Abstract
Histone lysines can be mono-, di-, or trimethylated, providing an ample magnitude of epigenetic information for transcription regulation. In fungi, SET2 is the sole methyltransferase responsible for mono-, di-, and trimethylation of H3K36. Here we show that in Arabidopsis thaliana, the degree of H3K36 methylation is regulated by distinct methyltransferases. The SET2 homologs SDG8 and SDG26 each can methylate oligonucleosomes in vitro, and both proteins are localized in the nucleus. While the previously reported loss-of-function sdg8 mutants have an early-flowering phenotype, the loss-of-function sdg26 mutants show a late-flowering phenotype. Consistently, several MADS-box flowering repressors are down-regulated by sdg8 but up-regulated by sdg26. The sdg8 but not the sdg26 mutant plants show a dramatically reduced level of both di- and trimethyl-H3K36 and an increased level of monomethyl-H3K36. SDG8 is thus specifically required for di- and trimethylation of H3K36. Our results further establish that H3K36 di- and tri- but not monomethylation correlates with transcription activation. Finally, we show that SDG8 and VIP4, which encodes a component of the PAF1 complex, act independently and synergistically in transcription regulation. Together our results reveal that the deposition of H3K36 methylation is finely regulated, possibly to cope with the complex regulation of growth and development in higher eukaryotes.
During the past few years, histone lysine (K) methylation has been viewed to play widespread roles in transcriptional regulation, DNA repair, and epigenetic inheritance (15, 32). It occurs on histone H3K4, H3K9, H3K27, H3K36, and H4K20 in several studied eukaryotes. In general, H3K4 and H3K36 methylation is associated with actively transcribed genes, whereas H3K9, H3K27, and H4K20 methylation is associated with transcriptional repression and silenced chromatin regions. Furthermore, K residues can be mono-, di- or trimethylated, and the degree of methylation on H3K4, H3K9, H3K27, and H4K20 has considerable influence on transcriptional activation or repression (15, 43, 55, 63). In comparison, methylation on H3K36 is less extensively characterized. In fungi, such as Saccharomyces cerevisiae, Schizosaccharomyces pombe, and Neurospora crassa, a sole histone-lysine-methyltransferase (HKMT), SET2, is responsible for mono-, di-, and trimethylation of H3K36 (1, 35, 49). In mammals, both the Sotos syndrome and leukemia-associated protein NSD1 and the Huntington disease protein HYPB can methylate H3K36 in vitro (42, 50). In Arabidopsis thaliana, the loss-of-function sdg8 (also named efs) mutants show a dramatically reduced level of H3K36 dimethylation and an early-flowering phenotype (25, 65). Because of these phenotype-associated crucial functions, unraveling the mechanism of deposition of H3K36 methylation in mammals and in plants is of particular importance.
Proper timing of flowering is pivotal for the reproductive success of plants and thus is controlled by complex genetic networks, which involve histone modifications and chromatin remodelling. In Arabidopsis, the MADS-box transcription repressor FLOWERING LOCUS C (FLC) plays a key role in flowering time control (34, 46). Both the vernalization pathway and the autonomous pathway act to repress FLC expression to induce flowering (6). The vernalization pathway represses FLC expression through H3K9 and H3K27 methylation and binding to LIKE-HETEROCHROMATIN PROTEIN 1 (5, 16, 28, 36, 51, 52, 58). The autonomous pathway genes FLOWERING LOCUS D (FLD) and FVE repress FLC expression through histone deacetylation (4, 18, 23). More recently, methylation on histone H4 arginine 3 by SHK1-BINDING PROTEIN 1 was also shown to repress FLC expression (57).
FRIGIDA (FRI), a coiled-coil protein, is a potent activator of FLC expression (19). The zinc-finger protein SUPPRESSOR OF FRIGIDA 4 (SUF4) was more recently identified to form a complex with FRI in activation of FLC expression (24, 26). However, a functional FRI gene is missing in many rapid-cycling accessions of Arabidopsis, including the Columbia (Col) ecotype. Independently of FRI, the activation of FLC expression implicates processes of ATP-dependent chromatin remodeling and exchange of conventional-with-variant histones in the nucleosome (8, 10, 11, 31, 33, 37). Several genes encoding homologues of the yeast RNA polymerase II-associated PAF1 complex, including VERNALIZATION INDEPENDENCE 4 (VIP4), VIP3, VIP5, VIP6/ELF8 (EARLY FLOWERING 8), and ELF7, also promote FLC expression (17, 38). The yeast PAF1 complex promotes transcription in part by recruiting the H3K4-specific HKMT SET1 and the H3K36-specific HKMT SET2 (29, 30). A similar mechanism seems to be conserved in Arabidopsis, because both H3K4 and H3K36 methylations at FLC are decreased in the paf1 mutants (17; this study).
Arabidopsis has five FLC paralogs, named MADS AFFECTING FLOWERING 1 (MAF1) to MAF5. Unlike FLC, MAF1 (also called FLM) functions independently of the vernalization and autonomous pathways but is involved in the photoperiod pathway (45). MAF2 expression is insensitive to vernalization, and MAF2 acts as a floral repressor to prevent precocious vernalization by short cold spells (41). Apart from this, the molecular mechanisms of regulation and function of the MAF genes are largely unknown.
In this study, we identify SET DOMAIN GROUP 26 (SDG26) as a novel regulator involved in flowering time control. We show that in contrast to the sdg8 mutants, the sdg26 mutants display increased levels of expression of FLC, MAF4, and MAF5 and show a late-flowering phenotype. More importantly, for the first time, we show that monomethylation, dimethylation, and trimethylation on H3K36 are differently deposited and that di- and tri- but not monomethylation on H3K36 associates with transcription stimulation of MADS-box genes.
MATERIALS AND METHODS
Phylogenetic analysis.
The entire protein sequences were aligned using the ClustalW multiple sequence alignment program and then optimized manually by removing poorly aligning regions, which leaves only the SET domain. The finalized file was subjected to phylogenic analysis using MEGA3.0 with bootstrapping set at 500 replicates. The resulting consensus tree was displayed using the TreeView program.
Plant materials and growth conditions.
All Arabidopsis mutants are in the Col background. The sdg8-1, sdg8-2, vip4, and vip4 sdg8-1 mutants were previously described (65). The sdg26-1 and sdg26-2 mutants correspond to SALK_013895 and SALK_075791, respectively, of the T-DNA insertion strains from the Arabidopsis Biological Resource Centre and Nottingham Arabidopsis Stock Center (http://www.Arabidopsis.org). Plant growth conditions and flowering-time measurements were performed as previously described (65).
RT-PCR.
Reverse transcription (RT)-PCR was used in isolation of SDG8 and SDG26 cDNA and in analysis of gene expression. Total RNA was extracted from 2-week-old Arabidopsis seedlings using TRI Reagent (Invitrogen, Cergy Pontoise, France), and cDNA synthesis was performed according to the manufacturer's recommendation (Pharmacia). PCR amplification from the cDNA template was performed using gene-specific primers (see Table S1 in the supplemental material for details of primers used in this study).
Recombinant-protein production.
The recombinant SDG714 protein was produced as previously described (12). For SDG8 expression vector construction, the 5′-end (about 3.2 kb in length) and the 3′-end (about 2.3 kb in length) coding regions of the SDG8 cDNA were separately PCR amplified using the primer pairs SDG8-RT5 with SDG8-M4 and SDG8-M3 with SDG8-RT3 (see Table S1 in the supplemental material), respectively. The two PCR products were ligated together using the restriction enzyme XhoI site, and the resulted fragment was digested with EcoRI and SalI and subsequently cloned into the EcoRI and XhoI sites of pGEX-4T1 (Pharmacia). The resulting vector, pGEX-SDG8, contains the entire coding sequence of SDG8 fused in frame with GST. The expression vectors pGEX-SDG8L and pGEX-SDG8S, containing a partial coding sequence of SDG8 fused in frame with GST, were obtained by PCR amplification of the cloned SDG8 cDNA using the primer pairs SDG8-A1 with SDG8-A2 and SDG8-B1 with SDG8-B2 (see Table S1 in the supplemental material) and subsequent cloning of the products into the EcoRI and BamHI sites of pGEX-2KT (Pharmacia, France). The expression vector pGEX-SDG26, containing the entire coding sequence of SDG26 fused in frame with GST, was obtained by RT-PCR amplification using the primer pair SDG26-RT5 with SDG26-RT3 (see Table S1 in the supplemental material) and subsequent cloning of the product into the EcoRI and XhoI sites of pGEX-4T1. Expression and purification of glutathione-S-transferase-fused proteins were performed according to the previously described procedure (14).
In vitro HKMT activity assay.
