Abstract
Neurofibromatosis type 1 (NF1) is one of the most common heritable autosomal dominant disorders. Alternative splicing modulates the function of neurofibromin, the NF1 gene product, by inserting the in-frame exon 23a into the region of NF1 mRNA that encodes the GTPase-activating protein-related domain. This insertion, which is predominantly skipped in neurons, reduces the ability of neurofibromin to regulate Ras by 10-fold. Here, we report that the neuron-specific Hu proteins control the production of the short protein isoform by suppressing inclusion of NF1 exon 23a, while TIA-1/TIAR proteins promote inclusion of this exon. We identify two binding sites for Hu proteins, located upstream and downstream of the regulated exon, and provide biochemical evidence that Hu proteins specifically block exon definition by preventing binding of essential splicing factors. In vitro analyses using nuclear extracts show that at the downstream site, Hu proteins prevent binding of U1 and U6 snRNPs to the 5′ splice site, while TIAR increases binding. Hu proteins also decrease U2AF binding at the 3′ splice site located upstream of exon 23a. In addition to providing the first mechanistic insight into tissue-specific control of NF1 splicing, these studies establish a novel strategy whereby Hu proteins regulate RNA processing.
Neurofibromatosis type I (NF1), which affects 1 in 3,500 individuals (11), is one of the most common dominantly inherited autosomal disorders. Loss-of-function mutations in the NF1 gene cause several abnormalities, including development of benign peripheral and optic nerve tumors (neurofibromas and gliomas) and abnormal distribution of melanocytes (café-au- lait spots). NF1 patients also have increased risk of developing malignant tumors of neuronal origin (11, 13). The tumor suppression function of NF1 was linked to a domain in its encoded protein, neurofibromin, which is structurally similar to the Ras GTPase-activating protein (GAP) family (13). In addition to its widely accepted tumor suppression function, NF1 also plays a significant role in brain development. About 30 to 60% of children with NF1 mutations develop learning disabilities, ranging from mild cognitive impairment to attention deficit disorders (15).
Exon 23a is an in-frame exon encoding 21 amino acids in the NF1 GAP region. This exon is alternatively included, producing two NF1 isoforms (5). The type I isoform does not contain this exon, while the type II isoform does. The ratio of the two isoforms varies in different tissues and during development. The type I isoform is predominantly expressed in neurons of the adult central nervous system (21, 25) and shows 10-times-higher activity in down-regulating Ras activity than the type II isoform (5, 48). In the pheochromocytoma cell line PC12, production of the NF1 type 1 isoform can be induced by nerve growth factor treatment (48). These lines of evidence suggest that a balance of the two isoforms is important during neuronal differentiation. Indeed, when exon 23a was deleted from the NF1 locus by gene targeting in mice, the mutant mice showed learning disabilities (16). To date, the molecular mechanism that controls this biologically important alternative splicing event has not been elucidated.
Here, we identify the Hu proteins as regulators of NF1 exon 23a splicing. Hu proteins are a family of highly conserved RNA-binding proteins that play an important role in neuronal differentiation (1, 2, 7, 46). Four members of the Hu protein family have been identified: HuA (also known as HuR), HuB (also known as Hel-N1), HuC, and HuD. With the exception of HuA, all of the Hu proteins are expressed predominantly in neurons (37). At the molecular level, Hu proteins, which all share a similar structure consisting of three RNA recognition motifs (RRMs) and a hinge domain between RRM2 and -3, regulate mRNA stability and translation in the cytoplasm (6, 8, 18, 27, 29, 31) and polyadenylation site selection in the nucleus (51, 53). In this report, we demonstrate that Hu proteins regulate skipping of NF1 exon 23a, through binding to intronic AU-rich sequence elements located on either side of the regulated exon. Overexpression of Hu proteins in nonneuronal cells promotes the inclusion of exon 23a from either the transfected reporter or the endogenously expressed NF1 pre-mRNA. More importantly, we elucidate the molecular mechanism by which Hu proteins suppress inclusion of this exon. We show that splicing repression requires at least two AU-rich sequences in the flanking introns. Upon binding at the AU-rich sequence downstream from the 5′ splice site of this exon, Hu proteins interfere with U1 and U6 snRNP binding by competing with the positive splicing factors TIA-1/TIAR, which promote inclusion of exon 23a. In addition, we demonstrate that Hu proteins reduce binding of U2AF65 to an RNA containing the 3′ splice site of exon 23a. In addition to providing new insights into the regulated expression of the NF1 gene, these studies provide definitive evidence to support a role for Hu proteins as splicing regulators and reveal a novel mechanism for regulating neuron-specific alternative splicing events.
MATERIALS AND METHODS
Bioinformatic analysis to search for potential binding sites of Hu proteins.
To search for potential binding sites of Hu proteins, we started with the Alternative Exon Database (http://www.ebi.ac.uk/asd/aedb/index.html). We first identified all of the known neuron/brain-specific exons and then looked for AU-rich sequences [(A/U)(A/U)UUU(A/U)(A/U)] on these exons and their flanking introns (200 nucleotides on each side). We next compared the identified AU-rich sequences among mammals (human, mouse, and rat). If the AU-rich sequence was conserved, we considered it a potential binding site of Hu proteins.
Immunoprecipitation (IP) of CA77 nuclear extract and RT-PCR analysis.
Eight hundred micrograms of CA77 cell nuclear extract proteins was immunoprecipitated with anti-Hu sera in NET supplemented with RNase Out (Invitrogen). The pellet was washed five times in NET, treated with proteinase K, and extracted with phenol-chloroform (1:1), followed by RNA precipitation. Reverse transcription-PCR (RT-PCR) was carried out as described previously (52). Oligonucleotides used for RT-PCR were NF1 5′-5 and NF1 3′-4 for rat NF1 (in intron 23 and intron 23a, respectively) and GAPDH 5′ and GAPDH 3′ for rat GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (in intron 3 and exon 5, respectively).
Plasmids.
The human NF1 reporter constructs used in transfection experiments consisted of NF1 exon 23a with part of the flanking introns inserted into the first intron of the human metallothionein (HMT) gene. To generate reporters HMT-NF1 863/499 and HMT-NF1 75/499, the human NF1 sequence was PCR amplified from HeLa cell genomic DNA using an upstream oligonucleotide (NF1 5′-1 or NF1 5′-2) and a downstream oligonucleotide (NF1 3′-1). The PCR products were digested with BglII and BamHI and cloned into the RSV-HMT reporter linearized with BglII. The mutant reporters with the AU-rich sequence mutated were generated by a PCR-mediated mutagenesis procedure. The plasmids used to generate in vitro transcribed RNA substrates for UV cross-linking and psoralen cross-linking assays were generated by PCR using the transfection reporter plasmid as the template and oligonucleotides NF1 5′-3 and NF1 3′-2. The PCR product was restriction digested with EcoRI and HindIII and cloned into pGEM-3Zf(+) (Promega) vector digested with EcoRI and HindIII. Oligonucleotide sequences are shown in Table 1.
TABLE 1.
Oligonucleotides used in this study
| Oligonucleotide | Sequence | Restriction site |
|---|---|---|
| NF1 5′-1 | GACAGATCTAGATGAAACCAATATGTGCC | BglII |
| NF1 5′-2 | GACAGATCTGTACAAGCCAACATT | BglII |
| NF1 3′-1 | GCGGATCCAGACTGCCTAGATAAAAATA | BamHI |
| NF1 5′-3 | AGAAGCTTCATTGTTTTTGTTGCTGTAT | HindIII |
| NF1 3′-2 | ATGAATTCATTTTACAGTGAAGGTCA | EcoRI |
| mHuB 5′ | GCGGATCCAATGGAAACACAACTGTCTAA | BamHI |
| mHuB 3′ | GCGAATTCTTAGGCTTTGTGCGTTTTGTT | EcoRI |
| mHuC 5′ | GCGGATCCAATGGTCACTCAGATACTGGG | BamHI |
| mHuC 3′ | GCGAATTCTCAGGCCTTGTGCTGCTTGC | EcoRI |
| DS8 | TTGACCATTCACCACATTGGTGTGC | |
| HMT3 | ATCTGGGAGCGGGGCTGT | |
| NF1 5′-4 | AAGTTCTTCCATGCCATCATCAG | |
| NF1 3′-3 | ATTCTAGGTGGTGGCTTTTTATCTA | |
| NF1 5′-5 | AAGGGACATCTTCAAAGA | |
| NF1 3′-4 | TTTATGTACAAGCCAACA | |
| GAPDH 5′ | ACCTCCAAACTGAAGAGC | |
| GAPDH 3′ | TGGCAGGTTTCTCCAGGCGGC |
Hu protein expression plasmids.
