Abstract
Merlin, the product of the NF2 tumor suppressor gene, is closely related to the ERM (ezrin, radixin, moesin) proteins, which provide anchorage between membrane proteins and the underlying cortical cytoskeleton; all four proteins are members of the band 4.1 superfamily. Despite their similarity, the subcellular distributions and functional properties of merlin and the ERM proteins are largely distinct. Upon cell-cell contact merlin prevents internalization of and signaling from the epidermal growth factor receptor (EGFR) by sequestering it into an insoluble membrane compartment. Here we show that the extreme amino (N) terminus directs merlin biochemically to an insoluble membrane compartment and physically to the cortical actin network, with a marked concentration along cell-cell boundaries. This insoluble-membrane distribution is required for the growth-suppressing function of merlin and for the functional association of merlin with EGFR and other membrane receptors. Our data support a model whereby locally activated merlin sequesters membrane receptors such as EGFR at the cortical network, contributing to the long-held observation that the cortical actin cytoskeleton can control the lateral mobility of and signaling from certain membrane receptors.
With the identification of the neurofibromatosis type 2 (NF2) tumor suppressor gene in 1993, it was immediately appreciated that the NF2-encoded protein, merlin, was a member of the band 4.1 superfamily of membrane-associated proteins and thus a novel type of tumor suppressor (40, 50). Although studies of NF2 in humans and mice suggest a broad role for NF2 loss in tumorigenesis and in tumor progression (9, 12, 19, 31, 32), the mechanism whereby merlin controls cell proliferation has only recently begun to be elucidated (reviewed in reference 33).
Merlin is closely related to the ERM (ezrin, radixin, moesin) proteins, which are thought to organize specific membrane domains by providing regulated anchorage between the membrane and underlying cortical actin cytoskeleton (reviewed in references 4 and 33). All four proteins contain an amino (N)-terminal FERM (four-point one, ezrin, radixin, moesin) domain, which mediates membrane association, are regulated by conformation-dependent changes in localization, and share common interacting partners. Nevertheless, the ERM proteins cannot functionally compensate for merlin, which is required for embryonic development in mice, flies, and worms (10, 31; J. Gervais, J. Satterlee, and A. I. McClatchey, unpublished data); similarly, in contrast to merlin, the ERM proteins are not known to function as tumor suppressors and loss of ERM function in mammals, flies, and worms is not associated with altered proliferation. While the subcellular distributions of merlin and the ERM proteins can overlap, they are often distinct. For example, in cultured cells, merlin and the ERM proteins are often concentrated in dynamic membrane structures, such as lamellipodia, filopodia, and sites of cell-cell adhesion (8, 14, 17, 25, 43). However, in highly polarized cells the localization of merlin and the ERM proteins is more discrete. In Drosophila melanogaster epithelial tissues, the single ERM orthologue moesin exhibits a uniform, apical and apicojunctional distribution while merlin exhibits a more punctate localization to the apical junction region and adjacent cytoplasm (30). In Caenorhabditis elegans ERM-1 distributes uniformly along the apical surfaces of non-cuticle-expressing tubular organ epithelia while NFM-1 (the product of the worm NF2 orthologue) is enriched along the basolateral surfaces (extending to the apical junction) of tubular epithelia (13). Furthermore, unlike ezrin, which is largely detergent soluble, a large fraction of merlin is insoluble (7, 48). Together these observations suggest that the distinct activities of merlin and the ERM proteins may be in part due to their partitioning within the cell.
The phenotypic consequences of loss of merlin or ERM function are consistent with functional partitioning of their activities. Thus, in mammalian, Drosophila, and C. elegans epithelial tissues ERM function is required for establishing or maintaining the integrity of the apical epithelial surface (2, 6, 13, 21, 42, 47). In contrast, accumulating evidence suggests that merlin mediates contact-dependent inhibition of proliferation (7, 18, 25, 35, 45), and we have found that merlin localizes to and stabilizes adherens junctions between cells (25). Thus, the ERM proteins appear to be dedicated to providing apical membrane-cytoskeleton linkage while merlin may perform a similar role in stabilizing the apical junction region.
Recent studies suggest that merlin and the related tumor suppressor, expanded, can control the surface abundance of certain membrane receptors including the epidermal growth factor receptor (EGFR) in Drosophila tissues (29). Similarly, we have found that, in contacting mammalian cells, merlin physically associates with the EGFR via the tandem PDZ domain-containing adaptor NHE-RF1 (Na+ hydrogen exchanger regulatory factor 1) and sequesters the EGFR into an insoluble membrane compartment from which it can neither internalize nor signal (7). Although these two studies seem to reach differing conclusions as to how merlin regulates membrane receptor surface availability—in one case stabilizing surface levels and the other promoting turnover—the primary function of merlin in both cases could be to retain receptors in a certain membrane compartment. To better delineate the molecular mechanism whereby merlin controls the surface availability of certain membrane receptors, we sought to understand how the membrane distribution of merlin itself is controlled. We have found that the N-terminal 17 amino acids of merlin, which form an extension not found in the ERM proteins, confer the distinct insoluble-membrane distribution of merlin relative to the ERM proteins. We also found that the ability of merlin to inhibit cell proliferation and to negatively regulate EGFR internalization and signaling depends on its insoluble-membrane distribution and its ability to stably decorate the cortical actin network. We propose a model wherein local activation of merlin from the cortical actin cytoskeleton controls the lateral mobility of and signaling from certain surface receptors (23, 24).
MATERIALS AND METHODS
Cell culture.
Mouse Nf2−/− immortalized fibroblasts and epithelial Nf2−/− liver-derived cells (LDCs) were generated as previously described (7, 45). Cell confluence is defined in reference 7. For jasplakinolide (Molecular Probes; J-7473) treatment, confluent LDCs were treated at 2 μM for 1 h at 37°C prior to fixation. Primary Schwann cells were isolated from embryonic day 13.5 Nf2lox/lox embryos, cultured as described in reference 38, infected with an adenovirus expressing the Cre recombinase (Ad-Cre), and reinfected with Ad-Nf2wt or Ad-Nf218-595.
Plasmids.
Generation of pCDNA3 (Invitrogen), encoding wild-type and L64P-containing mouse Nf2 cDNAs, was previously described (25, 45). Ezrin cDNA in pCDNA3.1 was a kind gift from Reuben Shaw. Both Nf218-595 and EzNf21-18 were created via PCR amplification of the mouse Nf2 and Ezrin coding regions and then cloned into pCDNA3 and the pBMN-GFP retroviral vector (Orbigen), respectively. For EzNf21-18, Nf2 residues 1 to 18 were appended to the N terminus of full-length ezrin in place of the N-terminal methionine. Nf215-17A, Nf2S7-13A, and Nf2S7-13D were generated via site-directed mutagenesis of a mouse Nf2wt pCDNA3 construct, according to the manufacturer's instructions (Stratagene). Finally, Nf2wt, Nf218-595, Nf2L64P, Nf215-17A, Nf2S7-13A, Nf2S7-13D, and Ezrin were subcloned into pBMN. Retroviral expression of pBMN-Nf2 and -Ezrin constructs was performed essentially according to the manufacturer's instructions (Orbigen). Nf2wt and Nf218-595 were also cloned into the adenoviral vector pAdEasy-1 (MP Biomedicals), and adenoviral infection with Ad-Nf2wt and -Nf218-595 was performed as described in reference 25.
Subcellular fractionation.
Subcellular fractionation was performed as previously described (25) with the final insoluble pellet boiled in modified radioimmunoprecipitation assay buffer containing 0.5% sodium dodecyl sulfate.
Antibodies.
