Abstract
Entry of wild-type lentivirus equine infectious anemia virus (EIAV) into cells requires a low-pH step. This low-pH constraint implicates endocytosis in EIAV entry. To identify the endocytic pathway involved in EIAV entry, we examined the entry requirements for EIAV into two different cells: equine dermal (ED) cells and primary equine endothelial cells. We investigated the entry mechanism of several strains of EIAV and found that both macrophage-tropic and tissue culture-adapted strains utilize clathrin-coated pits for entry. In contrast, a superinfecting strain of EIAV, EIAVvMA-1c, utilizes two mechanisms of entry. In cells such as ED cells that EIAVvMA-1c is able to superinfect, viral entry is pH independent and appears to be mediated by plasma membrane fusion, whereas in cells where no detectable superinfection occurs, EIAVvMA-1c entry that is low-pH dependent occurs through clathrin-coated pits in a manner similar to wild-type virus. Regardless of the mechanism of entry being utilized, the internalization kinetics of EIAV is rapid with 50% of cell-associated virions internalizing within 60 to 90 min. Cathepsin inhibitors did not prevent EIAV entry, suggesting that the low-pH step required by wild-type EIAV is not required to activate cellular cathepsins.
Enveloped virus infection is initiated by the viral glycoprotein binding to its cellular receptor. The binding event either triggers membrane fusion at the plasma membrane or internalization of the virus into an endosome. For those viruses that are endocytosed, subsequent endosomal events lead to fusion of the viral membrane with the vesicle, releasing the core particle into the cytoplasm. Cells utilize several endocytosis mechanisms to take up nutrients from their environment, and viruses usurp these mechanisms for internalization. Defined pathways of endocytosis include clathrin-mediated endocytosis, caveolae-mediated endocytosis, nonclathrin- noncaveolae-mediated endocytosis, and macropinocytosis (40). Many of these pathways traffic through acidic compartments. Viruses can take advantage of the pH decrease to stimulate events that trigger membrane fusion (1, 10, 65). Two mechanistically diverse examples of viral use of a low-pH step are influenza virus and Ebola virus. The vesicle-associated, low-pH environment initiates conformational changes in the influenza virus glycoprotein, leading to membrane fusion (65), whereas endosomal low-pH-activated proteases cleave the Ebola virus glycoprotein 1, allowing subsequent fusion events (14, 60).
An evolution in the understanding of retroviral entry has occurred and now incorporates a role for endocytosis in the internalization of many retroviruses. A general model for mammalian retroviral entry was initially proposed 17 years ago (45); however, the specific requirements for internalization of only a few viruses had been closely examined at that time. The model proposed that a prototypic retrovirus enters cells at the plasma membrane through a pH-independent fusion event (44, 45). As mechanisms of entry of more retroviruses have been examined, numerous retroviruses have been determined to utilize a low-pH-dependent mechanism of entry (8, 10, 19, 31, 47). In fact, within the family, a low-pH-dependent entry mechanism may be more commonly used than direct fusion with the plasma membrane. Retroviruses such as ecotropic murine leukemia virus, avian leukosis virus, and mouse mammary tumor virus use low-pH-dependent entry mechanisms (19, 47, 58, 59).
With the realization that many retroviruses exploit a low-pH entry mechanism, the method of endocytosis utilized by the viruses has been examined. Avian sarcoma and leukosis virus B enters through clathrin-coated pits, whereas avian sarcoma and leukosis virus A entry requires intact lipid rafts for efficient entry (20, 48). While human immunodeficiency virus (HIV) principally enters cells through direct fusion with the plasma membrane (44), HIV has been shown to productively enter CD4-expressing HeLa cells through clathrin-mediated endocytosis and into polarized trophoblastic cells through a clathrin-, caveolin-, and dynamin-independent endocytosis event (18, 71). The pH independence of fusion events associated with amphotropic murine leukemia virus (MLV) led investigators to believe that fusion occurred at the plasma membrane, but amphotropic MLV has since been shown to enter cells through caveolae endocytosis (2, 20, 48). Hence, within this single family of viruses, individual family members have evolved to utilize several different cellular mechanisms, presumably in order to most effectively take advantage of their targeted cellular receptor.
The lentivirus equine infectious anemia virus (EIAV) is responsible for the first-described retrovirus-mediated disease and was one of the first filterable agents described (37, 69). Although the disease that EIAV causes was initially characterized more than 150 years ago, little is known about its mechanism of entry. In vivo, EIAV is primarily if not exclusively macrophage tropic; however, in tissue culture the virus is able to adapt to infect additional cell types, including endothelial cells and fibroblasts, from not only equine species but also from feline and canine origins as well (51, 61). Changes within the long terminal repeat and envelope are associated with both altered virulence and cell tropism (13, 41, 55). Interaction with the cellular receptor equine lentiviral receptor 1 (ELR1) has been demonstrated to be responsible for EIAV internalization (74). EIAV entry into both primary cells and tissue culture cell lines has recently been shown to be dependent on a low-pH step (10, 31), implicating a requirement for endocytosis of the virus. However, the mechanism of endocytosis mediating productive entry of EIAV has not been investigated.
In this study we examine the pathway of EIAV internalization using the low-pH-dependent tissue culture strain EIAVMA-1. Parallel studies were performed to determine the internalization pathway used by a variant, superinfecting strain of EIAV that enters the cells it superinfects in a pH-independent manner (10, 42). Entry of EIAVMA-1, which is representative of wild-type strains of EIAV, was inhibited by treatments that blocked the formation of clathrin-coated pits. In contrast, entry of the superinfecting strain EIAVvMA-1c into target cells that it superinfects was not inhibited by these same treatments; however, inhibition of actin cytoskeletal rearrangements decreased entry. These latter findings indicate that EIAVvMA-1c superinfection does not require the same endocytosis pathway as wild-type EIAV and suggest that this variant virus is able to fuse with the plasma membrane of those target cells.
MATERIALS AND METHODS
Cells lines used.
ED cells, an equine fibroblastic cell line derived from dermal cells (ATCC CCL57), and primary equine umbilical vein endothelial cells (eUVEC) were used to characterize the entry requirements for EIAV. Human embryonic kidney 293T cells (22) were used for transfections to produce pseudotyped particles. Cells were maintained in high-glucose Dulbecco's modified Eagle's medium (DMEM) with penicillin and streptomycin. Medium was supplemented with 10% fetal calf serum for 293T cells, 15% for ED cells, and 40% for eUVEC.
Virus strains.
Various tissue culture strains of EIAV were used in this study. EIAVTh.1 is a macrophage-tropic strain obtained from the first viremic episode of a horse inoculated with a field isolate from Massachusetts (13). EIAVMA-1 is an avirulent, tissue culture-adapted strain of EIAVTh.1 (13). EIAVvMA-1c is a cytopathic, superinfecting strain that was derived by serial passages of EIAVMA-1 in ED cells, as described in reference 42. EIAVSP19 is a nonvirulent molecular clone of EIAV (56). EIAVWSU5 is a strain from Washington State University that was derived from the Wyoming strain and continues to have been passaged in equine fetal kidney cells and back-passaged through ponies to maintain virulence (52). EIAVUK is a mildly virulent molecular clone of EIAV (17).
Generation and titer determinations of viral stocks.
Viral stocks of EIAVSP19 and EIAVUK were produced by transfecting ED with the molecular clone using Amaxa transfections. The transfected populations were monitored for EIAV antigen, and stocks were collected when cells were >95% positive for EIAV antigen as determined by EIAV antigen immunostaining. Viral stocks of EIAVMA-1 and EIAVvMA-1c were produced by infecting ED cells, and EIAVTh.1 was produced by infecting eUVEC. Supernatants were harvested from cells that were >95% positive for EIAV antigen as determined by EIAV antigen immunostaining. Supernatants were centrifuged for 5 min at 13,500 × g to remove cell debris, aliquoted, and frozen at −80°C until needed. Viral titers were determined by infection of ED cells with serial dilutions of stock, followed by 75% acetone-25% water fixation at 40 h after infection, and anti-EIAV immunostaining of the cells as previously described (43). The EIAV antigen-positive cells within the infected cell monolayer were counted, and titers were determined. In all experiments, cells were infected with a multiplicity of infection (MOI) of 0.005. The titers were determined on the cell type used for the experiment.
