Abstract
Fanconi anemia (FA) is a cancer susceptibility syndrome characterized by defective DNA interstrand cross-link (ICL) repair. Here, we show that DOG-1 is the Caenorhabditis elegans homologue of FANCJ, a helicase mutated in FA-J patients. DOG-1 performs a conserved role in ICL repair, as dog-1 mutants are hypersensitive to ICL-inducing agents, but not to UVC irradiation or X rays. Genetic analysis indicated that dog-1 is epistatic with fcd-2 (C. elegans FANCD2) but is nonepistatic with brc-1 (C. elegans BRCA1), thus establishing the existence of two distinct pathways of ICL repair in worms. Furthermore, DOG-1 is dispensable for FCD-2 and RAD-51 focus formation, suggesting that DOG-1 operates downstream of FCD-2 and RAD-51 in ICL repair. DOG-1 was previously implicated in poly(G)/poly(C) (G/C) tract maintenance during DNA replication. G/C tracts remain stable in the absence of ATL-1, CLK-2 (FA pathway activators), FCD-2, BRC-2, and MLH-1 (associated FA components), implying that DOG-1 is the sole FA component required for G/C tract maintenance in a wild-type background. However, FCD-2 is required to promote deletion-free repair at G/C tracts in dog-1 mutants, consistent with a role for FA factors at the replication fork. The functional conservation between DOG-1 and FANCJ suggests a possible role for FANCJ in G/C tract maintenance in human cells.
Fanconi anemia (FA) is a rare chromosomal instability syndrome associated with various congenital abnormalities, bone marrow failure, and susceptibility to cancer (24). FA cells exhibit a characteristic cellular hypersensitivity to agents that cause DNA interstrand cross-links (ICLs), such as UV-activated trimethylpsoralen (TMP), nitrogen mustard, cisplatin, mitomycin C, and diepoxybutane, indicating that the FA proteins function in repair of ICLs (reviewed in references 24 and 31). Although ICLs are one of the most cytotoxic DNA lesions, the function of the FA factors in ICL repair is not well defined.
Currently, 13 FA complementation groups have been identified (A, B, C, D1/BRCA2, D2, E, F, G, I, J/BRIP1, L, M, and N) (34, 39), and more FA-associated genes are likely to be discovered, as a number of patients cannot be assigned to these groups. Eight of these proteins (FANCA, -B, -C, -E, -F, -G, -L, and -M) function in the FA core complex, along with additional proteins, such as FAAP100, for which no corresponding patients have been identified (27). The core complex is required for monoubiquitylation of FANCD2 and FANCI (38, 39). Monoubiquitylation appears to trigger the recruitment of FANCD2 and FANCI to DNA damage foci (24, 37, 38), where FANCD2 is known to interact with other repair proteins, including BRCA1, BRCA2, and the MRE11/RAD50/NBS1 complex (21). BRCA2 and FANCJ are thought to act downstream of FANCD2 monoubiquitylation in the FA pathway, as FANCD2 modification and recruitment are not affected in BRCA2- or FANCJ-deficient cells (24). While BRCA2/FANCD1 appear to regulate the assembly and disassembly of RAD51 onto single-stranded DNA during recombinational repair (17, 24, 33, 44), the function of FANCJ in ICL repair is not yet known.
FANCJ, also known as BRIP1 and BACH1, was first recognized as having a role in double-strand break (DSB) repair through its interaction with BRCA1 (8, 9). More recently, FANCJ was shown to be mutated in patients from the FA complementation group J (25, 26, 28), and individuals with monoallelic FANCJ mutations were shown to have increased susceptibility to breast cancer (35). Analysis of FANCJ indicated that the C-terminal region, containing the Ser990-X-X-Phe993 motif, is required for its interaction with BRCA1 and function in DSB repair (9, 36). The role of FANCJ in ICL repair is independent of BRCA1 and has recently been shown to require an interaction with the mismatch repair protein MLH1 that involves lysines 141 and 142 of FANCJ (32). The chicken homologue of FANCJ appears to function only in ICL repair, as the protein lacks the BRCA1 interaction motif and FANCJ DT40 cells are not significantly sensitive to DSBs induced by X rays (6). In DT40 cells, FANCD2 monoubiquitylation is not affected by the absence of FANCJ, suggesting that FANCJ functions downstream of FANCD2 (6). However, the same study showed that FANCJ FANCC double-mutant cells exhibit greater sensitivity to cisplatin treatment than FANCJ or FANCC single mutants (6), raising the possibility that FANCJ might function in a pathway parallel to the FA core complex.
As the involvement of FA factors in human ICL repair is very complex, the use of a model organism, such as Caenorhabditis elegans, provides a simpler means to elucidate function. C. elegans is particularly useful for the study of DNA repair because many of the repair proteins and pathways present in human cells are conserved in the nematode. For example, brc-1 and brc-2 are the C. elegans orthologues of BRCA1 and BRCA2/FANCD1, respectively (4, 30). Furthermore, the C. elegans homologue of the key FA factor, FANCD2, has recently been identified as fcd-2 (13, 14). It is likely that a simplified FA pathway exists in the nematode, as sequence homologues have not been found for all of the FA genes. Here, we show that the previously identified mutator dog-1 (deletions of guanine-rich DNA) is the C. elegans FANCJ homologue and is required for the repair of DNA ICLs.
MATERIALS AND METHODS
Strains.
Nematode strains were maintained as described previously (5). The strains used in these experiments included VC13 dog-1(gk10), FX1298 fcd-2(tm1298), RB1128 fcd-2(ok1145), DW101 atl-1(tm853), SP506 clk-2(mn159), FX1086 brc-2(tm1086), and RB1572 mlh-1(ok1917). dog-1(gk10), fcd-2(ok1145), and mlh-1(ok1917) were generated by the International C. elegans Gene Knockout Consortium. fcd-2(tm1298), atl-1(tm853), and brc-2(tm1086) were generated by S. Mitani and the National Bioresource Project of Japan.
DNA damage sensitivity assays.
L4 stage animals were picked to fresh plates and aged for 24 hours so that the animals were 1-day-old adults on the day of the experiment. For UVC experiments, a UV cross-linker (Spectronics Corporation; spectrolinker XL-1000) with 254-nm bulbs was used, and the animals were exposed to either 50 or 100 J/m2 UV. For X ray treatment, the animals were exposed to 3,000 or 4,500 rads of X rays. For TMP-UVA treatment, animals were immersed in 10 μg/ml TMP (also known as trioxsalen) (Sigma) in M9 buffer for 1 h. Two different UVA apparatuses were used. Following TMP treatment, the animals were exposed to UVA (55 to 165 J) at a dose of 550 μW/cm2 or to UVA (80 to 120 J) at a dose of 1 mW/cm2. Following treatment, the animals were allowed to recover overnight at 20°C. Animals were singled out or plated at five per plate and allowed to lay for a 4-h interval (22 to 26 h posttreatment). The number of dead eggs versus hatching larvae was scored 24 to 48 h after the laying in order to calculate the percentage of progeny surviving the treatment. All results were replicated in at least two independent experiments.