The HuR expression vector was generated by subcloning the HuR cDNA (a gift from Ann-Bin Shyu, University of Texas-Houston Medical School) into the pcDNA3.1HisB (Invitrogen) vector. To generate cDNA sequences of the mouse HuB (mHuB) and mHuC, RT-PCR was carried out using RNA isolated from mouse F9 cells and mHuB- or mHuC-specific oligonucleotides (mHuB 5′ and mHuB 3′ for mHuB and mHuC 5′ and mHuC 3′ for mHuC [Table 1]). The PCR products were digested with BamHI and EcoRI and cloned into the BamHI and EcoRI sites in the pcDNA3.1HisB (Invitrogen) vector for mammalian cell transfection. All of the mHuB and mHuC isoforms were PCR cloned into the BamHI and EcoRI sites in the pGEX-2TK (Amersham) vector for glutathione S-transferase (GST) recombinant protein production. GST-HuR and GST-hHuD were gifts from Imed-Eddine Gallouzi (McGill University, Canada) and Henry Furneaux (University of Connecticut Health Center), respectively.
Cell culture, cell transfection, and antibiotic selection.
HeLa and PC12 cell lines were maintained according to the ATCC instructions. CA77 cells (a gift from Alison Hall and Andrew Russo) were cultured in Dulbecco's modified Eagle medium/F-12 (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen) and 1% penicillin/streptomycin (Invitrogen). HeLa cells were transfected as previously described (52). Transfection of CA77 cells was carried out essentially as for HeLa cells except that Lipofectamine 2000 (Invitrogen) was used and cells were grown for 72 h instead of 48 h after transfection. Cotransfections were carried out using 1 μg of the NF1 reporter plasmid and 0.5 to 1 μg of polypyrimidine tract binding protein (PTB), TIAR, or mHuC plasmids. Stable transfection of PC12 cells was carried out using 5 μg of pcDNA3.1HisB vector, PTB, or mHuC. Forty-eight hours after transfection, the PC12 cells were selected with 500 μg/ml Geneticin (Invitrogen) for 7 days. The surviving cells were collected for further analysis.
RNA and protein analysis.
Procedures for total RNA and protein isolation and RT-PCR analysis were described previously (52). Oligonucleotide pairs DS8/HMT3 and NF1 5′-4/NF1 3′-3 were used to analyze the NF1 reporter RNA and endogenous NF1 RNA, respectively. Sixteen to 20 and 19 to 21 PCR cycles were used to analyze reporter RNA isolated from HeLa or CA77 cells, respectively. Endogenous NF1 RNA in all of the cell lines was analyzed using 26 PCR cycles. Quantification of exon inclusion was determined using a PhosphorImager. The results shown are representative of at least three independent transfections for each experiment. The effect of PTB, TIAR, and Hu proteins on RNA processing of the reporter pre-mRNA was calculated as a percentage of the NF1 exon 23a inclusion [exon 23a inclusion/(exon 23a inclusion + exon 23a exclusion)]. Western blot analysis using the proteins isolated from the transfected cells was carried out with anti-Xpress antibody (Invitrogen), anti-Myc antibody (Invitrogen), or antihemagglutinin antibody (Covance).
siRNA-mediated knockdown of TIA-1, TIAR, or HuC.
The TIA-1 and TIAR small interfering RNA (siRNA) duplexes were synthesized based on the information described by Izquierdo et al. (26) (Dharmacon). The target sequences are AAGCUCUAAUUCUGCAACUCUUU (TIA-1) and AACCAUGGAAUCAACAAGGAUUU (TIAR). One hundred twenty-five picomoles of each or both of the two siRNAs was used in these transfections, using DharmaFECT 1 (Dharmacon). The target sequence of HuC is AAUGAAUCCUGCAAGUUGGUU. Increasing amounts (150 and 300 pmol) of this siRNA were used to cotransfect the CA77 cells with the NF1 reporter.
In vitro assays.
UV cross-linking reactions were carried out as described previously (52). Cross-linked polypeptides were immunoprecipitated using monoclonal antibodies against TIA-1/TIAR, Hu sera (a gift from Jerome Posner), or anti-U2AF65 antibody (Sigma U4758). Psoralen cross-linking assays were carried out as described in a previous report (52). GST-TIAR and GST-mHuB SV4 recombinant proteins were added to the HeLa nuclear extract.
The RNA GST pull-down assay was carried out in a volume of 100 μl containing 44% HeLa cell nuclear extract, 2 mM ATP, 20 mM creatine phosphate, 0.15 mM dithiothreitol, 4 μg of either GST or GST-mHuB proteins, and 1 × 106 cpm of 32P-labeled RNA substrate. Reaction mixtures were incubated at 30°C for 20 min and then incubated with glutathione-Sepharose beads (Amersham) in NET supplemented with RNase Out (Invitrogen). The pellet was washed five times in NET and treated with proteinase K, followed by phenol-chloroform extraction and ethanol precipitation. The isolated RNA was analyzed on a 5% polyacrylamide gel containing 8.3 M urea.
Preparation of nuclear extracts from HeLa and CA77 cells and immunodepletion of TIA-1/TIAR proteins from HeLa nuclear extract.
HeLa cell nuclear extracts were prepared using S3 suspension culture and standard techniques (52). To make nuclear extracts from CA77 cells, 100 100-mm dishes of CA77 monolayer cells were collected and used according to a standard procedure (52).
TIA-1 and TIAR proteins were depleted from HeLa nuclear extract by incubating nuclear extract with GammaBind G Sepharose (Amersham) coated with antibodies against TIA-1 and TIAR at 4°C for 30 min on a rocker. After a quick spin, the supernatant was transferred to a fresh tube. This procedure was carried out three times in total to achieve significant depletion.
RESULTS
Our laboratory recently discovered the first nuclear role for Hu proteins in regulating alternative RNA processing of the human calcitonin/CGRP pre-mRNA by suppressing inclusion of an alternative 3′-terminal exon (51). In this case, Hu proteins function as polyadenylation regulators (53). To search for potential examples in which Hu proteins function as splicing regulators, we combined computational and conventional molecular biology methods to identify NF1 exon 23a as a potential target. A bioinformatic analysis to search for the well-characterized AU-rich Hu protein binding site in proximity to alternatively spliced exons that show neuron specificity identified more than 20 potential targets of Hu proteins. Of these potential targets, we chose exon 23a of the NF1 pre-mRNA to study the role of Hu proteins in splicing regulation because of the clear tissue specificity and biological importance of this particular alternative splicing event. NF1 exon 23a is surrounded by several AU-rich sequences (Fig. 1a). The sequence shown in Fig. 1a is 96% identical between human and mouse, indicating that not only the alternative splicing pattern of NF1 (5) but also the AU-rich sequences surrounding the cassette exon (not shown) are conserved.
FIG. 1.
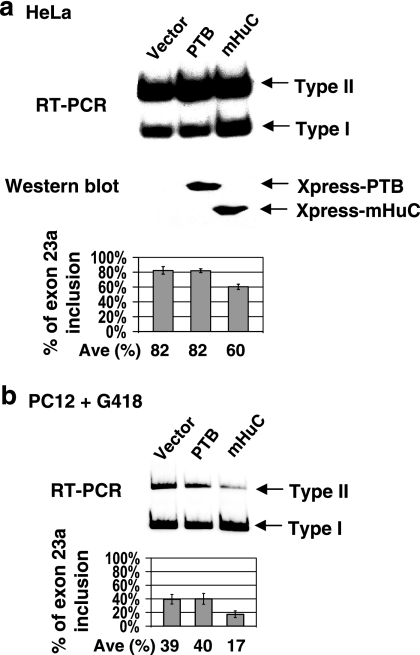
Hu proteins suppress inclusion of NF1 exon 23a. (a) Diagram depicting the alternative inclusion of exon 23a in the NF1 pre-mRNA. The 63-nucleotide (nt) in-frame exon 23a is specifically excluded in neurons. The arrows indicate the oligonucleotides used to analyze endogenous NF1 splicing by RT-PCR. The sequence of exon 23a and its surrounding introns is shown, with the exon sequence capitalized. AU-rich sequences are underlined. (b) Association of Hu proteins and the NF1 pre-mRNA in CA77 cells. Nuclear extract prepared from CA77 cells was immunoprecipitated with anti-Hu sera. RNA was isolated from either total nuclear extract or immunoprecipitated pellet and analyzed by RT-PCR. Lanes without RT (−) are included as controls. The oligonucleotides used to amplify the NF1 pre-mRNA anneal to sequences in introns upstream and downstream of exon 23a, and the resulting PCR product is 250 nucleotides, while the oligonucleotides used to amplify the GAPDH pre-mRNA anneal to sequences in intron 3 and exon 5, and the resulting PCR product is 585 nucleotides. (c) Correlation of skipping of exon 23a and Hu protein expression. Inclusion of exon 23a in the endogenous NF1 pre-mRNA in HeLa and CA77 cells was detected by RT-PCR. Amplification bands resulting from exon 23a inclusion (267 nucleotides) or exclusion (204 nucleotides) are indicated. Expression of neuron-specific Hu proteins in the two cell lines was detected by Western blot analysis using anti-Hu patient sera. (d) RT-PCR analysis of the NF1 reporter pre-mRNA. HeLa or CA77 cells were cotransfected with the NF1 reporter construct shown in the top panel and increasing amounts (0.5 and 1 μg) of PTB or mHuC. RT-PCR was carried out using total RNA isolated from the transfected cells and the oligonucleotides indicated in the diagram. Amplification products resulting from exon 23a inclusion (309 nucleotides) or exclusion (246 nucleotides) are indicated. The percentage of NF1 exon 23a inclusion is displayed in the bar graphs. The expression of transfected PTB or mHuC in HeLa cells was verified by Western blot analysis. Error bars indicate standard deviations.
