Abstract
The innate immune response in human tuberculosis is not completely understood. To improve our knowledge regarding the role of cathelicidin hCAP-18/LL37 in the innate immune response to tuberculosis infection, we used immunohistochemistry, immunoelectron microscopy, and gene expression to study the induction and production of the antimicrobial peptide in A549 epithelial cells, alveolar macrophages (AM), neutrophils, and monocyte-derived macrophages (MDM) after infection with Mycobacterium tuberculosis. We demonstrated that mycobacterial infection induced the expression and production of LL-37 in all cells studied, with AM being the most efficient. We did not detect peptide expression in tuberculous granulomas, suggesting that LL-37 participates only during early infection. Through the study of Toll-like receptors (TLR) in MDM, we showed that LL-37 can be induced by stimulation through TLR-2, TLR-4, and TLR-9. This last TLR was strongly stimulated by M. tuberculosis DNA. We concluded that LL-37 may have an important role in the innate immune response against M. tuberculosis.
Tuberculosis (TB) represents a global public health problem, with 3 million deaths per year worldwide. Our knowledge of protective immunity in human TB is still fragmentary. A better understanding of the protective human immune response against Mycobacterium tuberculosis is necessary for the development of new antituberculosis treatments and tuberculosis vaccinations (11, 12, 38). In particular, the role of the innate immune response against M. tuberculosis is poorly understood (17). While systemic immunity plays a dominant role in preventing mycobacterial dissemination, the pulmonary innate immune response determines the outcome of primary infection (29).
When bacilli enter the airways, the first cells that encounter the bacteria are epithelial cells (ECs) and alveolar macrophages (AMs) (1). After infection, ECs produce human β-defensin-2 (HBD-2) and several different interleukins and chemokines (5, 9, 23, 30, 39). At the same time, the bacilli encounter AMs, which phagocytose and eliminate bacilli (30). There is information about the mechanisms by which AMs destroy M. tuberculosis, but other immune mechanisms in this process participate, and it is important to characterize, in particular, the contribution of antimicrobial peptides (AMPs). Most multicellular organisms produce AMPs, which can kill viruses, fungi, and bacteria, including M. tuberculosis (35). These AMPs are produced by ECs, blood cells, and macrophages (28, 42). Some AMPs are constitutively expressed, while others are expressed only in response to stimuli, such as cytokines and microbial components. Among the better-studied inducible AMPs are β-defensin and LL-37, which is a member of the cathelicidins (1). We recently showed that lung ECs express HBD-2 after M. tuberculosis infection, which is associated with the mycobacterial cell wall in intracellular bacteria (30). Moreover, in a mouse model of progressive pulmonary tuberculosis, there was an initial rapid expression of β-defensins by respiratory ECs correlating with control of bacilli proliferation, followed by a profound decrease during the later progressive phase of the disease. In a latent-infection model, β-defensins were continuously expressed, but they were suppressed after reactivation of the disease. Thus, it seems that mycobacterial infection can efficiently induce the expression of β-defensins, which may participate in the control of mycobacterial growth (29).
LL-37, also known as human cationic antimicrobial protein (hCAP18), is the only member of the cathelicidin family identified in humans so far and is expressed in ECs and macrophages. Besides their direct antimicrobial function, cathelicidins have multiple roles as mediators of inflammation (10). These AMPs have been implicated in the immunopathogenesis of several diseases (16, 21). In the case of tuberculosis, there are no reports about the role of LL-37 during primary infection and the cell types that are responsible for its production. The aim of this study was to determine LL-37 gene expression and protein production in several human cell types after mycobacterial infection in vitro, as well as to carry out in situ detection by immunohistochemistry in lung tissue from patients with TB.
MATERIALS AND METHODS
Donors.
Healthy subjects were recruited at the Instituto Nacional de Enfermedades Respiratorias in Mexico City. All had a normal chest X-ray and negative HIV serology. All individuals were positive by the tuberculin skin test (≥10 mm), none of them had a history of prior exposure to TB patients, and all were BCG positive. After written informed consent was obtained, subjects underwent venipuncture and fiber optic bronchoscopy with bronchoalveolar lavage (BAL). Approval to perform BALs and venipunctures was given by the Institutional Review Boards at the Instituto Nacional de Enfermedades Respiratorias.
Bacteria.
M. tuberculosis strain H37Rv (ATCC 25618) was grown for 28 days in Middlebrook 7H9 broth, counted, and stored at −70°C. Salmonella enterica serovar Typhi, a clinical isolate from a patient with typhoid fever, was grown in BHI broth, counted, and stored at −70°C until use.
M. tuberculosis DNA preparation.
M. tuberculosis H37Rv bacteria (1 × 109) were digested in lysis buffer (50 mM Tris, 1 mM EDTA, 0.5% Tween 20) containing 2 mg/ml proteinase K. Total DNA was extracted using a chloroform-isoamyl alcohol mixture (49:1), precipitated with a sodium acetate-ethanol mixture (1:30), dissolved in pyrogen-free sterile water, aliquoted, and stored at −20°C. Human DNA was prepared by the same protocol using peripheral blood mononuclear cells (PBMCs) and was used as a negative stimulation control. Less than 12.6 pg/ml of lipopolysaccharide (LPS) was found in both DNA preparations as determined with a Limulus assay (PyrogentPlus; Cambrex, Walkersville, MD).
Human cell preparation and infection.
