Abstract
The product of the Snail1 gene is a transcriptional repressor required for triggering the epithelial-to-mesenchymal transition. Furthermore, ectopic expression of Snail1 in epithelial cells promotes resistance to apoptosis. In this study, we demonstrate that this resistance to γ radiation-induced apoptosis caused by Snail1 is associated with the inhibition of PTEN phosphatase. In MDCK cells, mRNA levels of the p53 target gene PTEN are induced after γ radiation; the transfection of Snail1 prevents this up-regulation. Decreased mRNA levels of PTEN were also detected in RWP-1 cells after the ectopic expression of this transcriptional factor. Snail1 represses and associates to the PTEN promoter as detected both by the electrophoretic mobility shift assay and chromatin immunoprecipitation experiments performed with either endogenous or ectopic Snail1. The binding of Snail1 to the PTEN promoter increases after γ radiation, correlating with the stabilization of Snail1 protein, and prevents the association of p53 to the PTEN promoter. These results stress the critical role of Snail1 in the control of apoptosis and demonstrate the regulation of PTEN phosphatase by this transcriptional repressor.
Epithelial-to-mesenchymal transition (EMT) is a complex process that occurs during embryonic development and tumor progression (19, 36, 43). During EMT, cells undergo a switch from a polarized epithelial phenotype to a motile fibroblastoid morphology. These changes are accompanied by the loss of epithelium-specific genes, such as E-cadherin, and increased expression of mesenchymal markers.
The Snail family members Snail (Snail1) and Slug (Snail2) are essential for triggering EMTs during embryonic development (3, 9, 31). Both genes encode transcriptional repressors capable of binding and inhibiting E-cadherin promoter activity (4, 5, 6). Snail1 expression is necessary for EMT at early phases of embryonic development, since mice deficient in Snail1 fail to down-regulate E-cadherin levels and to complete gastrulation (7). Other genetic studies carried out for Drosophila, Xenopus, and zebrafish have clearly shown that Snail1 is required for EMT “in vivo” (36).
In cell lines, the induction of Snail1 transcription is detected under conditions that promote EMT (9). Moreover, ectopic expression of Snail1 in epithelial cell lines represses E-cadherin and induces a complete EMT, with decreased expression of epithelial genes and up-regulated levels of mesenchymal markers (4, 6, 18). Previous reports have identified several epithelial genes as direct targets of Snail1. This list includes E-cadherin; Muc1; vitamin D receptor; Na+,K+ ATPase; cytokeratin 18; occludin; claudins; and others (4, 6, 10, 12, 18, 20, 28, 41). All these genes contain the Snail1 consensus binding sequence 5′-CACCTG-3′ in their promoters.
Snail family genes also perform additional roles, such as protection against cell death induced by the loss of survival factors or by apoptotic stimuli (3). Studies performed with hematopoietic cells indicate that Snail2 works as a survival factor, protecting normal progenitor cells from DNA damage (21). Snail2 represses the transcription of Puma, a critical mediator in p53-induced apoptosis (46). An interference with p53 function has also been reported for epithelial tumor cell lines, since it has previously been reported that Snail1 can repress the expression of p53 (24). However, other authors have not observed effects of Snail1 on p53 activity, even in cells where Snail1 induces radioprotection (33). The resistance to apoptosis induced by Snail1 has also been associated with its ability to up-regulate the activity of the phosphatidylinositol 3-kinase (PI3K) pathway (44). All these results suggest that Snail1 is a factor necessary for the survival of migrating cells (3).
We have investigated the molecular mechanisms responsible for this higher resistance to apoptosis of cells expressing Snail1. Our results indicate that Snail1 blocks the transcription of the phosphatase and tensin homolog deleted from the chromosome 10 (PTEN) gene, a negative effector of the PI3K pathway (40, 48). Snail1 binds to the PTEN promoter and represses its activity, contributing to the resistance to apoptosis detected in cells expressing Snail1.
MATERIALS AND METHODS
Cell lines.
Madin-Darby canine kidney (MDCK) cells were chosen for this study since they represent a well-established model of epithelial cells expressing wild-type PTEN. Ectopic expression of Snail1 in this cell line has been reported to increase resistance to apoptosis induced by tumor necrosis factor α (44) as well as cause a complete EMT (4, 6). As an alternative system, the human pancreatic cancer cell line RWP-1 was stably transfected with pcDNA3 or pcDNA3-Snail1-HA by following standard procedures (4). Parental or pcDNA3-transfected cells showed high E-cadherin expression (11), whereas clones with ectopic expression of Snail1-hemagglutinin (HA) displayed morphological features characteristic of an EMT and down-regulated levels of E-cadherin (data not shown).
MDCK cells stably transfected with a short hairpin RNA (shRNA) specific for Snail1 (shSnail1), PTEN (shPTEN), or the corresponding control (shCtl) were established as follows. DNA from each of the five mission Snail1 shRNA, PTEN short hairpin RNA (shRNA), or nontarget control vectors (Sigma) was obtained, and 2 μg of an equimolar mix was transfected to control or Snail1-expressing MDCK cells. Selection was performed for 5 days with puromycin (4 μg/ml). PTEN or Snail1-HA protein levels were analyzed by Western blotting as described below. Clones showing the lowest levels of ectopic Snail1-HA or endogenous PTEN proteins were selected for further studies. When indicated, RWP-1 cell clones were transfected with DNA plasmids encoding human PTEN or murine Snail1-HA in pcDNA3 plasmid cDNA by using the Lipofectamine Plus reagent and cell transfectants were selected by treatment with G418 as previously reported (4). Alternatively, MDCK control and MDCK-Snail1-HA cells were cotransfected with DNA plasmids carrying green fluorescent protein (GFP) cDNA alone or together with human PTEN cDNA by using the Lipofectamine Plus reagent. Twenty-four hours after transfection, the cells were irradiated and collected at the indicated times. After 48 h, cells were subjected to flow cytometry to determine the number of GFP-positive cells.
Fluorescence-activated cell sorter (FACS) analysis and determination of cell death.
Cells were irradiated in a Schering (IBL 473C) 137cesium irradiator. An initial dose-response study determined that a 20-Gy γ radiation dose was required to induce apoptosis in 30 to 40% of control MDCK cells 48 h after irradiation. RWP-1 cells were subjected to the same dose, but they were analyzed at 24 h since these cells are more sensitive to γ radiation. After the indicated times, cells were washed with ice-cold phosphate-buffered saline and fixed by adding ice-cold 70% ethanol. Fixed cells were treated with 5 μg/ml RNase A, labeled with 10 μg/ml propidium iodide, and analyzed with a FACScan flow cytometer.
Constructs.
The human PTEN promoter (position numbers −883/+305; GeneCards database, NCBI:chromosome 10; positions 89612292 to 89613480) was cloned by PCR from HT-29 cell genomic DNA by using high-fidelity polymerase (Pfx; Invitrogen) in pGL3* basic (Promega) (a putative Snail1 binding site of the plasmid was eliminated). The sense oligonucleotide sequence was 5′-CGAGCTCCCGACGCCGCGAACC-3′, and the antisense sequence was 5′-GGAAGATCTGAGAGGGGCTCCGGGC-3′. A double-mutant PTEN promoter was obtained by using the QuikChange site-directed mutagenesis kit (Stratagene). The sense oligonucleotide sequences used for performing the mutations were 5′-TACACTGAGCAGCGTGGTAACCTAGTCCTTTTCACCTGTGCACA-3′ and 5′-AGCGTGGTCACCTGGTCCTTTTAACCTATGCACAGGTAACCTCAGACTC-3′ for Ebox1 and Ebox2, respectively (the mutated nucleotides are displayed in bold). The preparation and use of Snail2 (Slug) and Zeb1 have previously been reported (11, 18). The Snail1-HA S246A point mutant was obtained by using the QuikChange site-directed mutagenesis kit (Stratagene) and pcDNA3-Snail1 as the template. The sense primer used for the generation of the mutation was 5′-CGAACCTTCGCCCGCATGTCC-3′, where modified nucleotides with respect to the Snail1 sequence (GenBank accession number gi:6755586) are indicated in bold. All mutants were verified by sequencing.
Luciferase reporter assays.
Reporter assays were carried out for MDCK cells by using 50 ng of the human PTEN promoter (position numbers −883/+331) cloned in the pGL3* basic vector (Promega). Cells were cotransfected with Snail1-HA, either the wild type or the P2A mutant (4), Snail2, or Zeb1, all cloned in pcDNA3, together with 1 ng of simian virus 40-Renilla luciferase plasmid as the control for transfection efficiency. The expression of Firefly and Renilla luciferases was analyzed 48 h after transfection, according to the manufacturer's instructions.
ChIP assays.