Detection of EIAV replication.
EIAV infection was determined by immunostaining of viral antigens as previously described (43). Acetone-fixed cells were immunostained with polyclonal horse anti-EIAV antiserum (1:800) from a long-term-infected horse (WSU 2085; a kind gift from J. Lindsay Oaks, Washington State University). This antiserum primarily recognizes envelope (gp90 and gp46) and Gag proteins, as well as Gag precursor polyproteins. Primary antiserum was incubated for 3 h at 37°C, followed by several washes with phosphate-buffered saline. Peroxidase-conjugated goat anti-horse immunoglobulin (1:800; Jackson Immunoresearch) was incubated as the secondary antibody for 45 min at 37°C. Peroxidase activity was detected with the substrate 3-amino-9-ethylcarbazole (Sigma). Cells were enumerated by microscopic visual inspection.
Generating VSV-G- and EBOV-pseudotyped particles.
293T cells were transfected with a total of 75 μg of DNA consisting of vesicular stomatitis virus glycoprotein (VSV-G) expression construct, a pONY3.1gag-pol expression plasmid, and pONYψβgal (46) at a ratio of 1:2:3, respectively. The DNA was transfected into 80% confluent 15-cm dishes of 293T cells using calcium-phosphate transfection (32). Ebola virus glycoprotein lacking the mucin domain (EBOVΔO) pseudotyped onto feline immunodeficiency virus particles were produced as previously described (9). Supernatants were collected at 24, 36, 48, 60, and 72 h after transfection and frozen at −80°C. Supernatants were thawed and passed through a 0.45-μm filter, and virus was pelleted by a 16-h centrifugation step (7,000 rpm at 4°C in a Sorvall GSA rotor). The pellet was resuspended in 250 μl of DMEM for an approximate 200-fold concentration. Pseudotyped particles were aliquoted and stored at −80°C until use.
Detection of VSV-G and EBOV pseudotype transduction.
ED cells were plated in a 48-well format the night before the transduction. On the day of the transduction, medium was refreshed with either our standard DMEM or DMEM containing the appropriate concentration of the endocytosis inhibitors, and pseudotyped particles were added. Forty hours after transduction, the cells were fixed in 3.7% formalin and evaluated for β-galactosidase activity using the substrate 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside. The number of β-galactosidase-positive cells was enumerated by microscopic visual inspection.
Internalization assay.
ED cells were incubated with virions or pseudotyped particles at 4°C for 1 h to allow virus binding, but not internalization. The virus-containing medium was replaced with fresh ED medium and shifted to 37°C. At various time points following the 37°C shift, cells were treated with citric acid buffer (40 mM citric acid, 10 mM KCl, 135 mM NaCl, pH 3.0) for 1 min to inactivate any particles that remained on the surface. The cells were washed two times with medium to remove the acidic buffer, and fresh ED medium was added. Cells were fixed and stained for either EIAV antigens or β-galactosidase activity 40 h after infection, and the number of positive cells was determined. Control values represent the number of positive cells when there was no citric acid wash.
Cellular membrane fractionation.
A total of 5 × 105 ED cells were trypsinized and aliquoted into microcentrifuge tubes. Medium was added, and cells were incubated at 37°C for 1 h to allow reexpression of plasma membrane proteins. Cells were pelleted and resuspended in ice-cold medium in the presence or absence of EIAV (EIAVMA-1 or EIAVvMA-1c). Cells were held for 1 h on ice and washed with ice-cold phosphate-buffered saline twice to remove unbound virus. Separation of detergent-insoluble membranes was performed as previously described (49). Cells were lysed on ice with lysis buffer (1% Brij, 150 mM NaCl, and 10 mM Tris [pH 7.5]) for 50 min. Nycodenz medium (70% Nycodenz, 20 mM Tris [pH 7.5], 150 mM NaCl) was added to the lysed cells for a final concentration of 38.75% Nycodenz, and this was layered under a Nycodenz step gradient generated in a sterile polycarbonate ultracentrifuge tube by adding 200 μl each of 25%, 22.5%, 20%, 18%, 15%, 12%, and 8% Nycodenz medium to the top of the lysed cell mixture. Tubes were centrifuged for 4 h at 200,000 × g at 4°C and stopped without a brake. Two hundred-microliter aliquots were collected from the top of the tube and stored at −80°C until analyzed by immunoblotting.
Immunoblotting.
Proteins present in the membrane fractions were separated on a 4 to 20% Tris-glycine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel (Invitrogen) and transferred onto nitrocellulose. ELR1 was detected by incubating the membranes with rabbit anti-ELR1 polyclonal serum (1:1,000; a kind gift of Baoshan Zhang, University of Pittsburgh) for 3 h and with secondary peroxidase-conjugated goat anti-rabbit antiserum (1:20,000; Sigma) for 1 h. EIAV antigen was detected by incubating the membranes with equine antiserum 2085, with serum from an EIAV-infected horse (1:5,000) for 3 h, and with secondary peroxidase-conjugated goat anti-horse antiserum (1:10,000; Jackson Immunoresearch) for 1 h. Caveolin 1 (Cav1) was detected by incubating with a monoclonal anti-Cav1 antibody (1:1,000; BD Biosciences) overnight at 4°C and with secondary peroxidase-conjugated rabbit anti-mouse antiserum (1:20,000). Membranes were visualized by the chemiluminescence method according to the manufacturer's instructions (Pierce).
Drug studies.
All drugs were obtained from Sigma (St. Louis, MO) unless noted. The no-drug control contained the appropriate dilution of solvent. ED cells were seeded onto 48-well plates and allowed to grow to 90% confluence before treatment and infection. All drug-treated cells were fixed and stained for viral antigens or β-galactosidase activity 40 h after infection.
MβCD.
Methyl-β-cyclodextran (MβCD) was resuspended in DMEM with penicillin-streptomycin and without fetal calf serum as a 25 mM stock. MβCD was diluted into DMEM-penicillin-streptomycin in the absence of fetal calf serum and incubated on cells or with virus for 1 h. The drug was removed, fresh medium was replaced, and the cells were infected.
Genistein.
Genistein (GEN) was resuspended in dimethyl sulfoxide (DMSO) at 20 mg/ml. GEN was diluted into medium and preincubated with the cells for 1 h. Cells were infected in the presence of the drug, the GEN-containing inoculum was removed 24 h after infection, and fresh medium was replaced.
Chlorpromazine.
Chlorpromazine (CPZ) was resuspended in ethanol at 1 mg/ml. CPZ was diluted into the medium and preincubated with cells for 1 h. Cells were infected in the presence of the drug, the CPZ-containing inoculum was removed 6 h after infection, and fresh medium was replaced.
Cytochalasin D.
Cytochalasin D (CytoD) was resuspended in DMSO as a 25 mM stock. CytoD was diluted in medium and incubated with cells for 1 h. The drug was removed, fresh medium was replaced, and cells were infected.
Cathepsin inhibitors.
Cathepsin L inhibitor (219402; CalBiochem) and a pan-cathepsin inhibitor (219419; CalBiochem) were resuspended in DMSO as 10 mM stocks. Cathepsin inhibitors were diluted into the medium and preincubated with cells for 1 h. The cells were infected in the presence of the drug, the cathepsin inhibitor-containing inoculum was removed 16 h after infection, and fresh medium was replaced.
Cell viability assay.
ED cells were plated and treated with drugs as described above. Forty hours after treatment, cell viability was monitored in an ATPLite assay (Packard Biosciences) per the manufacturer's instructions.
ED cell transfection with Eps15 constructs.
ED cells were transfected with a control Eps15D3Δ2-GFP fusion protein (7) or a dominant negative Eps15Δ95-295-GFP expression plasmid (5) using Amaxa transfection reagents. Briefly, 1.5 million ED cells were resuspended in 100 μl Amaxa solution T containing 5 μg of plasmid DNA. ED cells were electroporated using the Amaxa nucleotransfection device and program T-30. The transfected cells were divided into 12 wells of a 48-well plate. Twenty-four hours after transfection, the cells were infected with EIAV, and three wells were analyzed by flow cytometry to determine transfection efficiency. Forty hours after infection, the cells were fixed and evaluated for EIAV antigen expression or β-galactosidase activity.
