Abstract
Bone morphogenic proteins (BMPs) play pleotrophic roles in nervous system development, and their signaling is highly regulated at virtually every step in the pathway. We have cloned a novel gene, Sizn1 (Smad-interacting zinc finger protein), which functions as a transcriptional coactivator of BMP signaling. It positively modulates BMP signaling by interacting with Smad family members and associating with CBP in the transcription complex. Sizn1 is expressed in the ventral embryonic forebrain, where, as we will show, it contributes to BMP-dependent, cholinergic-neuron-specific gene expression. These data indicate that Sizn1 is a positive modulator of BMP signaling and provide further insight into how BMP signaling can be modulated in neuronal progenitor subsets to influence cell-type-specific gene expression and development.
Bone morphogenic protein (BMP) signaling is initiated by dimeric ligand (BMP) binding to a type I and type II receptor complex, which activates the type I receptor serine/threonine kinase and results in the phosphorylation of receptor Smads (R-Smads) (3). Phosphorylated R-Smads bind with the common mediator Smad (Smad4) and translocate to the nucleus to regulate transcription. Inhibitory Smads (I-Smads), such as Smad6 and Smad7, can block the phosphorylation of R-Smads or prevent the translocation of the R-Smad/Smad4 complex to the nucleus (3, 8, 19). In the nucleus, Smads can interact with FaxH1/FAST; FoxO; Runx2; Dlx1; Hoxc-8; OAZ; GATA-2, -3, -4, and -5; and/or members of the nuclear receptor family to enhance or repress the transcription of target genes through direct DNA binding (8). Furthermore, Smads are able to recruit transcriptional coactivators or corepressors such as p300/CBP, P/CAF, Smad-interacting protein 1 (SIP-1), melanocyte-specific gene 1 (Msg1), nuclear oncogene Ski/SnoN, Smad4-interacting factor (SMIF), transforming growth factor β (TGF-β)-interacting factor, and Tob into the transcription machinery (8).
BMP signaling plays multiple roles in central nervous system development. Among the established functions are dorsal specifications of the neural tube, including the spinal cord and forebrain (1, 9, 10, 14, 15, 26). Interestingly, in the forebrain, BMPs also play a role in ventral development (5). BMP-7 plays inductive functions in ventral forebrain midline cells in early development (5), and BMP-9, expressed only in the ventral telencephalic neural tube, induces and maintains the expression of septal cholinergic-neuron-specific genes (17, 18). Little is known about how BMP signaling is regulated in a fashion that permits the specification of distinct cell types in spatially and temporally restricted patterns during neural development.
We report the identification of a new modulator of BMP signaling. In contrast to other known BMP signaling modulators, Sizn1 (Smad interacting zinc finger protein) is unique in having developmentally regulated spatial and temporal expression patterns. We show that Sizn1 is expressed in the ventral forebrain and functions as a transcriptional coactivator necessary for BMP-dependent, cholinergic-neuron-specific gene expression. These data provide mechanistic insight into how BMP signaling can be regulated in specific cell subpopulations.
MATERIALS AND METHODS
Subtractive-hybridization screen.
To identify novel genes involved in forebrain patterning and neuron specification, a PCR-based subtractive-hybridization screen (PCR-select cDNA subtraction kit; Clontech) was performed using pooled samples of dorsal and ventral (basal) mouse prosencephalons microdissected from embryonic day 11.5 (E11.5) and E12.5 embryos, according to the manufacturer's instructions. The full-length clone of Sizn1 was obtained by screening an E12.5 mouse cDNA library (provided by Doug Epstein, University of Pennsylvania), using the partial cDNA of Sizn1 derived from the subtractive-hybridization screen.
Yeast two-hybrid screening.
The full-length Sizn1 coding sequence was PCR amplified and cloned into pGBKT7 (Clontech) to generate the Sizn1 bait. The yeast cell line AH109(MATa), containing the Sizn1 bait construct, was mated with Y187(MATα) cells pretransformed with an E11 mouse embryo two-hybrid system Matchmaker3 library (Clontech) to screen for interaction partners of Sizn1, according to the manufacturer's instructions.
Northern blot analysis.
Northern blotting was performed with total RNAs (10 μg in each lane) taken from the dorsal or ventral forebrains of E11.5-to-E12.5 mice. For adult tissue Northern blotting, we used mouse multiple-tissue Northern blotting (Clontech), ULTRAhyb (Ambion) (according to the manufacturer's instructions), and the following probes: partial 3′ untranslated regions of Sizn1, Dlx5 (the coding region), and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (Clontech) and the coding region from the vesicular acetyl choline transporter (VaChT; provided by Jan Krzysztof Blusztajn) (17).
In situ hybridization.
Whole-embryo in situ hybridization was performed as previously described using a full-length Sizn1 clone to generate the riboprobe (Roche) (10, 11, 28). E9.5-to-E14.5 embryos from CD1 mice were used for all experiments.
DNA constructs.
pCMV/Sizn1-GFP was generated by subcloning the mouse Sizn1 coding region into pcDNA3-CTGFP (Invitrogen). pMIWIII/Sizn1-myc was generated by subcloning the PCR product containing the Sizn1 coding region into the HindIII and EcoRV sites of pMIWIII/myc as the myc fusion protein. pGST-Sizn1 was constructed by subcloning the Sizn1 PCR product into pGEX-5T (Amersham). pMIWIII/Gal4DB-Sizn1 was constructed by subcloning the PCR products from pGBK/T7-Sizn1, which has a Sizn1 insert at the EcoRI and XhoI sites of pGBKT7 (Clontech). The FLAG-Smad1 and Gal4DB-Smad1 expression constructs and Smad binding element x4-luciferase (SBEx4-luc) construct were obtained from Kohei Miyazono and Takeshi Imamura (JFCR Cancer Institute, Tokyo, Japan). Smad1(2D) and Smad4 fragments were generated by PCR, inserted into pcDNA3.1D-TOPO (Invitrogen), and fused to the V5 tag. The CBPs were obtained from Gerd Blobel (the Children's Hospital of Philadelphia). Choline acetyltransferase (ChAT)-luc deletion mutants were constructed with PCR products containing each fragment of the ChAT promoter as described in the legend to Fig. 4.