To verify the in silico result, we performed RNA IP with anti-Hu sera, which can recognize HuB, HuC, and HuD. Nuclear extract from CA77 cells, a Hu protein-expressing rat medullary thyroid carcinoma cell line displaying numerous neuronal features, was used in this analysis. As shown in Fig. 1b, a strong NF1 pre-mRNA signal was detected in the immunoprecipitate, while no GAPDH pre-mRNA was detectable. PhosphorImager measurement of radioactivity in the RT-PCR products indicates that 52% of the input NF1 pre-mRNA was pulled down by the anti-Hu sera. These experiments demonstrate a significant interaction between Hu proteins and NF1 pre-mRNA in CA77 cells.
The NF1 gene is widely expressed in normal adult human and rodent tissues tested, with most cells expressing the type II NF1 mRNA including exon 23a. The type I NF1 mRNA, in which exon 23a is skipped, is found predominantly in adult neurons (Fig. 1a) (21, 24). To study splicing regulation of NF1 exon 23a, we chose two cell lines, HeLa and CA77, to mimic the two splicing pathways. These cell lines are excellent models to study this alternative splicing event because the NF1 gene is endogenously expressed in both cell lines but its transcript is differentially processed. Exon 23a is predominantly included in HeLa cells to produce type II NF1 and excluded in CA77 cells to produce type I NF1 (Fig. 1c).
As shown in Fig. 1c, the neuron-specific HuB, HuC, and HuD proteins are expressed in CA77 cells but not in HeLa cells. The other member of the Hu protein family, HuA (HuR in human), is a ubiquitously expressed protein that shows similar levels of expression in both cell lines (not shown). These experiments indicate a correlation between neuron-specific Hu protein expression and exon 23a skipping.
Hu proteins suppress inclusion of NF1 exon 23a.
In order to study how NF1 exon 23a inclusion is regulated, an NF1 reporter was generated by inserting NF1 exon 23a with part of its flanking intronic sequences into the first intron of the HMT gene (Fig. 1d). When transfected into HeLa or CA77 cells, the pre-mRNA generated from this reporter is processed similarly to the endogenous NF1 (compare lane 1 in each panel of Fig. 1d to Fig. 1c). In both cell lines, cotransfection of an Xpress-tagged mHuC cDNA plasmid with the NF1 reporter decreased inclusion of exon 23a of the reporter (Fig. 1d, middle and right panels). In contrast, overexpression of PTB, a general splicing suppressor that binds to pyrimidine-rich sequences, did not affect inclusion of this exon (Fig. 1d, left panel).
Overexpression of Hu proteins also decreased inclusion of exon 23a of the endogenous NF1 pre-mRNA. In HeLa cells, in spite of the relatively low transfection efficiency (20 to 40%, determined by transfection of a LacZ expression plasmid followed by X-Gal [5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside] staining [data not shown]), mHuC consistently suppressed inclusion of exon 23a in the endogenous NF1 mRNA, leading to decreased production of the type II isoform (from 82% to 60%) (Fig. 2a). We also tested the effect of Hu proteins on endogenous NF1 splicing in the rat pheochromocytoma cell line PC12. This cell line was previously used to study the function of NF1 in regulating Ras activity during cell differentiation (48). In contrast to HeLa cells, PC12 cells favor skipping of NF1 exon 23a (Fig. 2b). In this experiment, PC12 cells were stably transfected with PTB, mHuC, or a control vector plasmid because the transfection efficiency is much lower in PC12 cells than in HeLa cells. After selection, the surviving cells were pooled and total RNA purified for RT-PCR splicing assays. Compared to the vector and PTB, introduction of mHuC significantly reduced inclusion of exon 23a from the endogenously expressed NF1 pre-mRNA (from 39% to 17%) (Fig. 2b).
FIG. 2.
Overexpression of Hu proteins in HeLa and PC12 cells reduces inclusion of the endogenous NF1 exon 23a. (a) Transient transfection of PTB or mHuC in HeLa cells. Inclusion of endogenous NF1 exon 23a was detected by RT-PCR using total RNA isolated from the transfected cells and the oligonucleotides shown in Fig. 1a. The level of overexpressed proteins was detected by Western blot analysis. (b) Stable transfection of PTB or mHuC in PC12 cells. Inclusion of endogenous NF1 exon 23a was detected by RT-PCR. Error bars indicate standard deviations.
Two AU-rich sequence elements are important for the regulation of NF1 splicing by Hu proteins.
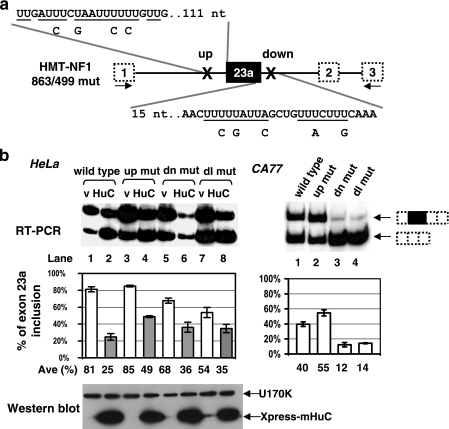
Exon 23a is surrounded by a number of AU-rich sequence blocks. To identify AU-rich sequence elements responsible for the shift in exon inclusion caused by overexpressing Hu proteins, we generated a number of deletion and point mutation reporter constructs and used them in cotransfection experiments. We identified two important sequence elements, one located 111 nucleotides upstream and one 15 nucleotides downstream of exon 23a (Fig. 1a). When the upstream AU-rich sequence was disrupted, the resulting reporter did not respond to the same amount of overexpressed mHuC as well as the wild-type reporter (49% versus 25% of exon 23a inclusion) (Fig. 3b). When the downstream AU-rich sequence was disrupted, the splicing suppression ability of HuC was only moderately reduced compared to the wild-type reporter (36% versus 25%) (Fig. 3b). When the upstream and downstream mutations were combined, the effect was again moderate, with 35% exon inclusion when mHuC was present. However, the double mutant alone led to reduced exon inclusion (from 81% to 54%) even without any mHuC, meaning that the difference between the presence and absence of mHuC is much smaller than that observed for the wild-type construct. Note that the lower RT-PCR signal in lane 6 of Fig. 3b is due to the reduced amount of total RNA (based on RT-PCR of β-actin [data not shown]).
FIG. 3.
Two AU-rich sequence elements flanking exon 23a are important for regulated splicing of NF1 exon 23a. (a) A diagram indicating mutated AU-rich sequences in the reporter. nt, nucleotides. (b) Left, the wild-type or mutant (mut) reporters were cotransfected with empty vector or mHuC cDNA in HeLa cells. Inclusion of exon 23a from the NF1 pre-mRNA was detected by RT-PCR. A Western blot showing expression of the Xpress-tagged mHuC is included. U170K protein is shown as a loading control for the Western blot analysis. Right, the wild-type or mutant reporters were transfected into CA77 cells. Error bars indicate standard deviations. dn, down; dl, double.
When the mutants were tested in CA77 cells, the upstream mutant led to increased exon 23a inclusion (from 40% to 55%), while the two mutants that have the downstream AU-rich sequence disrupted led to an unexpected decrease of exon inclusion (to 12% or 14%, respectively). The results of these experiments, although complicated, suggest that the upstream AU-rich sequence plays the major role in responding to overexpressed HuC protein, while the downstream sequence contributes in a more minor way to exon skipping promoted by Hu proteins. They also suggest that additional factors are involved in the regulation of exon 23a inclusion (see Discussion).
TIA-1/TIAR protein promotes inclusion of NF1 exon 23a.
