Abstract
Kaposi's sarcoma (KS), a vascular tumor associated with human immunodeficiency virus type 1 infection, is characterized by spindle-shaped endothelial cells, inflammatory cells, cytokines, growth and angiogenic factors, and angiogenesis. KS spindle cells are believed to be of the lymphatic endothelial cell (LEC) type. Kaposi's sarcoma-associated herpesvirus (KSHV, or human herpesvirus 8) is etiologically linked to KS, and in vitro KSHV infection of primary human dermal microvascular endothelial cells (HMVEC-d) is characterized by the induction of preexisting host signal cascades, sustained expression of latency-associated genes, transient expression of a limited number of lytic genes, sustained induction of NF-κB and several cytokines, and growth and angiogenic factors. KSHV induced robust vascular endothelial growth factor A (VEGF-A) and VEGF-C gene expression as early as 30 min postinfection (p.i.) in serum-starved HMVEC-d, which was sustained throughout the observation period of 72 h p.i. Significant amounts of VEGF-A and -C were also detected in the culture supernatant of infected cells. VEGF-A and -C were also induced by UV-inactivated KSHV and envelope glycoprotein gpK8.1A, thus suggesting a role for virus entry stages in the early induction of VEGF and requirement of KSHV viral gene expression for sustained induction. Exogenous addition of VEGF-A and -C increased KSHV DNA entry into target cells and moderately increased latent ORF73 and lytic ORF50 promoter activation and gene expression. KSHV infection also induced the expression of lymphatic markers Prox-1 and podoplanin as early as 8 h p.i., and a paracrine effect was seen in the neighboring uninfected cells. Similar observations were also made in the pure blood endothelial cell (BEC)-TIME cells, thus suggesting that commitment to the LEC phenotype is induced early during KSHV infection of blood endothelial cells. Treatment with VEGF-C alone also induced Prox-1 expression in the BEC-TIME cells. Collectively, these studies show that the in vitro microenvironments of KSHV-infected endothelial cells are enriched, with VEGF-A and -C molecules playing key roles in KSHV biology, such as increased infection and gene expression, as well as in angiogenesis and lymphangiogenesis, thus recapitulating the microenvironment of early KS lesions.
Kaposi's sarcoma (KS) is an AIDS-defining vascular tumor, and the pathogenesis of KS is under vigorous study. In the early stages, KS is characterized by inflammatory cell filtration, presence of cytokines and growth and angiogenic factors, endothelial cell activation, and angiogenesis. This is followed by the appearance of typical spindle-shaped cells that represent a heterogeneous population dominated by activated endothelial cells mixed with macrophages and dendritic cells. In advancing lesions, spindle cells tend to become the predominant cell type, and there is prominent angiogenesis (26, 33, 61). Available in vivo and in vitro evidence indicates that KS probably develops from nontumor cells (24, 44) that become characteristically “spindle”-shaped and induce angiogenesis under the influence of a variety of factors, including interleukin-1 (IL-1), IL-6, gamma interferon, granulocyte-macrophage colony-stimulating factor, tumor necrosis factor alpha, basic fibroblast growth factor (bFGF), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), chemokines, and transforming growth factor β (TGF-β) (23). It is believed that a cell clone probably assumes neoplastic features during the course of development of KS, followed by genotypic alterations causing KS hyperplastic lesions and transformation into sarcomas (4, 7, 42).
KS-associated herpesvirus (KSHV, also called human herpesvirus 8), first identified in an AIDS-KS lesion, is etiologically associated with the four epidemiologically distinct forms of KS, primary effusion lymphoma (PEL), and some forms of multicentric Castleman's disease (72). KSHV encodes more than 90 open reading frames (ORFs), which are designated as ORFs 4 to 75 by their homology to herpesvirus saimiri ORFs, and many of these KSHV-encoded proteins are homologs of host proteins (53, 67). These homologs include latency-associated proteins K13 (v-FLICE inhibitory protein) and ORF72 (v-cyclin D), as well as lytic cycle proteins such as ORF16 (vBcl-2), K2 (viral IL-6 [vIL-6]), K4 (viral macrophage inhibitory protein II), K3 and K5 (immunomodulatory proteins 1 and 2), K6 (viral macrophage inflammatory protein 1A), K7 (antiapoptotic protein), K9 (viral interferon regulatory factor [vIRF]), K11.1 (vIRF2), K14 (vOX-2), and ORF74 (viral G protein-coupled receptor). These viral proteins are believed to play roles in evading host intrinsic, innate, and adaptive immune defense mechanisms, blocking apoptosis and the induction of neoplasia (53, 67, 72).
In vivo, KSHV DNA and transcripts have been detected in human B cells, macrophages, keratinocytes, endothelial cells, and epithelial cells (11, 20, 72). In vitro, KSHV infects a variety of human cells, such as human B, macrophage, endothelial, fibroblast, keratinocyte, and epithelial cells (1-3, 10, 11, 19, 40, 63). In contrast to members of the alpha- and betaherpesviruses, which initiate the lytic cycle soon after infection, the γ2-KSHV infection of cultured cells results only in the establishment of latency (64). Infection of human dermal microvascular endothelial cells (HMVEC-d) and human foreskin fibroblast cells (HFF) is characterized by the expression of latency-associated ORF73, ORF72, and K13 genes as well as transient expression of a very limited number of early lytic genes, such as lytic cycle switch protein ORF50, K5, K8, and v-IRF2 (36, 38). However, infected cells do not support serial propagation of KSHV, and the viral genome is lost during successive passages (29, 63).
The roles of KSHV genes and the potential interplay between viral and host genes in endothelial cell transformation and establishment of KS angioproliferative lesions are under study. Angiogenesis is the outgrowth of new capillaries from preexisting vessels, and it is essential for embryonic development, organ formation, tissue regeneration, and remodeling (14, 27). Among angiogenic factors, VEGF-A is the best-characterized positive regulator, with its distinct specificity for vascular endothelial cells. VEGF-A is a proangiogenic molecule and has been reported to play a crucial role in the development of KSHV-associated diseases by acting as a mediator of angiogenesis and vascular permeability, factors that are central to the development of KS and PEL, and may potentially be involved in multicentric Castleman's disease. VEGF-A stimulates endothelial cell proliferation, migration, differentiation, tube formation, increased vascular permeability, and maintenance of vascular integrity (12, 14, 25, 50). VEGF-A is a 46-kDa protein that dissociates on reduction into two apparently identical 23-kDa subunits. Alternative splicing of VEGF-A mRNA transcripts results in five different isoforms: VEGF121, VEGF145, VEGF165, VEGF189, and VEGF206 (22, 54). Three of these isoforms of VEGF (VEGF121, VEGF145, and VEGF165) are secreted by a broad variety of cells, including vascular smooth muscle cells, monocytes, mesangial cells, endothelial cells, and megakaryocytes (48, 65, 82). The angiogenic responses induced by VEGF-A are mediated by two structurally related tyrosine kinase receptors, VEGFR-1 (Flt-1) and VEGFR-2 (KDR/Flk-1), both of which are expressed primarily on vascular cells of the endothelial lineage (18, 50, 77).
VEGF-A is the prototype of an enlarging family of growth peptides that includes four other structurally related members. These are placenta growth factor (PlGF), VEGF-B, VEGF-C, and c-fos-induced growth factor (VEGF-D) (25, 55, 66). They show a similarity in their primary sequences, especially in the PDGF-like domain containing the conserved eight cysteine residues. PlGF is predominantly expressed in the placenta and binds to VEGFR-1, but not to VEGFR-2. VEGF-B has been identified as a weak mitogen for endothelial cells, and robust expression is particularly detected in skeletal and cardiac muscle tissues. Both PlGF and VEGF-B modulate VEGF activity via formation of heterodimers. VEGF-C is a ligand for two receptors, VEGFR-2 and VEGFR-3 (Flt-4). The latter differs from VEGFR-1 and VEGFR-2 by being predominantly expressed in lymphatic endothelial cells in adult tissues but at low levels in most other vascular endothelial cells (34, 39). VEGF-C recently has been characterized to be a fairly selective growth factor for lymphatic vessels (32, 56). In addition, proteolytic processing is involved in the regulation of VEGF-C activity. VEGF-C also is shown to be involved in the regulation of physiological and pathological blood vessel growth (14).
Several immunohistochemical studies have shown that the KS spindle cells are of endothelial origin, but it was not clear until recently whether these spindle cells are the blood endothelial cell (BEC) or lymphatic endothelial cell (LEC) type (60, 83). Based on the gene expression microarray profiles and expression of several lymphatic lineage-specific proteins, including VEGFR-3 and podoplanin, it has been proposed that KS spindle cells are of LEC origin (30). KSHV infection of BECs induces the expression of the homeobox gene Prox-1, a master gene that controls lymphatic vessel development and differentiation, and its expression leads to lymphatic endothelial reprogramming of blood endothelial cells (15, 30, 81). The lymphatic endothelium controls the fluid and lymphocyte uptake into lymph nodes and is also architecturally different from the blood vascular endothelium. Lymphangiogenesis is a different process from blood or hemangiogenesis, utilizing VEGF-C and -D and their receptor, VEGFR-3 (5, 15, 58). The lymphatic vessels provide a major pathway for tumor metastasis in many types of cancers (58).
KSHV-induced up-regulation of angiogenesis factors may potentially be involved in KS development and progression. Multiple KSHV lytic proteins, such as K1, vGPCR, and vIL-6, were shown to induce VEGF-A and have been postulated to play a role in KSHV pathogenesis (6, 7, 75, 82). However, since less than 1% of KS cells (inflammatory B cells/monocytes) express lytic cycle proteins (20, 68), we explored the other possibilities. We have shown that during in vitro infection of endothelial cells, KSHV induces the host cell preexisting FAK, Src, phosphatidylinositol 3-kinase, Rho-GTPases, Dia-2, Ezrin, protein kinase C ζ, extracellular signal-regulated kinase 1/2, and NF-κB signal pathways that are critical for virus entry, cytoplasmic transport, nuclear delivery of viral DNA, and initiation of viral gene expression. In addition, we also observed the reprogramming of host transcriptional machinery regulating a variety of cellular processes, including apoptosis, transcription, cell cycle regulation, signaling, the inflammatory response, and angiogenesis. Notable among the host cell genes modulated by KSHV is the strong induction of several proinflammatory cytokines and growth factors, such as IL-1β, IL-6, IL-8, prostaglandin E2 (PGE2), GRO, oncostatin M, bFGF, VEGF-A, VEGF-C, matrix metalloproteinases, pre-B-cell colony-enhancing factor, macrophage-specific colony-stimulating factor 1, etc. (52). This resemblance between the cytokines and growth and angiogenic factors detected during in vitro KSHV infection with that of KS lesions suggests that the early stages of KS lesion formation may represent widespread primary infection of endothelial cells occurring in virus reactivation during immunosuppression or immune imbalance.
Since VEGF is a key element in angiogenesis, in this report we examined VEGF-A and -C expression during de novo infection of primary endothelial cells and studied the implications in viral gene expression/promoter induction and lymphangiogenesis.
MATERIALS AND METHODS
Cells.
HMVEC-d cells (CC-2543; Clonetics, Walkersville, MD) and TIME cells (tert-immortalized dermal microvascular endothelial cells; a gift from D. Ganem, UCSF, San Francisco) were grown in endothelial basal medium 2 (EBM2) with growth factors (Clonetics) (40, 72). HEK 293 (human embryonic kidney) cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco BRL, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS), 2 mM l-glutamine, and antibiotics. KSHV-carrying human B cells (BCBL-1) were cultured in RPMI 1640 medium (Gibco) with 10% heat-inactivated FBS (HyClone, Logan, Utah), 2 mM l-glutamine, and antibiotics (1-3).
Antibodies, reagents, and growth factors.
Polyclonal rabbit immunoglobulin G antibodies against full-length ORF73 expressed as a glutathione S-transferase fusion protein in baculovirus (49) were used, and rat monoclonal anti-LANA-1 antibody was obtained from Advanced Biotechnologies Inc. (ABI; MD). Mouse monoclonal antibodies against human VEGF-A and goat polyclonal antibodies against human VEGF-C were from Santa Cruz Biotechnology, Inc., Santa Cruz, CA. Mouse monoclonal antibodies against human CD31, Prox-1, and podoplanin were obtained from AngioBio Co., Del Mar, CA. Antiluciferase polyclonal antibody raised in goats against recombinant firefly luciferase (Photinus pyralis) was purchased from Promega, Madison, WI. β-Actin (clone AC-40) was from Sigma, St. Louis, MO. Anti-goat, anti-rabbit, and anti-mouse antibodies linked to horseradish peroxidase, Alexa 488, or Alexa 594 and anti-fade 4′,6′-diamidino-2-phenylindole (DAPI) were purchased from KPL Inc., Gaithersburg, MD, or Molecular Probes, Eugene, OR. Bovine serum albumin (BSA), tetradecanoyl phorbol acetate, Triton X-100, heparin, and paraformaldehyde were from Sigma, St. Louis, MO. Recombinant human VEGF-A165, VEGF-C, and human epidermal growth factor (EGF) were from R&D Systems, Minneapolis, MN.
Virus.
Induction of the KSHV lytic cycle in BCBL-1 cells, supernatant collection, and virus purification procedures were described previously (36), and purity was assessed according to general guidelines established in our laboratory (2, 36, 51). KSHV DNA was extracted from the purified virus, and copy numbers were quantitated by real-time DNA PCR using primers amplifying the KSHV ORF73 gene (36, 72). To prepare replication-defective virus, KSHV was irradiated with UV light (365 nm) for 20 min at a 10-cm distance. KSHV DNA was extracted from the UV-irradiated virus, and the copy numbers were quantified by real-time DNA PCR as described previously (36).
Preparation of DNA and RNA.
Total DNAs from the viral stocks were prepared using a DNeasy tissue kit (Qiagen, Inc., Valencia, CA), and total RNA was isolated from infected or uninfected cells using an RNeasy kit as described previously (36, 71).