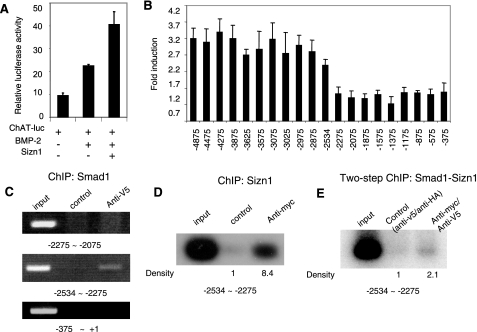
FIG. 4.
The ChAT promoter region from approximately −2534 to −2275 has Smad binding sites. (A) In C2C12 cells, the expression of ChAT-luc (kindly provided by Jan Krzysztof Blusztajn) (17) was induced by BMP-2 in the presence of Sizn1 (means ± SDs are shown [n = 4]). (B) Reporter gene assay of SN56 cells with serial deletion reporter constructs to identify the cis-acting element of the ChAT promoter interacting with Smad1(2D)/Smad4. The x axis is labeled with the 5′ extent of each construct, starting with the translational start site. The y axis values are induction rates over the baseline (means ± SDs [n = 7]). (C) The anti-V5 ChIP DNA pools contain only the promoter fragments from approximately −2534 to −2275 (n = 3). (D) Southern blot analysis shows that the anti-myc (4A12) ChIP DNA pools include the fragments from approximately −2534 to −2275 (n = 2). (E) Southern blot analysis with the two-step ChIP shows that the anti-V5 and anti-myc (4A12) ChIP DNA pools include the promoter fragment from approximately −2534 to −2275 (n = 2).
Cell culture, transfection, and luciferase assay.
HEK293T and C2C12 cell lines were cultured in Dulbecco's modified Eagle's medium containing 10% and 15% fetal bovine serum (HyClone), respectively, at 37°C and 5% CO2. The C2C12 cells were transfected with various combinations of the following plasmids using FuGene6 (Roche): reporter constructs (SBEx4-luc) (0.1 μg), a β-galactosidase (β-Gal) expression vector driven by the cytomegalovirus (CMV) promoter (CMV-β-Gal) (0.05 μg), constitutively active BMPR1a(QD) (0.2 μg), a CBP expression construct (0.5 μg), or pMIW/Sizn1-myc (each, 0.5 μg). In the Gal4x5 luciferase assays, the reporter construct (Gal4x5-luc) (0.1 μg), CMV-β-Gal (0.05 μg), the Gal4DB-Smad1 expression construct (0.2 μg), and pMIW/Sizn1-myc (each, 0.5 μg) were used with HEK293T cells. In the ChAT-luc reporter assay, a deletion series of ChAT-luc reporter constructs (0.1 μg), CMV-β-Gal (0.05 μg), or Smad1(2D) (0.3 μg)/Smad4 (0.3 μg) were used with SN56 cells. Empty vectors for each construct were used to allow the transfection of equal amounts of DNA. Cell extracts were prepared with Promega lysis buffer followed by centrifugation. Luciferase activity was measured by the Promega luciferase assay system. Transfection efficiency was standardized with β-Gal activity. All assays were performed each time in duplicate. The error bars in the graphs of the luciferase assay results in each figure indicate the standard deviations (SDs).
Immunostaining and antibody production.
The predicted amino acid sequences of Sizn1 proteins were used to design a peptide for generating antibody against Sizn1 (Fig. 1A). The C terminus (RARARGAPGEPIGLSE) was synthesized and injected into a rabbit after conjugation with keyhole limpet hemocyanin at Genosys, Inc. This antibody was purified with peptide-conjugated beads. Immunohistochemistry was performed on an E14.5, paraffin-embedded mouse brain. Antigen retrieval was accomplished by microwaving sections for 10 min in EDTA solution (Dako). After being blocked, the primary antibody (anti-Sizn1, 1/100, or anti-acetyl cholinesterase [AChE], 1/500) was applied overnight at 4°C. After being washed in phosphate-buffered saline, the appropriate secondary antibodies, conjugated to fluorescein isothiocyanate or Texas Red, were applied. The cells were washed, counterstained with DAPI (4′,6-′-diamidino-2-phenylindole), and viewed by indirect epifluorescence.
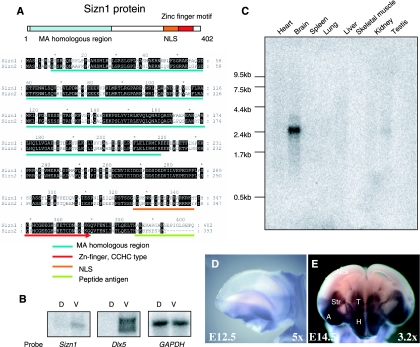
FIG. 1.
Sizn1 is a novel gene expressed in the ventral forebrain. (A) Schematic diagram of the Sizn1 protein and the amino acid sequence alignment of Sizn1 and Sizn2. The Zn finger motif, nuclear localization sequence (NLS), and MA-homologous domain are highlighted. The unique C-terminal sequence of Sizn1 used as the peptide antigen is also shown. (B) Northern blot analysis with E12.5 dorsal (D) and ventral (V) total RNAs shows that Sizn1 is expressed ventrally, similarly to the known ventrally expressed Dlx5 gene. GAPDH serves as a loading control. (C) Adult tissue Northern blot analysis shows that Sizn1 is expressed in the adult brain and testis. (D, E) Whole-mount in situ hybridization for Sizn1 in the embryonic mouse brain. Sizn1 expression is restricted to the ventral region of the forebrain, including the septum, amygdala, and caudal putamen. (D) Lateral view of an E12.5 telencephalon. (E) Coronal view of an E14.5 forebrain at the level where both the posterior telencephalon and anterior diencephalon can be recognized. Ncx, neocortex; Str, striatum; T, thalamus; H, hypothalamus; A, amygdala.
