Abstract
Transforming growth factor β (TGF-β) receptor (TβR) signaling contributes to normal development as well as tumorigenesis. Here we report that RIN1, a RAB5 guanine nucleotide exchange factor (GEF) and down regulator of receptor tyrosine kinases (RTKs), promotes TβR signaling through enhanced endocytosis. TβR activation induces SNAI1 (Snail), a transcription repressor that reduces RIN1 expression, providing a negative feedback mechanism to control TβR trafficking and downstream signaling. Persistent RAS signaling disrupts this equilibrium by stabilizing SNAI1 protein, resulting in strong silencing of RIN1 and stabilization of RTKs. TGF-β-induced RIN1 silencing in breast cancer cells prolonged sensitivity to hepatocyte growth factor, a ligand for the MET-type RTK, and enhanced growth factor-directed cell motility. We conclude that in some tumor cells TβR and RAS signals are integrated through the silencing of RIN1, leading to a reduction in RAB5-mediated endocytosis. These findings shed new light on the basis for distinct interpretations of TGF-β signaling by normal versus transformed cells.
Cell surface receptor internalization controls the intensity and duration of signaling and, as a consequence, regulates phenotypes such as differentiation, mitosis, and migration (36, 44). The clathrin-dependent endocytosis of receptor tyrosine kinases (RTKs), for example, typically results in down regulation and signal termination. The GTPase RAB5 is a key regulator of early endosome formation and trafficking and hence a control point for receptor function. This small GTPase is itself activated by the guanine nucleotide exchange factor (GEF) RIN1 (50) and other GEFs (9) and inhibited by GTPase-activating proteins (24, 39).
Transforming growth factor β (TGF-β), signaling through a receptor serine/threonine kinase complex (TβRI/TβRII or TβR), has antiproliferative effects on normal epithelial cells (48). Some late-stage tumor cells escape this cytostatic effect and instead respond to TGF-β with increased proliferation and enhanced motility that promote metastatic spread (16). Activated TβR phosphorylates associated SMAD2 and SMAD3 proteins that then accumulate in the nucleus, inducing expression of SNAI1 (Snail) (42), a promoter of cell motility in development and tumor progression (3), and other genes. This signaling pathway is enhanced by the recruitment of effector proteins during clathrin-mediated endocytosis (18, 27, 38, 43). An alternate, clathrin-independent, TβR internalization pathway targets TβR to a degradation shunt (46). We describe a dynamic role for RIN1 in TβR endocytosis and regulation of downstream signal intensity. In addition, we provide evidence that TβR and RTK signals integrate through down regulation of RIN1, contributing to the profound difference in TGF-β response between normal and transformed cells.
MATERIALS AND METHODS
Cell culture, transfections, and transductions.
MCF10A, MDA-MB-231, HeLa, and HepG2 cell lines were cultured under standard conditions. Transfections were performed using Polyfect (Qiagen) (MCF10A, HeLa, and HepG2) or calcium phosphate (MDA-MB-231). For lentivirus-mediated transductions, virus stocks were prepared and infections carried out as previously described (28). The glycogen synthase kinase 3β (GSK3β) inhibitor SB216763 (BioMol) and the proteosome inhibitor MG-132 (Sigma) were each used at 10 μM for 6 h. Wortmannin (BioMol) was used at 1 μM for 18 h. HMEC cells immortalized with simian virus 40 large T antigen and hTERT without (HMLE) or with (HMLER) HRASG12V (19) were subcloned and generously provided by Natalia Mitin and Channing Der (University of North Carolina).
Expression constructs.
All RAB5 constructs are derived from human RAB5A. Wild-type and RAB5S34N constructs were created by moving the two cDNAs (gifts of Guangpu Li, University of Oklahama) from pBl into an M4 lentiviral vector with Mlu and Nhe sites. The SNAI1 deletion mutant was created using PCR and pPGS-Snail (gift of Eric Fearon, University of Michigan). RIN1 mutants were constructed by QuikChange (Stratagene) site-directed mutagenesis (primer sequences available upon request). The epidermal growth factor receptor (EGFR) expression construct was purchased from Addgene, and RAS constructs were described previously (28). The Rin1 promoter-luciferase reporter construct will be described separately (B. Dzudzor, L. C. Huynh, and J. Colicelli, unpublished data).
RNA extraction and real-time PCR.
Total RNA was extracted from cell lines using TRIzol (Invitrogen Life Technologies) following the manufacturer's protocol. Isolated RNA was used to synthesize cDNA using an iScript cDNA synthesis kit (Bio-Rad). Real-time PCR was performed using the iCycler PCR platform (Bio-Rad) and the following conditions: 95°C for 10 min (initial incubation); 40 cycles of 95°C for 30 s, 55°C for 1 min, and 72°C for 30 s; and 1 cycle of 95°C for 1 min, 55°C for 30 s, and 95°C for 30 s. IQ Sybr green Supermix (Bio-Rad) was used according to the manufacturer's instructions. Primers were 5′-CATTGCCGACAGGATGCA and 5′-CGCTCAGGAGGAGCAATGAT (β-actin), 5′-GGCAGCAGAGGAGTAGCTTGA and 5′-GCTTGCTGGCGCTAAAAGG (RIN1), and 5′-GAAAGGCCTTCAACTGCAAA and 5′-TGACATCTGAGTGGGTCTGG (SNAI1).
Luciferase reporter assays and transcription factor analysis.
HeLa, HepG2, and MDA-MB-231 cells were transiently transfected with the luciferase reporter construct 3TP-Lux (gift of Joan Massague, Sloan-Kettering Cancer Center) or SBE-Lux (gift of Edward Leof, Mayo Clinic College of Medicine) and the indicated constructs. The RIN1 promoter-luciferase construct includes the 950-bp sequence upstream of the mouse RIN1 translation start site (Dzudzor et al., unpublished). Twenty-four hours after transfection, cells were incubated with or without 5 ng/ml TGF-β (R&D) for the indicated time. Luciferase activity was measured with the Dual-Glo luciferase assay system (Promega) in a Turner Design Model TD-20/20 luminometer. A cytomegalovirus-Renilla luciferase plasmid (Promega) was used to normalize transfections. Results were expressed as a normalized ratio of luciferase activity in each experiment. Transcription factor binding site analysis was done using the CONSITE database (http://asp.ii.uib.no:8090/cgi-bin/CONSITE/consite).
RNA interference.
RIN1 expression was attenuated using short interfering RNA (siRNA) as previously described (28) or short hairpin RNA (shRNA)-mediated RNA interference. The shRNA primers were designed using the BLOCK-iT RNAi Designer algorithm (Invitrogen). Primers were attached to a U6 promoter via PCR and subsequently cloned into the M4 lentiviral vector (28) modified to include a blasticidin resistance marker. Virus stocks were generated by standard techniques (28). Once infected, cells were selected using blasticidin (10 to 20 μg/ml). Cloning strategies and primer sequences are available upon request. SNAI1-directed siRNA (Ambion; catalog no. AM16708) and scrambled sequence control siRNA (Ambion; catalog no. 4615) were transfected using siPORT (Ambion).
Immunoprecipitation, immunoblotting, and immunofluorescence.
Immunoprecipitation and immunoblotting were performed as described previously (28). The following antibodies were used to precipitate proteins from cell extracts: rabbit anti-RIN1 (Transduction Laboratories), goat anti-MET (R&D Systems), rabbit anti-EGFR (Santa Cruz Biotechnology), and mouse anti-Flag beads (Sigma). Immunoblot analysis was performed using mouse anti-RIN1, mouse anti-RAB5, mouse anti-E-cadherin, mouse anti-SMAD2/3, and mouse anti-β-tubulin (all from BD Biosciences); mouse anti-p-Tyr, rabbit anti-pAKT, rabbit anti-AKT, mouse anti-p-Erk1/2, mouse anti-Erk1/2, and rabbit anti-pSMAD2/3 (all from Cell Signaling Technology); rabbit anti-SNAI1, rabbit anti-TβRI, and rabbit anti-TβRII (all from Santa Cruz Biotechnology); mouse anti-hepatocyte growth factor (anti-HGF; R&D Systems) and mouse anti-Myc (Upstate Biotechnology). Total extracellular signal-regulated kinase 1/2 (ERK1/2) was used as an alternative to TUBB for protein normalization in some cases, as both have been shown to give relatively even expression under a variety of growth conditions.