The observation that the mutant reporter carrying point mutations at the downstream sequence showed moderately reduced exon inclusion in HeLa cells in the absence of any overexpressed proteins compared to the wild-type reporter (81% versus 68%) suggests that this AU-rich sequence may also function as a positive element (compare lanes 1 to 5 in Fig. 3b, left). Since TIA-1/TIAR proteins are known to bind similar sequences as the Hu proteins and affect exon inclusion positively (17, 20, 32), we hypothesized that TIA-1/TIAR function as splicing enhancer proteins to promote exon 23a inclusion. To test this hypothesis, we carried out overexpression and siRNA knockdown experiments to manipulate the levels of these proteins. When TIAR protein was overexpressed, inclusion of exon 23a of the reporter pre-mRNA was increased from 82% to 96% in HeLa cells (Fig. 4a, left panel) and from 29% to 44% in CA77 cells (Fig. 4a, right panel). As expected, when the downstream element was disrupted, the mutant reporter transcript did not respond to an increased level of TIAR (Fig. 4a, middle panel).
FIG. 4.
TIA-1/TIAR proteins promote inclusion of exon 23a. (a) Overexpression of TIAR in HeLa cells increases exon 23a inclusion. HeLa cells were cotransfected with the wild-type (left panel) or mutant (see Fig. 3a for mutant sequence) (middle panel) NF1 reporter construct and increasing amounts (0.5 and 1 μg) of TIAR. RT-PCR was carried out using total RNA isolated from the transfected cells and the oligonucleotides indicated in the diagram in Fig. 1. The percentage of NF1 exon 23a inclusion is displayed in the bar graphs. The expression of transfected TIAR was verified by Western blot analysis. Overexpression of TIAR in CA77 cells increases exon 23a inclusion (right panel). (b) siRNA knockdown of TIA-1 and TIAR in HeLa cells reduces inclusion of exon 23a. HeLa cells were transfected with a control siRNA (250 pmol), an siRNA against TIA-1 (125 pmol TIA-1 plus 125 pmol control siRNA), an siRNA against TIAR (125 pmol TIAR plus 125 pmol control siRNA), or siRNAs against both TIA-1 and TIAR (125 pmol TIA-1 plus 125 pmol TIAR siRNA). Results of RT-PCR and Western blot analysis are shown. U170K protein served as a loading control for the Western blot analysis. WT, wild type. Error bars indicate standard deviations.
We next carried out an siRNA knockdown experiment in which we specifically reduced the level of TIA-1, TIAR, or both proteins and examined the effect on splicing of the endogenous NF1 pre-mRNA. As shown in Fig. 4b, when the TIA-1 or TIAR level was reduced individually, no apparent change in exon 23a inclusion was observed (lanes 1 to 4). However, when the levels of both proteins were reduced, exon 23a inclusion was decreased from 78% to 40% (compare lane 5 to lane 1). These results demonstrate not only that the TIA-1/TIAR proteins have a positive role in exon 23a inclusion but also that either protein is sufficient to promote exon inclusion.
To further establish the function mediated by Hu and TIA-1/TIAR proteins through binding at the downstream AU-rich sequence, we generated a shorter reporter that contains only 75 nucleotides of intron sequence upstream of exon 23a and the same amount of downstream intron sequence (Fig. 5a). Inspection of the remaining upstream intron sequence in this reporter reveals no AU-rich sequence, whereas at least three clusters of AU-rich sequence elements are present in the downstream 499-nucleotide intron sequence in the reporter. The RNA transcripts of this new short reporter still responded well to increased levels of either TIAR or mHuC protein (Fig. 5a). However, the transcript from the mutant reporter in which the downstream AU-rich element was disrupted did not respond to increased levels of either TIAR or mHuC (Fig. 5b), suggesting that the AU-rich sequence downstream of the 5′ splice site of exon 23a is a major determinant of the effect caused by either TIAR or mHuC in the short reporter background.
FIG. 5.
The AU-rich sequence element immediately downstream of exon 23a is important for regulated inclusion of NF1 exon 23a by TIA-1/TIAR and Hu proteins. (a) A shorter NF1 reporter that contains only 75 nucleotides of the upstream intron shown in the diagram was cotransfected with TIAR or mHuC protein in HeLa cells. Inclusion of exon 23a from this short reporter was analyzed by RT-PCR. (b) A short reporter carrying the same downstream mutations in AU-rich sequence as shown in Fig. 3a was cotransfected with TIAR or mHuC protein. Inclusion of exon 23a from this mutant short reporter was analyzed by RT-PCR. Error bars indicate standard deviations.
Hu and TIA-1/TIAR proteins bind to the AU-rich sequence in a competitive manner.
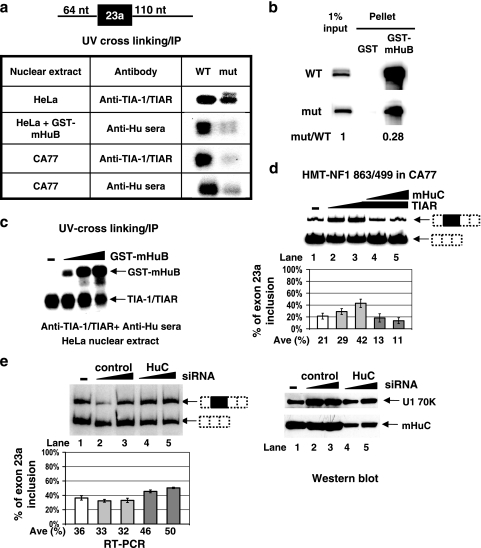
Because both Hu proteins and TIA-1/TIAR have a strong affinity for AU-rich sequences, we hypothesized that they compete for binding at the AU-rich sequence downstream of the 5′ splice site. We investigated binding of Hu proteins and TIA-1/TIAR to the downstream AU-rich sequence using a UV cross-linking/IP assay. A short RNA transcript containing the NF1 exon 23a with 64 nucleotides of upstream and 110 nucleotides of downstream intron sequence, including only the downstream AU-rich sequences, was used in this in vitro assay (Fig. 6a). Both HeLa and CA77 cell nuclear extracts were used to test binding of either Hu proteins or TIA-1/TIAR to the RNA. Because HeLa cells do not express any neuron-specific Hu proteins, recombinant GST-mHuB protein was added to the HeLa nuclear extract to test Hu binding to RNA. IP was performed with either Hu patient sera or anti-TIA-1/TIAR antibody. As shown in Fig. 6a, both TIA-1/TIAR and Hu proteins are capable of interacting with the RNA transcript in either HeLa or CA77 nuclear extract. Both TIA-1/TIAR and Hu proteins bind RNA directly, as indicated by RNA mobility shift assays (data not shown). When the AU-rich sequence was mutated, binding of both TIA and Hu proteins was dramatically reduced in all cases (Fig. 6a). The reduced binding of Hu proteins to the mutant RNA substrate was also observed in a completely different assay, shown in Fig. 6b. In this assay, we incubated wild-type and mutant RNAs with GST-mHuB protein in HeLa nuclear extract and carried out a pull-down analysis of the GST-tagged proteins with glutathione-Sepharose beads. We then purified the RNA coprecipitated with the GST-tagged proteins and analyzed it by denaturing polyacrylamide gel electrophoresis. Measuring the radioactivity in each band with a PhosphorImager indicated that binding of the mutant RNA substrate to GST-mHuB was reduced to 28%.
FIG. 6.
TIA-1/TIAR and Hu proteins compete with each other in regulating inclusion of the NF1 exon 23a. (a) Binding of TIA and Hu proteins to the wild-type (WT) or mutant RNA substrate was detected by a UV cross-linking/IP assay using an in vitro-transcribed RNA containing exon 23a and 64 nucleotides (nt) of the upstream and 110 nucleotides of the downstream intron sequences (see diagram) and HeLa or CA77 nuclear extract. GST-mHuB (1 μg) was added to HeLa nuclear extract to detect the binding of Hu proteins to NF1 RNA substrate. Anti-TIA-1/TIAR or anti-Hu serum was used in this IP experiment. (b) RNA GST pull-down assay using either wild-type or mutant in vitro NF1 RNA substrate and HeLa nuclear extract. (c) Competition between Hu and TIA-1/TIAR proteins was examined by UV cross-linking/IP using HeLa nuclear extract supplemented with increasing amounts (0.1, 0.5, and 2.5 μg) of GST-mHuB protein. Anti-TIA-1/TIAR antibody and anti-Hu sera were both included in the IP step. (d) In vivo competition of TIAR and Hu proteins. The NF1 reporter (HMT-NF1 863/499) was cotransfected with a control vector plasmid, increasing amounts of TIAR expression plasmid (1 and 2 μg) (lane 2 and lane 3), or 2 μg of TIAR plasmid together with increasing amount of mHuC (1 and 2 μg) (lane 4 and lane 5) in CA77 cells. Inclusion of the NF1 exon 23a was detected by RT-PCR. (e) siRNA knockdown of HuC in CA77 cells. CA77 cells were cotransfected with the NF1 reporter (HMT-NF1 863/499) in the absence of siRNA (lane 1) or with increasing concentrations (150 pmol and 300 pmol) of a control siRNA (lanes 2 and 3) or HuC siRNA (lanes 4 and 5). Inclusion of the endogenously expressed NF1 exon 23a was detected by RT-PCR (left panel). The same siRNAs were cotransfected with mHuC expression plasmid in HeLa cells, and a Western blot of the total protein lysate isolated from the transfected cells is shown in the right panel. Error bars indicate standard deviations.
