Abstract
Memory CD8+ T cells are essential for protective immunity against many intracellular pathogens; therefore, stimulation of this population of cells is an important goal of vaccination. We have previously shown that a detoxified derivative of Bacillus anthracis anthrax lethal toxin (LT) can deliver heterologous CD8+ T-cell epitopes to the major histocompatibility complex class I processing and presentation pathway of murine host cells and that immunization of mice with these LT-antigen fusion proteins leads to the induction of antigen-specific CD8+ T cells. In this report we extend these findings to include a detailed characterization of the phenotypic and functional properties of the T cells stimulated by the LT-based system. We found that after an initial period of expansion and contraction, antigen-specific CD8+ T cells differentiated into a pool of memory cells that produced gamma interferon and displayed in vivo cytotoxic activity. The transition to memory cells appeared to be quite rapid based on an analysis of the phenotypic marker CD127 and the effectiveness of a booster immunization administered early after the initial immunization. We also investigated the composition of the memory T-cell pool induced by this system and found that while one immunization induced a mixture of effector memory T cells (CD62Llow) and central memory T cells (CD62Lhigh), a second immunization preferentially elevated the effector memory T-cell frequency. Finally, we demonstrated that mice that received prime-boost immunizations of LT-antigen proteins were more protected in a Listeria monocytogenes challenge model than mice that received only one immunization.
Following infection or immunization, antigen-specific CD8+ T lymphocytes undergo extensive clonal expansion. Most of the effector T cells generated during this process die by apoptosis; however, a small population of antigen-specific CD8+ T cells remains in the host for an extended period of time as memory cells. Memory CD8+ T cells are a critical component of protective immunity because they can rapidly and extensively proliferate, secrete inflammatory cytokines, such as gamma interferon (IFN-γ), and lyse infected target cells upon reexposure to antigen (15). Consequently, vaccines are often specifically designed to stimulate this population of cells. To stimulate CD8+ T cells most effectively, the target antigen must be delivered not just into the bloodstream but into the cytosol of host cells. Once the antigen is delivered to the cytosol, it is broken down into peptides by the proteasome and presented on the cell surface by major histocompatibility complex class I (MHC-I) molecules to CD8+ T cells (13, 34).
One successful strategy that our laboratory has employed to induce CD8+ T-cell responses in mice is to fuse heterologous CD8+ T-cell epitopes to a detoxified derivative of Bacillus anthracis anthrax lethal toxin (LT) (6-8, 11, 26). LT is a bipartite toxin in which the first protein, protective antigen (PA), delivers the enzymatically active second protein, lethal factor (LF), across the host cell membrane into the cytosol (33). Entry into cells is initiated when PA binds one of its ubiquitously expressed cell surface receptors, ANTXR1 (10) or ANTXR2 (38), and forms a heptamer that can bind up to three LF molecules (31, 32). The entire toxin complex is then endocytosed by cells in a clathrin-dependent manner (1). Acidification of the endosome triggers a conformational change in PA leading to formation of a transmembrane pore (9, 24, 30). This PA pore facilitates translocation of catalytic LF molecules into the cytosol, where they can ultimately lead to host cell death (33). Importantly, the N-terminal 255 amino acids of LF (LFn) comprise a domain with no toxic activity that is still delivered into cells by PA (2). Therefore, CD8+ T-cell epitopes fused to LFn are also delivered into the host cell cytosol in a nontoxic manner.
We and others have previously shown that once in the cytosol, the heterologous antigen fused to LFn gains access to the MHC-I processing and presentation pathway (8, 12, 29). As a result, injecting mice intraperitoneally (i.p.) with picomole quantities of LFn-antigen fusion protein and PA leads to stimulation of antigen-specific CD8+ T cells in a PA-dependent manner (6-8, 11, 26). We have also demonstrated that these T cells are retained in the spleens of mice for at least 4 months after immunization (11) and that prior immunization does not interfere with the priming of antigen-specific T cells in a subsequent immunization with a different epitope (6). The data supporting these conclusions came from experiments in which splenocytes from immunized mice were restimulated for 5 days in vitro and tested for antigen-specific cytotoxic activity in standard 51Cr release assays (6-8, 11, 26). While these findings illustrated the ability of the LT-based system to induce CD8+ T-cell responses in mice, the myriad of other kinetic, phenotypic, and functional properties of the responding T cells have not been investigated or described.
It is becoming increasingly clear that CD8+ T cells exhibit diverse phenotypic and functional characteristics. Recent reports have classified long-lived memory T cells into two distinct groups, the lymph node-homing, highly proliferative, interleukin-2 (IL-2)-producing, central memory T cells (TCM) and the tissue-residing, cytotoxic, effector memory T cells (TEM) (18, 35, 36). Importantly, the TCM and TEM composition of an antigen-specific T-cell pool can change both with time and after successive immunizations (18, 19). It is also evident that circumstances surrounding the priming event, such as the nature of the vaccine vector or infectious agent, the inflammatory environment, and the antigen load, can greatly impact the phenotypic and functional properties of the resulting effector and memory T cells (18, 19, 39). This complexity suggested the need for a detailed analysis of the CD8+ T-cell response stimulated by LT-delivered antigen.
In this study we characterized the induction, effector functions, phenotypes, and protective capacities of memory CD8+ T cells induced by immunization of mice with LFn-antigen fusion proteins and PA. We showed that the CD8+ T cells stimulated by the fusion proteins rapidly transitioned to cells with a memory phenotype whose frequency could be increased by a second immunization. Furthermore, the memory cells stimulated by the LT-based system displayed protective effector functions and were a mixture of TCM and TEM. We also investigated how primary memory and secondary memory T cells stimulated by LFn fusion proteins and PA differed, both phenotypically and functionally.
MATERIALS AND METHODS
Mice and immunizations.
