Abstract
The heterotrimeric G proteins G12 and G13 link G-protein-coupled receptors to the regulation of the actin cytoskeleton and the induction of actomyosin-based cellular contractility. Here we show that conditional ablation of the genes encoding the α-subunits of G12 and G13 in the nervous system results in neuronal ectopia of the cerebral and cerebellar cortices due to overmigration of cortical plate neurons and cerebellar Purkinje cells, respectively. The organization of the radial glia and the basal lamina was not disturbed, and the Cajal-Retzius cell layer had formed normally in mutant mice. Embryonic cortical neurons lacking G12/G13 were unable to retract their neurites in response to lysophosphatidic acid and sphingosine-1-phosphate, indicating that they had lost the ability to respond to repulsive mediators acting via G-protein-coupled receptors. Our data indicate that G12/G13-coupled receptors mediate stop signals and are required for the proper positioning of migrating cortical plate neurons and Purkinje cells during development.
The cortical structures of the adult mammalian central nervous system are organized into cell layers. This laminar organization is the result of the precisely controlled migration of cells from distinct germinal layers to their final destination in the developing cortex (18, 20, 33, 48, 52). The mechanisms underlying the well orchestrated formation of cortical structures are only partially understood. Some of the molecules regulating the correct migration of neurons in the developing cortex have been discovered by analyzing mutations that disrupt neuronal migration and formation of neuronal layers (3, 9, 19). One of the best studied signaling systems involved in the proper positioning of neurons in the developing cerebral and cerebellar cortices consists of the extracellular protein reelin and its receptors VLDLR and ApoER2 (22, 42, 50). However, a variety of other proteins like integrins or β-amyloid precursor proteins have been shown to be required for the correct layering of cortical neurons (2, 12, 21), indicating that multiple mechanisms are involved in the migration and layering of neurons during cortical development (3, 19).
The intracellular signaling cascades involved in cell migration have been studied in various cells including neurons and fibroblasts (11, 19, 39, 41, 43). Monomeric GTPases of the Rho family have been shown to play a central role in the regulation of cytoskeletal rearrangements and regulation of adhesive functions underlying cell migration (43). While Rac and Cdc42 are involved in the formation of cell protrusions and the formation of adhesions, RhoA plays an important role in the retraction of cell protrusions. Multiple G-protein-coupled receptors have been shown to regulate the migratory activity of cells by modulating the activity of Rac/Cdc42 and/or Rho (44, 54). Activation of RhoA via G-protein-coupled receptors is primarily mediated by the ubiquitously expressed heterotrimeric G proteins G12 and G13 (5, 44). Mice lacking the α-subunit of G13 die in utero (38), whereas Gα12-deficient mice are viable (13). In the present study we generated conditional mouse mutants carrying inactivating mutations in the genes coding for Gα12 (Gna12) and Gα13 (Gna13) in the nervous system in order to study their role in neural development. Our study revealed an unexpected role of the G12/G13-mediated signaling pathway in the development of the cerebral and cerebellar cortices.
MATERIALS AND METHODS
Mice.
Mice in which the gene coding for Gα13, Gna13, is flanked by loxP sites (Gna13flox) (35) were crossed to the constitutively Gα12-deficient mouse line (13) and to mice which express the recombinase Cre under the control of the nestin promoter (51, 55) or the NEX promoter (10). Genotyping for the Gna13flox and Gna12− alleles was described previously (35). The primers used for detecting the nestin-Cre transgene were 5′-AGTGCTGACTCTCCTCGGCTT-3′ and 5′-CCAGACCTGTTCCACCTCTG-3′. Mice were housed under specific-pathogen-free conditions, and all animal experiments were performed in accordance with institutional animal care and use committee regulations. The genetic background of the mice was predominantly C57BL6/N (at least a sixth-generation backcross), and littermates with the nestin-Cre; Gna13fl/fl; Gna12+/+, Gna13fl/fl; Gna12−/−, or Gna13fl/fl; Gna12−/+ genotype were used as controls.
Western blot analysis.
Brain lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis. After blotting, nitrocellulose membranes were probed with antibodies against Gα12 (30), Gα13, or Gαq/11 (Santa Cruz Biotechnology).
Histology.
Mice were deeply anesthetized with pentobarbital (100 mg/kg of body weight, intraperitoneally) and perfused with 4% paraformaldehyde via the left cardiac ventricle. For histology, whole heads or dissected brains were postfixed overnight and then stored in 0.5% paraformaldehyde at 4°C for Vibratome sectioning or embedding in paraffin. Alternatively, fixed tissues were incubated for 24 h in 30% sucrose-phosphate-buffered saline (PBS) at 4°C before being frozen on dry ice. Vibratome sections (50 μm) were cut, paraffin-embedded material was sectioned at 6 μm, and frozen brains were sectioned at 20 μm. Sections were stained with cresyl violet following a standard protocol.
Immunohistochemistry.
Immunohistochemistry on Vibratome sections and paraffin-embedded material was performed with the following antibodies: anti-NeuN (Chemicon; 1:2,000) and anticalbindin (Chemicon; 1:2,000). Immunohistochemistry on paraffin-embedded material was performed with the following antibodies: anticalretinin (Chemicon; 1:250), antireelin (Chemicon; clone G10 at 1:50), and antibromodeoxyuridine (anti-BrdU) (Sigma; 1:100) diluted in PBS. Incubation with first antibodies was performed for 24 h at 4°C. For secondary antibodies, we used rabbit anti-mouse immunoglobulin G (Dako; diluted 1:50 in PBS) for 1 h at room temperature. Bound secondary antibodies were detected by the avidin-biotin peroxidase complex (Vector Laboratories, Burlingame, CA) and visualized with diaminobenzidine (Vector Laboratories). For anti-BrdU stainings, fluorescently labeled secondary antibodies were used (Dianova).
Immunohistochemistry on frozen sections was performed after fixation in 4% (wt/vol) paraformaldehyde in PBS (pH 7.4) overnight, followed by 30 min of incubation in blocking buffer (10% normal goat serum and 0.5% Triton X-100 in PBS). The first antibody incubation with anticalretinin (Chemicon; 1:500), anti-reelin (Chemicon; clone G10 at 1:500), and anti-RC2 antisera (1:100) was carried out overnight at 4°C at the indicated dilutions followed by incubation (1 h) with the secondary antibodies, which were conjugated either to Alexa TM 594 (red fluorescence) or Alexa TM 488 (green fluorescence) (Molecular Probes, Eugene, OR). Nuclei were counterstained with DAPI (4′,6′-diamidino-2-phenylindole; Sigma). Fluorescent specimens were viewed with a Nikon C1si confocal laser-scanning microscope.