Chromatin immunoprecipitation (ChIP) assays were performed as described previously (32). Cells (4 ×106) were cross-linked with 1% formaldehyde for 10 min. Cells were lysed in buffer IP1 (50 mM Tris [pH 8], 10 mM EDTA, 1% sodium dodecyl sulfate [SDS]) for 10 min at room temperature. Alternatively, cells were initially lysed in buffer IP2 (50 mM Tris [pH 8], 2 mM EDTA, 10% glycerol) supplemented with protease inhibitors and centrifuged for 15 min, and the pellet containing the nuclei was resuspended in buffer IP1. Sonication was performed five times at 40% for 10 s (in a Branson Sonicator) to generate 200 to 1,500 bp DNA fragments. Immunoprecipitation was carried out with antibodies against the HA epitope (Roche), monoclonal antibody (MAb) anti-Snail1 (13), anti-p53 (catalog no. sc-126X; Santa Cruz), or an irrelevant immunoglobulin G (IgG) (Sigma) in IP buffer (16.7 mM Tris [pH 8], 167 mN NaCl, 1.2 mM EDTA, 1.1% Triton X-100, 0.01% SDS). Samples were treated with elution buffer (100 mM Na2CO3, 1% SDS, proteinase K) and incubated at 65°C overnight to reverse formaldehyde cross-linking. DNA was purified by using the GFX PCR DNA and gel band purification kit (Amersham). Promoter regions were detected by quantitative PCR SYBR green (Qiagen). PCR and data collection were performed on the ABI Prism 7900HT system. All quantitations were normalized to input and calculated as a percentage of input. Where indicated, the data are presented as enrichment levels of Snail1 at the PTEN promoter, which correspond to the changes in the percentage of input over the control, the percentage obtained with an irrelevant IgG. The PCR was performed by the following specific primers. The PTEN promoter (GeneCards database, NCBI36:10) primers, 5′-CCGTGCATTTCCCTCTACAC-3′ and 5′-GAGGCGAGGATAACGAGCTA-3′, correspond to positions 89612787 to 89612807 and 89612979 to 89612959, respectively. These two oligonucleotides, corresponding to the human sequence, also amplify the Canis familiaris PTEN gene, as determined by sequencing the amplified fragment. The CDH1 human promoter (GeneCards database, NCBI:16) primers, 5′-ACTCCAGGCTAGAGGGTCAC-3′ and 5′-GTCGGGCCGGGCTGGAGC-3′, correspond to positions 67328516 to 67328536 and 67328774 to 67328756, respectively. For an irrelevant sequence, we used the following two oligonucleotides corresponding to the genomic sequence (GeneCards database, NCBI36:17), 5′-ACTCCAGGCTAGAGGGTCAC-3′ and 5′-CCGCAAGCTCACAGGTGCTTTGCAGTTCC-3′ (positions 7328681 to 7328700 and 7328744 to 7328724, respectively).
Quantitative RT-PCR analysis.
Total mRNA was extracted by using the GenElute mammalian total RNA kit (Sigma). Quantitative determination of RNA levels was performed in triplicate by using QuantiTect SYBR green reverse transcription-PCR (RT-PCR) (Qiagen). Canis familiaris PTEN mRNA (GeneCards database, BROADD1:26) was analyzed with the following primers: 5′-CTTTGAGTTCCCTCAGCCAT-3′ and 5′-GGTTTCCTCTGGTCCTGGTA-3′ (positions 39919229 to 39919249 and 39922770 to 39922750, respectively). Homo sapiens PTEN mRNA was analyzed with 5′-AATCCTCAGTTTGTGGTCT-3′ and 5′-GGTAACGGCTGAGGGAACT-3′ (chromosome 10; positions 89707598 to 89707614 and 89707699 to 89707675, respectively), and Canis familiaris Puma mRNA was analyzed with 5′-AGTGAGGGCTGAGGACCTG-3′ and 5′-TGACTGGAGGGAGGAAGAGA-3′ (chromosome 1, positions 111631415 to 111631434 and 111633041 to 111633022, respectively). Hypoxanthine-guanine phosphoribosyl transferase (HPRT) mRNA (GeneCards database, NCBI36:X) was analyzed as an internal control by using oligonucleotides 5′-GGCCAGACTTTGTTGGATTTG-3′ and 5′-TGCGCTCATCTTAGGCTTTGT-3′ (133460124 to 133460316 and 133461784 to 133461763, respectively). RT-PCR and data collection were performed on the ABI Prism 7900HT system. All quantitations were normalized to an endogenous control (HPRT). The relative quantitation value for each target gene compared to the calibrator for that target is expressed as 2−(Ct-Cc) (CT and CC are the mean threshold cycle differences after normalization to HPRT).
Immunohistochemistry.
Paraffin sections obtained from wild-type or Snail1-deficient (7) murine embryos (7.5 days postcoitum [dpc]) were deparaffined in xylene and rehydrated. The step of antigenic recovery was carried out in a pressure cooker for 15 min in Tris-EDTA buffer at pH 9. After we blocked the endogenous peroxidase with 4% H2O2 for 15 min, sections were blocked by incubation for 1 h in phosphate-buffered saline supplemented with 3% bovine seroalbumin, followed by incubation with the antibodies for 1 h at room temperature. The following MAbs were used: anti-phospho-Thr308 Akt, anti-PTEN (both from Cell Signaling), and anti-Snail1 (13). Immunohistochemical staining was performed by using the EnVision system (DakoCytomation) following the manufacturer's instructions.
Gel retardation assays.
Assays were performed essentially as described previously (4), by using recombinant proteins (glutathione S-transferase [GST]-Snail1 and GST as a control) and a 32P-labeled, double-stranded oligonucleotide corresponding to the −533/−557 positions in sequence of human PTEN promoter. Cold probes corresponding either to the wild-type human PTEN promoter or to a version where the two E boxes were mutated to 5′-AACCTA-3′ were used for the competition assays.
Western blotting and immunofluorescence.
Cells were lysed either in SDS buffer (1% SDS, 65 mM Tris-HCl [pH 8.8]) to obtain total extracts or in cytosol buffer (10 mM Tris-HCl [pH 6.5], 150 mM NaCl, 0.01% saponin, 2 mM EDTA, 5 mM EGTA, supplemented with protease inhibitors). In the latter case, the lysing was for 20 min on ice to obtain the cytosolic fraction. Lysates were clarified by centrifugation, and supernatants were collected. The purification of phosphorylated proteins was performed by using the PhosphoProtein purification kit (Qiagen) according to the manufacturer's instructions. Western blot analyses were performed according to standard procedures by using the following antibodies: PTEN, P-Akt (Thr308), Akt, p21, and P-p53 (Ser15) (all from Cell Signaling), HA (Roche Diagnostics), and β-actin (Sigma). An anti-annexin 2 polyclonal antiserum was a kind gift of Pilar Navarro (IMIM, Barcelona, Spain). Immunofluorescence was carried out as described previously (11).
RESULTS
Snail1 induces resistance to γ radiation-induced apoptosis.
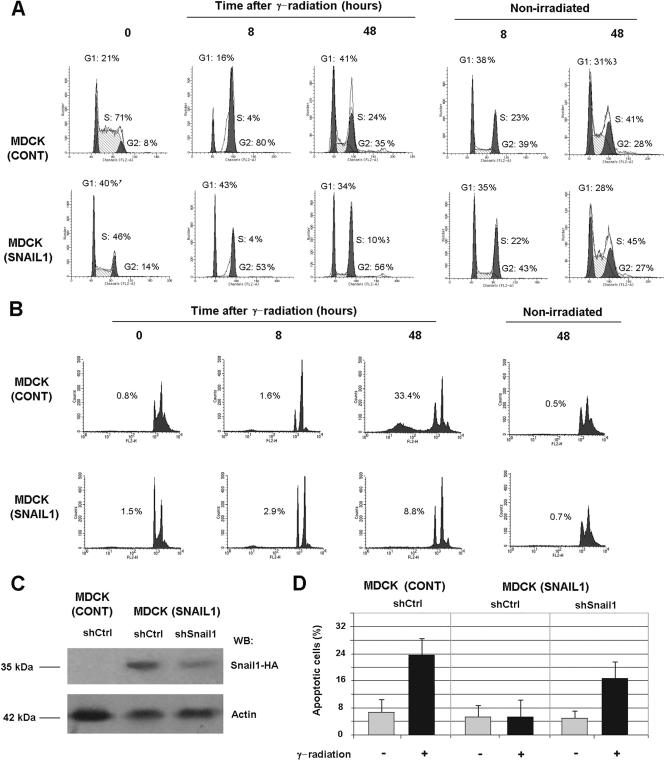
In order to analyze their resistance to apoptosis, MDCK control cells or stable clones overexpressing Snail1 cDNA were subjected to γ radiation. Cells were seeded on plastic plates and 15 h later challenged with a 20-Gy γ radiation. Initially, MDCK cells responded to this insult with an arrest in cell growth. After 8 h, 80% of the cells were observed in G2, as analyzed by FACS (Fig. 1A), suggesting that they had been arrested at the G2-M checkpoint, whereas nonirradiated cells progressed normally through the cell cycle. At longer times (48 h) after γ radiation, MDCK cells reentered the cell cycle (Fig. 1A) and started to undergo apoptosis (see below). As reported previously (44), MDCK-Snail1 clones display a higher number of cells in G1 in the moment of radiation than do control cells (40% versus 20%) (Fig. 1A, lower panel). Although delayed, the radiation of MDCK-Snail1 cells also induced a cell cycle arrest in G2-M. Even after 48 h of γ radiation, a significant percentage of the MDCK-Snail1 cell population rested in G2 phase (56% [Fig. 1A]), suggesting that this arrest was still active.
FIG. 1.