In order to test whether Hu and TIA-1/TIAR proteins compete with each other for binding to the AU-rich sequence, we performed UV cross-linking of in vitro-transcribed RNA in HeLa nuclear extract supplemented with recombinant GST-mHuB proteins. We then precipitated both endogenous TIA-1/TIAR proteins and recombinant Hu proteins at the same time using beads coated with antibodies against both proteins. As shown in Fig. 6c, with increasing amounts of GST-mHuB protein, binding of endogenous TIA-1/TIAR proteins to the RNA substrate was reduced, indicating that mHuB and TIA-1/TIAR proteins bind to the AU-rich sequence in a competitive manner in vitro. Importantly, the competition of the two groups of proteins in regulating inclusion of NF1 exon 23a was also observed in a cell transfection experiment. Overexpression of TIAR protein in CA77 cells increased exon 23a inclusion, and introduction of mHuC together with TIAR abolished the effect caused by TIAR, leading to more skipping of exon 23a (Fig. 6d).
Finally, we carried out an siRNA knockdown experiment in CA77 cells. In this experiment, when the NF1 reporter was cotransfected with siRNA against HuC, a small yet consistent increase of exon 23a inclusion was observed (Fig. 6e, left panel). Because of the extremely low transfection efficiency in CA77 cells, we performed a control experiment in which HeLa cells were transfected with the siRNA against HuC, which showed that it is capable of reducing the level of exogenously expressed HuC protein (Fig. 6e, right panel). Note that the absolute levels of exon 23a inclusion in CA77 cells vary from transfection to transfection (between 21 and 40%), but the relative levels of exon 23a inclusion between constructs containing a wild-type or mutant sequence remain the same.
TIA-1/TIAR and Hu proteins modulate the interaction of U1 snRNP and U6 snRNP with the 5′ splice site of exon 23a.
The experiments discussed above demonstrate that TIA-1/TIAR proteins are positive regulatory factors promoting exon 23a inclusion and that Hu proteins are negative regulatory factors that decrease exon 23a inclusion. We next wished to investigate the underlying mechanisms by which these proteins regulate inclusion of this exon. It is well established that TIA-1/TIAR proteins regulate a number of splicing events by facilitating the interaction of U1 snRNP with suboptimal 5′ splice sites (17, 20). The 5′ splice site sequence (UCA/GTAAGTT) of the NF1 exon 23a is relatively weak due to the presence of UCA instead of the consensus of CAG as the last three nucleotides of the exon. Thus, we tested whether TIA-1/TIAR promotes interaction of U1 snRNP with the 5′ splice site and, more importantly, whether Hu proteins suppress inclusion of NF1 exon 23a by interfering with binding of snRNPs to the 5′ splice site.
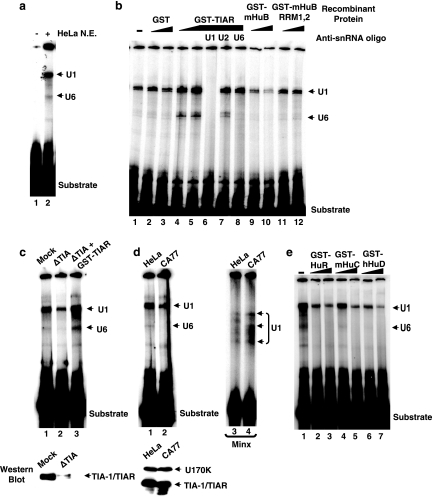
A psoralen cross-linking assay was used to examine the interaction of snRNPs with the in vitro-transcribed RNA substrate described in Fig. 6a, using HeLa cell nuclear extract with or without supplementary recombinant TIAR or Hu proteins. One major cross-linked product that is nuclear extract dependent was observed (Fig. 7a). When recombinant GST-TIAR was added to the nuclear extract, the yields of this cross-linked product and an additional cross-linked doublet that moved faster in the nondenaturing gel were significantly increased (Fig. 7b, compare lanes 4 and 5 to lane 1). The increase of these cross-linked products is specific to the TIAR portion of the recombinant protein because GST alone did not affect the cross-linking yield (Fig. 7b, compare lanes 4 and 5 to lanes 2 and 3). The identity of the cross-linked products was determined by pretreatment of the nuclear extract with anti-snRNA oligoribonucleotides to block binding by specific snRNAs. The appearance of both cross-linked products is abolished by anti-U1 snRNA oligoribonucleotide, while only the faster-moving product depends on U6 snRNA (Fig. 7b, lanes 6 and 8), indicating that the slower-moving product contains U1 snRNA and the faster-moving product contains U6 snRNA. Interestingly, Hu proteins had the opposite effect in modulating U1 and U6 snRNA binding. Addition of GST-mHuB led to reduced U1 and U6 snRNA binding (Fig. 7b, lanes 9 and 10). This effect is specific to the full-length mHuB protein. A truncated GST-mHuB that contains only the RRM1 and RRM2 domains of the three-RRM-domain-containing protein had no effect on snRNA binding (Fig. 7b, lanes 11 and 12).
FIG. 7.
TIAR and Hu proteins regulate binding of U1 and U6 snRNA to the 5′ splice site of NF1 exon 23a. (a) Psoralen cross-linking of the NF1 RNA substrate indicated in Fig. 6a in the presence (lane 2) or absence (lane 1) of HeLa nuclear extract (N.E.). (b) Psoralen cross-linking of the NF1 RNA substrate in HeLa nuclear extract without added protein (lane 1) or with with increasing amounts (0.5 and 2.0 μg) of GST (lane 2 and lane 3), GST-TIAR (lanes 4 to 8), GST-mHuB (lane 9 and lane 10), or a truncated Hu protein, GST-mHuB RRM1,2 (lane 11 and lane 12). A 2′-O′-methyl ribo-oligonucleotide that specifically blocks the pre-mRNA binding region of U1, U2, or U6 snRNA was included to identify the cross-linked species (lanes 6 to 8). (c) Psoralen cross-linking of NF1 RNA substrate in HeLa nuclear extract, in which the endogenous TIA-1 and TIAR proteins were removed by immunodepletion. Lane 1, mock-depleted nuclear extract; lane 2, TIA-1/TIAR-depleted nuclear extract; lane 3, TIA-1/TIAR-depleted nuclear extract supplemented with 1 μg GST-TIAR. The TIA-1/TIAR protein levels in the mock- and TIA-1/TIAR-depleted nuclear extracts were examined by Western blotting. (d) Psoralen cross-linking of NF1 RNA substrate in HeLa nuclear extract (lane 1) and CA77 nuclear extract (lane 2). A Minx RNA substrate that contains a 5′ splice site was used as a control in this experiment (lanes 3 and 4). The levels of TIA-1/TIAR proteins and a U1 snRNP protein were detected by Western blotting. U170K protein was used to indicate the levels of U1 snRNPs in these nuclear extracts. (e) Psoralen cross-liking of the NF1 RNA substrate in HeLa nuclear extract supplemented with increasing amounts (0.5 and 4 μg) of GST-HuR (lane 2 and lane 3), GST-mHuC (lane 4 and lane 5), or GST-HuD (lane 6 and lane 7).
These data indicate that TIAR protein promotes the interaction of U1 and U6 snRNAs with the RNA that contains the NF1 exon 23a, whereas mHuB protein down-regulates U1 and U6 snRNA binding. This conclusion was further strengthened by additional psoralen cross-linking analysis. When we immunodepleted TIA-1/TIAR proteins from the HeLa nuclear extract, both U1 and U6 snRNP binding were significantly compromised (Fig. 7c, compare lane 2 to lane 1). It is unlikely that the change in snRNA binding was caused by changes in the levels of these snRNAs after TIA-1/TIAR depletion, because this reduced binding was rescued by adding back recombinant GST-TIAR protein to the same TIA-1/TIAR-depleted extract (Fig. 7c, lane 3). CA77 cells express high levels of neuronal HuB, HuC, and HuD proteins compared to HeLa cells (Fig. 1c). The U1 snRNA binding to the NF1 RNA substrate in CA77 nuclear extract is significantly weaker than that in HeLa nuclear extract, and U6 snRNP binding is undetectable in CA77 cells (Fig. 7d, lanes 1 and 2). In a control experiment, the levels of U1 snRNA binding to the 5′ splice site of the Minx RNA, which contains a strong 5′ splice site, were similar in the two nuclear extracts (Fig. 7d, lanes 3 and 4). The multiple U1-containing cross-linked RNA species were identified by use of anti-U1 snRNA ribo-oligonucleotide (data not shown). Thus, these experiments argue that the balance between TIA-1/TIAR and Hu proteins modulates the interaction of U1 and U6 snRNAs with the 5′ splice site of exon 23a. The other three members of the Hu protein family are also capable of modulating snRNA binding in this in vitro analysis (Fig. 7e).
