryonated SPF chicken eggs (n = 5) were inoculated with 100 CD50 of an IBV strain and incubated, and virus titers (pool of five eggs) at either 48 h or 72 h p.i. were determined. Results from two separate experiments are given.
Tracheal tissues from 19-day-old SPF chicken embryos were cut into 1-mm-wide rings, and those with 100% ciliary activity (n = 5) were infected with 10 CD50 of IBV. Culture fluid was collected at 3 and 5 days after infection and titrated in TOCs. Complete ciliostasis, evaluated at 5 days after infection, indicated the presence of IBV.
SPF chicks were inoculated intraocularly with 103 CD50 of IBV, and virus titers in the tracheas of four chicks were determined at 3, 5, and 7 days p.i. The value given is the modal titer from four chicks.
Sera were collected 4 weeks after primary and secondary inoculations of SPF chickens with 100 CD50 of virus, and neutralizing antibody titers against 100 CD50 of homologous virus were determined using a pool of sera (n = 10) in TOCs. Titers are the reciprocals of the last dilution that completely neutralized viral infectivity.
NT, not tested.
Pathogenicity of novel IBVs.
As the novel strains all grew to much lower titers in the tracheas of infected chicks than did Q1/76 (Table 5), we compared the histopathological lesions induced by these strains in the tracheas and kidneys of infected chicks (Table 6). All the IBV strains induced histopathological changes in the trachea, and there were no marked differences in the severities of the lesions: levels of cellular infiltration in the tracheal epithelium were similar, with a modal lesion score of 2+. Although a loss of the cilia was less apparent, or absent, at day 3 p.i. in chicks infected with the novel strains, at day 7 p.i., the degrees of deciliation were similar in all groups. There were no obvious differences in levels of squamous metaplasia, although the loss of glands was more prominent and persistent in the tracheas of chicks infected with the classical strain. Prominent renal lesions typical of IBV were observed only in chicks inoculated with strain Q1/76.
TABLE 6.
Histological lesions in tracheas and kidneys of SPF chickens infected with different IBV strains
| IBV strain | Day p.i. | Histological lesion score in inoculated chicksa
|
||||
|---|---|---|---|---|---|---|
| Trachea
|
Kidney nephrosisc | |||||
| Cellular infiltration | Loss of cillia | Squamous metaplasiab | Loss of glands | |||
| Q1/76 | 3 | + | +++ | + | ++ | − |
| 5 | + | ++ | + | ± | ++ | |
| 7 | ++ | ++ | − | ± | ± | |
| N1/88 | 3 | + | ± | − | − | − |
| 5 | + | ++ | + | + | − | |
| 7 | + | + | − | − | − | |
| Q3/88 | 3 | + | + | ± | − | − |
| 5 | ++ | ++ | + | − | − | |
| 7 | ++ | ++ | − | − | − | |
| V18/91 | 3 | + | + | ± | ± | − |
| 5 | +++ | ++ | + | ++ | ± | |
| 7 | ++ | ± | − | − | − | |
| V6/92 | 3 | ± | − | − | − | − |
| 5 | ++ | ++ | ± | ++ | − | |
| 7 | ++ | ++ | − | − | − | |
SPF chickens were inoculated at 2 weeks of age with 103 CD50 of virus. At 3, 5, and 7 days p.i., four chicks from each group were euthanized, tracheas and kidneys were collected, and histopathological lesions were scored in formalin-fixed tissue following staining. Lesions were scored as follows: −, no lesions; +, mild lesions; 2++, moderate lesions; 3+, severe lesions; 4+, extensive lesions. The value given is the modal score from four chicks. ± indicates that two chicks had a score of + and that two had a score of −.
Squamous metaplasia of surface epithelium.
Edema and epithelial degeneration.
DISCUSSION
The 3′ 7.5-kb regions of the genomes of 17 Australian IBV strains were analyzed and compared to each other and to that of Beaudette, a reference strain for which the complete genome sequence is known. Growth properties, pathogenicities, and immunogenicities of six of these IBVs were also determined. This comparison revealed a number of aspects of IBV biology that had not previously been known: IBV strains with different genomic organizations can occur naturally in poultry; in addition to the more usual gene order of 5′-Pol-S-3a-3b-E-M-5a-5b-N-UTR-3′, IBV strains that have lost all, or most, of the accessory genes, with a gene order of either 5′-Pol-S-X1-E-M-5b-N-UTR-3′ or 5′-Pol-S-X1-E-M-N-UTR-3′, also exist; such IBVs have an additional ORF, designated X1, which has no homology with any other IBV gene; a loss of these accessory genes does not abolish virus replication or their pathogenicity for the tracheas of infected chicks; the lack of accessory genes may be responsible for slower growth in embryos and in infected chicks and poorer immunogenicity in chicks; and the nucleotide sequences of all essential genes of IBV strains that lack accessory genes differ significantly from those of their counterparts in IBV strains with a full accessory gene complement, suggesting that these novel viruses were not derived by mutation of or recombination with the more commonly isolated classical strains, mechanisms previously found to be involved in the generation of diversity among IBVs.