Snail1 prevents apoptosis of MDCK cells in response to γ radiation. (A) γ irradiation arrests MDCK control and MDCK-Snail1-HA cells at the G2/M checkpoint. Representative diagrams show the DNA content of MDCK control (cont) or MDCK-Snail1 at the times indicated after 20-Gy γ radiation. Cells were seeded on tissue culture plates, irradiated 15 h later, and at the indicated times after radiation, harvested, stained with propidium iodide and analyzed by flow cytometry. (B) MDCK-Snail1 cells are resistant to γ radiation-induced apoptosis. The induction of apoptosis was determined by flow cytometric analysis of cells stained with propidium iodide at the times indicated after 20-Gy γ radiation. The figure shows the result of a representative experiment of three performed. Similar results were obtained with another clone of MDCK-Snail1 for which a number of apoptotic cells after radiation was 5.6-fold lower than that of control cells. (C) MDCK control or MDCK-Snail1 cells were transfected with control or Snail1 shRNAs as indicated in Materials and Methods. A clone with decreasing expression of Snail1-HA was selected. The down-regulation of Snail1-HA expression with respect to a representative clone transfected with the control shRNA was analyzed by Western blotting (WB). The molecular masses of Snail1-HA and annexin 2, which was used as loading control, are indicated. (D) Control MDCK cells transfected with control shRNA or MDCK-Snail1 cells transfected with control or Snail1 shRNA were irradiated, and the percentage of apoptotic cells was determined after 48 h as indicated. The figure shows the averages ± ranges (error bars) of two experiments performed. −, absence of; +, presence of.
In a characteristic experiment, 48 h after radiation, 30 to 35% percent of the control MDCK cells were undergoing apoptosis. These cells presented traits of programmed cell death, such as increased staining with trypan blue and an elevated proportion of cells with a DNA content lower than 2n, as determined by FACS analysis (Fig. 1B). These two features were much less abundant in MDCK clones ectopically expressing Snail1, with only 8 to 10% of these cells suffering apoptosis 48 h after of γ radiation (Fig. 1B). To further demonstrate the role of Snail1 in the resistance to apoptosis, Snail1 protein was down-regulated by using an shRNA specific for this gene. As observed in Fig. 1C, the transfection of this interferent RNA significantly repressed the levels of Snail1-HA in MDCK clones. These cells with decreased levels of ectopic Snail1 displayed a higher sensitivity to γ radiation-induced apoptosis than did Snail1-expressing clones (Fig. 1D), indicating that the resistance to cell death is a consequence of Snail1 expression.
Snail1 prevents the decrease in Akt activity and the up-regulation of PTEN induced by γ radiation.
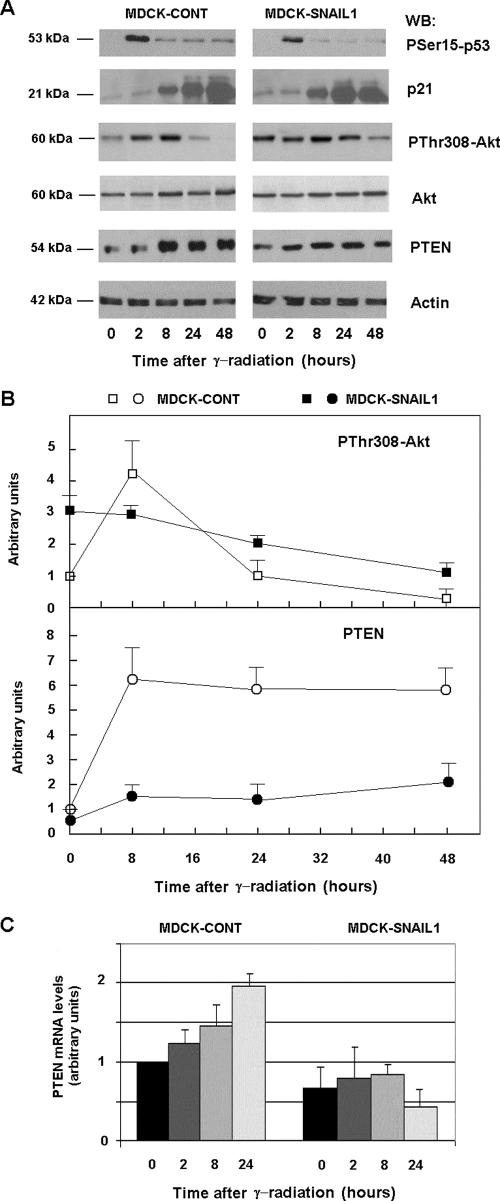
In MDCK cells, the induction of cell death by DNA damage is preceded by a rise in the activity of p53. DNA damage induces the phosphorylation of p53 at Ser15, reducing its interaction with its negative regulator MDM2 (37). As shown in Fig. 2, an increase in PSer15-p53 was detected in MDCK control cells 2 h after radiation. MDCK clones overexpressing Snail1 showed a similar increase in this parameter, suggesting that p53 activity is not altered by Snail1. Accordingly, after irradiation both in control cells and MDCK clones expressing Snail1, increases in a p53 target gene such as p21 were similar (Fig. 2A).
FIG. 2.
Snail1 inhibits PTEN up-regulation and prevents the decrease in Akt activity in response to γ radiation. (A) MDCK control (cont) and MDCK-Snail1 cells were irradiated at 20 Gy, and samples were collected at the times indicated. Cytosolic or total cell extracts were prepared, and p53 phosphorylation (PSer15-p53), Akt phosphorylation (PThr308-Akt), total AKT, p21, PTEN, and actin (as a loading control) levels were determined by Western blot (WB) analysis. (B) The figure shows the result of a representative experiment of three performed. The autoradiograms were scanned, and the measures obtained for PThr308-Akt and PTEN were represented in respect to the value in nonirradiated control MDCK cells. Averages ± ranges (error bars) are shown. A similar lack of increase of PTEN protein was also observed for another clone of MDCK-Snail1 cells. (C) Snail1 prevents PTEN mRNA up-regulation in response to irradiation in MDCK cells. Control and MDCK-Snail1 cells were irradiated, and RNAs were prepared at the indicated times. The levels of endogenous PTEN were detected by quantitative RT-PCR as described in Materials and Methods. Results are presented as the averages ± SDs (error bars) from a minimum of three independent experiments. Levels of PTEN mRNA in another clone of MDCK-Snail1 cells 24 h after γ radiation were 0.8 ± 0.15 (average ± range of two experiments; relative to the initial value of PTEN mRNA in control nonirradiated cells). With respect to the value at time zero, the increase of PTEN mRNA in control cells was significant at 8 h (P < 0.05) and at 24 h (P < 0.01); when we compared the levels of PTEN mRNA between control and Snail1 MDCK cells at the same time points, the differences were significant at 8 and 24 h with a P value of <0.01.
Control MDCK cells responded to radiation with a rapid increase in the activity of Akt, detected after 2 h with a specific MAb against P-Thr308. The phosphorylation of this amino acid by PDK is required for the activation of Akt (1). Later, the activity of Akt decayed and at 48 h was clearly lower than that of untreated cells (Fig. 2A and B). Prior to radiation, MDCK Snail1 cells presented higher levels of active Akt than did control cells (approximately threefold) (Fig. 2A and B). In these cells, the phosphorylation of Thr308 was slightly modified by DNA damage; the initial rise was not detected, and the amount of phosphorylated Thr308 decreased slowly. As a consequence, the activity of Akt was substantially higher in MDCK Snail1 than in control cells after 24 h of radiation (Fig. 2), providing an explanation for the higher resistance to apoptosis induced by Snail1.
An analysis of a human CpG-rich array with chromatin immunoprecipitated with a Snail1 antibody revealed that PTEN promoter sequences were highly enriched in this immunoprecipitate. The details of this ChIP-on-ChIP analysis are provided upon request. Because the regulation of Akt by PTEN phosphatase is crucial for the apoptotic response of cells after being exposed to γ radiation, we tested whether Snail1 was altering PTEN levels in MDCK cells. As shown in Fig. 2, γ radiation of control MDCK cells induced an increase of PTEN protein that was maximal after 8 h, preceding the down-regulation in Akt activity. The increase in PTEN protein in MDCK-Snail1 cells was much smaller than that in control MDCK cells and correlates with the higher persistence of active Akt in Snail1 transfectants with respect to the control cells.
Closely resembling the result obtained when we analyzed the protein levels, PTEN mRNA levels were also increased in irradiated MDCK cells (Fig. 2C). This up-regulation was not detected in Snail1 MDCK transfectants, suggesting that Snail1 affects PTEN transcription.
Up-regulated Akt activity and decreased levels of PTEN mRNA are also detected in RWP-1 cells transfected with Snail1.
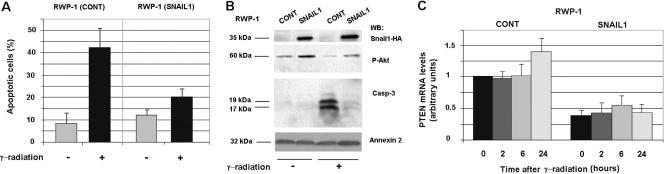
We checked whether the differences in Akt activity and PTEN expression were detected in other cell lines. As shown in Fig. 3A, RWP-1 cells stably transfected with Snail1 also presented a higher resistance to apoptosis after γ radiation than did control RWP-1 cells. Typically, 40 to 50% of control cells underwent apoptosis 24 h after γ radiation, whereas around 20% of RWP-1-Snail1 cells did. Accordingly, a characteristic marker of apoptosis, the processed form of caspase 3, was detected at higher levels in control cells than in Snail1-HA-expressing cells after γ radiation (Fig. 3B).