SYTO12 staining.
Apoptosis was measured in untreated worms or 24 h after treatment with 10 μg/ml TMP in M9 buffer and exposure to UVA (55 to 165 J) at a dose of 550 μW/cm2 (as described above). The animals were picked into 50 μl of 33 μM Syto12 (Molecular Probes) in M9 buffer and incubated for 3 h in the dark. The animals were destained by placing them on Escherichia coli NGM OP50-seeded plates to feed for 1 h. The animals were placed in 2 mM levamisole on 3% agarose pads for viewing on a Zeiss Axioscope fluorescence microscope with a 40× objective. The number of Syto12-stained bodies per gonad arm was determined.
Cytological preparation and immunostaining.
Gravid hermaphrodites were washed in 2 ml phosphate-buffered saline (PBS) and then transferred to 20 μl PBS in a well created using an ImmunEdge pen (Vector Laboratories, Burlington, CA) on a poly-L-lysine-coated slide (the slides were washed with 70% ethanol and subsequently given two coats of 100% poly-L-lysine with air drying between the coats). Twenty microliters of 10 mM levamisole was added, and the germ lines were then extruded by cutting off the head and tail of the animal with a fine-gauge (27-gauge) needle. The levamisole was subsequently replaced with 1% paraformaldehyde in PBS for 10 min. The paraformaldehyde was then removed, and the germ lines were permeabilized in Tris-buffered saline-0.5% bovine serum albumin (TBSB)-0.1% Triton X-100 for 5 min. Three 5-min washes with TBSB were performed, and then the germ lines were blocked with TBSB at room temperature for 30 min. Primary antibodies were diluted in TBSB (1:500 for RAD-51), and 25 μl per well was incubated in a humid chamber overnight at 4°C. The germ lines were then washed three times with TBSB for 30 min each time before they were incubated with secondary antibodies for 2 h at room temperature in a dark chamber (anti-rabbit Cy3 at 1:10,000 [Sigma] in TBSB). The germ lines were washed three times in TBSB for 30 min before being mounted on coverslips with Vectashield containing 1 μg/ml of DAPI (4′,6′-diamidino-2-phenylindole). Anti-FCD-2 staining was carried out as described by Collis et al. (13).
Fluorescence microscopy.
Deltavision microscopy was used to examine the germ lines with an ×63, 1.4-numerical-aperture Planapochromat lens on an Olympus inverted microscope (IX71), and images were captured using SoftWorx computer software (Applied Precision). Three-dimensional data sets were computationally deconvolved, and regions of interest were then projected into one dimension. Merged color images were recorded using GIMP software, and single-color images were created using Adobe Photoshop.
G/C tract deletion assay.
Poly(G)/poly(C) (G/C) tract deletions were examined by PCR as described by Youds et al. (45).
RESULTS
C. elegans DOG-1 exhibits significant sequence similarity to human FANCJ.
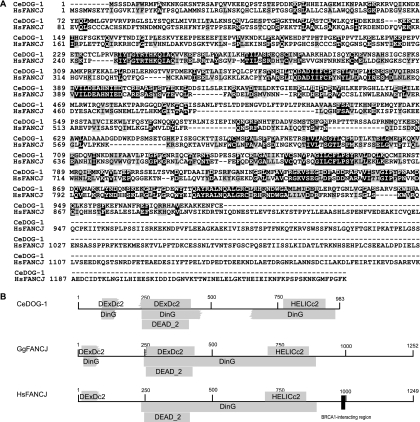
Our recent discovery that one of the key FA genes, FANCD2, is conserved in the nematode (13) led us to search for other genes within this largely uncharacterized DNA repair pathway. DOG-1 and FANCJ are reciprocal best-BLAST hits between C. elegans and humans, with 31% identity and 50% similarity (Fig. 1A). C. elegans DOG-1, Gallus gallus FANCJ, and Homo sapiens FANCJ are especially conserved within the functional domains (DExDc2, DEAD_2, HELICc2, and DinG) typical of RAD3-like helicases associated with DNA replication, transcription, recombination, and repair (29) (Fig. 1B). However, one notable difference between DOG-1 and human FANCJ is the protein size: FANCJ is 1,249 amino acids in length and contains 266 amino acids at its C-terminal end that are absent in the 983-amino-acid DOG-1 protein. The C-terminal region of human FANCJ interacts with the breast cancer-associated protein BRCA1 through the Ser990-X-X-Phe993 motif (36), and these residues are not conserved in DOG-1 or in chicken FANCJ (6). Peng et al. (32) have shown that lysines 141/142 are required for the interaction of human FANCJ with MLH1 and for the function of FANCJ in ICL repair. Based on the alignment in Fig. 1A, it appears that the binding site for MLH-1 is only weakly conserved in DOG-1, with only one of the two critical lysine residues present in the C. elegans protein.
FIG. 1.
(A) Protein sequence alignment of C. elegans DOG-1 (CeDOG-1) and human FANCJ (HsFANCJ). Identical amino acids are shown in white on a black background; conserved amino acids are shown in black on a gray background. The sequences were aligned using ClustalW 1.83 and shaded with BoxShade 3.21. (B) Schematic of conserved domains of C. elegans DOG-1, chicken FANCJ (GgFANCJ), and human FANCJ as determined by the Conserved Domains Database. The BRCA1-interacting region of human FANCJ, including Ser990 and Phe993 (shown by the black square), are not conserved in chicken FANCJ or C. elegans DOG-1.
dog-1 mutants are hypersensitive to ICL-inducing agents but not to UVC or X rays.
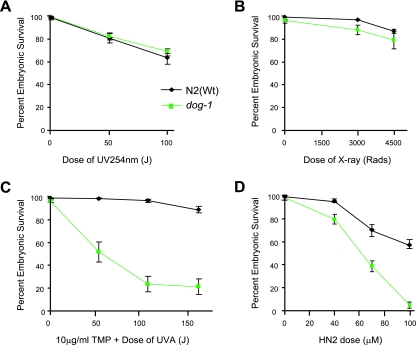
A diagnostic hallmark of FA cells is hypersensitivity to DNA ICL-inducing agents. This characteristic appears to be conserved, as chicken FANCJ cells are also highly sensitive to DNA damage in the form of ICLs but are not sensitive to DNA lesions generated by X-ray or UV irradiation treatment (6). If DOG-1 is a homologue of FANCJ, it would be expected that dog-1 mutants would show similar sensitivity to ICL-inducing agents. In order to test this hypothesis, we tested the sensitivity of the dog-1 strain to a range of different DNA-damaging agents. Exposure to UVC irradiation or X rays did not cause a significant difference in the embryonic survival of dog-1 mutants compared to N2(Wt) animals (Fig. 2A and B). However, following treatment with the ICL-inducing agent UVA-activated TMP (TMP plus UVA), embryonic survival of dog-1 mutants was significantly decreased compared to N2(Wt) (Fig. 2C). We did not observe significant sensitivity to either UVA or TMP alone, demonstrating that the sensitivity is specific to ICLs (data not shown). To confirm that dog-1 mutants were hypersensitive to cross-linking agents, we tested their sensitivity to other ICL-inducing agents, including nitrogen mustard and cisplatin. Embryonic survival was significantly decreased in dog-1 animals compared to N2(Wt) animals following treatment with either nitrogen mustard (Fig. 2D) or cisplatin (data not shown).
