Abstract
Antiviral immune defenses involve natural killer (NK) cells. We previously showed that the NK-activating receptor NKp44 is involved in the functional recognition of H1-type influenza virus strains by NK cells. In the present study, we investigated the interaction of NKp44 and the hemagglutinin of a primary influenza virus H5N1 isolate. Here we show that recombinant NKp44 recognizes H5-expressing cells and specifically interacts with soluble H5 hemagglutinin. H5-pseudotyped lentiviral particles bind to NK cells expressing NKp44. Following interaction with target cells expressing H5, pseudotyped lentiviral particles, or membrane-associated H5, NK cells show NKp44-mediated induced activity. These findings indicate that NKp44-H5 interactions induce functional NK activation.
Avian influenza is a highly contagious disease of birds that can easily reach epidemic proportions. Since 1997, influenza virus (IV) H5N1 has appeared in humans, causing severe viral pneumonia leading to acute respiratory dysfunction syndrome and carrying a high case fatality rate (9, 21). Between 2003 and October 2007, 332 human H5N1 infections have been reported to the World Health Organization. Of the 332 infected patients, 204 (61%) have died, and there has been no decline in the high mortality rate over time (22). Antiviral immune defenses involve natural killer (NK) cells (6). The imperative function of NK cells in virus defense in vivo was most concisely demonstrated in an adolescent who had no NK cells at all and was therefore susceptible to virus infections (5, 19). Natural cytotoxicity receptors (NCRs), expressed by NK cells, trigger the lysis of tumor and virus-infected cells upon interaction with surface ligands of these target cells. We and others have determined previously that H1- and H3-subtype hemagglutinins (HAs) expressed on the surfaces of IV-infected cells are involved in the functional recognition of these target cells by the human NCRs NKp44 and NKp46 (3, 4, 10, 15, 16). We further showed that IV infection in mice is lethal in the absence of murine Ncr1 (11). Recently, Owen et al. reported that alterations in HA binding properties of recent human influenza H3N2 viruses are associated with reduced NK cell lysis of infected cells (20). The pathogenicity of IV H5N1 strains in humans has been attributed mostly or partially to antigenic changes in the IV HA that render virus-specific immunity incapable of timely response to the developing infection (9, 21). Yet a successful immune response to virus involves an innate immunity arm in which NK cells play a critical role (6). Therefore, we aimed to explore the NCR-H5 interactions.
Infection with H5N1/PR8 virus enhanced lysis of infected cells by primary human NK lines.
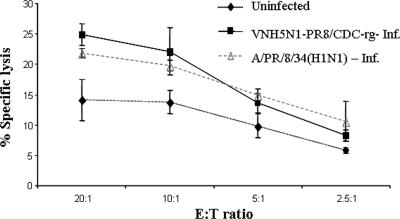
We first studied whether primary human NK lyse cells infected with the weakly pathogenic IV H5N1 strain VNH5N1-PR8/CDC-rg contain mutant H5 (with the deletion of the polybasic cleavage site motif), intact neuraminidase, and the internal genes from the A/PR/8/34 strain (23). Primary human NK lines were isolated from peripheral blood lymphocytes as described previously, and 1106mel cells were infected overnight (15). VNH5N1-PR8/CDC-rg infection of 1106mel cells enhanced NK-mediated lysis similarly to infection with A/PR/8/34 (H1N1) (Fig. 1).
FIG. 1.
Lysis of IV-infected 1106mel cells by a primary human NK line. The cytotoxic activity was assessed in a 5-h 35S release assay (15). 1106mel cells (106) were infected overnight with 200 hemagglutination units/ml (600 hemagglutination units total) and labeled with 35S. Following infection, all cells expressed membrane-associated HA as detected by antibodies to H1 or H5. NK cells were incubated with either uninfected 1106mel cells or VNH5N1-PR8/CDC-rg-infected (VNH5N1-PR8/CDC-rg-Inf.) or A/PR/8/34 (H1N1)-infected 1106mel cells at the indicated E:T ratios. Results are representative of three independent experiments performed with primary NK lines derived from different healthy donors. Bars, ± standard deviation (SD).
Cell-associated H5 and soluble H5 interact with NKp44-Ig.