Comparison of sequences of essential genes in IBV, in particular the S1 gene, has been commonly used to determine IBV strain relatedness and for elucidation of IBV evolution. In this study, comparisons of the entire 3′ 7.5 kb of the genome, a region that includes all the essential and accessory genes except that for the polymerase, demonstrated the value of this approach for the analysis of IBV strains and revealed major differences between IBV strains. The absence of the 3a, 3b, 5a, and 5b accessory genes has not been reported for any other naturally occurring IBV, and in experimentally constructed variants of IBV, only the gene 3 or the gene 5 accessory gene has been deleted. Thus, this study has confirmed that gene 3 (with the exception of the E gene) and gene 5 are indeed accessory genes, as previously suggested (1, 17).
Sequence analysis of genes 3 and 5 of several IBV strains, and also of turkey and pheasant coronaviruses, has indicated that their nucleotide sequences, locations, and consensus TRSs are highly conserved (6, 7, 25). This level of conservation has led to the suggestion that the presence of genes 3 and 5 can be taken as a distinguishing feature of group 3 coronaviruses, reflecting the close evolutionary relationship of all members (25). Members of coronavirus groups 1 and 2 also possess accessory genes, the size and location of which are group specific (16, 24, 35). Recently, it was demonstrated that the location and the number of accessory genes also differ in bat coronaviruses (33). Although the functions of accessory gene products have not been fully elucidated, to date no naturally occurring coronavirus that does not have a full complement of accessory genes has been found, although a derivative of strain Beaudette that contains a truncated 3b gene was obtained after at least 35 passages in embryonated chicken eggs (32). Recombinant IBVs that lack ORFs 3a and 3b, or in which the expression of a complete gene 5 is interrupted, have been generated recently using reverse genetics (1, 17). These recombinants replicated in cell culture, as did the parent virus, suggesting that genes 3 and 5 are accessory genes. Our study confirms this observation and extends it by showing that both genes can be absent simultaneously and that the viruses lacking these genes can replicate in all the substrates in which IBV grows: in embryonated eggs, in chickens, and in tracheal explants. However, in contrast to the studies that examined recombinant IBVs (1, 17), we found that all of the novel IBVs had a reduced capacity to replicate compared to that of a classical IBV with a full complement of ORFs within genes 3 and 5.
Our study has further shown that genes 3 and 5 may not be determinants of pathogenicity, at least for the tracheal mucosa. While the novel strains did not induce renal lesion changes, it is difficult to attribute this to the lack of accessory genes alone, as strains of IBV that are more similar to the classical Australian strains also fail to cause renal lesions (19). IBV tissue tropism is determined by the S1 gene (2). Since the S1 genes of classical and novel IBVs differ significantly (31), it is just as likely that the difference in tissue tropism between the classical and novel strains is a result of the absence of receptor binding sites in S1. Using reverse genetics, Haijema et al. (16) deleted the nonstructural genes 3a, 3b, and 3c or 7a and 7b in feline coronavirus and showed that these deletions resulted in viruses that grew well in cell culture and were immunogenic, inducing high levels of neutralizing antibodies in cats, but did not induce any clinical signs. However, in our study, the immunogenicity of the novel strains was markedly reduced compared with that of the classical strains. It is not clear if this reduced immunogenicity was the result of less efficient replication (possibly because of the lack of accessory genes) or the lack of tropism for kidney tissue and the restriction of viral replication to the tracheal mucosa. However, the phenotypic characteristics of the novel IBVs cannot be unequivocally ascribed to the lack of accessory proteins unless comparisons are made using isogenic viruses.