FIG. 2.
dog-1 mutants are not sensitive to DNA damage from UVC or X-ray irradiation but are hypersensitive to ICL-inducing agents. The sensitivities of N2(Wt) and dog-1 animals to UVC irradiation (A), X rays (B), UV-activated TMP (TMP-UVA) (C), and nitrogen mustard (HN2) (D) are shown. All values reported are ± standard error of the mean.
dog-1 mutants exhibit chromosomal abnormalities after DNA cross-linking.
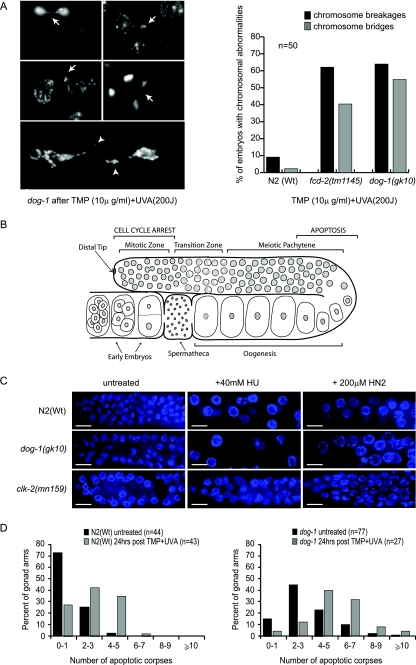
A further characteristic of FA cells is the accumulation of chromosomal aberrations following treatment with certain DNA-damaging agents, and this is used as a diagnostic tool (mitomycin C or diepoxybutane chromosome breakage test) (2, 7, 10, 18). Chromosomal abnormalities, including chromosome bridges and breaks, are also a hallmark of C. elegans ICL repair mutants, such as fcd-2 and rfs-1 (13, 23, 42). Because it is not possible to perform metaphase spreads in C. elegans as it is in human cells, we studied chromosome integrity in the germ lines of N2(Wt), fcd-2, and dog-1 animals by whole-mount DAPI staining 24 h after cross-linking treatment. Chromatin bridges and breaks were observed in the germ line at diakinesis, as well as in the early embryos of both fcd-2 and dog-1 mutants (Fig. 3A). Quantification of the chromosomal abnormalities arising after TMP-UVA treatment revealed that fcd-2 and dog-1 mutants exhibited a 6- to 10-fold increase in chromatin bridges and breaks compared to N2(Wt) animals (Fig. 3A). This phenotype might arise due to unrepaired ICLs in fcd-2 and dog-1 mutants, as they are rarely detected in N2(Wt) animals after TMP-UVA treatment. Furthermore, chromosomal abnormalities are not observed in N2(Wt) or in fcd-2 or dog-1 mutants under normal conditions, suggesting that they arise due to an inability to respond to replication-blocking lesions.
FIG. 3.
Chromosomal aberrations are present, and the DNA damage checkpoint is active, in dog-1 mutants after cross-linking treatment. (A) Representative chromatin bridges (arrows) and breaks (arrowheads) observed in the germ lines of dog-1 mutants following treatment with TMP (10 μg/ml) and UVA (200 J). Quantification of chromosome breakages and bridges in N2(Wt), fcd-2(ok1145), and dog-1(gk10) animals following treatment with TMP-UVA showed similar numbers of breaks and bridges in fcd-2 and dog-1 mutants. (B) Schematic of the C. elegans germ line. Nuclei in the mitotic zone serve as the stem cell compartment of the germ line and undergo cell cycle arrest following DNA damage. Nuclei move through the transition zone, where they take on a characteristic crescent shape as they begin the early stages of meiosis I. Meiosis progresses through pachytene, diplotene, and diakinesis, and completes once the oocyte has been fertilized. Cells in late pachytene/early diplotene undergo apoptosis in response to physiological cues or DNA damage. Early embryos are visible prior to ovulation. (C) Germ line mitotic zone nuclei of N2(Wt), dog-1, and clk-2 mutants stained with DAPI. Enlarged arrested nuclei were observed in the germ line mitotic zones of N2(Wt) and dog-1, but not in clk-2 mutants, following treatment with 40 mM HU or 200 μM nitrogen mustard (HN2). (D) Numbers of SYTO12-stained corpses in N2(Wt) and dog-1 animals with no treatment and 24 h after DNA cross-linking with TMP-UVA treatment (n, number of animals scored for apoptosis).
dog-1 mutants demonstrate an intact DNA damage checkpoint after cross-linking treatment.
The dog-1 hypersensitivity to ICL-inducing agents could be explained by two different hypotheses. One possibility is that the checkpoint that detects DNA ICLs might be compromised in dog-1 mutants. The other possibility is that lesion repair is impaired in the absence of DOG-1. The two major hallmarks of DNA damage-induced checkpoint activation in C. elegans are the accumulation of enlarged arrested nuclei in the mitotic compartment of the germ line and apoptosis of compromised pachytene nuclei (3) (Fig. 3B). In order to test the integrity of the DNA damage checkpoint after cross-linking treatment, we first examined cell cycle arrest in the mitotic compartment following nitrogen mustard or HU treatment. DAPI staining revealed that S-phase mitotic nuclei in N2(Wt) and dog-1 mutants exhibit the characteristic enlargement associated with checkpoint-induced cell cycle arrest (Fig. 3C). In contrast, clk-2, a known DNA damage checkpoint mutant, failed to arrest the cell cycle in S phase, as revealed by the absence of enlarged mitotic nuclei (19). To further test the integrity of the DNA damage checkpoint, staining with the apoptosis-specific dye Syto12 was carried out on N2(Wt) and dog-1 mutants after TMP-UVA treatment. As described previously (45), N2(Wt) animals under nondamaging conditions exhibited an average of 0.8 ± 0.2 Syto12-stained corpses per gonad arm observed in the pachytene stage of meiosis I, while dog-1 animals under normal conditions had an average of 3.3 ± 0.2 corpses present (Fig. 3D). Twenty-four hours after cross-linking treatment with TMP-UVA, the average number of corpses in N2(Wt) gonad arms had increased to 2.6 ± 0.2, while in dog-1 animals, the average number of corpses per gonad arm had increased to 5.3 ± 0.4 (Fig. 3D). Apoptosis increased in both N2(Wt) and dog-1 animals 24 h after TMP-UVA treatment. A similar increase in apoptosis was also measured in N2(Wt) and dog-1 mutants following treatment with nitrogen mustard (data not shown). These results, together with previous findings by Collis et al. (13), collectively demonstrate that both dog-1 and fcd-2 are dispensable for checkpoint-induced cell cycle arrest and apoptosis. It is therefore likely that defective ICL repair is the cause of lethality in dog-1 mutants following TMP-UVA treatment, as has been suggested previously for fcd-2 (13). Interestingly, the increase in apoptosis in dog-1 mutants after cross-link treatment is not as dramatic as that observed in brc-1 and brd-1 mutants following DNA damage arising from ionizing radiation (4), suggesting that cross-links do not generate a large number of substrates that trigger apoptosis in the germ line. Rather, the effects of cross-links are more evident in the arrested embryos laid by dog-1 animals after treatment.
dog-1 is epistatic to fcd-2, but not to brc-1.
