Abstract
β1Pix is a guanine nucleotide exchange factor (GEF) for the small GTPases Rac and Cdc42 which has been shown to mediate signaling pathways leading to cytoskeletal reorganization. In the present study, we show that the basal association between endogenous βPix and endogenous 14-3-3β was increased after forskolin stimulation and significantly inhibited by protein kinase A inhibitor. However, forskolin stimulation failed to increase the interaction between 14-3-3β and a β1Pix mutant that is insensitive to protein kinase A phosphorylation, β1Pix(S516A, T526A). We present evidence indicating that forskolin-induced binding of 14-3-3β to β1Pix results in inhibition of Rac1 GTP loading in 293 cells and in vitro. Furthermore, we show that deletion of 10 amino acid residues within the leucine zipper domain is sufficient to block β1Pix homodimerization and 14-3-3β binding and modulates β1Pix-GEF activity. These residues also play a crucial role in β1Pix intracellular localization. These results indicate that 14-3-3β negatively affects the GEF activity of dimeric β1Pix only. Altogether, these results provide a mechanistic insight into the role of 14-3-3β in modulating β1Pix-GEF activity.
Activation of Rho GTPases depends on the coordinated action of guanine nucleotide exchange factors (GEFs). β1Pix was identified as a p21-activated kinase (Pak)-interacting exchange factor and was shown to be a GEF for Cdc42 and Rac1 (2, 19). Rho-GEFs activate Rho GTPases by catalyzing the exchange of GDP with GTP at the nucleotide binding site. In addition to Dbl homology (DH) and plackstrin homology (PH) domains, β1Pix contains a Src homology 3 (SH3) domain responsible for binding Pak through a proline-rich region (1, 19). β1Pix also has a leucine zipper domain for homodimerization (16) and a GIT1 (G protein-coupled receptor kinase interactor 1) binding domain (1). β1Pix also regulates signaling pathways leading to cytoskeletal reorganization through its interaction with paxillin and other adhesion proteins (29). Furthermore, β1Pix has been shown to mediate reactive oxygen species generation through sequential activation of phosphatidylinositol 3-kinase and Rac1 (22). More recently we showed that PKA-dependent phosphorylation of β1Pix on Ser516 and Thr526 regulates β1Pix translocation to focal adhesion (7). The interaction of β1Pix with a variety of signaling molecules may be indicative of the important role of β1Pix in mediating different signaling pathways that convert extracellular stimuli to a biological response affecting cytoskeletal rearrangement. The activation of Rho-GEF by extracellular agonists has been studied extensively; however, little is known about how β1Pix-GEF activity is modulated to enable the propagation of the signal to downstream effectors.
Mass spectrometry analysis of proteins that associate with 14-3-3s revealed that βPix can bind 14-3-3 proteins (15). In our study, we have further explored the interaction between 14-3-3β and β1Pix using coimmunoprecipitation studies. Indeed, we show that endogenous 14-3-3β and βPix interact and this interaction is increased by forskolin through the protein kinase A (PKA)-dependent pathway. Most interestingly, we found that a mutant of β1Pix, β1Pix(S516A, T526A), impaired in its ability to undergo PKA-dependent phosphorylation, was also unable to bind 14-3-3β in response to forskolin. Homodimerization of β1Pix is required for 14-3-3β binding, and β1Pix dimerization plays a key role in its localization. Finally, we show that PKA-dependent recruitment of 14-3-3β inhibits both β1Pix-GEF activity in vitro and Rac1 signaling in 293 cells. These findings provide a mechanistic explanation on how PKA-dependent phosphorylation modulates β1Pix-GEF activity through 14-3-3β recruitment.
MATERIALS AND METHODS
Cell culture, transfection, and plasmids.
HEK-293 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) supplemented with 10% fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 μg/ml) in a 37°C humidified incubator with 5% CO2. Transient transfection of cells with mammalian expression vectors was performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. After transfection, cells were grown for 24 h in DMEM containing 3% serum before stimulation with forskolin (20 μM) for 15 min in the presence of 3-isobutyl-1-methylxanthine (IBMX) (200 μM).
The Myc-tagged β1Pix and β1Pix(S516A, T526A) plasmids have been described (7). β1Pix was cloned into the Flag pCMV vector (Stratagene). Flag-β1Pix(S516A, T526A), Myc-β1Pix(S516E, T526E), β1PixΔ(547-586), β1PixΔ(587-626), and β1PixΔ(602-611) were generated using the QuikChange site-directed mutagenesis kit (Stratagene). β1Pix and β1PixΔ(602-611) were cloned into the Flag pCMV vector. 14-3-3β was cloned into the Flag pCMV vector, and 14-3-3β(K51E, R58E, R62E) was generated using the QuikChange site-directed mutagenesis kit. The mutations were verified by automatic DNA sequencing (Applied Biosystems).
Purification of recombinant 14-3-3β and its mutant.
14-3-3β and 14-3-3β(K51E, R58E, R62E) were expressed as N-terminal His6 tag fusion proteins in bacteria [BL21(DE3)]. Recombinant proteins expressed in bacteria were purified using the nickel-nitrilotriacetic acid purification system (Invitrogen). Recombinant proteins were eluted from the resin with 250 mM imidazole at room temperature.
Immunoprecipitation and Western blot analysis.
Cells were transfected with the appropriate construct for 24 h. Cells were washed twice in phosphate-buffered saline (PBS) and lysed in lysis buffer containing 20 mM Tris, pH 7.5, 100 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 1% Triton X-100, 1 mM sodium fluoride, 1 mM sodium vanadate, 1 mM phenylmethylsulfonyl fluoride, 1 μg/ml pepstatin, and 1 μg/ml leupeptin. Equal amounts of proteins were separated by using 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, electrophoretically transferred onto a polyvinylidene difluoride membrane (Millipore), immunoblotted with the appropriate antibody, and visualized by enhanced chemiluminescence (ECL; Amersham Biosciences, Inc.). For immunoprecipitation, antibodies against 14-3-3β or Myc (Santa Cruz Biotechnologies) were added to the cell lysate (500 μg) for 2 h of incubation, followed by addition of protein A- or protein G-agarose beads for an additional hour. The beads were washed three times in PBS. The immunoprecipitated proteins were released from the beads by boiling in 1× sample buffer for 5 min and subsequently analyzed by Western blotting. Total cell lysate was run to assess the overexpression of the constructs. Expression of recombinant Myc-β1Pix and its mutants was verified by immunoblotting with anti-Myc antibody.