Primary antibodies were from Abcam (NHE-RF1, ab3452), Babco (ezrin, MMS-143R; 1:500), Becton Dickinson (5-bromo-2-deoxyuridine [BrdU], 347580), Biosource International (Src-pY418, 44-660; 1:1,000), Calbiochem (cyclin D1, CC12; 1:500), Cell Signaling (EGFR-pY845, 2231; EGFR-pY992, 2235; EGFR-pY1068, 2234; Raf-pS259, 9421; STAT3-pY705, 9138; all at 1:1,000), NeoMarkers (ezrin, Ab-1; EGFR, Ab-17), Santa Cruz (NF2, sc-331 and sc-332; 1:20,000; anti-EGFR, sc-03; 1:1,000), Sigma (actin, A-4700; 1:5,000), Transduction Labs (p120ctn-pY228, 612537; 1:2,000; caveolin-pY14, 611338; 1:1,000; E-cadherin, 610182; 1:1,000; paxillin, P13520). Horseradish peroxidase-conjugated secondary anti-mouse and anti-rabbit antibodies were from Amersham. All dilutions are for immunoblotting.
Indirect immunofluorescence and confocal imaging.
Coverslips containing confluent LDCs were prepared in three ways. For fix 1, cells were fixed for 10 min in 3.7% formaldehyde in cytoskeletal buffer [CB; 10 mM 2-(N-morpholino)ethanesulfonic acid sodium salt (MES), pH 6.1, 138 mM KCl, 3 mM MgCl2, and 2 mM EGTA] and subsequently permeabilized for 5 min in 0.5% Triton X-100 in phosphate-buffered saline (PBS). For fix 2, cells were simultaneously fixed and permeabilized in 1% formaldehyde and 2% Triton X-100 in CB for 15 min. For fix 3, cells were washed in 1% Triton X-100 lysis buffer for 30 min on ice, washed once in PBS, and then fixed for 10 min in 3.7% formaldehyde in CB. All coverslips were processed by fix 1, unless noted otherwise. Coverslips were blocked in 10% goat serum in PBS for 30 min and incubated overnight at 4°C with anti-NF2 (sc-332; 1:1,000), -ezrin (Ab-1; 1:200), -NHE-RF1 (ab3452l; 1:100), or -paxillin (P13520; 1:100) antibodies in 10% goat serum in PBS. Coverslips were rinsed five times in PBS, incubated for 1 h at room temperature in fluorescein isothiocyanate- or Cy3-conjugated secondary antibodies (Jackson Immunoresearch Laboratories), and mounted (Vectashield; Vector). Nuclei were stained with DAPI (4′,6′-diamidino-2-phenylindole dihydrochloride) or TOTO-3 iodide (Molecular Probes). Two-dimensional images were acquired using a Zeiss Plan Fluor 63×, 1.4-numerical-aperture (NA) oil objective lens on a Zeiss Axioplan microscope with IP Lab software and a Sony charge-coupled device camera. Z sections were acquired using a Zeiss Plan Fluor 40×, 1.3-numerical-aperture oil objective lens on a Zeiss LSM 510 microscope. Final images were prepared using Adobe Photoshop 7.0. For cell surface concanavalin A binding, fixed confluent LDCs were labeled with 50 μg/ml of concanavalin A prior to permeabilization (Molecular Probes; C11253) for 30 min at room temperature.
BrdU incorporation and Tr-EGF internalization.
For BrdU experiments, coverslips containing confluent LDCs were labeled with 20 μM BrdU for 12 h and DNA was denatured and prepared for immunofluorescence. To monitor Texas Red-labeled epidermal growth factor (Tr-EGF) internalization, coverslips containing confluent LDCs were serum starved in 1% bovine serum albumin in Dulbecco's modified Eagle medium for 4 h, incubated for 1 h at 37°C with 2 μg/ml Tr-EGF (Molecular Probes; E3480), and prepared for immunofluorescence.
IP.
Immunoprecipitation (IP) of late confluent LDCs was performed as described in reference 7. To facilitate the coimmunoprecipitation of insoluble membrane proteins, we included n-octyl-β-d-glucopyranoside (Calbiochem) in the extraction buffer to further extract insoluble-membrane domains. Total membrane extracts and total cell lysates were isolated via lysis of total membrane pellets and total cell pellets, respectively, in Triton X-100 lysis buffer containing 60 mM n-octyl-β-d-glucopyranoside. The following amounts of antibody were used for each IP: 3 μg anti-NHE-RF1 (ab3452), 2 μg anti-E-cadherin (610182), 6 μg anti-EGFR (Ab-17), and 5 μl antiezrin (ascites MMS-143R).
Cell surface biotinylation.
Late confluent LDCs were cooled at 4°C for 15 min and then incubated with 0.5 mg/ml of biotin {EZ-Link sulfo-NHS-LC-biotin [sulfosuccinimidyl-6-(biotinamido)hexanoate]; Pierce} in PBS for 1 h at 4°C. After LDCs were washed in PBS, the remaining biotin was quenched in 50 mM NH4Cl, 1 mM MgCl2, and 0.1 mM CaCl2 buffer in PBS for 10 min, washed with PBS, and either harvested immediately (time zero) or shifted to 37°C in full serum for 30 min and harvested on ice. Biotin pull-downs were performed with total membrane extracts by first preclearing extracts in 20 μl of protein G beads and then using 25 μl immobilized neutravidin biotin binding protein beads (Pierce).
RESULTS
The extreme N terminus of merlin is required for correct membrane distribution.
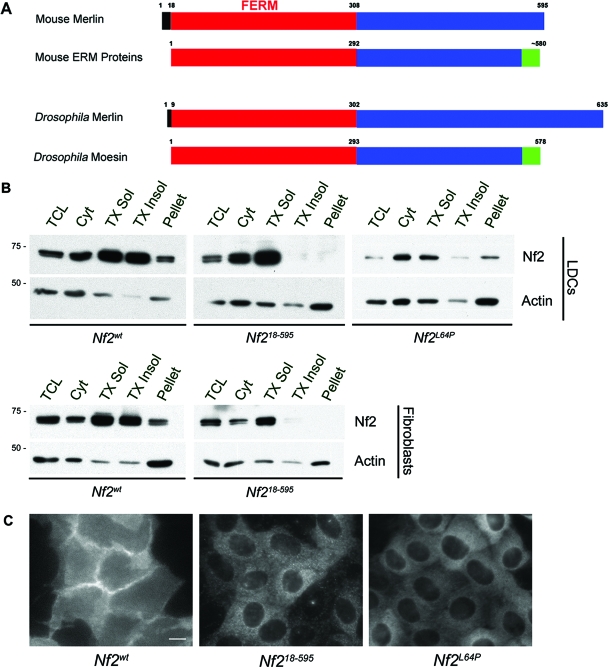
The FERM domain is known to mediate association with membrane proteins. Despite strong conservation across their N-terminal FERM domains merlin and the ERM proteins exhibit distinct localizations and solubilities, suggesting that other features within the protein could confer discrete membrane distribution. Closer inspection of the sequences of merlin and the ERM proteins revealed an N-terminal extension that is present, with various sizes, in the proteins encoded by all known NF2 orthologues but none of the ERM orthologues (Fig. 1A; see Fig. S1A in the supplemental material). Moreover, we noted that this segment shared several features with the N terminus of Src, which is known to mediate association with the lipid bilayer (see below). To ascertain whether these residues (amino acids 1 to 17 in mouse Nf2), which are not part of the FERM domain, are important for merlin localization, we created a version of merlin lacking these residues, Nf218-595, and examined its localization in confluent mouse Nf2−/− epithelial LDCs (see Materials and Methods). Biochemical fractionation revealed that substantial pools of merlin (Nf2wt) are present in both the soluble and insoluble membrane compartments (Fig. 1B). In contrast, although well represented in the soluble membrane, Nf218-595 is conspicuously absent from the Triton X-100-insoluble membrane and remaining insoluble pellet (Fig. 1B). The levels of Nf2L64P, a version of merlin carrying a patient-derived missense mutation that disrupts the architecture of the FERM domain, are diminished in both the soluble- and insoluble-membrane compartments, and the protein is largely cytosolic (Fig. 1B; see below) (7, 25). Similar distributions were seen in Nf2−/− immortalized fibroblasts (see Materials and Methods) into which Nf2wt, Nf218-595, and Nf2L64P were reintroduced (Fig. 1B; not shown). It is unlikely that the behavior of Nf218-595 is due to an improperly folded FERM domain, as structural analysis reveals that residues 1 to 19 of merlin form a disordered N-terminal extension (20, 46).