BrdU birthdating and morphometric analysis.
Pregnant females were injected at 11:00 a.m. either on embryonic day 12.5 (E12.5) or on E15.5, with a single dose of BrdU (Sigma) at a concentration of 100 μg/g of body weight. Females were killed 24 h later on E18.5, and pups were genotyped by PCR as described above. Brains from pups were processed for paraffin embedding, and incorporated BrdU was detected by immunohistochemistry (see above). Cells with dense staining of more than half of the nucleus were considered BrdU positive.
Primary cultures from mouse cortex.
Cultures of cortical and cerebellar neurons were prepared with some minor modifications according to the procedure described by Banker and Goslin (1) for hippocampal neurons. Cerebral and cerebellar cortices from E16.5 or E17.5 mouse embryos were prepared in ice-cold dissection solution (PBS with 30 mM HEPES and 33 mM glucose, pH 7.38) and washed once in PBS. After incubation with 0.05% trypsin (Invitrogen) for 15 min at 37°C, the tissues were washed with PBS and triturated with fire-polished Pasteur pipettes in plating medium (minimal essential medium supplemented with 10% fetal bovine serum, 1 mM sodium pyruvate [Invitrogen], 25 mM glucose, and 25 μM glutamic acid [Sigma]). Cells were seeded in plating medium on poly-l-lysine (Sigma)-coated glass slides (Nunc) or plastic dishes (Falcon) at a density of 1.3 × 105 cells/well (∼7.3 × 104 cells/cm2, for live-cell imaging) or 1 × 106 cells/well (∼1 × 105 cells/cm2, for RhoA activation assay), respectively, and maintained at 37°C in 5% CO2. After 4 h, plating medium was replaced by growth medium (neurobasal medium with B-27 supplement and 0.5 mM l-glutamine [Invitrogen]), and the cells were cultured for 20 h without changing the medium. Transfection of primary neurons with a eukaryotic expression plasmid encoding Gα13 was performed with an Amaxa (Cologne, Germany) mouse neuron transfection kit according to the manufacturer's instructions.
Live-cell imaging.
Live-cell images were recorded on a Leica DM IRE2 microscope equipped with a 37°C/5% CO2 environmental control chamber using a Leica DC 350 FX camera and Leica FW4000 software. Randomly selected cerebral and cerebellar neurons were imaged before and 5, 15, and 20 min after addition of 10 μM lysophosphatidic acid (LPA) and 1 μM sphingosine-1-phosphate (S1P) (Biomol) or 20 min after addition of 2 μg/ml ephrin-A5-Fc (R&D Systems) preclustered with 20 μg/ml anti-human immunoglobulin G-Fc (Sigma) as described previously (28). Cerebellar neuronal cells were acetone-fixed and stained with an anticalbindin antibody (Chemicon; 1:2,000) to identify Purkinje cells. Neurite retraction (percent of retracted neurites) was calculated from 15 to 45 cells per embryo, and the number of embryos analyzed was 3 to 6.
Determination of RhoA activity.
RhoA activation assay was performed using a G-Lisa RhoA activation assay kit (Cytoskeleton) according to the manufacturer's instructions. Briefly, cells were incubated for 30 s with vehicle alone (PBS) or 10 μM LPA, immediately washed with ice-cold PBS, and prepared in ice-cold lysis buffer. Lysates were incubated in microplate wells coated with a RhoA-GTP-binding protein and the bound active RhoA was detected using a RhoA-specific antibody and chemoluminescence.
Statistical analysis.
The statistical analyses were performed using the Mann-Whitney U test.
RESULTS
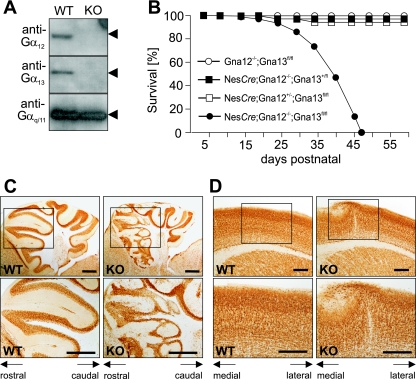
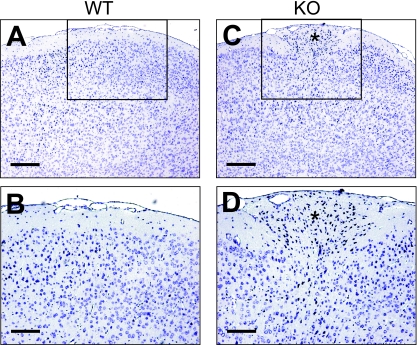
To analyze the role of the G12/G13-mediated signaling pathway in the development of the nervous system, we crossed Gα12-deficient mice which were homozygous for a floxed Gα13 allele (35) with a transgenic mouse line expressing Cre under the control of the neuron-specific enhancer of the nestin promoter (51). The nestin-Cre allele has been shown to result in a very efficient recombination in neuronal and glial precursor cells starting at E10.5. Nonneuronal/nonglial cells of the nervous system, like those of blood vessels and meninges, are not recombined (12, 55). When nestin-Cre; Gna12−/+; Gna13flox/flox mice were crossed with Gna12−/+; Gna13flox/flox animals, all genotypical combinations were obtained in the living offspring with the expected frequencies. Western blot analyses of whole-brain lysates of nestin-Cre; Gna12−/−; Gna13flox/flox mice showed the absence of both Gα12 and Gα13 proteins (Fig. 1A). The postnatal growth of nestin-Cre; Gna12−/−; Gna13flox/flox mice was retarded and resulted in premature death between postnatal day 10 (P10) and P40 (Fig. 1B). At about 2 weeks of age, surviving double mutants exhibited obvious abnormalities like a reduced body size and ataxia. In contrast, mice with one intact Gna12 or Gna13 allele (nestin-Cre; Gna12−/+; Gna13flox/flox or nestin-Cre; Gna12−/−; Gna13+/flox) showed normal postnatal development and had a normal life expectancy. When brains of P21 nestin-Cre; Gna12−/−; Gna13flox/flox mice were analyzed histologically, severe malformations of their cerebellar and cerebral cortices were observed in all animals analyzed (23) (Fig. 1C and D). The cerebellar cortex of the rostral part of the vermis was highly disorganized, while the caudal part and the hemispheres appeared to be grossly normal (Fig. 1C). The cerebral cortices of double knockouts were convoluted and marked by invasions of neurons into layer I as well as by ectopia (Fig. 1D). The cortical structures of mice carrying one intact Gna12 allele (nestin-Cre; Gna12−/+; Gna13flox/flox) or one intact Gna13 allele (nestin-Cre; Gna12−/−; Gna13+/flox) were morphologically normal (data not shown). This indicates that the loss of all four alleles of the Gna12 and Gna13 genes is required for the observed phenotypical abnormalities.