The novel viruses had a novel ORF, designated X1, in the gene 3 region. The size of this ORF, 264 nucleotides, was significantly greater than the size of either ORF 3a (174 nucleotides) or 3b (195 nucleotides), and it has no sequence similarity to any other coronaviral structural or nonstructural gene, or any other gene in the database, including the novel ORF recently identified in bat coronaviruses. However, this ORF was conserved in all novel IBVs and had its own TRS, which differed from that of the 3a gene in the classical strains, but was located almost the same distance (24 nucleotides) upstream of the initiation codon for gene 3 as the TRS for gene 3 (23 nucleotides) in other group 3 coronaviruses (25).
It is not possible to determine whether the accessory genes were never present in the novel strains or whether they were deleted during their circulation in commercial poultry or some other host. All the novel strains appeared suddenly and simultaneously in three different locations in Australia, suggesting their introduction from an as-yet-unidentified source into commercial poultry. If deletions occurred following their introduction into commercial poultry, it is difficult to explain how very similar deletions occurred simultaneously in poultry reared at distant locations. The introduction of a progenitor virus that already lacked the genes would thus seem more likely. However, as some variants retained one of the four accessory proteins, and as there was already substantial variation between the protein sequences of some of the novel IBVs in 1988, it could be argued that either there were three (or more) independent introductions of the novel IBVs into commercial poultry in Australia or the introduction of a single variant (possibly initially associated with another avian species) occurred well before 1988.
Phylogenetic analysis of sequences of each of the structural genes suggested the same phylogenetic relationships between the classical and novel strains, indicating that all the structural genes of the novel IBVs evolved in parallel. Therefore, it seems that the origin of the novel strains involved neither mutation from, nor recombination with, classical strains.
In IBV, the spike protein, and especially the S1 part, interacts with host cell surface receptors and is thus a target for protective antibody responses, with resultant selection for amino acid changes. Therefore, this protein would be expected to be under continuous positive selection. Molecular evolutionary analysis of the structural genes of two vaccine strains and three closely related field isolates showed that only the S1 gene of these viruses was under positive selection, as might be expected if the field isolates were reisolates of vaccine strains that had been subject to immune selection during circulation in poultry flocks. In contrast, evolutionary analysis of the novel IBVs detected evidence of purifying selection in all the structural genes. The sudden appearance of these novel IBVs suggests that these viruses were introduced into chickens from another species and that the purifying selection observed may have been a consequence of their subsequent adaptation to chickens.
The origin of the novel IBVs has not yet been determined. The most likely explanation is that they were introduced into commercial poultry from another avian species. After their introduction, probably in 1988, when they were first detected, N1/88- and Q3/88-like strains circulated for a period of 4 years. Since the introduction of two vaccines based on strains N1/88 and Q3/88, the novel strains have not been detected. The disappearance of the novel strains in commercial poultry is not unexpected; most IBV variants persist only transiently, particularly if they are targeted by vaccination. A factor contributing to the disappearance of the novel strains could have been their reduced capacity for replication in chickens. In addition, the novel IBVs may have disappeared from the poultry scene because the deletions of the accessory genes are strongly attenuating, as was demonstrated in animal studies of murine hepatitis virus A59 and feline infectious peritonitis virus (11, 16). Hence, these viruses may have lost the competition with the “fitter” classical viruses.
Comparative sequence analyses of the S, E, M, and N structural proteins of three groups of coronaviruses, aimed at a revision of the taxonomy of the Coronaviridae, found that frequency distributions of the identities derived from comparisons of groups 1 and 2 generated two main clusters based on all the structural proteins for each group (15). Pairwise identities within group 3 (IBV and turkey coronavirus) were high, and three proteins, S, E, and M, were closely clustered. However, comparisons of N protein sequences revealed two clearly separated clusters, with the second cluster resulting from comparisons involving the three novel Australian IBV strains N1/88, Q3/88, and V18/91 (15). The results from our study, which has added the sequences of the S, M, and E proteins of N1/88, Q3/88, and V18/91 to the analyses, has confirmed that the group 3 coronaviruses contain two main clusters and reinforce suggestions for revision of Coronavirus taxonomy, possibly requiring the classification of these novel IBVs as distinct types of coronavirus.
Acknowledgments
K.M. was supported by a scholarship from the University of Urmia, Urmia, Iran.
We thank Philip Markham and Sen-Lin Tang for assistance with methods.
Footnotes
Published ahead of print on 28 November 2007.