Immunoprecipitation and glutathione S-transferase (GST) pull-down assay.
After transfection, cells were lysed with TNE buffer (20 mM Tris-HCl [pH 7.4], 150 mM NaCl, 0.5% Triton X-100, 5% glycerol) containing a protease inhibitor cocktail (Roche). Anti-Smad1 monoclonal antibody (A-4; Santa Cruz Biotechnology, Inc.) was used for endogenous Smad1 protein immunoprecipitation. In order to avoid the possibility that cytoplasmic Smad proteins might interact with nuclear Sizn1 protein by the contamination of two fractions during preparation of the crude extracts, the nuclear extracts were prepared as described previously (2) after 40-min treatments with BMP-2 (20 ng/ml; R&D Systems). For preclearance, lysates were incubated with protein G-conjugated beads (Invitrogen). Each primary antibody (2 μg) defined by the individual experiment was added, incubated at 4°C for 2 h, and then incubated with the protein G-conjugated beads for an additional hour. The beads were washed with TNE buffer twice and with TNE buffer containing 500 mM NaCl two more times, followed by a final wash with TNE buffer. Bound protein was eluted using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. To detect the associated protein in the immunoprecipitate, SDS-PAGE followed by Western blotting was performed. Anti-Flag (M2, 1/500; Sigma), anti-CBP (A-22, 1/1,000; Santa Cruz), anti-myc (9E10, 1/100; Sigma), anti-Smad1 (1/500), and anti-Sizn1 (1/1,000) antibodies were used for immunoblotting.
The GST pull-down assay was performed with in vitro-translated Smad1 (labeled with [35S]methionine) and GST-Sizn1 protein from Escherichia coli. The glutathione bead coated with GST-Sizn1 or GST protein (each, 2 μg) was incubated in TNE buffer in the presence of in vitro-translated products of Smad1 for 1 h at 4°C. After washing of the beads five times, the eluated samples were loaded and run on SDS-PAGE gels, followed by autoradiography on X-ray film.
Primary septum culture, shRNA knockdown, and qRT-PCR.
Dissociated cells (1.5 × 106 cells per well) obtained from E14.5 mouse septum were treated with trypsin for 20 min, plated onto poly-d-lysine-laminin-coated 24-well plates, and incubated with 10% fetal bovine serum-DMEM in the presence of basic fibroblast growth factor (bFGF) (20 ng/ml). Short hairpin RNA (shRNA) expression constructs against Sizn1 were generated by subcloning the annealed oligonucleotide into pSUPER.neo (Oligoengine). The sequences used included the nontargeting negative control (5′-CAGTCGCGTTTGCGACTGG-3′; Dharmacon), target I (5′-GGAGTCTTGGCCCTTTAAT-3′), target II (5′-GGAGGAAAAGGTCAGACGT-3′), and target III (5′-GGAGACTTTTATTAATCCA-3′). A mouse neuron kit (Amaxa Biosystems) was used to introduce shRNA into primary septal neuron cultures. After incubation for 5 days with bFGF (20 ng/ml) with and without BMP-2 (10 ng/ml) and G418 (240 ng/ml), total RNA was extracted with an RNeasy mini kit (Qiagen) and used for real-time quantitative reverse transcription (qRT)-PCR to measure Sizn1, ChAT, and VaChT mRNA levels. The cultures were treated with G418 (400 μg/ml) to eliminate nontransfected cells. Real-time qRT-PCR was performed with Sybr green PCR reagent (Qiagen) and the Abi Prism 7000 sequence detection system. Total RNA was extracted from two independent experiments for each real-time RT-PCR. Each PCR was done in triplicate. All mRNA levels were normalized to the 18S rRNA level. The mRNA expression levels were calculated by relative quantification. The data were statistically analyzed by analysis of variance, followed by paired Student t tests for individual comparison when the analysis of variance showed significance. The PCR primers included Sizn1 forward (5′-TTTGGTGCAGCTGGTTGTAG-3′), Sizn1 reverse (5′-ACACGAAGTTCAAGGCGTTT-3′), ChAT forward (5′-AGGGCAGCCTCTCTGTATGA-3′), ChAT reverse (5′-GAGACGGCGGAAATTAATGA-3′), VaChT forward (5′-ACACGTCTGGCATTGCCAT-3′), and VaChT reverse (5′-CCACTAGGCTTCCAAAGCTGA-3′).
ChIP.
Since both the Smad1 and Sizn1 antibodies did not have sufficient affinity for the chromatin immunoprecipitation (ChIP) assays, we used cells transiently expressing these proteins for the ChIP assays. SN56 cells were transfected with expression constructs for Smad1 tagged with V5 and Smad4 or myc-Sizn1. After transfection, the cells were cultured for 2 days, harvested, and fixed with 1% formaldehyde for 10 min. ChIP was performed using an Upstate ChIP kit (Upstate, Inc.), according to the manufacturer's protocols, and anti-V5 (Invitrogen), anti-myc (4A12; Upstate, Inc.), and antihemagglutinin (as a negative control; Roche) antibodies. ChIP DNA was dissolved in 20 μl Tris-EDTA buffer and used as a template for the PCR (1 μl/reaction). Amplification was performed at 95°C for 20 s, 62°C for 30 s, and 68°C for 30 s for 27 cycles or 35 cycles (we tested the amplicons at the higher cycle number [data not shown]). For the two-step ChIP, anti-V5-immunoprecipitated complex was eluted with V5 peptide (100 μg/ml) and then anti-myc antibody (4A12), developed for ChIP, was applied. Southern blot analysis was performed for Sizn1 or the two-step ChIP because of a weak signal after common ethidium bromide staining in the agarose gel. The primer sets used were as follows: from approximately −2534 to −2275, 5′-GGGGCGGTGGGGAGGCAATGTTC-3′ and 5′-GCCGCCCCGACTGACTGACTG-3′; from approximately −2275 to −2015, 5′-CGGGGCGGCTGCTGGGATCT-3′ and 5′-CCTGGGTCCGCGGGCTCTTCA-3′; and from approximately −375 to 1, 5′-GGGAGTGTGCAACCAGCCCAGCT-3′ and 5′-GCTGCCGACCTGGGAACAGAGGA-3′.