BALB/cBy/J (H-2d) mice were obtained from the Jackson Laboratory. All animal experiments were approved by Harvard Medical School's Institutional Animal Care and Use Committee. LFn-NP118-126 was made and purified as described elsewhere (11). LFn-NP118-126 contains an amino-terminal His6 tag and a carboxyl-terminal fusion to NP118-126 (RPQASGVYM), an H-2Ld-restricted epitope from the nucleoprotein (NP) of lymphocytic choriomeningitis virus (LCMV). PA protein was a gift from R. J. Collier (Harvard Medical School, Boston, MA). Mice were immunized i.p. either with 30 pmol of LT-NP (30 pmol of LFn-NP118-126 [1 μg] plus 30 pmol of PA [2.5 μg]) or with 30 pmol of LFn-NP118-126 alone, both in 200 μl of phosphate-buffered saline (PBS). Booster immunizations were administered 9 or 21 days after the primary immunization as indicated below. In some experiments, control, unimmunized mice were also used. Listeria monocytogenes strain XFL303 (L. monocytogenes-NP) (40) was kindly provided by H. Shen (University of Pennsylvania Medical Center, Philadelphia). L. monocytogenes-NP was grown to stationary phase in brain heart infusion broth, aliquoted, titrated, and stored at −80°C. Before immunization, bacteria were thawed, grown to early exponential phase in brain heart infusion broth, and diluted in PBS. For CD127 or CD44 analysis mice were infected with approximately 0.1 50% lethal dose (LD50) of L. monocytogenes NP (1 × 103 CFU). For challenge experiments, mice were infected with 0.73 LD50 of L. monocytogenes-NP (7.3 × 103 CFU). In all cases, L. monocytogenes-NP was injected intravenously (i.v.) into the tail vein in 200 μl PBS. Approximately 72 h after challenge, spleens and livers were harvested and homogenized in 0.2% NP-40. Dilutions of the homogenate were made in PBS and plated on tryptic soy agar plates containing streptomycin (100 μg/ml).
IFN-γ enzyme-linked immunospot assay (ELISPOT) analysis.
Spleens from experimental mice were harvested, and red blood cells were lysed by hypotonic shock with ammonium chloride and potassium. Serial dilutions of splenocytes were added to nitrocellulose-backed 96-well plates previously coated with rat anti-mouse IFN-γ antibody (BD Biosciences). Each well also contained 1 × 105 irradiated, NP118-126 peptide-pulsed P815 (H-2d) mastocytoma cells. After 24 h, the plates were washed and treated with biotinylated rat anti-mouse IFN-γ antibody (BD Biosciences) for an additional 18 h. The plates were then washed, and streptavidin-horseradish peroxidase (BD Biosciences) was added. The plates were developed with 1 mg/ml 3,3′diaminobenzidine tetrahydrochloride dihydrate (Bio-Rad) in 50 mM Tris (pH 7.9). The number of NP-specific T cells was determined by subtracting the number of spots in the presence of unpulsed P815 cells from the number of spots observed when the T cells were incubated with peptide-pulsed P815 cells.
Intracellular cytokine staining.
Single-cell suspensions of splenocytes from immunized mice were stimulated for 6 h with 100 nM NP118-126 peptide in the presence of GolgiPlug (BD Biosciences). Cells were surface stained with anti-CD8α antibody (BD Biosciences), fixed and permeabilized using a Cytofix/Cytoperm Plus kit (BD Biosciences), and stained intracellularly with anti-IFN-γ antibody (BD Biosciences). To determine the percentage of IFN-γ+ NP-specific T cells in the total CD8+ T cells, the percentage of IFN-γ+ T cells in parallel unstimulated samples (no peptide) was subtracted from the peptide-stimulated value for the same mouse.
Flow cytometric analysis of NP-specific CD8+ T-cell frequency and phenotype.
Spleens were harvested from experimental mice on the indicated days after immunization, and red blood cells were lysed. Splenocyte suspensions were enriched for CD3+ T cells by negative selection using T-cell enrichment columns (R&D Systems) according to the manufacturer's recommendations. The resulting T-cell populations were surface stained with H-2Ld:NP118-126 (LdNP) MHC tetramers (allophycocyanin conjugated) that were generated at the National Institutes of Health Tetramer Facility (Bethesda, MD). Cells were also stained with anti-CD8α, anti-CD62L, anti-CD44, and anti-CD127 antibodies (all obtained from BD Biosciences) and analyzed with a BD Biosciences FACSCalibur flow cytometer. Flowjo software (Tree Star) was used for all data analysis.
In vivo lysis assay.
Splenocytes from naïve BALB/c mice (1 × 107 cells/100 μl) were pulsed with 5 μM NP118-126 peptide or without peptide in RP-10 (RPMI 1640 [Invitrogen], 10% fetal bovine serum, l-glutamine, HEPES, 50 μM 2-mercaptoethanol, 50 U/ml penicillin, 50 μg/ml streptomycin) for 1 h. Peptide-pulsed and unpulsed populations were differentially labeled with the intracellular fluorescent dye carboxyfluorescein diacetate succimidyl ester (CFSE) (0.5 μM CFSE for peptide-pulsed cells and 5 μM CFSE for unpulsed cells) in PBS with 0.1% bovine serum albumin for 10 min at 37°C. Labeling was stopped by addition of 5 volumes of cold RP-10. Cells were washed three times with PBS, and the two populations were mixed at a 1:1 ratio. A total of 5 × 106 cells (2.5 × 106 cells of each population) were injected i.v. into the tail veins of recipient BALB/c mice that had been previously immunized with LT-NP or that had received no prior immunization. Approximately 18 h later, splenocytes were harvested from recipient mice, and single-cell suspensions were analyzed by flow cytometry. Adoptively transferred cells were distinguished from host cells by CFSE staining, and peptide-pulsed cells (CFSElow) were distinguished from unpulsed cells (CFSEhigh) by the intensity of the CFSE staining. The percentage of specific lysis was calculated as follows: 100 − {[(percentage of peptide-pulsed cells in immunized mice/percentage of unpulsed cells in immunized mice)/(percentage of peptide-pulsed cells in unimmunized mice/percentage of unpulsed cells in unimmunized mice)] × 100}.
Statistical analysis.
Data are expressed below as means and standard deviations. The statistical significance of differences was analyzed using a two-tailed Student t test for independent samples (followed by Bonferroni's correction to adjust for multiple comparisons in Fig. 3 and 5B). A P value of <0.05 was considered statistically significant.
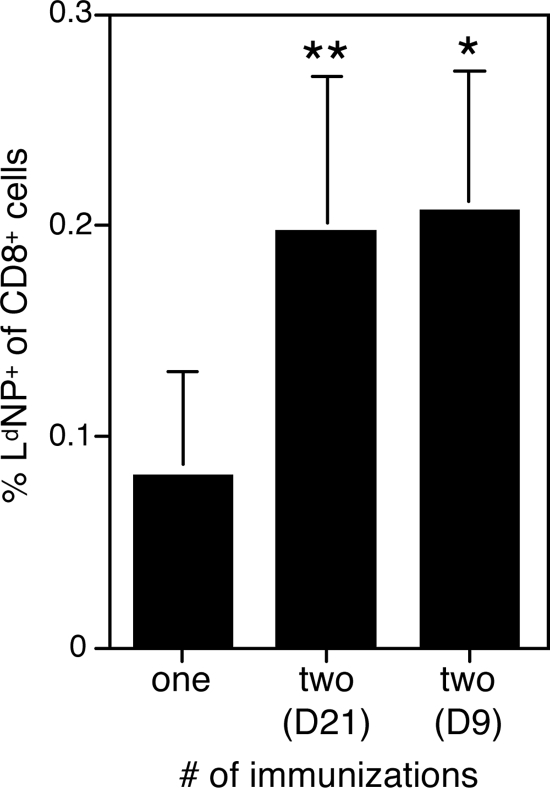
FIG. 3.