Hu proteins reduce U2AF65 binding to an RNA containing the upstream 3′ splice site.
The experiments discussed above indicate that Hu proteins affect recognition of the 5′ splice site of exon 23a. We next tested whether Hu proteins would affect binding of splicing factors to the 3′ splice site of this exon, using a longer RNA transcript this time to include the upstream AU-rich sequences. As shown in Fig. 8, addition of recombinant mHuB, but not a truncated mHuB that contains RRM1 and -2 only, to the HeLa nuclear extract reduced binding of U2AF65 to this RNA transcript. Note that the slight increase of U2AF65 binding in the presence of the truncated HuB at low concentration is not consistently observed. These results suggest that Hu proteins affect exon definition by preventing binding of splicing factors on both sides of exon 23a.
FIG. 8.
U2AF65 cross-linking with the RNA substrate indicated in the diagram. The 32P-labeled, in vitro-transcribed RNA transcripts were UV cross-linked in HeLa cell nuclear extract in the presence of increasing amounts of buffer alone or of GST-mHuB RRM1,2 or mHuB (50 and 200 ng) and immunoprecipitated with the anti-U2AF65 antibody. nt, nucleotides.
To determine if TIA-1/TIAR proteins promote binding of U2AF65, we carried out a similar UV cross-linking/IP assay using HeLa nuclear extract supplemented with recombinant GST-TIAR protein. Interestingly, addition of GST-TIAR did not increase U2AF65 binding (data not shown).
DISCUSSION
Hu proteins mediate alternative splicing of NF1 exon 23a.
Our finding that Hu proteins regulate alternative inclusion of exon 23a has significant implications for understanding NF1 biology. Exon 23a is located in the middle of the GAP-related domain of the neurofibromin protein previously shown to regulate Ras activity (13). The two neurofibromin isoforms generated by alternative inclusion of exon 23a show 10-fold-different potency in Ras-regulating activity (5, 48). Studies in human and mouse models indicate that the Ras-regulatory function of NF1 plays an important role in learning. In humans, a mutation in the NF1 gene that specifically abolishes the Ras-GAP function of neurofibromin without affecting its ability to bind Ras was shown to cause multiple NF1 symptoms, including cognitive dysfunction (30). In mice, learning deficits of Nf1+/− animals can be rescued by genetic and pharmacological manipulations that decrease Ras function (14). Most interestingly, genetic ablation of exon 23a (type II deletion) in mice resulted in learning disabilities (16). These studies show that abnormal Ras activity can disrupt learning, indicating that the precise Ras modulation by neurofibromin is crucial for learning and memory. Interestingly, a number of studies also implicate Hu proteins in learning (1, 38, 40). Our studies reported here provide a link between Hu proteins, a family of proteins also required for neuronal differentiation, and the function of NF1. Expression of Hu proteins, detected as early as embryonic day 10 (37), slightly precedes production of the type I NF1 mRNA during mouse fetal development (25). The Hu proteins are expressed in neurons migrating out of the peri-ventricular zone into the intermediate zone (37). While type II NF1 is dominant in the ventricular zone, type I NF1 is the major form in intermediate zone and neocortex neurons (25). Taken together, these findings support a role for Hu proteins in controlling the Ras-regulating function of NF1 through modulation of alternative splicing involving exon 23a.
Regulation of neuron-specific alternative splicing.
The studies presented in this report provide compelling evidence to support a role for Hu proteins in regulating alternative splicing and strongly suggest that Hu proteins are important splicing regulators in neurons. Hu proteins are one of only a few groups of neuron-specific splicing regulators identified thus far, including nPTB/brPTB, NAPOR/CUGBP2, Fox-1/Fox-2, Nova, and QK1 (9, 19, 28, 35, 39, 42, 44, 45, 47, 49, 50; reviewed in reference 33). We predict that, like Nova-2, which was shown to coordinately regulate alternative splicing of a network of genes related to synapsis (44), Hu proteins may regulate exon inclusion of numerous mRNAs by affecting alternative splicing of their pre-mRNAs. It is therefore of particular importance to identify additional targets of Hu proteins. Interestingly, in a recent report, HuD was implicated in regulation of Ikaros alternative splicing mediated by Notch3 signaling. In that study, HuD was shown to be up-regulated through T-cell receptor signaling, which is correlated with changes in several alternatively spliced isoforms of Ikaros (10). It is possible that HuD plays a direct role in splicing of the Ikaros pre-mRNA, although this remains to be definitively shown (26).
We demonstrate that inclusion of NF1 exon 23a is regulated in part by competition between Hu proteins and a group of previously characterized proteins, TIA-1/TIAR. TIA-1/TIAR proteins have been shown to bind to U-rich sequences immediately downstream of 5′ splice sites of internal exons (17, 20, 26, 32). Although one study demonstrated that binding of TIA-1 is U1 dependent, other studies showed that TIA-1/TIAR proteins promote the interaction of U1 snRNP and 5′ splice sites that contain suboptimal splicing signals (17, 20, 26, 32). In the NF1 system, we found that TIA-1/TIAR promote U1 snRNP interaction with the 5′ splice site of exon 23a, consistent with the idea that TIA-1/TIAR proteins help define 5′ splice sites. More interestingly, we found a novel function for these proteins in modulating U6 snRNP interaction with the same 5′ splice site (Fig. 7). Furthermore, the interaction of U6 snRNP with the NF1 RNA substrate appears to be independent of U2 snRNP (Fig. 7b). This result suggests that TIA-1/TIAR proteins may promote splicing at multiple stages during spliceosome assembly, at the E complex and formation of a later complex, most likely the B complex. It was shown previously that B complex formation is blocked by an exonic splicing silencer in the CD45 system (23). Additional experiments using complete splicing substrates need to be carried out to provide definitive mechanistic insights.
Importantly, we show that by binding to the AU-rich sequence downstream of the 5′ splice site of exon 23a, Hu proteins inhibit the function of TIA-1/TIAR, thereby reducing the interaction of U1 and U6 snRNPs with the 5′ splice site. The nature of the competition of Hu and TIA-1/TIAR proteins is not clear at present. Although the data in Fig. 6c suggest competitive binding between the two groups of proteins at some level, we cannot rule out the possibility that they can bind to the AU-rich sequence simultaneously. Collectively, the results of these experiments and previous studies that demonstrate competition between TIA-1/TIAR and other splicing regulators such as PTB (26, 41, 54) strongly suggest that the balance between alternative splicing regulators represents a key theme of alternative splicing regulation in neurons.
Complex regulation of inclusion of exon 23a.
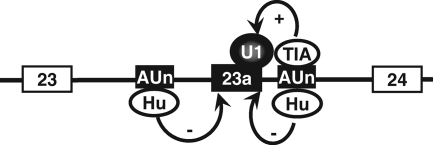
Our results indicate that, in addition to competing with TIA-1/TIAR to regulate inclusion of exon 23a, Hu proteins suppress the inclusion of NF1 exon 23a through an additional mechanism. When the AU-rich sequence upstream of the 5′ splice site of exon 23a was mutated, the mutant primary transcripts of the HMT-NF1 reporter responded to the increased Hu protein level to a much lesser extent (Fig. 3b). It has been observed in a number of systems, including Nova, PTB, and hnRNP A1, that splicing regulators function through binding at multiple sites (3, 4, 22, 36, 43). ELAV, the Drosophila homolog of the Hu proteins, also regulates alternative splicing by binding to multiple separate sites of the Neuroglian pre-mRNA (34). In the case of NF1 exon 23a, it is possible that the downstream binding site of Hu proteins plays a major role in controlling 5′ splice site recognition, while the upstream binding site regulates 3′ splice site recognition (Fig. 9). As a result, Hu proteins block exon definition of exon 23a during splicing.
FIG. 9.
A proposed model indicating how Hu proteins may regulate the neuron-specific exclusion of NF1 exon 23a.
How do Hu proteins regulate inclusion of exon 23a by binding to the sequence upstream of the exon? A simple model is that Hu proteins may directly interfere with the splicing factors that recognize the 3′ splice site, such as U2AF. Indeed, we observed reduction of U2AF65 binding to the RNA transcript when Hu proteins were added to the HeLa cell nuclear extract. They may also interfere with the function of other splicing enhancers that modulate the recognition of the 3′ splice site by splicing factors. Another possibility is that Hu protein binding at multiple sites both upstream and downstream of exon 23a forms a structure in which exon 23a is looped out, similar to the one that mediates skipping of the c-Src N1 exon induced by PTB proteins (36).