Histone methyltransferase assays were performed essentially according to the method of Rayasam et al. (42). Briefly, a mixture with a volume of 30 to 50 μl containing the substrate, enzyme, and 250 nCi S-adenosyl-l-[methyl-14C]methionine (Amersham) in the reaction buffer (50 mM Tris-HCl [pH 8.8], 1 mM phenylmethylsulfonyl fluoride, and 0.5 mM dithiothreitol) was incubated for 60 min at 37°C. For the substrate, the free histones were purchased (Sigma); the mononucleosomes and oligonucleosomes prepared from HeLa cells were gifts from Laszlo Tora and Yi Zhang, respectively. The reaction products were separated by 15% polyacrylamide gel electrophoresis and visualized for total proteins by staining with Coomassie brilliant blue R-250 and for methylated proteins by autoradiography.
Plant expression vector construction and transformation.
The plant expression vector pER8-YFP:SDG8 was constructed by PCR amplification of the entire coding sequence of the cloned SDG8 cDNA using the primer pair SDG8-RT5 with SDG8-Y3 (see Table S1 in the supplemental material) and ligation of the resulting product after digestion with SpeI and EcoRI together with the EcoRI-XhoI yellow fluorescent protein (YFP) fragment from pEYFP-EYFP (60) into the SpeI and XhoI sites of pER8 (67). Similarly, the vector pER8-YFP:SDG26 was constructed by PCR amplification of the entire coding sequence of SDG26 cDNA using the primer pair SDG26-Y5 with SDG26-Y3 (see Table S1 in the supplemental material) and ligation of the resulting product after digestion with XbaI and BamHI together with the BamHI-XhoI YFP fragment from pEYFP-EYFP into the SpeI and XhoI sites of pER8. These vectors, expressing YFP-fused SDG8 and SDG26 under the control of the estradiol-inducible promoter, were transformed into Agrobacterium tumefaciens, and the resulting strains were used to transform Arabidopsis plants and tobacco BY2 cells as described previously (61). Induction of transgene expression was performed with 4 μM estradiol according to Zuo et al. (67).
For rescue of the sdg26 mutant phenotype, the vector pCAMBIA-SDG26 was constructed by PCR amplification of the entire coding sequence of SDG26 cDNA using the primer pair SDG26-F with SDG26-R (see Table S1 in the supplemental material) and subsequent cloning of the resulting product into the BamHI and XhoI sites of pCAMBIA1300. The SDG26 cDNA under the control of the constitutive 35S promoter from pCAMBIA-SDG26 was introduced into the sdg26-1 and sdg26-2 mutant plants via Agrobacterium-mediated transformation.
Microscopy.
The epifluorescence and differential interference contrast images were taken using a confocal laser scanning microscope, Zeiss model LSM510 (Carl Zeiss, Jena, Germany), as previously described (47).
Histone extraction and Western blot analysis.
Arabidopsis histones were extracted from 15-day-old seedlings as previously described (60), separated by 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred to an Immobilon-P polyvinylidene difluoride transfer membrane (Millipore). Western blot was performed using the following antibodies: anti-trimethyl-H3K4 (07-473; Upstate), anti-trimethyl-H4K20 (07-463; Upstate), anti-trimethyl-H3K36 (ab9050; Abcam), anti-dimethyl-H3K36 (07-369; Upstate), anti-monomethyl-H3K36 (ab9048; Abcam), and anti-H3 (05-499; Upstate).
ChIP.
Chromatin immunoprecipitation (ChIP) assays were performed as previously described (25, 65). The antibodies against trimethyl-H3K4 and monomethyl-, dimethyl-, and trimethyl-H3K36 were the same as described for Western blot analysis. PCR primers used in this study are listed in Table S1 in the supplemental material.
Microarray analyses.
Microarray analysis was performed as described previously (66). Briefly, wild-type and mutant seeds were germinated under the same growth conditions. Three independently derived sets of 6-day-old seedlings, 30 to 40 plants per set, were pooled for each genotype. Total RNA was isolated from each sample and used for hybridization of the CATMA slides. To further enrich for biologically relevant changes linked with a mutant genotype, a second experiment with new sets of seeds was performed. Genes were considered significantly perturbed in the mutant if the change was at least 1.5-fold and the P values inferior to 0.05 from the two independent experiments.
Microarray data accession numbers.
Microarray data were deposited in the Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/) (accession no. GSE8427 and GSE8429) and at CATdb (http://urgv.evry.inra.fr/CATdb/) (project RS05-12_SETII) according to the “Minimum Information About a Microarray Experiment” standards.
RESULTS
SDG8 and SDG26 are histone methyltransferases.
The evolutionarily conserved SET [Su(var)3-9, E(Z), Trithorax-conserved] domain, a domain of approximately 130 amino acids in length, forms a knot-like structure and constitutes the catalytic core of HKMTs (for a recent review, see reference 40). The activity and specificity of an HKMT largely depend on the amino acid sequences within its SET domain. Therefore, SET-domain sequences are generally used to classify SET-domain proteins (48). Among the annotated 43 SDG proteins in the Arabidopsis genome (http://www.chromdb.org), 5 proteins (SDG4, SDG7, SDG8, SDG24, and SDG26) are grouped by phylogenetic analysis in a clade together with the H3K36-specific HKMTs so far found in fungi and mammals and 6 proteins (SDG14, SDG16, SDG25, SDG27, SDG29, and SDG30) in a clade with the H3K4-specific HKMTs (Fig. 1). Currently only SDG27 (ATX1) was experimentally demonstrated to possess HKMT activity on H3K4 (3). Although the level of dimethyl-H3K36 was dramatically reduced in the sdg8 mutant plants (65), the enzyme activity of SDG8 was not directly tested.
FIG. 1.
Cladogram of Arabidopsis SDG proteins that are homologous to H3K4- and H3K36-specific HKMTs of other organisms. The phylogenic analysis was performed using the MEGA3.0 package. GenBank accession numbers are as follows: NP_011987 and NP_012367 for the Saccharomyces cerevisiae proteins ScSET1 and ScSET2, respectively; NP_587812 and NP_594980 for the Schizosaccharomyces pombe proteins SpSET1 and SpSET2, respectively; XP_957740 for Neurospora crassa NsSET-2; NP_524160 and P20659 for the Drosophila melanogaster proteins DmASH1 and DmTRX, respectively; NP_055527, NP_005924, and NP_054878 for the Homo sapiens proteins HsSET1, HsMLL, and HsHYPB, respectively; and ABV68921 for SDG8. For the other SDG proteins, the sequences were as reported in the Plant Chromatin Database (http://www.chromdb.org).
The SDG26 protein shows a high level of homology with SDG8 in the SET domain and its surrounding AWS and C domains but is much smaller and lacks sequences from both the N- and C-terminal ends compared to SDG8 (Fig. 2A) (see Fig. S1 in the supplemental material for sequence alignment). To test whether SDG8 and SDG26 possess HKMT activity, recombinant SDG8 and SDG26 were produced and utilized in in vitro HKMT activity assays. The full-length form and two fragments of SDG8, SDG8L (residues 174 to 1284), and SDG8S (residues 938 to 1212), and the full-length SDG26 protein were tested as enzymes. As substrates, free histones and mononucleosomes were first successively used, but both failed to show detectable methylation by any of the recombinant SDG8 or SDG26 proteins (data not shown). Only when oligonucleosomes were used as substrates, methylations on histone H3 and on H4 were observed for the recombinant SDG8S and SDG26 proteins (Fig. 2B). This activity was abolished when the oligonucleosomes were pretreated by incubation at 60°C (Fig. 2B), which presumably altered the structure of oligonucleosomes. As a positive control, the H3K9-specific HKMT SDG714 (12, 13) methylated histone H3 in all tested substrates, including oligonucleosomes (Fig. 2B), mononucleosomes, and free histones (data not shown). SDG8 and SDG8L gave results similar to those of SDG8S, except that their expression levels were lower and consistently their histone methylation signals were weaker (data not shown). The previously reported H3K36-specific HKMTs from fungi and mammals could methylate free histones in vitro (1, 35, 42, 49, 50). The SDG8 and SDG26 HKMTs thus appear specific for native oligonucleosomes as substrates. This dissuaded us from using recombinant histones to determine specific methylation sites. Our analyses of methylation sites from in vitro-methylated oligonucleosomes also failed, likely because of the relative low level of methylation achieved in vitro. Nevertheless, our in vivo analyses, as shown below, support SDG8 as a major H3K36-specific HKMT in Arabidopsis.
FIG. 2.
Both SDG8 and SDG26 can methylate in vitro histones H3 and H4 using oligonucleosomes as substrates and are localized in the nucleus. (A) Schematic diagrams of the SDG8, SDG8L, SDG8S, and SDG26 proteins. The color boxes represent the conserved protein domains: CW (cysteine and tryptophan conserved), AWS (associated with SET), SET, and C (cysteine rich). (B) In vitro methylation assay. The upper panel shows a Coomassie blue-stained gel, and the lower panel shows corresponding autoradiography. The tested recombinant proteins as enzymes are indicated on top of the lanes. For the first four lanes on the left, native oligonucleosomes were used as substrates. For the other four lanes, close to the molecular size marker (M), oligonucleosomes were preincubated at 60°C for 10 min and then used as substrates. Positions of nucleosome core histones H2A, H2B, H3, and H4 are indicated. (C) Localization of YFP-SDG8 in an epidermal cell of the transgenic Arabidopsis root. (D) Localization of YFP-SDG26 in cortex cells of the transgenic Arabidopsis root. (E and F) Localization of YFP-SDG8 and YFP-SDG26 in transgenic tobacco BY2 cells, respectively. A fluorescence confocal image is shown together with a differential interference contrast image. Note that fluorescence is particularly strong in the spherical nucleus.