Cdc42/Rac1 activity assay.
Cells were transfected with empty vector, Myc-tagged β1Pix, or its mutants for 24 h. After stimulation with forskolin, cells were lysed in lysis/wash buffer (25 mM HEPES, pH 7.5, 150 mM NaCl, 1% Igepal CA-630, 10 mM MgCl2, 1 mM EDTA, 1% glycerol, 10 μg/ml leupeptin, and 10 μg/ml aprotinin). To measure the active GTP-bound form of Cdc42 or Rac1 GTPases in the cell lysates, we performed a pulldown assay (Upstate) using recombinant glutathione S-transferase (GST)-p21 binding domain (PBD). Aliquots (500 μg) of the supernatants were incubated with 10 μg of GST-PBD for 1 h and precipitated by centrifugation. Complexes were boiled in a Laemmli sample buffer and then separated on 15% sodium dodecyl sulfate-polyacrylamide gels. The separated proteins were immunoblotted using a monoclonal anti-Cdc42 or anti-Rac1 antibody.
GDP/GTP exchange assays.
The exchange assays were performed as previously described (10). For γ-35S-GTP binding, 2 μg of recombinant His-Rac1 (Cytoskeleton) was initially incubated for 5 min in 60 μl of loading buffer containing 10 μM of GDP at room temperature. MgCl2 was then added to a final concentration of 5 mM and the incubation continued for an additional 15 min. Twenty microliters of GDP-loaded Rac1 was mixed with immunoprecipitates from 250 μg of lysates from cells expressing Myc-β1Pix or Myc-β1Pix(S516A, T526A) diluted in reaction buffer containing γ-35S-GTP to initiate the exchange reaction at room temperature. Twenty microliters of each sample was taken at various time points from the reaction mixture and added to 10 ml of ice-cold PBS. After washing, the filters were placed in scintillation fluid (Fisher Scientific) and counted with the use of a TRI-CARB 2100TR liquid scintillation counter (Packard).
Immunofluorescence.
Cells were seeded on glass coverslips housed in a 12-well plate for 2 days and transfected with Myc-β1Pix or Myc-β1Pix(S516A, T526A) in serum-free DMEM for 24 h. Cells were left unstimulated or stimulated with forskolin (20 μM) for 15 min, washed in PBS, and fixed in 4% paraformaldehyde for 15 min. Cells were then washed, permeabilized with 0.2% Triton X-100 for 5 min, and incubated in 10% goat serum for 45 min. Primary antibody against Myc (Santa Cruz) was diluted 1:1,000 and incubated with cells for 1 h. Cells were washed several times with PBS, followed by a 1-h incubation with Alexa Fluor 488 secondary antibody (Molecular Probes) diluted 1:1,000. For F-actin staining, cells were incubated with rhodamine-phalloidin for 45 min. Cells were equilibrated and mounted in ProLong antifade reagent (Molecular Probes). Cells were visualized under a fluorescence microscope (E600; Nikon) and photographed using the SPOT system (Diagnostic Instruments). Cells of roughly equal and average fluorescent intensities were chosen as comparative examples.
RESULTS
Identification of 14-3-3β binding sites on β1Pix.
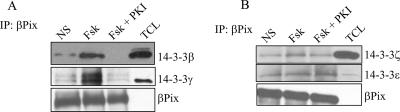
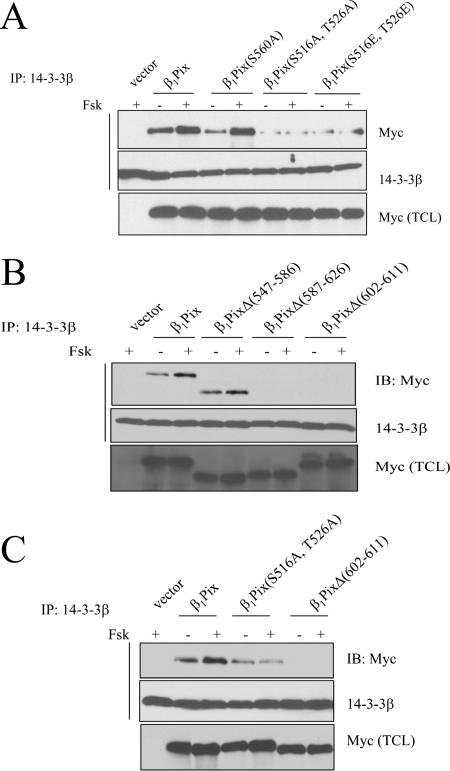
Using coimmunoprecipitation experiments with HEK293 cells, we showed that endogenous βPix was able to coimmunoprecipitate endogenous 14-3-3β, -γ, -ζ, and -ɛ (Fig. 1). Forskolin stimulation increased the interaction of βPix with 14-3-3β and -γ (Fig. 1A) but not that of βPix with 14-3-3ζ or 14-3-3ɛ (Fig. 1B), indicating that this increase is mediated by PKA. Indeed, the myristoylated PKA inhibitor, PKI, strongly inhibited the interaction between βPix and 14-3-3β and -γ (Fig. 1A) but had no effect on the interaction between βPix and 14-3-3ζ and -ɛ (Fig. 1B). Using HEK293 cells transiently transfected either with empty vector or with vector encoding Myc-tagged β1Pix, we confirmed that β1Pix interaction with 14-3-3β is increased after forskolin stimulation and strongly inhibited by PKA inhibitor (data not shown). The interaction of 14-3-3s with target proteins is often mediated by the recognition of consensus-binding motifs, RSXpSXP and RXY/FXpSXP (where pS is the phosphorylated amino acid) (31). However, several 14-3-3 binding sites have been shown to be different from these consensus motifs (23). A different recognition motif also exists when 14-3-3s bind unphosphorylated proteins (11, 13, 24). Analysis of the primary sequence of β1Pix showed Ser560 (RKES560AP) as a potential 14-3-3β binding site. The fact that forskolin increased binding of both wild-type β1Pix and β1Pix(S560A) to 14-3-3β (Fig. 2A, compare lane 3 to lane 5) indicates that this consensus motif does not mediate forskolin-induced 14-3-3β binding to β1Pix. We have previously shown that PKA phosphorylates β1Pix on Ser516 and Thr526 and regulates its signaling (7). In the present study, the mutation of these amino acids to Ala abolished the forskolin-induced increase of 14-3-3β binding to β1Pix, although it did not completely inhibit the basal interaction between 14-3-3β and β1Pix (Fig. 2A, lanes 6 and 7). Therefore, our results indicate that the stimulated increase of 14-3-3β binding to β1Pix is mediated through PKA phosphorylation of β1Pix