Real-time DNA PCR.
Real-time PCR was performed to quantify the internalized viral DNA in the infected cells as described previously (36).
Real-time RT-PCR.
To monitor the induction of VEGF-A by KSHV, a real-time reverse transcription-PCR (RT-PCR) assay was done as described previously (55). VEGF-A mRNA was amplified in the presence of TaqMan probe (5′-6-carboxyfluorescein-AGTTCATGGATGTCTATCAGCGCAGCT-6-carboxytetramethylrhodamine-3′) and VEGF-A-specific primers (forward, 5′-CTTGCCTTGCTGCTCTACC-3′; reverse, 5′-CACACAGGATGGCTTGAAG-3′). The primers for VEGF-A span exon 1 to exon 3 and thus detect all isoforms of VEGF-A. The following VEGF-C-specific primers were designed and used (forward, 5′AGATGCCTGGCTCAGGAAGA3′; reverse 5′ TGTCATGGAATCCATCTGTTGA 3; TaqMan probe, 5′-6-carboxyfluorescein-TCATCTCCAGCATCCGAGGAAAA-6-carboxytetramethylrhodamine-3′). The RT-PCR cycle parameters were as follows: 50°C for 2 min, 60°C for 30 min, and 95°C for 5 min, followed by 44 cycles at 95°C for 15 s and 60°C for 1 min.
Total RNA was isolated from uninfected and KSHV-infected HMVEC-d or TIME cells by using the RNeasy kit. RNA was normalized to contain 250 ng/5 μl, and different dilutions of known amounts (106, 105, 104, 103, 102, and 101 copies) of in vitro-transcribed human VEGF-A or VEGF-C transcripts were used for copy estimation. The human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was used as the internal housekeeping gene control. The relative copy numbers of the transcripts were calculated from the standard graph plotted using the threshold concentration (CT) values for different dilutions of in vitro-transcribed transcripts. These values were normalized to each other using the values of the GAPDH control reactions. The number of GAPDH transcripts in each sample was calculated from the standard graph plotted using copy numbers from different dilutions of known concentrations of human RNA and Taqman GAPDH reagents (Applied Biosystems, Foster City, CA). The ORF50 and ORF73 transcripts were detected by real-time RT-PCR using gene-specific real-time primers and specific TaqMan probes (Applied Biosystems) as described previously (36).
Prox-1 expression was detected by real-time RT-PCR using Sybr green chemistry. Two micrograms of total RNA was treated with DNase for 1 h at 37°C and then reverse transcribed into cDNA by using a mix of oligo(dT)12-18 and random primer with the SuperScript First-Strand synthesis system for RT-PCR (Invitrogen, Carlsbad, CA). cDNA was used as a template with primers specific for Prox-1 (forward, 5′-TGTTCACCAGCACACCCG-3′; reverse, 5′-ACTCGGACTTTATGAGCGACAAG-3′). Primers for GAPDH (forward, 5′-GGAAGGTGAAGGTCGGAGTC-3′; reverse, 5′-CTGGAAGATGGTGATGGGATTTC-3′) were used as an internal control. PCR was performed using the Applied Biosystems 7500 real-time PCR system with Sybr green PCR master mix (Applied Biosystems). The standard amplification program included 40 cycles of two steps each, comprised of heating to 95°C and 60°C. Fluorescent product was detected at the last step of each cycle. The final mRNA levels of the genes studied were normalized using the comparative CT method (41).
Vascular endothelial growth factor A and C protein quantitation.
The levels of VEGF-A and -C in the culture supernatant of uninfected or KSHV-infected HMVEC-d cells were quantitated using QuantiGlo human VEGF-A and VEGF-C enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN). Briefly, HMVEC-d cells, at 80 to 90% confluence, were serum starved for 8 h and infected with KSHV at a multiplicity of infection (MOI) of 10. Supernatants were collected at various times postinfection, spun at 1,000 rpm for 10 min at 4°C to remove the particulates, and stored at −80°C until use. Total soluble protein was quantified by the bicinchoninic acid protein assay (Pierce, Rockford, IL) to normalize ELISA results.
KSHV gB and gpK8.1A.
The generation and purification of baculovirus-expressed purified ΔTMgB and ΔTMgpK8.1A have been described in detail previously (72, 79, 80). Recombinant protein purifications were carried out in buffers prepared with lipopolysaccharide (LPS)-free water, and stock preparations of proteins were monitored for endotoxin contamination by standard Limulus assay (Limulus amebocyte lysate endochrome; Charles River Endosafe, Charleston, SC) as recommended by the manufacturer.
Immunofluorescence assay (IFA).
Confluent HMVEC-d cells in eight-well chamber slides (Nalge Nunc International, Naperville, IL) were either uninfected or infected with KSHV at an MOI of 10 DNA copies per cell at 37°C for 72 h. Immunofluorescent antibody detection of KSHV ORF73, Prox-1, podoplanin, or infection-induced VEGF-A and -C protein expression in target cells was done after fixing cells with 4% paraformaldehyde for 10 min at room temperature, permeabilizing with 0.4% Triton X-100 for 10 min at room temperature, and staining with primary antibody overnight at 4°C. Target cells were washed and incubated with an appropriate dilution of secondary antibodies for 1 h at room temperature. Nuclei were visualized by using DAPI (Molecular Probes, Eugene, OR) as a counterstain. Stained cells were washed and viewed with appropriate filters under a fluorescence microscope with the Nikon Metamorph digital imaging system.
Flow cytometry.
HMVEC-d and TIME cells were infected with KSHV (MOI, 10) and harvested for flow cytometry at the indicated times. Cells were detached from the plate with 0.25% trypsin-EDTA, and viability was assessed by trypan blue exclusion. For podoplanin, samples were washed twice with 1× phosphate-buffered saline and blocked for 30 min with 3% BSA in phosphate-buffered saline, and the cell surface staining was carried out for 1 h at 4°C. Nuclear staining to identify KSHV-infected cells was performed using a rat monoclonal antibody to the KSHV LANA-1 protein (ABI) using BD Pharmingen Perm/Wash buffer. Flow cytometry was performed with a FACSCalibur flow cytometer and analyzed with CellQuest Pro software (Becton Dickinson, Bedford, MA) in the Rosalind Franklin University of Medicine and Science flow cytometry core facility. Prior to analysis, all samples were gated by forward and side scatter to eliminate dead cells.
Plasmids.
The 774-bp full-length KSHV ORF73 promoter (70, 72) was cloned in a pGL3 vector (Promega, Madison, WI) to create the pGL3.6-Luciferase reporter construct. The p2500-Luc construct, a 2,500-bp ORF50 promoter-luciferase reporter cloned in the pcDNA3.1 vector (Invitrogen) (72, 86) was a kind gift from George Miller, Yale University School of Medicine, New Haven, CT.
Luciferase reporter assays.
The effects of VEGF-A and VEGF-C on the ORF73 promoter (pORF73-Luc) and ORF50 promoter (pORF50-Luc) were measured using the dual luciferase kit according to the manufacturer's protocol (Promega). Briefly, 293 cells (1 × 105) seeded in a 24-well tissue culture plate were fed with antibiotic-free, low-serum (0.5% FBS) DMEM for 12 to 18 h before transfection. Low-serum conditions were maintained throughout the experiment. Transfection of 293 cells was performed with 0.5 μg of ORF73 or ORF50 promoter luciferase constructs and with 50 ng of Renilla luciferase as a transfection efficiency control and using Lipofectamine 2000 (Invitrogen). After 24 h, cells were either uninfected or infected with KSHV at an MOI of 10 or treated with exogenous VEGF-A and -C (100 ng/ml and 250 ng/ml) for 4 h, 8 h, and 24 h. Cells were harvested at the indicated time points after KSHV infection in 1× passive lysis buffer, and luciferase assays were carried out in triplicate following the manufacturer's procedure in a Synergy HT multidetection microplate reader (Bio-Tek Instruments, Inc., VT). Firefly luciferase activities for each time point were normalized to those of parallel controls for each time point. Alterations in promoter activities of ORF73 or ORF50 as a result of KSHV infection or VEGF-A or VEGF-C treatment were determined after normalization, using the Renilla luciferase activity as a control.
Western blotting for LANA-1.
The effects of VEGF-A and VEGF-C on the ORF73 promoter (pORF73-Luc) and ORF50 promoter (pORF50-Luc) were then confirmed by the detection of LANA-1 by Western blotting. HMVEC-d cells (80 to 90% confluence) were serum starved for 8 h, either untreated or treated with VEGF-A or VEGF-C (250 ng/ml) for 1 h at 37°C, and infected with KSHV at an MOI of 10. Expression of ORF73 protein was monitored at the indicated time points. Cells were harvested at the indicated time points in radioimmunoprecipitation assay lysis buffer with protease inhibitor cocktail. Cellular debris was removed by centrifugation at 13,000 × g for 20 min at 4°C, and equal amounts of protein samples were resolved by sodium dodecyl sulfate-7.5% polyacrylamide gel electrophoresis (SDS-PAGE), subjected to Western blotting, and reacted with rabbit polyclonal antibody against KSHV LANA-1. To confirm equal protein loading, blots were also reacted with antibodies against human β-actin. Secondary antibodies conjugated either to horseradish peroxidase or to alkaline phosphatase were used for detection. Immunoreactive bands were developed by either the enhanced chemiluminescence reaction (NEN Life Sciences Products, Boston, MA) or CDP-Star (Roche Diagnostics Corp., Indianapolis, IN) and quantified by following standard protocols (72).
Western blotting for luciferase.
The effects of VEGF-A and VEGF-C on the ORF73 promoter (pORF73-Luc) and ORF50 promoter (pORF50-Luc) were also confirmed by the detection of luciferase by Western blot assay. Briefly, 293 cells (4 × 105) seeded in a six-well tissue culture plate were fed with antibiotic-free, low-serum (0.5% FBS) DMEM for 12 to 18 h before transfection. Low-serum conditions were maintained throughout the experiment. Transfection of 293 cells was performed with 1.0 μg of ORF73 or ORF50 promoter luciferase constructs and with 100 ng of pcDNA-GFP as a transfection efficiency control, using Lipofectamine 2000 (Invitrogen). After 24 h, cells were either untreated or treated with exogenous VEGF-A and -C (250 ng/ml) for 4 h, 8 h, and 24 h. Cells were harvested at the indicated time points in radioimmunoprecipitation assay lysis buffer with protease inhibitor cocktail. Total cell lysates prepared were resolved on 10% SDS-polyacrylamide gels, and the resolved proteins were transferred onto a nitrocellulose membrane and immunoblotted with antiluciferase primary antibodies. Transfection efficiency was detected by using anti-green fluorescent protein (GFP) mouse monoclonal antibodies. To confirm equal protein loading, blots were also reacted with antibodies against human β-actin. Secondary antibodies conjugated either to horseradish peroxidase or to alkaline phosphatase were used for detection. Immunoreactive bands were developed by either an enhanced chemiluminescence reaction (NEN Life Sciences Products, Boston, MA) or CDP-Star (Roche Diagnostics Corp., Indianapolis, IN) and quantified by following standard protocols (72).
RESULTS
In vitro infection of HMVEC-d cells by KSHV induces VEGF-A and VEGF-C gene expression.
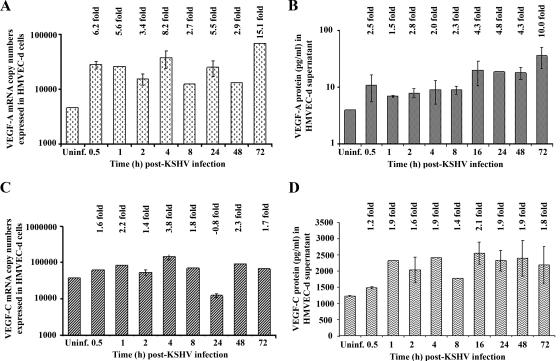
We have previously analyzed the transcriptional reprogramming of 22,283 host genes from KSHV-infected HMVEC-d and HFF cells at 2 and 4 h p.i. With a criterion of twofold gene induction as significant, transcriptional modulation of several genes was detected, including VEGF-A and VEGF-C genes. In HMVEC-d cells, we observed 2.59- and 2.62-fold up-regulation of VEGF-A RNA at 2 h and 4 h p.i., respectively, and 3.4- and 2.15-fold induction of the VEGF-C gene was observed at 2 h and 4 h p.i., respectively (52). Here, we used real-time RT-PCR to examine the kinetics of VEGF-A and VEGF-C mRNA induction in serum-starved HMVEC-d cells infected with KSHV (10 DNA copies/cell). A significant level of VEGF-A mRNA over uninfected cells was detected as early as 0.5 h p.i., which increased steadily with about 6.2-, 5.6-, 3.4-, 8.2-, 2.7-, 5.5-, 2.9-, and 15.1-fold induction at 0.5 h, 1 h, 2 h, 4 h, 8 h, 24 h, 48 h, and 72 h p.i., respectively (Fig. 1A). Peak induction (15-fold) was observed at 72 h p.i. When VEGF-A protein expression in the culture supernatant was quantitated by ELISA, compared to VEGF-A protein levels in the uninfected HMVEC-d cells (Fig. 1B), VEGF-A levels in KSHV-infected cells increased by about 2.5-, 1.5-, 2.8-, 2.0-, 2.3-, 4.3-, 4.8-, 4.3-, and 10-fold at 0.5 h, 1 h, 2 h, 4 h, 8 h, 16 h, 24 h, 48 h, and 72 h p.i., respectively.
FIG. 1.