FIG. 3.
Transfection of Snail1 to RWP-1 cells up-regulates Akt activity and decreases PTEN mRNA levels. Control (cont) RWP-1 cells stably transfected with pcDNA3 or with pcDNA3-Snail-HA were irradiated and analyzed. (A) Apoptosis was determined as described previously for nonirradiated cells (−) or for cells 24 h after γ radiation (+). The figure shows the averages ± ranges of three experiments performed. (B) Total cell extracts were prepared from nonirradiated cells (−) or 24 h after γ radiation (+) and analyzed by Western blotting (WB) with MAb against HA, PThr308-Akt, caspase-3 (Casp-3) or annexin 2 as a loading control. (C) mRNA was prepared from RWP-1 cells at the indicated time points, and levels of PTEN mRNA were detected by quantitative RT-PCR. Results are presented as the averages ± SDs (error bars) of three independent experiments. When we compared the levels of PTEN mRNA between control and Snail1-expressing cells at the same time points, the differences were significant at all time points with a P value of <0.01.
In a manner similar to that of MDCK cells, RWP-1 cells transfected with Snail1 presented higher levels of active Akt, both before and after 24 h of γ radiation (Fig. 3B). In the same way, PTEN mRNA was down-regulated in RWP-1 Snail1 cells with respect to the control (Fig. 3C). In a manner different from that of control MDCK cells, the increase in PTEN mRNA after irradiation was very minor in RWP-1 cells; however, since the down-regulation by Snail1 of this mRNA was more marked before the insult, the differences in PTEN mRNA between control and Snail1 transfectants were significant at all times examined.
Relevance of PTEN repression in the resistance to γ irradiation-induced apoptosis caused by Snail1.
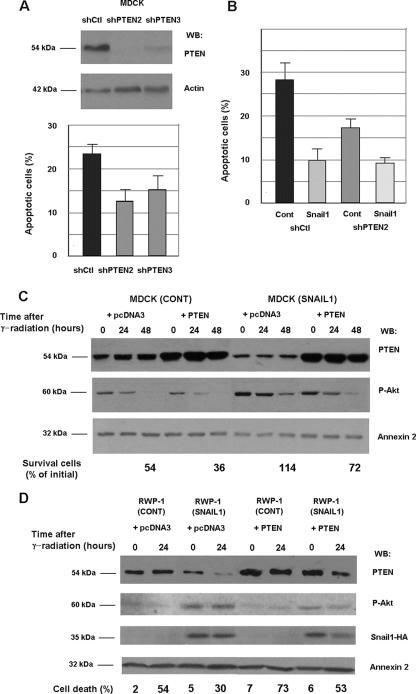
We determined the relevance of PTEN repression in the resistance to γ radiation-induced apoptosis. Cell clones showing down-regulated expression of PTEN were generated by transfecting MDCK cells with a plasmid that generates a shRNA specific for PTEN. As shown in Fig. 4A, the depletion of PTEN protein correlated with an increased resistance to apoptosis, since 48 h after γ radiation, the number of apoptotic cells was lower in MDCK cells expressing shPTEN than in the corresponding controls. This result indicates that as in other cell systems, PTEN plays a relevant role in the promotion of cell death in MDCK cells. However, the protection conferred by this shPTEN was not as complete as that provided by the expression of Snail1, suggesting that this factor also acts on elements other than PTEN. Accordingly, the expression of Snail1 increased the resistance to apoptosis even in cells with undetectable levels of PTEN (Fig. 4B).
FIG. 4.
Ectopic manipulation of PTEN protein levels affects cell death and Snail1 response. Stable clones expressing a shRNA specific for PTEN or expressing a shRNA control were generated in MDCK cells. (A) Expression of PTEN was checked by Western blot (WB) analysis (top panels). The indicated cells were irradiated and cultured for 48 h. The percentage of apoptotic cells was analyzed by flow cytometry (bottom panel). The averages ± SDs (error bars) of three experiments performed are shown. (B) MDCK shCtl or shPTEN (clone 2) cells were transfected with pcDNA3 or pcDNA3-Snail-HA and selected, and the resistance to apoptosis of the four different subpopulations was analyzed. The averages ± ranges (error bars) of two experiments performed are shown. (C) MDCK control or Snail1 cells were transfected with a GFP expression plasmid and PTEN cDNA or a control (cont) plasmid. After 24 h of expression, the cells were irradiated and cultured for an additional 48 h. The percentage of apoptotic cells was analyzed by determining the number of GFP-positive cells by flow cytometry and referred to the number of cells at the time of γ radiation. In parallel, cell extracts were prepared and analyzed by Western blotting with the indicated antibodies. (D) RWP-1 control or RWP-1 Snail1-HA cells were transfected with pcDNA3 or pcDNA3-PTEN plasmids, and the double-transfectant populations were selected. Cells were irradiated and the percentage of apoptotic cells was determined. The figure shows the result of one experiment of two performed with similar results.
To confirm this result, PTEN was overexpressed in MDCK or RWP-1 cells either in control cell populations or in cell populations ectopically expressing Snail1. As shown in Fig. 4C and D, the transfection of PTEN slightly increased the amount of control cells undergoing cell death after irradiation. For instance, in RWP-1 cells, at 24 h the number of dead cells increased from 54% to 73% in the representative experiment shown in Fig. 4D. Similarly, the number of surviving cells decreased in MDCK cells after ectopic expression of PTEN from 54% to 36% (Fig. 4C). As shown above, the expression of Snail1 decreased the extent of apoptosis in cells transfected with pcDNA3 (from 54% to 30% in RWP-1 cells) and also did so in cells overexpressing PTEN (from 73% to 53%). An analysis of Akt activity also correlated with these data. The expression of Snail1 up-regulated the levels of active Akt (Fig. 4C and D), whereas PTEN ectopic expression accelerated the decrease in Akt activity observed after γ radiation (Fig. 4C, compare lanes 2 and 5). However, even in cells with a high expression of PTEN, Snail1 transfectants presented higher levels of active Akt than did control cells (Fig. 4C, compare lanes 4 to 6 with lanes 10 to 12, or D, compare lanes 5 to 6 with 7 to 8).
Altogether, these results suggest that although PTEN down-regulation caused by Snail1 expression affects resistance to apoptosis and Akt activity, additional elements also contribute to the full Snail1 response.
Snail1 binds to the PTEN promoter and represses its activity.
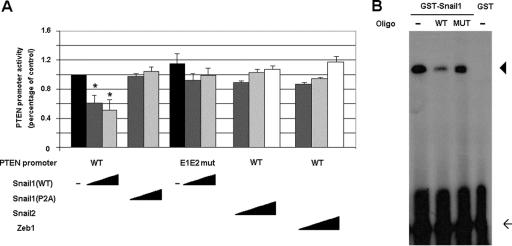
In order to analyze the mechanism responsible for the lower expression of PTEN in Snail1 transfectants, we cloned a fragment of the human PTEN promoter (positions −883/+331). This DNA fragment presents high activity in epithelial cells, such as the MDCK cells used in our assays. The activity of the PTEN promoter was lower (61% ± 3%, shown as mean ± standard deviation [SD]) in MDCK cells stably transfected with Snail1, indicating that the effect of Snail1 on PTEN mRNA levels is transcriptional. Similar results were obtained when the activity of this promoter fragment was examined in RWP-1 Snail1 transfectants. Transient transfections of Snail1 cDNA in MDCK cells substantially repressed the activity of the PTEN promoter in a dose-response manner (Fig. 5A). A P2A Snail1 mutant, a form of this protein deficient in the repression of genes, such as E-cadherin or vitamin D receptor (4, 28), was unable to repress PTEN promoter activity in this assay (Fig. 5A).
FIG. 5.
Snail1 represses PTEN promoter activity. (A) Snail1 represses the PTEN promoter in a dose-dependent manner. The activity of the wild type or the E1E2-mutant PTEN promoter was determined for MDCK cells by transient transfection. When indicated, wild-type or P2A mutant Snail1, wild-type Snail2, or Zeb1 cDNAs were cotransfected at several concentrations: 0.1 (dark gray bars), 1 (light gray bars), and 10 ng (white bars). Black bars correspond to the activity of each promoter in the absence of repressors. The figure shows the averages ± SDs (error bars) of three independent experiments performed in triplicate. Asterisks indicate differences that were significant at a P value of <0.05. (B) Snail1 binds to the PTEN promoter. The GST-Snail1 fusion protein or the GST protein was incubated with a double-stranded 32P-radiolabeled probe corresponding to the two E boxes of PTEN promoter. Binding experiments were carried out with 150 ng of GST-Snail1 without competitor (−) or competing with an excess of unlabeled wild-type (WT) or mutant oligonucleotide (MUT). Arrow, free probe; arrowhead, specific shifted band.