The human lung epithelial cell line A549 (ATCC CCL185, referred to here as ECs), was cultured in 75-cm2 culture flasks (Costar, Ontario, Canada) to semiconfluence. A549 cells were seeded in a 24-well plate and maintained for 24 h until infection with M. tuberculosis or S. enterica serovar Typhi.
To isolate monocytes, heparinized blood was obtained from 10 donors. PBMCs were isolated by Ficoll-Hypaque (Nycomed Pharma AS, Oslo, Norway). PBMCs were cultured in 962-mm2 polystyrene dishes (Costar, Ontario, Canada). After 2 h, nonadherent cells were removed. The remaining adherent cells were incubated for 1 h in Hanks’ solution (BioWhittaker, Walkersville, MD), removed with a scraper, counted, and allowed to readhere in a 24-well plate or in chamber slides (Costar, Corning, NY). Cells were incubated for 7 days, and MDMs were used for infection or stimulation.
Neutrophils were isolated from whole blood by using Polymorphprep (Axis-Shield, Oslo, Norway). After purification, neutrophils were plated for 1 h in chamber slides and infected with M. tuberculosis or S. enterica serovar Typhi.
To obtain bronchoalveolar cells (BACs), BAL was performed on four healthy donors as previously described (6). BACs were obtained by BAL fluid centrifugation. AMs were then enriched from the BACs by removal of alveolar lymphocytes with sheep red blood cell rosetting. Ninety-five percent of the cells had characteristics of AMs by microscopic observation using Cytospin preparations.
The in vitro infection was done as previously reported (30). Briefly, A549 cells, AMs, MDMs, and neutrophils were plated separately in 24-well dishes in addition to three-well chamber slides at a concentration of 106 cells per well and were allowed to adhere for 2 h (AMs, MDMs, and neutrophils). The cells were infected with M. tuberculosis or S. enterica serovar Typhi in RPMI with 10% non-heat-inactivated pooled human AB serum. All cells were infected at multiplicities of infection (MOIs) of 1:1, 5:1, and 10:1. Cells were then incubated for 1 and 18 h.
LL-37 production after cell stimulation with mycobacterial DNA, LAM, and LPSs.
In order to investigate whether some specific mycobacterial moieties can induce LL-37, MDMs and BACs were stimulated with various groups of stimuli, including 5 μg/ml of mycobacterial DNA or human DNA (control DNA), 3 μg/ml of type C CpG oligodeoxynucleotides (ODN) or type C non-CpG ODN as a control (Coley, Wellesley, MA), 2 μg/ml of M. tuberculosis H37Rv lipoarabinomannan (LAM) or 1 ng/ml of the synthetic lipoprotein palmitoylated N-acyl-S-diacylglyceryl cysteine (Pam3Cys; EMC Microcollections, Tuebingen, Germany), and 100 ng/ml of LPS (Sigma, St. Louis, MO) for 1 or 18 h, and compared to nonstimulated cells. Supernatants were discarded, and cells were prepared for immunocytochemical analysis.
LL-37 gene expression in infected cells was determined by real-time PCR.
Cells were resuspended in Trizol reagent and stored at −20°C until use. RNA was extracted as previously described (29). LL-37 reverse mRNA transcription was performed using 5 μg RNA, 2 μM oligo(dT), 15 μM primer (Promega), 10 units RNase inhibitor (10 units/μl) (Invitrogen), 1× reverse transcription buffer, 0.5 mM of each deoxynucleoside triphosphate, and 4 units Omniscript reverse transcriptase (Qiagen Inc.). Real-time PCR was performed with a 7500 real-time PCR system (Applied Biosystems). For 1 μl of cDNA, we added 12.5 μl of Quantitect SYBR green, PCR master mix {Quantitect SYBR green PCR buffer [Tris-Cl, KCl, (NH4)2SO4, 5 mM MgCl2 (pH 8.7)], deoxynucleoside triphosphate mix [dATP, dCTP, dGTP, dTTP/dUTP], SYBR green I, and ROX}, 0.5 μl forward primer (10 pmol/μl), 0.5 μl reverse primer (10 pmol/μl), and 10.5 μl nuclease-free solution. Standard curves of quantified and diluted PCR products, as well as negative controls, were included in each PCR run. All primers were designed with Primer Express 2.0 software from Applied Biosystems for the targets LL-37 (or hCAP-18) (5′-GAA GAC CCA AAG GAA TGG CC-3′ and 5′CAG AGC CCA GAA GCC TGA GC-3) and glyceraldehyde 3-phosphate dehydrogenase (constitutive gene expression control) as previously described (29).
LL-37 protein detection in cell suspensions and lung tissue.
Lung ECs (A549), neutrophils, monocytes, MDMs, and AMs were infected with different MOIs of S. enterica serovar Typhi or M. tuberculosis for 1 h or 18 h. LL-37 immunoreactivity was detected and expressed as a percentage of the value for positive cells. Cells were plated on four-well chamber slides (Costar, Ontario, Canada) at 1 × 106 cells/well. Following M. tuberculosis infection, cells were fixed with 4% paraformaldehyde for 1 h and stored at 4°C in phosphate-buffered saline.
Lung tissue samples from eight necropsies from patients who died of tuberculosis or died for other reasons but also had clinical tuberculosis were used to study LL-37 expression as determined by immunohistochemistry. Samples from three cases of gram-negative acute pneumonia (Pseudomonas aeruginosa) were used as positive controls. Five patients exhibited pulmonary tuberculosis (positive M. tuberculosis culture, histopathological analysis, and in situ PCR). Another three lung samples that did not have any histological abnormality were used as negative controls.