Immunofluorescence cell staining was performed as previously described (28) with the following modifications. MDA-MB-231/sh-ctr and MDA-MB-231/sh-RIN1 cells grown on coverslips were TGF-β stimulated (5 ng/ml, 5 or 15 min), washed with phosphate-buffered saline (PBS), fixed (4% paraformaldehyde, 10 min), and permeabilized (0.5% Triton X-100, 10 min). After blocking (2% bovine serum albumin), cells were first incubated with mouse anti-SMAD2/3 (BD Biosciences), washed, and then incubated with fluorescein isothiocyanate-conjugated anti-mouse immunoglobulin G (Sigma). Coverslips were mounted using Vectashield with DAPI (4′,6′-diamidino-2-phenylindole; Vector Laboratories). Images were captured with fluorescent in situ hybridization analysis software connected to a Leica DM RXA automated microscope equipped with Photometrix SenSyn. At least 20 fields (about 800 cells per condition) were counted per condition.
RAB5 guanine nucleotide occupancy assays.
Glutathione S-transferase (GST)-R5BD (gift of Jose Esteban, University of Michigan) (6) was expressed in BL21 bacterial cells and purified using glutathione beads (Amersham). For Rab5-GTP assays, cells were lysed in buffer (10% glycerol, 50 mM Tris, pH 7.4, 100 mM NaCl, 1% NP-40, 2 mM MgCl2 with protease inhibitor cocktail [Roche Diagnostics]) and incubated with GST-R5BD-coated beads for 1 h at 4°C, followed by three washes with lysis buffer. The bound material was analyzed by immunoblotting with mouse anti-RAB5 (BD Biosciences).
Migration assays.
Assays were performed with 8-μm-pore-size transwell chambers (BD Biosciences), and the surface was coated with fibronectin (10 ng/ml). Cells (5 × 104) were seeded in serum-free medium with or without 5 ng/ml TGF-β in the upper chamber and migrated toward 20 ng/ml HGF as a chemoattractant in the lower chamber for the indicated time periods. Cells in the upper chamber were carefully removed with a cotton swab, and cells at the bottom of the membrane were fixed in 4% polyformaldehyde and stained with 0.2% crystal violet in 20% methanol. Cells from at least 10 microscopic fields (20×) were counted, and data presented are means ± standard deviations (SDs) of duplicate wells from two experiments.
Internalization assays.
Subconfluent cells were cultured in serum-free medium overnight, washed twice with cold PBS, and incubated with 0.25-mg/ml EZ-link-sulfo-N-hydroxysuccinimide-SS-biotin (Pierce) in PBS for 30 min on ice. After three washes with cold PBS, cells were rewarmed to 37°C and treated with TGF-β (5 ng/ml) for 5 min to stimulate receptor internalization. Cells were either directly lysed with radioimmunoprecipitation assay buffer (control) or incubated at 4°C with glutathione solution (50 mM glutathione, 75 mM NaCl, 75 mM NaOH, 10 mM EDTA, and 1% bovine serum albumin) washed, scraped, and lysed in radioimmunoprecipitation assay buffer. Biotinylated proteins were purified from cell extracts using Neutravidin beads (Pierce) and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and anti-TβRII immunoblotting.
RESULTS
RIN1 is a positive regulator of TGF-β signaling.
To test whether RIN1, a GEF for RAB5, regulates TβR signaling, we measured activation of the SMAD-dependent promoter 3TP (35). TGF-β treatment induced reporter gene expression in HeLa cells, which have moderate levels of endogenous RIN1 (17). The level of reporter induction was increased by transfection with a RIN1 construct (Fig. 1A, left), and this was concentration dependent. We noted that baseline signals also increased with more RIN1 (Fig. 1A, right). Baseline effects have been seen following other manipulations of TGF-β signaling components (7, 12, 20, 47, 55, 57, 59) and typically follow the same trend as responses to added TGF-β. This likely reflects normal receptor signaling caused by low levels of TGF-β in the serum-supplemented medium (reference 22 and references within) and/or a TGF-β autocrine response (56; reviewed in reference 15). We next created a stable RIN1 overexpression HeLa subline and employed an alternate SMAD-responsive promoter, SBE (32), to monitor TGF-β induction. After normalization of baseline signals, we observed a small but still statistically significant RIN1-mediated increase (Fig. 1B). We confirmed this effect using another cell line, MDA-MB-231. Again, stable RIN1 overexpression led to an enhanced TGF-β-induced response (Fig. 1C).
FIG. 1.
RIN1 is a positive regulator of TGF-β signaling. (A) HeLa cells were transiently transfected with 3TP-Lux and either vector (ctr) or RIN1 and then incubated without or with TGF-β (5 ng/ml) for 18 h. Increased amounts of transduced RIN1 expression construct (wedge) were used to demonstrate concentration dependence. (Right side) Baseline luciferase activity (no added TGF-β) increased with the amount of transduced RIN1. (B) HeLa cells were transduced with ctr or RIN1 lentivirus. Stable, selected populations were then transfected with SBE-Lux and stimulated (or not) by TGF-β (5 ng/ml) for 18 h. Immunoblots for RIN1 and TUBB (normalization control) are shown above the graph. *, P = 0.02. (C) MDA-MB-231 cells were transduced with ctr or RIN1 lentivirus. Stable, selected populations were transfected with 3TP-Lux and stimulated (or not) by TGF-β (5 ng/ml) for 5 h. Immunoblots for RIN1 and TUBB (normalization control) are shown above the graph. *, P = 0.01. (D) MDA-MB-231 cells were transduced with shRNA lentivirus (ctr or RIN1). Stable, selected populations were transfected with SBE-Lux and stimulated (or not) by TGF-β (5 ng/ml, 18 h). Immunoblots for RIN1 and TUBB (normalization control) are shown above graph. *, P = 0.02. (A to D) Luciferase activities were normalized to Renilla luciferase activity and plotted as means ± SDs of triplicates from a representative of at least two independent experiments. (E) RIN1 silencing dampens SMAD2 nuclear localization in response to TGF-β. (Left) MDA-MB-231 cells transduced with ctr or RIN1 shRNA lentivirus (same as panel D) were induced with TGF-β (5 ng/ml, 15 min) and then fixed and stained with anti-SMAD2/3 and DAPI. (Right) Nuclear SMAD staining after 5 or 15 min of TGF-β treatment (mean of 10 fields counted; *, P < 0.001).
Consistent with RIN1 enhancement of TGF-β receptor signaling, silencing of endogenous RIN1 decreased TGF-β-stimulated activation of an SBE reporter (Fig. 1D). Parallel experiments using the 3TP reporter in this cell line also showed significant signal reduction (38%; P < 0.01) associated with RIN1 silencing (data not shown). As an independent assessment, we examined SMAD2/3 nuclear localization, an early step in TGF-β receptor signaling. RIN1 silencing led to a marked reduction in SMAD2/3 nuclear localization following TGF-β treatment (Fig. 1E).
RIN1 promotes TGF-β signaling through activation of RAB5.