The PTEN promoter contains two putative binding elements for the Snail1 transcriptional factor, characterized by a 5′-CACCTG-3′ core. These two E boxes are placed at positions −351/−346 and −337/−332 from the transcription start. As shown by electrophoretic gel shift assays (Fig. 5B), a Snail1 recombinant protein strongly bound an oligonucleotide containing both E boxes. Such binding was competed by a 10-fold excess of unlabeled oligonucleotide but not by a mutant oligonucleotide in which the two boxes had been replaced by 5′-AACCTA-3′. A similar mutation has been reported to block Snail1 binding to E-cadherin and other target promoters (4, 18, 28).
We checked whether the two E boxes were relevant for Snail1-repression of the PTEN promoter. As shown in Fig. 5A, a promoter form in which the two boxes were mutated to 5′-AACCTA-3′ was insensitive to Snail1 expression in the reporter assays, indicating that these sequences were mediating the effects of Snail1 on PTEN transcription.
Snail2 and Zeb1 are two transcriptional factors that also repress E-cadherin through binding to the same elements as Snail1, although with lower potency (5, 18). The transfection of these two cDNAs did not significantly repress PTEN promoter activity (Fig. 5A), indicating that Snail1 is much more efficient for the control of PTEN expression than these two other repressors are.
Binding of Snail1-HA to the PTEN promoter is modulated after γ radiation.
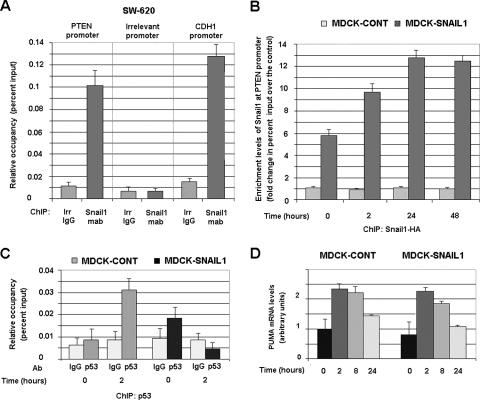
The binding of Snail1-HA to the PTEN promoter was also determined by ChIP assays (Fig. 6). PTEN promoter sequences were detected in the fraction precipitated with an MAb specifically detecting the HA tag labeling the ectopically expressed Snail1 in MDCK cells. As shown in Fig. 6A, binding of the PTEN promoter to Snail1 was also detected when endogenous Snail1 was immunoprecipitated from SW-620 cells (4), with a Snail1 MAb recently prepared in our lab (13). Therefore, the binding of Snail1 to the PTEN promoter was not due to the overexpression of this protein. The association of Snail1 with this promoter was comparable to that detected with another promoter of a well-established target of this transcriptional factor, E-cadherin (4, 30) (Fig. 6A). ChIP assays determined that the binding of Snail1-HA to the PTEN promoter was modulated by γ radiation. As shown in Fig. 6B, the amount of the PTEN promoter immunoprecipitated with Snail1-HA from MDCK cells was significantly increased 2 h after irradiation.
FIG. 6.
Snail1 is recruited to the PTEN promoter “in vivo” in response to γ radiation. (A) Snail1 binds to the PTEN promoter in SW-620 cells. ChIP assays were carried out by using an MAb specific for Snail1 or an irrelevant (irr) IgG as described in Materials and Methods. The presence of sequences corresponding to PTEN, E-cadherin (CDH1), or an irrelevant promoter was analyzed and represented as relative occupancy (percent input). (B) Binding of Snail1 to the PTEN promoter is up-regulated after irradiation. ChIP assays were performed by immunoprecipitating Snail1-HA with an anti-HA MAb or an irrelevant IgG from MDCK-Snail1 cells at different times after γ radiation. Enrichment levels in Snail1 at the PTEN promoter correspond to the change of the percent input calculated with respect to the amount detected in the immunoprecipitation carried out with an irrelevant IgG. In a representative experiment, the percentages of input obtained with the IgG or with anti-HA MAb in the control (cont) clones (not expressing Snail1) varied between 0.005 and 0.007; the values obtained with anti-HA MAb in MDCK-Snail1 cells at 0, 2, 24, and 48 h after γ radiation were 0.035, 0.07, 0.08, and 0.07, respectively. Similar results were obtained when the binding of Snail1-HA to the PTEN promoter was analyzed in another clone of MDCK-Snail1 cells. (C) Snail1 prevents the interaction of p53 with PTEN promoter in response to γ radiation. ChIP assays were performed by immunoprecipitating p53 from the indicated cells before or 2 h after γ radiation. Data are represented as described for panel A. Panels A to C of this figure show the averages ± SDs (error bars) of three independent experiments. (D) Snail1 does not prevent Puma up-regulation in response to γ radiation in MDCK cells. The levels of endogenous Puma were detected by quantitative RT-PCR, as described in Materials and Methods, by using RNA isolated from the indicated cells prior and after γ radiation. Results are presented as the averages ± SDs (error bars) from three independent experiments.
Since PTEN expression has been reported to be dependent on p53 activity and the binding of this protein to the PTEN promoter (39), we checked whether Snail1 could prevent p53 association to this promoter. By ChIP assays, very little binding of p53 to the PTEN promoter was detected prior to irradiation, either in control or in Snail1-expressing MDCK cells (Fig. 6C). This association was greatly increased in control cells 2 h after γ radiation (Fig. 6C), correlating with the detected up-regulation of PSer15-p53 (Fig. 2A). However, this binding was not observed in MDCK-Snail1 cells, indicating that Snail1 inhibits p53 interaction to the PTEN promoter (Fig. 6C).
We also analyzed whether Snail1 affected the expression of the apoptosis regulator Puma, another p53 target gene reported to be sensitive to Snail2 in hematopoietic cells (46). Puma mRNA levels were not significantly different in MDCK-Snail1 versus MDCK cells and were increased in similar manners after γ irradiation in both cell types (Fig. 6D). Moreover, the presence of Puma promoter sequences was not detected in Snail1 immunoprecipitates (data not shown), further suggesting that Snail1 does not bind to Puma promoter.
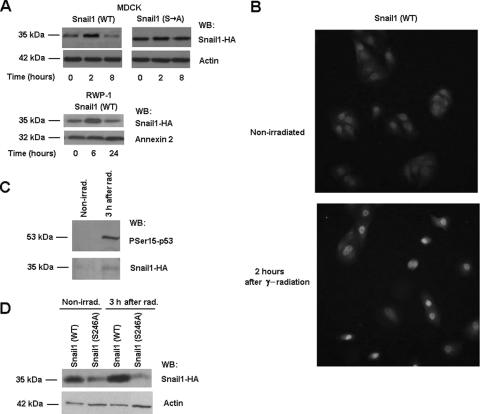
The mechanism leading to the up-regulated binding of Snail1 to the PTEN promoter after γ radiation was also investigated. A time course analysis of Snail1 in MDCK transfectants showed that the levels of this protein rose after γ radiation, temporally correlating with the increased binding to the PTEN promoter (Fig. 7A). This increase in Snail1 protein was transient and was also detected in RWP-1 cells (Fig. 7A). The enhanced expression of Snail1 was detected in the nucleus (Fig. 7B), suggesting that the process of export and the subsequent degradation of Snail1 protein (50, 51) is blocked in the irradiated cells. To further test that Snail1 protein was being stabilized, we used a Snail1-HA mutant (Snail1Ser→Ala) in which the Ser residues required for the export and degradation of the protein, placed in the Pro-Ser-rich domain, were replaced by Ala (11). Therefore, this protein is more stable than the wild-type form. As shown, the levels of this mutant were not significantly increased after radiation, further supporting our conclusion that γ radiation inhibits the degradation of Snail1 protein (Fig. 7A).
FIG. 7.
Snail1 protein is stabilized in response to γ radiation. Protein levels (A) and cellular distribution (B) of Snail1 were determined at the indicated times after irradiation in MDCK-Snail1 (wild-type [WT]), MDCK-Snail1 (Ser→Ala mutant [S→A]) or RWP-1-Snail1 cells by Western blotting (A) or immunofluorescence (B). Similar results were observed for another clone of MDCK-Snail1 cells. (C) Phosphorylation of Snail1 proteins was determined for stable MDCK-Snail1 transfectants 3 h after γ radiation (rad) and compared with the phosphorylation of nonirradiated (nonirrad) cells. Purification of phosphorylated Snail-HA was performed as indicated in Materials and Methods. (D) Snail1-HA protein levels in MDCK cells transiently transfected with wild-type or S246A Snail1-HA were determined as described above. The figure shows the result of a representative experiment of three (panels A and B for MDCK) or two (panel A for RWP-1 or panels C and D) performed. WB, Western blot.
We also analyzed the cause of the increase in Snail1 protein. We checked whether the up-regulation of Snail1 protein correlated with a decrease in the amount of phosphorylated Snail1 by using affinity chromatography. The validity of this system (the PhosphoProtein purification kit from Qiagen) was demonstrated by the increase in PSer15-p53 detected in the column-bound fraction (Fig. 7C), in accordance to our previous observations, indicating that the phosphorylation of p53 was stimulated shortly after radiation (Fig. 2C). Contrary to our expectations, the amount of phospho-Snail1 was increased after radiation (Fig. 7C). This result suggests that the stabilization of Snail1 protein is not due to the dephosphorylation of residues in the Pro-Ser-rich domain.