Immunocytochemistry for LL-37 and H37Rv was performed as previously described (29). Necropsy samples were fixed by immersion with 10% formaldehyde and embedded in paraffin (14). Lung sections were incubated for 18 h with anti-human LL-37 polyclonal antibody, which recognizes mature LL-37 (catalog no. Sc-21578; Santa Cruz Biotechnology, Santa Cruz, CA). The slides were incubated for 2 h with a goat anti-mouse immunoglobulin G (IgG) biotin-labeled antibody. Bound antibodies were detected with avidin-biotin peroxidase (Vector, Burlingame, CA) and counterstained with hematoxylin. A similar procedure was also used to stain uninfected and infected cells. For quantification purposes, samples were prepared in duplicate. Five random microscopic fields were selected. At a ×100 magnification, at least 400 negative or positive cells per field were identified and counted using an image analyzer, and the percentage of positive cells at each time point was determined. In order to identify any cross-reactivity between the anti-human LL-37 polyclonal antibody and M. tuberculosis, immunocytochemistry experiments with M. tuberculosis in the absence of human cells were performed. We did not observe any specific binding of this antibody to M. tuberculosis.
Subcellular detection of LL-37 by immunoelectron microscopy.
AMs were infected with M. tuberculosis as described above. After 18 h, infected and uninfected (control) AMs were treated as described previously (30), and the grids were incubated overnight at 4°C with polyclonal goat anti-LL-37 antibody (Santa Cruz Biotechnology) diluted 1:200 in phosphate-buffered saline. After rinsing, the grids were incubated for 1 h with rabbit anti-goat IgG (Sigma Co, St. Louis, MO) conjugated to 10-nm gold particles (Sigma).
To detect the subcellular colocalization of LL-37 with mycobacterial moieties, we performed double immunolabeling using a second incubation with specific polyclonal rabbit antibodies against LAM and a secondary anti-rabbit antibody conjugated with 20-nm gold particles. The grids were stained with uranium salts (Electron Microscopy Sciences, Fort Washington, PA) and examined with an M-10 Zeiss electron microscope (Carl Zeiss, Jena, Germany). As a negative control, the primary LL-37 antibody was replaced with normal rabbit serum. Also, experiments using M. tuberculosis alone incubated with LL-37 antibody were done to exclude cross-reactivity.
Statistical analysis.
To identify significant differences in LL-37 expression between S. enterica serovar Typhi and M. tuberculosis infection and stimuli, a two-tailed Wilcoxon signed rank test was used. Means and standard deviations (SDs) are presented. A statistically significant difference was defined as a P value of <0.05. Analysis was performed using the software SPSS 13.0 for Windows (SPSS, Chicago, IL).
RESULTS
Detection of LL-37 in infected cells by immunocytochemistry.
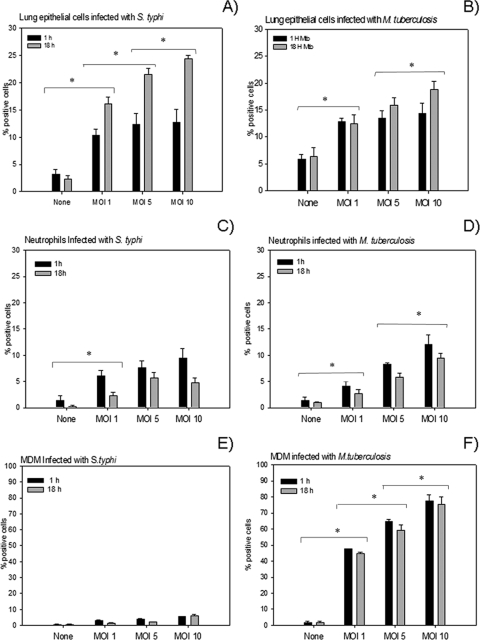
Lung ECs infected with S. enterica serovar Typhi or with M. tuberculosis showed positive immunostaining in a dose-dependent manner, which was higher after 18 h with an MOI of 10 (means ± SDs, 24.32% ± 0.62% and 18.82% ± 1.53%, respectively) (Fig. 1A and B). There was not a statistically significant difference between S. enterica serovar Typhi and M. tuberculosis infection (P < 0.05).
FIG. 1.
Production of LL-37 during M. tuberculosis and S. enterica serovar Typhi infection was determined by immunocytochemistry in different cell types at 1 h and 18 h postinfection. While cells infected with S. enterica serovar Typhi showed a low percentage of positive cells (A, C, and E), those infected with M. tuberculosis showed a high percentage of positive cells (B, D, and F). LL-37 production in both S. enterica serovar Typhi- and M. tuberculosis-infected cells was dose dependent. Data are means ± SDs from 10 independent experiments. *, P < 0.05.
Neutrophils showed strong immunostaining, but the percentage of positive cells were less than that of ECs, and the difference between S. enterica serovar Typhi and M. tuberculosis infections (9.46% ± 1.97% and 12.04% ± 1.81%, respectively) was not statistically significant. The percentage of immunostained cells showed a dose-dependent relation, with an MOI of 10 yielding the highest value after 1 h of infection (Fig. 1C and D).