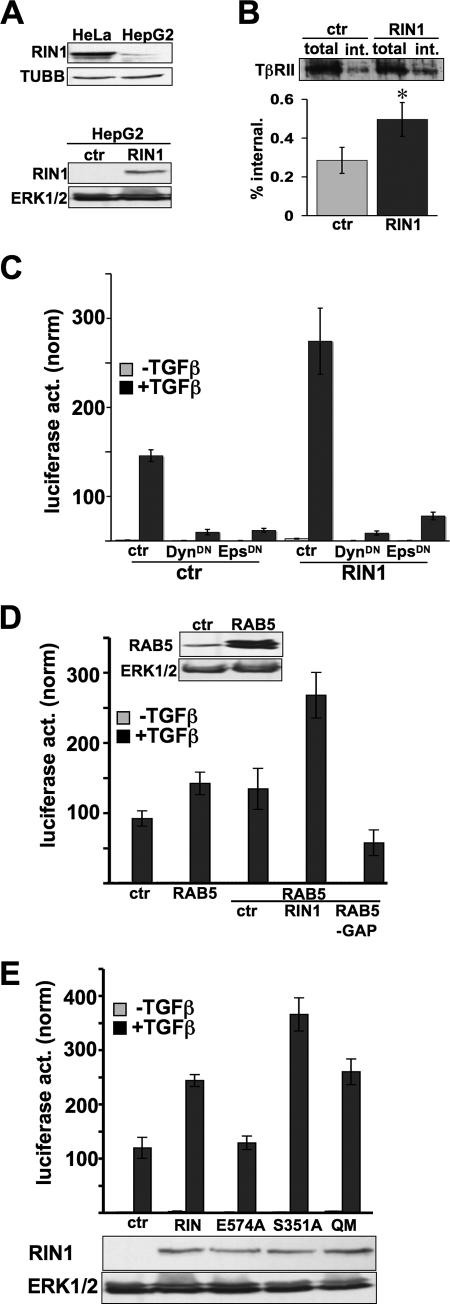
We next examined whether RIN1 might act by enhancing TGF-β endocytosis, which promotes TβR signaling (18). These experiments were performed in HepG2 cells because of their high endogenous TβR levels. HepG2 cells express RIN1, though at lower levels than HeLa cells do (Fig. 2A). We found that elevated levels of RIN1 led to enhanced ligand-induced TβR internalization (Fig. 2B). To test the possible connection between greater receptor internalization and elevated signaling, we blocked clathrin-mediated endocytosis using dominant-negative dynamin 1 (13, 49, 51) and dominant-negative EPS15 (4, 45). Both treatments inhibited TGF-β-induced signaling, even in the presence of RIN1 overexpression (Fig. 2C). These results suggested that RIN1 enhances TβR signaling through an endocytosis-dependent mechanism.
FIG. 2.
RIN1 enhancement of TGF-β signaling is endocytosis dependent. (A) Normalized immunoblots showing endogenous RIN1 expression levels in HeLa and HepG2 cells (top) and ectopic expression of RIN1 in HepG2 (bottom). (B) Control and RIN1 overexpression. HepG2 cells were surface biotinylated and cultured with TGF-β to stimulate receptor endocytosis. TβRII was recovered on avidin beads and quantified. (Top) Total and internalized (int.) TβRII immunoblot. (Bottom) Internalization levels, normalized to total TβRII, from four experiments (*, P < 0.05). (C) HepG2 transduced cell lines (ctr or RIN1) were transfected with 3TP-Lux and either vector (ctr) or the indicated construct. Cells were then incubated without or with TGF-β (5 ng/ml) for 18 h. DynDN, dominant-negative dynamin; EpsDN, dominant-negative EPS15. (D) HepG2 cells previously transduced with vector (ctr) or RAB5 were transfected with the TGF-β reporter (3TP-Lux) alone or in combination with RIN1 or RAB5-GAP as indicated. Where indicated, TGF-β treatment was for 18 h. Immunoblots for RAB5 and total ERK1/2 (normalization control) are shown above. (E) HepG2 transduced cell lines (ctr or RIN1, wild type or mutant) were transfected with the TGF-β reporter and stimulated with TGF-β as indicated. Immunoblots for RIN1 and total ERK1/2 (normalization control) are shown below. (C to E) Luciferase activities were normalized to Renilla luciferase activity and plotted as means ± SDs of triplicates from a representative of at least two independent experiments.
Because RIN1 can function as a RAB5 GEF (50), we considered whether RAB5 activity is rate limiting for clathrin-mediated endocytosis and signaling through TβRs. Overexpression of RAB5 caused a small increase in TGF-β response, and this effect was amplified by RIN1 overexpression (Fig. 2D). Conversely, ectopic expression of Rab5GAP, a negative regulator of RAB5 (24), led to a drop in TGF-β signaling (Fig. 2D). These results suggested that RAB5 activation is an important control point for TβR signaling.
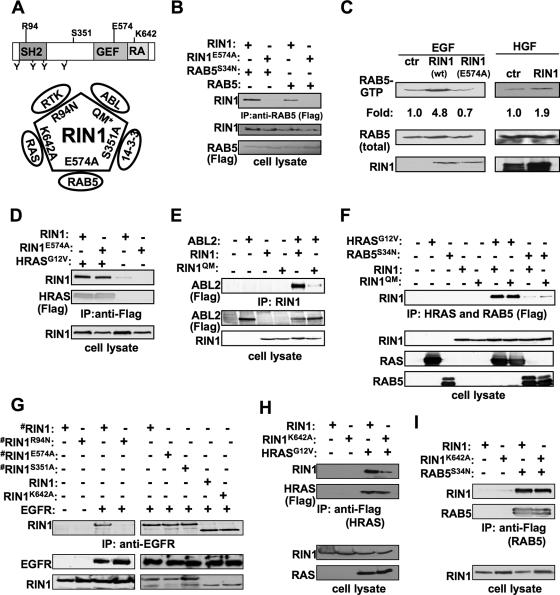
The GEF domain of RIN1 is most similar to the VPS9 family of RAB5 GEFs. We mutated RIN1 at a conserved glutamic acid residue believed to be critical for RAB5 binding and GEF activity of a related VPS9 domain (14). RIN1E574A (Fig. 3A) showed reduced binding to wild-type RAB5 and RAB5S34N, a dominant-negative mutant with high affinity for exchange factors (37) (Fig. 3B). RIN1E574A also had reduced RAB5 GEF activity; following stimulation with EGF, an RTK ligand, cells overexpressing RIN1 showed elevated levels of RAB5-GTP while cells expressing RIN1E574A did not (Fig. 3C). This effect was not caused by reduced association with RAS, which normally promotes the GEF activity of RIN1 (50), as both wild-type RIN1 and RIN1E574A bound well to activated RAS (Fig. 3D). The GEF-defective RIN1E574A did not increase TβR signaling (Fig. 2E), suggesting that enhancement by RIN1 requires activation of RAB5. RIN1S351A, a mutant with increased plasma membrane association due to reduced 14-3-3 binding (52) (Fig. 3A), displayed a superenhancement of TGF-β signaling (Fig. 2E), as would be expected for a mechanism requiring membrane localization.
FIG. 3.
RAB5 GEF activity is required for RIN1 enhancement of TGF-β signaling. (A) RIN1 signaling mutants. (Top) RIN1 diagram showing SH2, GEF, and RAS association (RA) domains (positions of key residues indicated). (Bottom) Diagram illustrates known direct interactions of RIN1 with RAS (26, 52), ABL (28), 14-3-3 (26, 52), and RAB5 (50). RIN1 interaction with EGFR is likely to be direct also (2). Mutations blocking each protein interaction are shown. The quadruple mutant (QM) carries four ABL kinase substrate residue mutations (Y36F, Y121F, Y148F, and Y295F). (B) RIN1E574A mutant shows reduced RAB5 binding. Expression constructs of RIN1 or RIN1E574A were cotransfected with Flag-RAB5 or Flag-RAB5S34N (as indicated) into HEK293T cells. RAB5 was immunoprecipitated using anti-Flag agarose beads, and this material was immunoblotted with anti-RIN1 (upper panel). Whole-cell lysates were immunoblotted with anti-RIN1 or anti-Flag to confirm expression. (C) RIN1E574A mutant, unlike wild-type RIN1, does not increase RAB5-GTP levels. Vector (ctr) and expression constructs of RIN1 or RIN1E574A were transfected into HeLa cells, which were then stimulated with EGF (20 ng/ml; 15 min) or HGF (50 ng/ml; 15 min). (Top) RAB5-GTP was precipitated with GST-R5BD and then immunoblotted with anti-RAB5. (Bottom) Whole-cell lysate was immunoblotted with anti-RAB5 or anti-RIN1. Change in RAB5-GTP (normalized to control) is indicated. (D) RIN1E574A mutant retains RAS binding property. Expression constructs of RIN1 or RIN1E574A were cotransfected with Flag-HRASG12V (as indicated) into HEK293T cells. RAS protein was immunoprecipitated using anti-Flag agarose beads, and this material was immunoblotted with anti-RIN1 or anti-RAS (upper panels). Whole-cell lysates were immunoblotted with anti-RIN1 (lower panel) to confirm expression. (E) RIN1QM mutant shows reduced ABL2 (Arg) binding. HEK293T cells were transfected with Flag-ABL2, RIN1, and RIN1QM constructs as indicated. RIN1 protein was immunoprecipitated, and this material was immunoblotted with anti-Flag. The bottom panels show immunoblots of whole-cell lysates with anti-Flag and anti-RIN1. (F) RIN1QM mutant binds normally to RAS and RAB5. HEK293T cells were transfected with Flag-HRASG12V, Flag-RAB5S34N, RIN1, and RIN1QM constructs as indicated. Whole-cell lysates (100 μg) were immunoblotted with RAS, RAB5, and RIN1 antibodies (bottom). RAS and RAB5 proteins were immunoprecipitated with anti-Flag and immunoblotted with anti-RIN1 (top). (G) RIN1R94N mutant is defective for EGFR binding. HEK293T cells were transfected with RIN1-HTM, RIN1R94N-HTM, RIN1E574A-HTM, RIN1S351A-HTM, RIN1, RIN1K642A, and EGFR as shown (“#” indicates HTM epitope tag causing slower migration). (Top) EGFR was immunoprecipitated with anti-EGFR and immunoblotted with anti-RIN1. (Bottom) Whole-cell lysates were immunoblotted with anti-EGFR and anti-RIN1. (H) RIN1K642A mutant shows reduced RAS binding. HEK293T cells were transfected with Flag-HRASG12V, RIN1, and RIN1K642A constructs as indicated. (Top) HRAS was immunoprecipitated with anti-Flag and immunoblotted with anti-RIN1 or anti-Flag. (Bottom) Whole-cell lysates were immunoblotted with anti-RAS and anti-RIN1. (I) RIN1K642A mutant retains RAB5 binding property. Expression constructs of RIN1 or RIN1K642A were cotransfected with Flag-RAB5S34N into HEK293T cells. RAB5 protein was immunoprecipitated using anti-Flag agarose beads, and this material was immunoblotted with anti-RIN1 or anti-RAB5 (upper panels). Whole-cell lysates were immunoblotted with anti-RIN1 (lower panel) to confirm expression.