Detection of VEGF-A and VEGF-C mRNA and protein in KSHV-infected HMVEC-d cells. HMVEC-d cells grown to 80 to 90% confluence were serum starved for 8 h and infected with KSHV at an MOI of 10. (A and C) Infected and uninfected cells were washed and lysed, and total RNA was prepared. DNase I-treated RNA (250 ng) was subjected to real-time RT-PCR with VEGF-A and VEGF-C gene-specific primers. Known concentrations of DNase I-treated, in vitro-transcribed VEGF-A and VEGF-C transcripts were used in a real-time RT-PCR to construct a standard graph from which the relative copy numbers of transcripts were calculated and normalized, with GAPDH used as the internal control. Each reaction was done in duplicate, and each bar represents the average ± standard deviation from three independent experiments. The VEGF-A and VEGF-C levels normalized to GAPDH in the uninfected cells were considered as 1 for comparison. (B and D) The levels of VEGF-A and VEGF-C proteins released in the cell-free culture supernatants were measured by enzyme-linked immunosorbent assay. The data were normalized to a 1-mg/ml total protein concentration in the supernatant. Each reaction was done in duplicate, and each point represents the average ± standard deviation from three independent experiments. VEGF-A and VEGF-C released from uninfected cells were considered as 1 for comparison, and the induction levels in infected cells are indicated.
The RNA samples and culture supernatants from the above experiments were also used for quantification of VEGF-C mRNA and protein expression levels. Like VEGF-A, significant levels of VEGF-C mRNA induction were observed as early as 0.5 h p.i. (1.6-fold), which increased to about 2.2-, 1.4-, 3.8-, 1.8-, 2.3-, and 1.7-fold at 1 h, 2 h, 4 h, 8 h, 48 h, and 72 h p.i., respectively (Fig. 1C). In contrast to VEGF-A, maximum induction in VEGF-C expression (3.8-fold) was observed at 4 h p.i. Similarly, VEGF-C protein levels were elevated by about 1.2-, 1.9-, 1.6-, 1.9-, 1.4-, 2.1-, 1.9-, 1.9-, and 1.8-fold at 0.5 h, 1 h, 2 h, 4 h, 8 h, 16 h, 24 h, 48 h, and 72 h p.i., respectively (Fig. 1D).
Specificity of KSHV-induced VEGF expression.
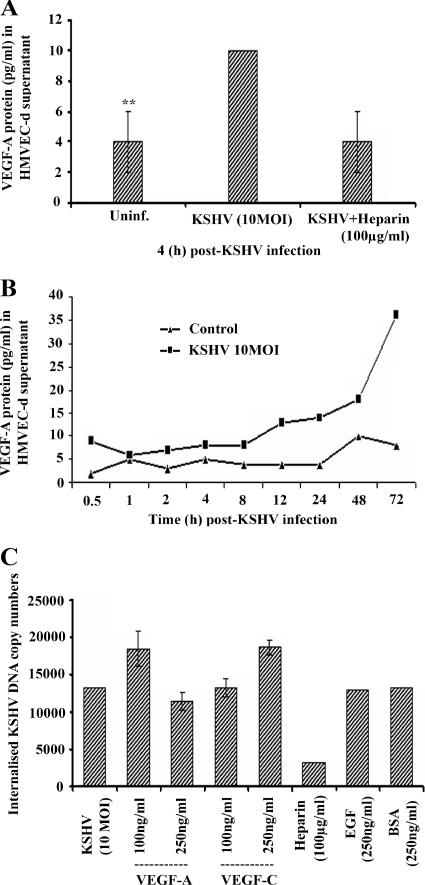
Incubation of KSHV with 100 μg/ml of heparin reduced the VEGF-A expression to the basal level (Fig. 2A). In a parallel experiment, serum-starved HMVEC-d cells were incubated for various durations with and without KSHV infection, and VEGF-A protein levels in the culture supernatants were then measured. As shown in the Fig. 2B, unlike KSHV-infected cells, VEGF-A levels in uninfected cells did not increase significantly with an increase in time, indicating that KSHV infection up-regulates VEGF-A expression. Further, a Limulus amebocyte lysate assay confirmed that all of the reagents used in the viral stock preparation were free of bacterial LPS (data not shown). These results demonstrated that VEGF-A and -C were specifically induced by KSHV infection and not by contaminating host cell factors and/or LPS, or any other factor(s). These results suggested that KSHV strongly up-regulates VEGF-A and VEGF-C mRNA and protein expression at sustained levels during infection of endothelial cells, thus supporting and extending our gene array analysis (52).
FIG. 2.
Blocking KSHV binding by heparin inhibits VEGF-A expression. (A) KSHV was incubated at 37°C for 1 h with DMEM containing 100 μg/ml of soluble heparin. This mixture was then added to a serum-starved (8 h) HMVEC-d cell monolayer and incubated for 4 h at 37°C. The levels of VEGF-A released in the cell-free culture supernatants were measured by enzyme-linked immunosorbent assay. **, statistically significant (P < 0.02). (B) HMVEC-d cells grown to 80 to 90% confluence were serum starved for 8 h and either uninfected (control) or infected with KSHV at an MOI of 10. The levels of VEGF-A proteins released in the cell-free culture supernatants collected at the indicated time points were measured by ELISA. The data were normalized to a 1-mg/ml total protein concentration in the supernatant. Each reaction was done in duplicate, and each point represents the average ± standard deviation from three independent experiments. (C) VEGF-A and VEGF-C enhance KSHV entry. HMVEC-d cells were serum starved for 8 h and either untreated or treated with VEGF-A or VEGF-C (100 and 250 ng/ml) or with human EGF or BSA (250 ng/ml) for 1 h at 37°C in serum-free EBM2 medium and infected (10 DNA copies per cell) for 2 h. Cells were washed, treated with trypsin-EDTA (0.25% trypsin and 5 mM EDTA) for 5 min, washed, and collected, and total DNA was prepared. The KSHV ORF73 gene in 100 ng of DNA was amplified by real-time DNA PCR, and the copy numbers were calculated from the standard graph generated by the real-time PCR using known concentrations of a cloned ORF73 gene. Each reaction was done in duplicate, and each point represents the average ± standard deviation of three experiments.
VEGF-A and -C augment KSHV entry.
The presence of VEGF-A and VEGF-C in KSHV-infected HMVEC-d cell supernatants prompted us to determine their role in KSHV biology. We examined KSHV infection in the presence of VEGF-A and VEGF-C. Serum-starved HMVEC-d cells were pretreated with 100 and 250 ng/ml of VEGF-A and VEGF-C for 1 h at 37οC and infected with KSHV at 10 MOI. We observed an approximately 30% increase in KSHV DNA entry in cells treated with 100 ng/ml of VEGF-A and about a 10% reduction in DNA entry at 250-ng/ml concentrations (Fig. 2C). VEGF-C treatment resulted in a dose-dependent increase in KSHV, with about 30% more viral DNA entry at 250 ng/ml of VEGF-C compared to untreated infected cells (Fig. 2C). We did not observe any change in virus entry in cells pretreated with 250 ng/ml of EGF or BSA. About 80 to 90% reduction in DNA entry was observed when cells were infected with heparin (100 μg/ml)-treated virus, thus demonstrating the specificity of infection procedures. Since VEGF-A is known to bind cell surface HS molecules, inhibition of virus entry at higher concentrations of VEGF-A could be due to saturation of HS by the VEGF165 isoform, resulting in HS unavailability for KSHV initial binding. These results suggested that VEGF-A and VEGF-C play a role in virus entry and infection.
Detection of KSHV infection-induced VEGF-A and VEGF-C by IFA.
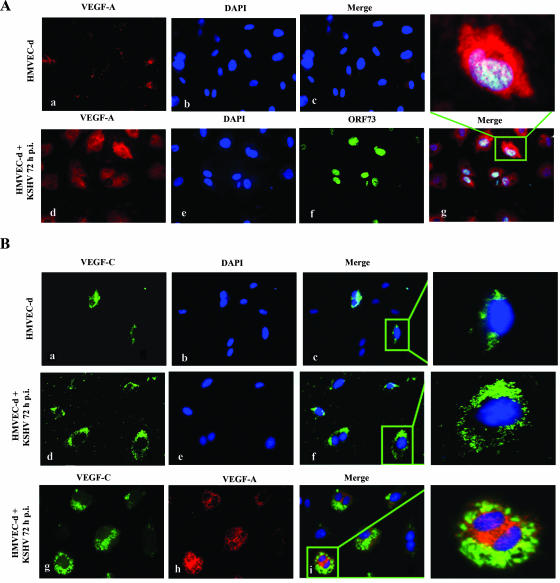
VEGF-A is a dimeric glycoprotein with structural homology to PDGF. Several variants of VEGF-A have been described that arise by alternative mRNA splicing (9). The anti-human VEGF-A mouse monoclonal antibody used in our study detects all isoforms, particularly the most commonly expressed 189-, 165-, and 121-amino-acid splice variants. VEGF-C is produced as a precursor protein and is proteolytically activated in the extracellular space by proteases to generate a homodimeric protein with high affinity for both VEGFR-2 and VEGFR-3 (66). In the IFA, uninfected HMVEC-d cells showed a very low level of expression of VEGF-A (Fig. 3A, panels a to c). In contrast, at 72 h p.i., several cells were positive for cytoplasmic staining of VEGF-A (Fig. 3A, panel d). We observed about 46% of cells expressing ORF73 protein (LANA-1) in these cells (Fig. 3A, panel f), and all LANA-1-positive cells, as well as the majority of the neighboring uninfected cells, were positive for VEGF-A (Fig. 3A, panel g).
FIG. 3.
Immunofluorescent detection of KSHV infection-induced VEGF-A and -C protein expression in HMVEC-d cells. (A and B) Uninfected HMVEC-d cells (panels a, b, and c) or cells infected with KSHV (MOI, 10) for 72 h (panels d, e, and f) were permeabilized and stained with anti-VEGF-A mouse monoclonal or VEGF-C goat polyclonal antibody, respectively, and detected by Alexa-594-coupled anti-mouse antibody or Alexa-488-coupled anti-goat antibody. Infected HMVEC-d cells were permeabilized and also stained with anti-ORF73 rabbit polyclonal antibody (A, panel f). Nuclei were counterstained with DAPI (b and e). The inset in panel g indicates nuclear staining for ORF73 in a cell with cytoplasmic staining for VEGF-A. KSHV-infected HMVEC-d cells express both VEGF-C (B, panel g) and VEGF-A (B, panel h). The inset demonstrates coexpression of VEGF-C and VEGF-A in the cytoplasm (i) of infected HMVEC-d cells. Magnification, ×40.
Like VEGF-A, VEGF-C was also up-regulated upon KSHV infection. Only a few uninfected cells stained positive for VEGF-C (Fig. 3B, panels a to c), and the infected HMVEC-d cells showed an increased cytoplasmic staining for VEGF-C (Fig. 3B, panels d to f). We also observed the coexpression of VEGF-A and VEGF-C in KSHV-infected cells (Fig. 3B, panels g to i). These results strongly demonstrated that KSHV infection of primary endothelial cells up-regulates expression of both VEGF-A and VEGF-C.
Initial induction of VEGF depends upon KSHV binding and entry into the target cells, while sustained levels require viral gene expression.
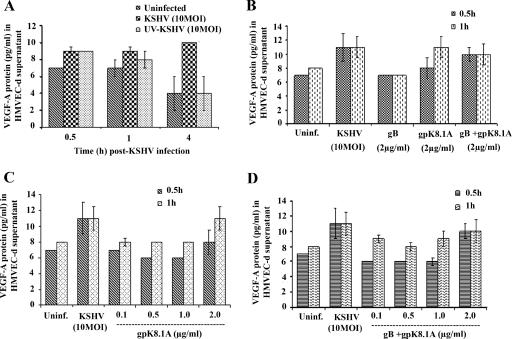
To determine whether KSHV gene expression is essential for VEGF-A or VEGF-C up-regulation, replication-incompetent KSHV was prepared by UV irradiation. We have previously demonstrated that KSHV exposed to UV for 20 min binds and enters HMVEC-d and HFF cells as efficiently as the untreated virus (71). In addition, our studies have shown that UV-inactivated KSHV does not induce any KSHV gene expression, whereas an equal MOI of live virus induced a quantitative increase in the latency-associated ORF73 gene expression as well as lytic cycle-associated ORF50, K8, and K5 gene expression (71). To determine whether VEGF induction requires KSHV-induced signal cascades and/or viral gene expression, we examined the secreted protein levels of VEGF-A in HMVEC-d cells infected with live or UV-inactivated KSHV at an MOI of 10. Live KSHV induced about 2.5-, 1.5-, and 2.8-fold-increased VEGF-A expression at 0.5 h, 1 h, and 4 h p.i., respectively. In contrast, UV-inactivated KSHV induced VEGF-A about 2.5- and 1.5-fold at 0.5 h and 1 h p.i., respectively, which decreased to uninfected cell levels at 4 h p.i. (Fig. 4A). We observed similar results for VEGF-C (data not shown), demonstrating that VEGF-A or -C expression is partially dependent upon virus binding and entry stages. However, KSHV viral gene expression appears to be necessary for the sustained expression of VEGF.
FIG. 4.
Induction of VEGF-A by UV-inactivated KSHV and viral glycoproteins gB and gpK8.1A. (A) HMVEC-d cells (80 to 90% confluence) serum starved for 8 h were uninfected or infected with either live KSHV or UV-irradiated KSHV for the indicated times at an MOI of 10 per cell. (B) Serum-starved HMVEC-d cells were uninfected, infected with KSHV (MOI, 10), or induced with KSHV glycoproteins gB and gpK8.1A alone or together for the indicated times. (C) Serum-starved HMVEC-d cells were uninfected, infected with KSHV (MOI, 10), or induced with KSHV glycoprotein gpK8.1A at the indicated concentrations for 0.5 and 1 h. (D) Serum-starved HMVEC-d cells were uninfected, infected with KSHV (MOI, 10), or induced with KSHV glycoproteins gB and gpK8.1A together at the indicated concentrations for 0.5 and 1 h. The levels of VEGF-A released in the cell-free culture supernatants were measured by ELISA. Each reaction was done in duplicate, and each point represents the average ± standard deviation from three independent experiments.