RESULTS
Identification and expression pattern of Sizn1.
A screen to identify modulators of dorsal-ventral patterning in the mammalian forebrain was performed with mice of E11.5 to E12.5 (see Materials and Methods for details). One of the candidate molecules identified in this screen was a 2.4-kb cDNA predicted to encode a novel 402-amino-acid protein containing an N-terminal paraneoplastic antigen (MA)-homologous region, a single zinc finger motif (C2HC type), and a putative nuclear localization sequence (Fig. 1A). A search for homologous genes in GenBank revealed that the N-terminal domain of Sizn1 shares approximately 50 to 60% similarity with the C-terminal region of the paraneoplastic antigen family members (MA-1, -2, and -3) and modulator of apoptosis 1 (MAP-1) (MA4) proteins (4, 6, 23, 25) (see the supplemental material). Based on these findings, the gene was named Sizn1 (Zcchc12) (see below for Smad interactions; GenBank accession no. AY466375). Northern blot analyses of the total RNAs taken from the dorsal or ventral forebrains of E11.5-to-E12.5 mice demonstrate that Sizn1 expression is restricted to the ventral forebrain (Fig. 1B). In the adult mouse, Sizn1 transcripts were detected mainly in the brain and at lower levels in the testis by Northern blot analysis (Fig. 1C). Consistent with the Northern blot analyses, in situ hybridization also showed Sizn1 expression in the ventral forebrain at E12.5 and E14.5, including the septal region, amygdala, putamen, thalamus, and hypothalamus (Fig. 1D and E). Using the mouse genome database (Ensembl), we localized Sizn1 and a gene (Sizn2 [Zcchc18]; GenBank accession no. AY650116) homologous to the X chromosome. Sizn1 was further mapped to X A3.1, whereas Sizn2 was mapped to chromosome X F1. These genes have 76% homology to each other along their entire coding sequences (Fig. 1A). The human ortholog of Sizn1 was identified by homology and found to map to Xq24. Mouse Sizn1 has 78% homology to human SIZN1. A human ortholog of Sizn2, based on domain structure, was also identified on human chromosome Xq22.2. No homologous genes were found in invertebrate genomes, including those of Caenorhabditis elegans and Drosophila melanogaster, suggesting that Sizn1 is a vertebrate-specific gene.
Sizn1 functions as a transcriptional coactivator in the BMP signaling pathway.
We next sought to identify Sizn1-interacting molecules. We first examined whether Sizn1 binds to nucleic acids, given its nuclear localization. However, the direct interaction of Sizn1 with DNA or RNA was not detected by nucleic acid binding studies (data not shown). The absence of nucleic acid binding suggested that Sizn1 may function by interacting with other nuclear proteins. To identify Sizn1-interacting proteins, 5 × 106 clones from an E11 mouse library (GAL4 DB Matchmaker library; Clontech) were screened by using Sizn1 as a bait in a yeast two-hybrid system. Most of the positive clones were nuclear proteins, including members of the Smad family (data not shown). To confirm the yeast two-hybrid system result, we directly tested the interaction between Sizn1 and Smad1. Using a GST pull-down assay with Sizn1 expressed in E. coli and in vitro-translated Smad1, we detected the direct binding of Sizn1 to Smad1 (Fig. 2A; see also the supplemental material). This interaction between Sizn1 and Smad1 was further confirmed by coimmunoprecipitation of Sizn1 with Smad1 in HEK293T cells transiently expressing myc-Sizn1 and FLAG-Smad1 (Fig. 2B). In addition, we tested the interaction of endogenous Sizn1 and Smad1 in mouse septal cell line SN56. In the presence of BMP-2, Sizn1 protein was detected in anti-Smad1 immunoprecipitates (Fig. 2C). To verify the functional interaction between Sizn1 and Smad1 in vivo, Gal4DB-Smad1 was generated and cotransfected with the Gal4x5-luc reporter construct with or without myc-Sizn1 in C2C12 cells. Sizn1 enhanced Gal4DB-responsive Gal4x5-luc reporter gene expression, indicating that it interacts with Smad1 in vivo and enhances Smad1-mediated transcription (Fig. 2D). However, Sizn1 is not able to activate the Gal4x5-luc reporter gene without Gal4DB-Smad1 (see the supplemental material).
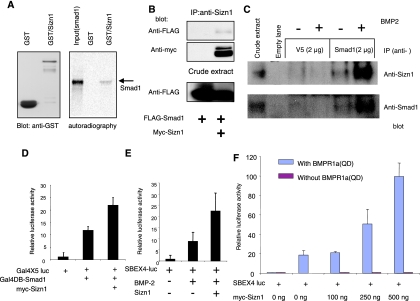
FIG. 2.
Sizn1 up-regulates BMP signaling through interaction with Smad1. (A) GST pull-down assay shows that the GST-Sizn1 fusion protein purified from E. coli binds with in vitro-translated Smad1 protein (a reverse GST pull-down assay using GST-Smad1 confirms this interaction [see the supplemental material]). (B) Immunoprecipitation (IP) and immunoblot analysis of HEK293T cells, transfected with pMIWIII/Sizn1-myc and pCMV/Flag-Smad1, indicate that Sizn1 can interact with Smad1. Sizn1 protein expressed in HEK293T cells showed two bands on the Western blot, the upper band reflecting a phosphorylated form (data not shown). (C) Endogenous Sizn1 interacts with Smad1 that has translocated to the nucleus. A nuclear fraction of BMP-2 (20 ng/ml for 40 min)-treated SN56 cells, which express both Sizn1 and Smad1, was immunoprecipitated with anti-Smad1 antibody (A-4), followed by Western blotting with anti-Sizn1 or anti-Smad1 antibody. The first lane shows that Sizn1 and Smad1 are present in whole lysates. The remaining lanes are immunoblots of nuclear extracts. Anti-V5 antibody was used as a negative control for immunoprecipitation. (D) The Gal4x5-luc reporter assay shows that Sizn1 enhances Smad1 function in the nucleus and that Sizn1 interacts with Smad1 in the nucleus (n = 8). (E) Sizn1 can activate the BMP-2-responsive induction of SBEx4-luc. C2C12 cells were transfected with SBEx4-luc and mock DNA or myc-Sizn1 in the presence or absence of BMP-2 (10 ng/ml) (n = 6). (F) The Sizn1-mediated activation of BMP signaling is dose dependent. C2C12 cells were cotransfected with and without SBEx4-luc, a constitutively active form of BMPR1a(QD), and with various concentrations of myc-Sizn1 (n = 3) [data not shown for a constitutively active mutant such as BMPR1b(QD) (n = 2)].