FIG. 1.
Postnatal lethality and cortical dysplasia in nestin-Cre; Gna12−/−; Gna13flox/flox mice. (A) Western blot analysis of Gα12, Gα13, and Gαq/Gα11 expression in brain extracts prepared from wild-type (WT) and nestin-Cre; Gna12−/−; Gna13flox/flox (KO) mice. (B) Postnatal survival of nestin-Cre; Gna12−/−; Gna13flox/flox, nestin-Cre; Gna12−/+; Gna13flox/flox, nestin-Cre; Gna12−/−; Gna13+/flox, and Gna12−/−; Gna13flox/flox mice; the total number of analyzed animals was 54, 51, 38, and 29, respectively. (C) Sagittal section through the cerebellum of wild type (WT) and nestin-Cre; Gna12−/−; Gna13flox/flox (KO) animals at P21 stained with an anti-NeuN antibody. Cortical malformations in the knockout cerebella were restricted to the rostral part of the vermis. (D) Frontal sections of the cerebral cortex from wild type (WT) and nestin-Cre; Gna12−/−; Gna13flox/flox (KO) P21 brains stained with an anti-NeuN antibody. Scale bars are 250 μm (panel C) and 125 μm (panel D).
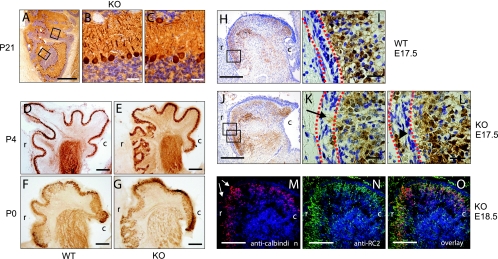
In the postnatal rostral cerebellum, the normal lobulation was absent. Instead, the cortex appeared to be thinner and convoluted with heterotopia and ectopia. Despite these severe irregularities, Purkinje cells had basically developed their dendritic tree, which filled the molecular layer, and granule cells had migrated inward past the Purkinje cell layer and had formed an inner granule cell layer (Fig. 2A to C). To determine the developmental stage during which the severe malformation of the rostral cerebellar cortex started, we analyzed serial sagittal sections at P7, P4, and P0 as well as at E18.5, E17.5, E15.5, and E13.5. Visualization of Purkinje cells by staining of sections for calbindin showed a severe mislocalization of Purkinje cells already at P4 and P0, indicating that Purkinje cells of the rostral cerebellar cortex had migrated abnormally (Fig. 2D to G). The first signs of abnormal Purkinje cell migration could be observed at E17.5. Purkinje cells of the developing anterior vermis migrated into the external granular layer (EGL) and formed nests of cells below the pia mater. A transmigration of Purkinje cells into the tectum was not observed (Fig. 2H to L). The overmigration of Purkinje cells into the EGL of the anterior vermis of the cerebellum was not accompanied by any obvious change in the EGL structure or by alterations in the architecture of the radial glia fibers (Fig. 2M to O).
FIG. 2.
Development of malformations of the cerebellar cortex of nestin-Cre; Gna12−/−; Gna13flox/flox mice. (A to C) Sagittal section through the rostral part of a mutant cerebellum at P21 stained with cresyl violet and an anticalbindin antibody. The high-power images (B and C; boxes in panel A) show that despite the severe disorganization of the rostral cerebellar cortex, the basal lamination into an internal granule cell layer, Purkinje cell layer, and molecular layer was largely intact. (D to G) Sagittal sections of wild-type (D and F) and nestin-Cre; Gna12−/−; Gna13flox/flox (E and G) mice at P4 (D and E) and at P0 (F and G) stained with anticalbindin antibody. Sections are shown with the rostral part of the cerebellum facing the left side. (H to L) Sagittal sections of a wild-type (WT) (H and I) and a nestin-Cre; Gna12−/−; Gna13flox/flox (KO) cerebellum (J to L) at E17.5 stained with cresyl violet and an anticalbindin antibody. While the granule cell layer and the Purkinje cell layer are clearly separated in the wild type, clusters of Purkinje cells invade the external granule cell layer in mutant cerebella (arrow) and nests of Purkinje cells can be seen in the EGL (arrowhead). The EGL is marked by dotted red lines. (M to O) Sagittal section of a nestin-Cre; Gna12−/−; Gna13flox/flox cerebellum at E18.5 stained with anti-calbindin (red) and anti-RC2 antibodies (radial glia; green). Arrows in panel M indicate overmigrating Purkinje cells entering the EGL. r, rostral; c, caudal. Scale bars are 500 μm (A), 31.25 μm (B and C), 250 μm (D, E, H, J, and M to O), 125 μm (F and G), and 25 μm (I, K, and L).