Booster LT-NP immunizations increase the frequency of NP-specific memory T cells. BALB/c mice were immunized i.p. either once or twice (with the second immunization 21 [D21] or 9 [D9] days after the first immunization, as indicated) with 30 pmol of LT-NP. Twenty-eight days after the final immunization the frequency of splenic, NP-specific CD8+ T cells was determined using LdNP tetramers and flow cytometry. The data represent 4 to 16 mice per group, pooled from several independent experiments. For comparisons with mice immunized once, one asterisk indicates that the P value is <0.05, and two asterisks indicate that the P value is <0.01.
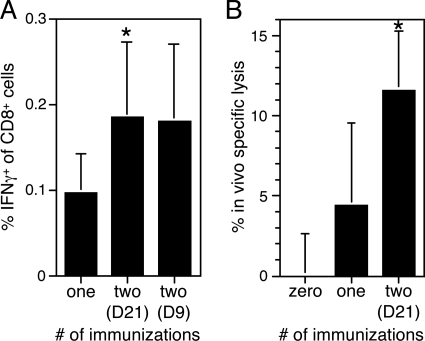
FIG. 5.
LT-NP induces IFN-γ-producing, cytotoxic memory T cells that can be boosted by a second immunization. Groups of mice were immunized i.p. with 30 pmol of LT-NP either once or twice (9 [D9] or 21 [D21] days after the first immunization, as indicated) or were not immunized. (A) Splenocytes were harvested 28 days later, and the percentage of CD8+ T cells that produced IFN-γ in response to the NP peptide was determined by intracellular cytokine staining. (B) Twenty-eight days after the final immunization, mice were injected i.v. with a 1:1 mixture of CFSElow NP peptide-pulsed splenocytes and CFSEhigh unpulsed splenocytes. The percentage of in vivo specific lysis of the peptide-pulsed splenocytes was determined by flow cytometry 18 h later. The data are the means and standard deviations for 4 to 16 mice (A) or 10 to 15 mice (B) from several independent experiments. An asterisk indicates that the P value is <0.01 for a comparison with mice immunized once (A) or for a comparison with either unimmunized mice or mice immunized once (B).
RESULTS
Single LT-NP immunization induces clonal expansion and contraction of NP-specific CD8+ T cells.
In this study we characterized the CD8+ T-cell response induced by PA plus nontoxic LFn fused to NP118-126. NP118-126 is a well-characterized H-2Ld-restricted CD8+ T-cell epitope from the nucleoprotein of LCMV. We have previously shown that NP118-126-specific T cells with cytotoxic activity can be cultured from the spleens of BALB/c mice injected with LFn-NP118-126 plus PA (LT-NP) 2 weeks earlier (6, 11). However, we were interested in a more detailed kinetic analysis of the primary T-cell response to this toxin fusion protein. Therefore, we immunized mice i.p. with LT-NP and determined the number of NP-specific T cells in the spleens of mice over the subsequent month by IFN-γ ELISPOT analysis (Fig. 1A). As expected, the number of NP-specific T cells was below the limit of detection in control, unimmunized mice. In mice immunized with LT-NP, however, these cells rapidly expanded and reached a peak frequency of more than 104 cells/spleen 6 days later. Like many pathogen-specific CD8+ T-cell responses, the effector population then underwent a period of contraction, in this case contraction by 73%. By day 28 the frequency of NP-specific T cells had stabilized at approximately 3 × 103 cells/spleen. These results not only illustrate the magnitude and kinetics of the T-cell response stimulated by LT-NP but also show that the responding T cells produced the inflammatory cytokine IFN-γ. We also analyzed the NP-specific T-cell frequency in a parallel group of LT-NP-immunized mice using H-2Ld:NP118-126 (LdNP) tetramers and flow cytometry and detected similar levels of expansion (0.4% of the total splenic CD8+ T cells) and contraction (80%) (Fig. 1B). Furthermore, both ELISPOT and tetramer analyses of splenocytes from mice immunized with LFn-NP118-126 (without PA) (Fig. 1A and data not shown) confirmed our previously published finding that CD8+ T-cell stimulation is entirely dependent on PA-mediated delivery of antigen into the cytosol of host cells (8).
FIG. 1.
NP-specific CD8+ T cells expand and contract following LT-NP immunization. Groups of BALB/c mice were immunized i.p. with 30 pmol of LT-NP, 30 pmol of PA, or 30 pmol of LFn-NP118-126 in the absence of PA or were not immunized. The frequencies of splenic NP-specific T cells on the indicated days after immunization were determined by (A) IFN-γ ELISPOT analysis or (B) flow cytometry using LdNP tetramers. Data were compiled from several independent experiments, and the values are the results for 3 to 25 mice (A) and 7 to 10 mice (B). The dotted line indicates the limit of detection for the ELISPOT assay. (C) Expression of the T-cell activation marker CD44 on CD8+ LdNP+ splenocytes isolated at the peak of the response to either LT-NP (day 6) or L. monocytogenes-NP (LM-NP) (day 7) or on naïve CD8+ cells from unimmunized mice.
To ensure that the T cells identified by tetramer staining were in fact cells that had encountered the NP antigen, we used flow cytometry to measure the expression of the T-cell activation marker CD44 on LdNP+ cells isolated from mice at the peak of the LT-NP-induced primary response. The vast majority of CD8+ LdNP+ cells expressed elevated levels of CD44 compared to naïve CD8+ cells from unimmunized mice (Fig. 1C). The extent of CD44 upregulation was similar to that on CD8+ LdNP+ cells isolated from mice infected 7 days earlier with L. monocytogenes-NP118-126 (L. monocytogenes-NP), a recombinant strain of L. monocytogenes that was engineered to express the NP peptide and that stimulates a robust NP-specific CD8+ T-cell response in mice (40). These results demonstrate that tetramer-positive cells from LT-NP-immunized mice were, in fact, antigen experienced.
NP-specific T cells rapidly transition to CD127high memory cells.