The interaction of Sizn1 with Smad1 led us to hypothesize that Sizn1 may have a function in BMP signaling. To test this hypothesis, we took advantage of a previously characterized in vitro assay (29). C2C12 cells, known to be BMP responsive, were transfected with SBEx4-luc and then exposed to recombinant BMP-2 protein. BMP-2 treatment activated SBEx4-luc expression as expected (Fig. 2E). Interestingly, Sizn1 cotransfection to this system enhanced the BMP-responsive expression of SBEx4-luc (Fig. 2E). Similar results were obtained when a constitutively active mutant BMP receptor construct, BMPR1a(QD) (16, 26), was used instead of BMP-2 treatment (Fig. 2F). This enhancement of BMP-responsive gene activation by Sizn1 is dose dependent, and Sizn1 alone cannot activate SBEx4-luc (Fig. 2F). Taken together, these data indicate that Sizn1 can positively modulate BMP signaling via interacting with Smad1 in the nucleus.
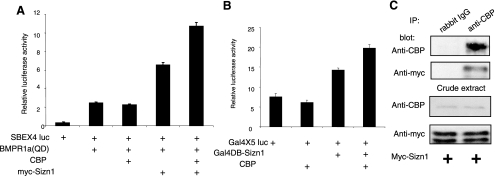
Transcriptional coactivation occurs through multiprotein complexes that dock on transcription factors and recruit chromatin-modifying enzymes, such as CBP/p300 or SWI/SNF family members. BMP-responsive transcriptional activation requires R-Smad-dependent recruitment of CBP (21). Thus, we hypothesized that Sizn1 may function in BMP signaling by associating with CBP in the transcription complex. To test this hypothesis, we cotransfected the CBP expression construct with SBEx4-luc and BMPR1a(QD) into C2C12 cells. Although CBP alone cannot activate the SBEx4-luc expression induced by BMPR1a(QD), the Sizn1-dependent activation of BMP signaling was further enhanced by the presence of CBP (Fig. 3A). Based on these data, we propose that the Sizn1-dependent activation of BMP signaling functions through the association of CBP with the Smad transcription complex and, thus, that Sizn1 is a transcriptional coactivator. To test for a possible interaction between Sizn1 and CBP, we transfected HEK293 cells with Gal4x5-luc and Gal4DB-Sizn1 or Gal4DB, with or without the CBP expression construct. Gal4DB-Sizn1 alone shows slight Gal4x5-luc induction, suggesting that it has inherent transcription coactivator activity (Fig. 3B). In addition, together with CBP, it can exert strong induction of the reporter gene, indicating that Sizn1 can associate CBP with the transcription complex. Consistent with this result, Sizn1 can be coimmunoprecipitated with CBP in C2C12 cells transfected with Sizn1 (Fig. 3C). Thus, at least one function of Sizn1 in the cell is to associate CBP with the transcription complex and, in so doing, to potentiate BMP signaling.
FIG. 3.
Sizn1 positively modulates BMP signaling by recruiting CBP. (A) C2C12 cells were transfected with SBEx4-luc, BMPR1a(QD), CBP, and Sizn1 as indicated. BMP signaling is up-regulated in the presence of Sizn1 and CBP (n = 8). (B) CBP can enhance reporter gene expression driven by Gal4DB-Sizn1. HEK293 cells were transfected with Gal4x5-luc with Gal4DB, in combination with Gal4DB-Sizn1, or with Gal4DB-Sizn1 and CBP expression constructs (n = 3). (C) C2C12 cells were transfected with Sizn1 expression constructs and immunoprecipitated with anti-CBP antibody or a control antibody (rabbit immunoglobulin G [IgG]). Immunoprecipitates were analyzed by Western blotting with an anti-myc antibody, which showed that Sizn1 interacts with CBP in both crude extracts and by immunoprecipitation (IP).
Smad1 can directly bind to the ChAT promoter and induce its expression.
BMP signaling contributes to basal-forebrain cholinergic-neuron development (17, 18). However, whether cholinergic-neuron differentiation is a direct or indirect target of BMP signaling is unknown. ChAT is a cholinergic-neuron-specific gene. We explored whether BMP signaling directly regulates ChAT expression. First, we used a previously reported ChAT-luc construct containing ∼5-kb sequences upstream of the ChAT start codon as a putative ChAT promoter (17). BMP-2 treatment of C2C12 cells (that do not express Sizn1 [data not shown]) transfected with ChAT-luc exhibited a modest up-regulation of luciferase activity, and this induction was enhanced by the coexpression of Sizn1, indicating that BMP signaling can induce ChAT promoter activity and that Sizn1 can enhance this induction (Fig. 4A; see also the supplemental material).