Since VEGF-A induction by UV-irradiated KSHV suggested initial stimulation during the binding and entry stages of infection, we next examined the ability of KSHV envelope glycoproteins gB and gpK8.1A to induce VEGF-A. Incubation of HMVEC-d cells with 2 μg/ml of gB for 0.5 h or 1 h did not induce any significant levels of VEGF-A as detected by ELISA (Fig. 4B); in contrast, incubation with 2 μg/ml of gpK8.1A for 0.5 h and 1 h induced a significant up-regulation of VEGF-A protein levels (Fig. 4B). When cells were treated with gB and gpK8.1A proteins together at 2 μg/ml, VEGF-A induction of about twofold at 0.5 h and 1 h of incubation, respectively, was observed (Fig. 4B). We then determined the minimal effective concentration of KSHV glycoprotein required for VEGF-A induction by using a series of concentrations of gpK8.1A (0.1, 0.5, 1.0, and 2.0 μg/ml) alone or together with gB. As shown in Fig. 4C, maximum induction of VEGF-A was observed only with 2.0 μg/ml of gpK8.1A. However, the combination of gB and gpK8.1A was more effective in inducing VEGF-A protein expression (Fig. 4D), as the combination induced VEGF-A at 1 h even at a 0.1 μg/ml concentration. The absence of LPS contamination in our purified protein preparations and inhibition of VEGF-A induction by preincubation of virus with heparin clearly demonstrated the specificity of VEGF induction by the glycoprotein gpK8.1A. These data further verified the conclusion that VEGF-A induction in KSHV-infected cells was initiated by the binding and entry of virions into the target cells and demonstrated that KSHV-gpK8.1A plays a role in this induction.
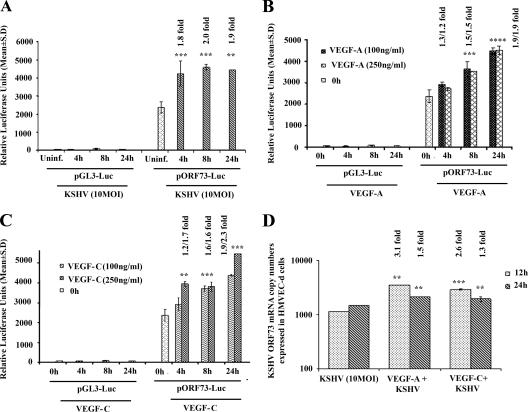
Exogenous VEGF-A and -C modulate KSHV latent ORF73 promoter and gene expression.
To determine further the potential role of VEGF-A and -C in infection, we next examined KSHV latent (ORF73) and lytic (ORF50) promoter activation. 293 cells were transfected with control luciferase vector (pGL3) or ORF73 promoter-luciferase (pORF73-Luc) constructs, and transfected cells were either uninfected or infected with KSHV at an MOI of 10 for 4 h, 8 h, and 24 h or treated with exogenous VEGF-A and VEGF-C at 100 and 250 ng/ml for the indicated times (Fig. 5). The mean relative luciferase activities of the pGL3-Luc and pORF73-Luc constructs were normalized based on cotransfected Renilla activity at all time points. The luciferase activity from the basic reporter pGL3-Luc construct was not affected by KSHV infection at any time during infection (Fig. 5A). In contrast, ORF73 promoter activity increased by about 1.8-, 2.0-, and 1.9-fold at 4 h, 8 h, and 24 h post-KSHV infection, respectively, compared to the activities in the uninfected cells transfected with pORF73-Luc (Fig. 5A). VEGF-A treatment alone increased ORF73 promoter activity by 1.3-, 1.5-, and 1.9-fold at 100 ng/ml and 1.2-, 1.5-, and 1.9-fold at 250 ng/ml at 4, 8, and 24 h, respectively. The addition of 250 ng/ml VEGF-A did not have any added effect over 100 ng/ml on ORF73 promoter activity (Fig. 5B). Similarly, VEGF-C treatment increased ORF73 promoter activity by 1.2-, 1.6-, and 1.9-fold at 100 ng/ml at 4 h, 8 h, and 24 h, respectively, and 1.7-, 1.6-, and 2.3-fold at 250 ng/ml at the corresponding time points. Unlike VEGF-A, an increase in ORF73 promoter activity by VEGF-C was dose dependent (Fig. 5C). The luciferase activity from the basic reporter pGL3-Luc construct was not affected by VEGF-A or VEGF-C addition (Fig. 5B and C).
FIG. 5.
Exogenous addition of VEGF-A or VEGF-C activates KSHV ORF73 promoter and gene expression. 293 cells were transfected with control pGL3-Luc or ORF73 promoter-luciferase construct and after 24 h cells were either uninfected or infected with KSHV at an MOI of 10 for 4 h, 8 h, and 24 h (A) or treated with exogenous VEGF-A (B) and -C (C) (100 ng/ml and 250 ng/ml). At the indicated time points, cells were harvested, lysed, and assayed for firefly luciferase activity. The data represent the mean relative luciferase units after normalizing with the cotransfected Renilla luciferase activity. (D) HMVEC-d cells (80 to 90% confluence) were serum starved for 8 h, either untreated or treated with VEGF-A or VEGF-C (100 ng/ml) for 1 h at 37°C, and infected with KSHV at an MOI of 10. Expression of ORF73 mRNA was monitored at the indicated time points. DNase I-treated RNA (250 ng) was subjected to real-time RT-PCR with an ORF73 gene-specific primer. Known concentrations of DNase I-treated, in vitro-transcribed ORF73 transcripts were used to construct a standard graph from which the relative copy numbers of transcripts were calculated and normalized, with GAPDH used as the internal control. Each reaction was done in duplicate, and each bar represents the average ± standard deviation from three independent experiments. **, ***, ****, statistically significant at P < 0.02, P < 0.01, and P < 0.001, respectively.
To further confirm the effect of VEGF-A or VEGF-C on KSHV ORF73 promoter activation, real-time RT-PCR was carried out to quantify KSHV ORF73 mRNA (36) from RNA isolated from cells that were either untreated or pretreated with VEGF-A or VEGF-C (100 ng/ml; 37°C, 1 h) and then infected with KSHV for 12 and 24 h p.i. (Fig. 5D). Significant increases of about 3.1- and 1.5-fold in ORF73 gene expression at 12 and 24 h p.i., respectively, were observed in VEGF-A-pretreated cells. Similarly, VEGF-C induced about 2.6- and 1.3-fold increases in ORF73 gene expression (Fig. 5D). These results suggested that KSHV infection-induced VEGF-A and VEGF-C play a moderate role(s) in viral latent gene expression.
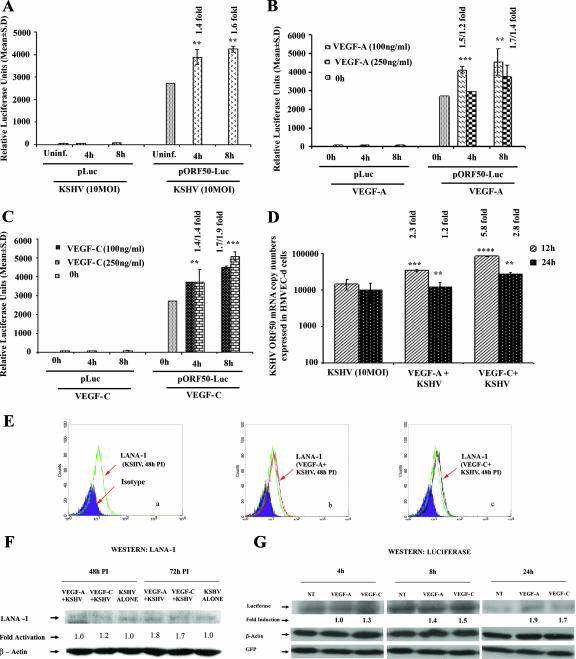
Exogenous VEGF-A and VEGF-C modulate KSHV lytic ORF50 promoter and gene expression.
We next examined the role of VEGF-A and VEGF-C in ORF50 (RTA) expression. 293 cells transfected with pLuc or ORF50 promoter construct (pORF50-Luc) were either uninfected or infected with KSHV at an MOI of 10 or treated with exogenous VEGF-A and VEGF-C at 100 and 250 ng/ml for 4 and 8 h. The luciferase activity from the basic reporter pLuc construct was not affected by KSHV infection at any time after infection (Fig. 6A). In contrast, ORF50 promoter activity increased by about 1.4- and 1.6-fold at 4 h and 8 h p.i., respectively, compared to the uninfected cells transfected with pORF50-Luc (Fig. 6A). VEGF-A treatment increased ORF50 promoter activity by about 1.5- and 1.7-fold at 100 ng/ml and about 1.2- and 1.4-fold at 250 ng/ml (Fig. 6B). The addition of 250 ng/ml VEGF-A did not have any added effect on ORF50 promoter activity (Fig. 6B). VEGF-C increased ORF50 promoter activity by about 1.4- and 1.7-fold at 100 ng/ml at 4 and 8 h, respectively, and about 1.4- and 1.9-fold at 250 ng/ml (Fig. 6C). The luciferase activity from the basic reporter pLuc construct was not affected by VEGF-A or VEGF-C addition (Fig. 6B and C). When ORF50 gene transcripts were examined by real-time RT-PCR in HMVEC-d cells pretreated with VEGF-A and infected with KSHV, a significant increase of about 2.3- and 1.2-fold in ORF50 gene expression at 12 and 24 h p.i., respectively, was observed (Fig. 6D), whereas a 5.8- and 2.8-fold increase in ORF50 gene expression was observed in VEGF-C (100 ng/ml)-treated cells (Fig. 6D), thus suggesting a potential role of VEGF-A and VEGF-C in KSHV lytic gene expression.
FIG. 6.
Exogenous addition of VEGF-A or VEGF-C activates KSHV ORF50 promoter and gene expression. 293 cells were transfected with control p-Luc or ORF50 promoter-Luciferase constructs and after 24 h, cells were either uninfected or infected with KSHV at an MOI of 10 for 4 h and 8 h (A) or treated with exogenous VEGF-A (B) and C (C) (100 ng/ml and 250 ng/ml). At the indicated time points cells were harvested, lysed, and assayed for firefly luciferase activity. The data represent the mean relative luciferase units after normalizing with the cotransfected Renilla luciferase activity. (D) HMVEC-d cells (80 to 90% confluence) were serum starved for 8 h, either untreated or treated with VEGF-A or VEGF-C (100 ng/ml) for 1 h at 37°C, and infected with KSHV at an MOI of 10. Expression of ORF50 mRNA was monitored at the indicated time points. DNase I-treated RNA (250 ng) was subjected to real-time RT-PCR with an ORF50 gene-specific primer. Known concentrations of DNase I-treated, in vitro-transcribed ORF50 transcripts were used to construct a standard graph from which the relative copy numbers of transcripts were calculated and normalized, with GAPDH used as the internal control. Each reaction was done in duplicate, and each bar represents the average ± standard deviation from three independent experiments. **, ***, ****, -statistically significant at P < 0.02, P < 0.01, and P < 0.001, respectively. (E) Exogenous addition of VEGF-A or VEGF-C induces KSHV LANA-1 expression at the protein level. HMVEC-d cells (80 to 90% confluence) were serum starved for 8 h, either untreated or treated with VEGF-A or VEGF-C (250 ng/ml) for 1 h at 37°C, and infected with KSHV at an MOI of 10. Cells were then harvested for flow cytometry after 48 h postinfection and detached from the plate with 0.25% trypsin-EDTA, and viability was assessed by trypan blue exclusion. Nuclear staining to identify KSHV-infected cells was performed using rat monoclonal antibody (ABI) to the KSHV LANA-1 protein. Flow cytometry was performed with a FACSCalibur flow cytometer and analyzed with CellQuest Pro software. Expression of LANA-1 at 48 h p.i. is shown as a green line for KSHV alone (a), a red line for VEGF-A-treated and KSHV-infected cells (b), a black line for VEGF-C-treated and KSHV-infected cells (c) compared to the isotype control, which is shown in a shaded purple histogram. (F) HMVEC-d cells (80 to 90% confluence) were serum starved for 8 h, either untreated or treated with VEGF-A or VEGF-C (250 ng/ml) for 1 h at 37°C, and infected with KSHV at an MOI of 10. Expression of LANA-1 protein was monitored at the indicated time points by Western blotting. Equal amounts of protein samples were resolved by SDS-7.5% PAGE, subjected to Western blotting, and reacted with rabbit polyclonal antibody against KSHV LANA-1. To confirm equal protein loading, blots were also reacted with antibodies against human β-actin. Each blot is representative of at least three independent experiments. The LANA-1 level in the untreated but KSHV-infected cells was considered as 1 for comparison. (G) Exogenous addition of VEGF-A or VEGF-C activates KSHV ORF73-luciferase expression. 293 cells were transfected with control pGL3-Luc or the ORF73 promoter-luciferase construct along with pcDNA-GFP as a transfection efficiency control. After 24 h, cells were either untreated (NT) or treated with exogenous VEGF-A or VEGF-C (250 ng/ml). At the indicated time points, cells were harvested and lysed, and total cell lysates were immunoblotted with antiluciferase primary antibodies. Transfection efficiency was detected by using anti-GFP mouse monoclonal antibody. To confirm equal protein loading, blots were also reacted with antibodies against human β-actin. Each blot is representative of at least three independent experiments. The luciferase level in the untreated cells was considered as 1 for comparison.