The results of the mutagenesis study shown in Fig. 3 suggest that very complex regulatory mechanisms control the cell-specific inclusion of the NF1 exon 23a. The fact that mutants in which the downstream AU-rich sequence is disrupted have significantly reduced inclusion of exon 23a in CA77 cells but not in HeLa cells suggests the involvement of additional factors. We predict the existence of either additional positive factors present in HeLa cells or additional negative factors in CA77 cells. In the future, more detailed mutagenesis analysis will be carried out to address this issue.
It is commonly observed that alternatively spliced exons are subject to complex control. In fact, the term “splicing code” was recently used to describe the ever-growing number of examples in which multiple splicing enhancer and silencer elements coexist to fine-tune the regulated inclusion of a particular exon. This paradigm is supported by a large number of conventional gene-specific studies as well as recent global splicing analyses using computational and array-type approaches (12). A remaining challenge is to decipher the details of the “splicing code,” identify the corresponding tissue-specific trans-acting factors, and define how the activities of different factors are coordinated to achieve tissue- and/or developmental stage-specific regulation for each alternative splicing event.
Acknowledgments
We thank the following individuals for providing antibodies and plasmids: Henry Furneaux at the University of Connecticut Health Center (GST-hHuD), Imed-Eddine Gallouzi at McGill University (GST-HuR), Alison Hall at Case Western Reserve University and Andrew Russo at the University of Iowa (CA77 cell line), Jerome Posner at the Sloan Kettering Cancer Center (Hu patient sera), and Ann-Bin Shyu at the University of Texas-Houston Medical School (HuR cDNA). Greg Matera's laboratory provided help with the siRNA knockdown experiments. We thank Helen Salz and Jo Ann Wise for critical reading of the manuscript.
This work was supported by an NSF grant (MCB-0237685) and an NIH grant (NS-049103-01) to Hua Lou. Hui Zhu was supported by a predoctoral fellowship from the American Heart Association (0415086B). Melissa Hinman is supported by a genetics training grant from NIH (T32GM008613).
Footnotes
Published ahead of print on 17 December 2007.
REFERENCES
- 1.Akamatsu, W., H. Fujihara, T. Mitsuhashi, M. Yano, S. Shibata, Y. Hayakawa, H. J. Okano, S. Sakakibara, H. Takano, T. Takano, T. Takahashi, T. Noda, and H. Okano. 2005. The RNA-binding protein HuD regulates neuronal cell identity and maturation. Proc. Natl. Acad. Sci. USA 1024625-4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akamatsu, W., H. J. Okano, N. Osumi, T. Inoue, S. Nakamura, S. Sakakibara, M. Miura, N. Matsuo, R. B. Darnell, and H. Okano. 1999. Mammalian ELAV-like neuronal RNA-binding proteins HuB and HuC promote neuronal development in both the central and the peripheral nervous systems. Proc. Natl. Acad. Sci. USA 969885-9890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amir-Ahmady, B., P. L. Boutz, V. Markovtsov, M. L. Phillips, and D. L. Black. 2005. Exon repression by polypyrimidine tract binding protein. RNA 11699-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.An, P., and P. J. Grabowski. 2007. Exon silencing by UAGG motifs in response to neuronal excitation. PLoS Biol. 5e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersen, L. B., R. Ballester, D. A. Marchuk, E. Chang, D. H. Gutmann, A. M. Saulino, J. Camonis, M. Wigler, and F. S. Collins. 1993. A conserved alternative splice in the von Recklinghausen neurofibromatosis (NF1) gene produces two neurofibromin isoforms, both of which have GTPase-activating protein activity. Mol. Cell. Biol. 13487-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson, K. D., M. A. Morin, A. Beckel-Mitchener, C. D. Mobarak, R. L. Neve, H. M. Furneaux, R. Burry, and N. I. Perrone-Bizzozero. 2000. Overexpression of HuD, but not of its truncated form HuD I+II, promotes GAP-43 gene expression and neurite outgrowth in PC12 cells in the absence of nerve growth factor. J. Neurochem. 751103-1114. [DOI] [PubMed] [Google Scholar]
- 7.Anderson, K. D., J. Sengupta, M. Morin, R. L. Neve, C. F. Valenzuela, and N. I. Perrone-Bizzozero. 2001. Overexpression of HuD accelerates neurite outgrowth and increases GAP-43 mRNA expression in cortical neurons and retinoic acid-induced embryonic stem cells in vitro. Exp. Neurol. 168250-258. [DOI] [PubMed] [Google Scholar]
- 8.Antic, D., N. Lu, and J. D. Keene. 1999. ELAV tumor antigen, Hel-N1, increases translation of neurofilament M mRNA and induces formation of neurites in human teratocarcinoma cells. Genes Dev. 13449-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ashiya, M., and P. J. Grabowski. 1997. A neuron-specific splicing switch mediated by an array of pre-mRNA repressor sites: evidence of a regulatory role for the polypyrimidine tract binding protein and a brain-specific PTB counterpart. RNA 3996-1015. [PMC free article] [PubMed] [Google Scholar]
- 10.Bellavia, D., M. Mecarozzi, A. F. Campese, P. Grazioli, C. Talora, L. Frati, A. Gulino, and I. Screpanti. 2007. Notch3 and the Notch3-upregulated RNA-binding protein HuD regulate Ikaros alternative splicing. EMBO J. 261670-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernards, A. 1995. Neurofibromatosis type 1 and Ras-mediated signaling: filling in the GAPs. Biochim. Biophys. Acta 124243-59. [DOI] [PubMed] [Google Scholar]
- 12.Blencowe, B. J. 2006. Alternative splicing: new insights from global analyses. Cell 12637-47. [DOI] [PubMed] [Google Scholar]
- 13.Cichowski, K., and T. Jacks. 2001. NF1 tumor suppressor gene function: narrowing the GAP. Cell 104593-604. [DOI] [PubMed] [Google Scholar]
- 14.Costa, R. M., N. B. Federov, J. H. Kogan, G. G. Murphy, J. Stern, M. Ohno, R. Kucherlapati, T. Jacks, and A. J. Silva. 2002. Mechanism for the learning deficits in a mouse model of neurofibromatosis type 1. Nature 415526-530. [DOI] [PubMed] [Google Scholar]
- 15.Costa, R. M., and A. J. Silva. 2002. Molecular and cellular mechanisms underlying the cognitive deficits associated with neurofibromatosis 1. J. Child Neurol. 17622-629, 646-651. [DOI] [PubMed] [Google Scholar]
- 16.Costa, R. M., T. Yang, D. P. Huynh, S. M. Pulst, D. H. Viskochil, A. J. Silva, and C. I. Brannan. 2001. Learning deficits, but normal development and tumor predisposition, in mice lacking exon 23a of Nf1. Nat Genet. 27399-405. [DOI] [PubMed] [Google Scholar]
- 17.Del Gatto-Konczak, F., C. F. Bourgeois, C. Le Guiner, L. Kister, M. C. Gesnel, J. Stevenin, and R. Breathnach. 2000. The RNA-binding protein TIA-1 is a novel mammalian splicing regulator acting through intron sequences adjacent to a 5′ splice site. Mol. Cell. Biol. 206287-6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deschenes-Furry, J., N. Perrone-Bizzozero, and B. J. Jasmin. 2006. The RNA-binding protein HuD: a regulator of neuronal differentiation, maintenance and plasticity. Bioessays 28822-833. [DOI] [PubMed] [Google Scholar]
- 19.Dredge, B. K., and R. B. Darnell. 2003. Nova regulates GABA(A) receptor γ2 alternative splicing via a distal downstream UCAU-rich intronic splicing enhancer. Mol. Cell. Biol. 234687-4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forch, P., O. Puig, N. Kedersha, C. Martinez, S. Granneman, B. Seraphin, P. Anderson, and J. Valcarcel. 2000. The apoptosis-promoting factor TIA-1 is a regulator of alternative pre-mRNA splicing. Mol. Cell 61089-1098. [DOI] [PubMed] [Google Scholar]
- 21.Gutmann, D. H., Y. Zhang, and A. Hirbe. 1999. Developmental regulation of a neuron-specific neurofibromatosis 1 isoform. Ann. Neurol. 46777-782. [DOI] [PubMed] [Google Scholar]
- 22.Han, K., G. Yeo, P. An, C. B. Burge, and P. J. Grabowski. 2005. A combinatorial code for splicing silencing: UAGG and GGGG motifs. PLoS Biol. 3e158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.House, A. E., and K. W. Lynch. 2006. An exonic splicing silencer represses spliceosome assembly after ATP-dependent exon recognition. Nat. Struct. Mol. Biol. 13937-944. [DOI] [PubMed] [Google Scholar]