MDMs infected with M. tuberculosis showed a very high percentage of cells positive for LL-37 (77.29% ± 3.94%) in comparison with those infected with S. enterica serovar Typhi (5.62% ± 0.14%) (Fig. 1E and F). The highest percentage of positive cells was seen after 1 h of infection in both cases. In contrast, monocytes showed low LL-37 immunoreactivity when infected with either S. enterica serovar Typhi or M. tuberculosis (1.32% ± 0.16%) (data not shown).
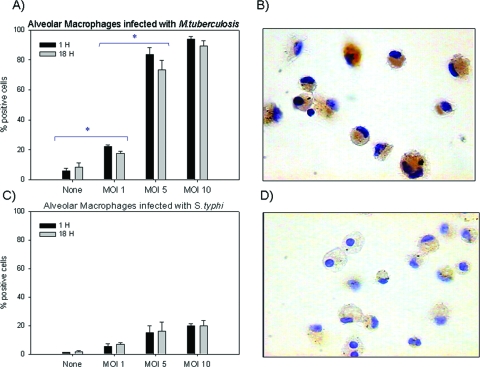
AMs infected with M. tuberculosis showed the highest percentage of LL-37-immunostained cells in a dose-dependent manner and was highest at 1 h postinfection with an MOI of 10 (93.99% ± 1.87%) (Fig. 2A). Immunostaining was very strong in AMs (Fig. 2B), even more so than in any other cell type infected with M. tuberculosis or S. enterica serovar Typhi. AMs infected with S. enterica serovar Typhi had lower percentage of immunostained cells (Fig. 2C), and immunostaining was also weaker in these cells (Fig. 2D).
FIG. 2.
(A and B) AMs showed abundant positive cells by immunocytochemistry when infected with M. tuberculosis. Production was dose dependent, and all infected cells showed high immunostaining for LL-7. (C and D) AMs infected with S. enterica serovar Typhi. Data are means ± SDs from four independent experiments. Magnification (B and D), ×200. *, P < 0.05.
Detection of LL-37 by electron microscopy.
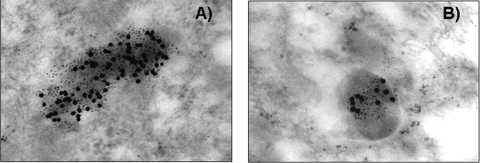
The AM immunoelectron microscopy study showed specific LL-37 labeling on the surfaces and in the cytoplasms of phagocytosed mycobacteria. Double staining showed the colocalization of LL-37 and LAM inside phagosomal vesicles (Fig. 3A). The same pattern of subcellular LL-37 distribution was found in intracellular mycobacteria (Fig. 3B). All mycobacteria phagocytosed by AMs had immunolabeling for LL-37. Uninfected control cells or M. tuberculosis-infected cells that were incubated without the primary anti-LL-37 antibody did not have any immunolabeling for LL-37.
FIG. 3.
Representative subcellular detection of cathelicidin (LL-37) and LAM in infected macrophages. Human AMs were collected by bronchial lavage and infected with M. tuberculosis strain H37Rv. After 18 h, cells were fixed and embedded in hydrosoluble resin in preparation for immunoelectron microscopy using specific antibodies and secondary antibodies labeled with different sizes of colloidal gold. (A) Intracellular bacilli showed specific immunolabeling of LAM (small dots) and LL-37 (large dots). (B) Numerous phagosomes also showed specific double immunolabeling, demonstrating the coexistence of both LAM and cathelicidin in the same cytoplasmic vesicles. Magnification, ×75,000.
Detection of LL-37 in tissue.
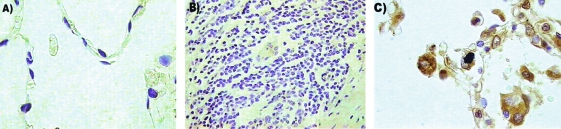
The detection of LL-37 by immunohistochemistry did not show any immunostaining in noninfected, control lung tissue (Fig. 4A). In contrast, strong and extensive LL-37 immunostaining was seen in macrophages and neutrophils, which constitute the inflammatory infiltrate in the pneumonia patches caused by gram-negative bacteria, as well as in lung ECs from airways affected by pneumonia (Fig. 4C). Interestingly, tissue samples with classical granulomatous lesions from TB patients also did not show LL-37 immunostaining (Fig. 4B), and only occasionally did activated alveolar or interstitial macrophages have any immunolabeling.
FIG. 4.
(A) Immunohistochemistry for LL-37 in healthy lung tissue showed no staining. (B) A similar result was obtained with lung granuloma tissue, where LL-37 was not detected. (C) In tissue from a patient with acute pneumonia, which was used as a positive control, immunostaining for LL-37 was intense, especially in AMs. Magnifications, ×400 (A and C) and ×200 (B).
Quantitative gene expression analysis.
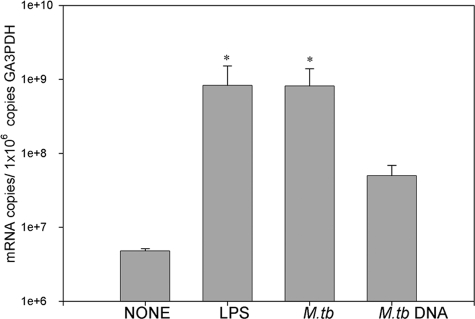
The quantitative analysis of LL-37 gene expression correlated with our immunohistochemical results. When MDMs were stimulated with LPS, gene expression was threefold higher than in unstimulated MDMs. Similar results were observed when MDMs were infected with M. tuberculosis at an MOI of 10. Interestingly, when MDMs were stimulated with purified M. tuberculosis DNA, gene expression was twofold higher than in unstimulated cells (P < 0.05) (Fig. 5). Similar results were observed for cells stimulated for 1 h and 18 h.