SDG8 and SDG26 proteins are localized in the nucleus.
The subcellular localization of SDG8 and SDG26 was examined by expression of YFP fusion proteins. In transgenic Arabidopsis, both YFP-SDG8 and YFP-SDG26 are localized in the nucleus of root cells (Fig. 2C and D). A similar nuclear localization was also observed in the other examined tissues, including those in coleoptiles and leaves (data not shown). In transgenic tobacco BY2 cells, both YFP-SDG8 and YFP-SDG26 are also localized in the nucleus (Fig. 2E and F). The YFP-SDG8 and YFP-SDG26 proteins distribute uniformly in the nucleoplasm but are absent from the nucleolus. The nuclear localization of SDG8 and SDG26 is consistent with the proposed role for these proteins in histone methylation in chromatin.
In contrast to the early-flowering phenotype of the sdg8 mutants, the sdg26 mutants show a late-flowering phenotype.
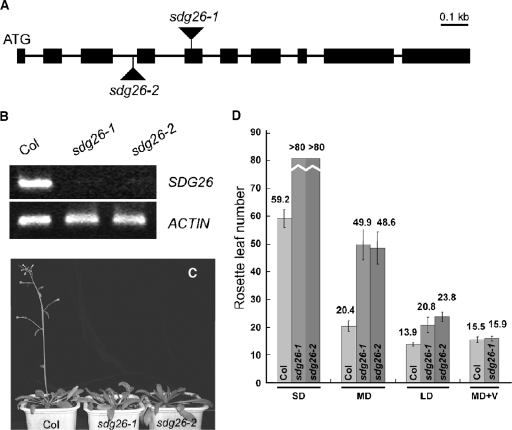
The loss-of-function sdg8-1 and sdg8-2 mutant plants show an early-flowering phenotype (65). To investigate SDG26 function, we searched for lines with T-DNA insertions within the SDG26 gene. From the SALK collection (2), two independent lines were identified, hereinafter named sdg26-1 and sdg26-2. PCR analysis confirmed that sdg26-1 contains a T-DNA insertion in the fifth exon of SDG26 and sdg26-2 contains a T-DNA insertion in the third intron of SDG26 (Fig. 3A; also data not shown). Homozygous (hereinafter called mutant) plants were obtained for both T-DNA insertion lines by self-pollination. RT-PCR analysis showed that the full-length SDG26 transcripts were absent in the mutant plants (Fig. 3B), indicating that both sdg26-1 and sdg26-2 are loss-of-function mutations. The two mutants displayed a similar late-flowering phenotype (Fig. 3C). Heterozygous plants did not show any phenotype, indicating that both the sdg26-1 and sdg26-2 mutations are recessive. Expression of SDG26 cDNA in the mutants could rescue the late-flowering phenotype (data not shown). We conclude that the loss of function of SDG26 had caused the late-flowering phenotype. The late-flowering phenotype of the sdg26-1 and sdg26-2 mutants is photoperiod independent and can be effectively suppressed by vernalization (Fig. 3D). These properties are characteristic of autonomous-pathway mutants and subsequently define SDG26 as an autonomous-pathway gene.
FIG. 3.
Loss of SDG26 function causes late flowering. (A) Exon-intron structure of the SDG26 gene. Boxes represent exons, lines represent introns, and triangles indicate T-DNA insertions in the sdg26-1 and sdg26-2 mutants. (B) RT-PCR analysis of SDG26 expression in wild-type Col and mutant sdg26-1 and sdg26-2 plants. ACTIN serves as an internal control. (C) Wild-type Col and mutant sdg26-1 and sdg26-2 plants 35 days after germination grown under long-day (16 h light and 8 h dark) conditions. (D) Rosette leaf number at flowering of wild-type Col and mutant sdg26-1 and sdg26-2 plants. SD, short day (8 h light and 16 h dark); MD, medium day (12 h light and 12 h dark); LD, long day (16 h light and 8 h dark); +V, with vernalization. The mean value for 10 to 15 plants is shown. Vertical bars represent standard deviations.
The FLC and MAF genes are down-regulated in the sdg8 mutants but up-regulated in the sdg26 mutants.
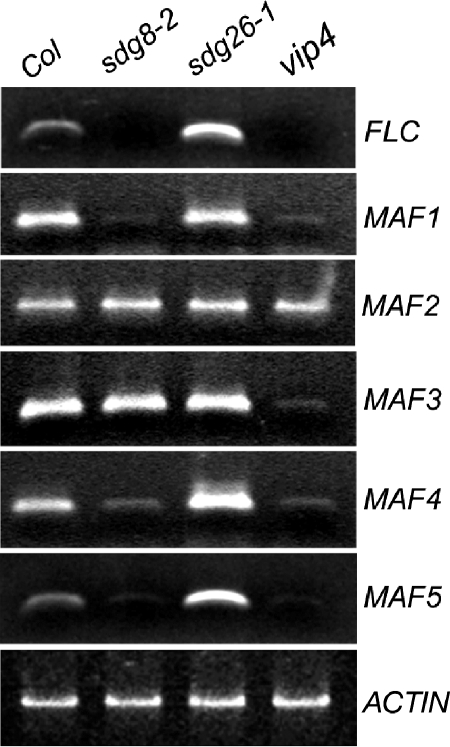
The opposite flowering phenotypes between the sdg8 and sdg26 mutants had not been predicted. To examine the molecular basis of the flowering phenotype, we performed RT-PCR analysis and compared the mRNA levels of the FLC and MAF genes in the sdg8-2 and sdg26-1 mutant plants (Fig. 4). In agreement with previous reports (25, 65), the expression levels of both FLC and MAF1 were found to be strongly reduced in the sdg8-2 mutant compared to levels in the wild-type plants. In addition, we found that the expression of MAF4 and MAF5 is also suppressed whereas that of MAF2 and MAF3 is unchanged in the sdg8-2 mutant plants. Very interestingly, the expression levels of FLC, MAF4, and MAF5 were found to be increased whereas those of MAF1, MAF2, and MAF3 remained unchanged in the sdg26-1 mutant compared to levels in the wild-type plants. VIP4 encodes a homologue of yeast LEO1, one of the PAF1 subunits that interacts with SET2 (30). The vip4 mutant has an early-flowering phenotype (62). We found that in the vip4 mutant, the expression levels of FLC, MAF1, MAF3, MAF4, and MAF5 were dramatically decreased while that of MAF2 was only slightly decreased. These gene expression patterns in the different mutants were reproducible in three independent experiments. Taken together, these data show that SDG8 and SDG26 antagonistically regulate FLC, MAF4, and MAF5 expression and that SDG8 and VIP4 synergistically regulate FLC, MAF1, MAF4, and MAF5 expression. MAF3, and to a lesser extent MAF2, is specifically regulated by VIP4 but not by SDG8 and SDG26.
FIG. 4.
MADS-box flowering repressors are down-regulated in sdg8-2 and vip4 mutants but up-regulated in the sdg26-1 mutant. RT-PCR analysis was performed on plants grown under medium-day (12 h light and 12 h dark) conditions 15 days after germination. ACTIN serves as an internal control.
The sdg8 mutant plants contain reduced levels of di- and trimethyl-H3K36 but an increased level of monomethyl-H3K36.
We analyzed histone methylation in the sdg8-2, sdg26-1, and vip4 mutant plants, first by Western blotting using antibodies specifically recognizing monomethyl-H3K36, dimethyl-H3K36, trimethyl-H3K36, trimethyl-H3K4, or trimethyl-H4K20 (Fig. 5). Both dimethyl-H3K36 and trimethyl-H3K36 were dramatically reduced in the sdg8-2 mutant plants. To our surprise, monomethyl-H3K36 was increased in the sdg8-2 mutant plants. No change was observed for trimethyl-H3K4 and trimethyl-H4K20 in the sdg8-2 mutant plants. The levels of dimethyl-H3K4, dimethyl-H3K9, and dimethyl-H3K27 also were previously reported to be unchanged in the sdg8-1 and sdg8-2 mutant plants (65). In the sdg26-1 and vip4 mutants, no change was detected for all the studied forms of histone methylation. Taken together, these data indicate that in Arabidopsis the SDG8 HKMT is primarily responsible for the di- and trimethylation on H3K36 whereas for SDG26 HKMT and the VIP4-containing PAF1 complex no effects on the global level of H3K36 and H3K4 methylation could be observed.
FIG. 5.
Western blot analysis detects changed levels of global H3K36 methylation in the sdg8-2 mutant but not in the sdg26-1 and vip4 mutants. Histone-enriched protein extracts from plants 15 days after germination grown under medium-day (12 h light and 12 h dark) conditions were analyzed by Western blotting using antibodies that specifically recognize the indicated forms of histones.