on Ser516 and Thr526. In order to confirm the role of this phosphorylation in 14-3-3β binding, we generated a β1Pix(S516E, T526E) mutant that mimics the negatively charged residues of phosphorylated Ser516 and Thr526. The amount of 14-3-3β bound to β1Pix(S516E, T526E) was comparable to that bound to β1Pix(S516A, T526A) (Fig. 2A), indicating that the phosphorylation of these amino acid residues is required for 14-3-3β binding. It appears that in the case of β1Pix, phosphorylation at serine and threonine rather than a negative charge is necessary for stable interaction with 14-3-3β. In order to identify 14-3-3β binding site responsible for the basal interaction between β1Pix and 14-3-3β, several truncated β1Pix mutants were generated. Coimmunoprecipitation studies showed that while deletion of amino acids 547 to 586 had no effect on β1Pix-14-3-3β interaction, deletion of amino acids 587 to 626 or 602 to 611 completely inhibited the interaction between 14-3-3β and β1Pix (Fig. 2B). Our results showed that forskolin-induced binding of 14-3-3β requires the presence of amino acids 602 to 611 and Ser516 and Thr526 on β1Pix. Ser516 and Thr526 are located within an amino acid sequence that is different from the 14-3-3 binding consensus motif indicated above. While deletion of amino acids 602 to 611 completely prevented the binding of 14-3-3β to β1Pix, the mutation of Ser516 and Thr526 did not completely abolish the interaction between β1Pix and 14-3-3β (Fig. 2C).
FIG. 1.
Interaction between endogenous βPix and 14-3-3 isoforms. HEK293 cells were treated with IBMX (200 μM) for 30 min and then stimulated with forskolin (20 μM) for 15 min in the presence or absence of myristoylated PKI (2 μM). Cells lysates were subjected to immunoprecipitation using anti-βPix antibody, and the membranes were blotted using antibodies against 14-3-3β and -γ (A) or 14-3-3ζ and -ɛ (B). Immunoprecipitated 14-3-3 and bound βPix were detected using anti-14-3-3s and anti-βPix antibodies, respectively. TCL, total cell lysate.
FIG. 2.
Identification of 14-3-3β binding site on β1Pix. (A) Lysates from cells expressing β1Pix, β1Pix(S560A), β1Pix(S516A, T526A), or β1Pix(S516E, T526E) were immunoprecipitated with anti-14-3-3β and subjected to Western blot analysis with anti-Myc or anti-14-3-33-3β. (B) Lysates from cells expressing β1Pix, β1PixΔ(547-586), β1PixΔ(587-626), or β1PixΔ(602-611) were immunoprecipitated with anti-14-3-3β, and Western blots were analyzed as indicated above. (C) Cells expressing β1Pix, β1Pix(S516A, T526A), or β1PixΔ(602-611) were immunoprecipitated with anti-14-3-3β, and the Western blots were revealed as indicated above. The experiment was repeated two additional times with similar results.
14-3-3β binding requires β1Pix homodimerization.
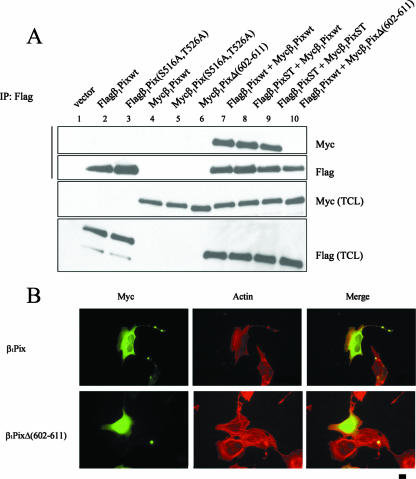
Based on the fact that the amino acid sequence of positions 602 to 611 is located within the LZ domain, which mediates β1Pix homodimerization (16), we tested the hypothesis that the deletion of this 14-3-3β binding site would also prevent β1Pix homodimerization. To answer this question, we transfected HEK293 cells with Flag-β1Pix, Myc-β1Pix, or both. We found that Myc-β1Pix coimmunoprecipitated with Flag-β1Pix only when both epitope-tagged forms of β1Pix were coexpressed (Fig. 3A, lane 7), confirming that β1Pix forms dimers (16). However, expression of Flag-β1Pix and the 14-3-3β binding-deficient mutant, Myc-β1PixΔ(602-611), failed to form a dimer (Fig. 3A, compare lanes 7 and 10). This result indicates that 14-3-3β binds only to the dimeric form of β1Pix and that deletion of these residues, 602 to 611, is sufficient to abolish β1Pix homodimerization. The expression of β1Pix(S516A, T526A) did not block the dimerization (Fig. 3A, compare lane 7 to lanes 8 and 9), indicating that β1Pix dimerization is independent of its phosphorylation status. A previous study showed that the coiled-coil region including the LZ domain is responsible for β1Pix dimerization and its localization to the cell periphery (17). Therefore, we sought to examine the effect of the dimerization-deficient β1Pix mutant on its localization. β1Pix showed a homogenous cytoplasmic distribution and also localized to the cell periphery at the membrane ruffles (Fig. 3B, upper panels), whereas β1PixΔ(602-611) was found in the cytoplasm and nucleus (Fig. 3B, lower panels). Our results confirm that β1Pix dimerization is essential to its subcellular localization.
FIG. 3.
14-3-3β binding requires β1Pix homodimerization. (A) HEK293 cells were transfected with different cDNA plasmids as indicated. Lysates were immunoprecipitated using anti-Flag antibody, and Western blots were analyzed with anti-Myc and anti-Flag antibodies. (B) Cells were transfected with Myc-β1Pix or Myc-β1PixΔ(602-611) in serum-free DMEM for 24 h. Myc distribution was visualized using an anti-Myc antibody, 9E10, and fluorescence-conjugated secondary antibody. Actin distribution was visualized with rhodamine-conjugated phalloidin. The bar represents 10 μM.