FIG. 5.
Gene expression analysis of LL-37. MDMs infected with M. tuberculosis, as well as those stimulated with LPS for 18 h, had the highest expression level, while those stimulated with M. tuberculosis DNA had twofold-higher expression than unstimulated cells. Data are means ± SDs from four independent experiments. *, P < 0.05.
Immunocytochemistry for LL-37 with different TLR ligands.
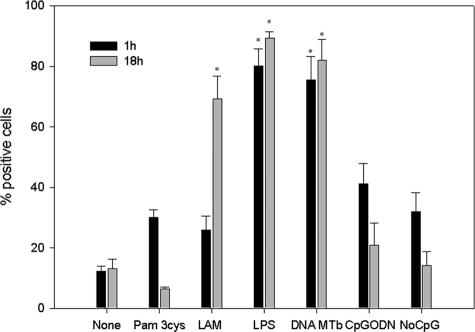
Immunocytochemistry of MDMs stimulated with different Toll-like receptor (TLR) ligands revealed that MDMs stimulated with LPS showed the highest percentage of positive cells at both 1 h and 18 h after stimulation (means ± SDs, 80.12% ± 5.58% and 89.39% ± 1.96%, respectively). Interestingly MDMs stimulated with purified M. tuberculosis DNA had the highest production level of LL-37 (1 h, 79.60% ± 7.75%, and 18 h, 82.03% ± 6.92%). When MDMs were stimulated with LAM, immunostaining showed a high percentage of LL-37-positive cells (1 h, 25.89% ± 4.70%; 18 h, 69.28% ± 7.42%). Statistical analysis for stimuli such as Pam3Cys, CpG ODN, and DNA with no CpG motifs showed no statistical significance with regard to unstimulated cells (P < 0.05) (Fig. 6).
FIG. 6.
Percentage of cells positive for LL-37 detected by immunocytochemistry. MDMs were stimulated through different specific TLR ligands for 18 h. Data are means ± SDs from four independent experiments. *, P < 0.05.
DISCUSSION
Innate immunity is the first line of defense against invading microorganisms in vertebrates and triggers an antigen-specific adaptive immune response (38). AMPs are thought to be significant effectors of innate immunity through their immunomodulatory activity and direct killing of microorganisms (2). AMPs are divided into two families: defensins and cathelicidins (1, 42). Both kinds of AMPs have been involved in the immunopathogenesis of several infectious diseases (3, 7, 16, 20-22, 25, 29). Abnormalities in HBD-2 production, have been involved in lung infections exhibited by patients with cystic fibrosis (3, 8). On the other hand, LL-37 is the only cathelicidin identified in humans so far and has been linked to skin and infectious bowel diseases (1, 16, 20). LL-37 can be induced by different molecules and has been used as a treatment for shigellosis in a rabbit model (27).
Scant information is available on the role of AMPs in TB. We showed that during mycobacterial infection of lung ECs, there is high expression of HBD-2 (30). Moreover, studies with experimental models revealed that mice with uncontrolled infection produced scant quantities of mBD-3 and -4, while animals that controlled mycobacterial growth produced abundant quantities of both defensins (29).
Whether cathelicidin LL-37 is involved in innate immunity against intracellular pathogens such as M. tuberculosis has not been determined in the human lung. To our knowledge, this is the first report showing gene induction and production of LL-37 in human cells after infection with M. tuberculosis. We demonstrated that when lung ECs were infected with M. tuberculosis, high levels of LL-37 were produced, primarily after 18 h, in a dose-dependent manner, and the same response was induced by S. enterica serovar Typhi. This result is in agreement with our previous study, which demonstrated the highest HBD-2 production by the same lung EC cell line after 18 h of mycobacterial infection (30), suggesting that both peptides, and probably other molecules, may act synergistically against M. tuberculosis during primary lung epithelial infection (9, 23, 39).
Neutrophils are one of the first cells to arrive at the infection site during tuberculosis, thus preventing early mycobacterial blood dissemination (4, 19). Moreover, it has been reported that phagocytosis of apoptotic neutrophils and granules by macrophages results in decreased viability of intracellular M. tuberculosis (36). Therefore, the transfer of AMPs from neutrophils to macrophages provides a cooperative defense strategy between innate immune cells against intracellular pathogens. We infected neutrophils with M. tuberculosis to assess whether these cells produced LL-37 during in vitro infection and compared it to salmonella infection, which has been reported to induce the production of this peptide (32, 40). Our results showed that neutrophils efficiently produced LL-37 when infected with M. tuberculosis. and may indicate early bactericidal activity of this peptide against M. tuberculosis infection.
It has been reported that AMs and MDMs are important participants in the innate immune response against M. tuberculosis (6, 31, 38). We investigated the production of LL-37 in AMs and MDMs during M. tuberculosis infection. Our results showed that MDMs infected with M. tuberculosis expressed LL-37 at high levels, and this expression was related to the MOI. Gene expression in MDMs correlated with the percentage of immunostained cells. Intriguingly, infected monocytes did not show such expression; thus, it appears that the level of macrophage differentiation is associated with the ability to produce LL-37.