The cytokine IL-7 is required for the survival and maintenance of CD8+ T cells (37). The alpha chain of its receptor, CD127, is constitutively expressed on the surface of naïve CD8+ T cells, downregulated on recently activated effector cells, and expressed by long-lived memory cells (37). In several infection models, including L. monocytogenes, investigators have shown that the small population of effector T cells retaining or regaining CD127 expression are the cells destined to survive the contraction phase and populate the memory pool (16, 22). We wanted to investigate the expression of CD127 on T cells induced by the LT-based antigen delivery system. To do this, we used flow cytometry to analyze CD127 expression on NP-specific T cells isolated from mice previously immunized with LT-NP. We compared the results to measurements of CD127 expression on NP-specific T cells isolated from mice inoculated with a sublethal dose of L. monocytogenes-NP. Even 28 days after L. monocytogenes-NP infection, only 63% (on average) of NP-specific T cells expressed high levels of surface CD127 (CD127high) (Fig. 2). This highlights the finding that enrichment of memory T cells can be a relatively slow process, often taking many weeks (21, 22). In marked contrast, more than 95% of NP-specific T cells were CD127high at the same time point after LT-NP immunization (Fig. 2). This difference suggests that compared to the T cells responding to L. monocytogenes-NP, the T cells responding to LT-NP either evolved toward a CD127high phenotype more quickly or did not undergo the same initial level of CD127 downregulation after priming. To investigate these possibilities, we monitored CD127 expression on NP-specific T cells at earlier times after L. monocytogenes-NP or LT-NP immunization. As expected, at the peak of the response to L. monocytogenes-NP (day 7), CD127low cells predominated, but as time progressed, the proportion of CD127high cells slowly increased (Fig. 2). The T cells stimulated by LT-NP were also enriched for CD127high cells over time, but strikingly, by 9 days after immunization approximately 90% of the NP-specific T cells already displayed a CD127high phenotype (Fig. 2). In contrast, 9 days after L. monocytogenes-NP infection, the majority of NP-specific T cells still expressed low levels of surface CD127 (CD127low) (Fig. 2). These results demonstrate that the enrichment of CD127high cells is more rapid after LT-NP immunization than after L. monocytogenes-NP infection and suggest that memory cell development may also be accelerated.
FIG. 2.
CD8+ T cells induced by LT-NP rapidly display a CD127high phenotype. Groups of BALB/c mice were immunized with 30 pmol of LT-NP i.p. or 0.1 LD50 of L. monocytogenes-NP (LM-NP) i.v. At the indicated days after immunization (days 6, 9, 12, and 28 for LT-NP and days 7, 9, 12, and 28 for L. monocytogenes-NP) splenocytes were enriched for T cells and analyzed by flow cytometry. NP-specific T cells (CD8+ LdNP+, as defined by the gate shown in the dot plots on the left, where the numbers indicate the percentages of CD8+ cells that are LdNP+ for the representative mice shown) were analyzed for CD127 expression. Representative dot plots indicate the percentages of CD127high and CD127low cells from individual mice. The graph shows the average percentage of CD127high cells in the total CD8+ LdNP+ cells from 3 to 10 mice per time point.
Another interesting finding was that at the peak of the LT-NP-induced T-cell response (day 6), the majority (63%, on average) of NP-specific T cells already displayed a CD127high memory phenotype (Fig. 2). Therefore, CD127 may be retained on many NP-specific effector T cells or is downregulated very early after priming and is reexpressed by day 6. Importantly, although 63% of NP-specific T cells were CD127high at the peak of the LT-NP-induced T-cell response, only 20 to 27% were expected to survive the contraction phase (Fig. 1A and B). These results demonstrate that CD127 expression by LT-NP-induced T cells is not sufficient to ensure the survival of these cells during the contraction phase and therefore that CD127 cannot be used as a marker to identify the precursors of long-term memory cells among the large pool of effectors.
Memory T-cell frequency is elevated by an early booster immunization.
Because the degree of protective immunity afforded by pathogen-specific CD8+ T cells is directly correlated to the frequency of these cells at the time of challenge, the efficacy of a vaccine is often linked to the number of memory T cells that it induces (23, 39). Unfortunately, many vaccines do not stimulate a robust T-cell response after one injection and thereby provide only limited protection in challenge models. However, administering a temporally spaced homologous or heterologous booster vaccine can be a successful strategy for expanding the pool of memory CD8+ T cells and therefore providing more protective immunity (39, 44). We wanted to determine whether sequential immunizations with the LT-based system would boost the antigen-specific T-cell pool. To test this possibility, we immunized mice with LT-NP, waited 21 days, and then reimmunized the same mice with LT-NP a second time. Twenty-eight days later we determined the frequency of NP-specific T cells in the spleens of these mice using LdNP tetramers and compared the results to the frequency in a parallel group of mice that were immunized only once, 28 days earlier. We found that on average, the second immunization boosted the splenic NP-specific memory T-cell population more than twofold (Fig. 3).
A substantial rest period (weeks to months) between the initial and booster immunizations is often required for optimal reexpansion of memory CD8+ T cells, most likely because the transition from effector T cells to memory T cells is slow (21). However, our finding that NP-specific T cells stimulated by LT-NP are predominantly CD127high after 9 days (Fig. 2) suggested that memory T-cell development may be faster in this system. Therefore, we hypothesized that we could administer the booster immunization less than 21 days after the first immunization, yet achieve the same substantial secondary expansion. To test this hypothesis, we again immunized a group of mice twice with LT-NP, but this time the injections were separated by only 9 days. Twenty-eight days later we observed the same sizeable increase in the NP-specific memory T-cell pool (Fig. 3). Of note, the vast majority (>95%) of the secondary memory T cells also expressed high levels of CD127 (Fig. 4A and data not shown). In conclusion, the time between the first and second LT-NP immunizations needs to be at most 9 days for effective boosting. Again, this suggests that the antigen-specific T cells stimulated by the LT-based system rapidly develop into memory cells capable of secondary expansion.
FIG. 4.
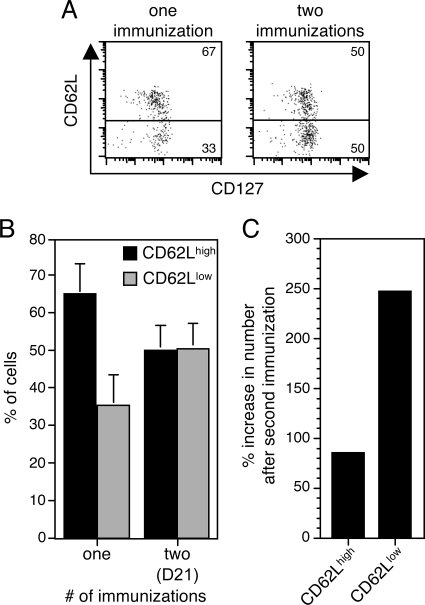
Reduced CD62L expression on secondary memory cells compared to primary memory cells. BALB/c mice were immunized i.p. either once or twice (21 days after the first immunization [D21]) with 30 pmol of LT-NP. Splenocytes were harvested 28 days later, enriched for T cells, and analyzed by flow cytometry. (A) Representative dot plots from individual mice demonstrating the distribution of CD62Lhigh and CD62Llow cells. Plots were gated on CD8+ LdNP+ cells. (B) Percentages of CD62Lhigh and CD62Llow cells among CD8+ LdNP+ cells from 10 to 16 mice per group compiled from three independent experiments. CD62Lhigh and CD62Llow were defined based on the gates indicated in panel A. (C) Calculated percent increases in the numbers of CD62Lhigh and CD62Llow cells 28 days after a second LT-NP immunization compared to the numbers 28 days after a first immunization.