We previously showed that NKp44 interacts with the H1-type IV HAs (4, 13). To study the binding of NKp44 with H5-type IV HA, we employed NKp44-immunoglobulin (Ig) (4, 13) and HA of the primary human isolate A/Cambodia/408008/05 (a clade 1 H5N1 virus with no known mutations at the sites known to be relevant for receptor binding) (18). We first tested the binding of NKp44-Ig to target cells transfected with pCDNA-synH5, encoding the full-length H5 tagged with C-terminal FLAG (18). Half of the H5-transfected 293T cells (H5-293T) expressed membrane-associated H5. As a negative control, we employed 293T cells transfected with pCDNA only (EM-293T) or exposed to a blank transfection procedure (BL-293T). The staining of the different cells with human IgG1 (hIgG1) or with NKp30-Ig, which does not interact with H1 (4), revealed no difference in the staining of H5-293T, BL-293T, and EM-293T cells (Fig. 2A). In contrast, NKp44-Ig showed a substantially higher level of binding to half of the H5-293T cells than to BL-293T and EM-293T cells (Fig. 2A). Double staining of H5-293T cells with NKp44-Ig and anti-H5 revealed that H5-positive cells manifested enhanced staining with NKp44-Ig (data not shown).
FIG. 2.
Binding of NKp44-Ig to 293T cells transfected with H5 and to soluble H5. (A) 293T cells were transiently transfected with pCDNA-synH5 (H5-293T) or with pCDNA (EM-293T) or exposed to a blank transfection procedure (BL-293T). Thirty-six hours after transfection, the cells were harvested using 5 mM EDTA in phosphate-buffered saline and stained with purified NCR-Ig proteins as follows. Three micrograms of NCR-Ig was incubated with 105 cells on ice for 2 h, followed by incubation with allophycocyanin-conjugated goat anti-human IgG Fcγ fragment-specific antibody. Cells were analyzed in the BD LSR II flow cytometer. Results are presented as primary fluorescence-activated cell sorter histograms. Values at the top left of the histograms represent the geometric mean fluorescence intensities of the populations, in arbitrary units. Percentages represent the fractions of cells included in the marker area. Results are representative of three independent experiments with duplicate staining in each experiment. (B) ELISA plates were coated with 25, 50, 100, and 200 ng of recombinant soluble H5 protein and then incubated with 100 ng of LIR1-Ig or NKp44-Ig fusion protein. The bound fusion proteins were incubated with biotin spacer-conjugated goat anti-human IgG Fcγ fragment-specific antibody. Binding was visualized with streptavidin-conjugated horseradish peroxidase. (C) ELISA plates were coated with 200, 400, 800, and 1,600 ng of NKp44-Ig or hIgG1 and then incubated with 100 ng of recombinant soluble H5. The bound H5 was detected with anti-H5N1 duck serum followed by horseradish peroxidase-conjugated rabbit anti-chicken IgG. The data in panels B and C represent optical density (OD) absorbance (415 nm). Results are representative of two independent experiments with triplicates in each experiment. The 100-ng-coating condition was repeated in six independent experiments. Bars, ± SD.
To specifically detect the binding of NKp44-Ig to H5, we used recombinant soluble H5 produced in BHK cells (18). This soluble H5 was observed also in a trimeric form (data not shown). NKp44-Ig bound to enzyme-linked immunosorbent assay (ELISA) wells coated with the H5 protein; NKp44-Ig binding was correlated with the amount of H5 coating, while the weak background binding of leukocyte Ig-like receptor 1 (LIR1)-Ig was not affected (Fig. 2B). Similar to LIR1-Ig, NKp30-Ig and hIgG1 did not bind to H5 (data not shown). We further analyzed NKp44-H5 interaction by coating ELISA wells with a titrated quantity of NKp44-Ig or hIgG1 and assaying the binding of H5. H5 manifested binding to NKp44-Ig which correlated with the quantity of NKp44 coating. In contrast, H5 did not show specific binding to hIgG1-coated ELISA wells (Fig. 2C). Studies were performed mostly with NKp44-Ig produced in CHO cells. Yet 293T cell-produced NKp44-Ig also bound recombinant H5. We previously reported that the binding of NKp44 and H1-type HAs is dependent upon the sialylation of the NKp44 glycans. We treated 293T cell-produced NKp44-Ig with neuraminidase as described in reference 4. This treatment resulted in sialic acid removal from NKp44-Ig (4); indeed, the interaction of NKp44-Ig and recombinant H5 was significantly reduced following the enzymatic removal of sialic acids from the NKp44 (data not shown). CHO cell-produced recombinant NKp46 interacts poorly with H1-type HA (3). This observation corresponds to the reported reduced activity of α(2,6)-sialyltransferases in CHO cells and the enhanced affinity of H1-type HA for Neu5Ac α(2,6)-Gal linkages. H5-type HAs have enhanced affinity for Neu5Ac α(2,3)-Gal linkages (17). In accordance, recombinant NKp44 produced in either CHO or 293T cells interacted efficiently with the H5 (Fig. 2 and data not shown). To summarize, we showed that NKp44-Ig interacts specifically with H5.