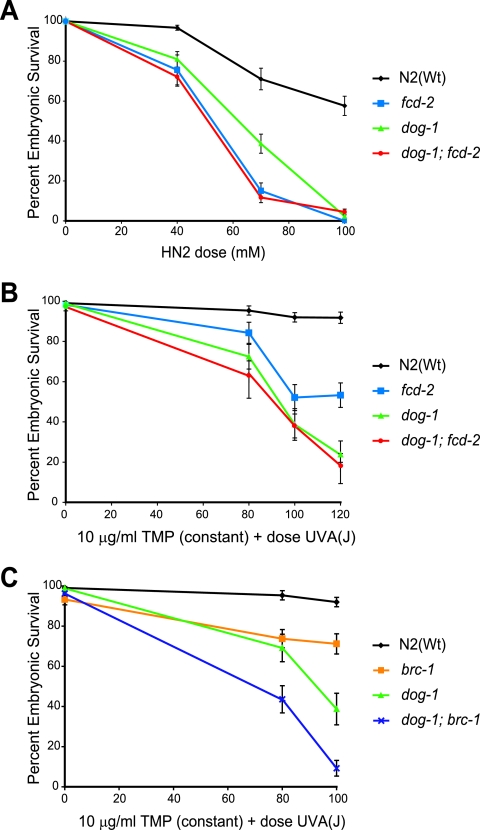
Given the hypersensitivity to ICL-inducing agents and characteristic chromosomal aberrations, it would seem that dog-1 functions similarly to FANCJ. We therefore tested whether DOG-1 acts in the same pathway as the C. elegans FANCD2 homologue, FCD-2. The dog-1; fcd-2 double-mutant strain was constructed and tested for sensitivity to both TMP-UVA and nitrogen mustard. dog-1; fcd-2 double mutants were no more sensitive to cross-linking treatment than either of the single mutants (Fig. 4A and B), suggesting that DOG-1 and FCD-2 function in the same pathway for ICL repair.
FIG. 4.
dog-1 is epistatic with fcd-2, but not with brc-1, for sensitivity to ICL-inducing agents. (A) TMP-UVA sensitivities of N2(Wt), dog-1, and fcd-2 mutants and dog-1; fcd-2 double mutants. (B) Nitrogen mustard (HN2) sensitivities of N2(Wt), dog-1, and fcd-2 mutants and dog-1; fcd-2 double mutants. (C) TMP-UVA sensitivities of N2(Wt), dog-1, and brc-1 mutants and dog-1; brc-1 double mutants. All values reported are ± standard error of the mean.
Previous studies using human cells and chicken DT40 cell lines indicated that FANCJ functions independently of BRCA1 in ICL repair (6, 32). To determine if this is also the case in C. elegans, we tested the sensitivities of a dog-1; brc-1 double-deletion strain and the respective single mutants to TMP-UVA treatment. brc-1 mutants showed moderate sensitivity to ICLs, while dog-1; brc-1 double mutants were more sensitive than either single mutant (Fig. 4C). Thus, dog-1 and brc-1 are not epistatic, suggesting these two proteins function in different pathways for the repair of ICLs.
DOG-1 is dispensable for FCD-2 focus formation.
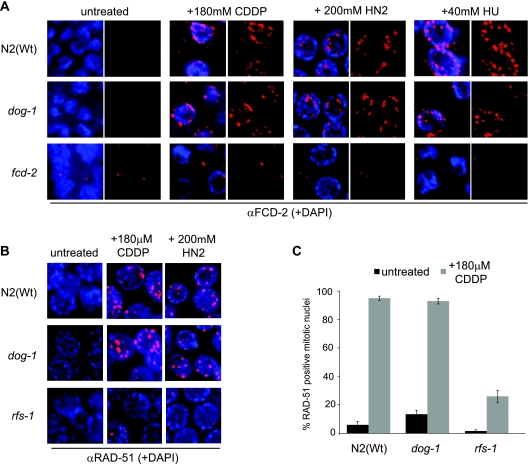
In response to ICL-inducing agents or replication stress, FCD-2 becomes monoubiquitylated and localizes to repair foci (13). The absence of upstream components of the FA pathway, including activators, such as ATL-1 and CLK-2, causes defects in FCD-2 focus formation in response to cross-linking treatment (12). It follows that the timing of DOG-1 activity within this pathway can be determined through characterization of FCD-2 recruitment to foci. We therefore stained dog-1 mutants with an anti-FCD-2 antibody in order to observe any change in FCD-2 focus formation after cross-linking or replication fork stalling in the absence of DOG-1. FCD-2 foci were not observed in untreated N2(Wt) or dog-1 animals but were observed in both N2(Wt) and dog-1 animals after treatment with cisplatin, nitrogen mustard, or HU (Fig. 5A). These data indicate that DOG-1 is not required for FCD-2 recruitment to sites of replication stress.
FIG. 5.
FCD-2 and RAD-51 focus formation after DNA damage is unaffected by the absence of DOG-1. (A) Representative images of mitotic zone anti-FCD-2 staining (red) on N2(Wt), dog-1, and fcd-2 mutants following no treatment or treatment with 180 μM cisplatin (CDDP), 200 μM nitrogen mustard (HN2), or 40 mM HU. (B) Representative images of mitotic zone anti-RAD-51 staining (red) on N2(Wt), dog-1, and rfs-1 mutants following no treatment or treatment with 180 μM cisplatin or 200 μM nitrogen mustard. (C) Quantification of mitotic nuclei with RAD-51 foci in N2(Wt), dog-1, and rfs-1 mutants before and after treatment with 180 μM cisplatin.
DOG-1 is dispensable for RAD-51 focus formation.