It has previously been reported that the phosphorylation of Ser246, placed in the C terminus of Snail1 protein, promotes effects contrary to those of the serine residues situated in the Pro-Ser-rich domain; the modification of this Ser246 retains Snail1 in the nucleus and stabilizes it (49). The phosphorylation of this Ser is catalyzed by PAK1 kinase, a protein kinase known to be activated by ionizing radiation (34). Therefore, we determined whether a Snail1 mutant unable to be phosphorylated in this residue (Snail1 Ser246Ala) was still sensitive to γ radiation. As shown in Fig. 7D, this mutant was not accumulated in response to this insult.
Contrary distribution of Snail1 and PTEN in murine embryos.
Since Snail1 is expressed more extensively in tissue at early stages of development than in adult tissue, we analyzed the expression of Snail1 and PTEN proteins in mouse embryos by using immunohistochemistry. As shown in Fig. 8A, Snail1 was expressed in the mesoderms of wild-type murine embryos (7.5 dpc), whereas, as expected, no signal was observed in Snail1 null embryos. PTEN presented an inverse distribution to Snail1 at this stage of development, being restricted to the ectoderm (Fig. 8A, upper middle panel). In Snail1-deficient embryos, PTEN was observed in cells not clearly corresponding to this tissue, although the alterations in the embryo architecture observed in this mutant precluded a more definitive conclusion. In these mutant embryos, fewer cells were stained by anti-PThr308-Akt MAb (only 36% ± 5% [average ± SD of four determinations] with respect to the number observed in control embryo sections), indicating that the activation of this kinase is also reduced in Snail1 mutants.
FIG. 8.
Snail1 and PTEN display an inverse expression pattern in murine embryos. (A) Presence of Snail1, PTEN, or P308-Akt was determined for wild-type or Snail1-null 7.5 dpc embryos (E7.5) by immunohistochemistry using specific MAbs and the conditions indicated in Materials and Methods. Original magnification, ×150. (B) Expression of Snail1 and PTEN was analyzed in wild-type 15.5 dpc embryos (E15.5) by immunohistochemistry using specific MAbs. The figure shows two representative areas where consecutive sections were analyzed with both antibodies. Lower panels show details of the upper panels. Original magnification, ×400.
In order to study the relationship between Snail1 and PTEN expression, we analyzed these two proteins in another system in which Snail1 plays an important role: hair follicle morphogenesis (22). As reported previously (13), in 15.5 dpc embryos, Snail1 was detected in the dermal mesenchymal cells adjacent to the hair bud (Fig. 8B). These dermal condensate cells correspond to cells that have undergone an EMT (22). PTEN was located in the epithelial cells situated in the deepest layer of the dermis, identifying the interface between the epithelial and mesenchymal compartments (Fig. 8B) These PTEN-positive cells were not labeled with Snail1 MAb, although some Snail1 reactivity could also be detected in the PTEN-positive cells located in deepest layer, in areas where hair buds were originating. These cells presumably correspond to cells that have just undergone EMT. Other cells showing Snail1 expression and situated more internally were not labeled by PTEN MAb. Therefore, these results suggest that Snail1-dependent EMT associated to the generation of the dermal condensate happens concomitantly with PTEN downregulation. Altogether, these data (i) indicate that in embryos, Snail1 expression is contrary to that of PTEN and (ii) suggest that the Snail1 regulation of PTEN expression is also active during embryonic development.
DISCUSSION
The expression of Snail family members has been associated with the acquisition of resistance to several types of programmed cell death. For instance, both Snail1 and Snail2 (Slug) protect hematopoietic cells from γ radiation-induced apoptosis (21, 33). We show here that the overexpression of Snail1 also preserves epithelial MDCK and RWP-1 cells from this type of apoptosis. The percentage of cells presenting characteristics of programmed cell death 48 h after inducing DNA damage is much lower in MDCK cells overexpressing Snail1 than in control cells. Similar results have been published by other authors using the same cellular model, which induced apoptosis by the withdrawal of survival factors or by other proapoptotic signals (44). These authors have also shown that Snail1 induces an activation of PI3K and Akt, a pathway that confers resistance to apoptosis. However, the mechanism underlying such a Snail1 effect has not been clarified yet. Our data show increased levels of active Akt in MDCK-Snail1 and RWP-1-Snail1 cells with regard to the respective controls and identify a critical effector of this pathway, PTEN, as a direct Snail1 target gene.
In our assays, MDCK-Snail1 cells respond normally to γ radiation, with an arrest in cell proliferation, and accumulate in G2 phase. This arrest is accompanied by an up-regulation of active p53 that is not affected by the expression of Snail1. These results are in agreement with previous results showing that ectopic expression of Snail1 in mouse embryo fibroblasts does not modify the expression of p53 after γ radiation (33). Moreover, in our MDCK cells, the transfection of Snail1 does not prevent the up-regulation of two p53 target genes, p21 (Fig. 2A) and Puma (Fig. 6D), further indicating that p53 activity is not affected by Snail1. However, other authors have indicated that Snail1 can alter the response of MCF-7 cells to the genotoxic stress induced by adriamycin, preventing the increase in p53 (24). The reasons for this discrepancy are unknown, although it is possible that Snail1 might act on genes involved in adriamycin export. Alternatively, it is possible that the factor responsible for p53 repression is not Snail1 by itself, but another transcriptional repressor specifically induced by Snail1 in MCF-7 and not in other cells.
Our results show that Snail1 prevents the up-regulation of PTEN phosphatase, an inhibitor of the PI3K/Akt pathway (48). The role of this pathway and PTEN in the modulation of apoptosis has been clearly demonstrated by several studies. The reexpression of PTEN in several carcinoma cell lines can induce apoptosis directly or in cooperation with apoptotic stimuli (38); therefore, the ectopic manipulation of PTEN levels in MDCK cells affects the capability of the cells to undergo apoptosis. For instance, the depletion of PTEN by an interferent RNA increases the resistance of MDCK cells to γ radiation-induced apoptosis (Fig. 4A), indicating the role of PTEN and, therefore, of Snail1 in the control of cell death. However, even in cells without PTEN expression, Snail1 causes a further increase in resistance to apoptosis, indicating that Snail1 is also acting on another cellular element. Experiments performed with RWP-1 cells also confirmed this conclusion. Therefore, our studies indicate that although the repression of PTEN by Snail1 contributes to the resistance to cell death, Snail1 is also acting on other factors involved in the regulation of this cellular event.
We have also determined that Snail1 repression of the PTEN promoter is specific, since Snail2 (Slug) presents very little activity on this promoter and other repressors with a similar specificity, such as Zeb1, are also inactive. Like what has been reported for other genes controlled by Snail1 (4, 28), the effect of Snail1 on this promoter is dependent on the integrity of two 5′-CACCTG-3′ boxes present in the proximal human promoter. The mutation of these two elements precludes not only the association of recombinant Snail1 to this sequence but also the repression by Snail1 of PTEN promoter activity. One box was present in PTEN promoters from all mammals studied, such as mouse, rat, rabbit, dog, and others, suggesting that probably only one of these elements present in the human PTEN promoter is involved in repression. Perhaps because of the existence of just one functional binding element, the effect of Snail1 is lower than that measured on the E-cadherin promoter (4) (contains three E boxes) but comparable to the effect of this repressor on the promoter of other targets such as the vitamin D receptor (28).
ChIP analysis demonstrated that Snail1 binds to the PTEN promoter. The association of Snail1 to this promoter precludes the binding of p53, a transcriptional activator of PTEN during apoptosis. The similarities existent between the regulation of PTEN and that of Puma during the process of cell death are noteworthy, although they also present relevant differences. Both genes are induced after γ irradiation by p53, and this induction is prevented in cells expressing Snail1 (in the case of PTEN) or expressing Snail2 (in the case of Puma) (46). It is also remarkable that both genes show a high specificity for their corresponding repressors, since Snail2 cannot repress the PTEN promoter (Fig. 5) and Snail1 neither binds to the Puma promoter nor affects Puma mRNA up-regulation in response to γ irradiation (Fig. 6 and data not shown).
Snail1 protein is up-regulated in response to DNA damage at a posttranslational level. Our results indicate that the accumulation of Snail1 is mainly a consequence of the modification of Ser246. The phosphorylation of this residue by PAK1 prevents Snail1 export from the nucleus and its subsequent degradation (49). This protein kinase (the γ isoform) is activated after DNA damage in fibroblasts (34). Moreover, PAK1 has been shown to down-regulate several proapoptotic pathways (reviewed in reference 25). Therefore, it is likely that the modification of Ser246 in Snail1 after γ irradiation is catalyzed by this kinase.
It is noteworthy that this increase in Snail1 protein is transient. For instance, in MDCK cells 8 h after irradiation, the total levels of the protein are lower than those before this insult. However, this down-regulation is not reflected in a concomitant decrease in Snail1-PTEN promoter association. This apparent discrepancy might be explained by the fact that the binding of Snail1 to DNA stabilizes this protein (M. Escrivà and A. Garcia de Herreros, unpublished observations), probably because it prevents its export from the nucleus. Therefore, we expect that PTEN promoter-bound Snail1 is not an efficient target for the nuclear export machinery and the repression of the activity of this promoter is maintained even after the cellular levels of Snail1 have returned to the basal levels.