REFERENCES
- 1.Casais, R., M. Davies, D. Cavanagh, and P. Britton. 2005. Gene 5 of the avian coronavirus infectious bronchitis virus is not essential for replication. J. Virol. 798065-8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casais, R., B. Dove, D. Cavanagh, and P. Britton. 2003. Recombinant avian infectious bronchitis virus expressing a heterologous spike gene demonstrates that the spike protein is a determinant of cell tropism. J. Virol. 779084-9089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cavanagh, D. 2005. Coronaviridae: a review of coronaviruses and toroviruses, p. 1-54. In A. Schmidt, M. H. Wolff, and O. Weber (ed.), Coronaviruses with special emphasis on first insights concerning SARS. Birkhauser Verlag, Berlin, Germany.
- 4.Cavanagh, D. 2003. Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus. Avian Pathol. 32567-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavanagh, D., P. J. Davis, J. H. Darbyshire, and R. W. Peters. 1986. Coronavirus IBV: virus retaining spike glycopolypeptide S2 but not S1 is unable to induce virus-neutralizing or haemagglutination-inhibiting antibody, or induce chicken tracheal protection. J. Gen. Virol. 671435-1442. [DOI] [PubMed] [Google Scholar]
- 6.Cavanagh, D., K. Mawditt, M. Sharma, S. E. Drury, H. L. Ainsworth, P. Britton, and R. E. Gough. 2001. Detection of a coronavirus from turkey poults in Europe genetically related to infectious bronchitis virus of chickens. Avian Pathol. 30355-368. [DOI] [PubMed] [Google Scholar]
- 7.Cavanagh, D., K. Mawditt, B. W. Dde, P. Britton, and R. E. Gough. 2002. Coronaviruses from pheasants (Phasianus colchicus) are genetically closely related to coronaviruses of domestic fowl (infectious bronchitis virus) and turkeys. Avian Pathol. 3181-93. [DOI] [PubMed] [Google Scholar]
- 8.Cavanagh, D., and S. A. Naqi. 2003. Infectious bronchitis, p. 101-119. In Y. M. Saif, Barnes, J. R. Glisson, A. M. Fadly, L. R. McDougald, and D. E. Swayne (ed.), Diseases of poultry, 11th ed. Iowa State University Press, Ames, IA.
- 9.Chousalkar, K. K., and J. R. Roberts. 2007. Ultrastructural study of infectious bronchitis virus infection in infundibulum and magnum of commercial laying hens. Vet. Microbiol. 122223-236. [DOI] [PubMed] [Google Scholar]
- 10.Collisson, E. W., A. K. Williams, R. Vonder Haar, W. Li, and L. W. Sneed. 1990. Sequence comparisons of the 3′ end of the genomes of five strains of avian infectious bronchitis virus. Adv. Exp. Med. Biol. 276373-377. [DOI] [PubMed] [Google Scholar]
- 11.de Haan, C. A., P. S. Masters, X. Shen, S. Weiss, and P. J. Rottier. 2002. The group-specific murine coronavirus genes are not essential, but their deletion, by reverse genetics, is attenuating in the natural host. Virology 296177-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Haan, C. A., and P. J. Rottier. 2005. Molecular interactions in the assembly of coronaviruses. Adv. Virus Res. 64165-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhinaker-Raj, G., and R. C. Jones. 1997. Infectious bronchitis virus: immunopathogenesis of infection in the chicken. Avian Pathol. 26677-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felsenstein, J. 1981. Evolutionary trees from DNA sequences: a maximum likelihood approach. J. Mol. Evol. 17368-376. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez, J. M., P. Gomez-Puertas, D. Cavanagh, A. E. Gorbalenya, and L. Enjuanes. 2003. A comparative sequence analysis to revise the current taxonomy of the family Coronaviridae. Arch. Virol. 1482207-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haijema, B. J., H. Volders, and P. J. Rottier. 2004. Live, attenuated coronavirus vaccines through the directed deletion of group-specific genes provide protection against feline infectious peritonitis. J. Virol. 783863-3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hodgson, T., P. Britton, and D. Cavanagh. 2006. Neither the RNA nor the proteins of open reading frames 3a and 3b of the coronavirus infectious bronchitis virus are essential for replication. J. Virol. 80296-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holmes, K. V., and M. M. C. Lai. 1996. Coronaviridae: the viruses and their replication, p. 541-559. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, PA.
