Abstract
Janus kinases are essential for signal transduction by a variety of cytokine receptors and when inappropriately activated can cause hematopoietic disorders and oncogenesis. Consequently, it can be predicted that the interaction of the kinases with receptors and the events required for activation are highly controlled. In a screen to identify phosphorylation events regulating Jak2 activity in EpoR signaling, we identified a mutant (Jak2-Y613E) which has the property of being constitutively activated, as well as an inactivating mutation (Y766E). Although no evidence was obtained to indicate that either site is phosphorylated in signaling, the consequences of the Y613E mutation are similar to those observed with recently described activating mutations in Jak2 (Jak2-V617F and Jak2-L611S). However, unlike the V617F or L611S mutant, the Y613E mutant requires the presence of the receptor but not Epo stimulation for activation and downstream signaling. The properties of the Jak2-Y613E mutant suggest that under normal conditions, Jak2 that is not associated with a receptor is locked into an inactive state and receptor binding through the FERM domain relieves steric constraints, allowing the potential to be activated with receptor engagement.
The tyrosine kinase Jak2 is the essential component of erythropoietin receptor (EpoR) signal transduction (26-28), and deregulation of the enzyme has been associated with hematopoietic disorders and oncogenesis (10, 13, 18-19). Under normal conditions, various levels of regulation maintain Jak2 in its inactive form until receptor activation occurs and involve interactions of the pseudokinase domain (JH2) and FERM domain (JH4 to -7) with the kinase domain (JH1). One of the essential events involves tyrosine phosphorylation within the activation loop (Y1007-Y1008) (5). This phosphorylation event allows the kinase to be fully active, most likely by promoting conformational changes which removes steric constraints imposed by the nonphosphorylated form.
Another level of regulation involves the pseudokinase domain (or JH2 domain). Theoretical models of Jak2 structure suggest that the JH1 and JH2 domains of the kinase are facing each other and the activation loop of Jak2 is buried at this interface (8). Phosphorylation of the activation loop is believed to prevent this JH1-JH2 interaction and therefore relieves inhibition. Whether phosphorylation of the activation loop occurs before or after the changes in the JH1-JH2 interactions is unknown. The requirement for the JH2 domain in preventing Jak2 from being inappropriately activated has been well illustrated by the increased catalytic activity of a JH2 deletion mutant in the absence of receptor engagement (31) and by the observations that JH2 domain mutations are associated with various myeloproliferative diseases (10, 13, 19).
A third level of regulation has been ascribed to the FERM domain. Not only is this domain required for receptor association, but it is proposed to also play a role in the overall organization of the tyrosine kinase structure and its subsequent activation. Naturally occurring mutations in the FERM domain of Jak3 have been identified, and some mutants have the property of maintaining association with the receptor but are inactive due to the lack of activation of the kinase domain (35).
In addition, postactivation mechanisms exist that down-regulate enzyme activity. In particular, activation of Jak2 results in phosphorylation of a tyrosine residue located in the FERM domain (Y119), promoting the dissociation of the kinase from the receptor and its subsequent degradation through a yet-to-be-identified proteolytic pathway (7). Other sites of phosphorylation include both tyrosine and serine residues in Jak2, but site-directed mutagenesis studies suggest that these events have relatively minor effects on the biological activity of the kinase (1, 4, 12, 16, 17, 24). Postactivation regulation also includes dephosphorylation, possibly through various protein tyrosine phosphatases, such as SH-PTP1, PTP1B, and CD45, and association of suppressors of cytokine signaling, which are proposed to bind activated Jaks and target them for degradation (2, 11, 14, 25, 32).
In the course of studies designed to identify regulatory phosphorylation events on Jak2, we identified a mutant of Jak2 that had the property of being constitutively active in the presence of the EpoR without receptor activation by its ligand Epo. The properties of this mutant allow us to propose a model for Jak2 activation by EpoR in which EpoR-unbound Jak2 is maintained in an inactive state and to propose that receptor binding is the first step in unlocking the potential to activate the kinase.
MATERIALS AND METHODS
Antibodies and reagents.
Reagents used in this study were from Fisher Scientific and Sigma Chemicals. The anti-Flag M2 antibody and agarose-conjugated Flag antibody were from Sigma Chemicals. The monoclonal antiphosphotyrosine antibody 4G10 was purchased from Upstate Biotechnologies. The rabbit polyclonal antibodies directed against phospho-Jak2 (Y1007/Y1008) and phospho-STAT5 (Y694) were from Cell Signaling Technologies. Antisera against STAT5A and STAT5B were produced in-house and described elsewhere (29). Peroxidase-conjugated anti-rabbit and anti-mouse antibodies were purchased from Amersham Life Technologies. Human retronectin was purchased from Takara, Inc. Recombinant human Epo was purchased from AMGEN, and recombinant SCF, interleukin 6 (IL-6), and IL-3 were purchased from R&D Systems.
Plasmids.
The cDNA encoding murine Jak2 containing the C-terminal hemagglutinin (HA) tag was subcloned into MSCV-IRES-GFP (7). Mutagenesis of amino acid residues in Jak2 (K882R, Y570F, Y570E, Y590F, Y590E, Y613F, Y613E, Y637F, Y637E, Y760F, Y760E, Y790F, Y790E, Y813F, Y868F, Y570F, Y913F, Y918F, Y931F, Y934F, Y940F, Y956F, Y966F, Y972F, Y1007F, Y1008F, Y1021F, Y1045F, Y1050F, and Y1099F) was performed using a site-directed mutagenesis kit according to the manufacturer's instructions (Stratagene). The cDNA encoding the murine EpoR containing a c-terminal Flag tag was described previously (28).
Cell culture.
Murine embryonic fibroblasts (MEFs) derived from wild-type (WT) or Jak2−/− embryos (Jak2−/− MEFs) and human epithelial kidney cells (Hek293T) were cultured in DMEM (high glucose) supplemented with fetal bovine serum (10% [vol/vol]), l-glutamine, penicillin, and streptomycin (Invitrogen/Gibco). The Il-3-dependent murine hematopoietic cell line Ba/F3 was cultured in RPMI-1640 supplemented with fetal bovine serum (10% [vol/vol]), l-glutamine, penicillin, and streptomycin and 2.5 ng/ml murine IL-3. The cells were maintained in exponentially growing phase unless otherwise mentioned. E12.5 Jak2−/− and EpoR−/− fetal liver cells were isolated and cultured as described previously (27, 28).