Adenovirus DN dynamin expression.
ED cells were plated in a 48-well plate. Adenoviral vectors expressing green fluorescent protein (GFP) and dynamin 1 (K44A) (a gift from Jeff Pessin, SUNY-Stony Brook) were incubated with the cells for 24 h (33). Adenoviral vector transduction efficiency was determined by the percentage of cells expressing GFP, which was greater than 95%. At 24 h after transduction, cells were infected with EIAVMA-1 or EIAVvMA-1c or transduced with VSV-G. Cells were fixed and stained 40 h after infection.
Statistical analysis.
All studies were performed at least three independent times. Means and standard errors of the means are shown. Student's t test was used to evaluate the statistical differences between treatments, utilizing the two-tailed distribution and two-sample equal variance conditions. P values were assessed by comparing the level of infectivity with treatment to the level of cytotoxicity seen with that treatment. A significant difference was determined by a P value of < 0.05, and significance is identified in each figure. If the P value was >0.05, the data were not considered significant.
RESULTS
EIAV internalization occurs rapidly.
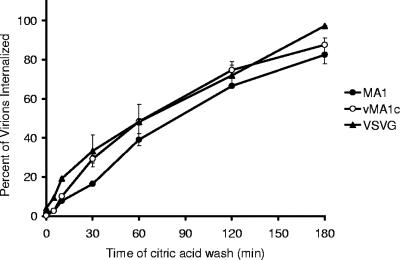
Viral entry kinetics provide insights into the pathway(s) of internalization. Clathrin-mediated endocytosis is known to occur very quickly after ligand binding, whereas caveolae uptake occurs more slowly (16, 40). Documented examples of this are the clathrin-mediated internalization of VSV-G-pseudotyped particles that occurs in minutes, whereas caveolae-mediated internalization of amphotropic murine leukemia virus requires hours (2). To examine the kinetics of EIAV internalization, a tissue culture-derived strain of EIAV, EIAVMA-1, was bound to ED cells at 4°C for 1 hour. The unbound virus and supernatant were removed, replaced with warmed cell medium, and shifted to 37°C to promote internalization. At various time points following the temperature shift, the cells were washed with citric acid to inactivate virion particles that had not been internalized. Cells were examined for EIAV antigen expression 40 h after infection. Internalization of EIAVMA-1 occurred rapidly, with 50% of the virions internalized within 1 h (Fig. 1). VSV-G-pseudotyped EIAV particle entry occurred at a similar rate. These data are consistent with previously published internalization kinetics for virions and ligands that enter through clathrin-coated pits (2, 16). The variant strain EIAVvMA-1c, which has been shown to primarily enter ED cells through a pH-independent pathway resulting in superinfection (10), was observed to have a similar, rapid rate of uptake.
FIG. 1.
Virion internalization kinetics. EIAVMA-1, EIAVvMA-1c, or VSV-G-pseudotyped EIAV particles were bound to ED cells at 4°C for 1 h. The virus was removed, and medium was refreshed. Cells were washed with citric acid buffer at the times indicated to inactivate all virions that had yet to be internalized. Cells were stained 40 h after infection and compared to the number of infected cells when no citric acid wash was performed. Data represent the averages and standard errors of three experiments performed in triplicate.
Wild-type EIAV, but not EIAVvMA-1c, is dynamin dependent.
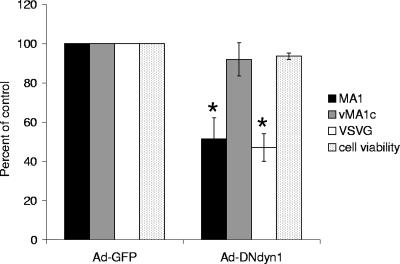
Dynamin is a GTPase required for the cellular membrane to pinch off endosomes from the plasma membrane and is needed for clathrin- and caveolae-mediated endocytosis and phagocytosis, but it is not required for macropinocytosis (25, 27, 63). Dominant negative dynamin [DNdyn1(K44A)] has decreased GTPase activity that results in reduced endocytosis (26, 70). While dynamin 2 is ubiquitously expressed in cells, dynamin 1 is specific for neuronal cells (63). However, overexpression of the dominant negative K44A mutation of either dynamin 1 or 2 has been shown to impact endocytosis in a variety of cells (21, 53, 62, 63). Utilizing adenovirus-transducing particles that express either GFP or DNdyn1(K44A) followed by EIAV infection or VSV-G-pseudotyped virion transduction, we were able to test the dynamin dependence of EIAV infection. An adenovirus MOI of 10 transduced more than 94% of the ED cells as evidenced by GFP expression (data not shown), and the same MOI was used for all the adenoviral transductions. VSV-G-pseudotyped MLV particle entry has been previously shown to be inhibited by DNdyn; thus, VSV-G-pseudotyped EIAV particles were used as a positive control (35). EIAVMA-1 entry was significantly inhibited by expression of DNdyn1 (Fig. 2). Similar trends were seen with expression of DNdyn2 (data not shown), but DNdyn2 expression in ED cells resulted in high levels of cell toxicity. EIAVvMA-1c entry into ED cells was not affected by DNdyn1, suggesting that neither clathrin-coated pits nor caveolae are important for entry of EIAVvMA-1c into these cells.
FIG. 2.
EIAV entry is dynamin dependent. ED cells were transduced with an adenoviral vector that expressed either GFP or DN dynamin 1. Twenty-four hours after transduction, cells were infected with EIAVMA-1 or EIAVvMA-1c or transduced with VSV-G-pseudotyped EIAV particles. Cells were stained 40 h after infection and compared to the number of infected cells transduced with Ad-GFP. Data represent the averages and standard errors of three experiments performed in triplicate. *, P < 0.05.
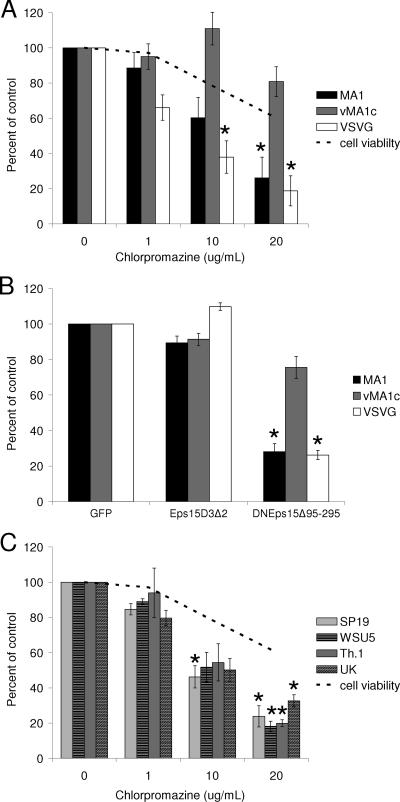
Role for actin rearrangement in EIAVvMA-1c entry, but not in EIAVMA-1 entry.