Booster immunization preferentially increases TEM frequency.
Although memory CD8+ T cells are quite heterogeneous, they can be grouped into two functionally distinct subsets that are distinguishable by expression of the lymph node homing marker CD62L (36, 42). While CD62Lhigh TCM traffic to lymph nodes and CD62Llow TEM do not, both subsets are found in the spleen and blood (18). To determine the composition of antigen-specific memory cells stimulated by the LT-based system, we used flow cytometry to measure CD62L expression on NP-specific memory T cells isolated from the spleens of mice immunized 28 days earlier with LT-NP. At this time point, the NP-specific memory pool was approximately 65% CD62Lhigh cells and 35% CD62Llow cells (Fig. 4A and B). These results show that the LT-based system induces a mixture of TCM and TEM, a potentially beneficial property of a vaccine because both classes of memory cells have been shown to contribute to protective immunity under different circumstances (18).
It has recently been demonstrated that the phenotype of memory CD8+ T cells is influenced by the number of times that they have encountered their cognate antigen (19). In other words, booster immunizations can affect the TCM and TEM composition of an antigen-specific memory T-cell population and most often shift the balance toward TEM (20, 27). To determine whether the phenotype of LT-NP-induced T cells changes after a second immunization, we immunized mice twice (with the second immunization 21 days after the first immunization) and analyzed the expression of CD62L on NP-specific memory T cells 28 days later. Interestingly, we found that the secondary memory T-cell pool was comprised of 50% CD62Lhigh cells and 50% CD62Llow cells (Fig. 4A and B). Therefore, the second LT-NP immunization preferentially expanded the CD62Llow TEM population. Because the second immunization also boosted the frequency of NP-specific memory T cells (Fig. 3), the result was an approximately 80% increase in the total number of CD62Lhigh cells and a 250% increase in the total number of CD62Llow cells (Fig. 4C). These data demonstrate that the primary and secondary memory T-cell pools differed in phenotype as well as in frequency. Importantly, the TCM and TEM composition of an antigen-specific memory pool is not normally static. Instead, the proportion of CD62Lhigh cells often increases with time, while the proportion of CD62Llow cells decreases. Therefore, it is possible that the second LT-NP immunization did not directly stimulate more CD62Llow cells but instead slowed the conversion to a population of cells with high levels of CD62L.
Memory T cells induced by LT-NP have effector functions.
We wanted not only to enumerate NP-specific memory CD8+ T cells in mice immunized with LT-NP but also to directly assess their effector functions. Therefore, we immunized groups of mice with LT-NP either once or twice (with the second immunization either 9 or 21 days after the first immunization), harvested splenocytes 28 days later, and used flow cytometry to determine the percentage of CD8+ T cells that produced IFN-γ in response to the NP peptide. There were approximately twofold more IFN-γ-producing, NP-specific CD8+ T cells in the mice immunized twice, independent of the time of the second immunization (Fig. 5A). Therefore, the T cells stimulated by both the early and late booster immunizations were functional. When the primary and secondary memory T-cell populations were analyzed, it became clear that the percentage of NP-specific CD8+ T cells in the spleen (measured by tetramer staining) was roughly equivalent to the percentage of IFN-γ-producing CD8+ T cells in the spleen (compare Fig. 3 to 5A). In other words, essentially all of the NP-specific CD8+ T cells induced by LT-NP were capable of producing IFN-γ after antigen reencounter, regardless of whether they displayed a CD62Lhigh or CD62Llow phenotype.
An effector function of CD8+ T cells that is especially critical for protection against intracellular pathogens is the ability to lyse infected target cells. Although we have previously shown that the antigen-specific T cells stimulated by the LT-based system have cytotoxic activity after in vitro restimulation (6, 11), we wanted to more quantitatively measure their direct in vivo cytolytic potential. We were additionally interested in how cytotoxicity was affected by a booster immunization. Therefore, we immunized groups of mice either once or twice with LT-NP (with the second immunization 21 days after the first immunization), allowed them to rest for 28 days, and then injected NP peptide-pulsed syngeneic splenocytes and unpulsed syngeneic splenocytes at a ratio of 1:1 into the tail veins of all immunized mice, as well as into a group of unimmunized mice. Because the peptide-pulsed and unpulsed target cells were labeled with different concentrations of the fluorescent dye CFSE, we were able to use flow cytometry to measure any specific lysis of the peptide-pulsed cells 18 h later. On average, 12% of the peptide-pulsed target cells were lysed in mice immunized twice with LT-NP, which was three times the level observed in mice immunized once (Fig. 5B). Taken together, these data suggest that when LT-NP-induced memory T cells reencounter antigen, they produce IFN-γ and lyse target cells, and that the frequency of cells with these effector functions is amplified by a booster immunization.
Two LT-NP immunizations are more protective than one immunization against L. monocytogenes-NP challenge.
We next wanted to determine whether the elevated number of NP-specific memory T cells in mice immunized twice with LT-NP provided more protection against a challenge with an intracellular pathogen. To test this, we gave groups of mice either one or two LT-NP immunizations (with the second immunization 21 days after the first immunization). Twenty-eight days later we challenged both groups of mice, as well as a third group of naïve, unimmunized mice, with 0.73 LD50 of L. monocytogenes-NP. Three days after the challenge we determined the number of L. monocytogenes-NP organisms in the spleens and livers of all mice. As expected, there was a high L. monocytogenes-NP titer in the spleens of unimmunized mice (Fig. 6). In contrast, there were many fewer bacteria in the spleens of mice immunized either once or twice with LT-NP (Fig. 6). In fact, the mice immunized twice had almost 10-fold-fewer organisms than the unimmunized control mice and 2-fold-fewer organisms than the mice immunized once (Fig. 6). We observed a similar level of protection when we analyzed the titers in the livers (data not shown). These results suggest that a booster immunization with LT-NP stimulated a more protective CD8+ T-cell response than a single immunization, most likely by inducing a larger number of antigen-specific T cells with protective effector functions. Perhaps not surprisingly, the level of protection seen with the LT-NP system did not equal the level afforded by immunization with a live, replicating vaccine. Specifically, infection of mice with 3 × 106 PFU of recombinant vaccinia virus expressing NP provided approximately 3 logs of protection in the same L. monocytogenes-NP challenge model, most likely due to the extremely robust NP-specific CD8+ T-cell response induced by recombinant vaccinia virus expressing NP (data not shown).