Retroviral infections.
Retroviruses expressing the various cDNAs were derived as described previously (7, 28). Ba/F3 cells were infected three times at 3-h intervals with 2 ml of fresh virus-containing supernatants in complete medium containing 8 μg/ml polybrene (Sigma Chemical). Twenty-four hours after infection, Ba/F3 cells were sorted based on green fluorescent protein expression and selected with puromycin (5 μg/ml) for 2 days, and therewith growth assays were performed. Exponentially growing Jak2−/− MEFs cells were infected three times at 3-h intervals with 2 ml of fresh virus-containing supernatant in complete medium containing 8 μg/ml polybrene.
Ba/F3 cell growth assays.
Transduced and exponentially growing Ba/F3 cells were washed twice with phosphate-buffered saline (PBS) and then left untreated or stimulated with IL-3 (2.5 ng/ml) or Epo (5 U/ml) for the appropriate times. Living cells were counted using a VI-Cell counter (Beckman Coulter, Fullerton, CA).
Colony assays.
EpoR−/− and Jak2−/− fetal liver cells (2 × 105 cells/ml) were isolated and transduced with the various viruses as described previously (7, 28). Twenty-four hours after infection, fetal liver cells were washed twice, resuspended in MethoCult M3334, plated in 3.5-cm dishes in duplicate, and cultured at 37°C, 5% CO2. For the CFU-E assay, 2 × 105 cells/dish were cultured with or without 0.2 U/ml Epo (Amgen), and benzidine-positive CFU-E colonies were scored at day 3. For the BFU-E assay, 2 × 105 cells/dish were cultured in 3 U/ml recombinant human Epo and 10 ng/ml recombinant murine IL-3, and benzidine-positive BFU-E colonies were scored at day 8.
Immunoprecipitation and Western blotting.
After stimulation, the cells were washed twice in ice-cold buffer and lysed in lysis buffer (10 mM sodium phosphate [pH 7.2], 150 mM NaCl, 1% sodium deoxycholate, 1% NP-40, 10 mM sodium phosphate, 2 mM EDTA, 50 mM NaF, 0.2 mM Na3VO4) supplemented with protease inhibitors (Complete; Roche). Lysates were cleared by centrifugation at 4°C, 12,000 RPM, for 5 min. The cleared lysates were mixed with an equal volume of 2× Laemmli buffer and denatured at 95°C for 5 min. Samples were run on either a 7.5 or 10% sodium dodecyl sulfate-polyacrylamide gel and transferred to nitrocellulose membranes. For immunoprecipitation assays, cells were washed twice with ice-cold PBS and lysed in lysis buffer. Lysates were cleared by centrifugation as described above and subjected to immunoprecipitation using the indicated antibodies. Immunoprecipitated protein complexes were eluted using the Flag peptide (for M2 immunoprecipitation assays; Sigma) as described by the manufacturer or with 2× Laemmli buffer. Samples were run on either a 7.5 or 10% sodium dodecyl sulfate-polyacrylamide gel, transferred to nitrocellulose or polyvinylidene difluoride membranes, and analyzed by Western blotting. For Western blotting, membranes were blocked at room temperature for 1 h with 5% nonfat milk (5% bovine serum albumin fraction V was used to probe with the phospho-specific antibodies) in TBST (50 mM Tris-Cl, pH 7.6, 150 mM NaCl, 0.1% Tween). Membranes were washed three times with TBST and probed with the various phospho-specific antibodies overnight at 4°C or 2 h at room temperature for other antibodies.
RESULTS
Activation of Jak2 by EpoR engagement leads to the tyrosine phosphorylation of the receptor and Jak2. While the biological activities ascribed to the tyrosine phosphorylation sites on the receptor have been well documented (28, 30, 33), the role for the sites of tyrosine phosphorylation on Jak2 are only beginning to be explored in detail (1, 4, 5, 7, 12, 16, 23, 24). To address this issue, we have been systematically mutating Jak2 tyrosine residues to phenylalanine in order to identify mutants that would affect the biological activity of the kinase in the context of EpoR signaling (7). Mutated kinases are assessed by cotransfection of the kinase with a retrovirus encoding wild-type EpoR into Jak2-deficient MEFs and measurement of Epo-induced tyrosine phosphorylation of the activation loop of Jak2 or tyrosine phosphorylation of Stat5.
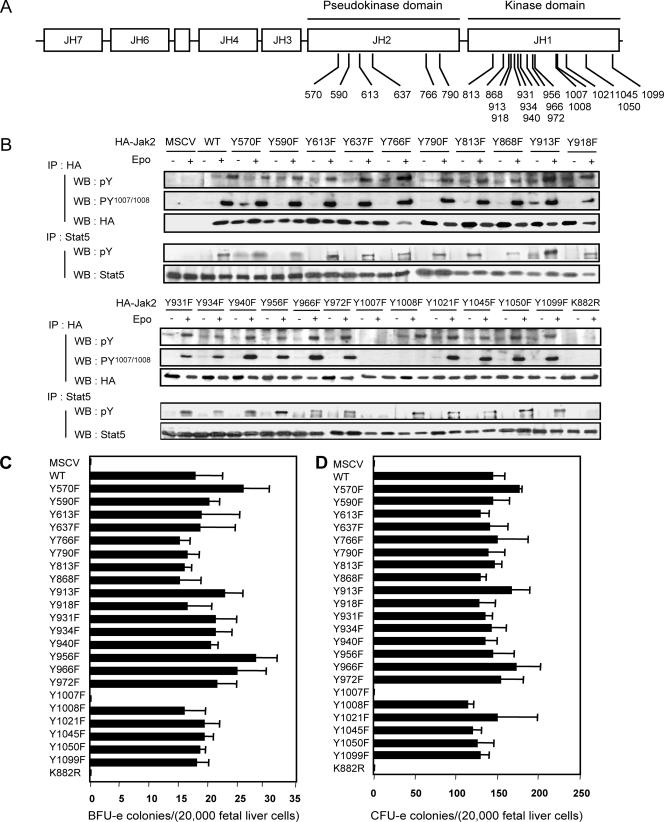
The results obtained with 22 mutants (Fig. 1A), in which each of the tyrosines in the pseudokinase (JH2) and kinase (JH1) domains was mutated to phenylalanine, are illustrated in Fig. 1B. Somewhat remarkably, with the exception of the activation loop tyrosine (Y1007) mutant, none of the mutants were significantly altered in their ability to function in Epo responses. As a more physiologically relevant assessment of function, we also determined the abilities of the mutants to rescue Epo or Epo/IL-3-induced BFU-e or CFU-e colony formation, respectively (Fig. 1C and D). Consistent with the results obtained in Jak2-deficient MEFs, only the activation loop mutant (Y1007F) lost activity. Of particular note is the lack of consequences of the Y813F and Y570F mutations for biological activity of Jak2 in the context of EpoR signaling, since previous studies had implicated these sites of tyrosine phosphorylation in regulation of Jak2 function (1, 4, 16-17).