H3K36 methylation at the FLC and MAF genes is differentially affected in the sdg8, sdg26, and vip4 mutants.
We investigated mono-, di-, and trimethylation on H3K36 and trimethylation on H3K4 at gene-specific regions by ChIP analysis. Previous studies revealed that regions proximal to the translation initiation codon ATG of FLC contain dimethyl-H3K36 and trimethyl-H3K4, whose levels positively correlate with FLC expression (17, 65). We chose to analyze two regions, one upstream and the other downstream of the ATG, for each of the FLC and MAF genes (detailed information and PCR primers are available in Table S1 in the supplemental material). The results are shown in Fig. 6. In the sdg8-2 mutant, compared to results for the wild-type plants, the level of monomethyl-H3K36 was increased whereas the levels of dimethyl-H3K36 and trimethyl-H3K36 were decreased in all analyzed gene-specific regions. The level of trimethyl-H3K4 was unchanged. These data are consistent with the previous Western analysis and support the conclusion that SDG8 HKMT is specific for di- and trimethylation on H3K36. In the sdg26-1 mutant, compared to the wild-type plants, the levels of monomethyl-H3K36, dimethyl-H3K36, trimethyl-H3K36, and trimethyl-H3K4 all were unchanged in the analyzed gene-specific regions. We extended our analysis to more regions of FLC for different histone methylations. We found that the levels of monomethyl-H3K4, trimethyl-H3K4, monomethyl-H3K36, trimethyl-H3K36, and trimethyl-H4K20 were all unchanged in the studied FLC regions in the sdg26-1 mutant (see Fig. S2 in the supplemental material). It is likely that in the sdg26 mutant, the expression of FLC, MAF1, MAF4, and MAF5 was upregulated through a novel, as yet unknown mechanism. In the vip4 mutant, compared to the wild-type plants, the levels of dimethyl-H3K36 and trimethyl-H3K36, as well as trimethyl-H3K4, were decreased whereas the level of monomethyl-H3K36 was unchanged. This was the case for all analyzed gene-specific regions excepting the upstream region of MAF2, which showed unchanged levels of monomethyl-H3K36, dimethyl-H3K36, trimethyl-H3K36, and trimethyl-H3K4. This observation is consistent with the weakly affected MAF2 expression in the vip4 mutant. Together with the previous Western data showing no detectable change of global H3K36 and H3K4 methylation in the vip4 mutant, it appears that VIP4-associated changes of dimethyl-H3K36, trimethyl-H3K36, and trimethyl-H3K4 are restricted to transcriptional activity of the FLC and MAF genes. This differs from the broad HKMT activity of SDG8 on the genome. The finding that methylation of both H3K36 and H3K4 is affected in the vip4 mutant suggests that the VIP4-containing PAF1 complex recruits both H3K36 and H3K4 HKMTs in transcription of the FLC and MAF genes. Our ChIP results were reproducible in three independent experiments.
FIG. 6.
ChIP analysis uncovers differences in changed levels of H3K4 and H3K36 methylation at MADS-box flowering repressor genes in sdg8-2, sdg26-1, and vip4 mutants. Fifteen-day-old plants grown under medium-day (12 h light and 12 h dark) conditions were analyzed with antibodies that specifically recognize methylated histone H3, as indicated at the top of each panel. Input and no antibody (−) are shown as positive and negative controls, respectively. Two regions, one upstream (u) and the other downstream (d) of ATG, were analyzed for each of the MADS-box genes (FLC and MAF1 to MAF5). ACTIN serves as an internal control of an actively expressed gene.
Taken together, our results show that di- and tri- but not monomethylation on H3K36 associates with transcription stimulation of the FLC and MAF genes. Nonetheless, changes in H3K36 methylation did not systematically affect levels of gene expression, e.g., decreased levels of di- and trimethylation on H3K36 were detected at MAF2 and MAF3, whereas these genes did not show detectable changes of expression in the sdg8-2 mutant. It is likely that H3K36 methylation is not the sole determinant of the transcription rate.
sdg8 has a pleiotropic phenotype and shows a degree of synergistic interaction with vip4.
Besides late flowering, the sdg26-1 and sdg26-2 mutants did not show any additional visible phenotype. In contrast, the sdg8-1 and sdg8-2 mutants showed not only early flowering but also a dramatically reduced plant size and fertility (Fig. 7A). An increased number of primary and secondary inflorescence branches were observed for the sdg8-1 and sdg8-2 mutant plants (Fig. 7B), suggesting a reduced apical dominance in the mutant plants. Some extra branches were observed to be formed at the same position of an existing branch in the mutant plants, whereas such events were not observed in the wild-type plants. It suggests that some auxiliary neomeristems are formed in the mutant. Microscopic examination revealed that the cell sizes were the same in the wild-type (Fig. 7D) and the sdg8-1 mutant (Fig. 7E) mature petals, indicating that the smaller mutant petals (Fig. 7C) were composed of fewer total cells rather than a normal number of smaller cells. Examinations of leaf veins and stem sections also showed a reduced number of cells in the mutant compared to the wild-type plants (not shown). Taken together, it appears that SDG8 is a critical positive regulator of cell proliferation for maintaining plant size.
FIG. 7.
The sdg8 mutants show pleiotropic phenotypes and synergistic interactions with vip4. (A) sdg8-1 mutant compared to wild-type Col plants 40 days after germination and grown under long-day (16 h light and 8 h dark) conditions. (B) Comparison of branch number of 40-day-old sdg8-1 and sdg8-2 mutants with wild-type Col plants. Values shown represent the means and standard deviations for at least 10 plants of each genotype. (C) Comparison of petal size of sdg8-1 mutant with that of wild-type Col plants. (D and E) Comparison of cell size by differential interference contrast images of wild-type Col and mutant sdg8 petals, respectively. (F) Comparison of sdg8-1 single mutant, vip4 mutant, and vip4 sdg8-1 double mutant with wild-type Col plants 30 days after germination and grown under long-day (16 h light and 8 h dark) conditions. (G) Closeup of the vip4 sdg8-1 plant. (H) Comparison of sdg8-1, vip4, and vip4 sdg8-1 mutant flowers with wild-type Col flowers. Arrowheads indicate defects of sepals that fail to enclose the flower.
The vip4 sdg8-1 double mutant flowered earlier than either of the single mutants (65) and showed a more pronounced reduction in size (Fig. 7F and G), suggesting synergistic action by sdg8-1 and vip4. Interestingly, the vip4 sdg8-1 double mutant was similar to the vip4 single mutant, showing a specific morphology defect of sepals that fail to enclose flower buds in the latest stages of development (Fig. 7H). This defect is specific to vip4 and independent of sdg8-1.
Transcriptome analysis reveals overlapping and specific genes affected in the sdg26, sdg8, and vip4 mutants.
To systematically examine and compare gene expression patterns of the sdg26, sdg8, and vip4 mutants at the global genome level, we performed transcriptome profiling experiments using “The Complete Arabidopsis Transcriptome Microarray” (CATMA), which contains 24,576 genes of the Arabidopsis genome (9). We choose to use 6-day-old seedlings for analysis because at this early stage, plants did not show any visible mutant phenotype. We also reasoned that secondary transcriptional changes caused by SDG26-, SDG8-, or VIP4-dependent differentially expressed genes would be minimal at this early developmental stage. For comparison, the mutant and wild-type seedlings were grown side by side under the same growth conditions. About 100 seedlings were collected and used to prepare each RNA extract. Two-color microarray experiments were performed with each RNA extract. Two biological repeats were performed with independently collected seeds. Genes showing greater than 1.5-fold changes in expression from the four replicates of hybridization with the Bonferroni P values inferior to 0.05 in statistic analysis were considered to be differentially expressed in the mutant.
Our first series of analyses identified 28 down-regulated genes and 123 up-regulated genes in the sdg26-1 mutant (see Table S2 in the supplemental material) and 88 down-regulated genes and 54 up-regulated genes in the sdg8-2 mutant (see Table S3 in the supplemental material). It suggests that SDG26 is primarily involved in maintaining the repressed state of genes whereas SDG8 is primarily involved in maintaining the activated state of genes. Comparison of differentially expressed genes between the sdg26-1 and sdg8-2 mutants revealed only a weak overlap of genes regulated in the opposite or the same direction (Fig. 8A), indicating that SDG26 and SDG8 independently regulate gene expression.
FIG. 8.
Venn diagram shows the number and overlap of differentially expressed genes found in the sdg26-1, sdg8-2, sdg8-1, and vip4 mutants. Microarray analyses were performed on the CATMA 24k chip using 6-day-old seedlings. The differentially expressed genes in the mutant compared to the wild type are validated by a change of at least 1.5-fold and a Bonferroni P value inferior to 0.05 from four replicates of hybridization. The arrows indicate the up-regulated (red color) and down-regulated (black color) categories of differentially expressed genes. The pink, green, blue, and white filled circles represent the sdg26-1, sdg8-2, sdg8-1, and vip4 mutants, respectively.