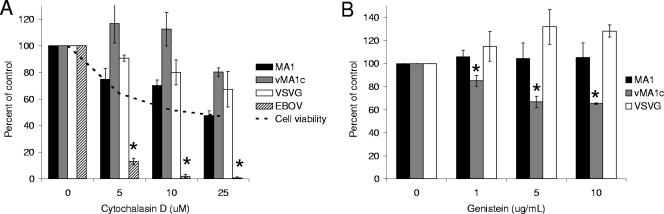
Actin rearrangement is necessary for some endocytosis mechanisms, such as caveolae-mediated uptake and macropinocytosis, as well as direct virus fusion with the plasma membrane (12, 38, 66). In order to determine if EIAV entry requires an intact actin cytoskeleton, we utilized CytoD, a drug that depolymerizes actin (38). Immunofluorescence images of actin staining demonstrate that the concentrations of CytoD that were used effectively depolymerized the actin network in ED cells (data not shown). As with some of the other endocytosis inhibitors used in this study, CytoD was cytotoxic and caused cells to round up and detach from the surface of the plate. Consequently, cell viability assays were performed to measure the impact of the drug on the cells over the 40-h experiment. The effect of CytoD on EIAV entry was evaluated, and results were compared to the drug's impact on cell viability. VSV-G-dependent transduction is not affected by CytoD and served as a negative control, whereas EBOV glycoprotein-dependent entry is dependent on actin polymerization and served as a positive control (11, 73). EIAVMA-1 entry was diminished by CytoD in a dose-dependent manner, and in parallel, a loss of cell viability occurred (Fig. 3A). The decrease in entry mediated by both EIAVMA-1 and VSV-G glycoproteins could be accounted for by the loss of cell numbers in the culture dish. EBOV-GP-mediated entry, into SNB-19 cells, was significantly decreased compared to the cell viability (Fig. 3A). The EBOV-GP transduction studies were performed in SNB-19 cells, because ED cells were not permissive for EBOV-GP-dependent transduction. We concluded that actin depolymerization does not directly impact entry of EIAVMA-1. Despite the loss of cells in the presence of CytoD, low concentrations of CytoD appeared to slightly enhance EIAVvMA-1c entry into ED cells, although this was not statistically significant.
FIG. 3.
EIAVvMA-1c requires dynamic actin rearrangement for efficient entry. CytoD- (A) or GEN- (B) treated cells were infected with EIAVMA-1 or EIAVvMA-1c or transduced with VSV-G- or EBOV-pseudotyped particles. Cells were stained 40 h after infection and compared to the number of infected cells treated with the DMSO control. Data represent the averages and standard errors of three experiments performed in triplicate. *, P < 0.05.
Signal transduction events mediated by tyrosine kinases induce actin rearrangements and can play a role in caveolae endocytosis (54, 57). These events can be blocked by the tyrosine kinase inhibitor GEN (57). Inhibition of tyrosine kinase activity by GEN also inhibits HIV-1 entry into primary macrophages by preventing virus fusion with the plasma membrane (67). EIAVMA-1 and EIAVvMA-1c entry into ED cells was examined in the presence of GEN (Fig. 3B). No cytotoxicity was observed with the concentrations of GEN used (data not shown). EIAVMA-1 entry was not inhibited by GEN, but EIAVvMA-1c infectivity did show a dose-dependent decrease. Loss of EIAVvMA-1c infectivity by the inhibition of tyrosine kinase activity would suggest that actin rearrangement and/or caveolae-mediated endocytosis is important for EIAVvMA-1c entry events into ED cells, and these findings are consistent with the trend of enhanced EIAVvMA-1c entry in the presence of the lower concentrations of CytoD.
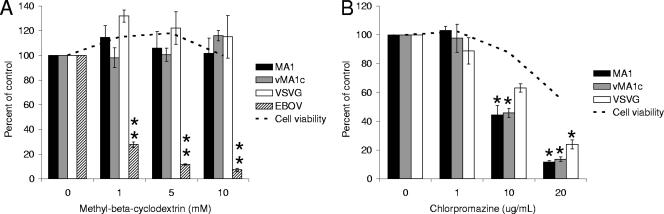
Cellular cholesterol is not required for EIAV infection.
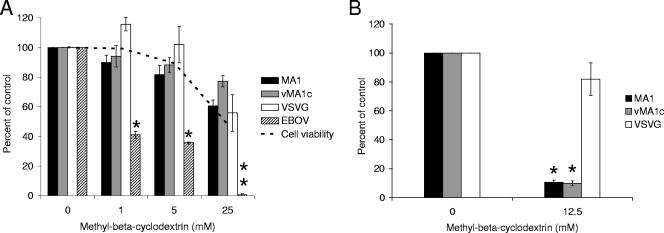
MβCD removes the cholesterol from membranes and sequesters it within the cell (30, 34). Removal of cholesterol disrupts lipid rafts and has been shown to inhibit caveolae-mediated endocytosis as well as lipid raft-dependent internalization (63). Treatment of ED cells with MβCD did not inhibit entry of EIAVMA-1, EIAVvMA-1c, or VSV-G-pseudotyped EIAV particles (Fig. 4A). Similar treatment of SNB-19 cells inhibited EBOV-GP-mediated entry, which has previously been shown to be dependent on lipid rafts (23).
FIG. 4.
Cholesterol in the target cell is not required for EIAV infectivity. ED cells were treated with MβCD for 1 h and infected with EIAVMA-1 or EIAVvMA-1c or transduced with VSV-G- or EBOV-pseudotyped EIAV particles (A). EIAVMA-1 and EIAVvMA-1c viral particles or VSV-G-transducing particles were incubated with MβCD for 1 h. The particles were diluted into ED medium and used to infect ED cells (B). Cells were stained 40 h after infection and compared to the number of infected cells when particles were treated with the control. Data represent the averages and standard errors of three experiments performed in triplicate. *, P < 0.05; **, P < 0.001.
To further demonstrate the efficacy of MβCD at the concentrations used in this study, we sought to determine if treatment of viral particles with MβCD to eliminate cholesterol from the viral membrane would reduce infectivity. Other retroviruses, such as HIV-1, have been shown to bud through lipid rafts, and the cholesterol in the viral membrane is important for viral infectivity (3, 36). Virus was incubated with MβCD for 1 hour, diluted approximately 200-fold, and added to cells. This treatment effectively reduced the infectivity of HIV virions (more than 90% decrease) known to require cholesterol in the viral membrane (data not shown). MβCD treatment dramatically inhibited the entry of EIAVMA-1 and EIAVvMA-1c, but it did not significantly inhibit the transduction of VSV-G-pseudotyped particles (Fig. 4B).
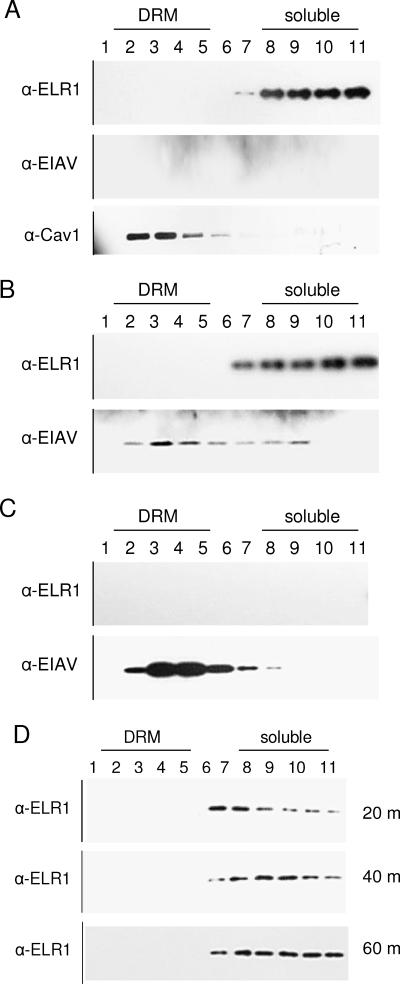
ELR1 is not present in lipid rafts.
If lipid rafts were not important for EIAV entry, we would anticipate that the cellular receptor for EIAV, ELR1, would not colocalize with detergent-resistant membranes. Consequently, we next determined the location of ELR1 within the plasma membrane both before and after virion binding. ED cells incubated in the presence or absence of EIAVMA-1 virions at 4°C were lysed in a Brij lysis buffer that solubilizes the non-lipid raft membranes but keeps the lipid rafts intact. Cell lysates were subjected to membrane flotation on a Nycodenz gradient. Centrifugation caused the lipid raft-containing membranes to float to the top of the gradient, whereas the solubilized proteins remained at the bottom (28). The gradient was fractionated into 11 fractions and subjected to Western blot analysis to determine which fraction contained ELR1 and EIAV proteins. Caveolin 1, a lipid raft-associated protein, was found in the detergent-resistant fractions (Fig. 5A), validating our experimental protocol. EIAV capsid protein was found primarily, but not exclusively, in fractions containing detergent-resistant membranes when the virions were examined by themselves or after they were bound to cells, suggesting that EIAV's lipid membrane contains lipid rafts (Fig. 5B and C). EIAV matrix protein was observed in a similar fraction (data not shown). ELR1 was only found in the lower fractions that are not associated with detergent-resistant membranes, suggesting the protein was solubilized by the lysis buffer and was not found in lipid rafts even after virus binding (Fig. 5B).