We next considered the possible involvement of another RIN1 effector, the tyrosine kinase ABL, in modulating TβR signaling. Based on the model for ABL activation (28), mutation of four amino-terminal tyrosines in RIN1 should prevent association with the SH2 domain of ABL and subsequent activation of the tyrosine kinase domain. A quadruple tyrosine mutation, RIN1QM (Fig. 3A), severely reduced ABL binding (Fig. 3E) and ABL activation (41). The multiple tyrosine mutations did not, however, adversely affect the interaction of RIN1 with RAB5 or RAS (Fig. 3F). We found that RIN1QM was fully capable of enhancing TGF-βR signaling (Fig. 2E), indicating that ABL activation by RIN1 does not contribute to this function.
These data support the model that RIN1, by activation of RAB5, drives clathrin-mediated TGF-β receptor internalization through an early endosome pathway, facilitating signal transduction through SMADs.
RIN1 is a negative regulator of epithelial cell RTK signaling.
RIN1 overexpression enhances EGFR internalization (1, 50) and degradation (34), inhibiting cytoplasmic signaling through ERK. Downstream signaling from MET, an RTK activated by the epithelial chemoattractant HGF, was similarly inhibited by RIN1 (Fig. 4A). Inhibition of downstream signaling is likely initiated through increased receptor internalization driven by RIN1 binding to activated RTK and stimulating GTP loading on RAB5. RIN1 silencing caused an elevation of EGFR signaling, as judged by ERK1/2 phosphorylation (Fig. 4B). This result suggests that other RAB5 GEFs cannot fully compensate for RIN1 loss and that ERK-targeted phosphatases cannot counterbalance enhanced signaling in this time frame.
FIG. 4.
RIN1 down regulates EGFR and MET signaling. (A) HeLa cells overexpressing RAB5 were transfected with vector (ctr) or RIN1 and subsequently treated with EGF (20 ng/ml) or HGF (50 ng/ml) for the indicated times. Cell extracts were analyzed by immunoblotting for phospho-ERK1/2 and total ERK1/2. Normalized changes in phospho-ERK levels are shown graphically. (B) HeLa cells stably transduced with control (ctr-shRNA) or RIN1-directed (RIN1-shRNA) silencing constructs were treated with EGF (50 ng/ml) for the indicated times. Cell extracts were analyzed by immunoblotting for phospho-ERK1/2 and total ERK1/2. Change in signal intensity is shown below (normalized to control cells after 5 min of induction). (C) HeLa cells transfected with vector or RIN1 (wild type or mutant) were treated with EGF (20 ng/ml) for the indicated time. Extracts were immunoblotted for ERK1/2 (phosphorylated and total) or RIN1. “#” indicates HTM epitope tag causing slower migration. Normalized phospho-ERK levels are shown graphically. The last three samples include SDs from three independent experiments to assess the minor effect of RIN1K642A (*, P < 0.03).
To evaluate further the endocytosis dependence of RIN1's effect on RTK signaling, we used the RIN1R94N mutant (28). This mutation disrupts the RIN1 SH2 domain, which mediates interaction with the phosphorylated form of EGFR and other RTKs (2) (Fig. 3A). RIN1R94N had no inhibitory effect on ERK phosphorylation (Fig. 4C), consistent with the fact that RIN1R94N was compromised for binding to EGFR (Fig. 3G). Similarly, RIN1E574A, the GEF domain mutant that blocks RAB5 stimulation function without affecting EGFR binding (Fig. 3G) or RAS binding (Fig. 3D), could not inhibit stimulation of phospho-ERK (Fig. 4C). Thus, both RTK binding and RAB5 GEF activity are required for the negative effects of RIN1 on downstream signaling by EGFR.
RAS binding unleashes the full RAB5 GEF activity of RIN1 (50). To test whether RIN1 overexpression could circumvent this requirement, we mutated a lysine residue needed for high-affinity binding of RIN1 to RAS (53) (Fig. 3A). As expected, RIN1K642A showed reduced affinity for a constitutively active RAS mutant (HRASG12V) (Fig. 3H) but could still bind EGFR (Fig. 3G) and RAB5 (Fig. 3I). The RIN1K642A mutant retained the ability to inhibit ERK phosphorylation in response to EGF stimulation. This mutant appeared to be a less potent signal inhibitor than wild-type RIN1 (Fig. 4C), however, perhaps reflecting lower GEF efficiency due to reduced RAS binding, despite high RIN1 levels. We cannot rule out the possibility that the ability of RIN1 to inhibit ERK phosphorylation is due in part to interference with the RAS effector RAF (52) and that this competitive binding to RAS is compromised by the K642A mutation. Nevertheless, taken together, the results suggest that RTK signaling is reduced by RIN1 primarily through RAB5 activation.
TGF-β and RTK/RAS signal integration silences RIN1 expression via Snail.
In some late-stage tumors, TGF-β promotes growth and dissemination (30). In these TGF-β-responsive tumor cells, the proliferation and metastatic phenotypes may in part reflect enhanced RTK signaling. We therefore tested whether TGF-β might facilitate RTK signaling by repressing RIN1. Indeed, TGF-β lowered RIN1 mRNA and protein levels in MCF10A cells, and this response was more rapid in MDA-MB-231, a breast tumor cell line (Fig. 5A and B). The reduction in RIN1 protein levels persisted for at least 36 h, and the level failed to be fully restored 18 h after removal of TGF-β (data not shown). MDA-MB-231 has, among other genetic changes, a mutationally activated RAS gene (KRASG13D). To test whether RAS contributed to an enhanced TGF-β response, we employed an immortalized human mammary epithelial cell line, HMLE. Treatment with TGF-β for 18 h showed no significant change in RIN1 protein levels in these cells, but treatment of HMLER cells (HMLE expressing HRASG12V) resulted in a large decrease in RIN1 levels (Fig. 5C). The same experiment carried out in HeLa cells with or without HRASG12V gave a similar outcome (data not shown). These results are consistent with RIN1 being among the transcripts most strongly silenced following TGF-β treatment of MCF10A cells overexpressing HRAS and ERBB2 (10).
FIG. 5.