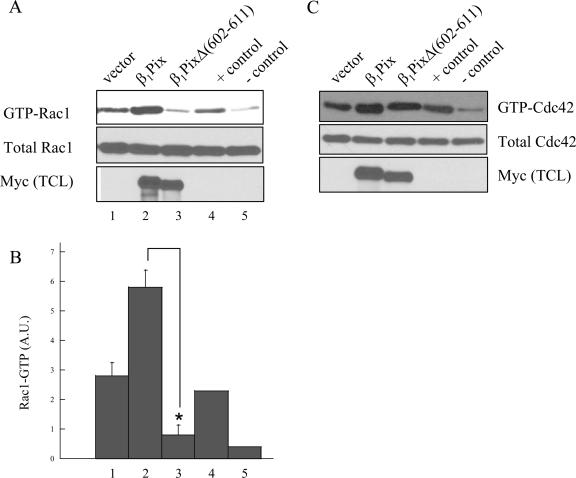
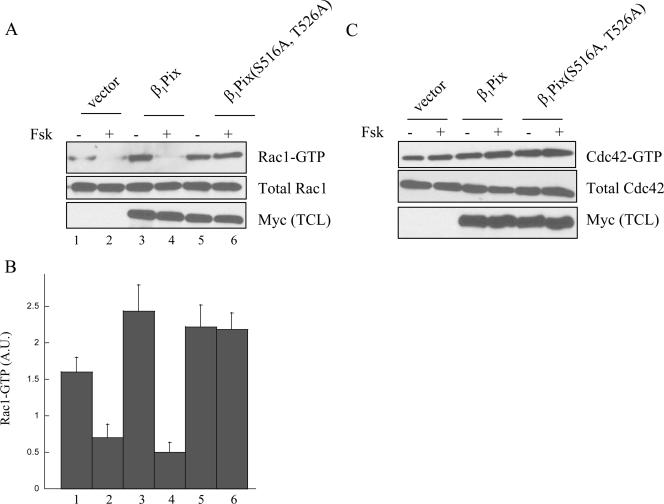
Recent reports showed that the dimerization could either activate or inhibit the activities of some GEFs. Dimerization is required for αPix to act as a GEF for Rac (12), while it inhibits the activity of AKAP-Lbc toward Rho (3). In order to assess the effect of dimerization on β1Pix-GEF activity toward Rac1, cells were transfected with β1Pix or β1PixΔ(602-611) under serum-free conditions and their basal GEF activity was determined using the GST-PBD pulldown assay. Cells expressing empty vector displayed a relatively high basal level of active Rac1, which was further increased in cells expressing β1Pix (Fig. 4A). However, in cells expressing the dimerization-deficient β1Pix mutant, β1PixΔ(602-611), the basal β1Pix-GEF activity is significantly inhibited. Interestingly, a similar experiment showed that the dimerization-deficient β1Pix mutant did not inhibit Cdc42 activity (Fig. 4C). This result shows that dimerization is required to maintain a high basal β1Pix-GEF activity toward Rac1 but not that of Cdc42.
FIG. 4.
Rac1 activation requires β1Pix homodimerization. Cells were transfected with empty vector, Myc-β1Pix, or Myc-β1PixΔ(602-611) in serum-free DMEM for 24 h. GTP-bound Rac1 (A) and GTP-bound Cdc42 (C) were measured using a pulldown assay as described in Materials and Methods. Positive and negative controls show the GTP-bound Rac1 (or Cdc42) in the presence of 100 μM γ-S-GTP or GDP, respectively. Anti-Myc (bottom) shows the expression of β1Pix constructs. (B) Quantitative analysis of Rac1-GTP bands was obtained by densitometry. Results are expressed as means ± standard errors for three independent experiments. Statistical significance was analyzed by a paired Student's t test. *, P < 0.05 compared with Rac1-GTP levels measured in cells transfected with β1Pix.
14-3-3β binding inhibits β1Pix-GEF activity in vitro and inside cells.
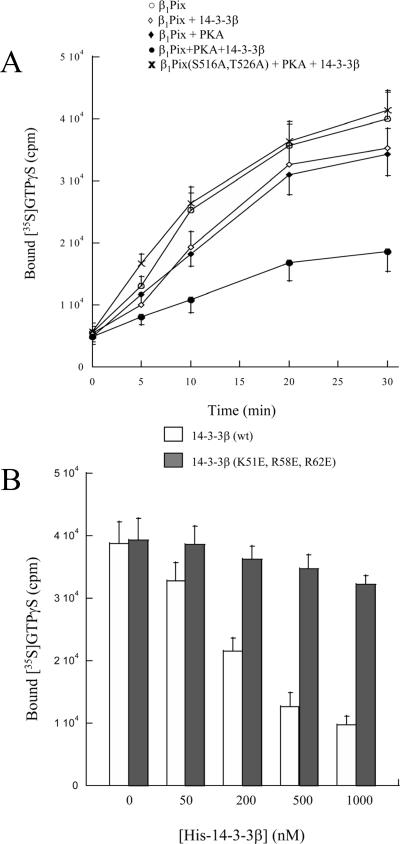
To determine the biological significance of 14-3-3β binding to β1Pix and its regulation through PKA-dependent phosphorylation, we sought to investigate the effect of forskolin stimulation on Cdc42 and Rac1 activity inside the cells. Cells expressing β1Pix or β1Pix(S516A, T526) were left untreated or stimulated with forskolin. To measure Cdc42/Rac1 activity, we used GST-PBD beads to pull down the GTP-bound form of these GTPases (active). Forskolin stimulation strongly decreased Rac1 activation both in control cells and in cells expressing β1Pix, whereas forskolin had no effect on Rac1 activity in cells expressing β1Pix(S516A, T526A), impaired in its ability to undergo PKA-dependent phosphorylation (Fig. 5). Cdc42 activity was not affected by forskolin stimulation in cells expressing β1Pix or β1Pix(S516A, T526A), indicating that in HEK293 cells, forskolin had no effect on Cdc42 activity. Our result indicates that forskolin-induced Rac1 inhibition is mediated by PKA-dependent phosphorylation of β1Pix on Ser516 and Thr526. It also suggests that 14-3-3β binding results in the inhibition of β1Pix-GEF activity. In the presence of PKA, addition of purified 14-3-3β resulted in a time-dependent inhibition of β1Pix-GEF activity (Fig. 6A). In the absence of PKA, addition of GST-14-3-3β had almost no inhibitory effect on β1Pix-GEF activity, indicating the role of PKA-dependent phosphorylation of β1Pix. The GEF activity of the β1Pix mutant, β1Pix(S516A, T526A), was unaffected by addition of purified GST-14-3-3β (Fig. 6A), confirming the role of Ser516 and Thr526 in PKA-mediated 14-3-3β binding to β1Pix. Moreover, addition of increasing concentrations of purified His-14-3-3β inhibited β1Pix guanine nucleotide exchange activity in vitro in a dose-dependent manner (Fig. 6B). To demonstrate that the binding is the mechanism by which 14-3-3β inhibits β1Pix-GEF activity, we used a dominant-negative form of 14-3-3β that is unable to bind its target proteins (36). Dominant-negative forms of 14-3-3s were first identified by using loss of function of 14-3-3ɛ mutants (8). The mutation of the conserved arginines at positions 56 and 60 on 14-3-3η results in the loss of Raf-1 phosphorylation (28). In addition, lysine at position 49 is also important in Raf-1 interaction (36). The corresponding lysine and arginine residues are found on 14-3-3β at positions 51, 58, and 62, respectively (32). Addition of the purified 14-3-3β dominant-negative mutant His-14-3-3β(K51E, R58E, R62E) had almost no inhibitory effect on β1Pix-GEF activity, confirming that 14-3-3β binding modulates β1Pix-GEF activity toward Rac1.