FIG. 1.
The extreme N-terminal residues of merlin confer insoluble-membrane localization. (A) Merlin orthologues (shown are mouse and Drosophila orthologues) contain an N-terminal sequence (black) that precedes the FERM domain (red) and is not present in the ERM proteins. The Drosophila N-terminal extension is smaller than that in mammals but contains both potential phosphorylation sites and charged residues (see Fig. 6A; see also Fig. S1A in the supplemental material). The ERM proteins contain a carboxy (C)-terminal F-actin binding domain (green). (B) Subcellular fractionation of confluent Nf2−/− mouse LDCs and Nf2−/− mouse immortalized fibroblasts expressing the indicated versions of Nf2 revealed that little or no Nf218-595 is present in the Triton X-100-insoluble fractions while Nf2L64P is enriched in the cytosol. TCL, total cell lysate; Cyt, cytosol; TX Sol, Triton X-100 soluble; TX Insol, Triton X-100 insoluble; pellet, remaining insoluble pellet. Samples were probed with an antiactin antibody to control for loading. (C) Immunostaining of confluent Nf2−/− LDCs infected with pBMN-Nf2wt, -Nf218-595, or -Nf2L64P revealed that, in contrast to Nf2wt, both Nf218-595 and Nf2L64P fail to localize to cell-cell boundaries. Bar = 10 μm; n ≥ 3.
As in other cell types, merlin exhibits a diffuse localization with marked concentration along cell-cell boundaries in LDCs (Fig. 1C). Notably, both the diffusely distributed and cell-junctional Nf2wt are completely resistant to detergent extraction or simultaneous fixation/extraction (see Fig. 4A, E, and H and Materials and Methods). In contrast, Nf218-595 exhibits a diffuse distribution across the cell but fails to concentrate at cell-cell boundaries in LDCs (Fig. 1C; see below). This pattern of localization is similar to that of Nf2L64P (Fig. 1C). However, while Nf218-595 is partially resistant to simultaneous fixation/extraction, Nf2L64P is completely removed by these conditions (see Fig. 4D; not shown); this is consistent with the biochemical analysis described above and suggests that Nf218-595 can localize to the membrane but remains soluble while Nf2L64P is largely cytosolic. Thus, amino acids 1 to 17 are not required for localization of merlin to the membrane per se, but they are required for the retention of merlin in a Triton X-100-resistant (insoluble) membrane compartment and for consequent junctional enrichment. In fact, junctional enrichment of merlin is dependent upon an intact FERM domain, as neither Nf2L64P nor a fusion protein consisting of Nf2 residues 1 to 17 appended to the N terminus of green fluorescent protein is enriched at cell-cell boundaries (Fig. 1C; not shown). It is formally possible that disruption of the FERM domain, as in Nf2L64P, impairs the ability of residues 1 to 17 to confer proper membrane localization to merlin.
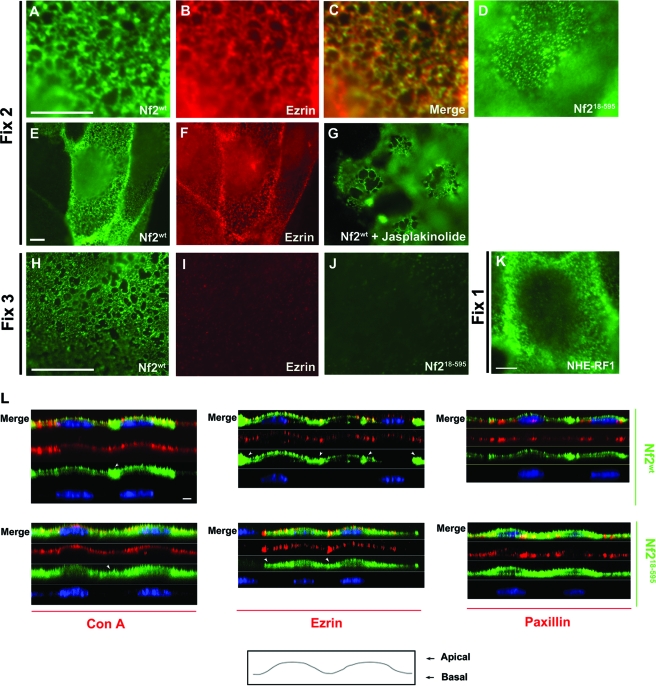
FIG. 4.
The N terminus directs merlin to an insoluble apical network. Simultaneous fixation and permeabilization (fix 2) revealed that Nf2wt (A) and ezrin (B) decorate the same cortical network in LDCs. Merge of the images is shown in panel C; lower magnification of images in panels A and B are represented in panels E and F, respectively. (D) Nf218-595 exhibits a fragmented localization to this network (see also Fig. S2D in the supplemental material). (E) The network localization of ezrin is not altered in the presence or absence of Nf2wt (E and F). (G) LDCs expressing Nf2wt were treated with jasplakinolide (2 μM, 1 h, 37°C). Jasplakinolide treatment yielded markedly enlarged compartments within the Nf2wt-decorated cortical actin network. Upon detergent extraction prior to fixation (fix 3), Nf2wt (H), but not ezrin (I) or Nf218-595 (J), retained network staining. (K) Immunofluorescence localization of NHE-RF1 in LDCs fixed and then permeabilized (fix 1) revealed that NHE-RF1 localizes to a similar cortical network. (L) Confocal imaging of confluent LDCs expressing various versions of Nf2 (fix 1) revealed that Nf2wt colocalizes with apically distributed concanavalin A (ConA; a lectin that binds polysaccharides on the cell surface) and ezrin (an established apical marker); Nf2wt is also enriched at cell-cell boundaries (arrowheads). However, careful examination revealed that Nf218-595 is not retained at the apical compartment and instead extends basally; Nf218-595 also fails to be enriched at cell-cell junctions (arrowheads). Little overlap is seen between apically distributed Nf2wt and the basal marker, paxillin; in contrast, Nf218-595 does overlap with paxillin. Nuclei in panel L are stained with TOTO 3 (blue). Bars = 5 μm. n ≥ 3.
Correct membrane distribution of merlin is required for contact-dependent inhibition of proliferation.