TGF-β and RAS cooperatively down regulate RIN1 expression via Snail. (A) MCF10A and MDA-MB-231 cells were stimulated with TGF-β (5 ng/ml) for the indicated time or left unstimulated. Levels of endogenous RIN1 mRNA were determined in triplicate using quantitative real-time PCR and normalized to unstimulated cells. (B) Cells and conditions as in panel A were used, and changes in endogenous RIN1 protein were quantified by immunoprecipitation and immunoblotting and normalized to total TUBB (β-tubulin). (C) Immortalized human mammary epithelial cells (HMLE) and equivalent cells expressing HRASG12V (HMLER) were treated with TGF-β (5 ng/ml, 18 h). Endogenous RIN1 protein was immunoprecipitated and immunoblotted. Cell lysates were probed with antibodies to RAS and TUBB (β-tubulin). Normalization to TUBB levels was used to calculate change in RIN1.
We considered the epithelial transcription repressor SNAI1 (also known as Snail) as a possible mediator of RIN1 silencing. SNAI1 is induced by SMAD2/3 following activation of TβRs (42) and promotes mesenchymal phenotypes in tumor cells. Snail binding sites are the most common transcription elements in the human RIN1 promoter (21 sites), with one of these sites well conserved in mammals. Indeed, TGF-β-induced SNAI1 expression appeared to be more robust in MDA-MB-231 cells than in MCF10A cells (Fig. 6A). This suggested that greater repression of RIN1 in MDA-MB-231 cells (Fig. 5A and B) might be the result of elevated and/or prolonged detectable levels of SNAI1.
FIG. 6.
Snail mediates control of RIN1 expression by TGF-β. (A) Cells and conditions as in Fig. 5A were used, and levels of endogenous SNAI1 (Snail) mRNA were determined by quantitative real-time PCR. (B) A mouse Rin1 promoter-luciferase construct (pRPluc) was transfected into HeLa cells together with vector (ctr), SNAI1 (SNAI), or a repressor domain deletion mutant of SNAI1 (SNAIm). Luciferase activity was measured and normalized to Renilla luciferase activity and plotted as means ± SDs of triplicates from a representative of at least two independent experiments. (C) Normalized real-time PCR measurements of SNAI1 and RIN1 mRNAs following treatment of MDA-MB-231 cells with SNAI1-directed or control siRNA. (D) MDA-MB-231 cells were treated with SB216763 (GSK3β inhibitor) and MG-132 (proteosome inhibitor) for 6 h or left untreated (ctr). Immunoblots of endogenous SNAI1, RIN1, CDH1, and TUBB are shown (CDH1 was immunoprecipitated prior to immunoblotting). (E) EGF (100 ng/ml, 18 h) stimulation of MDA-MB-231 cells in the absence (−) or presence (+) of wortmannin (Wort). Levels of SNAI1, phospho-AKT, and total AKT protein were measured by immunoblotting (left). Normalized levels of RIN1 mRNA were measured by real-time PCR (right).
To examine this further, we used several methods to directly manipulate SNAI1 expression. Ectopic SNAI1 repressed the RIN1 promoter while a mutant SNAI1 missing the transcription repressor domain could not block expression (Fig. 6B). Conversely, SNAI1 silencing led to an increase in RIN1 mRNA levels (Fig. 6C).
Rapid turnover of endogenous SNAI1 results from GSK3β-mediated phosphorylation followed by proteosome-mediated degradation (58). Treatment of MDA-MB-231 cells with GSK3β and proteosome inhibitors increased SNAI1 protein levels with a corresponding decrease in the levels of RIN1 and CDH1 (E-cadherin), an established target of SNAI1 (8) (Fig. 6D). Individually, the inhibitors had only minor effects on SNAI1 levels (data not shown), consistent with other reports (58). Because GSK3β is inhibited by RAS-phosphatidylinositol 3-kinase-AKT signaling (58), our results suggested that active RTKs or mutant RAS in tumor cells enhances TGF-β-induced SNAI1 expression through protein stabilization. We therefore employed a phosphatidylinositol 3-kinase inhibitor to block this pathway. Treatment of cells with wortmannin lowered AKT activity while at the same time reducing the level of SNAI1 protein and increasing the level of RIN1 expression (Fig. 6E).
RIN1 represses RTK-directed cell migration.
RIN1 promotion of RTK down regulation (Fig. 4) suggested that TGF-β-mediated RIN1 silencing might contribute to the invasive migration of tumor cells toward HGF, a mesenchyme-derived growth factor that binds to MET and promotes epithelial cell scattering and migration (5). Overexpression of RIN1 in MDA-MB-231 cells decreased HGF-induced cell migration (Fig. 7A), consistent with the ability of RIN1 to activate RAB5 and to suppress ERK1/2 phosphorylation following HGF stimulation (Fig. 4A). The RTK interaction-defective mutant RIN1R94N was compromised in this assay (Fig. 7B), suggesting that RIN1 impedes migration by directly binding activated MET and facilitating receptor down regulation. Indeed, silencing of RIN1 increased cell migration toward HGF (Fig. 7A). RIN1 overexpression and silencing did not alter cell proliferation noticeably within the time course of these assays (data not shown).
FIG. 7.
RIN1 negatively regulates RTK-mediated cell migration. (A) MDA-MB-231 cells transduced with vector (ctr) or an RIN1 expression construct and MDA-MB-231 cells transfected with siRNA (ctr or RIN1) were allowed to migrate toward HGF. Migration values in all panels represent the means of at least 30 independent field counts. Immunoblots for RIN1 and TUBB (normalization control) are shown above the graph. (B) MDA-MB-231 cells transduced with a vector (ctr), wild-type RIN1, or RIN1R94N were allowed to migrate toward HGF-containing medium for 6 h. Immunoblots for RIN1 and TUBB (normalization control) are shown above the graph. RIN1R94N migrates more slowly due to a carboxy-terminal HTM fusion (as in Fig. 3 and 4) that does not alter function (28). (C) MDA-MB-231 cells were seeded in medium with or without TGF-β (5 ng/ml) and allowed to migrate for 6 h toward target chamber medium with or without HGF (20 ng/ml). “*” indicates that the combined effect is statistically greater than the additive effect by two-way analysis of variance. (D) Conditioned medium (CM) from MDA-MB-231 cells treated, or not, with TGF-β was loaded onto a nylon membrane using a dot blot apparatus. Two independent experiments (#1 and #2) are shown. Standard amounts of HGF were loaded as controls (top row). HGF was visualized using anti-HGF. (E) MDA-MB-231 cells transduced with a control vector (ctr) or a RIN1-targeted shRNA construct and resuspended in medium with or without TGF-β (5 ng/ml) were allowed to migrate for 6 h toward HGF-containing medium. Immunoblots for RIN1 and TUBB (normalization control) are shown above the graph. (F) MDA-MB-231 cells transduced with a control vector (ctr) or a RIN1-targeted shRNA construct were allowed to migrate for 6 h before counting. Where indicated, cells were treated with TGF-β (5 ng/ml; 18 h). Where indicated, the target chamber medium contained IL-8 (100 ng/ml), an epithelial cell chemoattractant.
TGF-β has been shown to stimulate the migration of some tumor cells (23). Indeed, TGF-β not only increased the general motility of MDA-MB-231 cells, it also significantly enhanced migration toward HGF (Fig. 7C). We ruled out the possibility that TGF-β treatment somehow induced HGF secretion (Fig. 7D) and considered whether enhanced migration reflected silencing of RIN1 by TGF-β. As expected, shRNA-mediated silencing of RIN1 increased HGF-directed migration, but this had no effect on migration that was already enhanced by TGF-β (Fig. 7E). These results suggested that TGF-β increases HGF-directed migration primarily through SNAI1-mediated repression of RIN1 and persistence of RTK signaling. The specificity of these effects was confirmed by the observation that RIN1 silencing did not alter cell migration toward interleukin-8 (IL-8), a chemoattractant that utilizes a G-protein-coupled receptor, even in the presence of TGF-β (Fig. 7F).