We next examined the serum-starved untreated and VEGF-A- or -C-treated (250 ng/ml) and KSHV-infected HMVEC-d cells for the expression of LANA-1 by flow cytometry and by Western blot reactions (Fig. 6E and F). In VEGF-A- and VEGF-C-treated cells infected with KSHV, we observed a shift in the geometric mean fluorescent index (GMFI, which is the test mean fluorescence divided by the mean fluorescence of the isotype control). At 48 h p.i., we observed a LANA GMFI of 10.54 in untreated infected cells (Fig. 6E, panel a) which increased to statistically significant GMFI levels of 12.24 (P < 0.03) and 12.39 (P < 0.02) in VEGF-A- and VEGF-C-treated cells, respectively (Fig. 6E, panels b and c). These results suggested that VEGF treatment increases ORF73 expression. When HMVEC-d cells untreated or VEGF treated and KSHV infected were lysed, resolved by SDS-7.5% PAGE, and subjected to Western blotting using rabbit polyclonal antibody against KSHV LANA-1, at 72 h p.i. we observed about 1.8-fold and 1.7-fold increases in LANA-1 expression in VEGF-A- and C-treated cells, respectively (Fig. 6F). These observations clearly demonstrated that VEGF-A and -C indeed increase ORF73 gene expression.
The ORF73 and ORF50 promoter activations by VEGF-A and -C were also further confirmed by detecting luciferase enzyme in Western blot assays. After 24 h of transfection with pORF73-Luc plasmid, 293 cells were either untreated or treated with exogenous VEGF-A and -C at a concentration of 250 ng/ml for 4 h, 8 h, and 24 h and assayed. Transfection efficiency was examined by using anti-GFP antibodies, and equal protein loading was confirmed with anti-human β-actin antibodies. An increase in activation of luciferase over untreated cells was observed (Fig. 6G). As detected in the luciferase assay, VEGF-A increased the luciferase expression by about 1.4-fold and 1.9-fold at 8 h and 24 h, respectively, and VEGF-C treatment enhanced the luciferase expression by about 1.3-, 1.5-, and 1.7-fold at 4 h, 8 h, and 24 h posttreatment, respectively (Fig. 6G). In contrast, there was no change in GFP and β-actin expression levels. The Western blotting findings were in consensus with our promoter luciferase assay, confirming that VEGF-A and -C indeed activate the ORF73 and ORF50 promoters (data not shown).
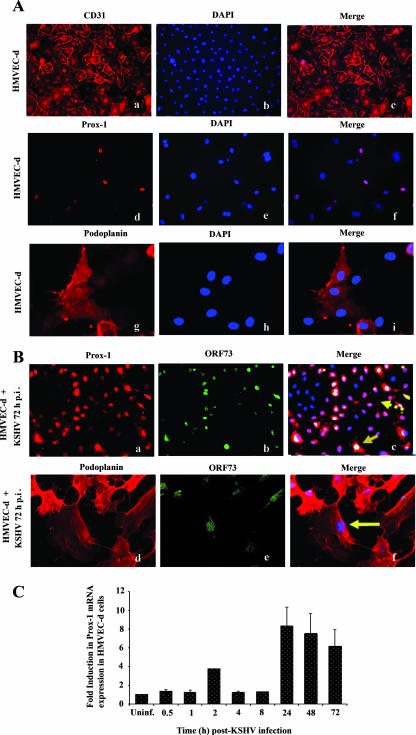
KSHV infection of HMVEC-d cells induces the expression of the lymphatic lineage-specific markers Prox-1 and podoplanin.
To further examine the biological relevance of VEGF in KSHV-infected cell supernatants, we next examined their role in lymphangiogenesis. Evidence suggests that KS spindle cells are of endothelial origin and predominately consist of KSHV-infected lymphatic endothelium (30, 60). VEGF-C is a ligand for VEGFR-3, a known lymphangiogenic marker and enhancer of lymphangiogenesis and lymphatic metastasis (43, 74, 84). When we examined the influence of KSHV infection on the expression of the lymphatic endothelial markers Prox-1 and podoplanin in confluent HMVEC-d cells, all uninfected HMVEC-d cells stained positive for CD31, the pan-endothelial cell marker, confirming the endothelial cell lineage of HMVEC-d cells (Fig. 7A, panels a to c). Only a small percentage (20 to 30%) of uninfected cells expressed Prox-1 and podoplanin (Fig. 7A, panels d to i). This is because the commercially available HMVEC-d cells are mixtures of BEC and LEC cells (70:30 ratio). In contrast, after 72 h post-KSHV infection, >80% cells stained positive for Prox-1 and podoplanin (Fig. 7B panels a and d, respectively). The expression levels of BEC markers like CD34 in the same cells were not tested in this study. The infected cells were also stained for KSHV ORF73/LANA-1, and colocalization of Prox-1 and podoplanin with ORF73 is shown in Fig. 7B (panels c and f). Most of the LANA-1-positive cells were also positive for Prox-1 and podoplanin (Fig. 7B, panels c and f). Interestingly, Prox-1 was detected in many cells that did not exhibit LANA-1 (Fig. 7B, panel c). Since there was no increase in the number of cells on the infected cover slides, these findings suggest that KSHV infects both BEC and LEC and up-regulates the expression of LEC markers in BEC, thus changing the cell lineage. These observations are in concordance with previous reports (15, 30, 81) and demonstrate that KSHV infection induces the LEC markers, switching the BEC phenotype to LEC.
FIG. 7.
KSHV infection of HMVEC-d cells induces expression of lymphatic lineage-specific markers Prox-1 and podoplanin. (A) Uninfected HMVEC-d cells (a to i) or (B) cells infected with KSHV (MOI, 10) for 72 h (a to f) were permeabilized and stained with anti-CD31, Prox-1, or podoplanin antibodies and detected with Alexa 594-coupled anti-mouse antibodies. Nuclei were counterstained with DAPI. (B) Infected HMVEC-d cells were permeabilized and also stained with anti-ORF73 polyclonal antibody and detected by Alexa 488-coupled anti-rabbit antibodies. Solid arrows indicate nuclei demonstrating positive staining for ORF73 in cells showing nuclear staining for Prox-1 (B, panel c). The solid arrow (B, panel f) shows nuclei demonstrating positive staining for ORF73 in cells showing staining for podoplanin. The broken arrow (B, panel c) indicates ORF73-negative cells positive for Prox-1 staining. Magnification, ×20 (A, panels a to f; B, panels a to c) or ×40 (A, panels g to i; B, panels d to f). (C) HMVEC-d cells grown to 80 to 90% confluence were serum starved for 8 h and infected with KSHV at an MOI of 10. Infected and uninfected cells were washed and lysed, and total RNA was prepared. After cDNA synthesis, real-time RT-PCR was performed for Prox-1 gene expression using the Sybr green detection protocol. The data were normalized with the expression of GAPDH. The data shown represent the average ± standard deviation from three independent experiments. The Prox-1 level normalized to GAPDH in the uninfected cells was considered 1 for comparison.
Kinetics of KSHV-induced Prox-1 expression in HMVEC-d cells.
Prox-1 is a homeobox gene that controls lymphatic vessel development and differentiation. It is the earliest lymphatic marker expressed, and its expression indicates the onset of lymphatic lineage transformation (30). We next examined the kinetics of Prox-1 mRNA expression following KSHV infection by real-time RT-PCR. Prox-1 expression increased to about fourfold over uninfected cells at 2 h p.i., reached a peak of about eightfold at 24 h p.i., and slowly declined to about sevenfold at 48 h and about sixfold level at 72 h p.i. (Fig. 7C). This is the first report showing the kinetics of Prox-1 expression at earlier time points immediately after KSHV infection and demonstrated that KSHV induces the blood endothelial-to-lymphatic endothelial lineage switch early during de novo infection.
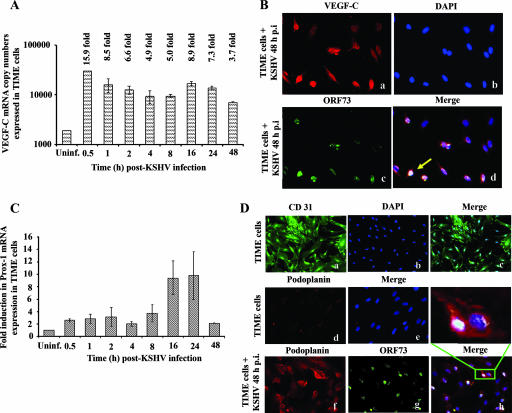
KSHV infection of TIME cells induces the expression VEGF-C and lymphatic markers.
To further confirm the above findings, serum-starved TIME cells, pure blood vascular endothelial cells (15), were infected with KSHV, and the kinetics of VEGF-C mRNA induction was examined (Fig. 8A). A significant increase in VEGF-C mRNA was detected as early as 0.5 h p.i., and this was maintained relatively steadily with about 15.9-, 8.5-, 6.6-, 4.9-, 5.0-, 8.9-, 7.3-, and 3.7-fold increases at 0.5 h, 1 h, 2 h, 4 h, 8 h, 24 h, and 48 h p.i., respectively (Fig. 8A). By IFA, only a few of the uninfected TIME cells were positive for VEGF-C expression (data not shown). In contrast, a significant increase in VEGF-C-positive cells expressing high levels of VEGF-C was readily detected after 48 h post-KSHV infection (Fig. 8B, panels a to d).
FIG. 8.
KSHV infection of TIME cells induced the expression VEGF-C and lymphatic markers. (A) TIME cells grown to 80 to 90% confluence were serum starved for 8 h and infected with KSHV at an MOI of 10, and the kinetics of VEGF-C mRNA induction was quantitated as per procedures described in the Fig. 1 legend. The VEGF-C level normalized to GAPDH in the uninfected cells was considered 1 for comparison. (B) Cells infected with KSHV (MOI, 10) for 48 h (a, b, c, and d) were permeabilized and stained with goat anti-VEGF-C antibodies and detected by Alexa 594-coupled anti-goat antibodies. Infected cells were also permeabilized and stained with anti-ORF73 polyclonal antibody and detected by Alexa 488-coupled anti-rabbit antibodies (c). Nuclei were counterstained with DAPI (b). The solid arrow indicates cytoplasmic staining for VEGF-C in cells infected with KSHV (d). Magnification, ×40. (C) Prox-1 mRNA from uninfected and KSHV-infected TIME cells was quantified by real-time RT-PCR as per the procedures described in the Fig. 7C legend. The Prox-1 level normalized to GAPDH in the uninfected cells was considered 1 for comparison. (D) Uninfected TIME cells (a to e) or cells infected with KSHV (MOI, 10) for 48 h (f to h) were permeabilized, stained with anti-CD31, and detected by Alexa 488- or Alexa 594-coupled anti-mouse antibodies. Magnification, ×20 (a to c) or ×40 (d and e). Infected cells were permeabilized and also stained with anti-ORF73 polyclonal antibody and detected by Alexa 488-coupled anti-rabbit antibodies (g). Nuclei were counterstained with DAPI (b). The inset in panel h indicates membrane staining for podoplanin in infected TIME cells. Magnification, ×40.
We next examined the kinetics of Prox-1 mRNA expression in TIME cells following KSHV infection (Fig. 8C). Prox-1 expression increased to about 2.5-fold over uninfected cells as early as 0.5 h p.i., reached a peak of 11- to 12-fold at 12 and 24 h p.i., and declined to 2-fold at 48 h p.i. (Fig. 8C). By IFA, all uninfected TIME cells were positive for the pan-endothelial cell marker CD31 (Fig. 8D, panels a to c), and only very low background staining for the LEC marker podoplanin was observed (Fig. 8D, panels d and e). In contrast, KSHV-infected TIME cells showed clear membranous expression of podoplanin in about 60% of cells (Fig. 8D, panels f to h). Most of the LANA-1-positive cells were also positive for podoplanin (Fig. 8D, panel h). Since there was no increase in the number of cells, these findings demonstrated that KSHV infection of BEC and VEGF-C induction lead to the expression of LEC markers, thus changing the cell lineage.
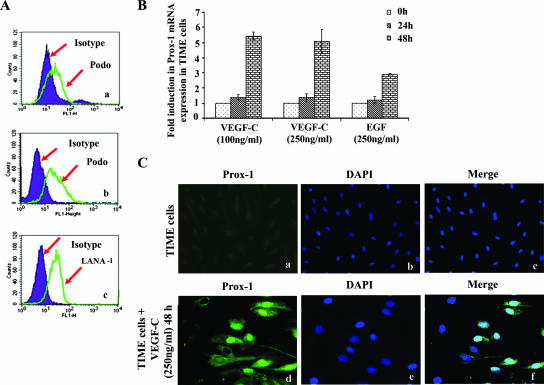
To further confirm the expression of lymphatic marker expression early during infection, TIME cells were infected with KSHV (MOI, 10) and harvested at 24 h and 48 h postinfection for flow cytometry analysis. As shown in Fig. 9A, panel a, about 7.2% of infected cells showed expression of podoplanin at 24 h p.i. and about 52.2% (Fig. 9A, panel b) after 48 h p.i. compared to the isotype control. Nuclear staining to identify KSHV-infected cells was performed using a rat monoclonal antibody to the KSHV LANA-1 protein, and about 25.0% of the cells were positive for LANA-1 at 24 h p.i. (Fig. 9A, panel c).
FIG. 9.
Exogenous VEGF-C induces Prox-1 mRNA in TIME cells. (A) TIME cells were infected with KSHV (MOI, 10) and harvested for flow cytometry at 24 h (a) and 48 h postinfection (b). Cells were detached from the plate with 0.25% trypsin-EDTA, and viability was assessed by trypan blue exclusion. For podoplanin, cell surface staining was carried out for 1 h at 4°C. Nuclear staining to identify KSHV-infected cells was performed using rat monoclonal antibody to the KSHV LANA-1 protein (c). Flow cytometry was performed with a FACSCalibur flow cytometer and analyzed with CellQuest Pro software. Expression of podoplanin and LANA-1 at 24 h and 48 h p.i. is shown by the green line and compared to the isotype control, which is shown in the shaded purple histogram. (B) TIME cells were incubated with serum-free EBM2 containing different concentrations (100 and 250 ng/ml) of recombinant VEGF-C for 24 h and 48 h at 37°C. As a control, cells were treated for corresponding times with 250 ng/ml human EGF. Prox-1 mRNA was then quantified by real-time RT-PCR as per the procedures described in the Fig. 7C legend. The Prox-1 level normalized to GAPDH in the untreated cells was considered 1 for comparison. (C) Untreated (a to c) or VEGF-C-treated (250 ng/ml for 48 h) TIME cells (d to f) were permeabilized and stained with Prox-1 antibodies and detected with Alexa 488-coupled anti-mouse antibodies. Nuclei were counterstained with DAPI. Magnification, ×20 (a to c).