FIG. 6.
LT-NP immunizations protect mice against L. monocytogenes-NP challenge. Groups of BALB/c mice were immunized with 30 pmol of LT-NP once or twice (21 days after the first immunization [D21]) or were not immunized. After 28 days all mice were injected i.v. with 0.73 LD50 of L. monocytogenes-NP. The number of bacteria per spleen was determined 72 h later. The lines indicate the mean numbers of CFU/spleen for the groups. The results are representative of three independent experiments. The asterisk indicates that the P value is <0.01 for a comparison with unimmunized mice.
DISCUSSION
Memory CD8+ T cells are important mediators of protection against pathogens, especially pathogens that reside inside host cells. The circumstances surrounding the initial priming events can greatly affect the properties of the resulting memory T cells and consequently the extent of protective immunity that they provide. Therefore, it is imperative to study and analyze CD8+ T cells stimulated by each antigen delivery system of interest. Here, we performed a detailed phenotypic and functional characterization of the endogenous, antigen-specific, memory CD8+ T cells stimulated by antigen fused to a nontoxic derivative of anthrax LT. We found that a single immunization with LT-NP stimulated NP-specific T-cell expansion and contraction, as well as rapid differentiation into CD127high TCM and TEM with protective effector functions that include the production of IFN-γ and in vivo lysis of target cells.
There has been much effort to identify a marker that can distinguish memory T-cell precursors from effector T cells during the acute phase of infection. CD127 expression has proven to be such a marker during infections with L. monocytogenes (16) and LCMV (22), among other organisms. At the peak of the T-cell response induced by these organisms, a small fraction of antigen-specific CD8+ T cells expresses high surface levels of CD127, and it is these cells that are selectively enriched during the contraction phase and persist into the memory phase (16, 22). Consequently, the percentage of CD127high cells at the peak of the pathogen-specific T-cell response is tightly correlated with the number of memory T cells remaining once contraction is complete. We did not find this to be true following immunization with LT-NP. Instead, the average percentage of CD127high NP-specific T cells at the peak of the response (63%) was much greater than the percentage of NP-specific T cells that survived the contraction phase (20 to 27%). Similar results have been observed following immunization with peptide-pulsed dendritic cells (5, 25). One theory is that the extent of CD127 downregulation on effector T cells is related to the extent of antigen-specific T-cell expansion (25). In other words, T cells from a large effector pool are more likely to be CD127low, whereas T cells from a smaller effector pool are more likely to be CD127high. In any case, our results add to the growing evidence that CD127 expression is not sufficient for a T cell to survive the contraction phase and suggest that factors in addition to IL-7 are important for long-term survival. Identification of these other factors should help increase our understanding of how cells avoid death signals and populate the memory pool.
We went on to demonstrate that a booster LT-NP immunization significantly increased the frequency of NP-specific memory CD8+ T cells. The ability to administer LT-NP multiple times and effectively boost the memory pool is beneficial in light of the fact that the frequency of antigen-specific memory T cells is often directly linked to the degree of protective immunity that they afford (39). A potential disadvantage with many prime-boost regimens is the relatively long time that is required between immunizations in order to induce robust secondary responses. For example, rechallenge during the effector phase of the L. monocytogenes-induced CD8+ T-cell response does not result in a substantial increase in the number of secondary memory T cells (5). However, we determined that by 9 days after LT-NP immunization the NP-specific CD8+ T-cell pool had undergone substantial contraction and was comprised largely of CD127high cells, suggesting that the LT-based antigen delivery system accelerates the rate of memory CD8+ T-cell development. Accordingly, a booster immunization administered at this early time point was as effective (as measured by the frequency of secondary memory T cells) as a booster immunization administered 21 days after the initial immunization. Rapid memory development and a corresponding ability for fast and effective boosting are desirable characteristics for vaccines, especially in circumstances where protective immunity must be acquired in a short time. Badovinac et al. demonstrated that CD8+ T cells stimulated by peptide-coated dendritic cells or L. monocytogenes coadministered with antibiotics also rapidly differentiated into T cells with phenotypic and functional memory properties and could be effectively boosted just days after the initial injection (4, 5). Together with our data, these results suggest that the rate of transition from effector T cells to memory T cells is not fixed but is influenced by the form in which the antigen is introduced.
One factor that has been shown to control the rate of memory cell generation is the inflammatory environment present during antigen encounter (19). Specifically, Badovinac et al. found that in cases of immunization with peptide-coated dendritic cells or L. monocytogenes plus antibiotics, the lack of overt inflammation, in particular IFN-γ, was responsible for accelerated memory generation (4, 5). In light of these findings, it will be interesting to investigate the role of inflammation in the development of LT-NP-induced memory T cells. In our system, the NP peptide antigen is conjugated to purified LFn protein and administered along with PA. Therefore, it is certainly possible that this immunization does not induce a robust inflammatory response. Interestingly, inflammation and IFN-γ also seem to influence the extent of CD8+ T-cell contraction (14). Here we demonstrated that the NP-specific effector T-cell pool induced by LT-NP contracted by at least 73%, which suggests that the immunization delivered an inflammatory signal sufficient to initiate substantial contraction. However, it is worth noting that the contraction that we observed in this system was not as extensive as the 90 to 95% contraction usually observed after infections with replicating organisms. Therefore, it is possible that the LT-based system delivers more modest inflammatory signals. In addition, we would not expect LFn-NP118-126 or PA proteins to persist for an extended period of time in mice. This may mean that NP-specific T cells are activated only very early after immunization, which could also contribute to accelerated memory development.
We also assessed the phenotypic and functional properties of the memory T cells stimulated by LT-NP. Using the expression of CD62L to distinguish between TCM (CD62Lhigh) and TEM (CD62Llow), we observed an approximately 3:2 ratio of TCM to TEM in the spleens of mice immunized 4 weeks previously with LT-NP. Notably, a booster LT-NP immunization not only increased the frequency of NP-specific memory T cells but also preferentially expanded the CD62Llow TEM population, or at least delayed its conversion to a CD62Lhigh TCM population. Maintenance of substantially larger numbers of CD62Llow cells after secondary or even tertiary immunizations has also been reported when viral and bacterial pathogens have been used in prime-boost combinations (20, 27). We further observed that essentially all NP-specific memory CD8+ T cells produced IFN-γ in response to the NP peptide, supporting the finding that production of this cytokine is independent of CD62L expression (3, 42). The NP-specific memory T cells stimulated by LT-NP also displayed in vivo cytotoxic activity. Interestingly, while there were approximately twice as many secondary memory cells as primary memory cells, the secondary memory cells were able to lyse about threefold more peptide-pulsed target cells. One possible explanation for this is that, on a per-cell basis, the secondary memory cells were more cytotoxic than the primary memory cells. Increased production of granzyme B and increased cytotoxicity of secondary TEM have been demonstrated with viral and bacterial prime-boost vaccination strategies (20, 27). The preferential increase in TEM after two LT-NP immunizations also may have contributed to the total increase in lytic capacity since TEM are more immediately cytotoxic than TCM (28, 43). In summary, these studies show that prime-boost immunizations affect not only the quantity but also the quality of memory T cells.