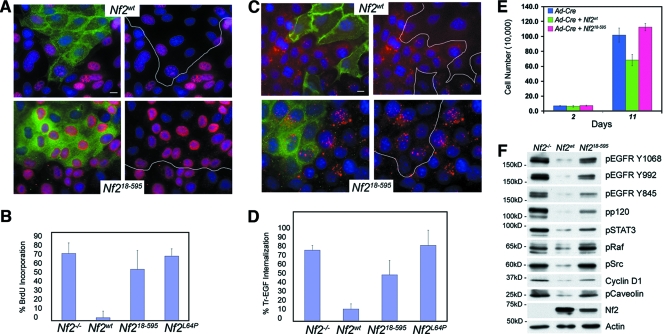
Increasing evidence indicates that merlin confers growth suppression in a contact-dependent manner (7, 18, 25, 35, 45). To determine whether localization of merlin to the insoluble membrane is necessary for contact-dependent inhibition of proliferation, we examined the ability of Nf218-595 to restore contact-dependent inhibition of proliferation to Nf2−/− LDCs. In contrast to Nf2wt, which almost completely prevented BrdU incorporation in confluent LDCs, Nf218-595, like Nf2L64P, had little to no effect on BrdU incorporation in contacting Nf2−/− LDCs or immortalized fibroblasts (Fig. 2A and B; not shown). Importantly, reintroduction of Nf2wt, but not Nf218-595 also restored contact-dependent inhibition of proliferation to primary Nf2−/− Schwann cells, the key cell type affected in human NF2 patients (Fig. 2E). Thus, localization to the insoluble-membrane compartment is necessary for merlin-mediated contact-dependent inhibition of proliferation in both epithelial and mesenchymal cells.
FIG. 2.
Nf218-595 cannot restore contact-dependent inhibition of proliferation or prevent EGFR internalization in Nf2−/− cells. (A) Confluent mosaic populations of Nf2-expressing and Nf2-deficient LDCs were scored for BrdU incorporation (20 μM for 12 h) as a measure of contact-dependent inhibition of proliferation (Nf2, green; BrdU, red; DAPI, blue). White lines demarcate the interface between Nf2-expressing and Nf2-deficient cells. Bars = 10 μm. (B) Quantitation reveals that both Nf2L64P and Nf218-595 are defective in restoring contact-dependent inhibition of proliferation. Approximately 300 cells were scored in each of five experiments. (C) Confluent mosaic populations of LDCs were serum starved and treated with Tr-EGF (2 μg/ml, 1 h, 37°C), and ligand internalization was monitored as a measure of EGFR internalization (Nf2, green; Tr-EGF, red; DAPI, blue). (D) Quantitation reveals that, in contrast to Nf2wt, neither Nf2L64P nor Nf218-595 prevented EGFR internalization in confluent cells. Approximately 300 cells were scored in each of five experiments. Data represent means ± standard deviations. (E) In contrast to Nf2wt, Nf218-595 failed to restore contact-dependent inhibition of proliferation to primary Nf2−/− Schwann cells. (F) Persistent activation of EGFR and its downstream effectors is apparent in the membranes of LDCs expressing Ad-Nf218-595 but not Ad-Nf2wt. Phosphorylated proteins are indicated by the letter “p” preceding the name. Membrane pellets including nuclei were extracted in 0.5% sodium dodecyl sulfate in radioimmunoprecipitation assay buffer.
Merlin associates with and downregulates EGFR from an insoluble-membrane compartment.
Our recent studies suggest that, upon cell contact, merlin retains EGFR in an insoluble-membrane compartment from which it can neither signal nor internalize (7). This predicts that merlin itself must reside in the insoluble membrane in order to affect EGFR membrane distribution and signaling; Nf218-595 provides a valuable tool with which to test this hypothesis directly. Therefore, we asked whether Nf218-595, like Nf2wt, can prevent EGFR internalization. As we had previously observed, Nf2wt expression prevented Tr-EGF internalization in confluent LDCs; however, Nf218-595, like Nf2L64P, had little effect on Tr-EGF internalization (Fig. 2C and D) (7). As shown below, Nf2wt, but not Nf218-595, downregulates the steady-state levels of active EGFR and its key downstream effectors, including cyclin D1, a known target of merlin (Fig. 2F) (51).
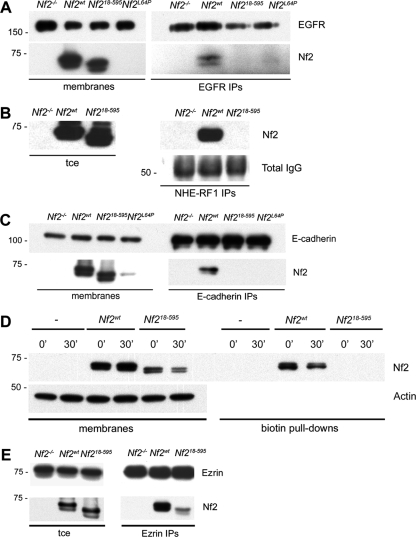
Importantly, Nf218-595 also failed to associate with EGFR or with NHE-RF1, which mediates the association between merlin and EGFR by interacting directly with both (Fig. 3A and B) (7, 26, 37). The residues required for ERM-NHE-RF1 interaction have been mapped to the surface of the F3 lobe of the FERM domain (11, 49), indicating that residues 1 to 17 of merlin do not interact directly with NHE-RF1 but instead likely direct merlin to a membrane compartment where it can associate with NHE-RF1 and consequently with EGFR. We found that Nf218-595 also does not associate with E-cadherin, suggesting that the association between merlin and E-cadherin, which may precede the association of merlin with NHE-RF1-EGFR, normally occurs in the insoluble-membrane compartment (Fig. 3C). In fact, in contrast to Nf2wt, Nf218-595 failed to associate with any biotinylated surface proteins in confluent LDCs (Fig. 3D), suggesting that residues 1 to 17 are required for the association of merlin with any surface receptors. Notably, Nf218-595 does retain the ability to associate with ezrin, suggesting that Nf2-ezrin heterodimerization is not limited to the insoluble-membrane compartment and can occur in the absence of association with surface receptors (Fig. 3E). Regulated phosphorylation of serine 518 (S518) has been shown to control the localization and activity of merlin (22, 44, 45). However, we found that both hyper- and hypophosphorylated versions of merlin are associated with EGFR and with biotinylated surface proteins, suggesting that binary models of phosphorylation- or dephosphorylation-induced translocation to the membrane are oversimplified for merlin. Indeed, unlike Nf2L64P, Nf218-595 can be phosphorylated at residue S518 (hyperphosphorylated), indicating that neither localization to the insoluble membrane nor association with surface receptors is required for S518 phosphorylation. Together, these data are consistent with a model wherein recruitment of merlin to an insoluble surface membrane compartment via residues 1 to 17 facilitates interaction with NHE-RF1-EGFR, which, in turn, prevents the internalization of and signaling from EGFR.
FIG. 3.
The N terminus of merlin is essential for merlin interaction with membrane-associated proteins. (A) IP of EGFR from total membrane extracts revealed that Nf2wt, but not Nf218-595 or Nf2L64P, associates with the EGFR. Both Nf2wt and Nf218-595 are present in the total membrane extracts, but Nf2L64P, which is largely cytosolic, is not. (B) IP of NHE-RF1 from total cell extracts (tce) revealed that Nf2wt, but not Nf218-595, associates with NHE-RF1. Total immunoglobulin G (IgG), representing the NHE-RF1 IPs, is shown as NHE-RF1 runs at the same 50-kDa molecular mass as the IgG heavy chain. (C) IP of E-cadherin from total membrane extracts revealed that Nf2wt, but not Nf218-595 or Nf2L64P, associates with E-cadherin. (D) Surface proteins in confluent Nf2−/− LDCs or in LDCs expressing pBMN-Nf2wt or -Nf218-595 were labeled with biotin and collected at 0 or 30 min after release to 37°C. Biotin pull-downs from total membrane extracts revealed that, in contrast to Nf2wt, Nf218-595 fails to associate with any surface proteins at either time point. (E) IP of ezrin from total cell extracts revealed that Nf218-595 retains the ability to interact with ezrin. n ≥ 3.
Merlin localizes to the apical cortical actin network.