Exogenous VEGF-C induces Prox-1 mRNA in TIME cells.
Since VEGF-C is a lymphangiogenic growth factor and was up-regulated by KSHV infection in TIME cells (BEC), we next examined Prox-1 expression by incubating cells for 24 and 48 h in medium containing VEGF-C. Minimal Prox-1 induction was observed after 24 h of treatment; however, after 48 h with VEGF-C, about fivefold higher Prox-1 expression was observed (Fig. 9B) by real-time PCR. Prox-1 induction by VEGF-C was not dose dependent. EGF, used as a control, had a moderate induction of Prox-1 (Fig. 9B). It is interesting that the level of Prox-1 expression in KSHV-infected TIME cells at 24 h p.i. (Fig. 8C) was much higher than with VEGF-C treatment alone (Fig. 9B). By IFA, about 50% of TIME cells were positive for Prox-1 after 48 h of treatment with 250 ng/ml of VEGF-C (Fig. 9C, panels d to f). These studies further suggest that KSHV infection-induced VEGF-C in the culture supernatant probably plays an important role in lymphangiogenesis.
DISCUSSION
The present study is the first comprehensive study detailing the up-regulation of VEGF-A and VEGF-C upon primary KSHV infection of endothelial cells, a mixture of BEC and LEC, and the roles of these molecules in KSHV latent and lytic gene expression in addition to induction of a lymphatic lineage switch. Further study is required to understand how latency is favored despite the induction of the lytic ORF50 gene by both VEGF-A and VEGF-C.
VEGF-A exists as a number of isoforms derived from mRNA splice variants. The isoforms differ at the C terminus, with the larger variants exhibiting substantial affinity for heparin (13, 66). The smallest isoform, VEGF121, does not bind heparin and is freely soluble upon secretion, whereas the higher-molecular-weight isoforms bind to the cell surface/extracellular matrix (ECM) HS proteoglycans (31, 57). It has been proposed that the ECM-bound forms of VEGF represent a pool of available growth factors, which can be activated in the course of tissue remodeling in conjunction with the degradation of ECM components necessary for neovascularization (31, 54, 57).
The earlier onset of VEGF-A and -C expression could be due to binding and entry of KSHV, as evidenced from the induction by UV-inactivated virus (Fig. 4A). Since target cell infection by KSHV starts with its binding to HS molecules, VEGF induction at the entry stage could also be attributed to the release of sequestered, extracellular bound VEGF into a soluble form by viral glycoproteins competing for HS binding sites of VEGF-A (8). In contrast to KSHV, viral gene expression appears to be unnecessary for the expression of VEGF-A by HCMV (62). Sustained VEGF-A and -C expression requires KSHV gene expression. Available evidence suggests that this induction must be occurring in conjunction with the many cytokines produced during primary infection by KSHV (68).
In addition to that, the VEGF mRNAs possess adenylate-uridylate-rich elements in their 3′ untranslated regions (17), and so it is very much possible that the VEGF-A mRNA is stabilized by kaposin B, a latent protein of KSHV, as it reportedly stabilizes the cytokines which have this adenylate-uridylate-rich element in their 3′ untranslated region (47).
VEGF-A expression is regulated by a variety of external factors. Angiogenic cytokines, growth factors, and gonadotropins that do not stimulate angiogenesis directly also can modulate angiogenesis by modulating VEGF-A expression in specific cell types and thus exert an indirect angiogenic or antiangiogenic effect. For example, factors that can potentiate VEGF-A production include FGF-4, PDGF, tumor necrosis factor, TGF-β, keratinocyte growth factor, insulin-like growth factor I, IL-1β, IL-6 (54), and PGE2. Among these, FGF-4, PDGF, and TGF-β are up-regulated following KSHV infection of HMVEC-d cells (72). Furthermore, COX-2, an inducible component of the inflammatory prostaglandin synthesis pathway, is also up-regulated by KSHV infection in HMVEC-d cells (52, 72). VEGF-A expression was reduced by COX-2 inhibition (N. Sharma-Walia, B. Chandran, et al., unpublished data), indicating that COX-2 partially regulates VEGF-A expression. It is interesting that KSHV also induces the expression of cytokines such as IL-10 and IL-13 in HMVEC-d cells (68), and these cytokines can inhibit the release of VEGF (46). These results indicate that KSHV has evolved to utilize multiple pathways and inflammatory responses for the regulation of VEGF-A levels in target cells. The cellular response to hypoxia is mediated by the hypoxia-inducible transcription factor 1 (HIF1), a heterodimeric protein that binds to hypoxia response elements in the promoter/regulatory regions of hypoxia-inducible genes, including the VEGF-A gene, and initiates transcription by recruitment of transcriptional activators such as CREB/p300 (21). Since latent KSHV infection of HMVEC-d cells leads to increased expression of HIF1α and HIF2α (16), hypoxia could also be one of the factors inducing VEGF-A in the infected target cells.
Compared to VEGF-A, we observed a moderate VEGF-C expression level. Several mechanisms have been reported for the regulation of VEGF-C expression, and KSHV probably utilizes a few or all of these. VEGF-C is synthesized as a proprotein, with the central receptor binding VEGF homology domain (VHD) flanked by N- and C-terminal propeptides. The propeptides are cleaved, yielding the mature VHD, and VEGF-C acquires the capacity to bind to VEGFR-2. Full-length forms of the growth factors bind to VEGFR-3 but do so with greater affinity after proteolytic maturation. The marked effects of the proteolytic activation of VEGF-C and VEGF-D on their affinity for VEGFR-2 and VEGFR-3 indicate that the enzymes carrying out this processing are important regulators of lymphangiogenesis and angiogenesis. The fibrinolytic serine protease plasmin was recently shown to generate the fully processed, mature forms of the VHD with greatly enhanced capacities to activate both VEGFR-2 and VEGFR-3. Plasmin can also release ECM-bound VEGF and activates both angiogenic and lymphangiogenic growth factors. It is interesting that the two enzymes that regulate plasmin activation, tissue plasminogen activator and plasminogen activator inhibitor 2, are also up-regulated during the in vitro infection of HMVEC-d cells by KSHV (52). COX-2 has also been shown to up-regulate VEGF-C expression in human lung adenocarcinoma cells (76). In addition to VEGF-C, COX-2 activity and some prostaglandins produced by COX-2 (59) also elevate angiopoietin-2, another protein required for lymphangiogenesis. COX-2 is therefore an inducer of two proteins integral to lymphangiogenesis, and induction of sustained COX-2 and PGE2 in KSHV-infected HMVEC-d cells may also be responsible for the sustained VEGF-C induction observed here.
The lymphatic vessels develop subsequent to the formation of the blood vasculature, leading to the suggestion that they are derived from the blood vessels. Recent advances in the understanding of lymphatic development have shown that expression of the homeobox transcription factor Prox-1 is a defining characteristic of differentiation to the lymphatic phenotype. Subsequent budding and sprouting of Prox-1-positive cells gives rise to the lymphatic vasculature (84, 85). Two members of the VEGF family, VEGF-C and VEGF-D, have been shown to act as lymphangiogenic growth factors. Disruption of the VEGF-C gene demonstrates that the growth factor is indispensable for embryonic lymphangiogenesis (35). Prox-1 expression was detected in endothelial cells of VEGF-C-deficient embryos, but the Prox-1-positive cells failed to migrate from the cardinal vein (35), indicating that VEGF-C is required for migration of the endothelial cells which go on to form the lymphatic system. IL-3 has been known to induce the lymphatic markers Prox-1 and podoplanin in HMVEC-d cells (28), and induction of IL-3 by KSHV infection of HMVEC-d cells (68) suggests that besides VEGF-C, KSHV-induced IL-3 could also be one of the factors playing a role in Prox-1 expression.
Our demonstration of KSHV infection induction of the LEC markers and switching from the BEC to the LEC phenotype confirms other earlier studies (15, 30, 81). More importantly, we extend these studies by the following important observations: (i) the in vitro microenvironment of KSHV-infected HMVEC-d and TIME cells is rich with VEGF-A and -C, which are known to play key roles in angiogenesis and lymphangiogenesis. (ii) Induction of Prox-1 occurs early during infection (as early as 8 h p.i.), thus suggesting that commitment to the LEC phenotype is induced early during KSHV infection of HMVEC-d cells. This is in contrast to other studies, where Prox-1 induction was examined only at 48 h p.i. in TIME cells, at 7 days p.i. in HMVEC-d cells, and at 2 and 7 days p.i. of BEC and LEC in studies by Carroll et al. (15), Hong et al. (30), and Wang et al. (81), respectively. VEGF-C, a key molecule of the BEC-to-LEC switch, is induced by KSHV infection early during infection of HMVEC-d cells, which was sustained throughout our observation period of 72 h p.i. We made similar observations in pure BEC-TIME cells. This finding is further strengthened by the induction of Prox-1 mRNA and protein expression in BEC-TIME cells. This is in contrast to other studies, where VEGF-C induction was examined only at 48 h p.i. in TIME cells (15). Significant amounts of VEGF-C mRNA and protein expression were observed in vivo in blood endothelial cells adjacent to KS lesions (73), demonstrating a paracrine function of VEGF-C. Our observations of VEGF detection in LANA-1-positive cells as well as the majority of the neighboring uninfected cells suggest a similar paracrine effect during the in vitro KSHV infection. These results suggest that the in vitro microenvironments of KSHV-infected HMVEC-d and TIME cells, which are enriched with VEGF-A and -C, recapitulate the microenvironment of early KS lesions.
KS is considered a chronic inflammation-associated malignancy due to the presence of spindle-shaped endothelial cells, slit-like neovascular structures, variable quantities of infiltrating inflammatory cells, growth and angiogenic factors, and inflammatory cytokines, such as VEGF, PDGF, bFGF, PGE2, TGF-β, IL-1β, IL-6, and gamma interferon. The driving force behind the initiation and maintenance of the cytokine and growth factor cascade remains to be elucidated thoroughly. VEGF-A has been identified in KS lesions, and VEGF-C has gained attention because of the presence of its receptor VEGFR-3 in KS lesions and its ability to induce lymphangiogenesis (44). Skobe et al. (73) reported the expression of flt-4, the receptor for VEGF-C in KS cells, and strong expression of VEGF-C in KS blood vessels. Coexpression of VEGF-A and bFGF has been shown in AIDS-KS and classic KS lesions, and the production of these factors is believed to be induced synergistically by inflammatory cytokines (69). Masood et al. (45) reported that AIDS-KS cell lines express higher levels of VEGF-A and its receptor than normal HUVEC, and AIDS-KS cell growth was inhibited by blocking VEGF-A expression with antisense oligonucleotides. However, KSHV's role in VEGF-A up-regulation was not clear, since these cells were devoid of KSHV. VEGF-A has been shown to be induced by KSHV lytic gene products such as vGPCR (a homolog of the human chemokine IL-8) (7, 75), vIL-6 (6), and K1 (82). While these findings suggest that KSHV has devised multiple ways to induce VEGF-A, the actual scenario in KS lesions appears to be different, since KS lesion endothelial cells express latent gene products and only <1% of inflammatory cells have been shown to express KSHV lytic cycle proteins (20, 68).
Demonstration of a moderate increase in viral entry and viral gene expression in VEGF-pretreated endothelial cells suggests that besides the induction of BEC to LEC, VEGF-A and -C must be playing important roles in KSHV biology. The increase in viral entry could possibly be due to induction of host signal molecules, such as FAK, Src, phosphatidylinositol 3-kinase, and Rho GTPases, which have been shown to be essential for KSHV entry (37, 71, 78). In the microenvironment of KS lesions, the presence of VEGF-A and -C may potentially facilitate an increase in infection of uninfected cells by KSHV released from the B cells and monocytes in the inflammatory cell pool. These effects, though moderate, suggest that besides playing a major role in the lymphatic lineage switch through activation of prox-1, VEGF induced by KSHV may also be contributing an active role in an increase of KSHV infection in neighboring cells.
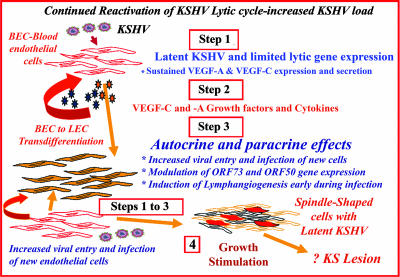
Several facts, such as requirements of lytic KSHV infection for KS lesion formation and KSHV reactivation leading to increased virus load under immunosuppression conditions (20), together with our studies demonstrating a robust induction of cytokines, growth factors, and angiogenic factors including COX-2 by KSHV at 4 h, 8 h, and 24 h p.i. of endothelial cells (72), and sustained activation of NF-κB, a key inflammatory induction molecule (68), suggest that primary infection of endothelial cells could create the microenvironment observed in early KS lesions and could be the initiating factor for KS lesion formation. This notion is further strengthened by the present study, which demonstrated that KSHV infection stimulates the transcription of VEGF-A and VEGF-C early during infection, resulting in the subsequent secretion of the protein in culture supernatants. In the microenvironment of KS lesions, sustained low levels of VEGF-A and VEGF-C expression could potentially increase entry of newly arriving KSHV into additional new target endothelial cells, increase virus gene expression, increase cytokine and growth factor induction including VEGF-C and -A, and induce lymphangiogenesis. The repeated cycle of the above processes in an immunosuppressed individual could lead to the formation of the highly angiogenic and hyperplastic multifocal KS lesions (Fig. 10). In the absence of a robust immune system response, such as that seen in human immunodeficiency virus type 1-infected individuals, reduced host immune regulation, inability to control inflammation, and elimination of infected cells could lead to clinical manifestations of Kaposi's sarcoma. Further studies are essential to determine the significance of the BEC-to-LEC switch in KSHV biology and the role of various host and viral factors involved in KSHV-induced lymphatic reprogramming, all of which will lead to a better understanding of KSHV biology and KS pathogenesis.