FIG. 1.
Structure and biological activity associated with the various tyrosine-to-phenylalanine mutations in the JH1 and JH2 domains of Jak2. (A) Structure of Jak2 tyrosine kinase, the Jak homology domain (JH), and putative tyrosine phosphorylation sites. JH1, kinase domain; JH2, pseudokinase domain; JH3, SH2-like domain; JH4 to -7, FERM domain. Numbers indicate the amino acid positions of the various tyrosine residues located in the JH1 and JH2 domains. (B) Jak2-deficient mouse embryonic fibroblasts were infected with retroviruses encoding EpoR together with retroviruses encoding HA-tagged Jak2 (WT), the indicated mutants of Jak2, or retroviruses not encoding Jak2 (murine stem cell virus [MSCV]). Forty-eight hours after infection, cells were serum starved for 16 h and then stimulated or not with Epo (5 U/ml) for 15 min before solubilization. Cell lysates were subjected to immunoprecipitation using an anti-HA or anti-STAT5 antibody. Immune complexes were analyzed by Western blotting using antibodies directed against phosphotyrosines (4G10 and pY), activating tyrosine phosphorylation of Jak2 (pY1007/Y1008), the HA epitope on Jak2 (HA), and STAT5. These experiments and the similar experiments shown in subsequent figures have been done at least three times. (C and D) Biological activity ascribed to the various Jak2 tyrosine mutants. Jak2-deficient fetal liver cells were infected with retroviruses encoding the indicated Jak2 mutants and then subjected to in vitro colony formation assays in the absence or presence of Epo (5 U/ml) (C) or Epo (5 U/ml) and IL-3 (1.8 ng/ml) (D). The benzidine-positive CFU-e and BFU-e colonies were scored at day 3 and day 8, respectively. In this and subsequent figures, the error bars indicate the standard deviation in colony numbers among the replicates.
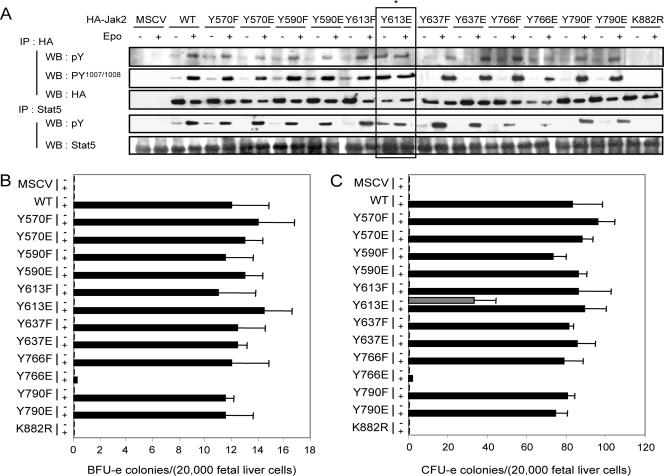
In the course of these studies, we also examined the consequences of mutating tyrosines within the pseudokinase domain to glutamic acid (Fig. 2). As illustrated, Jak2-deficient MEFs expressing WT Jak2, as well as virtually all the Y-E mutants examined, reconstituted Epo-dependent EpoR signal transduction as shown by comparable levels of Jak2 and Stat5 activation. It should also be noted that with Epo stimulation, the total levels of HA-tagged Jak2 are frequently decreased. This is consistent with our previous studies that demonstrated that activated Jak2 is more rapidly degraded (7). One mutation, Y766E, was an inactivating mutation. The properties of the corresponding mutant have not been further investigated.
FIG. 2.
Biological activity associated with various tyrosine-to-phenylalanine or -glutamic acid mutants of the JH2 domain of Jak2. (A) Biological activity associated with mutants of Jak2 in which the various tyrosines have been mutated for either a phenylalanine or a glutamic acid. Jak2-deficient mouse embryonic fibroblasts were infected with retroviruses encoding EpoR together with retroviruses encoding HA-tagged Jak2 (WT), the indicated mutants of Jak2, or retroviruses not encoding Jak2 (murine stem cell virus [MSCV]). Forty-eight hours after infection, cells were serum deprived for 16 to 24 h and then stimulated or not with Epo (5 U/ml) for 15 min before solubilization. Cell lysates were subjected to immunoprecipitation using an anti-HA or anti-STAT5 antibody. Immunoprecipitated proteins were then analyzed by Western blotting using antibodies directed against phosphorylated tyrosines (4G10 and pY), activating tyrosine phosphorylation of Jak2 (pY1007/Y1008), the HA epitope on Jak2 (HA), and STAT5. Note the constitutive global tyrosine phosphorylation of Jak2, activating tyrosine phosphorylation of Jak2, and the phosphorylation of STAT5 in cells expressing EpoR together with Jak2-Y613E in the absence of Epo stimulation (*). (B and C) Epo-independent formation of CFU-e from Jak2-deficient fetal liver cells expressing Jak2-V613E. Jak2-deficient fetal liver cells were infected with retroviruses encoding the indicated Jak2 mutants and then subjected to in vitro colony formation assays in the absence or presence of Epo (0.2 U/ml) (B) or Epo (3 U/ml) and IL-3 (10 ng/ml) (C). The benzidine-positive CFU-e and BFU-e colonies were scored at day 3 and day 8, respectively.
Unique among the mutants, the Y613E mutant showed constitutive, Epo-independent activation of Jak2 activity as assessed by tyrosine phosphorylation within the activation loop and phosphorylation of Stat5. Also consistent with constitutive activation, there were reduced levels of the Y613E mutant relative to either wild-type Jak2 or the Y613F mutant (Fig. 2A). Furthermore, treatment of the Y613E mutant-expressing cells with Epo only marginally enhanced tyrosine phosphorylation of the activation loop of Jak2 or Stat5, suggesting that the mutant enzyme is nearly fully active (Fig. 2A).