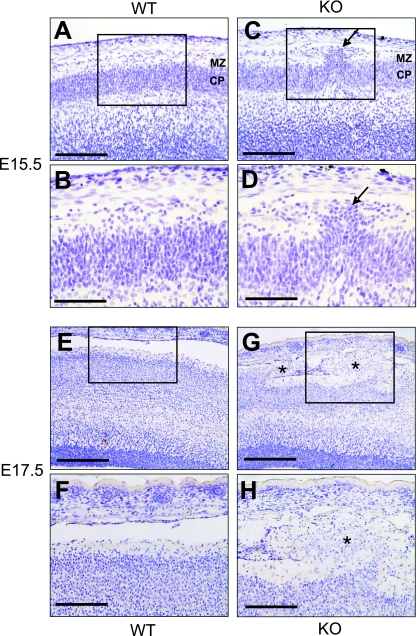
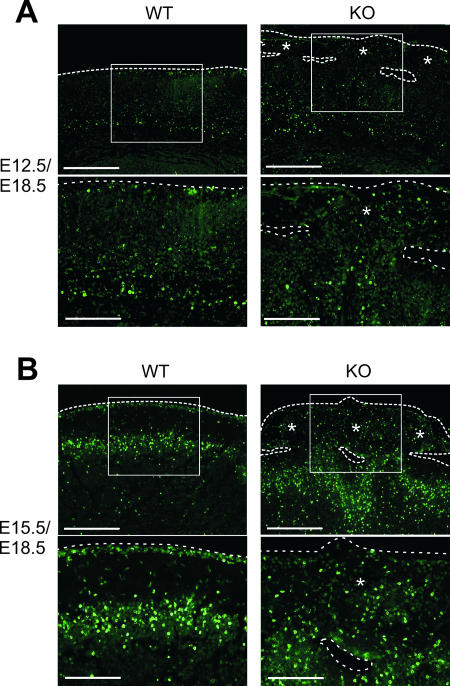
In order to determine the time point at which the first obvious defects in the development of the cerebral cortex occurred, nestin-Cre; Gna12−/−; Gna13flox/flox animals were analyzed at different stages before and after birth. The earliest time point at which careful analysis of the cerebral morphology of mutant animals consistently revealed abnormalities was E15.5. At this stage, cells migrated at some places into the marginal zone (Fig. 3A to D). Two days later, ectopic clusters of cells which had migrated through the marginal zone could be seen in the subarachnoidal space (Fig. 3E to H). Ectopic clusters were preferentially found in the frontal areas of the cortex. Neurons were the major constituent of the ectopia, as demonstrated by staining with anti-NeuN antibodies (data not shown).
FIG. 3.
Development of cortical ectopia in nestin-Cre; Gna12−/−; Gna13flox/flox embryos. Shown are coronal sections of wild-type (WT) (A, B, E, and F) and mutant (KO) (C, D, G, and H) cortices at E15.5 (A to D) and E17.5 (E to H). Overmigration of cortical plate neurons in mutant cortices was first seen at E15.5 (arrow in C and D). At E17.5, huge areas of ectopic neurons had formed which filled the whole molecular layer and reached into the subarachnoidal space (indicated by stars in G and H). MZ, marginal zone; CP, cortical plate. Scale bars are 125 μm (A, C, F, and H), 62.5 μm (B and D), and 250 μm (E and G).
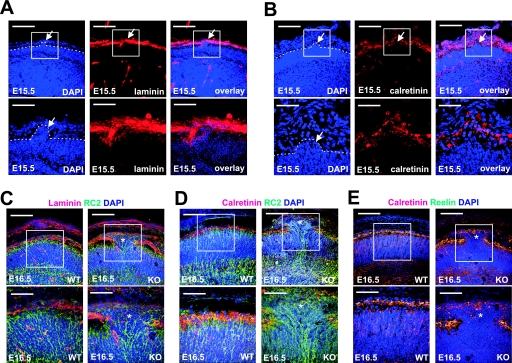
To further characterize the cortical defects in nestin-Cre; Gna12−/−; Gna13flox/flox animals, we analyzed various components of the cortex at different stages during development. To test whether defects in the basal lamina preceded the neuronal overmigration, we stained the developing cortex with anti-laminin antibodies at E14.5, a time point at which no ectopia could be observed in nestin-Cre; Gna12−/−; Gna13flox/flox animals, as well as at E15.5, the earliest time point when overmigration of cortical plate neurons could be seen. Despite an extensive analysis of the basal lamina morphology at E14.5, no defects were observed at this stage (data not shown). At E15.5, the basal lamina in regions of overmigrating neurons appeared also to be normal (Fig. 4A). At E16.5, when neuronal ectopia was more prominent, some fragmentation of the basal lamina could be observed (Fig. 4C). Scattered fragments of laminin could also be seen on the surface of the ectopia (Fig. 4C).
FIG. 4.
Immunohistochemical analysis of the cerebral ectopia in nestin-Cre; Gna12−/−; Gna13flox/flox embryos. (A and B) Cerebral cortices of E15.5 mice. nestin-Cre; Gna12−/−; Gna13flox/flox embryos were sectioned coronally and stained with antilaminin (A) or anticalretinin (B) antibodies. Sections were counterstained with DAPI. Shown are areas similar to those shown in Fig. 3C and D in which cortical plate neurons have invaded the molecular layer (arrows). Boxes indicate magnified areas, broken lines mark the outer border of the cortical plate. (C to E) Cerebral cortices of E16.5 wild-type (WT) and nestin-Cre; Gna12−/−; Gna13flox/flox (KO) embryos were sectioned coronally and stained with antilaminin (C, red), anti-RC2 (C and D, green), or anticalretinin (D and E, red) antibodies. Sections were counterstained with DAPI. Shown are representative areas of mutant cortices with ectopic neurons (marked by stars) and corresponding regions of cortices from wild-type embryos. Boxes indicate magnified areas. Bar lengths are 125 μm (upper panels in A to E), 41.5 μm (lower panels in A and B), and 62.5 μm (lower panels in C to E).
Cajal-Retzius cells which secrete reelin have been shown to play an important role in the regulation of neuronal migration in the developing cortex (34, 42, 47). Staining of Cajal-Retzius cells with an antibody directed against calretinin in areas in which cortical plate neurons started to invade the molecular layer at E15.5 showed that the number and localization of Cajal-Retzius cells were not altered (Fig. 4B). By using anti-reelin and anti-calbindin antibodies, we observed that Cajal-Retzius cells and reelin were still present on the surface of neuronal ectopia (Fig. 4D and E). These findings indicate that overmigrating neurons do not move through the Cajal-Retzius cell layer and suggest that a defect in the proper localization and function of Cajal-Retzius cells is unlikely to cause the overmigration of cortical neurons in nestin-Cre; Gna12−/−; Gna13flox/flox mice.
To analyze the morphology of radial glia, we immunostained embryonic cortical sections with an antibody directed against the radial glia marker RC2. Radial glia fibers showed a parallel organization both in wild-type as well as in mutant cortices (Fig. 4C and D). In regions with ectopia, radial glia fibers often reached into the ectopic clusters of neurons, suggesting that a detachment of radial glia fibers from the basal membrane is unlikely to have caused the formation of ectopia.