Given the role of Smad1 as a transcriptional activator in BMP signaling, we predict that ChAT promoter activation by BMP-2 is mediated through Smad1 binding to the ChAT promoter. To identify possible Smad binding sites in the ChAT promoter, we generated serial promoter deletions and tested for their induction ability in SN56 cells, immortalized mouse forebrain septal cells (17) that express Sizn1. In this assay, Smad4, required to bind Smad1 for cotranslocation to the nucleus, and the constitutively active mutant Smad1(2D) were used together to induce reporter gene expression with the promoters with serial deletions (22). Deletion of the region from approximately −2534 to −2275 resulted in the loss of Smad1(2D)-induced transcription (Fig. 4B). Based on sequence homology, four other candidate Smad binding sites are present in the region from approximately −4875 to −2875. However, mutations in these sites showed no change in transcription activity (data not shown). These data indicate that a functional Smad binding site exists in the region from approximately −2534 to −2275 of the ChAT promoter.
To confirm a direct interaction between Smad1 and the DNA fragment from approximately −2534 to −2275, a ChIP assay was performed with SN56 cells. V5-tagged Smad1(2D) and FLAG-tagged Smad4 were cotransfected into SN56 cells. The candidate region in the ChAT promoter was interrogated by PCR with anti-V5 ChIP DNA (Fig. 4C). The PCR amplified the fragment from approximately −2534 to −2275 (Fig. 4E), indicating that Smad1 can occupy this site in the ChAT promoter. Based on our model for Sizn1 function, we predict that Sizn1 up-regulates the transcription of the ChAT gene through its interaction with Smad proteins. To test this, we expressed myc-Sizn1 and V5-Smad1(2D)/FLAG-Smad4 in SN56 cells and performed a modified ChIP assay by pulling DNA down with myc antibody (Sizn1). As shown in Fig. 4D, PCR amplified the fragment from approximately −2534 to −2275 from Sizn1-ChIP DNA pools. Since Sizn1 does not directly bind DNA, this result may be due to Sizn1 occupying DNA through its interaction with Smad1. To prove this, we performed a two-step ChIP, sequentially pulling down Smad1 and Sizn1. As expected, the two-step ChIP also resulted in the amplification of the fragment from approximately −2534 to −2275 (Fig. 4E), providing evidence that Sizn1 exists in a protein-DNA complex through its association with Smad1.
Sizn1 is necessary for BMP-dependent, basal-forebrain cholinergic-neuron-specific gene expression.
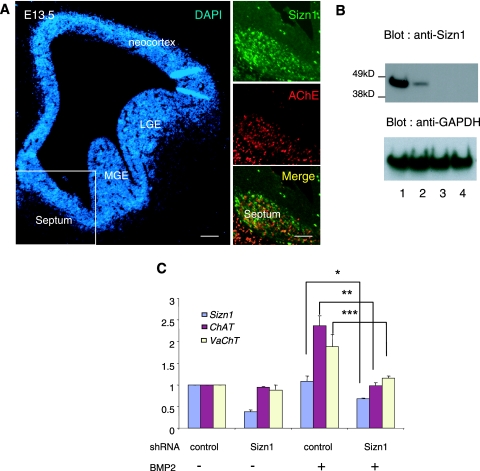
Coimmunostaining of Sizn1 with AChE in an E13.5 mouse brain section reveals that Sizn1 is expressed in the basal-forebrain cholinergic neurons (BFCN) (Fig. 5A). Given that Sizn1 modulates BMP signaling and is expressed in BFCN and that BMP signaling contributes to BFCN development (17, 18), we hypothesized that Sizn1 would play a role in BFCN-specific gene expression. To test this hypothesis, we generated shRNA expression constructs against three target sites in Sizn1. The knockdown efficiency of shRNAs was first tested in SN56 cells. We found that all target shRNAs efficiently inhibited the expression of Sizn1, although target I showed the weakest inhibition (Fig. 5B). Target II shRNA was selected for knocking down primary septum neurons. We next assayed mRNA levels for Sizn1, ChAT, and VaChT, which are preferentially expressed in cholinergic neurons, by real-time qRT-PCR of E13.5 primary septal cell cultures transfected with the target shRNA expression constructs (Fig. 5C; see also the supplemental material). Sizn1 expression in these cultures showed a slight induction by BMP-2. The cultures transfected with the shRNA to Sizn1 showed a repression of Sizn1 expression (Fig. 5C). ChAT and VaChT were up-regulated by BMP, and both transcripts were repressed in the presence of shRNA to Sizn1 (Fig. 5C), indicating that Sizn1 is necessary for the normal BMP-dependent induction of ChAT and VaChT in the cholinergic neurons of the basal forebrain.
FIG. 5.
Sizn1 plays a role in BMP-dependent, cholinergic-neuron-specific gene expression. (A) Immunostaining of Sizn1 and AChE in a coronal section of an E13.5 mouse brain, indicating that Sizn1 is expressed in cholinergic neurons. LGE, lateral ganglionic eminence; MGE, medial ganglionic eminence. Scale bar = 100 μm. (B) Knockdown of Sizn1 in SN56 septum cells after transfection of three kinds of shRNA. Lane 1 contains the control (nontargeting negative-control shRNA), and lanes 2 to 4 contain shRNA against Sizn1 (targets I to III). Target II was used for the introduction of shRNA to primary septum cells for qRT-PCR. (C) qRT-PCR analysis of relative mRNA levels for Sizn1, ChAT, and VaChT after Sizn1 knockdown with shRNA (target II) in the primary septal culture in the presence of BMP-2. The Sizn1 mRNA level is significantly reduced (by ∼60%) by treatment with Sizn1 shRNA (n = 3). ChAT and VaChT expression is also repressed by Sizn1 shRNA in the presence of BMP-2 (means ± SDs). *, P = 0.02; **, P = 0.007; ***, P = 0.016. bFGF (20 ng/ml) was added to each sample to maintain high precursor cell numbers.
DISCUSSION
We have identified a novel protein that functions as a transcriptional coactivator interacting with Smad to modulate BMP signaling. Our data support a model where Sizn1 associates with Smads in the nucleus upon BMP signaling activation. Sizn1 also recruits, or stabilizes, CBP to the transcription complex to positively regulate signaling. We have also demonstrated that BMP signaling can directly regulate ChAT gene transcription, a necessary factor for the cholinergic phenotype. Furthermore, we have found that Sizn1 knockdown reduces the BMP-dependent induction of cholinergic-neuron-specific genes (ChAT and VaChT). Finally, we have shown that the coactivator function of Sizn1 is dependent on R-Smads entering the nucleus.