To further analyze the properties of the Y613E mutant and the consequences of its expression in a physiological setting, we tested its ability to restore CFU-e and BFU-e colony formation in Jak2-deficient fetal liver cells. Expression of wild-type Jak2, Jak2-Y613F, and Jak2-Y613E but not the kinase-inactive mutant of Jak2 restored BFU-e and CFU-e colony formation in the presence of Epo or Epo/IL-3 (Fig. 2B and C). Consistent with its constitutive activation in Jak2-deficient MEFs, the Y613E mutant restored CFU-e colony formation in the absence of Epo (Fig. 2C). Interestingly, however, the Y613E mutant was unable to restore BFU-e colony formation in the absence of Epo/IL-3 stimulation (Fig. 2B).
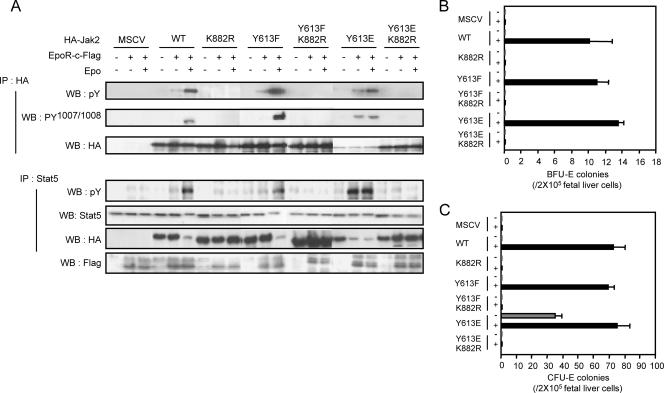
At least two mechanisms could be envisioned for the ability of the Y613E mutation to cause constitutive activation of the kinase domain. First, a conformational change in the pseudokinase/kinase domains could allow more-efficient auto/transphosphorylation in the activation loop. Alternatively, this mutation could allow activation loop phosphorylation by other cellular tyrosine kinases. To distinguish between these possibilities, we generated a Jak2 mutant that contained both the Y613E or Y613F mutation and the K882R mutation to inactivate kinase activity. Expression of these mutants in Jak2-deficient MEFs revealed that tyrosine phosphorylation of the activation loop relies on Jak2 catalytic activity, since no Y1007/Y1008 tyrosine phosphorylation was observed with the Y613E/K882R mutation (Fig. 3A). In addition, it was confirmed that these mutants were not able to induce the phosphorylation of Stat5 (Fig. 3A). Also, as illustrated in Fig. 3B and C, the mutants were unable to induce either BFU-e or CFU-e colony formation even in the presence of cytokine stimulation. In these experiments, we also assessed the requirement for EpoR for the constitutive activation of the Y613E mutant (Fig. 3A). As illustrated, activation of Jak2-Y613E was not observed in Jak2-deficient MEFs in the absence of EpoR.
FIG. 3.
The constitutive activity of Jak2-Y613E requires the expression of the EpoR and its kinase activity but not Epo stimulation. (A) Jak2-deficient MEFs were infected with retroviruses encoding the indicated Jak2 mutants together (+) or not (−) with viruses encoding EpoR-c-Flag. Forty-eight hours after infection, Jak2-deficient fibroblasts were left untreated or incubated with Epo (5 U/ml) for 15 min before solubilization. Cell lysates were subjected to immunoprecipitation using antibodies against HA (3F10) or Stat5. Cell lysates and immune complexes were then analyzed by Western blotting using antibodies directed against phosphotyrosine (4G10 and pY), activating tyrosine phosphorylation on Jak2 or the HA epitope. Lysates were also analyzed by Western blotting using the anti-Flag antibody M2 to detect EpoR expression (Flag). MSCV, empty retrovirus; K882R, Jak2 kinase dead mutant; Y613Y, Jak2-Y613F; Y613F K882R, Jak2 bearing the Y613F and K882R mutations; Y613E, Jak2-Y613E; Y613E K882R, Jak2 bearing the Y613E and K882R mutations. (B and C) Kinase activity and phosphorylation in the activation loop are required for the biological activity of Jak2-V613E mutant. Jak2-deficient fetal liver cells were infected with retroviruses encoding the indicated Jak2 mutants and then subjected to in vitro colony formation assays in the absence or presence of Epo (0.2 U/ml) (B) or Epo (3 U/ml) and IL-3 (10 ng/ml) (C). The benzidine-positive CFU-e (C) and BFU-e (B) colonies were scored at day 3 and day 8, respectively.
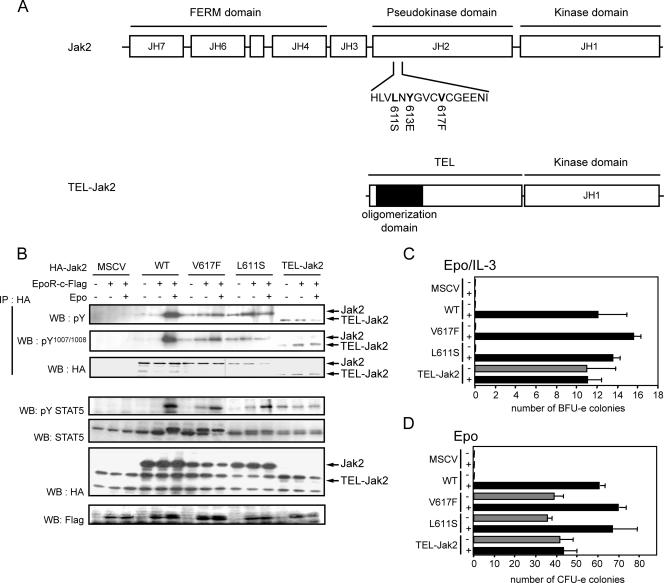
Recently two point mutations in Jak2, illustrated in Fig. 4, have been associated with constitutive activation of Jak2 kinase activity (13, 19). The two mutations are located in the JH2 domain of Jak2, in close proximity to Y613 (Fig. 4A). The similarities between the phenotypes associated with these various mutations and their proximity to Y613 prompted us to compare the properties of these mutants. We also included the Tel-Jak2 fusion protein, which has been shown to have constitutive kinase activity (18). Both V617F and L611S mutants have constitutive activity in the absence of Epo stimulation as assessed by tyrosine phosphorylation in the activation loop (Fig. 4B). In contrast to the Y613E mutant, however, activation of these mutants did not require EpoR expression (Fig. 4B). Similarly, activating tyrosine phosphorylation of TEL-Jak2 was also independent of Epo stimulation or EpoR expression. Both the V617F and L611S mutants were active as assessed by tyrosine phosphorylation of Stat5 in the absence of Epo stimulation, although, as expected, the efficient recruitment and phosphorylation of Stat5 was dependent upon EpoR with these mutants (Fig. 4B). In contrast, Tel-Jak2 is able to phosphorylate Stat5 via a receptor-independent mechanism. We also assessed their ability to rescue Jak2 deficiency in in vitro colony assays with Jak2-deficient fetal liver. Consistent with their constitutive activation in Jak2-deficient MEFs, all three mutants restored CFU-e colony formation in the absence of Epo (Fig. 4D) but only TEL-JAK2 had the ability to restore BFU-e colonies in the absence of Epo/IL-3 (Fig. 4C).