In order to determine the time point when ectopic neurons were born, we labeled proliferating cells with BrdU at E12.5 and E15.5 (Fig. 5). Areas of the cortex which did not show any obvious malformations were not significantly different from wild-type cortices with regard to the number or distribution of BrdU-positive cells at E18.5 (data not shown). This indicates that there were no general defects in neuronal proliferation or survival in nestin-Cre; Gna12−/−; Gna13flox/flox mice. When ectopic neurons were studied at E18.5, cells which were labeled at E12.5 as well as cells labeled at E15.5 were found in the ectopia (Fig. 5). Thus, in regions developing neuronal ectopia, neurons born at different stages of cortical development show alterations in migratory behavior, whereas no major layering defects could be observed in normal areas of the cortex.
FIG. 5.
Neuronal migration in the cerebral cortex of nestin-Cre; Gna12−/−; Gna13flox/flox embryos. Pregnant mice were injected at E12.5 (A) or E15.5 (B) with BrdU, and the distribution of BrdU-labeled neurons was determined in wild-type (WT) and nestin-Cre; Gna12−/−; Gna13flox/flox (KO) embryos at E18.5. Neurons born at both E12.5 and E15.5 took part in the formation of neuronal ectopia. The broken lines mark the surface of the developing brain. Areas containing ectopic neurons are marked by stars. Bar lengths are 125 μm (upper panels in A and B) and 62.5 μm (lower panels in A and B).
Since Gα12/Gα13 are ubiquitously expressed G protein α-subunits and since in nestin-Cre; Gna12−/−; Gna13flox/flox mice Gα12/Gα13 deficiency is induced both in neural and glial precursor cells, we cannot exclude that the lack of G12/G13-mediated signaling in radial glia cells rather than in migrating postmitotic neurons is responsible for the observed defects. To clarify this uncertainty, we used mice in which Cre expression is driven by the NEX promoter, which restricts recombination to principal neurons of the forebrain excluding glial cells and interneurons (10). Similar to nestin- Cre; Gna12−/−; Gna13flox/flox mice, NEX-Cre; Gna12−/−; Gna13flox/flox mice displayed multiple ectopia of the cerebral cortices (Fig. 6), thus strongly suggesting that lack of Gα12/Gα13 in cortical neurons is responsible for the observed neuronal overmigration.
FIG. 6.
Cortical ectopia in NEX-Cre; Gna12−/−; Gna13flox/flox animals. Shown are coronal sections of wild-type (A and B) and mutant (KO) (C and D) cortices at P21. Areas containing ectopic neurons are marked by stars. Scale bars are 250 μm (A and C) and 125 μm (B and D).
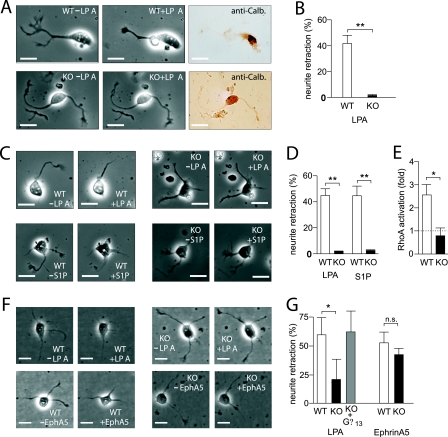
Based on the morphological analysis of the cerebral and cerebellar cortices, the overmigration of radially migrating neurons appears to be the earliest defect during the development of cortical structures in nestin-Cre; Gna12−/−; Gna13flox/flox embryos. We therefore hypothesize that Purkinje cells as well as neurons of the cerebral cortex which lack Gα12/Gα13 are unable to receive stop signals which under wild-type conditions ensure that neurons do not migrate into the EGL of the cerebellar cortex or into the marginal zone of the cerebral cortex. This hypothesis would be consistent with the cellular role of G12/G13, which have been shown to mediate receptor-dependent retraction of neurites and neurite-like structures (26, 29, 46), a process which would be expected to inhibit migratory activity. Lysophospholipids like LPA and S1P have been shown to induce neurite retraction by activation of G-protein-coupled receptors and RhoA (36) and have been demonstrated to regulate cortical development (23). To test whether cerebellar Purkinje cells and cortical neurons from nestin-Cre; Gna12−/−; Gna13flox/flox embryos were still able to respond with morphological changes to LPA and S1P, we isolated Purkinje cells and cortical neurons at E17.5. While both LPA and S1P induced neurite retraction in wild-type neurons (24, 40, 45, 49) (Fig. 7A to D), Purkinje cells and cortical neurons prepared from E17.5 nestin-Cre; Gna12−/−; Gna13flox/flox mice were completely unresponsive (Fig. 7A to D). The inability of Gα12/Gα13-deficient neurons to retract their neurites in response to LPA was accompanied by a lack of LPA-induced RhoA activation, while wild-type neurons showed robust activation of RhoA in response to LPA (Fig. 7E). The lack of LPA effects on cortical neurons was not due to a general defect in Gα12/Gα13-deficient neurons, since neurite retraction in response to ephrin-A5 was not affected by the lack of G12/G13-mediated signaling (Fig. 7F and G). The defect in LPA-induced neurite retraction in cortical neurons from NEX-Cre; Gna12−/−; Gna13flox/flox mice could be rescued by the transfection of cells with a eukaryotic expression plasmid encoding Gα13 (Fig. 7F and G). These data indicate that the retraction of neuronal processes induced by mediators acting through G-protein-coupled receptors is mediated by the G12/G13-Rho pathway and that defects in G-protein-coupled receptor-dependent retraction of cellular processes may underlie the observed phenotype of mice lacking Gα12/Gα13 in the nervous system.
FIG. 7.
Effect of LPA and S1P on neurite morphology of wild-type and nestin-Cre; Gna12−/−; Gna13flox/flox neurons. (A and B) Wild-type (WT) and nestin-Cre; Gna12−/−; Gna13flox/flox (KO) neurons from E17.5 cerebellar cortices were studied by live-cell imaging before (−LPA) and after (+LPA) addition of LPA. Thereafter, cells were stained for calbindin (anti-Calb.) to identify embryonic cerebellar Purkinje cells. Shown are representative images of embryonic Purkinje cells (A) as well as a statistical evaluation of neurite retraction in response to LPA (B). (C to G) Cortical plate neurons were isolated from wild-type (WT), nestin-Cre; Gna12−/−; Gna13flox/flox (KO; C and D), or NEX-Cre; Gna12−/−; Gna13flox/flox (KO; E to G) E16.5 embryonic brains. Isolated cells were then monitored by live-cell imaging before (−LPA/−S1P) or after addition of LPA (+LPA), S1P (+S1P), or ephrin-A5 (EphA5) as indicated. The gray bar in panel G (KO + Gα13) indicates LPA-induced neurite retraction in cortical neurons transfected with a plasmid encoding Gα13. Shown are representative images (C and F) as well as a statistical evaluation of the effects of 10 μM LPA, 1 μM S1P, and 2 μg/ml ephrin-A5 on neurite retraction in wild-type and mutant cortical plate neurons (D and G). Fifteen to forty-five cells per embryo were analyzed, and the total number of embryos per experiment was three to six. (E) Effect of 10 μM LPA on RhoA activity in cortical neurons from wild-type (WT) or NEX-Cre; Gna12−/−; Gna13flox/flox (KO) E16.5 embryonic brains. Shown is the RhoA activation relative to the basal activity (=1). All values are means ± standard deviations; *, P < 0.05; **, P < 0.01; n.s., not significant.