The studies described above indicate that localization to an insoluble-membrane compartment is critical for the growth-inhibiting activity of merlin. Insolubility within the membrane compartment can reflect residence in so-called lipid raft domains and/or association with the actin cytoskeleton (reviewed in reference 27). Indeed, merlin has been reported to localize to the cortical actin cytoskeleton, to detergent-resistant membranes, and to intracellular vesicles (17, 25, 28, 30, 41, 48). Merlin concentrates in cortical actin-rich structures such as cell-cell boundaries, filopodia, and membrane ruffles. In epithelial LDCs, Nf2wt exhibits both a concentrated localization along cell-cell boundaries and a diffuse detergent-resistant localization throughout the cell. Examination of the distribution of Nf2wt at higher resolution revealed that this diffuse appearance actually reflects localization to the cortical cytoskeletal “network” (membrane cytoskeleton) that band 4.1 and the ERM proteins anchor to the plasma membrane (Fig. 4A and E; see Fig. S2C in the supplemental material) (6, 16, 34). Consistent with this interpretation, the network localization of merlin does not coincide with markers of the endoplasmic reticulum, Golgi apparatus, intermediate filaments, or microtubules but instead substantially coincides with the cortical network decorated by green fluorescent protein-actin (see Fig. S3 in the supplemental material). Indeed, the integrity of the merlin-decorated cortical network is markedly altered by the actin-disrupting drug jasplakinolide (Fig. 4G). Endogenous ezrin decorates the same network although, in contrast to Nf2wt, ezrin is removed under more-stringent detergent conditions (Fig. 4B, C, F, and I). Merlin localizes to a similar network in fibroblasts, epithelial keratinocytes, and Madin-Darby canine kidney (MDCK) cells (see Fig. S2A and B in the supplemental material; not shown). As shown in Fig. 4K, NHE-RF1 localizes to a similar network although, like ezrin, its localization is detergent sensitive, necessitating the use of milder fixation conditions.
Confocal microscopy reveals the apical position of this network where Nf2wt colocalizes with both ezrin and concanavalin A (Fig. 4L). Notably, Nf2wt is markedly more concentrated in the apical junction region than ezrin in these cells (Fig. 4E, F, and L and 5B). The localization of ezrin to this network is not dependent on endogenous merlin or displaced by exogenous merlin, indicating that merlin is not required for network formation or integrity and that merlin and ezrin do not compete for network binding (Fig. 4E and F). Importantly, this network is distinct from the established localization of ezrin to apical microvilli as LDCs (and fibroblasts and keratinocytes) have few apical microvilli (not shown).
FIG. 5.
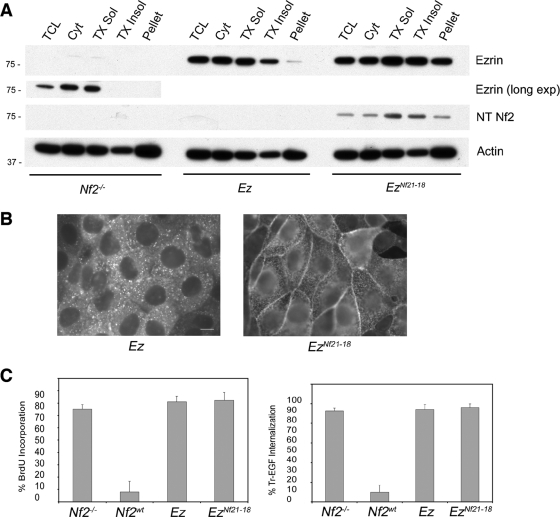
Residues 1 to 18 confer insoluble-membrane localization to ezrin. (A) Subcellular fractionation of immortalized Nf2−/− fibroblasts with and without exogenous ezrin (Ez) or EzNf21-18 revealed that neither endogenous (long exposure) nor exogenous ezrin is enriched in the insoluble fractions; however, EzNf21-18 is specifically enriched in the insoluble fractions. The same extracts were probed with an anti-Nf2 antibody (sc-331) that recognizes residues 1 to 18 of merlin (NT Nf2); only the EzNf21-18 protein is detected. TCL, total cell lysate; Cyt, cytosol, TX Sol, Triton X-100 soluble; TX Insol, Triton X-100 insoluble; pellet, remaining insoluble pellet. Samples were probed with an antiactin antibody to control for loading. (B) Immunostaining of confluent Nf2−/− LDCs expressing pBMN-Ez or EzNf21-18 revealed that EzNf21-18 is now enriched along cell-cell boundaries. Bar = 10 μm. (C) EzNf21-18 cannot restore contact-dependent inhibition of proliferation, as measured with BrdU (20 μM for 12 h), or prevent EGF internalization (Tr-EGF; 2 μg/ml, 1 h, 37°C) in Nf2−/− cells. Approximately 300 cells were scored in each experiment. Data represent means ± standard deviations. n ≥ 3.
Importantly, we found that Nf218-595 exhibits a more punctate localization to the cortical network (Fig. 4D; see Fig. S2D in the supplemental material). Furthermore, the network localization of Nf218-595, like that of ezrin but unlike that of Nf2wt, is removed by more-stringent detergent conditions (Fig. 4J). Interestingly, confocal microscopy reveals that Nf218-595 also fails to be apically restricted and, instead, extends basally without enrichment at cell-cell junctions, consistent with its inability to associate with surface receptors (Fig. 4L). Taken together, these results suggest that stable localization to the apical and apicojunctional cortical cytoskeleton is mediated by the N terminus of merlin and is important for the functional activity of merlin.
The N-terminal residues of merlin are sufficient to confer insoluble membrane localization but not tumor suppressor function to ezrin.
Given that removal of the N-terminal residues of merlin alters its membrane distribution and solubility, we asked whether those residues constitute a membrane localization determinant that is sufficient to alter the localization of ezrin. Subcellular fractionation reveals that both endogenous ezrin and exogenous ezrin are primarily soluble (Fig. 5A). However, expression of a version of ezrin onto which the N-terminal residues of merlin have been N-terminally appended, EzNf21-18, revealed that a substantial pool of this fusion protein is now present in the insoluble-membrane fractions and enriched at cell-cell boundaries (Fig. 5A and B), mirroring the distribution of Nf2wt (Fig. 1B and C). Importantly, EzNf21-18 was not able to restore contact-dependent inhibition of proliferation in Nf2−/− LDCs and immortalized Nf2−/− fibroblasts or prevent Tr-EGF internalization in Nf2−/− LDCs (Fig. 5C; not shown), suggesting that the growth-suppressing activity of merlin is mediated by additional features that are not conserved in the ERM proteins.
Membrane targeting via the N-terminal residues of merlin.
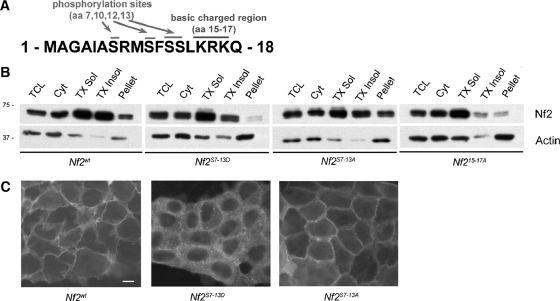
The N-terminal residues of merlin could mediate association with proteins that reside in the insoluble membrane or confer direct association with the insoluble membrane. Close inspection reveals a cluster of positively charged residues and several serines within this sequence (Fig. 6A; see Fig. S1A in the supplemental material); both features are conserved among merlin orthologues. Notably, both features are also present in the N-terminal residues of Src that are known to contribute to its membrane association (see Fig. S1B in the supplemental material). Indeed, electrostatic interaction between N-terminal positively charged amino acids and negatively charged membrane phospholipids has been shown to mediate the association of several proteins with the membrane including Src and the MARCKS (myristoylated alanine-rich C kinase substrate) protein (reviewed in reference 39). Reasoning that these residues could similarly be important for localization of merlin to the insoluble membrane, we generated a version of merlin in which these residues have been changed to neutral alanines (Nf215-17A) and compared its biochemical distribution to that of Nf2wt in LDCs. We found that Nf215-17A, like Nf218-595, was markedly underrepresented in the Triton X-100-insoluble fractions (Fig. 6B), suggesting that electrostatic interactions may play a role in targeting merlin to the membrane.