FIG. 5.
Forskolin induces Rac1 inhibition. Cells were transfected with empty vector, β1Pix, or β1Pix(S516A, T526A) in serum-free DMEM for 24 h. Cells were treated in the presence or absence of forskolin (20 μM) for 15 min. Cells lysates were incubated with GST-PBD beads. GTP-bound Rac1 (A) and GTP-bound-Cdc42 (C) were detected by anti-Rac1 and anti-Cdc42, respectively. Anti-Myc (bottom) shows the expression of β1Pix constructs. (B) Densitometric analysis of the bands corresponding to Rac1-GTP from cells treated as described in panel A, upper panel. Each column represents the average ± standard error of three determinations.
FIG. 6.
14-3-3β binding inhibits β1PixGEF activity. (A) Anti-Myc immunoprecipitates were treated with vehicle (○ and ⋄) or PKA catalytic subunit (⧫, •, and ×) and then incubated with GDP-loaded Rac1 and γ-35S-GTP in the presence (⋄, •, and ×) or absence (• and ⧫) of 200 nM of GST-14-3-3β. (B) Cells were transfected with Myc-β1Pix, and the cell lysates were immunoprecipitated using anti-Myc antibody. After incubation with the catalytic PKA subunit, immunoprecipitates were incubated with GDP-loaded Rac1 and γ-35S-GTP in the presence of different concentrations of His-14-3-3β or negative control His-14-3-3β(K51E, R58E, R62E). GDP/GTP exchange assays were carried out for 30 min. Experiments shown here are representative of three independent experiments carried out in duplicate.
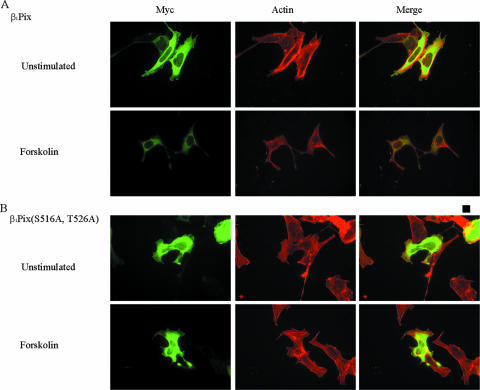
β1Pix has been shown to translocate to the cell periphery and colocalize with focal adhesion complexes to promote the formation of membrane ruffles (7, 17). In our study, expression of β1Pix induced formation of membrane ruffles and colocalized with F-actin filaments at the cell periphery (Fig. 7A, upper panels). Forskolin stimulation of cells expressing β1Pix showed a diffuse cytoplasmic localization but no membrane ruffle-like structures (Fig. 7A, lower panels). However, in cells expressing β1Pix(S516A, T526A), which is impaired in its ability to bind 14-3-3β, forskolin stimulation did not inhibit membrane ruffle formation (Fig. 7B), suggesting that this inhibition is mediated at least in part by 14-3-3β binding to β1Pix.
FIG. 7.
Forskolin induces inhibition of membrane ruffle formation. Cells were transfected with cDNAs encoding Myc-β1Pix (A) or Myc-β1Pix(S516A, T526A) (B) in serum-free DMEM for 24 h and stimulated with forskolin for 15 min. Cells were fixed, permeabilized, and incubated with anti-Myc antibodies and fluorescein isothiocyanate-conjugated secondary antibodies and stained using rhodamine-phalloidin to detect actin. The bar represents 10 μM.