We sought to detect the production of LL-37 in infected AMs, considering that this cell type is the main effector cell in M. tuberculosis destruction and antigen presentation in the lung (41). Our immunocytochemistry data showed that AMs infected with M. tuberculosis produced more LL-37 than any other cell type analyzed in our study. Indeed, the immunostaining was also stronger in AMs than in any of the other cells. In fact, all AMs positive for LL-37 showed phagocytosed mycobacteria as determined by Ziehl-Nielsen staining. This observation was corroborated and extended by immunoelectron microscopy, which showed LL-37 on the mycobacterial surface and cytoplasm and also in phagosomal vesicles. Interestingly, scarce bacilli were observed after 18 h of infection, but double immunolabeling of LAM and LL-37 was seen inside AM phagosomal vesicles. This observation suggests that at this time point of infection, many bacteria were killed and LL-37 could participate in this process.
Liu et al. showed that TLR activation of MDMs induced killing of M. tuberculosis through LL-37 participation and this induction is in part due to the vitamin D receptor and vitamin D hydroxylase gene activation (18a). During our experiments, no vitamin D supplementation was used to induce LL-37 production. However, we used human serum, which is a source of vitamin D, so we could not determine the real participation of this factor in LL-37 production. This interesting point will be explored in future experiments.
The histopathological hallmark of tuberculosis is granulomas, whose main function is to prevent dissemination (15). There is no information available about LL-37 production in tuberculous granulomas. Our immunohistochemistry results showed that LL-37 was not detected in lung granulomas. Considering that AMPs are produced during early infection and TB granulomas are typical of a chronic infection, this finding is not surprising. In contrast, pneumonia caused by gram-negative organisms showed strong LL-37 immunostaining in neutrophils and macrophages, as well as bronchial ECs. These results suggested that LL-37 is mainly produced during acute infection and probably early M. tuberculosis infection and has limited or no participation in the bactericidal activity in chronic granulomatous inflammation.
During the innate immune response, TLR activation triggers direct antimicrobial activity (41). Therefore, we studied LL-37 production after TLR stimulation. We stimulated MDMs with different TLR ligands, including TLR-9. In agreement with previous publications (37, 40), we found that LPS induced strong production of LL-37. However, other authors reported that LPS had minimal capacity to stimulate cathelicidin production after blood mononuclear cell activation (33). This difference could be explained by the different kinds of cells used in our experiments and also by the inherent responses produced by the study subjects. As previously reported, cathelicidin blocks and inactivates LPS, thus inhibiting septic shock (18, 34), and it has also been reported that some subjects are more susceptible to septicemia than others.
Interestingly, when cells were stimulated with purified DNA from M. tuberculosis, a ligand for TLR-9, cells responded with high LL-37 production. Some AMPs, such as HBD-2, can be induced through TLR-9 ligands in lung ECs (24). On the other hand, several reports suggested that TLR-9 is a very important receptor for optimal innate immune responses against M. tuberculosis (13, 24, 26). We found that AMs and MDMs expressed high levels of TLR-9, and this could be related to the inherent immune response against M. tuberculosis in some individuals (our unpublished data).
In summary, our results showed that AMs are most efficient at producing LL-37 after infection with M. tuberculosis, suggesting that cathelicidin from AMs may be an important participant in the innate immune response during early infection in humans, probably after TLR-9 activation with M. tuberculosis DNA. Other significant issues, such as the role of cathelicidin in the immunopathogenesis of TB, its direct bactericidal activity, and its participation in immunoregulation, should be studied in the future.
Acknowledgments
We are thankful to Cesar Rivas and Laura Galvan for their invaluable participation in this study.
This work was supported by the National Council of Science and Technology in Mexico (CONACYT, grant SEP-2004-C01-47745) and the European Community (INCO DC, grant ICA4-CT-2002-10063).
Editor: R. P. Morrison
Footnotes
Published ahead of print on 26 December 2007.