Given that DOG-1 acts downstream of FCD-2, we tried to establish the context of DOG-1 function. It has been shown that RAD51 focus formation occurs normally after X-ray or mitomycin C treatment in lymphoblasts derived from FA-J patients (20) and after HU treatment of FANCJ-deficient MCF7 cells (28). It therefore follows that if DOG-1 is the C. elegans homologue of FANCJ, it should be dispensable for RAD-51 loading in response to replication stress. It has been shown that the C. elegans Rad51 paralog RFS-1 is required for RAD-51 recruitment to replication forks blocked by ICLs (42). We therefore investigated whether RAD-51 foci are induced in N2(Wt) or in dog-1 or rfs-1 mutants after treatment with ICL-inducing agents. Few RAD-51 foci were observed in the mitotic zones of N2(Wt), dog-1, and rfs-1 mutants with no treatment. However, after cross-linking treatment with cisplatin or nitrogen mustard, RAD-51 focus formation was induced to similar extents in both N2(Wt) and dog-1 mutants, but not in rfs-1 mutants (Fig. 5B). Quantification of the mitotic nuclei with RAD-51 foci showed that N2(Wt) and dog-1 animals had similar levels of RAD-51-positive nuclei following cisplatin treatment (Fig. 5C). This demonstrates that DOG-1 is not required for generating the homologous recombination (HR) substrate at stalled replication forks or for the subsequent recruitment of RAD-51.
The absence of FA pathway components does not affect G/C tract stability.
Previous characterization of DOG-1 showed that it is required to prevent deletions that initiate at G/C tracts (11). Based on recent observations, it is likely that G/C tracts adopt secondary structures in DNA that, like ICLs, result in a barrier to replication fork progression (42). Because dog-1 functions in the C. elegans FA pathway with fcd-2, we questioned whether other activators or associated components of the FA pathway might also affect G/C tract stability. The C. elegans homologues of ATR and HCLK2 (ATL-1 and CLK-2, respectively) are known to be required for activation of the FA pathway (12, 19). G/C tracts were tested for deletions in strains lacking ATL-1 or CLK-2 using the vab-1 G/C tract assay (45). Similar to our previous findings (42, 45), we observed that 8.6% of dog-1 animals had deletions in the vab-1 G/C tract. In both atl-1 and clk-2 mutant strains, no G/C tract deletions were observed (Table 1). We also tested whether the absence of proteins functioning further downstream, such as FCD-2 and BRC-2, might affect G/C tract stability. FCD-2 plays a central role in the FA pathway in C. elegans (13), while BRC-2 is a homologue of human BRCA2/FANCD1 (30), which is known to function in ICL repair (22). G/C tract deletions were not detected in either fcd-2 or brc-2 mutants (Table 1). We also tested mlh-1 mutants for G/C tract deletions, as a recent study had demonstrated a direct connection between FANCJ and the mismatch repair complex; MLH1 interacts with the helicase domain of FANCJ, an association essential for resistance to ICL-inducing agents (32). G/C tract deletions were also absent from mlh-1 mutants (Table 1). Thus, of the FA pathway components tested in a wild-type background, only DOG-1 is required for G/C tract stability.
TABLE 1.
Assay for deletions of the vab-1 G/C tract in the absence of activators, components, or associated proteins of the FA pathwaya
| Genotype | No. of animals assayed | No. of animals with deletions | % of animals with deletions |
|---|---|---|---|
| dog-1(gk10) | 128 | 11 | 8.6 |
| atl-1(tm853) | 196 | 0 | 0 |
| clk-2(mn159) | 196 | 0 | 0 |
| fcd-2(ok1145) | 384 | 0 | 0 |
| fcd-2(tm1298) | 206 | 0 | 0 |
| brc-2(tm1086) | 288 | 0 | 0 |
| mlh-1(ok1917) | 196 | 0 | 0 |
| dog-1; fcd-2(tm1298) | 150 | 50 | 33.3 |
Greater than threefold more dog-1; fcd-2 animals than dog-1 single mutants had deletions (t test; P = 0.0021).
Previously, proteins involved in HR repair and translesion synthesis (TLS) were shown to prevent G/C tract deletion formation in the absence of DOG-1 (45). It is thought that FA factors may be required to promote the repair of ICLs encountered by the replication fork through HR or TLS. Therefore, we tested the frequency of G/C tract deletions in the absence of both DOG-1 and FCD-2. Similar to what was previously reported for dog-1; rad-51 double mutants (45), dog-1; fcd-2 double mutants had significantly more G/C tract deletions than did dog-1 single mutants (Table 1), indicating that while loss of FCD-2 does not affect G/C tract stability in a wild-type background, it is required for deletion-free repair at G/C tracts in the absence of DOG-1.
DISCUSSION
The findings reported here demonstrate that DOG-1, the C. elegans protein with the greatest similarity to human FANCJ, performs a conserved role in ICL repair. DOG-1 possesses the best identity and similarity to FANCJ (31% identity and 50% similarity) of any predicted helicase encoded by the C. elegans genome. We have shown that dog-1 mutants exhibit the major hallmark of FA-deficient human cells: mutations in dog-1 confer exquisite sensitivity to UV-activated TMP and nitrogen mustard (ICL agents), but not to ionizing radiation or UVC. Similar to FANCJ-deficient human cells (6, 28), the sensitivity of dog-1 mutants to ICL agents correlates with accumulation of chromosomal abnormalities. This phenotype is not due to a failure to sense or signal ICLs, as the DNA damage checkpoint is efficiently activated in dog-1 mutants in response to drug treatment, leading to cell cycle arrest and/or apoptosis. Rather, our data support a role for DOG-1 in promoting ICL repair, which suggests beyond any reasonable doubt that DOG-1 is the functional orthologue of FANCJ in C. elegans. It is therefore reasonable to predict that C. elegans dog-1 mutant strains can serve as an informative model for the study of FANCJ function in ICL repair.
The genetic relationship between FANCJ and other DNA repair genes is not well understood in any system and remains to be explored in detail. Perhaps surprisingly, only one genetic study of the relationship between the FA genes has been reported, and it revealed additive sensitivity of FANCC FANCJ double-mutant DT40 cells to ICL agents compared to the single mutants (6). This result questioned whether FA genes actually work in a common genetic pathway. By exploiting the genetics of C. elegans, we have established for the first time in any system that dog-1 (FANCJ) functions in the same epistasis group as fcd-2 (FANCD2) based on the observation that dog-1; fcd-2 double mutants are no more sensitive to UV-activated TMP or nitrogen mustard than their respective single mutants. Our data suggest that DOG-1 acts downstream of FCD-2 in ICL repair based on the fact that DOG-1 is dispensable for FCD-2 recruitment to sites of replication stress. Unlike RFS-1 (the sole C. elegans Rad51 paralog), which is essential for recruitment of RAD-51 to blocked replication forks (42), dog-1 and fcd-2 are dispensable for RAD-51 focus formation. Thus, dog-1 and fcd-2 are not required for generation of an HR substrate at blocked replication forks or for the subsequent recruitment and loading of RAD-51. These data are in agreement with previous studies in human cells demonstrating that FANCJ is dispensable for Rad51 focus formation in response to replication stress (20, 28). Our analysis of DOG-1 function in the FA pathway could not be extended to include FA core genes, as such components have yet to be definitively identified in C. elegans due a lack of sequence conservation. While a putative FANCM homologue does exist, mutants in the gene are nonviable, thus preventing us from assessing either its function in ICL repair or its relationship to dog-1 through genetic-epistasis analysis.