DISCUSSION
In this study we analyzed the consequences of Gα12/Gα13 double deficiency for the development of the mammalian brain. We report that lack of Gα12/Gα13 in cortical neurons results in the overmigration of cerebellar Purkinje cells and of postmitotic neurons of the cerebrum, resulting in the formation of ectopia and severe malformations of cortical structures. This indicates that G-protein-coupled receptors acting via G12/G13 are involved in the proper positioning of radially migrating cortical neurons.
Under normal conditions, radially migrating cortical neurons stop at defined sites. The signals which induce a stop of migration are not well defined. One of the best characterized factors regulating neuronal migration in the cortex is the extracellular protein reelin, which is produced by Cajal-Retzius cells (42, 50). Mislocalization of Cajal-Retzius cells and reelin expression have been described in several mutants with cortical ectopia (12, 16, 37). In contrast to these mutants, we found that both Cajal-Retzius cells and reelin showed normal localization at early stages of neuronal overmigration and were still covering the surface of ectopic clusters of neurons, suggesting that a defect in reelin function is unlikely to have caused the cortical defects in nestin-Cre; Gna12−/−; Gna13flox/flox embryos.
The radial glia fibers along which cortical neurons migrate outward are attached at the basal lamina, which is produced by meningeal fibroblasts. Defects in several proteins which are required for the proper assembly of the basal lamina or for the anchoring of the radial glia end feet have been shown to lead to defects in neuronal migration, resulting in ectopia (2, 8, 12, 15, 16, 21, 37). These migratory effects have been suggested to represent secondary effects due to the misplacement of Cajal-Retzius cells (4, 16, 17, 37, 53). In the nestin-Cre; Gna12−/−; Gna13flox/flox embryos, the basic architecture of radial glia fibers appeared to be undisturbed. In addition, the basal lamina appeared to be intact in areas where overmigration of neurons occurred at later stages of development. Once cells had overmigrated and had formed ectopic cell clusters, the basal lamina was rarefied or fragmented, which most likely resulted from the expansion of the cortical surface in ectopic areas. This strongly indicates that defects in the structure of the basal lamina or the radial glia are not primary causes of the defects in cortical development of nestin-Cre; Gna12−/−; Gna13flox/flox mice.
Based on the fact that G12/G13 couple receptors to the activation of RhoA and the stimulation of actomyosin-based contractility (5, 44), it is likely that the loss of this regulatory pathway interferes with the normal regulation of cell migration and may cause the observed overmigration phenotype. Studies of migratory processes in neurons and other cells have demonstrated that movement occurs by extension of a leading process, formation of new adhesion junctions, cell body contraction, and detachment of adhesions at the cell rear (7, 20, 31). These processes are regulated by various signaling pathways which involve Rho family GTPases (41, 43). While Rac and Cdc42 are responsible for the formation of the leading process, RhoA is required for the rear end contraction. In neurons, neurite-like protrusions which resemble the leading process of migrating neurons are retracted when RhoA is activated (6, 11, 14, 25, 32), and RhoA activation in leading processes of migrating neurons would be expected to be incompatible with proper migration and to act as a stop signal.
Several mediators such as the lysophospholipids S1P and LPA have been shown to induce neurite retraction via the activation of G-protein-coupled receptors and the activation of RhoA (23, 24, 29, 40). Consistent with this, we found that both LPA and S1P induced neurite retraction in embryonic cortical neurons and cerebellar Purkinje cells, whereas these effects were abrogated in neurons lacking Gα12/Gα13. The fact that mice lacking one or two of the various LPA and S1P receptors do not show overmigration of cortical neurons (23, 27) indicates that the lysophospholipids LPA or S1P alone are obviously not critically involved in the regulation of neuronal migration in the cortex. Nevertheless, our data suggest that mediators acting through G12/G13-coupled receptors act as stop signals for migrating postmitotic cortical neurons and thereby contribute to the establishment of properly laminated cerebellar and cerebral cortices.
Acknowledgments
We thank Melanie Bernhard for technical assistance, Andreas Faissner and Alexander von Holst for kindly providing the anti-RC2 antiserum, Elisabeth Pollerberg for help with the transfection of neurons, and Ulrike Engel and Christian Ackermann from the Nikon Imaging Center at the University of Heidelberg for expert technical support.
This study was supported by the Collaborative Research Center 488 (SFB 488) of the German Research Foundation.
Footnotes
Published ahead of print on 17 December 2007.