DISCUSSION
β1Pix is a GEF for small GTPases Cdc42 and Rac which has been shown to mediate signaling pathways leading to cytoskeletal reorganization (19, 27). The 14-3-3 proteins are highly conserved and are involved in pathways regulating proliferation, differentiation, and apoptosis (20, 30). 14-3-3 proteins have been shown to bind specific phosphoserine-containing motifs, RSXpSXP and RXY/FXpSXP, present in many of its binding partners (31). A previous report showed how binding of 14-3-3ξ to glycoprotein Ibα regulates Cdc42, Rac, and cytoskeletal reorganization (5). In our study, we show that endogenous 14-3-3β binds endogenous βPix through a PKA-dependent mechanism (Fig. 1A). The expression of a β1Pix mutant, β1Pix(S516A, T526A), impaired in its ability to undergo PKA-dependent phosphorylation, showed resistance to a forskolin-induced increase in 14-3-3β binding (Fig. 2A), demonstrating that 14-3-3β binding requires PKA-dependent phosphorylation of Ser516 and Thr526 on β1Pix. This confirms our previous finding that Ser516 and Thr526 are targets for PKA phosphorylation (7). More importantly, we found that 14-3-3β binds to dimeric β1Pix only (Fig. 2C). Indeed, deletion of amino acids residues 602 to 611, located within the LZ domain of β1Pix, is sufficient to abolish 14-3-3β binding and block β1Pix dimerization. This result is supported by the finding that monomeric 14-3-3s interact with their targets independently of phosphorylation while binding of dimeric 14-3-3s requires phosphorylated targets (25). A previous report showed that the LZ domain deletion blocks platelet-derived growth factor-induced membrane ruffle formation (16). In line with this, we found that a dimerization-deficient β1Pix mutant, β1PixΔ(602-611), which cannot bind 14-3-3β, also showed a significant decrease in its GEF activity toward Rac1 (Fig. 4A). What was particularly interesting was that under the same conditions where PKA-mediated β1Pix phosphorylation inhibited Rac1 in 293 cells, we have previously shown that PKA activation caused a strong stimulation of Cdc42 in mesangial cells (7). Besides the fact that these two experiments were performed with two different cell types, these findings have potentially important implications, since they would suggest a crucial role of 14-3-3β in the regulation of β1Pix-GEF activity toward Rac1 and Cdc42. Indeed, in the case of β1Pix-mediated Cdc42 activation, no specific interaction between β1Pix and 14-3-3β was found (data not shown), suggesting that Cdc42 activity is regulated by monomeric β1Pix. Under such conditions, 14-3-3β is unable to bind monomeric β1Pix, while binding of 14-3-3β to dimeric β1Pix results in inhibition of its GEF activity toward Rac1. In agreement with this, we found that Cdc42 activity was not dependent on β1Pix dimerization (Fig. 4C). Interestingly, a previous report showed that αPix dimerization is required for its function as a GEF for Rac, while the αPix monomer acts as a GEF for both Rac and Cdc42 (12). In contrast, the dimerization inhibits the activity of Rho-GEFs (3, 9).
We expected that binding of 14-3-3β to β1Pix would modulate its function and/or its localization. Indeed, PKA-dependent phosphorylation of β1Pix on Ser516 and Thr526 induced by forskolin results in Rac1 inhibition, whereas in cells expressing β1Pix(S516A, T526A), Rac1 activity was not affected. Furthermore, our in vitro experiments showed that addition of purified 14-3-3β inhibits β1Pix-GEF activity but not that of β1Pix(S516A, T526A). This confirms that forskolin-induced 14-3-3β recruitment results in Rac1 inhibition.
Experiments with transfected cells have shown that β1Pix has the potential to localize to focal adhesions and promotes the formation of membrane ruffles that colocalize with focal adhesions (7, 17, 19). Expression of β1Pix induced the formation of ruffles at the cell periphery that were inhibited upon exposure to forskolin stimulation (Fig. 7A). However, forskolin stimulation did not inhibit membrane ruffle formation in cells expressing β1Pix(S516A, T526A), which is insensitive to PKA phosphorylation. The fact that these mutations strongly inhibited 14-3-3β binding to β1Pix but did not block β1Pix dimerization strongly suggest that β1Pix translocation to the cell periphery does not require 14-3-3β binding. This hypothesis is supported by our findings showing that β1Pix(S516A, T526A), impaired in its ability to bind 14-3-3β, was still able to localize to the cell periphery. A recent report showed that PKA activity is enriched in cell protrusions formed during chemotaxis (14). Inhibition of PKA activity induced a decrease in Rac activity and an increase in GTPase-activating protein (GAP) activity. Adding to the complexity, other evidence shows that the Rac-dependent spatial localization of protrusive activity is mediated by active Pak through the recruitment of Pix (6, 35). Ser516 and Thr526 are located within the GIT1 binding domain, which couples β1Pix to GIT1, Pak, and paxillin (18, 21, 37), allowing localized Rac1 activation at the leading edge of migrating cells. It is tempting to speculate that PKA-dependent phosphorylation of β1Pix and the subsequent 14-3-3β recruitment might play a yet-unidentified role in the formation of this multimolecular complex and/or the regulation of its signaling. It can be speculated that external (Gαs-coupled receptor ligands) or internal (integrin-mediated) signals which increase PKA activity may induce β1Pix phosphorylation and its binding to 14-3-3β, which leads to suppression of the Rac1-related signal pathways, including cytoskeletal reorganization. The effect of PKA on Rac1 activation is likely to be cell type specific. A recent study showed that PKA activation by a parathyroid hormone-related peptide inhibits cell migration and angiogenesis by inhibiting the small GTPase Rac in endothelial cells (4).
Interestingly, 14-3-3β has been shown to interact with AKAP-Lbc, a Rho-GEF, and inhibits its GEF activity through a PKA-dependent mechanism (10). The binding of 14-3-3 to p190Rho-GEF regulates the formation of cytoplasmic aggregates of this exchange factor, although the biological consequences of this interaction remain to be elucidated (34). A recent study showed that Pak1-mediated phosphorylation of Rho-GEF-H1 regulates its interaction with 14-3-3 without affecting Rho-GEF-H1 exchange activity (33). Given that Pak1 is a well-established Pix partner (2, 19), it is tempting to speculate on the existence of a cross talk between the Rho-GEF-H1 and β1Pix signaling pathways. It is also interesting to note that a very recent study showed that β1Pix-induced Rac1 activation requires another GEF, smgGDS, to induce neurite outgrowth (26), suggesting that cross talk at the level of GEFs may add to the specificity of modulating different signaling pathways independently of the receptor activation.
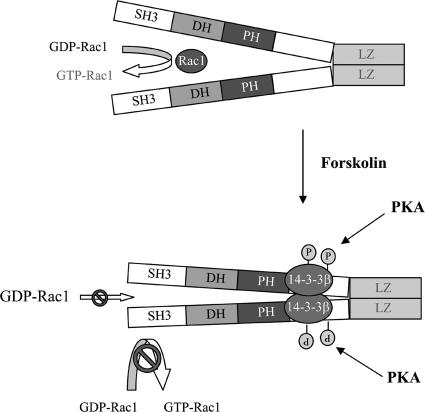
In summary, our results support a model (Fig. 8) in which binding of 14-3-3β to β1Pix is minimal and does not inhibit β1Pix-GEF activity, since basal Rac1 is high and β1Pix is able to promote membrane ruffle formation. However, forskolin stimulation induces β1Pix phosphorylation on Ser516 and Thr526 by PKA, resulting in increased 14-3-3β binding. Consequently, the binding of 14-3-3β inhibits β1Pix-GEF activity through a conformational change that would directly affect the DH domain or by blocking the interaction between β1Pix and Rac1.