Similar to the chicken FANCJ homologue, DOG-1 lacks the BRCA1-interacting domain and is therefore unlikely to interact with BRC-1. Interaction analysis has failed to detect a direct physical association between DOG-1 and BRC-1 in either yeast two-hybrid assays or pull-down experiments from cells (data not shown). In contrast to brc-1-deficient animals (4, 42), dog-1 mutants are not sensitive to X-ray treatment, indicating that DOG-1 does not play a major role in the BRC-1-dependent DSB repair pathway. Genetic analysis has also shown that dog-1 and brc-1 are nonepistatic with respect to ICL repair, as the dog-1; brc-1 double mutant exhibits additive sensitivity to ICLs compared to the single mutants. Based on these data, FANCJ has an evolutionarily conserved role in ICL repair (C. elegans, chicken, and human), but only the human protein, containing the BRCA1-interacting motif, appears to function in DSB repair, as well. Our data also allow us propose that at least two distinct pathways of ICL repair exist in C. elegans: (i) the FA pathway, comprising fcd-2 and dog-1, and (ii) a genetically distinct pathway that requires brc-1 and its heterodimeric partner, brd-1 (data not shown). The contributions and interplay of these two pathways in ICL repair remain to be elucidated.
DOG-1 was previously shown to play a role in the maintenance of G/C tracts, as dog-1 mutants display a mutator phenotype characterized by deletions initiating in G/C tracts throughout the genome (11). The G/C tract deletions observed in dog-1 mutants were typically a few hundred base pairs long and initiated in the 3′ end of the G tract, extending upstream for various distances; therefore, Cheung et al. (11) proposed that DOG-1 is involved in unwinding DNA secondary structures that occur in tracts of 18 or more guanines during lagging-strand replication. Recently, it has been shown that rfs-1; dog-1 mutants exhibit accelerated G/C tract deletions compared to dog-1 single mutants (42). Since rfs-1 is required exclusively for promoting RAD-51 loading at blocked replication forks (42), this result strongly suggested that G/C tracts form secondary DNA structures that impact on replication fork progression. Although DOG-1 is essential for G/C tract stability, our data indicate that this function is not shared by other FA proteins. The absence of any of the FA pathway activators or associated components, including ATL-1, CLK-2, FCD-2, BRC-2, and MLH-1, did not affect G/C tract stability in a wild-type background. Of those tested, DOG-1 was the only FA pathway component required for G/C tract stability. Previously, it was shown that both HR repair and TLS are required to prevent deletions at G/C tracts in the absence of DOG-1 (42, 45). We report here that the frequency of deletions in dog-1; fcd-2 mutants is similar to that previously detected in HR and TLS mutants in the dog-1 background. Our data raise the possibility that in the absence of DOG-1, replication forks stalled by G/C tract secondary structures are repaired through cooperation of FCD-2 with HR and/or TLS proteins. Although FCD-2 is dispensable for recruitment of HR proteins to sites of DNA damage, it may facilitate HR repair following RAD-51 nucleoprotein filament formation, as has been suggested in human cells (15, 40, 41, 43).
One intriguing possibility arising from our findings in the nematode is that FANCJ and FANCD2 (in the absence of FANCJ) are important for the maintenance of G-rich DNA in humans. Indeed, cells defective for ATR, RAD51, and FANCD2 show instability at fragile sites that, like G/C tracts, represent DNA regions that are difficult to replicate through and consequently are susceptible to sequence alterations and genomic instability (references 1 and 16 and references therein). However, it is important to note that G/C tracts are a source of genome instability only in dog-1 mutants and not in other C. elegans FA genes (e.g., fcd-2 or brc-2/C. elegans FANCD1). This implies that the role of DOG-1 in the maintenance of G/C tract sequences may be a function independent of its role in the C. elegans FA pathway during ICL repair. Based on our analysis in C. elegans, it is unlikely that secondary structures formed by G/C tracts represent a physiological lesion that will impact human FA patients in general, but such lesions may be a cause of genome instability in FA-J cells. In future studies, it will be important to assess G/C tract stability in FA-deficient cells to determine whether the genomic instability observed in FA patients arises at such sequences and, if so, if this instability is unique to cells deficient in FANCJ.
In summary, the data reported here support a role for DOG-1 in ICL repair that is functionally similar to that of human FANCJ. Given that the fundamental components of the FA pathway are conserved in C. elegans, including FANCD2, FANCI, FANCD1/BRCA2, FANCM, FANCL (13), and now FANCJ, the nematode will be a useful model for study of ICL repair. The identification of a FANCJ homologue in C. elegans also creates the potential to identify new components of the ICL repair pathway through genetic screens for functional interactors with DOG-1. Furthermore, the role of DOG-1 at G/C tracts implies that DNA secondary structures could be a potential contributor to the genomic instability observed in FA-J cells.
Acknowledgments
We thank S. Mitani and the International C. elegans Knockout Consortium for generating strains. Some strains were provided by the Caenorhabditis elegans Genetics Centre, which is supported by the National Institutes of Health Center for Research Resources. Don Riddle, Don Moerman, and David Baillie are acknowledged for sharing laboratory equipment.
This work was supported by a Natural Sciences and Engineering Research Council (NSERC) discovery grant and a Canadian Institutes of Health Research grant (A.M.R.), as well as an NSERC scholarship and Michael Smith Foundation for Health Research fellowship (J.L.Y.). Funds for this project were also provided by Cancer Research UK (S.J.B.) and the Fanconi Anemia Research Fund (S.J.C. and S.J.B.).
Footnotes
Published ahead of print on 17 December 2007.