Cell-associated H5 and H5pp are functionally recognized by NK cell-expressed NKp44.
H5-pseudotyped lentiviral particles (H5pp) were shown to reproduce IV H5N1 entry into target cells and provide a safe method to study the characteristics of H5-type HA in a viral particle setup (18). We thus employed these H5pp to study the functional interactions of NK cell-expressed NKp44 and H5. We first stained NK-92 and NK92-44 cells with H5pp. NK-92 cells express nonexistent to low levels of membranal NKp44 (Fig. 3A). NK92-44 cells express membrane-associated NKp44 following the retroviral transduction of NK-92 cells with NKp44-encoding retroviral vector (8) (Fig. 3A). H5pp did bind to NK92-44 cells, as detected by staining the cells, following incubation with H5pp with duck anti-H5N1 serum (Fig. 3B). Yet similar staining of NK-92 cells with H5pp revealed that H5pp did not bind to NK-92 cells (Fig. 3B). Therefore, wild-type NKp44 expression by NK cells directly correlated with H5pp attachment to these cells.
FIG. 3.
NKp44 expression and H5pp binding to NK92-44 cells. (A) NKp44 expression by NK-92 and NK92-44 cells. Cells (105) were stained with anti-NKp44 monoclonal antibody followed by allophycocyanin-conjugated goat anti-mouse IgG2a. 2Ab, staining with allophycocyanin-conjugated goat anti-mouse IgG2a only; geoMFI, geometric mean fluorescence intensity (in arbitrary units). (B) H5pp were produced using two plasmid lentivirus systems in 293T cells. Freshly produced H5pp in the culture supernatant were incubated with 105 NK cells (NK92-44 or NK-92) on ice for 2 h, followed by incubation with anti-H5N1 duck serum and then allophycocyanin-conjugated donkey anti-chicken IgG (black-filled histograms). For control staining (gray-line open histograms), the incubation step with H5pp was omitted. Results are presented as overlays of primary fluorescence-activated cell sorter histograms and are representative of five (A) and three (B) independent experiments with duplicate staining in each experiment.
To assess whether this H5pp recognition by NK92-44 cells can result in the functional activation of the NK cells, we further tested NK cell membrane-associated CD107a expression as a marker for NK activity. Lysosome-associated membrane protein 1 (LAMP-1, or CD107a) has been described previously to be a marker of NK cell functional activity in a multiparameter flow cytometry assay. CD107a is significantly up-regulated on the surfaces of NK cells following stimulation that results in NK cell-mediated lysis of target cells and/or cytokine production. As target cells, we employed H5pp-producing cells, which consisted of 293T cells transfected with human immunodeficiency virus (HIV) backbone plasmid and with pCDNA-synH5 plasmid. These H5pp-producing 293T cells expressed the H5pp attached to their cell membranes (18). Control target cells were 293T cells transfected with HIV backbone plasmid or with pCDNA only (EM-293T).