FIG. 6.
Membrane targeting via the N-terminal residues of merlin. (A) Schematic of residues 1 to 18 of merlin depicting putative sites of phosphorylation and basic charged residues. (B) Subcellular fractionation of confluent Nf2−/− LDCs expressing pBMN-Nf2wt, -Nf2S7-13D, -Nf2S7-13A, or -Nf215-17A revealed that both Nf2S7-13D and Nf215-17A are underrepresented in the insoluble fractions. TCL, total cell lysate; Cyt, cytosol; TX Sol, Triton X-100 soluble; TX Insol, Triton X-100 insoluble; pellet, remaining insoluble pellet. Immunoblotting with an antiactin antibody served as a loading control. (C) Immunostaining of confluent Nf2−/− LDCs expressing pBMN-Nf2wt, -Nf2S7-13D, or -Nf2S7-13A revealed that Nf2S7-13D failed to concentrate at cell-cell boundaries. Bar = 10 μm. n ≥ 3.
Membrane association of Src is disrupted by phosphorylation of this N-terminal membrane localization determinant (reviewed in reference 39). Indeed, mass spectrometry suggests that all of the four serines (S7, S10, S12, and S13) present in the first 17 residues of merlin can be phosphorylated (not shown). Therefore, we generated a version of merlin containing phosphomimetic aspartic acid replacements of all four serines (Nf2S7-13D). Immunofluorescence localization and subcellular fractionation of Nf2S7-13D-expressing LDCs revealed that Nf2S7-13D, like Nf218-595, exhibited decreased membrane staining and representation in the Triton X-100-insoluble pool (Fig. 6B and C). In contrast, a version of merlin containing nonphosphorylatable residues (Nf2S7-13A) exhibited a localization and profile of detergent solubility that are similar to those of Nf2wt (Fig. 6B and C). Thus, phosphorylation of this 17-residue membrane localization determinant may regulate the targeting of merlin to the insoluble-membrane compartment under certain conditions.
DISCUSSION
Recent evidence from Drosophila and mammalian cells supports a role for merlin in controlling the surface abundance of certain membrane receptors including EGFR (7, 29). However, the molecular basis of this activity is not yet clear. Our previous studies indicate that, upon cell contact, merlin associates with EGFR via NHE-RF1 and retains EGFR in an insoluble-membrane compartment. Here we report that the extreme N-terminal amino acids of merlin, which form an unstructured extension that is not part of the FERM domain, specifically direct merlin to the Triton X-100-insoluble membrane, providing a valuable tool with which to explore the molecular basis of how merlin controls EGFR from this compartment. Indeed, we found that a version of merlin lacking these residues (Nf218-595) fails to mediate contact-dependent inhibition of proliferation or to inhibit EGFR internalization and signaling. Importantly, the N-terminal residues of merlin conferred insoluble-membrane localization and junctional enrichment to ezrin, but EzNf21-18 could not functionally substitute for merlin, suggesting that differences in membrane distribution alone appear not to account for the functional distinctions between merlin and the ERM proteins.
Several models could explain how the N-terminal 17 residues of merlin confer localization to cell-cell boundaries and stable association with the cortical actin network. First, these residues could mediate association with certain adhesion receptors/complexes, which, in turn, stabilizes cortical cytoskeleton association. Alternatively, the requirement of the N-terminal residues for stable association with the cortical cytoskeleton could be a prerequisite for association with junctional components given the continuum between the apical and junctional cortical cytoskeletons. Notably, the apical terminal web of intestinal enterocytes is a specialized cortical cytoskeleton that is firmly anchored to apical junctions; ezrin is required for proper maintenance of this structure (42). Importantly, endogenous ezrin and NHE-RF1 also decorate the cortical actin cytoskeleton but in a detergent-sensitive manner, suggesting that association with the cortical actin cytoskeleton per se does not render merlin insoluble. Finally, direct association between residues 1 to 17 and the lipid bilayer could direct merlin to the insoluble apical and junctional membrane compartment, where it stably associates with the cortical cytoskeleton and with surface receptors. In fact, this stretch of amino acids bears striking similarity to the N terminus of Src, which is known to contribute to its direct anchorage in the insoluble membrane (reviewed in reference 39) (see Fig. S1B in the supplemental material). We found that, as for Src, several charged residues within this segment are important for merlin localization to the insoluble-membrane compartment and that resident serines can be phosphorylated, providing a potential mechanism for regulating the membrane distribution of merlin.
The ERM proteins associate with the actin cytoskeleton via a conserved C-terminal motif that is not present in merlin. Indeed, the nature of the physical association between merlin and the actin cytoskeleton is unclear; notably, one study reported that a peptide representing the N-terminal 27 amino acids of merlin can cosediment with actin, as could polypeptides representing other portions of merlin (3). Neither merlin nor the ERM proteins localize to actin stress fibers; instead, the ERM proteins provide flexible anchorage between the membrane and cortical actin cytoskeleton (4, 6). Our observation that merlin also decorates the cortical actin network in epithelial cells is consistent with the reported localization of merlin in cultured meningioma cells by electron microscopy (17). Indeed the ability to visualize this structure by immunofluorescence localization of merlin/ERMs will facilitate an investigation of its properties given that the thin filaments of the cortical cytoskeleton are not easily detected by phalloidin.
Many studies support a key role for this cortical network in controlling the lateral mobility of certain membrane receptors (reviewed in references 23, 24, and 34 and references therein). However, it is not clear whether it does so by physically tethering the receptors or by forming a physical barrier to the lateral diffusion of untethered receptors or both. The compartment diameter outlined by merlin/ERMs in these cells is ∼0.1 to 1 μM, which is within the range of sizes estimated by electron microscopy in other cell types (reviewed in references 6, 23, 24, and 34). Notably, it has been shown that the lateral mobility of fluorescently labeled lipids increases with jasplakinolide treatment in other cell types, and it was predicted that jasplakinolide treatment yielded aberrantly larger cortical network compartments (36). In perfect agreement with this, we discovered that jasplakinolide treatment led to markedly larger compartments within the merlin-decorated network (Fig. 4G). Jasplakinolide stabilizes actin filaments in vitro but exhibits more-complicated actin-disrupting properties in vivo (5); the cortical actin meshwork is particularly sensitive to the effects of jasplakinolide at the time point and concentration used here. Importantly, NHE-RF1 itself has been reported to slow the lateral diffusion of certain receptors, including the cystic fibrosis transmembrane conductance regulator, in a manner dependent upon its ERM (and merlin)-binding domain (1, 15). Perhaps by decorating the cortical network, merlin/ERMs are poised to transiently “trap” NHE-RF-associated receptors, preventing their lateral mobility and signaling. Local activation of merlin/ERMs would therefore be a powerful way to confer spatial and temporal regulation to certain membrane receptors in response to extracellular signals.
Supplementary Material
Acknowledgments
We would like to thank the members of the McClatchey laboratory, especially Jessica Casaletto, for valuable discussions and suggestions regarding the manuscript.