FIG. 5.
ELR1 is not found in lipid rafts. ED cells (A), ED cells bound with EIAVMA-1 particles (B), or EIAVMA-1 viral particles alone (C) were lysed and run on a Nycodenz gradient. Detergent-resistant membranes (DRM) floated to the top fractions (1 to 5), whereas solubilized protein can be seen in the bottom fractions (6 to 11). The fractions were analyzed for ELR1, EIAV capsid, and caveolin 1 by Western blotting. EIAVMA-1-bound ED cells were shifted to 37°C for 20, 40, or 60 min, and fractions were Western blotted for ELR1 (D).
Because membrane fluidity is reduced at 4°C, the experiment was repeated with an additional 37°C incubation. Once virus was bound to cells at 4°C, the cells were shifted to 37°C for 20, 40, or 60 min and lysed, and membrane flotation was performed. The ELR1 protein was found in the soluble fractions at each time point (Fig. 5D), indicating that ELR1 does not move into lipid rafts after viral glycoprotein-receptor interactions. The absence of ELR1 from lipid raft microdomains is consistent with the location of the human homolog of ELR1, the herpes virus entry mediator, which remains in the soluble membrane fractions even after virus binding (4).
EIAVMA-1 enters cells through clathrin-coated pits.
Clathrin-mediated endocytosis has been shown to be inhibited by the drug CPZ (63). CPZ redirects the adapter proteins that bind to the plasma membrane proteins to internal membranes, preventing clathrin-mediated endocytosis from the cell surface (72). VSV-G is known to enter through clathrin-mediated endocytosis (68) and therefore was utilized as a positive control. ED cells were treated with increasing amounts of CPZ and examined for their ability to inhibit EIAV entry. EIAVMA-1 virus was inhibited in a dose-dependent manner (Fig. 6A). Although the drug had some cytotoxicity, levels of EIAVMA-1 entry were significantly reduced compared to chlorpromazine's impact on cell viability, suggesting that wild-type EIAV enters cells through a clathrin-mediated pathway. CPZ did not impact EIAVvMA-1c entry into ED cells.
FIG. 6.
EIAVMA-1 enters ED cells through clathrin-mediated endocytosis. ED cells were treated with CPZ for 1 h and infected with EIAVMA-1 or EIAVvMA-1c or transduced with VSV-G-pseudotyped EIAV particles (A). ED cells were transfected with GFP, Eps15D3Δ2-GFP, or DNEps15Δ95-295-GFP. Twenty-four hours after transfection, the cells were infected with EIAVMA-1 or EIAVvMA-1c or transduced with VSV-G-pseudotyped EIAV particles (B). ED cells were treated with CPZ for 1 h and infected with EIAVSP19, EIAVTh.1, EIAVWSU5, or EIAVUK (C). Cells were stained 40 h after infection and compared to the number of infected cells when particles were treated with the control. Data represent the averages and standard errors of three experiments performed in triplicate. *, P < 0.05.
The clathrin pit-associated protein Eps15 serves as a linker between the clathrin coat and the receptor being internalized (6, 29). A dominant-negative form of Eps15 (DN-Eps15Δ95-295) lacks the Eps homology domains that are necessary for clathrin-coated pit targeting and thus inhibits clathrin uptake (5). ED cells were transfected with GFP, Eps15D3Δ2-GFP that does not interfere with clathrin uptake (6, 29), or DNEps15Δ95-295-GFP and infected with EIAVMA-1 or EIAVvMA-1c 24 h after transfection. Expression of the DN-Eps15Δ95-295-GFP fusion protein followed by infection with EIAVMA-1 confirmed that clathrin endocytosis is required for EIAV entry (Fig. 6B). The transfected cells were 57 to 65% GFP positive at 24 h after transfection, demonstrating a good efficiency of transfection. EIAVvMA-1c only showed minor inhibition, similar to the slight inhibition seen when a low-pH entry pathway is blocked during EIAVvMA-1c infection (10). These findings are consistent with the CPZ findings that EIAVvMA-1c does not require clathrin for entry into ED cells.
To understand whether entry via clathrin-mediated endocytosis occurs with other strains of EIAV, the infectivity of four additional strains of EIAV was examined in the presence of CPZ. These EIAV strains, which have previously been shown to enter cells through a low-pH-dependent mechanism, were inhibited by CPZ in a dose-dependent manner (Fig. 6C). These strains included EIAVWSU5, which retains the ability to infect horses, suggesting that both tissue culture-adapted strains and field isolates enter ED cells through clathrin-mediated endocytosis.
EIAVMA-1 and EIAVvMA-1c enter eUVEC through clathrin-mediated endocytosis.
EIAVvMA-1c superinfects ED cells in a pH-independent manner, but it does not superinfect equine endothelial cells (eUVEC) and enters eUVEC through a low-pH-dependent pathway (10). Thus, EIAVvMA-1c behaves like wild-type strains of EIAV in eUVEC. To determine if EIAV enters eUVEC through a clathrin-mediated endocytosis event, eUVEC were infected in the presence of MβCD or CPZ. As observed in ED cells, entry of neither EIAVMA-1 nor EIAVvMA-1c was significantly inhibited (Fig. 7A). Consistent with low-pH-dependent entry, entry of both EIAVMA-1 and EIAVvMA-1c into eUVEC was inhibited in a dose-dependent manner by CPZ (Fig. 7B).
FIG. 7.
EIAVvMA-1c enters eUVEC through clathrin-mediated endocytosis. eUVEC were treated with MβCD for 1 h and infected with EIAVMA-1 or EIAVvMA-1c or transduced with VSV-G- or EBOV-pseudotyped particles (A). eUVEC were treated with CPZ for 1 h and infected with EIAVMA-1 or EIAVvMA-1c or transduced with VSV-G-pseudotyped EIAV particles (B). Cells were stained 40 h after infection and compared to the number of infected cells when particles were treated with the control. Data represent the averages and standard errors of three experiments performed in triplicate. *, P < 0.05; **, P < 0.001.
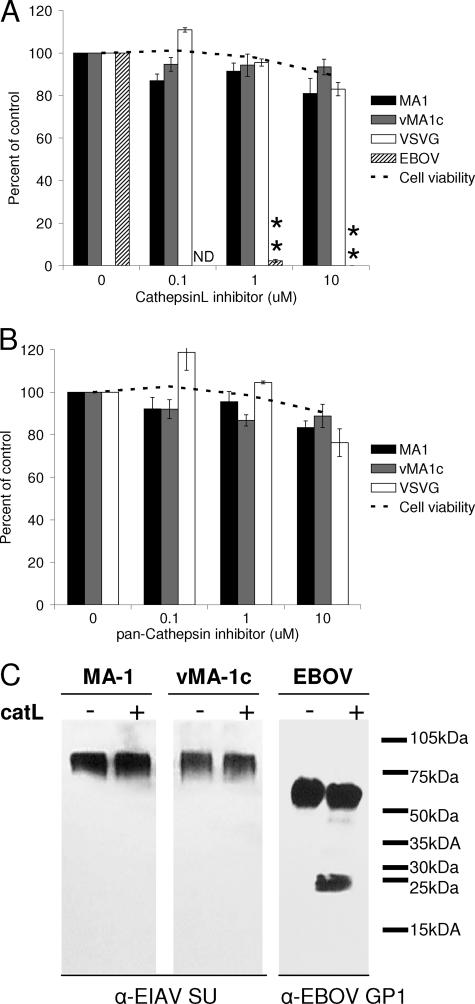
Cathepsin inhibitors do not inhibit EIAV entry.
Recently, the entry of several low-pH-dependent viruses has been shown to require viral glycoprotein processing by cellular, low-pH-dependent endosomal proteases, such as cathepsins (60, 64). To determine if wild-type EIAV's requirement for low-pH entry is dependent on glycoprotein processing by cellular cathepsin proteases, we infected ED cells in the presence of a pan-cathepsin inhibitor as well as a specific cathepsin L inhibitor. EBOV-pseudotyped particles served as a positive control for these studies, whereas VSV-G transduction served as a negative control. Cathepsin L is a ubiquitously expressed protease and has been shown to be involved in Ebola virus entry (60). Both the pan-cathepsin and cathepsin L inhibitor had no effect on EIAVMA-1 or EIAVvMA-1c entry (Fig. 8A and B). The cathepsin B inhibitor CA-074Me also did not inhibit EIAV entry into ED cells (data not shown).