Following 3 h of coincubation of NK cells with control targets or with H5pp-expressing 293T cells, cells were stained for membrane expression of CD107a. No substantial difference in CD107a expression in NK-92 cells incubated with different control 293T targets compared to those incubated with H5pp-producing 293T target cells was observed (Fig. 4A). Yet CD107a expression in NK92-44 cells incubated with H5pp-producing 293T target cells was considerably enhanced compared to that in NK92-44 cells incubated with the different control 293T target cells. The optimal effector-to-target cell (E:T) ratio for CD107a expression in our assays was 1:1 (1); CD107a expression in NK92-44 cells incubated with H5pp-expressing 293T target cells (at a 1:1 E:T ratio) was enhanced nearly threefold compared to that in NK92-44 cells incubated with control target cells (Fig. 4B). To summarize, CD107a-assayed NK activity was induced only when NK92-44 cells were coincubated with H5pp-expressing 293T cells. This result may be attributable to the secretion of the cytokine gamma interferon (IFN-γ) or lytic activity or both, yet CD107a was also expressed on a large subset of NK cells that did not secrete cytokine following stimulation (1). In this experimental system, IFN-γ was not secreted from H5pp-producing 293T cell-activated NK92-44 cells (data not shown). The inhibition of IFN-γ secretion from NK cells when the cells were incubated with 293T target cells is attributed to HLA class I expression by the 293T cells. Indeed, major histocompatibility complex-mediated differential regulation of natural cytotoxicity and IFN-γ release has been reported previously (14).
FIG. 4.
Assessment of NKp44-mediated NK activation. (A and B) Enhancement of NK membrane-associated CD107a expression following incubation with target cell-attached H5pp. Target cells were 293T cells expressing H5pp attached to their cell membranes (H5pp-293T), control 293T cells transfected with HIV backbone plasmid (BB-293T), and control EM-293T cells. Targets were incubated at the indicated E:T ratios with NK-92 (A) and NK92-44 (B) cells. After 3 h, the percentages of membrane-associated CD107a expression were detected by an anti-human CD107a monoclonal antibody followed by allophycocyanin-conjugated goat anti-mouse IgG1 Fcγ fragment-specific antibody. Cells were analyzed in the BD LSR II flow cytometer. (C) Induction of NKp44-mediated IFN-γ secretion upon coincubation of H5-expressing target cells and NK cells. Vero-E6 cells were transiently transfected with pCDNA-synH5 (H5-Vero). EM-Vero and BL-Vero cells were used as control target cells. Thirty-six hours posttransfection, target cells (104 per well in a 96-well plate) were coincubated with effector NK92-44 or NK-92 cells (2 × 104 per well) in a final volume of 200 μl of cell culture medium per well for 24 h. Then 100 μl of the culture medium was assayed for the presence of human IFN-γ. Results are representative of three independent experiments with duplicate or triplicate readings in each experiment. Bars, ± SD.
To further test H5-induced NKp44-mediated IFN-γ secretion, we employed a different experimental system in which Vero-E6 cells were used as targets for NK-92 and NK92-44 cells. H5-transfected Vero-E6 (H5-Vero) cells expressed membrane-associated H5. As negative controls, we used Vero-E6 cells transfected with empty vector (EM-Vero) and Vero-E6 cells exposed to a blank transfection procedure (BL-Vero). Different target Vero cells were coincubated with NK-92 or NK92-44 cells for 24 h, and IFN-γ levels in supernatants were assayed. Vigorous IFN-γ secretion into the supernatant was observed only when NK92-44 cells were coincubated with H5-expressing Vero targets (Fig. 4C). The assay for IFN-γ secretion into the supernatant was negative when NK92-44 cells were coincubated with control EM-Vero or BL-Vero cells. No substantial IFN-γ secretion was observed when NK-92 cells were coincubated with the different target Vero cells (Fig. 4C).
Conclusions.
The human pathogenicity of the H5N1 strain motivated us to explore NCR-HA interactions for the H5-type HA. We studied attenuated H5N1 and the HA of the primary human isolate A/Cambodia/408008/05 (H5N1). We concluded that the enhanced virulence of the influenza A virus H5N1 strain could not be attributed to changes in the functional interaction of NKp44 and H5-type HA. The tumoral and viral ligands recognized by NCRs are only partially defined (2, 7, 12, 13, 15, 24). Yet the functional interaction of NKp44 and NKp46 with different types of IV HAs (H1, H3, and H5) is well-defined (present study and references 3, 4, 10, 11, 15, and 16). This finding suggests a wider definition of the classical concept of pathogen recognition by innate immune receptors involving pathogen-associated molecular patterns. The NKp44- and NKp46-HA interaction suggests an innate recognition mechanism based on a pathogen-associated function pattern.