REFERENCES
- 1.Banker, G., and K. Goslin. 1988. Developments in neuronal cell culture. Nature 336185-186. [DOI] [PubMed] [Google Scholar]
- 2.Beggs, H. E., D. Schahin-Reed, K. Zang, S. Goebbels, K. A. Nave, J. Gorski, K. R. Jones, D. Sretavan, and L. F. Reichardt. 2003. FAK deficiency in cells contributing to the basal lamina results in cortical abnormalities resembling congenital muscular dystrophies. Neuron 40501-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bielas, S., H. Higginbotham, H. Koizumi, T. Tanaka, and J. G. Gleeson. 2004. Cortical neuronal migration mutants suggest separate but intersecting pathways. Annu. Rev. Cell Dev. Biol. 20593-618. [DOI] [PubMed] [Google Scholar]
- 4.Borrell, V., and O. Marin. 2006. Meninges control tangential migration of hem-derived Cajal-Retzius cells via CXCL12/CXCR4 signaling. Nat. Neurosci. 91284-1293. [DOI] [PubMed] [Google Scholar]
- 5.Buhl, A. M., N. L. Johnson, N. Dhanasekaran, and G. L. Johnson. 1995. G alpha 12 and G alpha 13 stimulate Rho-dependent stress fiber formation and focal adhesion assembly. J. Biol. Chem. 27024631-24634. [DOI] [PubMed] [Google Scholar]
- 6.Dickson, B. J. 2001. Rho GTPases in growth cone guidance. Curr. Opin. Neurobiol. 11103-110. [DOI] [PubMed] [Google Scholar]
- 7.Edmondson, J. C., and M. E. Hatten. 1987. Glial-guided granule neuron migration in vitro: a high-resolution time-lapse video microscopic study. J. Neurosci. 71928-1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Georges-Labouesse, E., M. Mark, N. Messaddeq, and A. Gansmuller. 1998. Essential role of alpha 6 integrins in cortical and retinal lamination. Curr. Biol. 8983-986. [DOI] [PubMed] [Google Scholar]
- 9.Gleeson, J. G., and C. A. Walsh. 2000. Neuronal migration disorders: from genetic diseases to developmental mechanisms. Trends Neurosci. 23352-359. [DOI] [PubMed] [Google Scholar]
- 10.Goebbels, S., I. Bormuth, U. Bode, O. Hermanson, M. H. Schwab, and K. A. Nave. 2006. Genetic targeting of principal neurons in neocortex and hippocampus of NEX-Cre mice. Genesis 44611-621. [DOI] [PubMed] [Google Scholar]
- 11.Govek, E. E., S. E. Newey, and L. Van Aelst. 2005. The role of the Rho GTPases in neuronal development. Genes Dev. 191-49. [DOI] [PubMed] [Google Scholar]
- 12.Graus-Porta, D., S. Blaess, M. Senften, A. Littlewood-Evans, C. Damsky, Z. Huang, P. Orban, R. Klein, J. C. Schittny, and U. Muller. 2001. Beta1-class integrins regulate the development of laminae and folia in the cerebral and cerebellar cortex. Neuron 31367-379. [DOI] [PubMed] [Google Scholar]
- 13.Gu, J. L., S. Muller, V. Mancino, S. Offermanns, and M. I. Simon. 2002. Interaction of G alpha(12) with G alpha(13) and G alpha(q) signaling pathways. Proc. Natl. Acad. Sci. USA 999352-9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guan, K. L., and Y. Rao. 2003. Signalling mechanisms mediating neuronal responses to guidance cues. Nat. Rev. Neurosci. 4941-956. [DOI] [PubMed] [Google Scholar]
- 15.Guenette, S., Y. Chang, T. Hiesberger, J. A. Richardson, C. B. Eckman, E. A. Eckman, R. E. Hammer, and J. Herz. 2006. Essential roles for the FE65 amyloid precursor protein-interacting proteins in brain development. EMBO J. 25420-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halfter, W., S. Dong, Y. P. Yip, M. Willem, and U. Mayer. 2002. A critical function of the pial basement membrane in cortical histogenesis. J. Neurosci. 226029-6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartmann, D., B. De Strooper, and P. Saftig. 1999. Presenilin-1 deficiency leads to loss of Cajal-Retzius neurons and cortical dysplasia similar to human type 2 lissencephaly. Curr. Biol. 9719-727. [DOI] [PubMed] [Google Scholar]
- 18.Hatten, M. E. 1999. Central nervous system neuronal migration. Annu. Rev. Neurosci. 22511-539. [DOI] [PubMed] [Google Scholar]
- 19.Hatten, M. E. 2002. New directions in neuronal migration. Science 2971660-1663. [DOI] [PubMed] [Google Scholar]
- 20.Hatten, M. E., and N. Heintz. 1995. Mechanisms of neural patterning and specification in the developing cerebellum. Annu. Rev. Neurosci. 18385-408. [DOI] [PubMed] [Google Scholar]
- 21.Herms, J., B. Anliker, S. Heber, S. Ring, M. Fuhrmann, H. Kretzschmar, S. Sisodia, and U. Muller. 2004. Cortical dysplasia resembling human type 2 lissencephaly in mice lacking all three APP family members. EMBO J. 234106-4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herz, J., and H. H. Bock. 2002. Lipoprotein receptors in the nervous system. Annu. Rev. Biochem. 71405-434. [DOI] [PubMed] [Google Scholar]
- 23.Ishii, I., N. Fukushima, X. Ye, and J. Chun. 2004. Lysophospholipid receptors: signaling and biology. Annu. Rev. Biochem. 73321-354. [DOI] [PubMed] [Google Scholar]
- 24.Jalink, K., E. J. van Corven, T. Hengeveld, N. Morii, S. Narumiya, and W. H. Moolenaar. 1994. Inhibition of lysophosphatidate- and thrombin-induced neurite retraction and neuronal cell rounding by ADP ribosylation of the small GTP-binding protein Rho. J. Cell Biol. 126801-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaibuchi, K., S. Kuroda, and M. Amano. 1999. Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu. Rev. Biochem. 68459-486. [DOI] [PubMed] [Google Scholar]
- 26.Katoh, H., J. Aoki, Y. Yamaguchi, Y. Kitano, A. Ichikawa, and M. Negishi. 1998. Constitutively active Galpha12, Galpha13, and Galphaq induce Rho-dependent neurite retraction through different signaling pathways. J. Biol. Chem. 27328700-28707. [DOI] [PubMed] [Google Scholar]
- 27.Kingsbury, M. A., S. K. Rehen, X. Ye, and J. Chun. 2004. Genetics and cell biology of lysophosphatidic acid receptor-mediated signaling during cortical neurogenesis. J. Cell. Biochem. 921004-1012. [DOI] [PubMed] [Google Scholar]