FIG. 4.
Properties of Jak2-V617F, Jak2-L611S, and TEL-Jak2 mutants. (A) Schematic representation of Jak2 mutants and fusion protein TEL-Jak2. JH1, kinase domain; JH2, pseudokinase domain; JH3, SH2-like; JH4 to -7, FERM domain; TEL-Jak2, gene product obtained from the translocation of the JAK2 gene with the translocated Ets leukemia gene TEL. The TEL-Jak2 fusion protein contains the N-terminal region of TEL, which comprises the oligomerization domain and the JH1 (kinase) domain of Jak2. (B) EpoR-independent activity of Jak2-V617F, Jak2-L611S, and TEL Jak2. Jak2-deficient MEFs were infected with retroviruses encoding the indicated Jak2 cDNAs. Forty-eight hours after infection, Jak2-deficient fibroblasts were left untreated or incubated with Epo (5 U/ml) for 15 min before solubilization. Cell lysates were subjected to immunoprecipitation using antibodies against HA (3F10). Cell lysates and immune complexes were then analyzed by Western blotting using antibodies directed against phosphotyrosine (4G10 and pY), activating tyrosine phosphorylation on Jak2, and the HA epitope, activating phosphorylation of Stat5 and Stat5. MSCV, murine stem cell virus. (C and D) Biological activities of the various Jak2 mutants and TEL-Jak2. Jak2-deficient fetal liver cells were infected with retroviruses encoding the indicated Jak2 mutants and then subjected to in vitro colony formation assays in the absence or presence of Epo (0.2 U/ml) or Epo (3 U/ml) and IL-3 (10 ng/ml) (C) or Epo alone (D). The benzidine-positive CFU-e (C) and BFU-e (D) colonies were scored at day 3 and day 8, respectively.
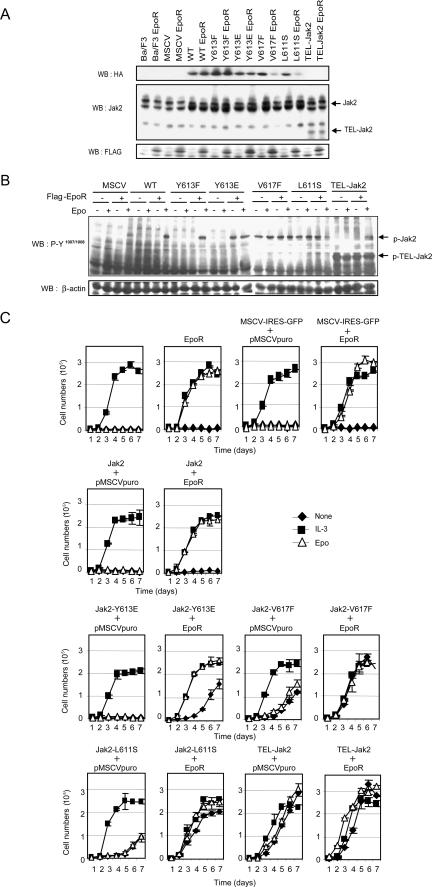
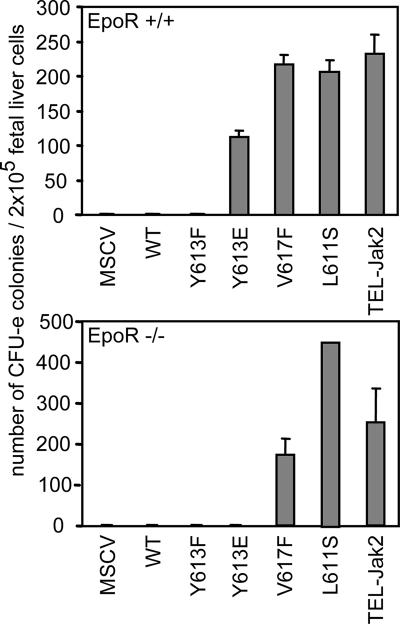
To compare the functions of the mutants in hematopoietic cells, we also expressed them alone or together with EpoR in BaF3 cells (Fig. 5A and B) and assessed their requirement for growth factors to support proliferation (Fig. 5C). Please note that the upper band is a nonspecific band that is detected by the antiserum that was utilized in this particular experiment. Coexpression of Jak2-Y613E together with EpoR conferred some growth factor independence to BaF3 cells, whereas alone this Jak2 mutant failed to support proliferation. The proliferation seen in cells expressing both EpoR and Jak2-Y613E was not as pronounced as the one seen in the presence of Epo or IL-3, however. In contrast, expression of either the Jak2-Y617F or Jak2-L611S mutant conferred growth factor independence to the BaF3 cells even in the absence of EpoR. Similarly, expression of TEL-Jak2 in BaF3 cells allowed these cells to grow with or without EpoR expression. Lastly, the requirement for EpoR for the biological activity of the Jak2 mutants was examined by expressing the mutants in EpoR-deficient fetal liver cells. As illustrated (Fig. 6), both the V617F and L611S mutants were able to rescue CFU-e colonies in the absence of EpoR, whereas the Y613E mutant required the receptor for activity.
FIG. 5.