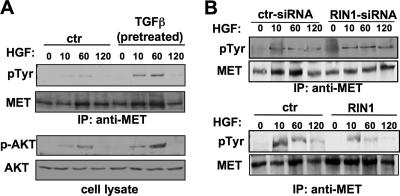
We next asked whether RIN1 could function as a negative regulator of MET in tumor cells. Pretreatment of MDA-MB-231 cells with TGF-β, which silences RIN1 expression (Fig. 5A and B), enhanced both autophosphorylation of MET and transphosphorylation of the downstream effector AKT (Fig. 8A) following HGF treatment. Indeed, in the absence of TGF-β, knockdown of RIN1 increased the levels of tyrosine-phosphorylated MET after HGF stimulation (Fig. 8B, top), a result consistent with the increased cell migration seen following RIN1 knockdown (Fig. 7A and E). Conversely, overexpression of RIN1 reduced levels of tyrosine-phosphorylated MET (Fig. 8B, bottom), again paralleling cell migration results (Fig. 7A and B). Taken together, these findings indicate that TGF-β increases growth factor-directed cell motility, at least in part, through silencing of the growth factor receptor down regulator RIN1.
FIG. 8.
HGF receptor (MET) signaling is affected by TGF-β and by altered RIN1 expression. (A) MDA-MB-231 cells were pretreated or not with TGF-β (5 ng/ml; 18 h) and switched to HGF medium for the indicated time. Lysates were immunoprecipitated with anti-MET and immunoblotted with anti-MET or anti-phosphotyrosine (pTyr). Lysate was also directly immunoblotted with anti-AKT or anti-phospho-AKT. (B) (Top) MDA-MB-231 cells were transfected with control (ctr) or RIN1-targeted siRNA (same as Fig. 7A, right) and treated with HGF for the indicated time. MET was immunoprecipitated and analyzed by immunoblotting for total MET and tyrosine-phosphorylated MET. (Bottom) MDA-MB-231 cells transduced with vector or a RIN1 expression construct (same as Fig. 7A, left) were analyzed for phospho-MET levels following HGF treatment.
DISCUSSION
Mechanism of receptor signaling regulation by RIN1.
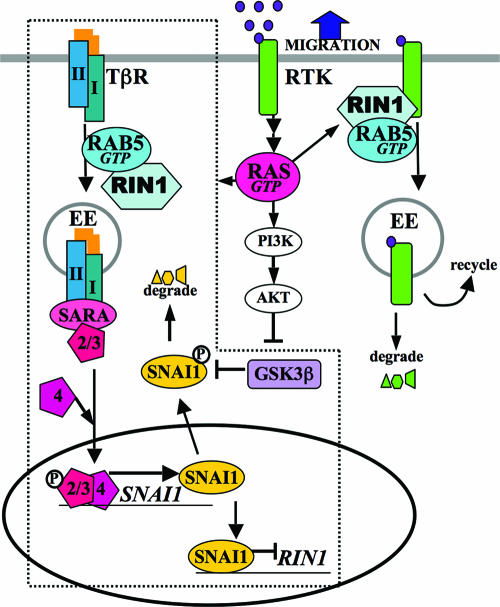
Our findings support a model in which RIN1, through the activation of RAB5, directs TβRs into an endocytic pathway that promotes TGF-β signaling through SMADs. This appears to be a self-regulated pathway, as increased SMAD2/3 activation leads to SNAI1 induction and RIN1 silencing (Fig. 9, dotted line). The SNAI1-mediated repression of a protein trafficking regulator such as RIN1, while novel, is consistent with the epithelial-to-meshenchymal transition program imposed by Snail proteins (3). Lower levels of RIN1, in turn, reduce TβR signaling efficiency. We propose that feedback regulation of TβR signaling is normally dynamic, with SNAI1 being phosphorylated and degraded to reset the cycle. In transformed cells, however, activated RAS decreases GSK3β activity, stabilizing SNAI1 and causing persistent RIN1 silencing.
FIG. 9.
Model for integration of TGF-β and RTK signaling through RIN1. RIN1 facilitates TβR internalization through RAB5-mediated, clathrin-dependent endocytosis, which enhances TβR signaling through the adaptor SARA, SMAD2/3 phosphorylation and association with SMAD4, and induction of SNAI1. SNAI1 represses RIN1, leading to reduced TβR signaling and creating a self-regulated circuit (enclosed by dotted lines). Endocytosis of RTKs (e.g., EGFR and MET), facilitated by RIN1 through RAB5, curtails downstream signaling through these receptors. TβR and RTK signals cooperate to strongly silence RIN1 expression through SNAI1 transcription (TβR-SMAD) and SNAI1 protein stabilization (GSK3β). Depletion of RIN1 enhances RTK signal intensity and promotes growth factor-directed migration of tumor cells. PI3K, phosphatidylinositol 3-kinase.
RIN1 promotes clathrin-dependent endocytosis of RTKs through direct binding to activated receptors and stimulation of RAB5 proteins. This serves principally as a down regulation pathway leading to reduced signaling by these receptors. Since RTKs activate RAS proteins, which further stimulate RIN1 function, this pathway has features of a self-regulating circuit. Indeed, the RAS interaction mutant RIN1K642A appears to be partly compromised for EGFR down regulation. In addition, our results suggest that RIN1-activated endocytosis of MET and EGFR primarily serves to down regulate RTK signaling and does not seem compatible with receptor recycling or endosome-based signaling. This is in agreement with previous studies that also suggest that RIN1 facilitates RTK signal attenuation (2, 34, 50). The enhancement of TβR signaling by RIN1, however, makes clear that RIN1-mediated endocytosis is not inextricably linked to receptor degradation. Multiple endocytosis complexes, each containing RIN1 and RAB5 but with other distinct components, may help explain different outcomes during and following internalization. Other RAB5 GEFs (9) are likely to add further nuances to the regulation of receptor signaling.
Integration of TGF-β and RTK signaling.
TGF-β is a key regulator of epithelial-mesenchymal transition, which is characterized by loss of polarity and increased cell motility. TβR/SMAD and RTK/RAS signaling pathways cooperate to regulate epithelial-mesenchymal transition through several different mechanisms (11, 31). We have uncovered a cooperative interaction between RTK/RAS and TGF-β pathways through RIN1-mediated receptor endocytosis. RAS signaling stabilizes TGF-β-induced SNAI1, leading to persistent silencing of RIN1 and prolonged RTK downstream signaling that promotes cell migration toward growth factors (Fig. 7). At the same time, silencing of RIN1 would reduce its contribution to ABL signaling pathways, which normally impede cytoskeleton remodeling required for cell movement (28) and alter tyrosine phosphorylation on a wider scale. The combined impact on directed migration may explain the high rate of RIN1 silencing in breast tumor cells (41).
Our observation that repression of RIN1 by TGF-β leads to less RTK endocytosis and more RTK signaling is consistent with a role for RIN1 silencing in tumor progression. Indeed, reduced endocytosis correlates with the role of MET in tumor progression (25) and may be a contributing factor in the tumorigenicity of amplified ERBB2 (54) and mutant EGFR (29). Conversely, increasing receptor internalization has been suggested as a form of cancer therapy (21).
TGF-β promotes the metastatic spread of late-stage tumor cells in part by stimulating secretion of metastatic factors such as IL-11 and CTGF (33) and by regulating the expression of genes involved in cell motility or invasion (40). RIN1 silencing appears to enhance factor-directed migration of tumor cells. This defines a new mechanism directly linking the promigratory effects of TGF-β to the growth factor responsiveness of RTKs. Our proposed model also suggests a mechanism through which TGF-β, by cooperating with RAS signaling, can evoke disparate effects in normal versus transformed cells.
Acknowledgments
We thank the following for technical assistance: Bartholomew Dzudzor, Roman Kleynberg, and Suman Machinani. We are also grateful to Guangpu Li, Ayyappan Rajasekaran, Greg Payne, Jose Esteban, Dafna Bar-Sagi, Sandy Schmid, Channing Der, and Francis Barr for sharing reagents and advice.
This work was supported by NIH grant NS046787 (J.C.), DoD grant W81XWH-04-1-0443 (J.C.), and CBCRP predoctoral fellowship 11GB-0038 (M.M.).
Footnotes
Published ahead of print on 26 December 2007.