From other series of analyses, we identified 92 down-regulated genes and 20 up-regulated genes in the vip4 mutant (see Table S4 in the supplemental material) and 84 down-regulated genes and 40 up-regulated genes in the sdg8-1 mutant (see Table S5 in the supplemental material). Fourteen genes were found to be down-regulated in both the vip4 and sdg8-1 mutants (Fig. 8B). These data are consistent with the idea that VIP4 and SDG8 are primarily involved in maintaining the activated state of genes and act synergistically at some genes. Meanwhile, a large number of differentially expressed genes did not overlap between the vip4 and sdg8-1 mutants, unraveling the other aspect for independent function of VIP4 and SDG8 in transcription regulation.
Cross-comparison of differentially expressed genes between the sdg8-1 and sdg8-2 mutants revealed that about 50% of genes overlap and are regulated in the same direction (Fig. 8C). It appears that the overlapping categories of differentially expressed genes are underestimated in our studies compared to the reality. This might be explained by the highly stringent parameters used in our analyses. The nonoverlap of differentially expressed genes in the cross-comparison between the sdg8-1 and sdg8-2 mutants might also be explained by the less-strict same growth conditions and by mutant allele differences. Indeed, the sdg8-1 mutant frequently shows very slightly earlier flowering and reduced seed production compared to the sdg8-2 mutant. Further supporting the good quality of our analysis, FLC was found among down-regulated genes in sdg8-1, sdg8-2, and vip4 mutants but among up-regulated genes in the sdg26-1 mutant (Table 1). This is in agreement with our previous RT-PCR analysis. The MAF1 to MAF5 sequences were not present on the CATMA chip and consequently were not included in our transcriptome analyses.
TABLE 1.
Overlap of differentially expressed genes found in the sdg26-1, sdg8-2, vip4, and sdg8-1 mutants
| Function | Probe set | Gene IDa | Description of protein | Direction of gene regulation for mutant with genotype:
|
|||
|---|---|---|---|---|---|---|---|
| sdg26-1 | sdg8-2 | vip4 | sdg8-1 | ||||
| Transcription | CATMA5A08895 | AT5G10140 | MADS-box protein flowering locus C (FLC) | Up | Down | Down | Down |
| CATMA1A70060 | AT1G80840 | WRKY family transcription factor (WRKY40) | Down | Down | |||
| CATMA4A01430 | AT4G01250 | WRKY family transcription factor (WRKY22) | Up | Up | |||
| CATMA3A22990 | AT3G23030 | Auxin-responsive/indoleacetic acid-induced protein 2 (IAA2) | Up | Down | Up | ||
| Metabolism | CATMA2A34120 | AT2G35930 | U-box domain-containing protein | Up | Down | Down | |
| CATMA2B42010 | AT2G43590 | Chitinase, putative | Up | Down | Down | Down | |
| CATMA2A42040 | AT2G43620 | Chitinase, putative | Down | Down | Down | ||
| CATMA4A02835 | AT4G02520 | Glutathione S-transferase, putative | Down | Down | Down | ||
| CATMA1A61750 | AT1G72520 | Lipoxygenase, putative | Down | Down | |||
| CATMA3A16260 | AT3G16860 | Phytochelatin synthetase related | Down | Down | |||
| CATMA2A38025 | AT2G39800 | Delta 1-pyrroline-5-carboxylate synthetase A/P5CS A (P5CS1) | Down | Down | |||
| CATMA2A34910 | AT2G36690 | Oxidoreductase, 2OG-Fe(II) oxygenase family protein | Down | Down | |||
| CATMA4A41195 | AT4G39800 | Inositol-3-phosphate/myo-inositol-1-phosphate synthase 1 | Down | Down | Down | ||
| CATMA1A16465 | AT1G17420 | Lipoxygenase, putative | Down | Down | |||
| CATMA5A36740 | AT5G41080 | Glycerophosphoryl diester phosphodiesterase family protein | Up | Up | Up | ||
| CATMA5A18330 | AT5G19890 | Peroxidase, putative | Down | Down | |||
| CATMA3A17550 | AT3G18080 | Glycosyl hydrolase family 1 protein | Down | Down | |||
| CATMA1A01610 | AT1G02660 | Lipase class 3 family protein | Up | Up | |||
| Others | CATMA3A43980 | AT3G50970 | Dehydrin xero2 (XERO2)/low-temp-induced protein (LTI30) | Up | |||
| CATMA1A45550 | AT1G54410 | Dehydrin family protein | Up | Down | Up | ||
| CATMA2A04295 | AT2G05520 | Glycine-rich protein (GRP) | Down | Down | Down | ||
| CATMA5A44520 | AT5G48540 | 33-kDa secretory protein-related | Down | Down | Down | ||
| CATMA4A31410 | AT4G29780 | Expressed protein | Down | Down | |||
| CATMA4A29280 | AT4G27652 | Expressed protein | Down | Down | Down | ||
| CATMA2A24855 | AT2G26530 | Expressed protein | Down | Down | |||
| CATMA2A12750 | AT2G14247 | Expressed protein | Down | Up | |||
| CATMA2A38220 | AT2G40000 | Expressed protein | Up | Up | |||
| CATMA3A05130 | AT3G06070 | Expressed protein | Up | Up | |||
ID, identifier.
Overall, no specific pathway, gene family, or gene cluster is overrepresented in the list of differentially expressed genes in the mutants, supporting the hypothesis that SDG26, SDG8, and VIP4 contribute to general transcriptional regulation. The differentially expressed genes include some that are potentially involved in transcription, signal transduction, transport, and metabolism (see Tables S2 to S5 in the supplemental material). The overlap category of differentially expressed genes includes a few transcription factor genes and a higher number of genes involved in metabolism (Table 1). These identified genes will be useful for in-depth characterization of the mutant phenotypes and the interaction of SDG26, SDG8, and VIP4 in transcription regulation.
DISCUSSION
Histone methylation serves as an epigenetic marker for the recruitment of specific effector proteins that direct downstream molecular and physiological processes. The binding of the effector protein is modulated by both the site and the degree of methylation on the histone molecule. Therefore, identification and characterization of HKMTs that target specific lysine residues and set different methylation states of a given lysine residue (mono-, di-, and trimethylation) are of great interest. For the first time, we have shown that H3K36 can be mono-, di-, and trimethylated by distinct HKMTs and that only di- and trimethylation on H3K36 are associated with transcription stimulation. Our results unravel that methylation states of H3K36 play critical functions in flowering-time control and in other processes in Arabidopsis.
SDG8 and SDG26 could methylate both H3 and H4 in vitro. Also, mouse NSD1 could methylate both H3K36 and H4K20 in vitro (42), though the human HYPB and fungal SET2 proteins show higher specificity for H3K36 methylation (1, 35, 49, 50). Differing from the NSD1, HYPB, and SET2 proteins, which could use free histones as substrates, SDG8 and SDG26 showed detectable HKMT activity in vitro only when we used oligonucleosomes as substrates. This suggests that the higher order of oligonucleosome structure effectively promotes HKMT activities of the SDG8 and SDG26 proteins. The observation of localization of the YFP-fused SDG8 and SDG26 proteins in the nucleus is consistent with their function as HKMT on the chromatin. Interestingly, loss-of-function mutations of SDG8 specifically decreased methylation on H3K36 but not that on H4K20. The in vitro HKMT activity on histone H4 detected for the recombinant SDG8 and SDG26 proteins could be explained by methylation on a residue different from K20. Alternatively, it may represent an artifact caused by in vitro assay conditions. Examples exist from previous studies showing differences between in vitro and in vivo HKMT specificities of some enzymes, e.g., the human G9a (39, 53, 54) and tobacco NtSET1 (60) proteins could methylate both H3K9 and H3K27 in vitro but only H3K9 in vivo. Enzyme activity/specificity in vivo might be regulated by cofactors associated with HKMT. This assumption might also explain the barely detectable activity in vivo of SDG26, whereas the recombinant SDG26 protein showed activity similar to that of SDG8 in vitro.
In yeast, H3K36 methylation is coupled to transcription by association of SET2 with the PAF1 complex and the elongating form of RNA polymerase II, which is phosphorylated on Ser2 of the C-terminal domain (27, 30). This association is independent of the conserved AWS, SET, and C domains but requires the C-terminal part of SET2. Sequence homology can be detected at the C terminus between SET2 and SDG8 but not between SET2 and SDG26, which contains a dramatically shortened C terminus (Fig. 2A). We hypothesize that SDG8 but not SDG26 acts similarly to SET2 in association of H3K36 methylation with transcription. This hypothesis is also supported by our following observations. Firstly, SDG8, like VIP4 (a homolog of the yeast PAF1 subunit LEO1), was primarily involved in maintaining activation of genome transcription, whereas SDG26 contributed essentially to maintaining repression of genome transcription. Secondly, SDG8 and VIP4 synergistically regulated a number of genes. Finally, both SDG8 and VIP4 were required for di- and trimethylation of H3K36 at the FLC and MAF genes. Our current study did not detect changes in the levels of histone methylation in the sdg26-1 mutant plants. Future experiments will be necessary to examine whether SDG26 methylates histones at some particular genome regions.