In spite of its important role in the modulation of apoptosis, not much is known about the mechanisms controlling the expression of PTEN, other than the transcription of this gene is sensitive to the direct binding and activation of p53 of its promoter (39). It has been also reported that PTEN levels are negatively regulated by transforming growth factor β and NF-κB (26, 47), two factors that stimulate Snail1 transcription in MDCK and other epithelial cells (2, 29). Mutations and deletions in the PTEN gene have previously been described for a wide variety of tumors, although in some advanced carcinomas, such as prostatic and endometrial carcinomas, as well as melanomas, the silencing of this gene seems to be controlled epigenetically (35, 45, 52). It is possible that Snail1 is responsible for this inhibition, considering that this gene is expressed in advanced tumors (3). However, the limited expression of Snail1 to specific areas of epithelial tumors (13) suggests that Snail1 might be involved in the down-regulation of PTEN in cells that are undergoing an EMT, more than in the permanent silencing of this gene. Similarly, since Snail1 is expressed only in a subset of fibroblasts (activated fibroblasts) (13), the negative effect of Snail1 on PTEN expression might be limited to these cells.
As indicated above, a consequence of PTEN repression is the activation of Akt observed after the transfection of Snail1. Curiously, the overexpression of Akt has also been shown to induce EMT through the NF-κB-dependent activation of Snail1 (16, 23). These results suggest the existence of a positive feedback loop wherein Snail1 might induce its own transcription. Actually, results from our lab indicate that Snail1 can stimulate the activity of its own promoter in a cell-specific manner (M. Escrivà, S. Peiró, and A. Garcia de Herreros, unpublished observations). This positive feedback loop would be coordinated with the negative self-regulation already described for this gene, since Snail1 is also capable of repressing its own synthesis, both directly, through the binding to its own promoter (32), and indirectly, through the inhibition of Egr-1, an activator of its transcription (17). The existence of transcriptional feedback loops has previously been described, and they seem to be particularly relevant for cell pathways implicated in embryo development (14). A possible consequence of the operation of the positive and negative feedback controls of a gene is the appearance of oscillatory patterns of expression (14). In this respect, it is remarkable that oscillations in the levels of Snail1 RNA have been detected in the presomitic mesoderm (8). In any case, this positive feedback loop might help coordinate and integrate the signals provided by factors of the fibroblast growth factor and transforming growth factor superfamilies required for the induction of Snail1 during the development and subsequent triggering of EMT (3, 9, 31).
Moreover, in addition to its role in the modulation of apoptosis, PTEN reconstitution or overexpression inhibits cell migration (48). PTEN-null mouse fibroblasts show increased rates of migration, a property that is reversed by the reintroduction of PTEN (27). PTEN also prevents tumoral cell invasion (42). It has been suggested that the effects of PTEN are due not only to its lipid phosphatase activity but also to its tyrosine phosphatase activity on focal adhesion kinase and Shc (48). In any case, since the overexpression of Snail1 in different cell lines induces migration and invasion, the possibility that the down-regulation of PTEN is relevant for these effects is worth being studied.
Acknowledgments
We greatly appreciate the advice and help of Gabriel Gil. We also thank Marta Garrido and Álvaro Jansà for technical assistance, Isabel Puig and Lionel Larue for PTEN cDNA, and Lauro Sumoy for his help in the interpretation of the ChIP-on-ChIP experiments.
This study was supported by grants from the Ministerio de Ciencia y Tecnología to A.G.H. (SAF2003-02324 and SAF2006-00339), from the American Cancer Society to S.A.M. (PF-04-245-01-DDC), and from the NIH to T.G. (HD034883). Partial support from Instituto Carlos III (RTICCC and C03710) and from the Generalitat de Catalunya (2005SGR00970) is also appreciated. M.E, N.H., and P.V. are recipients of predoctoral fellowships from the Ministerio de Educación. S.P. was supported by a La Cierva contract.
Footnotes
Published ahead of print on 2 January 2008.
REFERENCES
- 1.Alessi, D. R., M. Adjelkovic, B. Caudwell, P. Cron, N. Morrice, P. Cohen, and B. A. Hemmings. 1996. Mechanims of activation of protein kinase B by insulin and IGF-1. EMBO J. 156541-6551. [PMC free article] [PubMed] [Google Scholar]
- 2.Barberà, M. J., I. Puig, D. Domínguez, S. Julien-Grille, S. Guaita-Esteruelas, S. Peiró, J. Baulida, C. Francí, S. Dedhar, L. Larue, and A. García de Herreros. 2004. Regulation of Snail transcription during epithelial to mesenchymal transition of tumor cells. Oncogene 237345-7354. [DOI] [PubMed] [Google Scholar]
- 3.Barrallo-Gimeno, A., and M. A. Nieto. 2005. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development 1323151-3161. [DOI] [PubMed] [Google Scholar]
- 4.Batlle, E., E. Sancho, C. Francí, D. Domínguez, M. Monfar, J. Baulida, and A. García de Herreros. 2000. The transcription factor Snail is a repressor of E-cadherin gene expression in epithelial tumor cells. Nat. Cell Biol. 284-89. [DOI] [PubMed] [Google Scholar]
- 5.Bolos, V., H. Peinado, M. A. Perez-Moreno, M. F. Fraga, M. Esteller, and A. Cano. 2003. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J. Cell Sci. 116499-511. [DOI] [PubMed] [Google Scholar]
- 6.Cano, A., M. A. Pérez-Moreno, I. Rodrigo, A. Locascio, M. J. Blanco, M. G. del Barrio, F. Portillo, and M. A. Nieto. 2000. The transcription factor Snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 278-83. [DOI] [PubMed] [Google Scholar]
- 7.Carver, E. A., J. Rulang, Y. Lan, K. F. Oram, and T. Gridley. 2001. The mouse Snail gene encodes a key regulator of the epithelial-mesenchymal transition. Mol. Cell. Biol. 218184-8188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dale, J. K., P. Malapaert, J. Chal, G. Vilhais-Neto, M. Maroto, T. Johnson, S. Jayshinge, P. Trainor, B. Herrmann, and O. Pourquier. 2006. Oscillations of the snail genes in the presomitic mesoderm coordinate segmental patterning and morphogenesis in vertebrate somitogenesis. Dev. Cell 10355-366. [DOI] [PubMed] [Google Scholar]
- 9.De Crane, B., F. van Roy, and G. Berx. 2005. Unraveling signalling cascades from the Snail family of transcription factors. Cell. Signal. 17535-547. [DOI] [PubMed] [Google Scholar]
- 10.De Crane, B., B. Gilbert, C. Stove, E. Bruyneel, F. van Roy, and G. Berx. 2005. The transcription factor Snail induces tumor cell invasion through modulation of the epithelial cell differentiation program. Cancer Res. 656237-6244. [DOI] [PubMed] [Google Scholar]
- 11.Domínguez, D., B. Montserrat-Sentís, A. Virgós-Soler, S. Guaita, J. Grueso, M. Porta, I. Puig, J. Baulida, C. Francí, and A. García de Herreros. 2003. Phosphorylation regulates nuclear export and activity of Snail transcriptional repressor. Mol. Cell. Biol. 235078-5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Espineda, C. E., J. H. Chang, J. Twiss, S. A. Rajasekran, and A. K. Rajasekran. 2004. Repression of Na,K-ATPase b1 subunit by the transcription factor Snail in carcinoma. Mol. Biol. Cell 151364-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Francí, C., M. Takkunen, N. Dave, F. Alameda, S. Gómez, R. Rodríguez, M. Escrivà, B. Montserrat-Sentís, T. Baró, M. Garrido, F. Bonilla, I. Virtanen, and A. García de Herreros. 2006. Expression of Snail in tumor-stroma interface. Oncogene 255134-5144. [DOI] [PubMed] [Google Scholar]
- 14.Freeman, M. 2000. Feedback control of intercellular signalling in development. Nature 408313-319. [DOI] [PubMed] [Google Scholar]
- 15.Reference deleted.