Recent reports have shown that the degree of protection provided by memory CD8+ T cells is a function not only of their absolute frequency but also of their phenotype (18). The contribution of TCM and TEM to protective immunity appears to depend on many factors, including the site of infection, the replication rate of the pathogen, and the infectious dose (3, 18). Here we show that mice immunized either once or twice with LT-NP were protected from an L. monocytogenes-NP challenge and that mice that received the booster immunization had fewer recoverable organisms in their spleens and livers than mice that received one immunization. However, it is not clear whether the added protection was a result of the total increase in NP-specific memory T cells or specifically the large increase in TEM, the subset of memory cells that have been shown to provide more protection against L. monocytogenes (17). The ability of the LT-NP system to stimulate a mixture of antigen-specific TCM and TEM suggests that this antigen delivery vehicle may be effective at stimulating protective immunity against a range of pathogens. In support of this theory, we previously showed that mice immunized with LT-NP were protected from both acute and persistent LCMV infections (11).
In conclusion, the LT-based antigen delivery system is an attractive vaccine candidate because it can stimulate IFN-γ-producing, cytotoxic, memory T cells that display both TCM and TEM phenotypes. Our finding that this system rapidly induces CD127high memory cells that can be effectively boosted as early as 9 days after the initial immunization also demonstrates its utility for quickly generating a large pool of antigen-specific memory T cells. Despite these potential advantages, the magnitude of the T-cell response elicited by LT-NP is clearly less than the magnitude of the response elicited by live, replicating vaccines. This highlights a potential drawback to using nonreplicating vaccines to stimulate cell-mediated immunity, that is, their difficulty in delivering sufficient antigen to induce robust T-cell responses. It is possible that inclusion of adjuvants or other inflammatory mediators along with the LT-based protein immunization may result in an amplified antigen-specific CD8+ T-cell response and a corresponding increase in protective immunity. It will also be interesting to directly compare the properties of the T cells induced by the LT-based system to the properties of the T cells induced by other nonreplicating vaccine systems, particularly other bacterial toxin-based antigen delivery systems, such as that based on the adenlyate cyclase toxin produced by Bordetella pertussis (41). The knowledge gained should increase our understanding of how the priming environment affects the generation of different types of memory T cells and should help us engineer vaccines to stimulate potent and protective cell-mediated immune responses.
Acknowledgments
This work was supported by National Institutes of Health grant AI055962 to M.N.S.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 17 December 2007.
REFERENCES
- 1.Abrami, L., S. Liu, P. Cosson, S. H. Leppla, and F. G. van der Goot. 2003. Anthrax toxin triggers endocytosis of its receptor via a lipid raft-mediated clathrin-dependent process. J. Cell Biol. 160321-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arora, N., and S. H. Leppla. 1993. Residues 1-254 of anthrax toxin lethal factor are sufficient to cause cellular uptake of fused polypeptides. J. Biol. Chem. 2683334-3341. [PubMed] [Google Scholar]
- 3.Bachmann, M. F., P. Wolint, K. Schwarz, P. Jager, and A. Oxenius. 2005. Functional properties and lineage relationship of CD8+ T cell subsets identified by expression of IL-7 receptor alpha and CD62L. J. Immunol. 1754686-4696. [DOI] [PubMed] [Google Scholar]
- 4.Badovinac, V. P., and J. T. Harty. 2007. Manipulating the rate of memory CD8+ T cell generation after acute infection. J. Immunol. 17953-63. [DOI] [PubMed] [Google Scholar]
- 5.Badovinac, V. P., K. A. Messingham, A. Jabbari, J. S. Haring, and J. T. Harty. 2005. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nat. Med. 11748-756. [DOI] [PubMed] [Google Scholar]
- 6.Ballard, J. D., R. J. Collier, and M. N. Starnbach. 1998. Anthrax toxin as a molecular tool for stimulation of cytotoxic T lymphocytes: disulfide-linked epitopes, multiple injections, and role of CD4+ cells. Infect. Immun. 664696-4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballard, J. D., R. J. Collier, and M. N. Starnbach. 1996. Anthrax toxin-mediated delivery of a cytotoxic T-cell epitope in vivo. Proc. Natl. Acad. Sci. USA 9312531-12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ballard, J. D., A. M. Doling, K. Beauregard, R. J. Collier, and M. N. Starnbach. 1998. Anthrax toxin-mediated delivery in vivo and in vitro of a cytotoxic T-lymphocyte epitope from ovalbumin. Infect. Immun. 66615-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blaustein, R. O., T. M. Koehler, R. J. Collier, and A. Finkelstein. 1989. Anthrax toxin: channel-forming activity of protective antigen in planar phospholipid bilayers. Proc. Natl. Acad. Sci. USA 862209-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradley, K. A., J. Mogridge, M. Mourez, R. J. Collier, and J. A. Young. 2001. Identification of the cellular receptor for anthrax toxin. Nature 414225-229. [DOI] [PubMed] [Google Scholar]
- 11.Doling, A. M., J. D. Ballard, H. Shen, K. M. Krishna, R. Ahmed, R. J. Collier, and M. N. Starnbach. 1999. Cytotoxic T-lymphocyte epitopes fused to anthrax toxin induce protective antiviral immunity. Infect. Immun. 673290-3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goletz, T. J., K. R. Klimpel, N. Arora, S. H. Leppla, J. M. Keith, and J. A. Berzofsky. 1997. Targeting HIV proteins to the major histocompatibility complex class I processing pathway with a novel gp120-anthrax toxin fusion protein. Proc. Natl. Acad. Sci. USA 9412059-12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guermonprez, P., J. Valladeau, L. Zitvogel, C. Thery, and S. Amigorena. 2002. Antigen presentation and T cell stimulation by dendritic cells. Annu. Rev. Immunol. 20621-667. [DOI] [PubMed] [Google Scholar]