A significant number of up-regulated genes were also found in the sdg8-1 and sdg8-2 mutants. Both down-regulated and up-regulated categories of genes are found in the yeast set2 mutant (56). When SET2 is tagged with the LexA DNA-binding domain and introduced into yeast cells along with the lacZ reporter gene containing a LexA-binding site, it represses the reporter activity (49). The histone deacetylase complex Rpd3S is recruited to chromatin via binding of the chromodomain protein Eaf3 to methylated H3K36, which apparently represses transcription and prevents erroneous transcription initiation (7, 21, 22). Similar mechanisms are likely conserved in Arabidopsis. Independently, previous mass spectrometry analysis revealed a combination of monomethyl-H3K36 with the transcription repressive marker dimethyl-H3K27 on the Arabidopsis histone H3.2 (20). Our finding of increased levels of monomethyl-H3K36 in the sdg8-2 mutant prompted us to hypothesize that monomethylation of H3K36 acts together with dimethylation of H3K27 in transcription repression. In agreement with this hypothesis, sdg8-1 shows genetic interaction with clf (our unpublished result), a mutant exhibiting defects in H3K27 methylation in Arabidopsis (44).
Our finding that the sdg8-2 mutant specifically contained decreased levels of dimethyl- and trimethyl-H3K36 and an increased level of monomethyl-H3K36 indicates that SDG8 is a specific HKMT for di- and trimethylation on H3K36 and that monomethylation on H3K36 involve other HKMTs in Arabidopsis. This contrasts with the previous knowledge acquired for fungi, where each species contains a sole HKMT SET2 for mono-, di-, and trimethylation on H3K36 (1, 35, 49). Defects in converting monomethyl to di-/trimethyl in the sdg8-2 mutant might have elevated the level of H3K36 monomethylation deposited by a specific HKMT. Our studies of the sdg26-1 mutant exclude SDG26 serving as such an HKMT, because neither global nor FLC and MAF locus-specific levels of H3K36 monomethylation were changed in the mutant. Based on protein sequence conservation (Fig. 1), SDG4, SDG7, and SDG24 might also have HKMT activity on H3K36 and therefore contribute to the establishment of the H3K36 methylation pattern in Arabidopsis. Three-dimensional structures determined for several HKMTs from fungi and mammals reveal that the transfer of a methyl group onto H3K4, H3K9, H3K27, and H4K20 occurs in a channel formed by the SET domain of the enzymes (40). The diameter and the shape of the channel have a great impact on the number of methyl groups that can be transferred. Phenylalanine 281 in DIM-5 and tyrosine 305 in SET7/9, which are situated at the same position in the alignment of the conserved SET domain sequences, determine the trimethylation and monomethylation specificities of the two enzymes, respectively (59, 64). At this same position, SDG8 and SDG26 contain a threonine whereas SDG7 and SDG24 contain a methionine and SDG4 contains a leucine. It thus appears possible that SDG4, SDG7, and SDG24 meet the higher constraint of channel space for carrying out di- and trimethylation. Future studies will examine if these proteins act on H3K36 monomethylation and whether the predicted amino acids play a determinant role.
In agreement with a previous observation for the vip5 and vip6 mutants (38), the expression of FLC, MAF1, and MAF3 to MAF5 was strongly suppressed while that of MAF2 was weakly suppressed in the vip4 mutant. This suppression correlated with the decreased levels of dimethyl- and trimethyl-H3K36 observed at these genes in the vip4 mutant. In the case of MAF3 and MAF2, however, the observed decrease of dimethyl- and trimethyl-H3K36 did not appear to be the cause of suppression. This is because a similar or more-pronounced decrease of dimethyl- and trimethyl-H3K36 did not affect expression of MAF2 and MAF3 in the sdg8-2 mutant. We believe that the decreased levels of H3K4 methylation play a more important role in the suppression of MAF2 and MAF3 in the vip4 mutant. Our observation of decreased levels of both dimethyl/trimethyl-H3K36 and trimethyl-H3K4 at the FLC and MAF genes in the vip4 mutant is consistent with the knowledge that the yeast PAF1 complex successively recruits H3K4-specific HKMT SET1 and H3K36-specific HKMT SET2 during transcription (29, 30). The identity of the HKMT involved in H3K4 methylation at the FLC and MAF genes is currently unknown. H3K4 trimethylation has been observed to be reduced at a region covering the initiation codon ATG of FLC under a FRI-genetic background in efs (25), a loss-of-function mutant allele of SDG8. In the sdg8-1 mutant under the fri genetic background, this same region of FLC showed a slight increase in H3K4 dimethylation (65). We believe that these modest changes in H3K4 methylation are secondary effects caused by the dramatically changed levels of H3K36 methylation in the mutants. Our current analysis, extending to new regions of FLC and to the MAF genes, did not detect significant changes in H3K4 trimethylation in the sdg8-2 mutant and thus failed to support SDG8 as an H3K4 HKMT.
Our study demonstrated that SDG8 is involved in di- and trimethylation on H3K36 and in activation of transcription of FLC, MAF1, MAF4, and MAF5, which are required for preventing early flowering. Interestingly, SDG26 acts in an antagonistic pathway in repressing FLC, MAF4, and MAF5 expression. It is currently unclear whether SDG26 directly or indirectly (through an unknown factor) repressed FLC, MAF4, and MAF5 expression. The presently known regulatory genes of FLC were not among the differentially expressed genes identified in the sdg26-1 mutant by our transcriptome analyses. Future experiments will be required to uncover the pathway by which SDG26 represses FLC, MAF4, and MAF5 expression. In addition to the MADS box flowering repressor genes, a number of other genes potentially involved in transcription, signal transduction, transport, and metabolism were also identified as differentially expressed in the sdg26-1, sdg8-1, sdg8-2, and vip4 mutants. Our observation also revealed that SDG8 plays important roles in cell proliferation, organ size control, and fertility. It is likely that more functions of H3K36 methylation in epigenetic regulation will be revealed in the future.
Supplementary Material
Acknowledgments
We thank Denise Meyer, Ziqiang Liu, and Juan Gao for help in cytology, RNA preparation, and plasmid construction, respectively. We thank Manfred Heinlein and Alexandre Berr for critically reading the manuscript. We are very grateful to Ru Cao and Yi Zhang (UNC at Chapel Hill, NC) and to Adrien Eberlin and Laszlo Tora (IGBMC, Strasbourg, France) for kindly providing the oligonucleosomes and mononucleosomes, respectively. We thank the Arabidopsis Biological Resource Centre and the Nottingham Arabidopsis Stock Center for providing Arabidopsis seeds.
L.X. is supported by a research training fellowship from the Ministère de la Culture, de l'Enseignement Supérieur et de la Recherche, Luxembourg. Research in W.-H.S.'s laboratory is supported by the Centre National de la Recherche Scientifique.