FIG. 8.
Model depicting how binding of 14-3-3β inhibits dimeric β1Pix-GEF activity. Under basal conditions, the binding of 14-3-3β to β1Pix is minimal and does not affect its GEF activity. In the presence of forskolin, binding of 14-3-3β to β1Pix through S516 and T526 is increased. 14-3-3β binding may either block the interaction between Rac1 and the DH domain of β1Pix or induce a conformational change of the DH domain that would interfere with GTP binding.
Acknowledgments
This work was supported in part by an American Heart Association grant (0520114Z) to A.C. and by National Institutes of Health grant HL22563 to A.S.
Footnotes
Published ahead of print on 26 December 2007.
REFERENCES
- 1.Bagrodia, S., D. Bailey, Z. Lenard, M. Hart, J. J. Guan, R. T. Premont, S. J. Taylor, and R. A. Cerione. 1999. A tyrosine-phosphorylated protein that binds to an important regulatory region on the cool family of p21-activated kinase-binding proteins. J. Biol. Chem. 27422393-22400. [DOI] [PubMed] [Google Scholar]
- 2.Bagrodia, S., S. J. Taylor, K. A. Jordon, A. Van Aeslt, and R. A. Cerione. 1998. A novel regulator of p21-activated kinases. J. Biol. Chem. 27323633-23636. [DOI] [PubMed] [Google Scholar]
- 3.Baisamy, L., N. Jurisch, and D. Divani. 2005. Leucine zipper-mediated homo-oligomerization regulates the Rho-GEF activity of AKAP-Lbc. J. Biol. Chem. 28015405-154412. [DOI] [PubMed] [Google Scholar]
- 4.Bakre, M. M., Y. Zhu, H. Yin, D. W. Burton. R. Terkeltaub, L. J. Deftos, and J. A. Varner. 2002. Parathyroid-related peptide is a naturally occurring, protein kinase A-dependent angiogenesis inhibitor. Nat. Med. 8995-1003. [DOI] [PubMed] [Google Scholar]
- 5.Bialkowska, K., Y. Zaffran, S. C. Meyer, and J. E. Fox. 2003. 14-3-3 zeta mediates integrin-induced activation of Cdc42 and Rac. Platelet glycoprotein Ib-IX regulates integrin-induced signaling by sequestering 14-3-3 zeta. J. Biol. Chem. 27833342-33350. [DOI] [PubMed] [Google Scholar]
- 6.Cau, J., and A. Hall. 2005. Cdc42 controls the polarity of the actin and microtubule cytoskeletons through two distinct signal transduction pathways. J. Cell Sci. 1182579-2587. [DOI] [PubMed] [Google Scholar]
- 7.Chahdi, A., B. Miller, and A. Sorokin. 2005. Endothelin 1 induces beta 1Pix translocation and Cdc42 activation via protein kinase A-dependent pathway. J. Biol. Chem. 281578-584. [DOI] [PubMed] [Google Scholar]
- 8.Chang, H. C., and G. M. Rubin. 1997. 14-3-3ɛ positively regulates Ras-mediated signaling in Drosophila. Genes Dev. 111132-1139. [DOI] [PubMed] [Google Scholar]
- 9.Chikumi, H., A. Barac, B. Behbahani, Y. Gao, H. Teramoto, Y. Zheng, and J. S. Gutkind. 2004. Homo- and hetero-oligomerization of PDZ-RhoGEF, LARG and p115RhoGEF by their C-terminal region regulates their in vivo Rho GEF activity and transforming potential. Oncogene 23233-240. [DOI] [PubMed] [Google Scholar]
- 10.Diviani, D., L. Abuin, S. Cotecchia, and L. Pansier. 2004. Anchoring of both PKA and 14-3-3 inhibits the Rho-GEF activity of the AKAP-Lbc signaling complex. EMBO J. 232811-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du, X., J. E. Fox, and S. Pei. 1996. Identification of a binding sequence for the 14-3-3 protein within the cytoplasmic domain of the adhesion receptor, platelet glycoprotein Ib alpha. J. Biol. Chem. 2717362-7367. [DOI] [PubMed] [Google Scholar]
- 12.Feng, Q., D. Baird, and R. A. Cerione. 2004. Novel regulatory mechanisms for the Dbl family guanine nucleotide exchange factor Cool-2/alpha-Pix. EMBO J. 233492-3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu, H., R. R. Subramanian, and S. C. Masters. 2000. 14-3-3 proteins: structure, function, and regulation. Annu. Rev. Pharmacol. Toxicol. 40617-647. [DOI] [PubMed] [Google Scholar]
- 14.Howe, A. K., L. C. Baldor, and B. P. Hogan. 2005. Spatial regulation of the cAMP-dependent protein kinase during chemotactic cell migration. Proc. Natl. Acad. Sci. USA 10214320-14325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin, J., F. D. Smith, C. Stark, C. D. Wells, J. P. Fawcett, S. Kulkarni, P. Metalnikov, P. O'Donnell, P. Taylor, L. Taylor, A. Zougman, J. R. Woodgett, L. K. Langeberg, J. D. Scott, and T. Pawson. 2004. Proteomic, functional, and domain-based analysis of in vivo 14-3-3 binding proteins involved in cytoskeletal regulation and cellular organization. Curr. Biol. 141436-1450. [DOI] [PubMed] [Google Scholar]
- 16.Kim, S., S. H. Lee, and D. Park. 2001. Leucine zipper-mediated homodimerization of the p21-activated kinase-interacting factor, beta Pix. Implication for a role in cytoskeletal reorganization. J. Biol. Chem. 27610581-10584. [DOI] [PubMed] [Google Scholar]
- 17.Koh, C. G., E. Manser, Z. S. Zhao, C. P. Ng, and L. Lim. 2001. β1PIX, the PAK-interacting exchange factor, requires localization via a coiled-coil region to promote microvillus-like structures and membrane ruffles. J. Cell Sci. 1144239-4251. [DOI] [PubMed] [Google Scholar]