The EpoR is required for Jak2-Y613E biological activity but not for Jak2-V617F, Jak2-L611S, and TEL-Jak2 in vitro. Exponentially growing Ba/F3 cell lines were infected with retroviruses encoding the indicated constructs, and green fluorescent protein-positive cells were sorted and selected with puromycin as described in Materials and Methods. Ba/F3 cells were then washed twice with PBS and left untreated or stimulated with IL-3 (2.5 ng/ml) and Epo (5 U/ml), and living cells were counted using a Beckman Coulter VI-Cell analyzer at the indicated times (C). For panel A, the levels of Jak2 expression were determined by Western blotting with an antiserum against Jak2 (C-20, sc-294; Santa Cruz) that detects a nonspecific band that migrates above the Jak2 band. For panel B, the cell lysates were immunoblotted using antibodies directed against Jak2 pY1007/Y1008 to assess the levels of activated Jak2. MSCV, murine stem cell virus.
FIG. 6.
EpoR is required for Jak2-Y613E biological activity but not for Jak2-V617F, Jak2-L611S, and TEL-Jak2 activities ex vivo. Wild-type (upper panel) or EpoR-deficient (lower panel) fetal liver cells were infected with retroviruses encoding the indicated Jak2 mutants and then subjected to in vitro colony formation assays in the presence of Epo (5 U/ml). The benzidine-positive CFU-e colonies were scored at day 3. MSCV, murine stem cell virus.
DISCUSSION
The data presented demonstrate that a point mutation in the JH2 domain of Jak2 leads to the constitutive activation of Jak2. However, unlike the case with other naturally occurring Jak2 mutations which also lead to constitutive activation of Jak2 (13, 19), the Y613E mutant manifests its activity only when EpoR is present. Previous studies reported that expression of a homodimeric type 1 cytokine receptor was required for Jak2-V617F-mediated transformation of BAF3 cells (21). However, using BaF3 cells, we found that expression of Jak2-V617F or -L611S conferred growth factor independence even in the absence of EpoR expression. Coexpression of EpoR enhanced the transforming activity of Jak2-V617F and -L611S but was not absolutely required. Similarly, TEL-Jak2 expression conferred growth factor independence in BaF3 cells. The reasons for these differences are unknown; however, in the studies by Lu et al. (21), the expression levels of the various Jak2 mutants and cytokine receptors were not assessed, and consequently it is quite possible that differences in the levels of protein expression account for the differences.
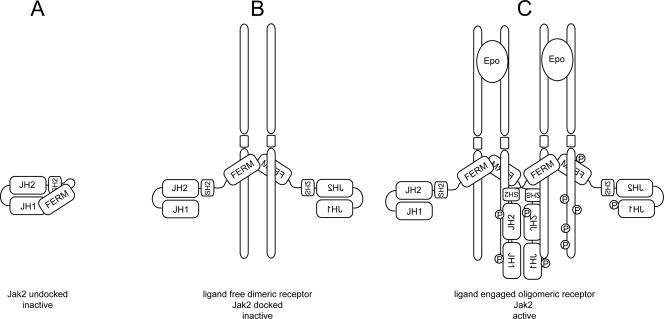
The results demonstrate that receptor expression is required not only for recruiting and activating downstream effectors but also for the activation of the kinase itself. This suggests that the structural changes produced by the Y613E mutation conferred an activated conformation only when Jak2 was tethered to the receptor. Since the FERM domain of Jak2 is involved in receptor binding (3, 6, 15, 34, 35), these observations support the model in which the unbound FERM domain may interact with the JH1/JH2 domains, thus preventing the inappropriate activation of the kinase. From these and other data, a model for activation of Jak2 by cytokine receptors can be envisioned in which receptor binding by the FERM domain of Jak2 (a binding that is enhanced by the Y613E mutation) recruits Jak2 to the receptor complex and leads to the relief of inhibitory constraints exerted by the FERM domain on the JH1/JH2 domains (Fig. 7). Therefore, the steps leading to the activation of Jak2 by EpoR can be seen as the gradual relief of multiple intramolecular constraints exerted in part by the FERM domain as presented in Fig. 7.
FIG. 7.
Proposed model for activation of Jak2 by EpoR derived from the properties of Jak2 mutants. In the unbound and inactive state, the FERM domain and the JH1 and JH2 domains of Jak2 are tightly folded together and prevent the Jak2 catalytic domain from being active (A). The first step toward activation of the kinase is the displacement of the FERM domain by its interaction with the receptor (B). The next step in activation occurs with ligand-induced receptor aggregation. In particular, as a consequence of aggregation, the probability of tyrosine phosphorylation within the active loop is greatly increased, which may be associated with changes in JH2-JH1 domain interactions. Once phosphorylation occurs within the activation loop, the kinase is fully activated (C). The active conformation of Jak2 is likely to be mimicked by the Jak2-V617F mutant, whereas the Y613E mutant failed to undergo complete conformational changes leading to its activation.
The role of the FERM domain in regulating kinase activity of Janus kinases has also been suggested by the identification of a mutation in the FERM domain of Hop which causes leukemia-like disease (9, 22). Similarly, studies of naturally occurring mutations in the FERM domain of Jak3 have been the basis of a model for the activation of Jak3 by the common γ chain. In this case the FERM domain of Jak3, through its interaction with receptors sharing the common γ chain, is proposed to affect the structure of the entire kinase and control the ability of the kinase to be activated (35). Of particular interest in this regard, the crystal structures representing both the active and inactive states of the focal adhesion kinase have been recently reported (20). The inactive state of the enzyme reveals that the kinase domain of Fak interacts with the N-terminal FERM domain in a way that blocks the access to the catalytic cleft by activating kinases such as Src, thus preventing phosphorylation in the activation loop and activation. In addition to blocking access to the catalytic cleft, the FERM domain of Fak also sequesters the Src binding site and a critical autophosphorylation site (Y397). Conversely, the active form of the kinase reveals that the kinase domain cleft is freed of FERM domain inhibition. However, in the steps leading to Jak2 activation, displacement of the FERM domain through its interaction with EpoR does not result in activation, but rather, further conformational changes, triggered by ligand binding to the receptor, are required. This second level of autoinhibition can be ascribed to the JH2 domain of Jak2, because previous studies have shown that deletion of the JH2 domain in Jak2 is associated with increased activity and because of the recent observations that mutation in this domain leads to the constitutive activation of the kinase (31).