- 28.Knöll, B., O. Kretz, C. Fiedler, S. Alberti, G. Schutz, M. Frotscher, and A. Nordheim. 2006. Serum response factor controls neuronal circuit assembly in the hippocampus. Nat. Neurosci. 9195-204. [DOI] [PubMed] [Google Scholar]
- 29.Kranenburg, O., M. Poland, F. P. van Horck, D. Drechsel, A. Hall, and W. H. Moolenaar. 1999. Activation of RhoA by lysophosphatidic acid and Galpha12/13 subunits in neuronal cells: induction of neurite retraction. Mol. Biol Cell 101851-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuner, R., J. M. Swiercz, A. Zywietz, A. Tappe, and S. Offermanns. 2002. Characterization of the expression of PDZ-RhoGEF, LARG and G(alpha)12/G(alpha)13 proteins in the murine nervous system. Eur. J. Neurosci. 162333-2341. [DOI] [PubMed] [Google Scholar]
- 31.Lambert de Rouvroit, C., and A. M. Goffinet. 2001. Neuronal migration. Mech. Dev. 10547-56. [DOI] [PubMed] [Google Scholar]
- 32.Luo, L. 2000. Rho GTPases in neuronal morphogenesis. Nat. Rev. Neurosci. 1173-180. [DOI] [PubMed] [Google Scholar]
- 33.Marin, O., and J. L. Rubenstein. 2003. Cell migration in the forebrain. Annu. Rev. Neurosci. 26441-483. [DOI] [PubMed] [Google Scholar]
- 34.Marin-Padilla, M. 1998. Cajal-Retzius cells and the development of the neocortex. Trends Neurosci. 2164-71. [DOI] [PubMed] [Google Scholar]
- 35.Moers, A., B. Nieswandt, S. Massberg, N. Wettschureck, S. Gruner, I. Konrad, V. Schulte, B. Aktas, M. P. Gratacap, M. I. Simon, M. Gawaz, and S. Offermanns. 2003. G13 is an essential mediator of platelet activation in hemostasis and thrombosis. Nat. Med. 91418-1422. [DOI] [PubMed] [Google Scholar]
- 36.Moolenaar, W. H. 2000. Development of our current understanding of bioactive lysophospholipids. Ann. N. Y. Acad. Sci. 9051-10. [DOI] [PubMed] [Google Scholar]
- 37.Niewmierzycka, A., J. Mills, R. St-Arnaud, S. Dedhar, and L. F. Reichardt. 2005. Integrin-linked kinase deletion from mouse cortex results in cortical lamination defects resembling cobblestone lissencephaly. J. Neurosci. 257022-7031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Offermanns, S., V. Mancino, J. P. Revel, and M. I. Simon. 1997. Vascular system defects and impaired cell chemokinesis as a result of Galpha13 deficiency. Science 275533-536. [DOI] [PubMed] [Google Scholar]
- 39.Park, H. T., J. Wu, and Y. Rao. 2002. Molecular control of neuronal migration. Bioessays 24821-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Postma, F. R., K. Jalink, T. Hengeveld, and W. H. Moolenaar. 1996. Sphingosine-1-phosphate rapidly induces Rho-dependent neurite retraction: action through a specific cell surface receptor. EMBO J. 152388-2392. [PMC free article] [PubMed] [Google Scholar]
- 41.Raftopoulou, M., and A. Hall. 2004. Cell migration: Rho GTPases lead the way. Dev. Biol. 26523-32. [DOI] [PubMed] [Google Scholar]
- 42.Rice, D. S., and T. Curran. 2001. Role of the reelin signaling pathway in central nervous system development. Annu. Rev. Neurosci. 241005-1039. [DOI] [PubMed] [Google Scholar]
- 43.Ridley, A. J., M. A. Schwartz, K. Burridge, R. A. Firtel, M. H. Ginsberg, G. Borisy, J. T. Parsons, and A. R. Horwitz. 2003. Cell migration: integrating signals from front to back. Science 3021704-1709. [DOI] [PubMed] [Google Scholar]
- 44.Sah, V. P., T. M. Seasholtz, S. A. Sagi, and J. H. Brown. 2000. The role of Rho in G protein-coupled receptor signal transduction. Annu. Rev. Pharmacol. Toxicol. 40459-489. [DOI] [PubMed] [Google Scholar]
- 45.Sato, K., H. Tomura, Y. Igarashi, M. Ui, and F. Okajima. 1997. Exogenous sphingosine 1-phosphate induces neurite retraction possibly through a cell surface receptor in PC12 cells. Biochem. Biophys. Res. Commun. 240329-334. [DOI] [PubMed] [Google Scholar]
- 46.Sayas, C. L., J. Avila, and F. Wandosell. 2002. Glycogen synthase kinase-3 is activated in neuronal cells by Galpha12 and Galpha13 by Rho-independent and Rho-dependent mechanisms. J. Neurosci. 226863-6875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soriano, E., and J. A. Del Rio. 2005. The cells of cajal-retzius: still a mystery one century after. Neuron 46389-394. [DOI] [PubMed] [Google Scholar]
- 48.Sotelo, C. 2004. Cellular and genetic regulation of the development of the cerebellar system. Prog. Neurobiol. 72295-339. [DOI] [PubMed] [Google Scholar]
- 49.Tigyi, G., D. J. Fischer, A. Sebok, C. Yang, D. L. Dyer, and R. Miledi. 1996. Lysophosphatidic acid-induced neurite retraction in PC12 cells: control by phosphoinositide-Ca2+ signaling and Rho. J. Neurochem. 66537-548. [DOI] [PubMed] [Google Scholar]
- 50.Tissir, F., and A. M. Goffinet. 2003. Reelin and brain development. Nat. Rev. Neurosci. 4496-505. [DOI] [PubMed] [Google Scholar]
- 51.Tronche, F., C. Kellendonk, O. Kretz, P. Gass, K. Anlag, P. C. Orban, R. Bock, R. Klein, and G. Schutz. 1999. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat. Genet. 2399-103. [DOI] [PubMed] [Google Scholar]
- 52.Wang, V. Y., and H. Y. Zoghbi. 2001. Genetic regulation of cerebellar development. Nat. Rev. Neurosci 2484-491. [DOI] [PubMed] [Google Scholar]
- 53.Wines-Samuelson, M., M. Handler, and J. Shen. 2005. Role of presenilin-1 in cortical lamination and survival of Cajal-Retzius neurons. Dev. Biol. 277332-346. [DOI] [PubMed] [Google Scholar]
- 54.Xu, J., F. Wang, A. Van Keymeulen, P. Herzmark, A. Straight, K. Kelly, Y. Takuwa, N. Sugimoto, T. Mitchison, and H. R. Bourne. 2003. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell 114201-214. [DOI] [PubMed] [Google Scholar]
- 55.Zimmerman, L., B. Parr, U. Lendahl, M. Cunningham, R. McKay, B. Gavin, J. Mann, G. Vassileva, and A. McMahon. 1994. Independent regulatory elements in the nestin gene direct transgene expression to neural stem cells or muscle precursors. Neuron 1211-24. [DOI] [PubMed] [Google Scholar]