- 19.Ignjatovic, J., D. F. Ashton, R. Reece, P. Scott, and P. Hooper. 2002. Pathogenicity of Australian strains of avian infectious bronchitis virus. J. Comp. Pathol. 126115-123. [DOI] [PubMed] [Google Scholar]
- 20.Ignjatovic, J., and P. G. McWaters. 1991. Monoclonal antibodies to three structural proteins of avian infectious bronchitis virus: characterization of epitopes and antigenic differentiation of Australian strains. J. Gen. Virol. 722915-2922. [DOI] [PubMed] [Google Scholar]
- 21.Ignjatovic, J., S. I. Sapats, and F. Ashton. 1997. A long term study of Australian infectious bronchitis viruses indicates a major antigenic change in recently isolated strains. Avian Pathol. 26535-552. [DOI] [PubMed] [Google Scholar]
- 22.Koch, G., L. Hartog, A. Kant, and D. J. van Roozelaar. 1990. Antigenic domains on the peplomer protein of avian infectious bronchitis virus: correlation with biological functions. J. Gen. Virol. 711929-1935. [DOI] [PubMed] [Google Scholar]
- 23.Kumar, S., K. Tamura, and M. Nei. 2004. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 5150-163. [DOI] [PubMed] [Google Scholar]
- 24.Lai, M. M. C., and D. Cavanagh. 1997. The molecular biology of coronaviruses. Adv. Virus Res. 481-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin, T. L., C. C. Loa, and C. C. Wu. 2004. Complete sequences of 3′ end coding region for structural protein genes of turkey coronavirus. Virus Res. 10661-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu, D. X., D. Cavanagh, P. Green, and S. C. Inglis. 1991. A polycistronic mRNA specified by the coronavirus infectious bronchitis virus. Virology 184531-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu, D. X., and S. C. Inglis. 1992. Internal entry of ribosomes on a tricistronic mRNA encoded by infectious bronchitis virus. J. Virol. 666143-6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mardani, K., A. H. Noormohammadi, J. Ignatovic, and G. F. Browning. 2006. Typing infectious bronchitis virus strains using reverse transcription-polymerase chain reaction and restriction fragment length polymorphism analysis to compare the 3′ 7.5 kb of their genomes. Avian Pathol. 3563-69. [DOI] [PubMed] [Google Scholar]
- 29.Niesters, H. G., N. M. Bleumink-Pluym, A. D. Osterhaus, M. C. Horzinek, and B. A. van der Zeijst. 1987. Epitopes on the peplomer protein of infectious bronchitis virus strain M41 as defined by monoclonal antibodies. Virology 161511-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sapats, S. I., F. Ashton, P. J. Wright, and J. Ignjatovic. 1996. Novel variation in the N protein of avian infectious bronchitis virus. Virology 226412-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sapats, S. I., F. Ashton, P. J. Wright, and J. Ignjatovic. 1996. Sequence analysis of the S1 glycoprotein of infectious bronchitis viruses: identification of a novel genotypic group in Australia. J. Gen. Virol. 77413-418. [DOI] [PubMed] [Google Scholar]
- 32.Shen, S., Z. L. Wen, and D. X. Liu. 2003. Emergence of a coronavirus infectious bronchitis virus mutant with a truncated 3b gene: functional characterization of the 3b protein in pathogenesis and replication. Virology 31116-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang, X. C., J. X. Zhang, S. Y. Zhang, P. Wang, X. H. Fan, L. F. Li, G. Li, B. Q. Dong, W. Liu, C. L. Cheung, K. M. Xu, W. J. Song, D. Vijaykrishna, L. L. M. Poon, J. S. M. Peiris, G. J. D. Smith, H. Chen, and Y. Guan. 2006. Prevalence and genetic diversity of coronaviruses in bats from China. J. Virol. 807481-7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 224673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiss, S. R., and S. Navas-Martin. 2005. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol. Mol. Biol. Rev. 69635-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams, A. K., L. Wang, L. W. Sneed, and E. W. Collisson. 1993. Analysis of a hypervariable region in the 3′ noncoding end of the infectious bronchitis virus genome. Virus Res. 2819-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams, A. K., L. Wang, L. W. Sneed, and E. W. Collisson. 1992. Comparative analyses of the nucleocapsid genes of several strains of infectious bronchitis virus and other coronaviruses. Virus Res. 25213-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zwaagstra, K. A., B. A. van der Zeijst, and J. G. Kusters. 1992. Rapid detection and identification of avian infectious bronchitis virus. J. Clin. Microbiol. 3079-84. [DOI] [PMC free article] [PubMed] [Google Scholar]