Acknowledgments
We thank all the partners of the DENFRAME program (EC-FP6) for their fruitful discussions. We thank Kerry S. Campbell (Fox Chase Cancer Center, Philadelphia, PA) for the NK92-44 cells. We also thank Gopesh Srivastava (University of Hong Kong) for useful discussions and technical support.
This study was supported by grants from the Israeli Ministry of Health, by the Research Fund for the Control of Infectious Disease (grant no. 07060042) from the Hong Kong government, and by Small Project Funding grant no. 10208008.25399.99816.323.01 from the University of Hong Kong.
Footnotes
Published ahead of print on 12 December 2007.
REFERENCES
- 1.Alter, G., J. M. Malenfant, and M. Altfeld. 2004. CD107a as a functional marker for the identification of natural killer cell activity. J. Immunol. Methods 29415-22. [DOI] [PubMed] [Google Scholar]
- 2.Arnon, T. I., H. Achdout, O. Levi, G. Markel, N. Saleh, G. Katz, R. Gazit, T. Gonen-Gross, J. Hanna, E. Nahari, A. Porgador, A. Honigman, B. Plachter, D. Mevorach, D. G. Wolf, and O. Mandelboim. 2005. Inhibition of the NKp30 activating receptor by pp65 of human cytomegalovirus. Nat. Immunol. 6515-523. [DOI] [PubMed] [Google Scholar]
- 3.Arnon, T. I., H. Achdout, N. Lieberman, R. Gazit, T. Gonen-Gross, G. Katz, A. Bar-Ilan, N. Bloushtain, M. Lev, A. Joseph, E. Kedar, A. Porgador, and O. Mandelboim. 2004. The mechanisms controlling the recognition of tumor- and virus-infected cells by NKp46. Blood 103664-672. [DOI] [PubMed] [Google Scholar]
- 4.Arnon, T. I., M. Lev, G. Katz, Y. Chernobrov, A. Porgador, and O. Mandelboim. 2001. Recognition of viral hemagglutinins by NKp44 but not by NKp30. Eur. J. Immunol. 312680-2689. [DOI] [PubMed] [Google Scholar]
- 5.Biron, C. A., K. S. Byron, and J. L. Sullivan. 1989. Severe herpesvirus infections in an adolescent without natural killer cells. N. Engl. J. Med. 3201731-1735. [DOI] [PubMed] [Google Scholar]
- 6.Biron, C. A., K. B. Nguyen, G. C. Pien, L. P. Cousens, and T. P. Salazar-Mather. 1999. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 17189-220. [DOI] [PubMed] [Google Scholar]
- 7.Bloushtain, N., U. Qimron, A. Bar-Ilan, O. Hershkovitz, R. Gazit, E. Fima, M. Korc, I. Vlodavsky, N. V. Bovin, and A. Porgador. 2004. Membrane-associated heparan sulfate proteoglycans are involved in the recognition of cellular targets by NKp30 and NKp46. J. Immunol. 1732392-2401. [DOI] [PubMed] [Google Scholar]
- 8.Campbell, K. S., S. Yusa, A. Kikuchi-Maki, and T. L. Catina. 2004. NKp44 triggers NK cell activation through DAP12 association that is not influenced by a putative cytoplasmic inhibitory sequence. J. Immunol. 172899-906. [DOI] [PubMed] [Google Scholar]
- 9.Claas, E. C., A. D. Osterhaus, R. van Beek, J. C. De Jong, G. F. Rimmelzwaan, D. A. Senne, S. Krauss, K. F. Shortridge, and R. G. Webster. 1998. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet 351472-477. [DOI] [PubMed] [Google Scholar]
- 10.Draghi, M., A. Pashine, B. Sanjanwala, K. Gendzekhadze, C. Cantoni, D. Cosman, A. Moretta, N. M. Valiante, and P. Parham. 2007. NKp46 and NKG2D recognition of infected dendritic cells is necessary for NK cell activation in the human response to influenza infection. J. Immunol. 1782688-2698. [DOI] [PubMed] [Google Scholar]
- 11.Gazit, R., R. Gruda, M. Elboim, T. I. Arnon, G. Katz, H. Achdout, J. Hanna, U. Qimron, G. Landau, E. Greenbaum, Z. Zakay-Rones, A. Porgador, and O. Mandelboim. 2006. Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nat. Immunol. 7517-523. [DOI] [PubMed] [Google Scholar]