REFERENCES
- 1.Arlt, M. F., S. G. Durkin, R. L. Ragland, and T. W. Glover. 2006. Common fragile sites as targets for chromosome rearrangements. DNA Repair 51126-1135. [DOI] [PubMed] [Google Scholar]
- 2.Auerbach, A. D., D. Warburton, A. D. Bloom, and R. S. Chaganti. 1979. Preliminary communication: prenatal detection of the Fanconi Anemia gene by cytogenetic methods. Am. J. Hum. Genet. 3177-81. [PMC free article] [PubMed] [Google Scholar]
- 3.Boulton, S. J., A. Gartner, J. Reboul, P. Vaglio, N. Dyson, D. E. Hill, and M. Vidal. 2002. Combined functional genomic maps of the C. elegans DNA damage response. Science 295127-131. [DOI] [PubMed] [Google Scholar]
- 4.Boulton, S. J., J. S. Martin, J. Polanowska, D. E. Hill, A. Gartner, and M. Vidal. 2004. BRCA1/BARD1 orthologs required for DNA repair in Caenorhabditis elegans. Curr. Biol. 1433-39. [DOI] [PubMed] [Google Scholar]
- 5.Brenner, S. 1974. The genetics of Caenorhabditis elegans. Genetics 7771-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bridge, W. L., C. J. Vandenberg, R. J. Franklin, and K. Hiom. 2005. The BRIP1 helicase functions independently of BRCA1 in the Fanconi anemia pathway for DNA crosslink repair. Nat. Genet. 37953-957. [DOI] [PubMed] [Google Scholar]
- 7.Cabuy, E., C. Newton, G. Joksic, L. Woodbine, B. Koller, P. A. Jeggo, and P. Slijepcevic. 2005. Accelerated telomere shortening and telomere abnormalities in radiosensitive cell lines. Radiat. Res. 16453-62. [DOI] [PubMed] [Google Scholar]
- 8.Cantor, S., R. Drapkin, F. Zhang, Y. Lin, J. Han, S. Pamidi, and D. M. Livingston. 2004. The BRCA1-associated protein BACH1 is a DNA helicase targeted by clinically relevant inactivating mutations. Proc. Natl. Acad. Sci. USA 1012357-2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cantor, S. B., D. W. Bell, S. Ganesan, E. M. Kass, R. Drapkin, S. Grossman, D. C. Wahrer, D. C. Sgroi, W. S. Lane, D. A. Haber, and D. M. Livingston. 2001. BACH1, a novel helicase-like protein, interacts directly with BRCA1 and contributes to its DNA repair function. Cell 105149-160. [DOI] [PubMed] [Google Scholar]
- 10.Cervenka, J., D. Arthur, and C. Yasis. 1981. Mitomycin C test for diagnostic differentiation of idiopathic aplastic anemia and Fanconi anemia. Pediatrics 67119-127. [PubMed] [Google Scholar]
- 11.Cheung, I., M. Schertzer, A. Rose, and P. M. Lansdorp. 2002. Disruption of dog-1 in Caenorhabditis elegans triggers deletions upstream of guanine-rich DNA. Nat. Genet. 31405-409. [DOI] [PubMed] [Google Scholar]
- 12.Collis, S. J., L. J. Barber, A. J. Clark, J. S. Martin, J. D. Ward, and S. J. Boulton. 2007. HCLK2 is essential for the mammalian S-phase checkpoint and impacts on Chk1 stability. Nat. Cell Biol. 9391-401. [DOI] [PubMed] [Google Scholar]
- 13.Collis, S. J., L. J. Barber, J. D. Ward, J. S. Martin, and S. J. Boulton. 2006. C. elegans FANCD2 responds to replication stress and functions in interstrand cross-link repair. DNA Repair 51398-1406. [DOI] [PubMed] [Google Scholar]
- 14.Dequen, F., J. F. St-Laurent, S. N. Gagnon, M. Carreau, and S. Desnoyers. 2005. The Caenorhabditis elegans FancD2 ortholog is required for survival following DNA damage. Comp. Biochem. Physiol. B 141453-460. [DOI] [PubMed] [Google Scholar]
- 15.Digweed, M., S. Rothe, I. Demuth, R. Scholz, D. Schindler, M. Stumm, M. Grompe, A. Jordan, and K. Sperling. 2002. Attenuation of the formation of DNA-repair foci containing RAD51 in Fanconi anaemia. Carcinogenesis 231121-1126. [DOI] [PubMed] [Google Scholar]
- 16.Durkin, S. G., and T. W. Glover. 2007. Chromosome fragile sites. Annu. Rev. Genet. Annu. Rev. Genet. 41169-192. [DOI] [PubMed] [Google Scholar]
- 17.Esashi, F., V. E. Galkin, X. Yu, E. H. Egelman, and S. C. West. 2007. Stabilization of RAD51 nucleoprotein filaments by the C-terminal region of BRCA2. Nat. Struct. Mol. Biol. 14468-474. [DOI] [PubMed] [Google Scholar]
- 18.Esmer, C., S. Sanchez, S. Ramos, B. Molina, S. Frias, and A. Carnevale. 2004. DEB test for Fanconi anemia detection in patients with atypical phenotypes. Am. J. Med. Genet. A 12435-39. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Muse, T., and S. J. Boulton. 2005. Distinct modes of ATR activation after replication stress and DNA double-strand breaks in Caenorhabditis elegans. EMBO J. 244345-4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Godthelp, B. C., W. W. Wiegant, Q. Waisfisz, A. L. Medhurst, F. Arwert, H. Joenje, and M. Z. Zdzienicka. 2006. Inducibility of nuclear Rad51 foci after DNA damage distinguishes all Fanconi anemia complementation groups from D1/BRCA2. Mutat. Res. 59439-48. [DOI] [PubMed] [Google Scholar]
- 21.Gurtan, A. M., and A. D. D'Andrea. 2006. Dedicated to the core: understanding the Fanconi anemia complex. DNA Repair 51119-1125. [DOI] [PubMed] [Google Scholar]
- 22.Howlett, N. G., T. Taniguchi, S. Olson, B. Cox, Q. Waisfisz, C. De Die-Smulders, N. Persky, M. Grompe, H. Joenje, G. Pals, H. Ikeda, E. A. Fox, and A. D. D'Andrea. 2002. Biallelic inactivation of BRCA2 in Fanconi anemia. Science 297606-609. [DOI] [PubMed] [Google Scholar]
- 23.Lee, K. Y., I. Yang, J. E. Park, O. R. Baek, K. Y. Chung, and H. S. Koo. 2007. Developmental stage- and DNA damage-specific functions of C. elegans FANCD2. Biochem. Biophys. Res. Commun. 352479-485. [DOI] [PubMed] [Google Scholar]
- 24.Levitus, M., H. Joenje, and J. P. de Winter. 2006. The Fanconi anemia pathway of genomic maintenance. Cell Oncol. 283-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levitus, M., Q. Waisfisz, B. C. Godthelp, Y. de Vries, S. Hussain, W. W. Wiegant, E. Elghalbzouri-Maghrani, J. Steltenpool, M. A. Rooimans, G. Pals, F. Arwert, C. G. Mathew, M. Z. Zdzienicka, K. Hiom, J. P. De Winter, and H. Joenje. 2005. The DNA helicase BRIP1 is defective in Fanconi anemia complementation group J. Nat. Genet. 37934-935. [DOI] [PubMed] [Google Scholar]