TGF-β family members, including BMPs and activin, play multiple roles in development, differentiation, and tumorigenesis through signaling (13, 20, 27). Given that TGF-β family members and their receptors show broad temporal and spatial expression patterns during development, the expression patterns of ligands and receptors alone are not sufficient to explain the distinct roles played by TGF-β family members in restricted cell populations. Thus, additional modulators are required to control TGF-β signaling levels. The regulation of pathway activation is found at many levels, including ligand availability, receptor binding and activation, the translocation of the activated Smad complex to the nucleus, and the recruitment of transcription factors and transcriptional coregulators (coactivators or corepressors). Sizn1 can now be added to the list of known transcriptional coregulators. Unlike most ubiquitously expressed transcriptional coregulators that have been reported so far, Sizn1 exhibits restricted spatial and temporal expression patterns in the brain (Msg1 also shows some restricted expression) (24), which can permit further refinement of BMP signaling. Cholinergic-neuron-specific expression of Sizn1 in the septal nucleus is a good example of this postulate. Once its own expression is induced by BMP signaling, Sizn1 can participate in the activation of other genes necessary for inducing and/or maintaining the cholinergic-neuron phenotype.
Although our studies have focused on Sizn1 expression in septal cholinergic neurons, our data also show that Sizn1 is expressed in the developing thalamus, amygdala, putamen (Fig. 1D and E), and brain stem (data not shown) as well as in the septum. Preliminary data indicate that its expression is not restricted to cholinergic neurons in other brain regions. Definition of these areas of expression, the cell types, and the function of Sizn1 in these cells is under active investigation. Outside the nervous system, we know that Sizn1 is also expressed in the testis (Fig. 1C). TGF-β signaling, including through BMP-7, BMP-4, BMP-8a, and BMP-8b, plays multiple roles in spermatogenesis and maintaining epididymal integrity (12). It is reasonable to consider that Sizn1 may also have some role in these processes.
Given the fact that Sizn1 is expressed in the forebrain and hindbrain regions, where cholinergic neurons do not reside, we can postulate that Sizn1 likely has roles in the development/maintenance of other types of neurons. It is also possible that Sizn1 may be involved in other signaling pathways, not only in the BMP pathway. We have some evidence supporting the idea that Sizn1 can modulate Wnt signaling but not Shh signaling (unpublished data). In this manner, Sizn1, along with other transcription factors and transcriptional coregulators, contributes to generating the complexity of neuronal subtypes in the mammalian nervous system. Theoretically, this may also account for the lack of a Sizn1 ortholog, as well as many other transcriptional coactivators/corepressors, in invertebrates, which have simpler nervous systems and fewer neuronal subtypes. It should be noted that Sizn1 is also expressed in the adult brain (Fig. 1C). We speculate that it may have a role in maintaining cholinergic-neuron-specific gene expression. Further studies are obviously required to define the expression of Sizn1 in the adult nervous system and to characterize any functions that it has in the mature brain.
BFCNs project to the cortex and limbic system, where they function in processes such as attention, learning, and memory (7). Previous studies have shown that BMP-9 contributes to the maturation of BFCNs by regulating multiple genes involved in the induction and maintenance of the cholinergic phenotype (17, 18). Our data support these findings and provide the mechanism of how BMP signaling can directly modulate the cholinergic phenotype. In summary, Sizn1 is a novel transcriptional coactivator in the BMP-signaling pathway, functioning by interacting with Smad1 and CBP. It contributes to the BMP-induced enhancement of cholinergic-neuron-specific gene expression, including that of ChAT and VaChT. Given the requirement of ventral-forebrain cholinergic neurons in cognition, future studies with animal models will provide further insight into the potential role of Sizn1 in memory and other related cognitive functions.
Supplementary Material
Acknowledgments
We are grateful to Thomas Curran, Craig B. Thompson, and Bryan Wolf for critically reviewing the manuscript. We thank Kohei Miyazono and Takeshi Imamura for sharing the Smad expression constructs and the SBEx4-luc reporter and Jan Krzysztof Blusztajn for ChAT-luc.
This work was supported by Public Health Service grant HD26979 (J.A.G.) from the National Institutes of Health.