Our studies also address another important issue of regulation of Jak2 activity by auto/transphosphorylation. Multiple sites of phosphorylation have been identified in Jak2. These include tyrosines 119, 221, 570, 813, 966, 1007, and 1008 and serine 523 (1, 5, 7, 12, 16, 23, 24). Although phosphorylation of these residues is well supported by published data, the consequences of these phosphorylation events on Jak2 biological activity in the context of receptor function have been reported only for two, Y1007 and Y119. Here we report that mutation of tyrosines 570, 813, and 966 and other putative phosphorylation sites in the JH1 and JH2 domains have no, or very subtle, consequences on Jak2 biological activity in the context of EpoR signaling under the various assay conditions that we have used. As shown here, activation of the kinase and downstream signaling pathways are not affected by the mutation of these tyrosine residues for phenylalanine, and all JH1/JH2 tyrosine mutants with the exception of Y1007 fully rescued Jak2 deficiency in colony assays. This suggests that besides phosphorylation on residues 119 and 1007, putative or characterized phosphorylation events in the JH1 and JH2 domains of Jak2 play no or very subtle roles in Jak2 function that will require additional assays to detect.
Acknowledgments
This work was supported by Cancer Center CORE grant CA21765, by grants RO1 DK42932 and PO1 HL53740 to J.N.I., and by the American Lebanese Syrian Associated Charities.
Footnotes
Published ahead of print on 26 December 2007.
REFERENCES
- 1.Argetsinger, L. S., J. L. Kouadio, H. Steen, A. Stensballe, O. N. Jensen, and C. Carter-Su. 2004. Autophosphorylation of JAK2 on tyrosines 221 and 570 regulates its activity. Mol. Cell. Biol. 244955-4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bourdeau, A., N. Dube, and M. L. Tremblay. 2005. Cytoplasmic protein tyrosine phosphatases, regulation and function: the roles of PTP1B and TC-PTP. Curr. Opin. Cell Biol. 17203-209. [DOI] [PubMed] [Google Scholar]
- 3.Chen, M., A. Cheng, Y. Q. Chen, A. Hymel, E. P. Hanson, L. Kimmel, Y. Minami, T. Taniguchi, P. S. Changelian, and J. J. O'Shea. 1997. The amino terminus of JAK3 is necessary and sufficient for binding to the common gamma chain and confers the ability to transmit interleukin 2-mediated signals. Proc. Natl. Acad. Sci. USA 946910-6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feener, E. P., F. Rosario, S. L. Dunn, Z. Stancheva, and M. G. Myers, Jr. 2004. Tyrosine phosphorylation of Jak2 in the JH2 domain inhibits cytokine signaling. Mol. Cell. Biol. 244968-4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng, J., B. A. Witthuhn, T. Matsuda, F. Kohlhuber, I. M. Kerr, and J. N. Ihle. 1997. Activation of Jak2 catalytic activity requires phosphorylation of Y1007 in the kinase activation loop. Mol. Cell. Biol. 172497-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frank, S. J., W. Yi, Y. Zhao, J. F. Goldsmith, G. Gilliland, J. Jiang, I. Sakai, and A. S. Kraft. 1995. Regions of the JAK2 tyrosine kinase required for coupling to the growth hormone receptor. J. Biol. Chem. 27014776-14785. [DOI] [PubMed] [Google Scholar]
- 7.Funakoshi-Tago, M., S. Pelletier, T. Matsuda, E. Parganas, and J. N. Ihle. 2006. Receptor specific downregulation of cytokine signaling by autophosphorylation in the FERM domain of Jak2. EMBO J. 254763-4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giordanetto, F., and R. T. Kroemer. 2002. Prediction of the structure of human Janus kinase 2 (JAK2) comprising JAK homology domains 1 through 7. Protein Eng. 15727-737. [DOI] [PubMed] [Google Scholar]
- 9.Harrison, D. A., R. Binari, T. S. Nahreini, M. Gilman, and N. Perrimon. 1995. Activation of a Drosophila Janus kinase (JAK) causes hematopoietic neoplasia and developmental defects. EMBO J. 142857-2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ihle, J. N., and D. G. Gilliland. 2007. Jak2: normal function and role in hematopoietic disorders. Curr. Opin. Genet. Dev. 178-14. [DOI] [PubMed] [Google Scholar]
- 11.Irie-Sasaki, J., T. Sasaki, W. Matsumoto, A. Opavsky, M. Cheng, G. Welstead, E. Griffiths, C. Krawczyk, C. D. Richardson, K. Aitken, N. Iscove, G. Koretzky, P. Johnson, P. Liu, D. M. Rothstein, and J. M. Penninger. 2001. CD45 is a JAK phosphatase and negatively regulates cytokine receptor signalling. Nature 409349-354. [DOI] [PubMed] [Google Scholar]
- 12.Ishida-Takahashi, R., F. Rosario, Y. Gong, K. Kopp, Z. Stancheva, X. Chen, E. P. Feener, and M. G. Myers, Jr. 2006. Phosphorylation of Jak2 on Ser(523) inhibits Jak2-dependent leptin receptor signaling. Mol. Cell. Biol. 264063-4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.James, C., V. Ugo, J. P. Le Couedic, J. Staerk, F. Delhommeau, C. Lacout, L. Garcon, H. Raslova, R. Berger, A. Bennaceur-Griscelli, J. L. Villeval, S. N. Constantinescu, N. Casadevall, and W. Vainchenker. 2005. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 4341144-1148. [DOI] [PubMed] [Google Scholar]
- 14.Klingmuller, U., U. Lorenz, L. C. Cantley, B. G. Neel, and H. F. Lodish. 1995. Specific recruitment of SH-PTP1 to the erythropoietin receptor causes inactivation of JAK2 and termination of proliferative signals. Cell 80729-738. [DOI] [PubMed] [Google Scholar]