FIG. 8.
Cathepsin inhibitors do not inhibit EIAV entry. ED cells were treated with a cathepsin L inhibitor (A) or a pan-cathepsin inhibitor (B) and infected with EIAVMA-1 or EIAVvMA-1c or transduced with VSV-G-pseudotyped EIAV particles. Cells were stained 40 h after infection and compared to the number of infected cells when particles were treated with the control. Data represent the averages and standard errors of three experiments performed in triplicate. Viral particles were incubated with cathepsin L protease for 1 h, and Western analysis was performed to examine cleavage of the viral glycoprotein (C). **, P < 0.001.
To further demonstrate that cathepsin L does not play a role in EIAV entry, we performed an in vitro protease cleavage assay. Viral particles were incubated in a low-pH acetate buffer with purified cathepsin L. Virions were analyzed by immunoblotting to determine if proteolysis of the glycoprotein had occurred. There was no detectable decrease in full-length glycoprotein and no cleavage products detected with EIAVMA-1 or EIAVvMA-1c, but the Ebola virus glycoprotein was efficiently cleaved (Fig. 8C). These findings indicate that EIAV glycoprotein does not require a low-pH step for proteolytic processing but perhaps for glycoprotein conformational changes that are needed to generate a fusion-ready state.
DISCUSSION
In summary, we have presented data that the wild-type EIAVMA-1 infects cells through clathrin-mediated endocytosis. The clathrin pathway was important for EIAVMA-1 entry into both the ED cell line and primary eUVEC. We also demonstrated that several other strains of EIAV enter ED cells through clathrin-coated pits. The low-pH requirement for EIAV entry is not required for glycoprotein cleavage by cellular endosomal proteases. Instead, we propose that the acidic conditions may be altering the conformation of the intact surface protein and thereby facilitating conditions that allow fusion to occur. In contrast, the superinfecting EIAV strain EIAVvMA-1c does not require clathrin-mediated endocytosis, lipid rafts, or dynamin, but active actin remodeling enhances entry into ED cells, suggesting that EIAVvMA-1c may fuse at the plasma membrane. However, in UVEC, which EIAVvMA-1c cannot superinfect, the entry pathway appears to be similar to that found for other EIAV strains.
The importance of actin remodeling in clathrin-mediated endocytosis remains controversial. Some reports demonstrated that dynamic actin polymerization is required for clathrin-mediated endocytosis (24), but other reports showed that this requirement is cell type specific (24, 73). As others have observed for VSV-G-dependent entry (73), our study demonstrates a clathrin-dependent and actin remodeling-independent entry of VSV-G. In a similar manner, we propose that wild-type EIAV also uses a clathrin-dependent and actin remodeling-independent mechanism for entry.
Conflicting data are present in the literature about VSV-G-mediated entry and dynamin dependence. Entry of VSV-G-pseudotyped MLV particles was shown to be inhibited by expression of dominant-negative dynamin (K44A) (35). In contrast, a recent report showed that VSV-G-pseudotyped HIV particles were not inhibited by expression of a different mutant dynamin (G273D) (18). Our results with the dynamin mutant K44A are consistent with the earlier study that used this same mutant and suggest that the discrepancy may be due to the different dynamin mutants used or the cell type.
While the presence of cholesterol in target cells is not important for EIAV entry, loss of cholesterol from the virion decreases virus infectivity. Other retroviruses, such as HIV and amphotropic MLV, have been shown to bud through lipid raft-containing membranes (2, 36, 50). Our data indicate that EIAV also buds through lipid raft domains in the plasma membrane.
In contrast to wild-type EIAV, the variant strain EIAVvMA-1c enters cells that it superinfects via a pH-independent pathway (10). The absence of a low-pH requirement for ED cell entry suggested that EIAVvMA-1c either fuses at the plasma membrane or enters through a pH-independent endocytosis mechanism. Studies presented here allowed us to exclude internalization of EIAVvMA-1c into ED cells by endocytosis. Our supporting evidence includes the absence of an effect on EIAVvMA-1c entry by DNdyn, MβCD, CPZ, or DNEps15 in ED cells. In contrast, our experiments support the hypothesis that EIAVvMA-1c fuses with the plasma membrane of ED cells. A trend for enhanced EIAVvMA-1c infection was observed at the lower concentrations of CytoD, and virus replication was inhibited by the tyrosine kinase inhibitor genistein. Genistein blocks signaling events that mediate actin cytoskeleton movement, whereas CytoD depolymerizes the actin cytoskeleton. HIV virions, which fuse at the plasma membrane, have a similar response to these drugs (12, 67). The prevention of actin reorganization by genistein may impede EIAVvMA-1c from passing through the actin network, whereas CytoD removes the actin cytoskeleton, facilitating virion delivery from the plasma membrane into the cytoplasm.
In contrast, EIAVvMA-1c entry into eUVEC, which was previously shown to be pH dependent, occurs through clathrin-mediated endocytosis. Pathways used by EIAVvMA-1c for entry into eUVEC were indistinguishable from other strains of EIAV, suggesting that EIAVvMA-1c uses ELR1 to gain entry into eUVEC. Conversely, the pH-independent plasma membrane fusion event that results in EIAVvMA-1c superinfection of ED cells profoundly differs from that observed with other EIAV strains. The different pathways by which EIAVvMA-1c enters fibroblasts and endothelial cells suggest the intriguing possibility that EIAVvMA-1c may utilize different receptors in these two cell types. Further studies are needed to explore this possibility.
The low-pH requirement for productive entry of many viruses has been investigated (8, 14, 15, 19, 39, 40, 45, 47, 63). Current evidence indicates that low-pH conditions are required to alter the viral surface glycoprotein conformation and thereby remove its steric hindrance of virus-cell fusion. Two different mechanisms of acid-induced conformational changes have been identified. In the case of influenza virus, the conformation of the intact viral glycoprotein is altered under low-pH conditions (65). In contrast, the viral surface glycoprotein of such viruses as Ebola virus and severe acute respiratory syndrome coronavirus is cleaved into small peptides by low-pH-activated cellular protease activities (14, 60, 64). Here we have demonstrated that EIAV entry is not inhibited by cathepsin inhibitors, and cathepsin L does not cleave the EIAV SU protein in vitro. Cathepsin-independent, low-pH-dependent surface glycoprotein alterations are consistent with our previously published findings that a low-pH shock of virus-bound cells increases infectivity (10). The increase in infectivity at the cell surface suggests that there are no endosome-specific enzymatic activities required for the fusion event to take place.
A previous study that used a sucrose shock to block clathrin-dependent entry did not inhibit entry of EIAV into ED cells, leading those authors to conclude that EIAV entry was independent of clathrin-mediated endocytosis (31). We also found that a similar sucrose treatment did not affect EIAV or VSV-G entry into ED cells (data not shown). The findings from the sucrose studies are inconsistent with our findings when more specific means of inhibiting the formation of clathrin-coated pits were used, such as dominant-negative Eps15 or chlorpromazine (63). Therefore, we conclude that under the conditions used, the sucrose shock did not impact clathrin-mediated entry.
Acknowledgments
We thank Chantal Allamargot at the University of Iowa Central Microscopy Research Facility for help with fluorescence microscopy.
This work was supported by NIH R21 AI064526 (W.J.M.).
Footnotes
Published ahead of print on 5 December 2007.