REFERENCES
- 1.Agerberth, B., and G. H. Gudmundsson. 2006. Host antimicrobial defence peptides in human disease. Curr. Top. Microbiol. Immunol. 30667-90. [DOI] [PubMed] [Google Scholar]
- 2.Bals, R. 2000. Epithelial antimicrobial peptides in host defense against infection. Respir. Res. 1141-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bals, R., D. J. Weiner, R. L. Meegalla, and J. M. Wilson. 1999. Transfer of a cathelicidin peptide antibiotic gene restores bacterial killing in a cystic fibrosis xenograft model. J. Clin. Invest. 1031113-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrios-Payan, J., D. Aguilar-Leon, R. Lascurain-Ledezma, and R. Hernandez-Pando. 2006. Neutrophil participation in early control and immune activation during experimental pulmonary tuberculosis. Gac. Med. Mex. 142273-281. [PubMed] [Google Scholar]
- 5.Bermudez, L. E., F. J. Sangari, P. Kolonoski, M. Petrofsky, and J. Goodman. 2002. The efficiency of the translocation of Mycobacterium tuberculosis across a bilayer of epithelial and endothelial cells as a model of the alveolar wall is a consequence of transport within mononuclear phagocytes and invasion of alveolar epithelial cells. Infect. Immun. 70140-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carranza, C., E. Juarez, M. Torres, J. J. Ellner, E. Sada, and S. K. Schwander. 2006. Mycobacterium tuberculosis growth control by lung macrophages and CD8 cells from patient contacts. Am. J. Respir. Crit. Care Med. 173238-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chromek, M., Z. Slamova, P. Bergman, L. Kovacs, L. Podracka, I. Ehren, T. Hokfelt, G. H. Gudmundsson, R. L. Gallo, B. Agerberth, and A. Brauner. 2006. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat. Med. 12636-641. [DOI] [PubMed] [Google Scholar]
- 8.Dauletbaev, N., R. Gropp, M. Frye, S. Loitsch, T. O. Wagner, and J. Bargon. 2002. Expression of human beta defensin (HBD-1 and HBD-2) mRNA in nasal epithelia of adult cystic fibrosis patients, healthy individuals, and individuals with acute cold. Respiration 6946-51. [DOI] [PubMed] [Google Scholar]
- 9.Debbabi, H., S. Ghosh, A. B. Kamath, J. Alt, D. E. Demello, S. Dunsmore, and S. M. Behar. 2005. Primary type II alveolar epithelial cells present microbial antigens to antigen-specific CD4+ T cells. Am. J. Physiol. Lung Cell Mol. Physiol. 289L274-L279. [DOI] [PubMed] [Google Scholar]
- 10.Durr, U. H., U. S. Sudheendra, and A. Ramamoorthy. 2006. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim. Biophys. Acta. 17581408-1425. [DOI] [PubMed] [Google Scholar]
- 11.Dye, C., S. Scheele, P. Dolin, V. Pathania, and M. C. Raviglione. 1999. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA 282677-686. [DOI] [PubMed] [Google Scholar]
- 12.Ellner, J. J., C. S. Hirsch, and C. C. Whalen. 2000. Correlates of protective immunity to Mycobacterium tuberculosis in humans. Clin. Infect. Dis. 30(Suppl. 3)S279-S282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fremond, C. M., V. Yeremeev, D. M. Nicolle, M. Jacobs, V. F. Quesniaux, and B. Ryffel. 2004. Fatal Mycobacterium tuberculosis infection despite adaptive immune response in the absence of MyD88. J. Clin. Invest. 1141790-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hernandez-Pando, R., H. Orozcoe, A. Sampieri, L. Pavon, C. Velasquillo, J. Larriva-Sahd, J. M. Alcocer, and M. V. Madrid. 1996. Correlation between the kinetics of Th1, Th2 cells and pathology in a murine model of experimental pulmonary tuberculosis. Immunology 8926-33. [PMC free article] [PubMed] [Google Scholar]
- 15.Houben, E. N., L. Nguyen, and J. Pieters. 2006. Interaction of pathogenic mycobacteria with the host immune system. Curr. Opin. Microbiol. 976-85. [DOI] [PubMed] [Google Scholar]
- 16.Islam, D., L. Bandholtz, J. Nilsson, H. Wigzell, B. Christensson, B. Agerberth, and G. Gudmundsson. 2001. Downregulation of bactericidal peptides in enteric infections: a novel immune escape mechanism with bacterial DNA as a potential regulator. Nat. Med. 7180-185. [DOI] [PubMed] [Google Scholar]
- 17.Kaufmann, S. H. 2006. Tuberculosis: back on the immunologists’ agenda. Immunity 24351-357. [DOI] [PubMed] [Google Scholar]
- 18.Larrick, J. W., M. Hirata, R. F. Balint, J. Lee, J. Zhong, and S. C. Wright. 1995. Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. Infect. Immun. 631291-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18a.Liu, P. T., S. Stenger, H. Li, L. Wenzel, B. H. Tan, S. R. Krutzik, M. T. Ochoa, J. Schauber, K. Wu, C. Meinken, D. L. Kamen, M. Wagner, R. Bals, A. Steinmeyer, U. Zügel, R. L. Gallo, D. Eisenberg, M. Hewison, B. W. Hollis, J. S. Adams, B. R. Bloom, and R. L. Modlin. 2006. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 241770-1773. [DOI] [PubMed] [Google Scholar]
- 19.Martineau, A. R., S. M. Newton, K. A. Wilkinson, B. Kampmann, B. M. Hall, N. Nawroly, G. E. Packe, R. N. Davidson, C. J. Griffiths, and R. J. Wilkinson. 2007. Neutrophil-mediated innate immune resistance to mycobacteria. J. Clin. Invest. 1171988-1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nizet, V., and R. L. Gallo. 2003. Cathelicidins and innate defense against invasive bacterial infection. Scand. J. Infect. Dis. 35670-676. [DOI] [PubMed] [Google Scholar]