- 12.Hershkovitz, O., M. Jarahian, A. Zilka, A. Bar-Ilan, G. Landau, S. Jivov, Y. Tekoah, R. Glicklis, J. T. Gallagher, S. C. Hoffmann, H. Zer, O. Mandelboim, C. Watzl, F. Momburg, and A. Porgador. 15 November 2007, posting date. Altered glycosylation of recombinant NKp30 hampers binding to heparan sulfate: a lesson for the use of recombinant immuno-receptors as an immunological tool. Glycobiology. doi: 10.1093/glycob/cwm125. [DOI] [PubMed]
- 13.Hershkovitz, O., S. Jivov, N. Bloushtain, A. Zilka, G. Landau, A. Bar-Ilan, R. G. Lichtenstein, K. S. Campbell, T. H. Kuppevelt, and A. Porgador. 2007. Characterization of the recognition of tumor cells by the natural cytotoxicity receptor, NKp44. Biochemistry 467426-7436. [DOI] [PubMed] [Google Scholar]
- 14.Kurago, Z. B., C. T. Lutz, K. D. Smith, and M. Colonna. 1998. NK cell natural cytotoxicity and IFN-gamma production are not always coordinately regulated: engagement of DX9 KIR+ NK cells by HLA-B7 variants and target cells. J. Immunol. 1601573-1580. [PubMed] [Google Scholar]
- 15.Mandelboim, O., N. Lieberman, M. Lev, L. Paul, T. I. Arnon, Y. Bushkin, D. M. Davis, J. L. Strominger, J. W. Yewdell, and A. Porgador. 2001. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature 4091055-1060. [DOI] [PubMed] [Google Scholar]
- 16.Mandelboim, O., and A. Porgador. 2001. NKp46. Int. J. Biochem. Cell Biol. 331147-1150. [DOI] [PubMed] [Google Scholar]
- 17.Matrosovich, M. N., T. Y. Matrosovich, T. Gray, N. A. Roberts, and H. D. Klenk. 2004. Human and avian influenza viruses target different cell types in cultures of human airway epithelium. Proc. Natl. Acad. Sci. USA 1014620-4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nefkens, I., J. M. Garcia, C. S. Ling, N. Lagarde, J. Nicholls, D. J. Tang, M. Peiris, P. Buchy, and R. Altmeyer. 2007. Hemagglutinin pseudotyped lentiviral particles: characterization of a new method for avian H5N1 influenza sero-diagnosis. J. Clin. Virol. 3927-33. [DOI] [PubMed] [Google Scholar]
- 19.Orange, J. S. 2002. Human natural killer cell deficiencies and susceptibility to infection. Microbes Infect. 41545-1558. [DOI] [PubMed] [Google Scholar]
- 20.Owen, R. E., E. Yamada, C. I. Thompson, L. J. Phillipson, C. Thompson, E. Taylor, M. Zambon, H. M. Osborn, W. S. Barclay, and P. Borrow. 2007. Alterations in receptor binding properties of recent human influenza H3N2 viruses are associated with reduced natural killer cell lysis of infected cells. J. Virol. 8111170-11178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park, A. W., and K. Glass. 2007. Dynamic patterns of avian and human influenza in east and southeast Asia. Lancet Infect. Dis. 7543-548. [DOI] [PubMed] [Google Scholar]
- 22.WHO. 25 October 2007, posting date. Cumulative number of confirmed human cases of avian influenza A/(H5N1) reported to WHO. World Health Organization, Geneva, Switzerland. http://www.who.int/csr/disease/avian_influenza/country/cases_table_2007_10_25/en/index.html
- 23.World Health Organization Global Influenza Program Surveillance Network. 2005. Evolution of H5N1 avian influenza viruses in Asia. Emerg. Infect. Dis. 111515-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zilka, A., G. Landau, O. Hershkovitz, N. Bloushtain, A. Bar-Ilan, F. Benchetrit, E. Fima, T. H. van Kuppevelt, J. T. Gallagher, S. Elgavish, and A. Porgador. 2005. Characterization of the heparin/heparan sulfate binding site of the natural cytotoxicity receptor NKp46. Biochemistry 4414477-14485. [DOI] [PubMed] [Google Scholar]