FIG. 10.
Schematic model depicting the potential implications of VEGF-A and VEGF-C induction during in vitro KSHV infection of endothelial cells. KSHV has been shown to be reactivated under immunosuppression conditions, resulting in increased circulating virus. Similar to in vitro infection of HMVEC-d cells, primary infection of endothelial cells in vivo could also result in the induction of preexisting host signal cascades, sustained expression of latency-associated genes, transient expression of a limited number of lytic genes, sustained induction of NF-κB and several cytokines and growth and angiogenic factors, including VEGF-A and -C (step 1). These factors when released to the extracellular environment of infected cells could act in an autocrine or paracrine fashion on the infected cells as well as neighboring cells (step 2). In the microenvironment of KS lesions, the presence of VEGF-A and -C may potentially be facilitating (i) an increase in infection of uninfected neighboring cells by KSHV released from the B cells and monocytes in the inflammatory cell pool, (ii) modulation of viral gene expression, and (iii) induction of lymphangiogenesis early during infection (step 3). Continual repetitions of these steps in an immunosuppressed individual could result in an increase in new infection of endothelial cells and increased autocrine actions of VEGF and other factors, along with viral gene expression, probably contributing to the maintenance of latency, angiogenesis, lymphatic lineage switch, neovascularization, inflammation, and growth stimulation. Reduced host immune regulation, an inability to control inflammation, and elimination of infected cells could ultimately lead to the formation of multifocal KS lesions (step 4).
Acknowledgments
This study was supported in part by Public Health Service grant CA 099925 and the Rosalind Franklin University of Medicine and Science H. M. Bligh Cancer Research Fund to B.C.
We thank Keith Philibert for critically reading the manuscript.
Footnotes
Published ahead of print on 5 December 2007.
REFERENCES
- 1.Akula, S. M., P. P. Naranatt, N.-S. Walia, F.-Z. Wang, B. Fegley, and B. Chandran. 2003. Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) infection of human fibroblast cells occurs through endocytosis. J. Virol. 777978-7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akula, S. M., N. P. Pramod, F.-Z. Wang, and B. Chandran. 2002. Integrin α3β1 (CD 49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 108407-419. [DOI] [PubMed] [Google Scholar]
- 3.Akula, S. M., F.-Z. Wang, J. Vieira, and B. Chandran. 2001. Human herpesvirus 8 interaction with target cells involves heparan sulfate. Virology 282245-255. [DOI] [PubMed] [Google Scholar]
- 4.Albini, A., I. Paglieri, G. Orengo, S. Carlone, M. G. Aluigi, R. DeMarchi, C. Matteucci, A. Mantovani, F. Carozzi, S. Donini, and R. Benelli. 1997. The beta-core fragment of human chorionic gonadotrophin inhibits growth of Kaposi's sarcoma-derived cells and a new immortalized Kaposi's sarcoma cell line. AIDS 11713-721. [DOI] [PubMed] [Google Scholar]
- 5.Alitalo, K., T. Tammela, and T. V. Petrova. 2005. Lymphangiogenesis in development and human disease. Nature 438946-953. [DOI] [PubMed] [Google Scholar]
- 6.Aoki, Y., E. S. Jaffe, Y. Chang, K. Jones, J. Teruya-Feldstein, P. S. Moore, and G. Tosato. 1999. Angiogenesis and hematopoiesis induced by Kaposi's sarcoma-associated herpesvirus-encoded interleukin-6. Blood 934034-4043. [PubMed] [Google Scholar]
- 7.Bais, C., B. Santomasso, O. Coso, L. Arvanitakis, E. G. Raaka, J. S. Gutkind, A. S. Asch, E. Cesarman, M. C. Gershengorn, and E. A. Mesri. 1998. G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature 39186-89. [DOI] [PubMed] [Google Scholar]
- 8.Barillari, G., C. Sgadari, V. Fiorelli, F. Samaniego, S. Colombini, V. Manzari, A. Modesti, B. C. Nair, A. Cafaro, M. Sturzl, and B. Ensoli. 1999. The Tat protein of human immunodeficiency virus type-1 promotes vascular cell growth and locomotion by engaging the α5β1 and αVβ3 integrins and by mobilizing sequestered basic fibroblast growth factor. Blood 94663-672. [PubMed] [Google Scholar]
- 9.Bermont, L., F. Lamielle, S. Fauconnet, H. Esumi, A. Weisz, and G. L. Adessi. 2000. Regulation of vascular endothelial growth factor expression by insulin-like growth factor-I in endometrial adenocarcinoma cells. Int. J. Cancer 85117-123. [DOI] [PubMed] [Google Scholar]
- 10.Blackbourn, D. J., E. Lennette, B. Klencke, A. Moses, B. Chandran, M. Weinstein, R. G. Glogau, M. H. Witte, D. L. Way, T. Kutzkey, B. Herndier, and J. A. Levy. 2000. The restricted cellular host range of human herpesvirus 8. AIDS 141123-1133. [DOI] [PubMed] [Google Scholar]
- 11.Boshoff, C., T. F. Schulz, M. M. Kennedy, A. K. Graham, C. Fisher, A. Thomas, J. O. McGee, R. A. Weiss, and J. J. O'Leary. 1995. Kaposi's sarcoma-associated herpesvirus infects endothelial and spindle cells. Nat. Med. 11274-1278. [DOI] [PubMed] [Google Scholar]
- 12.Breier, G., and W. Risau. 1996. The role of vascular endothelial growth factor in blood vessel formation. Trends Cell Biol. 6454-456. [DOI] [PubMed] [Google Scholar]
- 13.Byrne, A. M., D. J. Bouchier-Hayes, and J. H. Harmey. 2005. Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF). J. Cell Mol. Med. 9777-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao, Y., P. Linden, J. Farnebo, R. Cao, A. Eriksson, V. Kumar, J.-H. Qi, L. Claesson-Welsh, and K. Alitalo. 1998. Vascular endothelial growth factor C induces angiogenesis in vivo. Proc. Natl. Acad. Sci. USA 9514389-14394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carroll, P. A., E. Brazeau, and M. Lagunoff. 2004. Kaposi's sarcoma-associated herpesvirus infection of blood endothelial cells induces lymphatic differentiation. Virology 3287-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carroll, P. A., H. L. Kenerson, R. S. Yeung, and M. Lagunoff. 2006. Latent Kaposi's sarcoma-associated herpesvirus infection of endothelial cells activates hypoxia-induced factors. J. Virol. 8010802-10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Claffey, K. P., S. C. Shih, A. Mullen, S. Dziennis, J. L. Cusick, K. R. Abrams, S. W. Lee, and M. Detmar. 1998. Identification of a human VPF/VEGF 3′ untranslated region mediating hypoxia-induced mRNA stability. Mol. Biol. Cell 9469-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Vries, C., J. A. Escobedo, H. Ueno, K. Houck, N. Ferrara, and L. T. Williams. 1992. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science 255989-991. [DOI] [PubMed] [Google Scholar]
- 19.Dezube, B. J., M. Zambela, D. R. Sage, J.-F. Wang, and J. D. Fingeroth. 2002. Characterization of Kaposi sarcoma-associated herpesvirus/human herpesvirus-8 infection of human vascular endothelial cells: early events. Blood 100888-896. [DOI] [PubMed] [Google Scholar]
- 20.Dourmishev, L. A., A. L. Dourmishev, D. Palmeri, R. A. Schwartz, and D. M. Lukac. 2003. Molecular genetics of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) epidemiology and pathogenesis. Microbiol. Mol. Biol. Rev. 67175-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ema, M., K. Hirota, J. Mimura, H. Abe, J. Yodoi, K. Sogawa, L. Poellinger, and Y. Fujii-Kuriyama. 1999. Molecular mechanisms of transcription activation by HLF and HIF1α in response to hypoxia: their stabilization and redox signal-induced interaction with CBP/p300. EMBO J. 181905-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Enomoto, H., I. Inoki, K. Komiya, T. Shiomi, E. Ikeda, K.-I. Obata, H. Matsumoto, Y. Toyama, and Y. Okada. 2003. Vascular endothelial growth factor isoforms and their receptors are expressed in human osteoarthritic cartilage. Am. J. Pathol. 162171-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ensoli, B., G. Barillari, and R. C. Gallo. 1992. Cytokines and growth factors in the pathogenesis of AIDS-associated Kaposi's sarcoma. Immunol. Rev. 127147-155. [DOI] [PubMed] [Google Scholar]
- 24.Ensoli, B., S. Z. Salahuddin, and R. C. Gallo. 1989. AIDS-associated Kaposi's sarcoma: a molecular model for its pathogenesis. Cancer Cell 193-96. [PubMed] [Google Scholar]
- 25.Ferrara, N., and T. Davis-Smyth. 1997. The biology of vascular endothelial growth factor. Endocr. Rev. 184-25. [DOI] [PubMed] [Google Scholar]
- 26.Fiorelli, V., R. Gendelman, M. C. Sirianni, H. K. Chang, S. Colombini, P. D. Markham, P. Monini, J. Sonnabend, A. Pintus, R. C. Gallo, and B. Ensoli. 1998. γ-Interferon produced by CD8+ T cells infiltrating Kaposi's sarcoma induces spindle cells with angiogenic phenotype and synergy with human immunodeficiency virus-1 Tat protein: an immune response to human herpesvirus-8 infection? Blood 91956-967. [PubMed] [Google Scholar]
- 27.Folkman, J., and Y. Shing. 1992. Angiogenesis. J. Biol. Chem. 26710931-10934. [PubMed] [Google Scholar]
- 28.Groger, M., R. Loewe, W. Holnthoner, R. Embacher, M. Pillinger, G. S. Herron, K. Wolff, and P. Petzelbauer. 2004. IL-3 induces expression of lymphatic markers Prox-1 and podoplanin in human endothelial cells. J. Immunol. 1737161-7169. [DOI] [PubMed] [Google Scholar]
- 29.Grundhoff, A., and D. Ganem. 2004. Inefficient establishment of KSHV latency suggests an additional role for continued lytic replication in Kaposi sarcoma pathogenesis. J. Clin. Investig. 113124-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hong, Y. K., K. Foreman, J. W. Shin, S. Hirakawa, C. L. Curry, D. R. Sage, T. Libermann, B. J. Dezube, J. D. Fingeroth, and M. Detmar. 2004. Lymphatic reprogramming of blood vascular endothelium by Kaposi sarcoma-associated herpesvirus. Nat. Genet. 36683-685. [DOI] [PubMed] [Google Scholar]
- 31.Houck, K. A., D. W. Leung, A. M. Rowland, J. Winer, and N. Ferrara. 1992. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J. Biol. Chem. 26726031-26037. [PubMed] [Google Scholar]
- 32.Jeltsch, M., A. Kaipainen, V. Joukov, X. Meng, M. Lakso, H. Rauvala, M. Swartz, D. Fukumura, R. K. Jain, and K. Alitalo. 1997. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science 2761423-1425. [DOI] [PubMed] [Google Scholar]
- 33.Kaaya, E. E., C. Parravicini, C. Ordonez, R. Gendelman, E. Berti, R. C. Gallo, and P. Biberfeld. 1995. Heterogeneity of spindle cells in Kaposi's sarcoma: comparison of cells in lesions and in culture. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 10295-305. [PubMed] [Google Scholar]
- 34.Kaipainen, A., J. Korhonen, T. Mustonen, V. W. M. van Hinsbergh, G. Fang, D. Dumont, M. Breitman, and K. Alitalo. 1995. Expression of the Fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc. Natl. Acad. Sci. USA 923566-3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karkkainen, M. J., P. Haiko, K. Sainio, J. Partanen, J. Taipale, T. V. Petrova, M. Jeltsch, D. G. Jackson, M. Talikka, H. Rauvala, C. Betsholtz, and K. Alitalo. 2004. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat. Immunol. 574-80. [DOI] [PubMed] [Google Scholar]
- 36.Krishnan, H. H., P. P. Naranatt, M. S. Smith, L. Zeng, C. Bloomer, and B. Chandran. 2004. Concurrent expression of latent and a limited number of lytic genes with immune modulation and antiapoptotic function by Kaposi's sarcoma-associated herpesvirus early during infection of primary endothelial and fibroblast cells and subsequent decline of lytic gene expression. J. Virol. 783601-3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krishnan, H. H., N. Sharma-Walia, D. N. Streblow, P. P. Naranatt, and B. Chandran. 2006. Focal adhesion kinase is critical for entry of Kaposi's sarcoma-associated herpesvirus into target cells. J. Virol. 801167-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krishnan, H. H., N. Sharma-Walia, L. Zeng, S.-J. Gao, and B. Chandran. 2005. Envelope glycoprotein gB of Kaposi's sarcoma-associated herpesvirus is essential for egress from infected cells. J. Virol. 7910952-10967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kukk, E., A. Lymboussaki, S. Taira, A. Kaipainen, M. Jeltsch, V. Joukov, and K. Alitalo. 1996. VEGF-C receptor binding and pattern of expression with VEGFR-3 suggests a role in lymphatic vascular development. Development 1223829-3837. [DOI] [PubMed] [Google Scholar]