This work was supported by the National Institutes of Health (A.I.M.), Department of Defense Neurofibromatosis Research Program (A.I.M.), and the Mahoney Center for Neuro-Oncology, Charles A. Dana Foundation (A.W.C.).
Footnotes
Published ahead of print on 17 December 2007.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Bates, I. R., B. Hebert, Y. Luo, J. Liao, A. I. Bachir, D. L. Kolin, P. W. Wiseman, and J. W. Hanrahan. 2006. Membrane lateral diffusion and capture of CFTR within transient confinement zones. Biophys. J. 911046-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonilha, V. L., M. E. Rayborn, I. Saotome, A. I. McClatchey, and J. G. Hollyfield. 2006. Microvilli defects in retinas of ezrin knockout mice. Exp. Eye Res. 82720-729. [DOI] [PubMed] [Google Scholar]
- 3.Brault, E., A. Gautreau, M. Lamarine, I. Callebaut, G. Thomas, and L. Goutebroze. 2001. Normal membrane localization and actin association of the NF2 tumor suppressor protein are dependent on folding of its N-terminal domain. J. Cell Sci. 1141901-1912. [DOI] [PubMed] [Google Scholar]
- 4.Bretscher, A., K. Edwards, and R. G. Fehon. 2002. ERM proteins and merlin: integrators at the cell cortex. Nat. Rev. Mol. Cell Biol. 3586-599. [DOI] [PubMed] [Google Scholar]
- 5.Bubb, M. R., I. Spector, B. B. Beyer, and K. M. Fosen. 2000. Effects of jasplakinolide on the kinetics of actin polymerization. J. Biol. Chem. 2755163-5170. [DOI] [PubMed] [Google Scholar]
- 6.Charras, G. T., C. K. Hu, M. Coughlin, and T. J. Mitchison. 2006. Reassembly of contractile actin cortex in cell blebs. J. Cell Biol. 175477-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curto, M., B. K. Cole, D. Lallemand, C. Liu, and A. I. McClatchey. 2007. Contact-dependent inhibition of EGFR signaling by Nf2/merlin. J. Cell Biol. 177893-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.den Bakker, M. A., P. H. J. Riegman, R. A. C. P. Hekman, W. Boersma, P. J. A. Janssen, T. H. van der Kwast, and E. C. Zwarthoff. 1995. The product of the NF2 tumor suppressor gene localizes near the plasma membrane and is highly expressed in muscle cells. Oncogene 10757-763. [PubMed] [Google Scholar]
- 9.Evans, D. G., M. Sainio, and M. E. Baser. 2000. Neurofibromatosis type 2. J. Med. Genet. 37897-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fehon, R. G., T. Oren, D. R. LaJeunesse, T. E. Melby, and B. M. McCartney. 1997. Isolation of mutations in the Drosophila homologues of the human neurofibromatosis 2 and yeast CDC42 genes using a simple and efficient reverse-genetic method. Genetics 146245-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finnerty, C. M., D. Chambers, J. Ingraffea, H. R. Faber, P. A. Karplus, and A. Bretscher. 2004. The EBP50-moesin interaction involves a binding site regulated by direct masking on the FERM domain. J. Cell Sci. 1171547-1552. [DOI] [PubMed] [Google Scholar]
- 12.Giovannini, M., E. Robanus-Maandag, M. van der Valk, M. Niwa-Kawakita, V. Abramowski, L. Goutebroze, J. M. Woodruff, A. Berns, and G. Thomas. 2000. Conditional biallelic Nf2 mutation in the mouse promotes manifestations of human neurofibromatosis type 2. Genes Dev. 141617-1630. [PMC free article] [PubMed] [Google Scholar]
- 13.Göbel, V., P. L. Barrett, D. H. Hall, and J. T. Fleming. 2004. Lumen morphogenesis in C. elegans requires the membrane-cytoskeleton linker erm-1. Dev. Cell 6865-873. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Agosti, C., L. Xu, D. Pinney, R. Beauchamp, W. Hobbs, J. Gusella, and V. Ramesh. 1996. The merlin tumor suppressor localizes preferentially in membrane ruffles. Oncogene 131239-1247. [PubMed] [Google Scholar]
- 15.Haggie, P. M., J. K. Kim, G. L. Lukacs, and A. S. Verkman. 2006. Tracking of quantum dot-labeled CFTR shows near immobilization by C-terminal PDZ interactions. Mol. Biol. Cell 174937-4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hitt, A. L., and E. J. Luna. 1994. Membrane interactions with the actin cytoskeleton. Curr. Opin. Cell Biol. 6120-130. [DOI] [PubMed] [Google Scholar]
- 17.James, M. F., N. Manchanda, C. Gonzalez-Agosti, J. H. Hartwig, and V. Ramesh. 2001. The neurofibromatosis 2 protein product merlin selectively binds F-actin but not G-actin, and stabilizes the filaments through a lateral association. Biochem. J. 356377-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson, K. C., J. L. Kissil, J. L. Fry, and T. Jacks. 2002. Cellular transformation by a FERM domain mutant of the Nf2 tumor suppressor gene. Oncogene 215990-5997. [DOI] [PubMed] [Google Scholar]
- 19.Kalamarides, M., M. Niwa-Kawakita, H. Leblois, V. Abramowski, M. Perricaudet, A. Janin, G. Thomas, D. H. Gutmann, and M. Giovannini. 2002. NF2 gene inactivation in arachnoidal cells is rate-limiting for meningioma development in the mouse. Genes Dev. 161060-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang, B. S., D. R. Cooper, Y. Devedjiev, U. Derewenda, and Z. S. Derewenda. 2002. The structure of the FERM domain of merlin, the neurofibromatosis type 2 gene product. Acta Crystallogr. D Biol. Crystallogr. 58381-391. [DOI] [PubMed] [Google Scholar]
- 21.Karagiosis, S. A., and D. F. Ready. 2004. Moesin contributes an essential structural role in Drosophila photoreceptor morphogenesis. Development 131725-732. [DOI] [PubMed] [Google Scholar]
- 22.Kissil, J. L., K. C. Johnson, M. S. Eckman, and T. Jacks. 2002. Merlin phosphorylation by p21-activated kinase 2 and effects of phosphorylation on merlin localization. J. Biol. Chem. 27710394-10399. [DOI] [PubMed] [Google Scholar]
- 23.Kusumi, A., and Y. Sako. 1996. Cell surface organization by the membrane skeleton. Curr. Opin. Cell Biol. 8566-574. [DOI] [PubMed] [Google Scholar]
- 24.Kusumi, A., C. Nakada, K. Ritchie, K. Murase, K. Suzuki, H. Murakoshi, R. S. Kasai, J. Kondo, and T. Fujiwara. 2005. Paradigm shift of the plasma membrane concept from the two-dimensional continuum fluid to the partitioned fluid: high-speed single-molecule tracking of membrane molecules. Annu. Rev. Biophys. Biomol. Struct. 34351-378. [DOI] [PubMed] [Google Scholar]
- 25.Lallemand, D., M. Curto, I. Saotome, M. Giovannini, and A. I. McClatchey. 2003. NF2-deficiency promotes tumorigenesis and metastasis by destabilizing adherens junctions. Genes Dev. 171090-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lazar, C. S., C. M. Cresson, D. A. Lauffenburger, and G. N. Gill. 2004. The Na+/H+ exchanger regulatory factor stabilizes epidermal growth factor receptors at the cell surface. Mol. Biol. Cell 155470-5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lucero, H. A., and P. W. Robbins. 2004. Lipid rafts-protein association and the regulation of protein activity. Arch. Biochem. Biophys. 426208-224. [DOI] [PubMed] [Google Scholar]