- 14.Haring, J. S., V. P. Badovinac, and J. T. Harty. 2006. Inflaming the CD8+ T cell response. Immunity 2519-29. [DOI] [PubMed] [Google Scholar]
- 15.Harty, J. T., A. R. Tvinnereim, and D. W. White. 2000. CD8+ T cell effector mechanisms in resistance to infection. Annu. Rev. Immunol. 18275-308. [DOI] [PubMed] [Google Scholar]
- 16.Huster, K. M., V. Busch, M. Schiemann, K. Linkemann, K. M. Kerksiek, H. Wagner, and D. H. Busch. 2004. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc. Natl. Acad. Sci. USA 1015610-5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huster, K. M., M. Koffler, C. Stemberger, M. Schiemann, H. Wagner, and D. H. Busch. 2006. Unidirectional development of CD8+ central memory T cells into protective Listeria-specific effector memory T cells. Eur. J. Immunol. 361453-1464. [DOI] [PubMed] [Google Scholar]
- 18.Huster, K. M., C. Stemberger, and D. H. Busch. 2006. Protective immunity towards intracellular pathogens. Curr. Opin. Immunol. 18458-464. [DOI] [PubMed] [Google Scholar]
- 19.Jabbari, A., and J. T. Harty. 2006. The generation and modulation of antigen-specific memory CD8 T cell responses. J. Leukoc. Biol. 8016-23. [DOI] [PubMed] [Google Scholar]
- 20.Jabbari, A., and J. T. Harty. 2006. Secondary memory CD8+ T cells are more protective but slower to acquire a central-memory phenotype. J. Exp. Med. 203919-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaech, S. M., S. Hemby, E. Kersh, and R. Ahmed. 2002. Molecular and functional profiling of memory CD8 T cell differentiation. Cell 111837-851. [DOI] [PubMed] [Google Scholar]
- 22.Kaech, S. M., J. T. Tan, E. J. Wherry, B. T. Konieczny, C. D. Surh, and R. Ahmed. 2003. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 41191-1198. [DOI] [PubMed] [Google Scholar]
- 23.Kaech, S. M., E. J. Wherry, and R. Ahmed. 2002. Effector and memory T-cell differentiation: implications for vaccine development. Nat. Rev. Immunol. 2251-262. [DOI] [PubMed] [Google Scholar]
- 24.Koehler, T. M., and R. J. Collier. 1991. Anthrax toxin protective antigen: low-pH-induced hydrophobicity and channel formation in liposomes. Mol. Microbiol. 51501-1506. [DOI] [PubMed] [Google Scholar]
- 25.Lacombe, M. H., M. P. Hardy, J. Rooney, and N. Labrecque. 2005. IL-7 receptor expression levels do not identify CD8+ memory T lymphocyte precursors following peptide immunization. J. Immunol. 1754400-4407. [DOI] [PubMed] [Google Scholar]
- 26.Lu, Y., R. Friedman, N. Kushner, A. Doling, L. Thomas, N. Touzjian, M. Starnbach, and J. Lieberman. 2000. Genetically modified anthrax lethal toxin safely delivers whole HIV protein antigens into the cytosol to induce T cell immunity. Proc. Natl. Acad. Sci. USA 978027-8032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masopust, D., S. J. Ha, V. Vezys, and R. Ahmed. 2006. Stimulation history dictates memory CD8 T cell phenotype: implications for prime-boost vaccination. J. Immunol. 177831-839. [DOI] [PubMed] [Google Scholar]
- 28.Masopust, D., V. Vezys, A. L. Marzo, and L. Lefrancois. 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science 2912413-2417. [DOI] [PubMed] [Google Scholar]
- 29.McEvers, K., M. Elrefaei, P. Norris, S. Deeks, J. Martin, Y. Lu, and H. Cao. 2005. Modified anthrax fusion proteins deliver HIV antigens through MHC class I and II pathways. Vaccine 234128-4135. [DOI] [PubMed] [Google Scholar]
- 30.Milne, J. C., and R. J. Collier. 1993. pH-dependent permeabilization of the plasma membrane of mammalian cells by anthrax protective antigen. Mol. Microbiol. 10647-653. [DOI] [PubMed] [Google Scholar]
- 31.Milne, J. C., D. Furlong, P. C. Hanna, J. S. Wall, and R. J. Collier. 1994. Anthrax protective antigen forms oligomers during intoxication of mammalian cells. J. Biol. Chem. 26920607-20612. [PubMed] [Google Scholar]
- 32.Mogridge, J., K. Cunningham, and R. J. Collier. 2002. Stoichiometry of anthrax toxin complexes. Biochemistry 411079-1082. [DOI] [PubMed] [Google Scholar]
- 33.Mourez, M. 2004. Anthrax toxins. Rev. Physiol. Biochem. Pharmacol. 152135-164. [DOI] [PubMed] [Google Scholar]
- 34.Pamer, E., and P. Cresswell. 1998. Mechanisms of MHC class I-restricted antigen processing. Annu. Rev. Immunol. 16323-358. [DOI] [PubMed] [Google Scholar]
- 35.Sallusto, F., J. Geginat, and A. Lanzavecchia. 2004. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 22745-763. [DOI] [PubMed] [Google Scholar]
- 36.Sallusto, F., D. Lenig, R. Forster, M. Lipp, and A. Lanzavecchia. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401708-712. [DOI] [PubMed] [Google Scholar]
- 37.Schluns, K. S., and L. Lefrancois. 2003. Cytokine control of memory T-cell development and survival. Nat. Rev. Immunol. 3269-279. [DOI] [PubMed] [Google Scholar]
- 38.Scobie, H. M., G. J. Rainey, K. A. Bradley, and J. A. Young. 2003. Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor. Proc. Natl. Acad. Sci. USA 1005170-5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seder, R. A., and A. V. Hill. 2000. Vaccines against intracellular infections requiring cellular immunity. Nature 406793-798. [DOI] [PubMed] [Google Scholar]
- 40.Shen, H., J. F. Miller, X. Fan, D. Kolwyck, R. Ahmed, and J. T. Harty. 1998. Compartmentalization of bacterial antigens: differential effects on priming of CD8 T cells and protective immunity. Cell 92535-545. [DOI] [PubMed] [Google Scholar]
- 41.Simsova, M., P. Sebo, and C. Leclerc. 2004. The adenylate cyclase toxin from Bordetella pertussis—a novel promising vehicle for antigen delivery to dendritic cells. Int. J. Med. Microbiol. 293571-576. [DOI] [PubMed] [Google Scholar]
- 42.Wherry, E. J., V. Teichgraber, T. C. Becker, D. Masopust, S. M. Kaech, R. Antia, U. H. von Andrian, and R. Ahmed. 2003. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 4225-234. [DOI] [PubMed] [Google Scholar]
- 43.Wolint, P., M. R. Betts, R. A. Koup, and A. Oxenius. 2004. Immediate cytotoxicity but not degranulation distinguishes effector and memory subsets of CD8+ T cells. J. Exp. Med. 199925-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woodland, D. L. 2004. Jump-starting the immune system: prime-boosting comes of age. Trends Immunol. 2598-104. [DOI] [PubMed] [Google Scholar]