Footnotes
Published ahead of print on 10 December 2007.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Adhvaryu, K. K., S. A. Morris, B. D. Strahl, and E. U. Selker. 2005. Methylation of histone H3 lysine 36 is required for normal development in Neurospora crassa. Eukaryot. Cell 41455-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alonso, J. M., A. N. Stepanova, T. J. Leisse, C. J. Kim, H. Chen, P. Shinn, D. K. Stevenson, J. Zimmerman, P. Barajas, R. Cheuk, C. Gadrinab, C. Heller, A. Jeske, E. Koesema, C. C. Meyers, H. Parker, L. Prednis, Y. Ansari, N. Choy, H. Deen, M. Geralt, N. Hazari, E. Hom, M. Karnes, C. Mulholland, R. Ndubaku, I. Schmidt, P. Guzman, L. Aguilar-Henonin, M. Schmid, D. Weigel, D. E. Carter, T. Marchand, E. Risseeuw, D. Brogden, A. Zeko, W. L. Crosby, C. C. Berry, and J. R. Ecker. 2003. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301:653-657. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez-Venegas, R., S. Pien, M. Sadder, X. Witmer, U. Grossniklaus, and Z. Avramova. 2003. ATX-1, an Arabidopsis homolog of trithorax, activates flower homeotic genes. Curr. Biol. 13627-637. [DOI] [PubMed] [Google Scholar]
- 4.Ausin, I., C. Alonso-Blanco, J. A. Jarillo, L. Ruiz-Garcia, and J. M. Martinez-Zapater. 2004. Regulation of flowering time by FVE, a retinoblastoma-associated protein. Nat. Genet. 36162-166. [DOI] [PubMed] [Google Scholar]
- 5.Bastow, R., J. S. Mylne, C. Lister, Z. Lippman, R. A. Martienssen, and C. Dean. 2004. Vernalization requires epigenetic silencing of FLC by histone methylation. Nature 427164-167. [DOI] [PubMed] [Google Scholar]
- 6.Baurle, I., and C. Dean. 2006. The timing of developmental transitions in plants. Cell 125655-664. [DOI] [PubMed] [Google Scholar]
- 7.Carrozza, M. J., B. Li, L. Florens, T. Suganuma, S. K. Swanson, K. K. Lee, W. J. Shia, S. Anderson, J. Yates, M. P. Washburn, and J. L. Workman. 2005. Histone H3 methylation by Set2 directs deacetylation of coding regions by Rpd3S to suppress spurious intragenic transcription. Cell 123581-592. [DOI] [PubMed] [Google Scholar]
- 8.Choi, K., S. Kim, S. Y. Kim, M. Kim, Y. Hyun, H. Lee, S. Choe, S. G. Kim, S. Michaels, and I. Lee. 2005. SUPPRESSOR OF FRIGIDA3 encodes a nuclear ACTIN-RELATED PROTEIN6 required for floral repression in Arabidopsis. Plant Cell 172647-2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crowe, M. L., C. Serizet, V. Thareau, S. Aubourg, P. Rouze, P. Hilson, J. Beynon, P. Weisbeek, P. van Hummelen, P. Reymond, J. Paz-Ares, W. Nietfeld, and M. Trick. 2003. CATMA: a complete Arabidopsis GST database. Nucleic Acids Res. 31156-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deal, R. B., M. K. Kandasamy, E. C. McKinney, and R. B. Meagher. 2005. The nuclear actin-related protein ARP6 is a pleiotropic developmental regulator required for the maintenance of FLOWERING LOCUS C expression and repression of flowering in Arabidopsis. Plant Cell 172633-2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deal, R. B., C. N. Topp, E. C. McKinney, and R. B. Meagher. 2007. Repression of flowering in Arabidopsis requires activation of FLOWERING LOCUS C expression by the histone variant H2A.Z. Plant Cell 1974-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding, B., Y. Zhu, J. Gao, Y. Yu, K. Cao, W. H. Shen, and A. Dong. 2007. Molecular characterization of three rice SET-domain proteins. Plant Sci. 1721072-1078. [Google Scholar]
- 13.Ding, Y., X. Wang, L. Su, J. Zhai, S. Cao, D. Zhang, C. Liu, Y. Bi, Q. Qian, Z. Cheng, C. Chu, and X. Cao. 2007. SDG714, a histone H3K9 methyltransferase, is involved in Tos17 DNA methylation and transposition in rice. Plant Cell 199-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong, A., Z. Liu, Y. Zhu, F. Yu, Z. Li, K. Cao, and W. H. Shen. 2005. Interacting proteins and differences in nuclear transport reveal specific functions for the NAP1 family proteins in plants. Plant Physiol. 1381446-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuchs, J., D. Demidov, A. Houben, and I. Schubert. 2006. Chromosomal histone modification patterns—from conservation to diversity. Trends Plant Sci. 11199-208. [DOI] [PubMed] [Google Scholar]
- 16.Greb, T., J. S. Mylne, P. Crevillen, N. Geraldo, H. An, A. R. Gendall, and C. Dean. 2007. The PHD finger protein VRN5 functions in the epigenetic silencing of Arabidopsis FLC. Curr. Biol. 1773-78. [DOI] [PubMed] [Google Scholar]
- 17.He, Y., M. R. Doyle, and R. M. Amasino. 2004. PAF1-complex-mediated histone methylation of FLOWERING LOCUS C chromatin is required for the vernalization-responsive, winter-annual habit in Arabidopsis. Genes Dev. 182774-2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He, Y., S. D. Michaels, and R. M. Amasino. 2003. Regulation of flowering time by histone acetylation in Arabidopsis. Science 3021751-1754. [DOI] [PubMed] [Google Scholar]
- 19.Johanson, U., J. West, C. Lister, S. Michaels, R. Amasino, and C. Dean. 2000. Molecular analysis of FRIGIDA, a major determinant of natural variation in Arabidopsis flowering time. Science 290344-347. [DOI] [PubMed] [Google Scholar]
- 20.Johnson, L., S. Mollah, B. A. Garcia, T. L. Muratore, J. Shabanowitz, D. F. Hunt, and S. E. Jacobsen. 2004. Mass spectrometry analysis of Arabidopsis histone H3 reveals distinct combinations of post-translational modifications. Nucleic Acids Res. 326511-6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joshi, A. A., and K. Struhl. 2005. Eaf3 chromodomain interaction with methylated H3-K36 links histone deacetylation to Pol II elongation. Mol. Cell 20971-978. [DOI] [PubMed] [Google Scholar]
- 22.Keogh, M. C., S. K. Kurdistani, S. A. Morris, S. H. Ahn, V. Podolny, S. R. Collins, M. Schuldiner, K. Chin, T. Punna, N. J. Thompson, C. Boone, A. Emili, J. S. Weissman, T. R. Hughes, B. D. Strahl, M. Grunstein, J. F. Greenblatt, S. Buratowski, and N. J. Krogan. 2005. Cotranscriptional set2 methylation of histone H3 lysine 36 recruits a repressive Rpd3 complex. Cell 123593-605. [DOI] [PubMed] [Google Scholar]
- 23.Kim, H. J., Y. Hyun, J. Y. Park, M. J. Park, M. K. Park, M. D. Kim, H. J. Kim, M. H. Lee, J. Moon, I. Lee, and J. Kim. 2004. A genetic link between cold responses and flowering time through FVE in Arabidopsis thaliana. Nat. Genet. 36167-171. [DOI] [PubMed] [Google Scholar]
- 24.Kim, S., K. Choi, C. Park, H. J. Hwang, and I. Lee. 2006. SUPPRESSOR OF FRIGIDA4, encoding a C2H2-type zinc finger protein, represses flowering by transcriptional activation of Arabidopsis FLOWERING LOCUS C. Plant Cell 182985-2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim, S. Y., Y. He, Y. Jacob, Y. S. Noh, S. Michaels, and R. Amasino. 2005. Establishment of the vernalization-responsive, winter-annual habit in Arabidopsis requires a putative histone H3 methyl transferase. Plant Cell 173301-3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim, S. Y., and S. D. Michaels. 2006. SUPPRESSOR OF FRI 4 encodes a nuclear-localized protein that is required for delayed flowering in winter-annual Arabidopsis. Development 1334699-4707. [DOI] [PubMed] [Google Scholar]
- 27.Kizer, K. O., H. P. Phatnani, Y. Shibata, H. Hall, A. L. Greenleaf, and B. D. Strahl. 2005. A novel domain in Set2 mediates RNA polymerase II interaction and couples histone H3 K36 methylation with transcript elongation. Mol. Cell. Biol. 253305-3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krichevsky, A., H. Gutgarts, S. V. Kozlovsky, T. Tzfira, A. Sutton, R. Sternglanz, G. Mandel, and V. Citovsky. 2007. C2H2 zinc finger-SET histone methyltransferase is a plant-specific chromatin modifier. Dev. Biol. 303259-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krogan, N. J., J. Dover, A. Wood, J. Schneider, J. Heidt, M. A. Boateng, K. Dean, O. W. Ryan, A. Golshani, M. Johnston, J. F. Greenblatt, and A. Shilatifard. 2003. The Paf1 complex is required for histone H3 methylation by COMPASS and Dot1p: linking transcriptional elongation to histone methylation. Mol. Cell 11721-729. [DOI] [PubMed] [Google Scholar]
- 30.Krogan, N. J., M. Kim, A. Tong, A. Golshani, G. Cagney, V. Canadien, D. P. Richards, B. K. Beattie, A. Emili, C. Boone, A. Shilatifard, S. Buratowski, and J. Greenblatt. 2003. Methylation of histone H3 by Set2 in Saccharomyces cerevisiae is linked to transcriptional elongation by RNA polymerase II. Mol. Cell. Biol. 234207-4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.March-Diaz, R., M. Garcia-Dominguez, F. J. Florencio, and J. C. Reyes. 2007. SEF, a new protein required for flowering repression in Arabidopsis, interacts with PIE1 and ARP6. Plant Physiol. 143893-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin, C., and Y. Zhang. 2005. The diverse functions of histone lysine methylation. Nat. Rev. Mol. Cell Biol. 6838-849. [DOI] [PubMed] [Google Scholar]
- 33.Martin-Trillo, M., A. Lazaro, R. S. Poethig, C. Gomez-Mena, M. A. Pineiro, J. M. Martinez-Zapater, and J. A. Jarillo. 2006. EARLY IN SHORT DAYS 1 (ESD1) encodes ACTIN-RELATED PROTEIN 6 (AtARP6), a putative component of chromatin remodelling complexes that positively regulates FLC accumulation in Arabidopsis. Development 1331241-1252. [DOI] [PubMed] [Google Scholar]