- 28.Maeda, M., T. Matsui, M. Imamura, S. Tsukita, and S. Tsukita. 1999. Expression level, subcellular distribution and Rho-GDI binding affinity of merlin in comparison with ezrin/radixin/moesin proteins. Oncogene 184788-4797. [DOI] [PubMed] [Google Scholar]
- 29.Maitra, S., R. M. Kulikauskas, H. Gavilan, and R. G. Fehon. 2006. The tumor suppressors merlin and expanded function cooperatively to modulate receptor endocytosis and signaling. Curr. Biol. 16702-709. [DOI] [PubMed] [Google Scholar]
- 30.McCartney, B. M., and R. G. Fehon. 1996. Distinct cellular and subcellular patterns of expression imply distinct functions for the Drosophila homologues of moesin and the neurofibromatosis 2 tumor suppressor, merlin. J. Cell Biol. 133843-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McClatchey, A. I., I. Saotome, V. Ramesh, J. F. Gusella, and T. Jacks. 1997. The Nf2 tumor suppressor gene product is essential for extraembryonic development immediately prior to gastrulation. Genes Dev. 111253-1265. [DOI] [PubMed] [Google Scholar]
- 32.McClatchey, A. I., I. Saotome, K. Mercer, D. Crowley, J. F. Gusella, R. T. Bronson, and T. Jacks. 1998. Mice heterozygous for a mutation at the Nf2 tumor suppressor locus develop a range of highly metastatic tumors. Genes Dev. 121121-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McClatchey, A. I., and M. Giovannini. 2005. Membrane organization and tumorigenesis—the NF2 tumor suppressor, merlin. Genes Dev. 192265-2277. [DOI] [PubMed] [Google Scholar]
- 34.Morone, N., T. Fujiwara, K. Murase, R. S. Kasai, H. Ike, S. Yuasa, J. Usukura, and A. Kusumi. 2006. Three-dimensional reconstruction of the membrane skeleton at the plasma membrane interface by electron tomography. J. Cell Biol. 174851-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morrison, H., L. S. Sherman, J. Legg, F. Banine, C. Isacke, C. A. Haipek, D. H. Gutmann, H. Ponta, and P. Herrlich. 2001. The NF2 tumor suppressor gene product, merlin, mediates contact inhibition of growth through interactions with CD44. Genes Dev. 15968-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murase, K., T. Fujiwara, Y. Umemura, K. Suzuki, R. Iino, H. Yamashita, M. Saito, H. Murakoshi, K. Ritchie, and A. Kusumi. 2004. Ultrafine membrane compartments for molecular diffusion as revealed by single molecule techniques. Biophys. J. 864075-4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murthy, A., C. Gonzalez-Agosti, E. Cordero, D. Pinney, C. Candia, F. Solomon, J. Gusella, and V. Ramesh. 1998. NHE-RF, a regulatory cofactor for Na+-H+ exchange, is a common interactor for merlin and ERM (MERM) proteins. J. Biol. Chem. 2731273-1276. [DOI] [PubMed] [Google Scholar]
- 38.Ratner, N., J. P. Williams, J. J. Kordich, and H. A. Kim. 2005. Schwann cell preparation from single mouse embryos: analysis of neurofibromin function in Schwann cells. Methods Enzymol. 40722-33. [DOI] [PubMed] [Google Scholar]
- 39.Resh, M. D. 1994. Myristylation and palmitylation of Src family members: the fats of the matter. Cell 76411-413. [DOI] [PubMed] [Google Scholar]
- 40.Rouleau, G. A., P. Merel, M. Lutchman, M. Sanson, J. Zucman, C. Marineau, K. Hoang-Xuan, S. Demczuk, C. Desmaze, B. Plougastel, S. M. Pulst, G. Lenoir, E. Bijlsmaparallel, R. Fashold, J. Dumanski, P. de Jong, D. Parry, R. Eldrige, A. Aurias, O. Delattre, and G. Thomas. 1993. Alteration in a new gene encoding a putative membrane-organizing protein causes neuro-fibromatosis type 2. Nature 363515-521. [DOI] [PubMed] [Google Scholar]
- 41.Sainio, M., F. Zhao, L. Heiska, O. Turunen, M. den Bakker, E. Zwarthoff, M. Luthman, G. A. Rouleau, J. Jääskeläinen, A. Vaheri, and O. Carpén. 1997. Neurofibromatosis 2 tumor suppressor protein colocalizes with ezrin and CD44 and associates with actin-containing cytoskeleton. J. Cell Sci. 1102249-2260. [DOI] [PubMed] [Google Scholar]
- 42.Saotome, I., M. Curto, and A. I. McClatchey. 2004. Ezrin is essential for epithelial organization and villus morphogenesis in the developing intestine. Dev. Cell 6855-864. [DOI] [PubMed] [Google Scholar]
- 43.Shaw, R. J., A. I. McClatchey, and T. Jacks. 1998. Localization and functional domains of the neurofibromatosis type II tumor suppressor, merlin. Cell Growth Differ. 9287-296. [PubMed] [Google Scholar]
- 44.Shaw, R. J., A. I. McClatchey, and T. Jacks. 1998. Regulation of the neurofibromatosis type 2 tumor suppressor protein, merlin, by adhesion and growth arrest stimuli. J. Biol. Chem. 2737757-7764. [DOI] [PubMed] [Google Scholar]
- 45.Shaw, R. J., J. G. Paez, M. Curto, A. Yaktine, W. M. Pruitt, I. Saotome, J. P. O'Bryan, V. Gupta, N. Ratner, C. J. Der, T. Jacks, and A. I. McClatchey. 2001. The Nf2 tumor suppressor, merlin, functions in Rac-dependent signaling. Dev. Cell 163-72. [DOI] [PubMed] [Google Scholar]
- 46.Shimizu, T., A. Seto, N. Maita, K. Hamada, S. Tsukita, S. Tsukita, and T. Hakoshima. 2002. Structural basis for the neurofibromatosis type 2. J. Biol. Chem. 27710332-10336. [DOI] [PubMed] [Google Scholar]
- 47.Speck, O., S. C. Hughes, N. K. Noren, R. M. Kulikauskas, and R. G. Fehon. 2003. Moesin functions antagonistically to the Rho pathway to maintain epithelial integrity. Nature 42183-87. [DOI] [PubMed] [Google Scholar]
- 48.Stickney, J. T., W. C. Bacon, M. Rojas, N. Ratner, and W. Ip. 2004. Activation of the tumor suppressor merlin modulates its interaction with lipid rafts. Cancer Res. 642717-2724. [DOI] [PubMed] [Google Scholar]
- 49.Terawaki, S., R. Maesaki, and T. Hakoshimam. 2006. Structural basis for NHERF recognition by ERM proteins. Structure 14777-789. [DOI] [PubMed] [Google Scholar]
- 50.Trofatter, J. A., M. M. MacCollin, J. L. Rutter, J. R. Murrell, M. P. Duyao, D. M. Parry, R. Eldridge, N. Kley, A. G. Menon, K. Pulaski, V. H. Haase, C. M. Ambrose, D. Munroe, C. Bove, J. L. Haines, R. L. Martuza, M. E. MacDonald, B. R. Seizinger, M. P. Short, A. J. Buckler, and J. F. Gusella. 1993. A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell 72791-800. [DOI] [PubMed] [Google Scholar]
- 51.Xiao, G. H., R. Gallagher, J. Shetler, K. Skele, D. A. Altomare, R. G. Pestell, S. Jhanwar, and J. R. Testa. 2005. The NF2 tumor suppressor gene product, merlin, inhibits cell proliferation and cell cycle progression by repressing cyclin D1 expression. Mol. Cell. Biol. 252384-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.