REFERENCES
- 1.Barnard, R. J., and J. A. Young. 2003. Alpharetrovirus envelope-receptor interactions. Curr. Top. Microbiol. Immunol. 281107-136. [DOI] [PubMed] [Google Scholar]
- 2.Beer, C., D. S. Andersen, A. Rojek, and L. Pedersen. 2005. Caveola-dependent endocytic entry of amphotropic murine leukemia virus. J. Virol. 7910776-10787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beer, C., L. Pedersen, and M. Wirth. 2005. Amphotropic murine leukaemia virus envelope protein is associated with cholesterol-rich microdomains. Virol. J. 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bender, F. C., J. C. Whitbeck, M. Ponce de Leon, H. Lou, R. J. Eisenberg, and G. H. Cohen. 2003. Specific association of glycoprotein B with lipid rafts during herpes simplex virus entry. J. Virol. 779542-9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benmerah, A., M. Bayrou, N. Cerf-Bensussan, and A. Dautry-Varsat. 1999. Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J. Cell Sci. 1121303-1311. [DOI] [PubMed] [Google Scholar]
- 6.Benmerah, A., B. Begue, A. Dautry-Varsat, and N. Cerf-Bensussan. 1996. The ear of alpha-adaptin interacts with the COOH-terminal domain of the Eps 15 protein. J. Biol. Chem. 27112111-12116. [DOI] [PubMed] [Google Scholar]
- 7.Benmerah, A., C. Lamaze, B. Begue, S. L. Schmid, A. Dautry-Varsat, and N. Cerf-Bensussan. 1998. AP-2/Eps15 interaction is required for receptor-mediated endocytosis. J. Cell Biol. 1401055-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolander, F. F., Jr. 1996. Requirements for mouse mammary tumour virus internalization in mouse mammary epithelial cells. J. Gen. Virol. 77793-796. [DOI] [PubMed] [Google Scholar]
- 9.Brindley, M. A., L. Hughes, A. Ruiz, P. B. McCray, Jr., A. Sanchez, D. A. Sanders, and W. Maury. 2007. Ebola virus glycoprotein 1: identification of residues important for binding and postbinding events. J. Virol. 817702-7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brindley, M. A., and W. Maury. 2005. Endocytosis and a low-pH step are required for productive entry of equine infectious anemia virus. J. Virol. 7914482-14488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bukrinskaya, A., B. Brichacek, A. Mann, and M. Stevenson. 1998. Establishment of a functional human immunodeficiency virus type 1 (HIV-1) reverse transcription complex involves the cytoskeleton. J. Exp. Med. 1882113-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell, E. M., R. Nunez, and T. J. Hope. 2004. Disruption of the actin cytoskeleton can complement the ability of Nef to enhance human immunodeficiency virus type 1 infectivity. J. Virol. 785745-5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carpenter, S., and B. Chesebro. 1989. Change in host cell tropism associated with in vitro replication of equine infectious anemia virus. J. Virol. 632492-2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chandran, K., N. J. Sullivan, U. Felbor, S. P. Whelan, and J. M. Cunningham. 2005. Endosomal proteolysis of the Ebola virus glycoprotein is necessary for infection. Science 3081643-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chu, J. J., and M. L. Ng. 2004. Infectious entry of West Nile virus occurs through a clathrin-mediated endocytic pathway. J. Virol. 7810543-10555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conner, S. D., and S. L. Schmid. 2003. Regulated portals of entry into the cell. Nature 42237-44. [DOI] [PubMed] [Google Scholar]
- 17.Cook, R. F., C. Leroux, S. J. Cook, S. L. Berger, D. L. Lichtenstein, N. N. Ghabrial, R. C. Montelaro, and C. J. Issel. 1998. Development and characterization of an in vivo pathogenic molecular clone of equine infectious anemia virus. J. Virol. 721383-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daecke, J., O. T. Fackler, M. T. Dittmar, and H. G. Krausslich. 2005. Involvement of clathrin-mediated endocytosis in human immunodeficiency virus type 1 entry. J. Virol. 791581-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diaz-Griffero, F., S. A. Hoschander, and J. Brojatsch. 2002. Endocytosis is a critical step in entry of subgroup B avian leukosis viruses. J. Virol. 7612866-12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diaz-Griffero, F., A. P. Jackson, and J. Brojatsch. 2005. Cellular uptake of avian leukosis virus subgroup B is mediated by clathrin. Virology 33745-54. [DOI] [PubMed] [Google Scholar]
- 21.Duan, D., Q. Li, A. W. Kao, Y. Yue, J. E. Pessin, and J. F. Engelhardt. 1999. Dynamin is required for recombinant adeno-associated virus type 2 infection. J. Virol. 7310371-10376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DuBridge, R. B., P. Tang, H. C. Hsia, P. M. Leong, J. H. Miller, and M. P. Calos. 1987. Analysis of mutation in human cells by using an Epstein-Barr virus shuttle system. Mol. Cell. Biol. 7379-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Empig, C. J., and M. A. Goldsmith. 2002. Association of the caveola vesicular system with cellular entry by filoviruses. J. Virol. 765266-5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujimoto, L. M., R. Roth, J. E. Heuser, and S. L. Schmid. 2000. Actin assembly plays a variable, but not obligatory role in receptor-mediated endocytosis in mammalian cells. Traffic 1161-171. [DOI] [PubMed] [Google Scholar]
- 25.Henley, J. R., E. W. Krueger, B. J. Oswald, and M. A. McNiven. 1998. Dynamin-mediated internalization of caveolae. J. Cell Biol. 14185-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herskovits, J. S., C. C. Burgess, R. A. Obar, and R. B. Vallee. 1993. Effects of mutant rat dynamin on endocytosis. J. Cell Biol. 122565-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hinshaw, J. E. 2000. Dynamin and its role in membrane fission. Annu. Rev. Cell Dev. Biol. 16483-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hostager, B. S., I. M. Catlett, and G. A. Bishop. 2000. Recruitment of CD40 and tumor necrosis factor receptor-associated factors 2 and 3 to membrane microdomains during CD40 signaling. J. Biol. Chem. 27515392-15398. [DOI] [PubMed] [Google Scholar]
- 29.Iannolo, G., A. E. Salcini, I. Gaidarov, O. B. Goodman, Jr., J. Baulida, G. Carpenter, P. G. Pelicci, P. P. Di Fiore, and J. H. Keen. 1997. Mapping of the molecular determinants involved in the interaction between eps15 and AP-2. Cancer Res. 57240-245. [PubMed] [Google Scholar]
- 30.Ilangumaran, S., and D. C. Hoessli. 1998. Effects of cholesterol depletion by cyclodextrin on the sphingolipid microdomains of the plasma membrane. Biochem. J. 335433-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin, S., B. Zhang, O. A. Weisz, and R. C. Montelaro. 2005. Receptor-mediated entry by equine infectious anemia virus utilizes a pH-dependent endocytic pathway. J. Virol. 7914489-14497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnston, J. C., M. Gasmi, L. E. Lim, J. H. Elder, J. K. Yee, D. J. Jolly, K. P. Campbell, B. L. Davidson, and S. L. Sauter. 1999. Minimum requirements for efficient transduction of dividing and nondividing cells by feline immunodeficiency virus vectors. J. Virol. 734991-5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kao, A. W., B. P. Ceresa, S. R. Santeler, and J. E. Pessin. 1998. Expression of a dominant interfering dynamin mutant in 3T3L1 adipocytes inhibits GLUT4 endocytosis without affecting insulin signaling. J. Biol. Chem. 27325450-25457. [DOI] [PubMed] [Google Scholar]
- 34.Keller, P., and K. Simons. 1998. Cholesterol is required for surface transport of influenza virus hemagglutinin. J. Cell Biol. 1401357-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee, S., Y. Zhao, and W. F. Anderson. 1999. Receptor-mediated Moloney murine leukemia virus entry can occur independently of the clathrin-coated-pit-mediated endocytic pathway. J. Virol. 735994-6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liao, Z., D. R. Graham, and J. E. Hildreth. 2003. Lipid rafts and HIV pathogenesis: virion-associated cholesterol is required for fusion and infection of susceptible cells. AIDS Res. Hum Retrovir. 19675-687. [DOI] [PubMed] [Google Scholar]
- 37.Ligné, M. 1843. Mémoire et observations sur une maladie de sang, connue sous le nom d'anhémie hydrohémie, cachexie acquise du cheval. Rec. Med. Vet. Ec. Alfort. 184330-44. [Google Scholar]
- 38.Maniak, M. 2001. Macropinocytosis, p. 78-93. In M. Marsh (ed.), Endocytosis. Oxford University Press, Oxford, England.