- 34.Michaels, S. D., and R. M. Amasino. 1999. FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 11949-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morris, S. A., Y. Shibata, K. Noma, Y. Tsukamoto, E. Warren, B. Temple, S. I. Grewal, and B. D. Strahl. 2005. Histone H3 K36 methylation is associated with transcription elongation in Schizosaccharomyces pombe. Eukaryot. Cell 41446-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mylne, J. S., L. Barrett, F. Tessadori, S. Mesnage, L. Johnson, Y. V. Bernatavichute, S. E. Jacobsen, P. Fransz, and C. Dean. 2006. LHP1, the Arabidopsis homologue of HETEROCHROMATIN PROTEIN1, is required for epigenetic silencing of FLC. Proc. Natl. Acad. Sci. USA 1035012-5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noh, Y. S., and R. M. Amasino. 2003. PIE1, an ISWI family gene, is required for FLC activation and floral repression in Arabidopsis. Plant Cell 151671-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oh, S., H. Zhang, P. Ludwig, and S. van Nocker. 2004. A mechanism related to the yeast transcriptional regulator Paf1c is required for expression of the Arabidopsis FLC/MAF MADS box gene family. Plant Cell 162940-2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peters, A. H., S. Kubicek, K. Mechtler, R. J. O'Sullivan, A. A. Derijck, L. Perez-Burgos, A. Kohlmaier, S. Opravil, M. Tachibana, Y. Shinkai, J. H. Martens, and T. Jenuwein. 2003. Partitioning and plasticity of repressive histone methylation states in mammalian chromatin. Mol. Cell 121577-1589. [DOI] [PubMed] [Google Scholar]
- 40.Qian, C., and M. M. Zhou. 2006. SET domain protein lysine methyltransferases: structure, specificity and catalysis. Cell Mol. Life Sci. 632755-2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ratcliffe, O. J., R. W. Kumimoto, B. J. Wong, and J. L. Riechmann. 2003. Analysis of the Arabidopsis MADS AFFECTING FLOWERING gene family: MAF2 prevents vernalization by short periods of cold. Plant Cell 151159-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rayasam, G. V., O. Wendling, P. O. Angrand, M. Mark, K. Niederreither, L. Song, T. Lerouge, G. L. Hager, P. Chambon, and R. Losson. 2003. NSD1 is essential for early post-implantation development and has a catalytically active SET domain. EMBO J. 223153-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santos-Rosa, H., R. Schneider, A. J. Bannister, J. Sherriff, B. E. Bernstein, N. C. Emre, S. L. Schreiber, J. Mellor, and T. Kouzarides. 2002. Active genes are tri-methylated at K4 of histone H3. Nature 419407-411. [DOI] [PubMed] [Google Scholar]
- 44.Schubert, D., L. Primavesi, A. Bishopp, G. Roberts, J. Doonan, T. Jenuwein, and J. Goodrich. 2006. Silencing by plant Polycomb-group genes requires dispersed trimethylation of histone H3 at lysine 27. EMBO J. 254638-4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scortecci, K., S. D. Michaels, and R. M. Amasino. 2003. Genetic interactions between FLM and other flowering-time genes in Arabidopsis thaliana. Plant Mol. Biol 52915-922. [DOI] [PubMed] [Google Scholar]
- 46.Sheldon, C. C., J. E. Burn, P. P. Perez, J. Metzger, J. A. Edwards, W. J. Peacock, and E. S. Dennis. 1999. The FLF MADS box gene: a repressor of flowering in Arabidopsis regulated by vernalization and methylation. Plant Cell 11445-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shen, W. H. 2001. NtSET1, a member of a newly identified subgroup of plant SET-domain-containing proteins, is chromatin-associated and its ectopic overexpression inhibits tobacco plant growth. Plant J. 28371-383. [DOI] [PubMed] [Google Scholar]
- 48.Springer, N. M., C. A. Napoli, D. A. Selinger, R. Pandey, K. C. Cone, V. L. Chandler, H. F. Kaeppler, and S. M. Kaeppler. 2003. Comparative analysis of SET domain proteins in maize and Arabidopsis reveals multiple duplications preceding the divergence of monocots and dicots. Plant Physiol. 132907-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Strahl, B. D., P. A. Grant, S. D. Briggs, Z. W. Sun, J. R. Bone, J. A. Caldwell, S. Mollah, R. G. Cook, J. Shabanowitz, D. F. Hunt, and C. D. Allis. 2002. Set2 is a nucleosomal histone H3-selective methyltransferase that mediates transcriptional repression. Mol. Cell. Biol. 221298-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun, X. J., J. Wei, X. Y. Wu, M. Hu, L. Wang, H. H. Wang, Q. H. Zhang, S. J. Chen, Q. H. Huang, and Z. Chen. 2005. Identification and characterization of a novel human histone H3 lysine 36-specific methyltransferase. J. Biol. Chem. 28035261-35271. [DOI] [PubMed] [Google Scholar]
- 51.Sung, S., and R. M. Amasino. 2004. Vernalization in Arabidopsis thaliana is mediated by the PHD finger protein VIN3. Nature 427159-164. [DOI] [PubMed] [Google Scholar]
- 52.Sung, S., Y. He, T. W. Eshoo, Y. Tamada, L. Johnson, K. Nakahigashi, K. Goto, S. E. Jacobsen, and R. M. Amasino. 2006. Epigenetic maintenance of the vernalized state in Arabidopsis thaliana requires LIKE HETEROCHROMATIN PROTEIN 1. Nat. Genet. 38706-710. [DOI] [PubMed] [Google Scholar]
- 53.Tachibana, M., K. Sugimoto, T. Fukushima, and Y. Shinkai. 2001. Set domain-containing protein, G9a, is a novel lysine-preferring mammalian histone methyltransferase with hyperactivity and specific selectivity to lysines 9 and 27 of histone H3. J. Biol. Chem. 27625309-25317. [DOI] [PubMed] [Google Scholar]
- 54.Tachibana, M., K. Sugimoto, M. Nozaki, J. Ueda, T. Ohta, M. Ohki, M. Fukuda, N. Takeda, H. Niida, H. Kato, and Y. Shinkai. 2002. G9a histone methyltransferase plays a dominant role in euchromatic histone H3 lysine 9 methylation and is essential for early embryogenesis. Genes Dev. 161779-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tamaru, H., X. Zhang, D. McMillen, P. B. Singh, J. Nakayama, S. I. Grewal, C. D. Allis, X. Cheng, and E. U. Selker. 2003. Trimethylated lysine 9 of histone H3 is a mark for DNA methylation in Neurospora crassa. Nat. Genet. 3475-79. [DOI] [PubMed] [Google Scholar]
- 56.Tompa, R., and H. D. Madhani. 2007. Histone H3 lysine 36 methylation antagonizes silencing in Saccharomyces cerevisiae independently of the Rpd3S histone deacetylase complex. Genetics 175585-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang, X., Y. Zhang, Q. Ma, Z. Zhang, Y. Xue, S. Bao, and K. Chong. 2007. SKB1-mediated symmetric dimethylation of histone H4R3 controls flowering time in Arabidopsis. EMBO J. 261934-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wood, C. C., M. Robertson, G. Tanner, W. J. Peacock, E. S. Dennis, and C. A. Helliwell. 2006. The Arabidopsis thaliana vernalization response requires a polycomb-like protein complex that also includes VERNALIZATION INSENSITIVE 3. Proc. Natl. Acad. Sci. USA 10314631-14636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xiao, B., C. Jing, J. R. Wilson, P. A. Walker, N. Vasisht, G. Kelly, S. Howell, I. A. Taylor, G. M. Blackburn, and S. J. Gamblin. 2003. Structure and catalytic mechanism of the human histone methyltransferase SET7/9. Nature 421652-656. [DOI] [PubMed] [Google Scholar]
- 60.Yu, Y., A. Dong, and W. H. Shen. 2004. Molecular characterization of the tobacco SET domain protein NtSET1 unravels its role in histone methylation, chromatin binding, and segregation. Plant J. 40699-711. [DOI] [PubMed] [Google Scholar]
- 61.Yu, Y., A. Steinmetz, D. Meyer, S. Brown, and W. H. Shen. 2003. The tobacco A-type cyclin, Nicta;CYCA3;2, at the nexus of cell division and differentiation. Plant Cell 152763-2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang, H., and S. van Nocker. 2002. The VERNALIZATION INDEPENDENCE 4 gene encodes a novel regulator of FLOWERING LOCUS C. Plant J. 31663-673. [DOI] [PubMed] [Google Scholar]
- 63.Zhang, X., O. Clarenz, S. Cokus, Y. V. Bernatavichute, M. Pellegrini, J. Goodrich, and S. E. Jacobsen. 2007. Whole-genome analysis of histone H3 lysine 27 trimethylation in Arabidopsis. PLoS Biol. 5e129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang, X., Z. Yang, S. I. Khan, J. R. Horton, H. Tamaru, E. U. Selker, and X. Cheng. 2003. Structural basis for the product specificity of histone lysine methyltransferases. Mol. Cell 12177-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao, Z., Y. Yu, D. Meyer, C. Wu, and W. H. Shen. 2005. Prevention of early flowering by expression of FLOWERING LOCUS C requires methylation of histone H3 K36. Nat. Cell Biol. 71256-1260. [DOI] [PubMed] [Google Scholar]
- 66.Zhu, Y., A. Dong, D. Meyer, O. Pichon, J. P. Renou, K. Cao, and W. H. Shen. 2006. Arabidopsis NRP1 and NRP2 encode histone chaperones and are required for maintaining postembryonic root growth. Plant Cell 182879-2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zuo, J., Q. W. Niu, and N. H. Chua. 2000. Technical advance: an estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant J. 24265-273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.