- 40.Lagunoff, M., J. Bechtel, E. Venetsanakos, A.-M. Roy, N. Abbey, B. Herndier, M. McMahon, and D. Ganem. 2002. De novo infection and serial transmission of Kaposi's sarcoma-associated herpesvirus in cultured endothelial cells. J. Virol. 762440-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li, Q., Q. Lu, V. Bottero, G. Estepa, L. Morrison, F. Mercurio, and I. M. Verma. 2005. Enhanced NF-κB activation and cellular function in macrophages lacking IκB kinase 1 (IKK1). Proc. Natl. Acad. Sci. USA 10212425-12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lunardi-Iskandar, Y., P. Gill, V. H. Lam, R. A. Zeman, F. Michaels, D. L. Mann, M. S. Reitz, Jr., M. Kaplan, Z. N. Berneman, D. Carter, et al. 1995. Isolation and characterization of an immortal neoplastic cell line (KS Y-1) from AIDS-associated Kaposi's sarcoma. J. Natl. Cancer Inst. 87974-981. [DOI] [PubMed] [Google Scholar]
- 43.Mandriota, S. J., L. Jussila, M. Jeltsch, A. Compagni, D. Baetens, R. Prevo, S. Banerji, J. Huarte, R. Montesano, D. G. Jackson, L. Orci, K. Alitalo, G. Christofori, and M. S. Pepper. 2001. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J. 20672-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marchio, S., L. Primo, M. Pagano, G. Palestro, A. Albini, T. Veikkola, I. Cascone, K. Alitalo, and F. Bussolino. 1999. Vascular endothelial growth factor-C stimulates the migration and proliferation of Kaposi's sarcoma cells. J. Biol. Chem. 27427617-27622. [DOI] [PubMed] [Google Scholar]
- 45.Masood, R., J. Cai, T. Zheng, D. L. Smith, Y. Naidu, and P. S. Gill. 1997. Vascular endothelial growth factor/vascular permeability factor is an autocrine growth factor for AIDS-Kaposi sarcoma. Proc. Natl. Acad. Sci. USA 94979-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsumoto, K., H. Ohi, and K. Kanmatsuse. 1997. Interleukin 10 and interleukin 13 synergize to inhibit vascular permeability factor release by peripheral blood mononuclear cells from patients with lipoid nephrosis. Nephron 77212-218. [DOI] [PubMed] [Google Scholar]
- 47.McCormick, C., and D. Ganem. 2005. The kaposin B protein of KSHV activates the p38/MK2 pathway and stabilizes cytokine mRNAs. Science 307739-741. [DOI] [PubMed] [Google Scholar]
- 48.Mohle, R., D. Green, M. A. S. Moore, R. L. Nachman, and S. Rafii. 1997. Constitutive production and thrombin-induced release of vascular endothelial growth factor by human megakaryocytes and platelets. Proc. Natl. Acad. Sci. USA 94663-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moses, A. V., K. N. Fish, R. Ruhl, P. P. Smith, J. G. Strussenberg, L. Zhu, B. Chandran, and J. A. Nelson. 1999. Long-term infection and transformation of dermal microvascular endothelial cells by human herpesvirus 8. J. Virol. 736892-6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mustonen, T., and K. Alitalo. 1995. Endothelial receptor tyrosine kinases involved in angiogenesis. J. Cell Biol. 129895-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Naranatt, P. P., S. M. Akula, C. A. Zien, H. H. Krishnan, and B. Chandran. 2003. Kaposi's sarcoma-associated herpesvirus induces the phosphatidylinositol 3-kinase-PKC-ζ-MEK-ERK signaling pathway in target cells early during infection: implications for infectivity. J. Virol. 771524-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Naranatt, P. P., H. H. Krishnan, S. R. Svojanovsky, C. Bloomer, S. Mathur, and B. Chandran. 2004. Host gene induction and transcriptional reprogramming in Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8)-infected endothelial, fibroblast, and B cells: insights into modulation events early during infection. Cancer Res. 6472-84. [DOI] [PubMed] [Google Scholar]
- 53.Neipel, F., J. C. Albrecht, and B. Fleckenstein. 1997. Cell-homologous genes in the Kaposi's sarcoma-associated rhadinovirus human herpesvirus 8: determinants of its pathogenicity? J. Virol. 714187-4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neufeld, G., T. Cohen, S. Gengrinovitch, and Z. Poltorak. 1999. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 139-22. [PubMed] [Google Scholar]
- 55.Niki, T., S. Iba, M. Tokunou, T. Yamada, Y. Matsuno, and S. Hirohashi. 2000. Expression of vascular endothelial growth factors A, B, C, and D and their relationships to lymph node status in lung adenocarcinoma. Clin. Cancer Res. 62431-2439. [PubMed] [Google Scholar]
- 56.Oh, S.-J., M. M. Jeltsch, R. Birkenhager, J. E. G. McCarthy, H. A. Weich, B. Christ, K. Alitalo, and J. Wilting. 1997. VEGF and VEGF-C: specific induction of angiogenesis and lymphangiogenesis in the differentiated avian chorioallantoic membrane. Dev. Biol. 18896-109. [DOI] [PubMed] [Google Scholar]
- 57.Park, J. E., G. A. Keller, and N. Ferrara. 1993. The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol. Biol. Cell 41317-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Petrova, T. V., T. Makinen, T. P. Makela, J. Saarela, I. Virtanen, R. E. Ferrell, D. N. Finegold, D. Kerjaschki, S. Yla-Herttuala, and K. Alitalo. 2002. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J. 214593-4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pichiule, P., J. C. Chavez, and J. C. LaManna. 2004. Hypoxic regulation of angiopoietin-2 expression in endothelial cells. J. Biol. Chem. 27912171-12180. [DOI] [PubMed] [Google Scholar]
- 60.Pyakurel, P., F. Pak, A. R. Mwakigonja, E. Kaaya, T. Heiden, and P. Biberfeld. 2006. Lymphatic and vascular origin of Kaposi's sarcoma spindle cells during tumor development. Int. J. Cancer 1191262-1267. [DOI] [PubMed] [Google Scholar]
- 61.Regezi, J. A., L. A. MacPhail, T. E. Daniels, Y. G. DeSouza, J. S. Greenspan, and D. Greenspan. 1993. Human immunodeficiency virus-associated oral Kaposi's sarcoma. A heterogeneous cell population dominated by spindle-shaped endothelial cells. Am. J. Pathol. 143240-249. [PMC free article] [PubMed] [Google Scholar]
- 62.Reinhardt, B., P. Schaarschmidt, A. Bossert, A. Luske, G. Finkenzeller, T. Mertens, and D. Michel. 2005. Upregulation of functionally active vascular endothelial growth factor by human cytomegalovirus. J. Gen. Virol. 8623-30. [DOI] [PubMed] [Google Scholar]
- 63.Renne, R., D. Blackbourn, D. Whitby, J. Levy, and D. Ganem. 1998. Limited transmission of Kaposi's sarcoma-associated herpesvirus in cultured cells. J. Virol. 725182-5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Renne, R., W. Zhong, B. Herndier, M. McGrath, N. Abbey, D. Kedes, and D. Ganem. 1996. Lytic growth of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat. Med. 2342-346. [DOI] [PubMed] [Google Scholar]
- 65.Robinson, C. J., and S. E. Stringer. 2001. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J. Cell Sci. 114853-865. [DOI] [PubMed] [Google Scholar]
- 66.Roy, H., S. Bhardwaj, and S. Yla-Herttuala. 2006. Biology of vascular endothelial growth factors. FEBS Lett. 5802879-2887. [DOI] [PubMed] [Google Scholar]
- 67.Russo, J. J., R. A. Bohenzky, M.-C. Chien, J. Chen, M. Yan, D. Maddalena, J. P. Parry, D. Peruzzi, I. S. Edelman, Y. Chang, and P. S. Moore. 1996. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8). Proc. Natl. Acad. Sci. USA 9314862-14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sadagopan, S., N. Sharma-Walia, M. V. Veettil, H. Raghu, R. Sivakumar, V. Bottero, and B. Chandran. 2007. Kaposi's sarcoma-associated herpesvirus induces sustained NF-κB activation during de novo infection of primary human dermal microvascular endothelial cells that is essential for viral gene expression. J. Virol. 813949-3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Samaniego, F., P. D. Markham, R. Gendelman, Y. Watanabe, V. Kao, K. Kowalski, J. A. Sonnabend, A. Pintus, R. C. Gallo, and B. Ensoli. 1998. Vascular endothelial growth factor and basic fibroblast growth factor present in Kaposi's sarcoma (KS) are induced by inflammatory cytokines and synergize to promote vascular permeability and KS lesion development. Am. J. Pathol. 1521433-1443. [PMC free article] [PubMed] [Google Scholar]
- 70.Sarid, R., J. S. Wiezorek, P. S. Moore, and Y. Chang. 1999. Characterization and cell cycle regulation of the major Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) latent genes and their promoter. J. Virol. 731438-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sharma-Walia, N., H. H. Krishnan, P. P. Naranatt, L. Zeng, M. S. Smith, and B. Chandran. 2005. ERK1/2 and MEK1/2 induced by Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) early during infection of target cells are essential for expression of viral genes and for establishment of infection. J. Virol. 7910308-10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sharma-Walia, N., H. Raghu, S. Sadagopan, R. Sivakumar, M. V. Veettil, P. P. Naranatt, M. M. Smith, and B. Chandran. 2006. Cyclooxygenase 2 induced by Kaposi's sarcoma-associated herpesvirus early during in vitro infection of target cells plays a role in the maintenance of latent viral gene expression. J. Virol. 806534-6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Skobe, M., L. F. Brown, K. Tognazzi, R. K. Ganju, B. J. Dezube, K. Alitalo, and M. Detmar. 1999. Vascular endothelial growth factor-C (VEGF-C) and its receptors KDR and flt-4 are expressed in AIDS-associated Kaposi's sarcoma. J. Investig. Dermatol. 1131047-1053. [DOI] [PubMed] [Google Scholar]
- 74.Skobe, M., T. Hawighorst, D. G. Jackson, R. Prevo, L. Janes, P. Velasco, L. Riccardi, K. Alitalo, K. Claffey, and M. Detmar. 2001. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat. Med. 7192-198. [DOI] [PubMed] [Google Scholar]
- 75.Sodhi, A., S. Montaner, V. Patel, M. Zohar, C. Bais, E. A. Mesri, and J. S. Gutkind. 2000. The Kaposi's sarcoma-associated herpes virus G protein-coupled receptor up-regulates vascular endothelial growth factor expression and secretion through mitogen-activated protein kinase and p38 pathways acting on hypoxia-inducible factor 1α. Cancer Res. 604873-4880. [PubMed] [Google Scholar]
- 76.Su, J.-L., J.-Y. Shih, M.-L. Yen, Y.-M. Jeng, C.-C. Chang, C.-Y. Hsieh, L.-H. Wei, P.-C. Yang, and M.-L. Kuo. 2004. Cyclooxygenase-2 induces EP1- and HER-2/Neu-dependent vascular endothelial growth factor-C up-regulation: a novel mechanism of lymphangiogenesis in lung adenocarcinoma. Cancer Res. 64554-564. [DOI] [PubMed] [Google Scholar]
- 77.Terman, B. I., M. Dougher-Vermazen, M. E. Carrion, D. Dimitrov, D. C. Armellino, D. Gospodarowicz, and P. Bohlen. 1992. Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem. Biophys. Res. Commun. 1871579-1586. [DOI] [PubMed] [Google Scholar]
- 78.Veettil, M. V., N. Sharma-Walia, S. Sadagopan, H. Raghu, R. Sivakumar, P. P. Naranatt, and B. Chandran. 2006. RhoA-GTPase facilitates entry of Kaposi's sarcoma-associated herpesvirus into adherent target cells in a Src-dependent manner. J. Virol. 8011432-11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang, F.-Z., S. M. Akula, N. P. Pramod, L. Zeng, and B. Chandran. 2001. Human herpesvirus 8 envelope glycoprotein K8.1A interaction with the target cells involves heparan sulfate. J. Virol. 757517-7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang, F.-Z., S. M. Akula, N. Sharma-Walia, L. Zeng, and B. Chandran. 2003. Human herpesvirus 8 envelope glycoprotein B mediates cell adhesion via its RGD sequence. J. Virol. 773131-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang, H. W., M. W. Trotter, D. Lagos, D. Bourboulia, S. Henderson, T. Makinen, S. Elliman, A. M. Flanagan, K. Alitalo, and C. Boshoff. 2004. Kaposi sarcoma herpesvirus-induced cellular reprogramming contributes to the lymphatic endothelial gene expression in Kaposi sarcoma. Nat. Genet. 36687-693. [DOI] [PubMed] [Google Scholar]
- 82.Wang, L., N. Wakisaka, C. C. Tomlinson, S. M. DeWire, S. Krall, J. S. Pagano, and B. Damania. 2004. The Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) K1 protein induces expression of angiogenic and invasion factors. Cancer Res. 642774-2781. [DOI] [PubMed] [Google Scholar]
- 83.Weninger, W., T. A. Partanen, S. Breiteneder-Geleff, C. Mayer, H. Kowalski, M. Mildner, J. Pammer, M. Sturzl, D. Kerjaschki, K. Alitalo, and E. Tschachler. 1999. Expression of vascular endothelial growth factor receptor-3 and podoplanin suggests a lymphatic endothelial cell origin of Kaposi's sarcoma tumor cells. Lab. Investig. 79243-251. [PubMed] [Google Scholar]
- 84.Wigle, J. T., N. Harvey, M. Detmar, I. Lagutina, G. Grosveld, M. D. Gunn, D. G. Jackson, and G. Oliver. 2002. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 211505-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wigle, J. T., and G. Oliver. 1999. Prox1 function is required for the development of the murine lymphatic system. Cell 98769-778. [DOI] [PubMed] [Google Scholar]
- 86.Ye, J., D. Shedd, and G. Miller. 2005. An Sp1 response element in the Kaposi's sarcoma-associated herpesvirus open reading frame 50 promoter mediates lytic cycle induction by butyrate. J. Virol. 791397-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]