Footnotes
Published ahead of print on 26 December 2007.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Altmann, C. R., and A. H. Brivanlou. 2001. Neural patterning in the vertebrate embryo. Int. Rev. Cytol. 203447-482. [DOI] [PubMed] [Google Scholar]
- 2.Andrews, N. C., and D. V. Faller. 1991. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res. 192499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Attisano, L., and J. L. Wrana. 2002. Signal transduction by the TGF-beta superfamily. Science 2961646-1647. [DOI] [PubMed] [Google Scholar]
- 4.Barnett, M., J. Prosser, I. Sutton, G. M. Halmagyi, L. Davies, C. Harper, and J. Dalmau. 2001. Paraneoplastic brain stem encephalitis in a woman with anti-Ma2 antibody. J. Neurol. Neurosurg. Psychiatry 70222-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dale, J. K., C. Vesque, T. J. Lints, T. K. Sampath, A. Furley, J. Dodd, and M. Placzek. 1997. Cooperation of BMP7 and SHH in the induction of forebrain ventral midline cells by prechordal mesoderm. Cell 90257-269. [DOI] [PubMed] [Google Scholar]
- 6.Dalmau, J., S. H. Gultekin, R. Voltz, R. Hoard, T. DesChamps, C. Balmaceda, T. Batchelor, E. Gerstner, J. Eichen, J. Frennier, J. B. Posner, and M. R. Rosenfeld. 1999. Ma1, a novel neuron- and testis-specific protein, is recognized by the serum of patients with paraneoplastic neurological disorders. Brain 12227-39. [DOI] [PubMed] [Google Scholar]
- 7.Everitt, B. J., and T. W. Robbins. 1997. Central cholinergic systems and cognition. Annu. Rev. Psychol. 48649-684. [DOI] [PubMed] [Google Scholar]
- 8.Feng, X. H., and R. Derynck. 2005. Specificity and versatility in TGF-beta signaling through Smads. Annu. Rev. Cell Dev. Biol. 21659-693. [DOI] [PubMed] [Google Scholar]
- 9.Furuta, Y., D. W. Piston, and B. L. Hogan. 1997. Bone morphogenetic proteins (BMPs) as regulators of dorsal forebrain development. Development 1242203-2212. [DOI] [PubMed] [Google Scholar]
- 10.Golden, J. A., A. Bracilovic, K. A. McFadden, J. S. Beesley, J. L. Rubenstein, and J. B. Grinspan. 1999. Ectopic bone morphogenetic proteins 5 and 4 in the chicken forebrain lead to cyclopia and holoprosencephaly. Proc. Natl. Acad. Sci. USA 962439-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hargrave, M., and P. Koopman. 2000. In situ hybridization of whole-mount embryos. Methods Mol. Biol. 123279-289. [DOI] [PubMed] [Google Scholar]
- 12.Hu, J., Y. X. Chen, D. Wang, X. Qi, T. G. Li, J. Hao, Y. Mishina, D. L. Garbers, and G. Q. Zhao. 2004. Developmental expression and function of Bmp4 in spermatogenesis and in maintaining epididymal integrity. Dev. Biol. 276158-171. [DOI] [PubMed] [Google Scholar]
- 13.Larsson, J., and S. Karlsson. 2005. The role of Smad signaling in hematopoiesis. Oncogene 245676-5692. [DOI] [PubMed] [Google Scholar]
- 14.Liem, K. F., Jr., G. Tremml, and T. M. Jessell. 1997. A role for the roof plate and its resident TGFβ-related proteins in neuronal patterning in the dorsal spinal cord. Cell 91127-138. [DOI] [PubMed] [Google Scholar]
- 15.Liem, K. F., Jr., G. Tremml, H. Roelink, and T. M. Jessell. 1995. Dorsal differentiation of neural plate cells induced by BMP-mediated signals from epidermal ectoderm. Cell 82969-979. [DOI] [PubMed] [Google Scholar]
- 16.Lim, Y., G. Cho, J. Minarcik, and J. Golden. 2005. Altered BMP signaling disrupts chick diencephalic development. Mech. Dev. 122603-620. [DOI] [PubMed] [Google Scholar]
- 17.Lopez-Coviella, I., B. Berse, R. Krauss, R. S. Thies, and J. K. Blusztajn. 2000. Induction and maintenance of the neuronal cholinergic phenotype in the central nervous system by BMP-9. Science 289313-316. [DOI] [PubMed] [Google Scholar]
- 18.Lopez-Coviella, I., M. T. Follettie, T. J. Mellott, V. P. Kovacheva, B. E. Slack, V. Diesl, B. Berse, R. S. Thies, and J. K. Blusztajn. 2005. Bone morphogenetic protein 9 induces the transcriptome of basal forebrain cholinergic neurons. Proc. Natl. Acad. Sci. USA 1026984-6989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Massague, J., and D. Wotton. 2000. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 191745-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munoz-Sanjuan, I., and A. H. Brivanlou. 2002. Neural induction, the default model and embryonic stem cells. Nat. Rev. Neurosci. 3271-280. [DOI] [PubMed] [Google Scholar]
- 21.Pearson, K. L., T. Hunter, and R. Janknecht. 1999. Activation of Smad1-mediated transcription by p300/CBP. Biochim. Biophys. Acta 1489354-364. [DOI] [PubMed] [Google Scholar]
- 22.Qin, B. Y., B. M. Chacko, S. S. Lam, M. P. de Caestecker, J. J. Correia, and K. Lin. 2001. Structural basis of Smad1 activation by receptor kinase phosphorylation. Mol. Cell 81303-1312. [DOI] [PubMed] [Google Scholar]
- 23.Rosenfeld, M. R., J. G. Eichen, D. F. Wade, J. B. Posner, and J. Dalmau. 2001. Molecular and clinical diversity in paraneoplastic immunity to Ma proteins. Ann. Neurol. 50339-348. [PubMed] [Google Scholar]
- 24.Shioda, T., M. H. Fenner, and K. J. Isselbacher. 1996. msg1, a novel melanocyte-specific gene, encodes a nuclear protein and is associated with pigmentation. Proc. Natl. Acad. Sci. USA 9312298-12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan, K. O., K. M. Tan, S. L. Chan, K. S. Yee, M. Bevort, K. C. Ang, and V. C. Yu. 2001. MAP-1, a novel proapoptotic protein containing a BH3-like motif that associates with Bax through its Bcl-2 homology domains. J. Biol. Chem. 2762802-2807. [DOI] [PubMed] [Google Scholar]
- 26.Timmer, J. R., C. Wang, and L. Niswander. 2002. BMP signaling patterns the dorsal and intermediate neural tube via regulation of homeobox and helix-loop-helix transcription factors. Development 1292459-2472. [DOI] [PubMed] [Google Scholar]
- 27.Varga, A. C., and J. L. Wrana. 2005. The disparate role of BMP in stem cell biology. Oncogene 245713-5721. [DOI] [PubMed] [Google Scholar]
- 28.Wilkinson, D. G., and M. A. Nieto. 1993. Detection of messenger RNA by in situ hybridization to tissue sections and whole mounts. Methods Enzymol. 225361-373. [DOI] [PubMed] [Google Scholar]
- 29.Yoshida, Y., S. Tanaka, H. Umemori, O. Minowa, M. Usui, N. Ikematsu, E. Hosoda, T. Imamura, J. Kuno, T. Yamashita, K. Miyazono, M. Noda, T. Noda, and T. Yamamoto. 2000. Negative regulation of BMP/Smad signaling by Tob in osteoblasts. Cell 1031085-1097. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.