- 21.Nizet, V., T. Ohtake, X. Lauth, J. Trowbridge, J. Rudisill, R. A. Dorschner, V. Pestonjamasp, J. Piraino, K. Huttner, and R. L. Gallo. 2001. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 414454-457. [DOI] [PubMed] [Google Scholar]
- 22.Ong, P. Y., T. Ohtake, C. Brandt, I. Strickland, M. Boguniewicz, T. Ganz, R. L. Gallo, and D. Y. Leung. 2002. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N. Engl. J. Med. 3471151-1160. [DOI] [PubMed] [Google Scholar]
- 23.Pechkovsky, D. V., T. Goldmann, E. Vollmer, J. Muller-Quernheim, and G. Zissel. 2006. Interleukin-18 expression by alveolar epithelial cells type II in tuberculosis and sarcoidosis. FEMS Immunol. Med. Microbiol. 4630-38. [DOI] [PubMed] [Google Scholar]
- 24.Platz, J., C. Beisswenger, A. Dalpke, R. Koczulla, O. Pinkenburg, C. Vogelmeier, and R. Bals. 2004. Microbial DNA induces a host defense reaction of human respiratory epithelial cells. J. Immunol. 1731219-1223. [DOI] [PubMed] [Google Scholar]
- 25.Putsep, K., G. Carlsson, H. G. Boman, and M. Andersson. 2002. Deficiency of antibacterial peptides in patients with morbus Kostmann: an observation study. Lancet 3601144-1149. [DOI] [PubMed] [Google Scholar]
- 26.Quesniaux, V., C. Fremond, M. Jacobs, S. Parida, D. Nicolle, V. Yeremeev, F. Bihl, F. Erard, T. Botha, M. Drennan, M. N. Soler, M. Le Bert, B. Schnyder, and B. Ryffel. 2004. Toll-like receptor pathways in the immune responses to mycobacteria. Microbes Infect. 6946-959. [DOI] [PubMed] [Google Scholar]
- 27.Raqib, R., P. Sarker, P. Bergman, G. Ara, M. Lindh, D. A. Sack, K. M. Nasirul Islam, G. H. Gudmundsson, J. Andersson, and B. Agerberth. 2006. Improved outcome in shigellosis associated with butyrate induction of an endogenous peptide antibiotic. Proc. Natl. Acad. Sci. USA 1039178-9183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rivas-Santiago, B., E. Sada, R. Hernandez-Pando, and V. Tsutsumi. 2006. Antimicrobial peptides in the innate immunity of infectious diseases. Salud Publica Mex. 4862-71. [DOI] [PubMed] [Google Scholar]
- 29.Rivas-Santiago, B., E. Sada, V. Tsutsumi, D. Aguilar-Leon, J. L. Contreras, and R. Hernandez-Pando. 2006. β-Defensin gene expression during the course of experimental tuberculosis infection. J. Infect. Dis. 194697-701. [DOI] [PubMed] [Google Scholar]
- 30.Rivas-Santiago, B., S. K. Schwander, C. Sarabia, G. Diamond, M. E. Klein-Patel, R. Hernandez-Pando, J. J. Ellner, and E. Sada. 2005. Human β-defensin 2 is expressed and associated with Mycobacterium tuberculosis during infection of human alveolar epithelial cells. Infect. Immun. 734505-4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rivas-Santiago, B., P. Vieyra-Reyes, and Z. Araujo. 2005. Cell immunity response in human pulmonary tuberculosis. Invest. Clin. 46391-412. [PubMed] [Google Scholar]
- 32.Rosenberger, C. M., R. L. Gallo, and B. B. Finlay. 2004. Interplay between antibacterial effectors: a macrophage antimicrobial peptide impairs intracellular Salmonella replication. Proc. Natl. Acad. Sci. USA 1012422-2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schauber, J., R. A. Dorschner, K. Yamasaki, B. Brouha, and R. L. Gallo. 2006. Control of the innate epithelial antimicrobial response is cell-type specific and dependent on relevant microenvironmental stimuli. Immunology 118509-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scott, M. G., D. J. Davidson, M. R. Gold, D. Bowdish, and R. E. Hancock. 2002. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J. Immunol. 1693883-3891. [DOI] [PubMed] [Google Scholar]
- 35.Sharma, S., I. Verma, and G. K. Khuller. 2001. Therapeutic potential of human neutrophil peptide 1 against experimental tuberculosis. Antimicrob. Agents Chemother. 45639-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan, B. H., C. Meinken, M. Bastian, H. Bruns, A. Legaspi, M. T. Ochoa, S. R. Krutzik, B. R. Bloom, T. Ganz, R. L. Modlin, and S. Stenger. 2006. Macrophages acquire neutrophil granules for antimicrobial activity against intracellular pathogens. J. Immunol. 1771864-1871. [DOI] [PubMed] [Google Scholar]
- 37.Turner, J., Y. Cho, N. N. Dinh, A. J. Waring, and R. I. Lehrer. 1998. Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob. Agents Chemother. 422206-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Crevel, R., T. H. Ottenhoff, and J. W. van der Meer. 2003. Innate immunity to Mycobacterium tuberculosis. Adv. Exp. Med. Biol. 531241-247. [DOI] [PubMed] [Google Scholar]
- 39.Wickremasinghe, M. I., L. H. Thomas, and J. S. Friedland. 1999. Pulmonary epithelial cells are a source of IL-8 in the response to Mycobacterium tuberculosis: essential role of IL-1 from infected monocytes in a NF-κB-dependent network. J. Immunol. 1633936-3947. [PubMed] [Google Scholar]
- 40.Wu, H., G. Zhang, J. E. Minton, C. R. Ross, and F. Blecha. 2000. Regulation of cathelicidin gene expression: induction by lipopolysaccharide, interleukin-6, retinoic acid, and Salmonella enterica serovar Typhimurium infection. Infect. Immun. 685552-5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yew, W. W., and C. C. Leung. 2006. Update in tuberculosis 2005. Am. J. Respir. Crit. Care Med. 173491-498. [DOI] [PubMed] [Google Scholar]
- 42.Zasloff, M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415389-395. [DOI] [PubMed] [Google Scholar]