- 24.Huynh, D. P., T. Nechiporuk, and S. M. Pulst. 1994. Alternative transcripts in the mouse neurofibromatosis type 2 (NF2) gene are conserved and code for schwannomins with distinct C-terminal domains. Hum. Mol. Genet. 31075-1079. [DOI] [PubMed] [Google Scholar]
- 25.Huynh, D. P., T. Nechiporuk, and S. M. Pulst. 1994. Differential expression and tissue distribution of type I and type II neurofibromins during mouse fetal development. Dev. Biol. 161538-551. [DOI] [PubMed] [Google Scholar]
- 26.Izquierdo, J. M., N. Majos, S. Bonnal, C. Martinez, R. Castelo, R. Guigo, D. Bilbao, and J. Valcarcel. 2005. Regulation of Fas alternative splicing by antagonistic effects of TIA-1 and PTB on exon definition. Mol. Cell 19475-484. [DOI] [PubMed] [Google Scholar]
- 27.Jain, R. G., L. G. Andrews, K. M. McGowan, P. H. Pekala, and J. D. Keene. 1997. Ectopic expression of Hel-N1, an RNA-binding protein, increases glucose transporter (GLUT1) expression in 3T3-L1 adipocytes. Mol. Cell. Biol. 17954-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jensen, K. B., B. K. Dredge, G. Stefani, R. Zhong, R. J. Buckanovich, H. J. Okano, Y. Y. Yang, and R. B. Darnell. 2000. Nova-1 regulates neuron-specific alternative splicing and is essential for neuronal viability. Neuron 25359-371. [DOI] [PubMed] [Google Scholar]
- 29.Keene, J. D. 1999. Why is Hu where? Shuttling of early-response-gene messenger RNA subsets. Proc. Natl. Acad. Sci. USA 965-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klose, A., M. R. Ahmadian, M. Schuelke, K. Scheffzek, S. Hoffmeyer, A. Gewies, F. Schmitz, D. Kaufmann, H. Peters, A. Wittinghofer, and P. Nurnberg. 1998. Selective disactivation of neurofibromin GAP activity in neurofibromatosis type 1. Hum. Mol. Genet. 71261-1268. [DOI] [PubMed] [Google Scholar]
- 31.Kullmann, M., U. Gopfert, B. Siewe, and L. Hengst. 2002. ELAV/Hu proteins inhibit p27 translation via an IRES element in the p27 5′UTR. Genes Dev. 163087-3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Guiner, C., F. Lejeune, D. Galiana, L. Kister, R. Breathnach, J. Stevenin, and F. Del Gatto-Konczak. 2001. TIA-1 and TIAR activate splicing of alternative exons with weak 5′ splice sites followed by a U-rich stretch on their own pre-mRNAs. J. Biol. Chem. 27640638-40646. [DOI] [PubMed] [Google Scholar]
- 33.Li, Q., J. A. Lee, and D. L. Black. 2007. Neuronal regulation of alternative pre-mRNA splicing. Nat. Rev. Neurosci 8819-831. [DOI] [PubMed] [Google Scholar]
- 34.Lisbin, M. J., J. Qiu, and K. White. 2001. The neuron-specific RNA-binding protein ELAV regulates neuroglian alternative splicing in neurons and binds directly to its pre-mRNA. Genes Dev. 152546-2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Markovtsov, V., J. M. Nikolic, J. A. Goldman, C. W. Turck, M. Y. Chou, and D. L. Black. 2000. Cooperative assembly of an hnRNP complex induced by a tissue-specific homolog of polypyrimidine tract binding protein. Mol. Cell. Biol. 207463-7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oberstrass, F. C., S. D. Auweter, M. Erat, Y. Hargous, A. Henning, P. Wenter, L. Reymond, B. Amir-Ahmady, S. Pitsch, D. L. Black, and F. H. Allain. 2005. Structure of PTB bound to RNA: specific binding and implications for splicing regulation. Science 3092054-2057. [DOI] [PubMed] [Google Scholar]
- 37.Okano, H. J., and R. B. Darnell. 1997. A hierarchy of Hu RNA binding proteins in developing and adult neurons. J. Neurosci. 173024-3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pascale, A., P. A. Gusev, M. Amadio, T. Dottorini, S. Govoni, D. L. Alkon, and A. Quattrone. 2004. Increase of the RNA-binding protein HuD and posttranscriptional up-regulation of the GAP-43 gene during spatial memory. Proc. Natl. Acad. Sci. USA 1011217-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Polydorides, A. D., H. J. Okano, Y. Y. Yang, G. Stefani, and R. B. Darnell. 2000. A brain-enriched polypyrimidine tract-binding protein antagonizes the ability of Nova to regulate neuron-specific alternative splicing. Proc. Natl. Acad. Sci. USA 976350-6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quattrone, A., A. Pascale, X. Nogues, W. Zhao, P. Gusev, A. Pacini, and D. L. Alkon. 2001. Posttranscriptional regulation of gene expression in learning by the neuronal ELAV-like mRNA-stabilizing proteins. Proc. Natl. Acad. Sci. USA 9811668-11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shukla, S., F. Del Gatto-Konczak, R. Breathnach, and S. A. Fisher. 2005. Competition of PTB with TIA proteins for binding to a U-rich cis-element determines tissue-specific splicing of the myosin phosphatase targeting subunit 1. RNA 111725-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ule, J., K. B. Jensen, M. Ruggiu, A. Mele, A. Ule, and R. B. Darnell. 2003. CLIP identifies Nova-regulated RNA networks in the brain. Science 3021212-1215. [DOI] [PubMed] [Google Scholar]
- 43.Ule, J., G. Stefani, A. Mele, M. Ruggiu, X. Wang, B. Taneri, T. Gaasterland, B. J. Blencowe, and R. B. Darnell. 2006. An RNA map predicting Nova-dependent splicing regulation. Nature 444580-586. [DOI] [PubMed] [Google Scholar]
- 44.Ule, J., A. Ule, J. Spencer, A. Williams, J. S. Hu, M. Cline, H. Wang, T. Clark, C. Fraser, M. Ruggiu, B. R. Zeeberg, D. Kane, J. N. Weinstein, J. Blume, and R. B. Darnell. 2005. Nova regulates brain-specific splicing to shape the synapse. Nat. Genet. 37844-852. [DOI] [PubMed] [Google Scholar]
- 45.Underwood, J. G., P. L. Boutz, J. D. Dougherty, P. Stoilov, and D. L. Black. 2005. Homologues of the Caenorhabditis elegans Fox-1 protein are neuronal splicing regulators in mammals. Mol. Cell. Biol. 2510005-10016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wakamatsu, Y., and J. A. Weston. 1997. Sequential expression and role of Hu RNA-binding proteins during neurogenesis. Development 1243449-3460. [DOI] [PubMed] [Google Scholar]
- 47.Wu, J. I., R. B. Reed, P. J. Grabowski, and K. Artzt. 2002. Function of quaking in myelination: regulation of alternative splicing. Proc. Natl. Acad. Sci. USA 994233-4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yunoue, S., H. Tokuo, K. Fukunaga, L. Feng, T. Ozawa, T. Nishi, A. Kikuchi, S. Hattori, J. Kuratsu, H. Saya, and N. Araki. 2003. Neurofibromatosis type I tumor suppressor neurofibromin regulates neuronal differentiation via its GTPase-activating protein function toward Ras. J. Biol. Chem. 27826958-26969. [DOI] [PubMed] [Google Scholar]
- 49.Zhang, L., W. Liu, and P. J. Grabowski. 1999. Coordinate repression of a trio of neuron-specific splicing events by the splicing regulator PTB. RNA 5117-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang, W., H. Liu, K. Han, and P. J. Grabowski. 2002. Region-specific alternative splicing in the nervous system: implications for regulation by the RNA-binding protein NAPOR. RNA 8671-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu, H., R. A. Hasman, V. A. Barron, G. Luo, and H. Lou. 2006. A nuclear function of Hu proteins as neuron-specific alternative RNA processing regulators. Mol. Biol. Cell 175105-5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu, H., R. A. Hasman, K. M. Young, N. L. Kedersha, and H. Lou. 2003. U1 snRNP-dependent function of TIAR in the regulation of alternative RNA processing of the human calcitonin/CGRP pre-mRNA. Mol. Cell. Biol. 235959-5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu, H., H. L. Zhou, R. A. Hasman, and H. Lou. 2007. Hu proteins regulate polyadenylation by blocking sites containing U-rich sequences. J. Biol. Chem. 2822203-2210. [DOI] [PubMed] [Google Scholar]
- 54.Zuccato, E., E. Buratti, C. Stuani, F. E. Baralle, and F. Pagani. 2004. An intronic polypyrimidine-rich element downstream of the donor site modulates cystic fibrosis transmembrane conductance regulator exon 9 alternative splicing. J. Biol. Chem. 27916980-16988. [DOI] [PubMed] [Google Scholar]