- 15.Kohlhuber, F., N. C. Rogers, D. Watling, J. Feng, D. Guschin, J. Briscoe, B. A. Witthuhn, S. V. Kotenko, S. Pestka, G. R. Stark, J. N. Ihle, and I. M. Kerr. 1997. A JAK1/JAK2 chimera can sustain alpha and gamma interferon responses. Mol. Cell. Biol. 17695-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurzer, J. H., L. S. Argetsinger, Y. J. Zhou, J. L. Kouadio, J. J. O'Shea, and C. Carter-Su. 2004. Tyrosine 813 is a site of JAK2 autophosphorylation critical for activation of JAK2 by SH2-B beta. Mol. Cell. Biol. 244557-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurzer, J. H., P. Saharinen, O. Silvennoinen, and C. Carter-Su. 2006. Binding of SH2-B family members within a potential negative regulatory region maintains JAK2 in an active state. Mol. Cell. Biol. 266381-6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lacronique, V., A. Boureux, V. D. Valle, H. Poirel, C. T. Quang, M. Mauchauffe, C. Berthou, M. Lessard, R. Berger, J. Ghysdael, and O. A. Bernard. 1997. A TEL-JAK2 fusion protein with constitutive kinase activity in human leukemia. Science 2781309-1312. [DOI] [PubMed] [Google Scholar]
- 19.Levine, R. L., M. Wadleigh, J. Cools, B. L. Ebert, G. Wernig, B. J. Huntly, T. J. Boggon, I. Wlodarska, J. J. Clark, S. Moore, J. Adelsperger, S. Koo, J. C. Lee, S. Gabriel, T. Mercher, A. D'Andrea, S. Frohling, K. Dohner, P. Marynen, P. Vandenberghe, R. A. Mesa, A. Tefferi, J. D. Griffin, M. J. Eck, W. R. Sellers, M. Meyerson, T. R. Golub, S. J. Lee, and D. G. Gilliland. 2005. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 7387-397. [DOI] [PubMed] [Google Scholar]
- 20.Lietha, D., X. Cai, D. F. Ceccarelli, Y. Li, M. D. Schaller, and M. J. Eck. 2007. Structural basis for the autoinhibition of focal adhesion kinase. Cell 1291177-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu, X., R. Levine, W. Tong, G. Wernig, Y. Pikman, S. Zarnegar, D. G. Gilliland, and H. Lodish. 2005. Expression of a homodimeric type I cytokine receptor is required for JAK2V617F-mediated transformation. Proc. Natl. Acad. Sci. USA 10218962-18967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo, H., W. P. Hanratty, and C. R. Dearolf. 1995. An amino acid substitution in the Drosophila hopTum-l Jak kinase causes leukemia-like hematopoietic defects. EMBO J. 141412-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuda, T., J. Feng, B. A. Witthuhn, Y. Sekine, and J. N. Ihle. 2004. Determination of the transphosphorylation sites of Jak2 kinase. Biochem. Biophys. Res. Commun. 325586-594. [DOI] [PubMed] [Google Scholar]
- 24.Mazurkiewicz-Munoz, A. M., L. S. Argetsinger, J. L. Kouadio, A. Stensballe, O. N. Jensen, J. M. Cline, and C. Carter-Su. 2006. Phosphorylation of JAK2 at serine 523: a negative regulator of JAK2 that is stimulated by growth hormone and epidermal growth factor. Mol. Cell. Biol. 264052-4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myers, M. P., J. N. Andersen, A. Cheng, M. L. Tremblay, C. M. Horvath, J. P. Parisien, A. Salmeen, D. Barford, and N. K. Tonks. 2001. TYK2 and JAK2 are substrates of protein-tyrosine phosphatase 1B. J. Biol. Chem. 27647771-47774. [DOI] [PubMed] [Google Scholar]
- 26.Neubauer, H., A. Cumano, M. Muller, H. Wu, U. Huffstadt, and K. Pfeffer. 1998. Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell 93397-409. [DOI] [PubMed] [Google Scholar]
- 27.Parganas, E., D. Wang, D. Stravopodis, D. J. Topham, J. C. Marine, S. Teglund, E. F. Vanin, S. Bodner, O. R. Colamonici, J. M. van Deursen, G. Grosveld, and J. N. Ihle. 1998. Jak2 is essential for signaling through a variety of cytokine receptors. Cell 93385-395. [DOI] [PubMed] [Google Scholar]
- 28.Pelletier, S., S. Gingras, M. Funakoshi-Tago, S. Howell, and J. N. Ihle. 2006. Two domains of the erythropoietin receptor are sufficient for Jak2 binding/activation and function. Mol. Cell. Biol. 268527-8538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quelle, F. W., D. Wang, T. Nosaka, W. E. Thierfelder, D. Stravopodis, Y. Weinstein, and J. N. Ihle. 1996. Erythropoietin induces activation of Stat5 through association with specific tyrosines on the receptor that are not required for a mitogenic response. Mol. Cell. Biol. 161622-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richmond, T. D., M. Chohan, and D. L. Barber. 2005. Turning cells red: signal transduction mediated by erythropoietin. Trends Cell Biol. 15146-155. [DOI] [PubMed] [Google Scholar]
- 31.Saharinen, P., M. Vihinen, and O. Silvennoinen. 2003. Autoinhibition of Jak2 tyrosine kinase is dependent on specific regions in its pseudokinase domain. Mol. Biol. Cell 141448-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yasukawa, H., H. Misawa, H. Sakamoto, M. Masuhara, A. Sasaki, T. Wakioka, S. Ohtsuka, T. Imaizumi, T. Matsuda, J. N. Ihle, and A. Yoshimura. 1999. The JAK-binding protein JAB inhibits Janus tyrosine kinase activity through binding in the activation loop. EMBO J. 181309-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zang, H., K. Sato, H. Nakajima, C. McKay, P. A. Ney, and J. N. Ihle. 2001. The distal region and receptor tyrosines of the Epo receptor are non-essential for in vivo erythropoiesis. EMBO J. 203156-3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao, Y., F. Wagner, S. J. Frank, and A. S. Kraft. 1995. The amino-terminal portion of the JAK2 protein kinase is necessary for binding and phosphorylation of the granulocyte-macrophage colony-stimulating factor receptor beta c chain. J. Biol. Chem. 27013814-13818. [DOI] [PubMed] [Google Scholar]
- 35.Zhou, Y. J., M. Chen, N. A. Cusack, L. H. Kimmel, K. S. Magnuson, J. G. Boyd, W. Lin, J. L. Roberts, A. Lengi, R. H. Buckley, R. L. Geahlen, F. Candotti, M. Gadina, P. S. Changelian, and J. J. O'Shea. 2001. Unexpected effects of FERM domain mutations on catalytic activity of Jak3: structural implication for Janus kinases. Mol. Cell 8959-969. [DOI] [PubMed] [Google Scholar]