- 26.Levran, O., C. Attwooll, R. T. Henry, K. L. Milton, K. Neveling, P. Rio, S. D. Batish, R. Kalb, E. Velleuer, S. Barral, J. Ott, J. Petrini, D. Schindler, H. Hanenberg, and A. D. Auerbach. 2005. The BRCA1-interacting helicase BRIP1 is deficient in Fanconi anemia. Nat. Genet. 37931-933. [DOI] [PubMed] [Google Scholar]
- 27.Ling, C., M. Ishiai, A. M. Ali, A. L. Medhurst, K. Neveling, R. Kalb, Z. Yan, Y. Xue, A. B. Oostra, A. D. Auerbach, M. E. Hoatlin, D. Schindler, H. Joenje, J. P. de Winter, M. Takata, A. R. Meetei, and W. Wang. 2007. FAAP100 is essential for activation of the Fanconi anemia-associated DNA damage response pathway. EMBO J. 262104-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Litman, R., M. Peng, Z. Jin, F. Zhang, J. Zhang, S. Powell, P. R. Andreassen, and S. B. Cantor. 2005. BACH1 is critical for homologous recombination and appears to be the Fanconi anemia gene product FANCJ. Cancer Cell 8255-265. [DOI] [PubMed] [Google Scholar]
- 29.Marchler-Bauer, A., J. B. Anderson, P. F. Cherukuri, C. DeWeese-Scott, L. Y. Geer, M. Gwadz, S. He, D. I. Hurwitz, J. D. Jackson, Z. Ke, C. J. Lanczycki, C. A. Liebert, C. Liu, F. Lu, G. H. Marchler, M. Mullokandov, B. A. Shoemaker, V. Simonyan, J. S. Song, P. A. Thiessen, R. A. Yamashita, J. J. Yin, D. Zhang, and S. H. Bryant. 2005. CDD: a Conserved Domain Database for protein classification. Nucleic Acids Res. 33D192-D196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin, J. S., N. Winkelmann, M. I. Petalcorin, M. J. McIlwraith, and S. J. Boulton. 2005. RAD-51-dependent and -independent roles of a Caenorhabditis elegans BRCA2-related protein during DNA double-strand break repair. Mol. Cell Biol. 253127-3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mirchandani, K. D., and A. D. D'Andrea. 2006. The Fanconi anemia/BRCA pathway: a coordinator of cross-link repair. Exp. Cell Res. 3122647-2653. [DOI] [PubMed] [Google Scholar]
- 32.Peng, M., R. Litman, J. Xie, S. Sharma, R. M. Brosh, Jr., and S. B. Cantor. 2007. The FANCJ/MutLalpha interaction is required for correction of the cross-link response in FA-J. cells. EMBO J. 263238-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petalcorin, M. I., V. E. Galkin, X. Yu, E. H. Egelman, and S. J. Boulton. 2007. Stabilization of RAD-51-DNA filaments via an interaction domain in Caenorhabditis elegans BRCA2. Proc. Natl. Acad. Sci. USA 1048299-8304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reid, S., D. Schindler, H. Hanenberg, K. Barker, S. Hanks, R. Kalb, K. Neveling, P. Kelly, S. Seal, M. Freund, M. Wurm, S. D. Batish, F. P. Lach, S. Yetgin, H. Neitzel, H. Ariffin, M. Tischkowitz, C. G. Mathew, A. D. Auerbach, and N. Rahman. 2007. Biallelic mutations in PALB2 cause Fanconi anemia subtype FA-N and predispose to childhood cancer. Nat. Genet. 39162-164. [DOI] [PubMed] [Google Scholar]
- 35.Seal, S., D. Thompson, A. Renwick, A. Elliott, P. Kelly, R. Barfoot, T. Chagtai, H. Jayatilake, M. Ahmed, K. Spanova, B. North, L. McGuffog, D. G. Evans, D. Eccles, Breast Cancer Susceptibility Collaboration (UK), D. F. Easton, M. R. Stratton, and N. Rahman. 2006. Truncating mutations in the Fanconi anemia J. gene BRIP1 are low-penetrance breast cancer susceptibility alleles. Nat. Genet. 381239-1241. [DOI] [PubMed] [Google Scholar]
- 36.Shiozaki, E. N., L. Gu, N. Yan, and Y. Shi. 2004. Structure of the BRCT repeats of BRCA1 bound to a BACH1 phosphopeptide: implications for signaling. Mol. Cell 14405-412. [DOI] [PubMed] [Google Scholar]
- 37.Sims, A. E., E. Spiteri, R. J. Sims III, A. G. Arita, F. P. Lach, T. Landers, M. Wurm, M. Freund, K. Neveling, H. Hanenberg, A. D. Auerbach, and T. T. Huang. 2007. FANCI is a second monoubiquitinated member of the Fanconi anemia pathway. Nat. Struct. Mol. Biol. 14564-567. [DOI] [PubMed] [Google Scholar]
- 38.Smogorzewska, A., S. Matsuoka, P. Vinciguerra, E. R. McDonald III, K. E. Hurov, J. Luo, B. A. Ballif, S. P. Gygi, K. Hofmann, A. D. D'Andrea, and S. J. Elledge. 2007. Identification of the FANCI protein, a monoubiquitinated FANCD2 paralog required for DNA repair. Cell 129289-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taniguchi, T., and A. D. D'Andrea. 2006. Molecular pathogenesis of Fanconi anemia: recent progress. Blood 1074223-4233. [DOI] [PubMed] [Google Scholar]
- 40.Taniguchi, T., I. Garcia-Higuera, P. R. Andreassen, R. C. Gregory, M. Grompe, and A. D. D'Andrea. 2002. S-phase-specific interaction of the Fanconi anemia protein, FANCD2, with BRCA1 and RAD51. Blood 1002414-2420. [DOI] [PubMed] [Google Scholar]
- 41.Thompson, L. H., J. M. Hinz, N. A. Yamada, and N. J. Jones. 2005. How Fanconi anemia proteins promote the four Rs: replication, recombination, repair, and recovery. Environ. Mol. Mutagen. 45128-142. [DOI] [PubMed] [Google Scholar]
- 42.Ward, J. D., L. J. Barber, M. I. Petalcorin, J. Yanowitz, and S. J. Boulton. 2007. Replication blocking lesions present a unique substrate for homologous recombination. EMBO J. 263384-3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamamoto, K., S. Hirano, M. Ishiai, K. Morishima, H. Kitao, K. Namikoshi, M. Kimura, N. Matsushita, H. Arakawa, J. M. Buerstedde, K. Komatsu, L. H. Thompson, and M. Takata. 2005. Fanconi anemia protein FANCD2 promotes immunoglobulin gene conversion and DNA repair through a mechanism related to homologous recombination. Mol. Cell. Biol. 2534-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang, H., P. D. Jeffrey, J. Miller, E. Kinnucan, Y. Sun, N. H. Thoma, N. Zheng, P. L. Chen, W. H. Lee, and N. P. Pavletich. 2002. BRCA2 function in DNA binding and recombination from a BRCA2-DSS1-ssDNA structure. Science 2971837-1848. [DOI] [PubMed] [Google Scholar]
- 45.Youds, J. L., N. J. O'Neil, and A. M. Rose. 2006. Homologous recombination is required for genome stability in the absence of DOG-1 in Caenorhabditis elegans. Genetics 173697-708. [DOI] [PMC free article] [PubMed] [Google Scholar]