- 39.Marsh, M., and A. Helenius. 1989. Virus entry into animal cells. Adv. Virus Res. 36107-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marsh, M., and A. Helenius. 2006. Virus entry: open sesame. Cell 124729-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maury, W., R. J. Thompson, Q. Jones, S. Bradley, T. Denke, P. Baccam, M. Smazik, and J. L. Oaks. 2005. Evolution of the equine infectious anemia virus long terminal repeat during the alteration of cell tropism. J. Virol. 795653-5664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maury, W., P. J. Wright, and S. Bradley. 2003. Characterization of a cytolytic strain of equine infectious anemia virus. J. Virol. 772385-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maury, W. J., S. Carpenter, K. Graves, and B. Chesebro. 1994. Cellular and viral specificity of equine infectious anemia virus Tat transactivation. Virology 200632-642. [DOI] [PubMed] [Google Scholar]
- 44.McClure, M. O., M. Marsh, and R. A. Weiss. 1988. Human immunodeficiency virus infection of CD4-bearing cells occurs by a pH-independent mechanism. EMBO J. 7513-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McClure, M. O., M. A. Sommerfelt, M. Marsh, and R. A. Weiss. 1990. The pH independence of mammalian retrovirus infection. J. Gen. Virol. 71767-773. [DOI] [PubMed] [Google Scholar]
- 46.Mitrophanous, K., S. Yoon, J. Rohll, D. Patil, F. Wilkes, V. Kim, S. Kingsman, A. Kingsman, and N. Mazarakis. 1999. Stable gene transfer to the nervous system using a non-primate lentiviral vector. Gene Ther. 61808-1818. [DOI] [PubMed] [Google Scholar]
- 47.Mothes, W., A. L. Boerger, S. Narayan, J. M. Cunningham, and J. A. Young. 2000. Retroviral entry mediated by receptor priming and low pH triggering of an envelope glycoprotein. Cell 103679-689. [DOI] [PubMed] [Google Scholar]
- 48.Narayan, S., R. J. Barnard, and J. A. Young. 2003. Two retroviral entry pathways distinguished by lipid raft association of the viral receptor and differences in viral infectivity. J. Virol. 771977-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Naslavsky, N., R. Stein, A. Yanai, G. Friedlander, and A. Taraboulos. 1997. Characterization of detergent-insoluble complexes containing the cellular prion protein and its scrapie isoform. J. Biol. Chem. 2726324-6331. [DOI] [PubMed] [Google Scholar]
- 50.Nguyen, D. H., and J. E. Hildreth. 2000. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J. Virol. 743264-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oaks, J. L., T. C. McGuire, C. Ulibarri, and T. B. Crawford. 1998. Equine infectious anemia virus is found in tissue macrophages during subclinical infection. J. Virol. 727263-7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O'Rourke, K., L. E. Perryman, and T. C. McGuire. 1988. Antiviral, anti-glycoprotein and neutralizing antibodies in foals with equine infectious anaemia virus. J. Gen. Virol. 69667-674. [DOI] [PubMed] [Google Scholar]
- 53.Parker, J. S., and C. R. Parrish. 2000. Cellular uptake and infection by canine parvovirus involves rapid dynamin-regulated clathrin-mediated endocytosis, followed by slower intracellular trafficking. J. Virol. 741919-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parton, R. G., B. Joggerst, and K. Simons. 1994. Regulated internalization of caveolae. J. Cell Biol. 1271199-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Payne, S. L., X. F. Pei, B. Jia, A. Fagerness, and F. J. Fuller. 2004. Influence of long terminal repeat and Env on the virulence phenotype of equine infectious anemia virus. J. Virol. 782478-2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Payne, S. L., J. Rausch, K. Rushlow, R. C. Montelaro, C. Issel, M. Flaherty, S. Perry, D. Sellon, and F. Fuller. 1994. Characterization of infectious molecular clones of equine infectious anaemia virus. J. Gen. Virol. 75425-429. [DOI] [PubMed] [Google Scholar]
- 57.Pelkmans, L., D. Puntener, and A. Helenius. 2002. Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science 296535-539. [DOI] [PubMed] [Google Scholar]
- 58.Picard-Maureau, M., G. Jarmy, A. Berg, A. Rethwilm, and D. Lindemann. 2003. Foamy virus envelope glycoprotein-mediated entry involves a pH-dependent fusion process. J. Virol. 774722-4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ross, S. R., J. J. Schofield, C. J. Farr, and M. Bucan. 2002. Mouse transferrin receptor 1 is the cell entry receptor for mouse mammary tumor virus. Proc. Natl. Acad. Sci. USA 9912386-12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schornberg, K., S. Matsuyama, K. Kabsch, S. Delos, A. Bouton, and J. White. 2006. Role of endosomal cathepsins in entry mediated by the Ebola virus glycoprotein. J. Virol. 804174-4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sellon, D. C., S. T. Perry, L. Coggins, and F. J. Fuller. 1992. Wild-type equine infectious anemia virus replicates in vivo predominantly in tissue macrophages, not in peripheral blood monocytes. J. Virol. 665906-5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sieczkarski, S. B., and G. R. Whittaker. 2005. Characterization of the host cell entry of filamentous influenza virus. Arch. Virol. 1501783-1796. [DOI] [PubMed] [Google Scholar]
- 63.Sieczkarski, S. B., and G. R. Whittaker. 2002. Dissecting virus entry via endocytosis. J. Gen. Virol. 831535-1545. [DOI] [PubMed] [Google Scholar]
- 64.Simmons, G., D. N. Gosalia, A. J. Rennekamp, J. D. Reeves, S. L. Diamond, and P. Bates. 2005. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc. Natl. Acad. Sci. USA 10211876-11881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Skehel, J. J., and D. C. Wiley. 2000. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu. Rev. Biochem. 69531-569. [DOI] [PubMed] [Google Scholar]
- 66.Stahlhut, M., and B. van Deurs. 2000. Identification of filamin as a novel ligand for caveolin-1: evidence for the organization of caveolin-1-associated membrane domains by the actin cytoskeleton. Mol. Biol. Cell 11325-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stantchev, T. S., I. Markovic, W. G. Telford, K. A. Clouse, and C. C. Broder. 2007. The tyrosine kinase inhibitor genistein blocks HIV-1 infection in primary human macrophages. Virus Res. 123178-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun, X., V. K. Yau, B. J. Briggs, and G. R. Whittaker. 2005. Role of clathrin-mediated endocytosis during vesicular stomatitis virus entry into host cells. Virology 33853-60. [DOI] [PubMed] [Google Scholar]
- 69.Vallée H., C. H. 1904. Sur la nature infectieuse de l'anémie du cheval. C. R. Acad. Sci. 139331-333. [Google Scholar]
- 70.van der Bliek, A. M., T. E. Redelmeier, H. Damke, E. J. Tisdale, E. M. Meyerowitz, and S. L. Schmid. 1993. Mutations in human dynamin block an intermediate stage in coated vesicle formation. J. Cell Biol. 122553-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vidricaire, G., and M. J. Tremblay. 2007. A clathrin, caveolae, and dynamin-independent endocytic pathway requiring free membrane cholesterol drives HIV-1 internalization and infection in polarized trophoblastic cells. J. Mol. Biol. 3681267-1283. [DOI] [PubMed] [Google Scholar]
- 72.Wang, L. H., K. G. Rothberg, and R. G. Anderson. 1993. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 1231107-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yonezawa, A., M. Cavrois, and W. C. Greene. 2005. Studies of Ebola virus glycoprotein-mediated entry and fusion by using pseudotyped human immunodeficiency virus type 1 virions: involvement of cytoskeletal proteins and enhancement by tumor necrosis factor alpha. J. Virol. 79918-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang, B., S. Jin, J. Jin, F. Li, and R. C. Montelaro. 2005. A tumor necrosis factor receptor family protein serves as a cellular receptor for the macrophage-tropic equine lentivirus. Proc. Natl. Acad. Sci. USA 1029918-9923. [DOI] [PMC free article] [PubMed] [Google Scholar]