REFERENCES
- 1.Barbieri, M. A., S. Fernandez-Pol, C. Hunker, B. H. Horazdovsky, and P. D. Stahl. 2004. Role of rab5 in EGF receptor-mediated signal transduction. Eur. J. Cell Biol. 83305-314. [DOI] [PubMed] [Google Scholar]
- 2.Barbieri, M. A., C. Kong, P. I. Chen, B. F. Horazdovsky, and P. D. Stahl. 2003. The SRC homology 2 domain of Rin1 mediates its binding to the epidermal growth factor receptor and regulates receptor endocytosis. J. Biol. Chem. 27832027-32036. [DOI] [PubMed] [Google Scholar]
- 3.Barrallo-Gimeno, A., and M. A. Nieto. 2005. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development 1323151-3161. [DOI] [PubMed] [Google Scholar]
- 4.Benmerah, A., M. Bayrou, N. Cerf-Bensussan, and A. Dautry-Varsat. 1999. Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J. Cell Sci. 1121303-1311. [DOI] [PubMed] [Google Scholar]
- 5.Birchmeier, C., W. Birchmeier, E. Gherardi, and G. F. Vande Woude. 2003. Met, metastasis, motility and more. Nat. Rev. Mol. Cell Biol. 4915-925. [DOI] [PubMed] [Google Scholar]
- 6.Brown, T. C., I. C. Tran, D. S. Backos, and J. A. Esteban. 2005. NMDA receptor-dependent activation of the small GTPase Rab5 drives the removal of synaptic AMPA receptors during hippocampal LTD. Neuron 4581-94. [DOI] [PubMed] [Google Scholar]
- 7.Camoretti-Mercado, B., D. J. Fernandes, S. Dewundara, J. Churchill, L. Ma, P. C. Kogut, J. F. McConville, M. S. Parmacek, and J. Solway. 2006. Inhibition of transforming growth factor beta-enhanced serum response factor-dependent transcription by SMAD7. J. Biol. Chem. 28120383-20392. [DOI] [PubMed] [Google Scholar]
- 8.Cano, A., M. A. Perez-Moreno, I. Rodrigo, A. Locascio, M. J. Blanco, M. G. del Barrio, F. Portillo, and M. A. Nieto. 2000. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 276-83. [DOI] [PubMed] [Google Scholar]
- 9.Carney, D. S., B. A. Davies, and B. F. Horazdovsky. 2006. Vps9 domain-containing proteins: activators of Rab5 GTPases from yeast to neurons. Trends Cell Biol. 1627-35. [DOI] [PubMed] [Google Scholar]
- 10.Chen, C. R., Y. Kang, and J. Massague. 2001. Defective repression of c-myc in breast cancer cells: a loss at the core of the transforming growth factor beta growth arrest program. Proc. Natl. Acad. Sci. USA 98992-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cordenonsi, M., M. Montagner, M. Adorno, L. Zacchigna, G. Martello, A. Mamidi, S. Soligo, S. Dupont, and S. Piccolo. 2007. Integration of TGF-{beta} and Ras/MAPK signaling through p53 phosphorylation. Science 315840-843. [DOI] [PubMed] [Google Scholar]
- 12.Dai, F., C. Chang, X. Lin, P. Dai, L. Mei, and X. H. Feng. 2007. Erbin inhibits transforming growth factor beta signaling through a novel Smad-interacting domain. Mol. Cell. Biol. 276183-6194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Damke, H., T. Baba, D. E. Warnock, and S. L. Schmid. 1994. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J. Cell Biol. 127915-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delprato, A., E. Merithew, and D. G. Lambright. 2004. Structure, exchange determinants, and family-wide rab specificity of the tandem helical bundle and Vps9 domains of Rabex-5. Cell 118607-617. [DOI] [PubMed] [Google Scholar]
- 15.Derynck, R., and R. J. Akhurst. 2007. Differentiation plasticity regulated by TGF-beta family proteins in development and disease. Nat. Cell Biol. 91000-1004. [DOI] [PubMed] [Google Scholar]
- 16.Derynck, R., R. J. Akhurst, and A. Balmain. 2001. TGF-beta signaling in tumor suppression and cancer progression. Nat. Genet. 29117-129. [DOI] [PubMed] [Google Scholar]
- 17.Dhaka, A., R. M. Costa, H. Hu, D. K. Irvin, A. Patel, H. I. Kornblum, A. J. Silva, T. J. O'Dell, and J. Colicelli. 2003. The RAS effector RIN1 modulates the formation of aversive memories. J. Neurosci. 23748-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Guglielmo, G. M., C. Le Roy, A. F. Goodfellow, and J. L. Wrana. 2003. Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat. Cell Biol. 5410-421. [DOI] [PubMed] [Google Scholar]
- 19.Elenbaas, B., L. Spirio, F. Koerner, M. D. Fleming, D. B. Zimonjic, J. L. Donaher, N. C. Popescu, W. C. Hahn, and R. A. Weinberg. 2001. Human breast cancer cells generated by oncogenic transformation of primary mammary epithelial cells. Genes Dev. 1550-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Felici, A., J. U. Wurthner, W. T. Parks, L. R. Giam, M. Reiss, T. S. Karpova, J. G. McNally, and A. B. Roberts. 2003. TLP, a novel modulator of TGF-beta signaling, has opposite effects on Smad2- and Smad3-dependent signaling. EMBO J. 224465-4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedman, L. M., A. Rinon, B. Schechter, L. Lyass, S. Lavi, S. S. Bacus, M. Sela, and Y. Yarden. 2005. Synergistic down-regulation of receptor tyrosine kinases by combinations of mAbs: implications for cancer immunotherapy. Proc. Natl. Acad. Sci. USA 1021915-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gadir, N., D. N. Jackson, E. Lee, and D. A. Foster. 13 August 2007. Defective TGF-beta signaling sensitizes human cancer cells to rapamycin. Oncogene [Epub ahead of print.] doi: 10.1038/sj.onc.1210721. [DOI] [PubMed]
- 23.Grunert, S., M. Jechlinger, and H. Beug. 2003. Diverse cellular and molecular mechanisms contribute to epithelial plasticity and metastasis. Nat. Rev. Mol. Cell Biol. 4657-665. [DOI] [PubMed] [Google Scholar]
- 24.Haas, A. K., E. Fuchs, R. Kopajtich, and F. A. Barr. 2005. A GTPase-activating protein controls Rab5 function in endocytic trafficking. Nat. Cell Biol. 7887-893. [DOI] [PubMed] [Google Scholar]
- 25.Hammond, D. E., S. Carter, and M. J. Clague. 2004. Met receptor dynamics and signalling. Curr. Top. Microbiol. Immunol. 28621-44. [DOI] [PubMed] [Google Scholar]
- 26.Han, L., and J. Colicelli. 1995. A human protein selected for interference with Ras function interacts directly with Ras and competes with Raf1. Mol. Cell. Biol. 151318-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayes, S., A. Chawla, and S. Corvera. 2002. TGF beta receptor internalization into EEA1-enriched early endosomes: role in signaling to Smad2. J. Cell Biol. 1581239-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu, H., J. M. Bliss, Y. Wang, and J. Colicelli. 2005. RIN1 is an ABL tyrosine kinase activator and a regulator of epithelial-cell adhesion and migration. Curr. Biol. 15815-823. [DOI] [PubMed] [Google Scholar]
- 29.Huang, H. S., M. Nagane, C. K. Klingbeil, H. Lin, R. Nishikawa, X. D. Ji, C. M. Huang, G. N. Gill, H. S. Wiley, and W. K. Cavenee. 1997. The enhanced tumorigenic activity of a mutant epidermal growth factor receptor common in human cancers is mediated by threshold levels of constitutive tyrosine phosphorylation and unattenuated signaling. J. Biol. Chem. 2722927-2935. [DOI] [PubMed] [Google Scholar]
- 30.Jakowlew, S. B. 2006. Transforming growth factor-beta in cancer and metastasis. Cancer Metastasis Rev. 25435-457. [DOI] [PubMed] [Google Scholar]
- 31.Janda, E., M. Nevolo, K. Lehmann, J. Downward, H. Beug, and M. Grieco. 2006. Raf plus TGFbeta-dependent EMT is initiated by endocytosis and lysosomal degradation of E-cadherin. Oncogene 257117-7130. [DOI] [PubMed] [Google Scholar]