- 18.Manabe, R., M. Kovalenko, D. J. Webb, and A. R. Hoewitz. 2002. GIT1 functions in a motile, multi-molecular signaling complex that regulates protrusive activity and cell migration. J. Cell Sci. 1151497-1510. [DOI] [PubMed] [Google Scholar]
- 19.Manser, E., T. H. Loo, C. G. Koh, Z. S. Zhao, X. Q. Chen, L. Tan, I. Tan, T. Leung, and L. Lim. 1998. PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol. Cell 1183-192. [DOI] [PubMed] [Google Scholar]
- 20.Masters, S. C., H. Yang, S. R. Datta, M. E. Greenberg, and H. Fu. 2001. 14-3-3 inhibits Bad-induced cell death through interaction with serine-136. Mol. Pharmacol. 601325-1331. [DOI] [PubMed] [Google Scholar]
- 21.Nayal, A., D. J. Webb, C. M. Brown, E. M. Schaeffer, M. Vicente-Manzanares, and A. R. Horwitz. 2006. Paxillin phosphorylation at Ser273 localizes a GIT1-PIX-PAK complex and regulates adhesion and protrusion dynamics. J. Cell Biol. 173587-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park, H. S., S. H. Lee, D. Park, J. S. Lee, S. H. Ryu, W. J. Lee, S. G. Rhee, and Y. S. Bae. 2004. Sequential activation of phosphatidylinositol 3-kinase, beta Pix, Rac1, and Nox1 in growth factor-induced production of H2O2. Mol. Cell. Biol. 244384-4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pozuelo Rubio, M., D. G. Campbell, N. A. Morrice, and C. Mackintosh. 2003. 14-3-3s regulate fructose-2,6-bisphosphate levels by binding to PKB-phosphorylated cardiac fructose-2,6-bisphosphate kinase/phosphatase. EMBO J. 223514-3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pozuelo Rubio, M., K. M. Geraghty, B. H. Wong, N. T. Wood, D. G. Campbell, N. A. Morrice, and C. Mackintosh. 2004. 14-3-3-affinity purification of over 200 human phosphoproteins reveals new links to regulation of cellular metabolism, proliferation and trafficking. Biochem. J. 379395-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen, Y. H., J. Godlewski, A. Bronisz, J. Zhu, M. J. Comb, J. Avruch, and G. Tzivion. 2003. Significance of 14-3-3 self-dimerization for phosphorylation-dependent target binding. Mol. Biol. Cell 144721-4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shin, E. Y., C. S. Lee, G. C. Tae, Y. G. Kim, S. Song, Y. S. Juhnn, S. C. Park, E. Manser, and E. G. Kim. 2006. betaPak-interacting exchange factor-mediated Rac1 activation requires smgGDS guanine nucleotide exchange factor in basic fibroblast growth factor-induced neurite outgrowth. J. Biol. Chem. 28135954-35964. [DOI] [PubMed] [Google Scholar]
- 27.ten Klooster, J. P., Z. M. Jaffer, J. Chernoff, and P. L. Hordijk. 2006. Targeting and activation of Rac1 are mediated by the exchange factor βPix. J. Cell Biol. 172759-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thorson, J. A., L. W. Yu, A. L. Hsu, N. Y. Shih, P. R. Graves, J. W. Tanner, P. M. Allen, H. Piwnica-Worms, and A. S. Shaw. 1998. 14-3-3 proteins are required for maintenance of Raf-1 phosphorylation and kinase activity. Mol. Cell. Biol. 185229-5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turner, C. E., M. C. Brown, J. A. Perrotta, M. C. Riedy, S. N. Nokoloupulos, A. R. Mcdonald, S. Bagrodia, S. Thomas, and P. S. Leventhal. 1999. Paxillin LD4 motif binds PAK and PIX through a novel 95-kD ankyrin repeat, ARF-GAP protein: a role in cytoskeletal remodeling. J. Cell Biol. 145851-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tzivion, G., and J. Avruch. 2002. 14-3-3 proteins: active cofactors in cellular regulation by serine/threonine phosphorylation. J. Biol. Chem. 2773061-3064. [DOI] [PubMed] [Google Scholar]
- 31.Yaffe, M. B., K. Rittinger, S. Volinia, P. R. Caron, A. Aitken, H. Leffers, S. J. Gamblin, S. J. Smerdon, and L. C. Cantley. 1997. The structural basis for 14-3-3:phosphopeptide binding specificity. Cell 91961-971. [DOI] [PubMed] [Google Scholar]
- 32.Yaffe, M. B. 2002. How do 14-3-3 proteins work? Gatekeeper phosphorylation and the molecular anvil hypothesis. FEBS Lett. 51353-57. [DOI] [PubMed] [Google Scholar]
- 33.Zenke, F. T., M. Krendel, C. DerMardirossian, C. C. King, B. P. Bohl, and G. M. Bokoch. 2004. p21-activated kinase 1 phosphorylates and regulates 14-3-3 binding to GEF-H1, a microtubule-localized Rho exchange factor. J. Biol. Chem. 27918392-18400. [DOI] [PubMed] [Google Scholar]
- 34.Zhai, J., H. Lin, M. Shamim, W. W. Schlaepfer, and R. Conte-Soler. 2001. Identification of a novel interaction of 14-3-3 with p190RhoGEF. J. Biol. Chem. 27641318-41324. [DOI] [PubMed] [Google Scholar]
- 35.Zhang, H., D. J. Webb, H. Asmussen, S. Niu, and A. R. Horwitz. 2005. A GIT1/PIX/Rac/PAK signaling module regulates spine morphogenesis and synapse formation through MLC. J. Neurosci. 253379-3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang, L., H. Wang, D. Liu, R. Liddington, and H. Fu. 1997. Raf-1 kinase and exoenzyme S interact with 14-3-3ξ through a common site involving lysine 49. J. Biol. Chem. 2113717-13724. [DOI] [PubMed] [Google Scholar]
- 37.Zhao, Z. S., E. Manser, and L. Lim. 2000. Interaction between PAK and nck: a template for Nck targets and role of PAK autophosphorylation. Mol. Cell. Biol. 203906-3917. [DOI] [PMC free article] [PubMed] [Google Scholar]