- 16.Grille, S. J., A. Bellacosa, J. Upson, A. J. Klein-Szanto, F. Van Roy, W. Lee-Kwon, M. Donowitz, P. N. Tsichlis, and L. Larue. 2003. The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res. 632172-2178. [PubMed] [Google Scholar]
- 17.Gronegut, S., D. von Schweinitz, G. Christofori, and F. Lehembre. 2006. Hepatocyte growth factor induces cell scattering through MAPK/Egr-1 mediated upregulation of Snail. EMBO J. 253534-3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guaita, S., I. Puig, C. Francí, M. Garrido, D. Domínguez, E. Batlle, E. Sancho, S. Dedhar, A. García de Herreros, and J. Baulida. 2002. Snail induction of epithelial-to-mesenchymal transition in tumor cells is accompanied by MUC-1 repression and ZEB1 expression. J. Biol. Chem. 27730209-39216. [DOI] [PubMed] [Google Scholar]
- 19.Huber, M. A., N. Kraut, and H. Beug. 2005. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr. Opin. Cell Biol. 171-11. [DOI] [PubMed] [Google Scholar]
- 20.Ikenouchi, J., M. Matsuda, M. Furue, and S. Tsukita. 2003. Regulation of tight junctions during epithelium-mesenchymal transition: direct expression of gene expression of claudins/occludin by Snail. J. Cell Sci. 1161959-1967. [DOI] [PubMed] [Google Scholar]
- 21.Inoue, A., M. G. Seidel, W. Wu, S. Kamizono, A. A. Ferrando, R. T. Bronson, H. Iwasaki, K. Akashi, A. Morimoto, J. K. Hitzler, T. T. Pestina, C. W. Jackson, R. Tanaka, M. J. Chong, P. J. McKinnon, T. Inukai, G. C. Grosveld, and T. A. Look. 2002. Slug, a highly conserved zinc finger transcriptional repressor, protects hematopoietc progenitor cells from radiation-induced apoptosis in vivo. Cancer Cell 2279-288. [DOI] [PubMed] [Google Scholar]
- 22.Jamora, C., P. Lee, P. Kocieniewski, M. Azhar, R. Hosokawa, Y. Chai, and E. Fuchs. 2005. A signaling pathway involving TGF-β2 and Snail in hair follicle morphogenesis. PLoS Biol. 3e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Julien, S., I. Puig, E. Caretti, J. Bonaventure, L. Nelles, F. van Roy, C. Dargemont, A. García de Herreros, A. Bellacosa, and L. Larue. 2007. Activation of NF-κB by Akt upregulates Snail expression and induces epithelium-mesenchymal transition. Oncogene 267445-7456. [DOI] [PubMed] [Google Scholar]
- 24.Kajita, M., K. N. McClinic, and P. Wade. 2004. Aberrant expression of the transcriptional factors Snail and Slug alters the response to genotoxic stress. Mol. Cell. Biol. 247559-7566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar, R., A. E. Gururaj, and C. Barnes. 2006. p21-activated kinases in cancer. Nat. Rev. Cancer 6459-471. [DOI] [PubMed] [Google Scholar]
- 26.Li, D. M., and H. Sun. 1997. TEP1, encoded by a candidate tumor suppressor locus, is a novel protein tyrosine phosphates regulated by transforming growth factor beta. Cancer Res. 572124-2129. [PubMed] [Google Scholar]
- 27.Liliental, J., S. Y. Moon, R. Lesche, R. Mamillapalli, D. Li, Y. Zheng, H. Sun, and H. Wu. 2000. Genetic deletion of the Pten tumor suppressor gene promotes cell motility by activation of Rac1 and Cdc42 GTPases. Curr. Biol. 10401-404. [DOI] [PubMed] [Google Scholar]
- 28.Pálmer, H. G., M. J. Larriba, J. M. García, P. Ordóñez-Morán, C. Peña, S. Peiró, I. Puig, R. Rodríguez, R. De la Fuente, A. Bernad, M. Pollán, F. Bonilla, C. Gamallo, A. García de Herreros, and A. Muñoz. 2004. The transcription factor SNAIL represses vitamin D receptor expression and responsiveness in human colon cancer. Nat. Med. 10917-919. [DOI] [PubMed] [Google Scholar]
- 29.Peinado, H., M. Quintanilla, and A. Cano. 2003. Transforming growth factor beta-1 induces snail transcription factor in epithelial cell lines: mechanisms for epithelial mesenchymal transitions. J. Biol. Chem. 2782113-2123. [DOI] [PubMed] [Google Scholar]
- 30.Peinado, H., E. Ballestar, M. Esteller, and A. Cano. 2004. Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol. Cell. Biol. 24306-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peinado, H., D. Olmeda, and A. Cano. 2007. Snail, ZEB and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat. Rev. Cancer 7415-428. [DOI] [PubMed] [Google Scholar]
- 32.Peiró, S., M. Escrivà, I. Puig, M. J. Barberà, N. Dave, N. Herranz, M. J. Larriba, M. Takkunen, C. Francí, A. Muñoz, I. Virtanen, J. Baulida, and A. García de Herreros. 2006. Snail transcriptional repressor binds to its own promoter and controls its expression. Nucleic Acids Res. 342077-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pérez-Mancera, P. A., M. Pérez-Caro, I. González-Herrero, T. Flores, A. Orfao, A. García de Herreros, A. Gutiérrez-Adán, B. Pintado, A. Sagrera, M. Sánchez-Martín, and I. Sánchez-García. 2005. Cancer development induced by graded expression of Snail in mice. Hum. Mol. Genet. 143449-3461. [DOI] [PubMed] [Google Scholar]
- 34.Roig, J., and J. A. Traugh. 1999. p21-activated protein kinase γ-PAK is activated by ionizing radiation and other DNA-damaging agents. J. Biol. Chem. 27431119-31122. [DOI] [PubMed] [Google Scholar]
- 35.Salvesen, H. B., I. Stefansson, E. I. Kretzschmar, P. Gruber, N. D. MacDonald, A. Ryan, I. J. Jacobs, L. A. Akslen, and S. Das. 2001. PTEN methylation is associated with advanced stage and microsatellite instability in endometrial carcinoma. Int. J. Cancer 922-26. [DOI] [PubMed] [Google Scholar]
- 36.Savagner, P. 2001. Leaving the neighborhood: molecular mechanisms involved during epithelial-mesenchymal transition. Bioessays 23912-923. [DOI] [PubMed] [Google Scholar]
- 37.Shieh, S. Y., M. Ikeda, Y. Taya, and C. Prives. 1997. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell 91325-334. [DOI] [PubMed] [Google Scholar]
- 38.Simpson, L., and R. Parsons. 2001. Pten: life as a tumor suppressor. Exp. Cell Res. 26429-41. [DOI] [PubMed] [Google Scholar]
- 39.Stambolic, V., D. MacPherson, D. Sas, Y. Lin, B. Snow, Y. Jang, S. Benchimol, and T. W. Mak. 2001. Regulation of PTEN transcription by p53. Mol. Cell 8317-325. [DOI] [PubMed] [Google Scholar]
- 40.Sulis, M. L., and R. Parsons. 2003. PTEN: form pathology to biology. Trends Cell Biol. 13478-483. [DOI] [PubMed] [Google Scholar]
- 41.Taki, M., N. Kamata, K. Yokoyama, R. Fujimoto, S. Tsutsumi, and M. Nagayama. 2003. Down-regulation of Wnt-4 and up-regulation of Wnt-5A expresión by epithelial-mesenchymal transition in human squamous carcinoma cells. Cancer Sci. 94593-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tamura, M., J. Gu, T. Takino, and K. M. Yamada. 1999. Tumor suppressor PTEN inhibition of cell invasion, migration and growth: differential involvement of focal adhesion kinase and p120-Cas. Cancer Res. 59442-449. [PubMed] [Google Scholar]
- 43.Thiery, J. P. 2003. Epithelial-mesenchymal transitions in development and pathologies. Curr. Opin. Cell Biol. 15740-746. [DOI] [PubMed] [Google Scholar]
- 44.Vega, S., A. V. Morales, O. H. Ocaña, F. Valdés, I. Fabregat, and M. A. Nieto. 2004. Snail blocks the cell cycle and confers resistance to cell death. Genes Dev. 181131-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whang, Y. E., X. Wu, H. Suzuki, R. E. Reiter, C. Tran, R. L. Vessella, J. W. Said, W. B. Isaacs, and C. L. Sawyers. 1998. Inactivation of the tumor suppressor PTEN /MMAC1 in advanced human prostate cancer through loss of expression. Proc. Natl. Acad. Sci. 955246-5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu, W. S., S. Heirichs, D. Xu, S. P. Garrison, G. P. Zambetti, J. M. Adams, and A. T. Look. 2005. Slug antagonizes p53-mediated apoptosis of hematopoietic progenitors by repressing puma. Cell 123641-653. [DOI] [PubMed] [Google Scholar]
- 47.Xia, D., H. Srinivas, Y. Ahn, G. Sethi, X. Sheng, W. K. A. Yung, Q. Xia, P. J. Chiao, H. Kim, P. H. Brown, I. I. Wistuba, B. B. Aggarwal, and J. M. Kurie. 2007. Mitogen-activated protein kinase kinase-4 promotes cell survival by decreasing PTEN expression through and NFκB-dependent pathway. J. Biol. Chem. 2823507-3519. [DOI] [PubMed] [Google Scholar]
- 48.Yamada, K. M., and M. Araki. 2002. Tumor suppressor PTEN: modulator of cell signalling, growth, migration and apoptosis. J. Cell Sci. 1142375-2382. [DOI] [PubMed] [Google Scholar]
- 49.Yang, Z., S. Rayala, D. Nguyen, R. K. Vadlamundi, S. Chen, and R. Kumar. 2005. Pak1 phosphorylation of Snail, a master regulator of epithelial-to-mesenchymal transition, modulates Snail's subcellular localization and functions. Cancer Res. 653179-3184. [DOI] [PubMed] [Google Scholar]
- 50.Yook, J. I., X. Y. Li, I. Ota, E. Fearon, and S. J. Weiss. 2005. Wnt-dependent regulation of E-cadherin-repressor Snail. J. Biol. Chem. 28011740-11748. [DOI] [PubMed] [Google Scholar]
- 51.Zhou, B. P., J. Deng, W. Xia, J. Xu, Y. M. Li, M. Gunduz, and M. C. Hung. 2004. Dual regulation of Snail by GSK-3β-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat. Cell Biol. 6931-940. [DOI] [PubMed] [Google Scholar]
- 52.Zhou, X. P., O. Gimm, H. Hampel, T. Niemann, M. J. Walker, and C. Eng. 2000. Epigenetic PTEN silencing in malignant melanomas without PTEN mutation. Am. J. Pathol. 1571123-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]