- 32.Jonk, L. J., S. Itoh, C. H. Heldin, P. ten Dijke, and W. Kruijer. 1998. Identification and functional characterization of a Smad binding element (SBE) in the JunB promoter that acts as a transforming growth factor-beta, activin, and bone morphogenetic protein-inducible enhancer. J. Biol. Chem. 27321145-21152. [DOI] [PubMed] [Google Scholar]
- 33.Kang, Y., P. M. Siegel, W. Shu, M. Drobnjak, S. M. Kakonen, C. Cordon-Cardo, T. A. Guise, and J. Massague. 2003. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 3537-549. [DOI] [PubMed] [Google Scholar]
- 34.Kong, C., X. Su, P. I. Chen, and P. D. Stahl. 2007. RIN1 interacts with signal transducing adaptor molecule (STAM) and mediates epidermal growth factor receptor trafficking and degradation. J. Biol. Chem. 28215294-15301. [DOI] [PubMed] [Google Scholar]
- 35.Leong, G. M., N. Subramaniam, J. Figueroa, J. L. Flanagan, M. J. Hayman, J. A. Eisman, and A. P. Kouzmenko. 2001. Ski-interacting protein interacts with Smad proteins to augment transforming growth factor-beta-dependent transcription. J. Biol. Chem. 27618243-18248. [DOI] [PubMed] [Google Scholar]
- 36.Le Roy, C., and J. L. Wrana. 2005. Signaling and endocytosis: a team effort for cell migration. Dev. Cell 9167-168. [DOI] [PubMed] [Google Scholar]
- 37.Li, G., and P. D. Stahl. 1993. Structure-function relationship of the small GTPase rab5. J. Biol. Chem. 26824475-24480. [PubMed] [Google Scholar]
- 38.Lin, H. K., S. Bergmann, and P. P. Pandolfi. 2004. Cytoplasmic PML function in TGF-beta signalling. Nature 431205-211. [DOI] [PubMed] [Google Scholar]
- 39.Martinu, L., A. Santiago-Walker, H. Qi, and M. M. Chou. 2002. Endocytosis of epidermal growth factor receptor regulated by Grb2-mediated recruitment of the Rab5 GTPase-activating protein RN-tre. J. Biol. Chem. 27750996-51002. [DOI] [PubMed] [Google Scholar]
- 40.Michl, P., A. R. Ramjaun, O. E. Pardo, P. H. Warne, M. Wagner, R. Poulsom, C. D'Arrigo, K. Ryder, A. Menke, T. Gress, and J. Downward. 2005. CUTL1 is a target of TGF(beta) signaling that enhances cancer cell motility and invasiveness. Cancer Cell 7521-532. [DOI] [PubMed] [Google Scholar]
- 41.Milstein, M., C. K. Mooser, H. Hu, M. Fejzo, D. Slamon, L. Goodglick, S. Dry, and J. Colicelli. 2007. RIN1 is a breast cancer suppressor gene. Cancer Res. 6711510-11516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peinado, H., M. Quintanilla, and A. Cano. 2003. Transforming growth factor beta-1 induces snail transcription factor in epithelial cell lines: mechanisms for epithelial mesenchymal transitions. J. Biol. Chem. 27821113-21123. [DOI] [PubMed] [Google Scholar]
- 43.Penheiter, S. G., H. Mitchell, N. Garamszegi, M. Edens, J. J. Dore, Jr., and E. B. Leof. 2002. Internalization-dependent and -independent requirements for transforming growth factor beta receptor signaling via the Smad pathway. Mol. Cell. Biol. 224750-4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Polo, S., and P. P. Di Fiore. 2006. Endocytosis conducts the cell signaling orchestra. Cell. 124897-900. [DOI] [PubMed] [Google Scholar]
- 45.Puri, V., R. Watanabe, R. D. Singh, M. Dominguez, J. C. Brown, C. L. Wheatley, D. L. Marks, and R. E. Pagano. 2001. Clathrin-dependent and -independent internalization of plasma membrane sphingolipids initiates two Golgi targeting pathways. J. Cell Biol. 154535-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Razani, B., X. L. Zhang, M. Bitzer, G. von Gersdorff, E. P. Bottinger, and M. P. Lisanti. 2001. Caveolin-1 regulates transforming growth factor (TGF)-beta/SMAD signaling through an interaction with the TGF-beta type I receptor. J. Biol. Chem. 2766727-6738. [DOI] [PubMed] [Google Scholar]
- 47.Runyan, C. E., H. W. Schnaper, and A. C. Poncelet. 2005. The role of internalization in transforming growth factor beta1-induced Smad2 association with Smad anchor for receptor activation (SARA) and Smad2-dependent signaling in human mesangial cells. J. Biol. Chem. 2808300-8308. [DOI] [PubMed] [Google Scholar]
- 48.Siegel, P. M., W. Shu, R. D. Cardiff, W. J. Muller, and J. Massague. 2003. Transforming growth factor beta signaling impairs Neu-induced mammary tumorigenesis while promoting pulmonary metastasis. Proc. Natl. Acad. Sci. USA 1008430-8435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Skretting, G., M. L. Torgersen, B. van Deurs, and K. Sandvig. 1999. Endocytic mechanisms responsible for uptake of GPI-linked diphtheria toxin receptor. J. Cell Sci. 1123899-3909. [DOI] [PubMed] [Google Scholar]
- 50.Tall, G. G., M. A. Barbieri, P. D. Stahl, and B. F. Horazdovsky. 2001. Ras-activated endocytosis is mediated by the Rab5 guanine nucleotide exchange activity of RIN1. Dev. Cell 173-82. [DOI] [PubMed] [Google Scholar]
- 51.van der Bliek, A. M., T. E. Redelmeier, H. Damke, E. J. Tisdale, E. M. Meyerowitz, and S. L. Schmid. 1993. Mutations in human dynamin block an intermediate stage in coated vesicle formation. J. Cell Biol. 122553-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang, Y., R. T. Waldron, A. Dhaka, A. Patel, M. M. Riley, E. Rozengurt, and J. Colicelli. 2002. The RAS effector RIN1 directly competes with RAF and is regulated by 14-3-3 proteins. Mol. Cell. Biol. 22916-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wohlgemuth, S., C. Kiel, A. Kramer, L. Serrano, F. Wittinghofer, and C. Herrmann. 2005. Recognizing and defining true Ras binding domains I: biochemical analysis. J. Mol. Biol. 348741-758. [DOI] [PubMed] [Google Scholar]
- 54.Worthylake, R., L. K. Opresko, and H. S. Wiley. 1999. ErbB-2 amplification inhibits down-regulation and induces constitutive activation of both ErbB-2 and epidermal growth factor receptors. J. Biol. Chem. 2748865-8874. [DOI] [PubMed] [Google Scholar]
- 55.Wrighton, K. H., D. Willis, J. Long, F. Liu, X. Lin, and X. H. Feng. 2006. Small C-terminal domain phosphatases dephosphorylate the regulatory linker regions of Smad2 and Smad3 to enhance transforming growth factor-beta signaling. J. Biol. Chem. 28138365-38375. [DOI] [PubMed] [Google Scholar]
- 56.Wu, S. P., D. Theodorescu, R. S. Kerbel, J. K. Willson, K. M. Mulder, L. E. Humphrey, and M. G. Brattain. 1992. TGF-beta 1 is an autocrine-negative growth regulator of human colon carcinoma FET cells in vivo as revealed by transfection of an antisense expression vector. J. Cell Biol. 116187-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang, S., T. Fei, L. Zhang, R. Zhang, F. Chen, Y. Ning, Y. Han, X. H. Feng, A. Meng, and Y. G. Chen. 2007. Smad7 antagonizes transforming growth factor beta signaling in the nucleus by interfering with functional Smad-DNA complex formation. Mol. Cell. Biol. 274488-4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou, B. P., J. Deng, W. Xia, J. Xu, Y. M. Li, M. Gunduz, and M. C. Hung. 2004. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat. Cell Biol. 6931-940. [DOI] [PubMed] [Google Scholar]
- 59.Zhu, S., W. Wang, D. C. Clarke, and X. Liu. 2007. Activation of Mps1 promotes transforming growth factor-beta-independent Smad signaling. J. Biol. Chem. 28218327-18338. [DOI] [PubMed] [Google Scholar]