Abstract
Botulinum neurotoxins (BoNTs) are the most toxic proteins for humans and are classified as category A toxins. There are seven serotypes of BoNTs defined by the lack of cross-serotype toxin neutralization. Thus, an effective vaccine must neutralize each BoNT serotype. BoNTs are organized as dichain A-B toxins, where the N-terminal domain (light chain) is a zinc metalloprotease targeting soluble NSF attachment receptor proteins that is linked to the C-terminal domain (heavy chain [HC]) by a disulfide bond. The HC comprises a translocation domain and a C-terminal receptor binding domain (HCR). HCRs of the seven serotypes of BoNTs (hepta-HCR) were engineered for expression in Escherichia coli, and each HCR was purified from E. coli lysates. Immunization of mice with the E. coli-derived hepta-serotype HCR vaccine elicited an antibody response to each of the seven BoNT HCRs and neutralized challenge by 10,000 50% lethal doses of each of the seven BoNT serotypes. A solid-phase assay showed that the anti-hepta-serotype HCR sera inhibited the binding of HCR serotypes A and B to the ganglioside GT1b, the first step in BoNT intoxication of neurons. This is the first E. coli-derived vaccine that effectively neutralizes each of the seven BoNT serotypes.
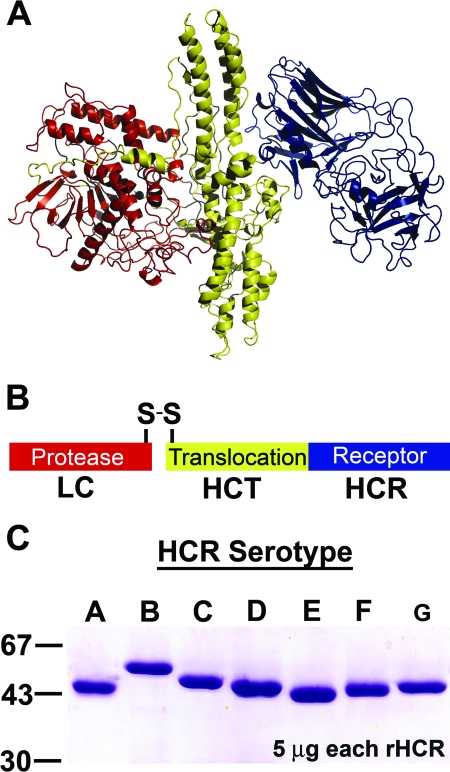
Clostridium botulinum neurotoxins (BoNTs) are the most potent protein toxins for humans and are category A toxins (9). There are seven serotypes of BoNTs, termed A to G (BoNT/A to BoNT/G), with serotypes A, B, E, and F being responsible for most natural human disease (1). BoNT serotypes are defined by antibody neutralization, where antibodies that neutralize BoNT/A do not neutralize BoNT serotypes B to G. BoNTs are dichain A-B toxins that are organized into three specific domains (Fig. 1A). The N-terminal zinc protease domain (light chain [LC]) is linked by a disulfide bond to the C-terminal domain (heavy chain [HC]). The HC is comprised of a translocation domain (HCT) and a C-terminal receptor-binding domain (HCR) (Fig. 1B) (25).
FIG. 1.
Structure-function properties of the BoNT. Ribbon (A) and line (B) diagrams of BoNT/A (Protein Data Bank entry no. 3BTA) are shown. BoNTs are organized into three domains: the N terminus, LC, encodes a zinc protease (red), and the C terminus, HC, encodes an HCT (yellow) and an HCR (blue). (C) Purification of HCR/A to HCR/G. Individual plasmids encoding HCR (serotypes A to G) were expressed as six-His-tag fusion proteins in E. coli. Proteins were purified by affinity- and size-exclusion chromatography. Five micrograms of each HCR was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The gel was stained with Coomassie blue and is shown. To the left are the migrations of three molecular size markers (kDa).
BoNT neurotoxicity is due to toxin affinity for neuron-specific receptors and substrate specificity of the protease activity toward either vesicle or target soluble NSF attachment receptors (26). The current model for BoNT binding to peripheral neurons was presented by Montecucco and colleagues (24). BoNTs bind neurons through coreceptor interactions that involve an initial binding to a lipid or a polysialoganglioside, which increases the local BoNT membrane concentration for subsequent binding to one or more protein receptor(s). The BoNT/B protein receptor was first identified by Kozaki et al., who showed that BoNT/B binds to lipid vesicles containing synaptotagmin II and the ganglioside GT1b (16). Chapman and coworkers (12) subsequently observed that the entry of BoNT/B into PC12 cells and isolated rat hemidiaphragms was activity dependent, which implicated a role for synaptic vesicle-associated proteins as BoNT receptors. The role of synaptotagmins I and II as protein receptors was confirmed by showing that luminal-domain peptides of synaptotagmins I and II competed with native synaptotagmin for binding to BoNT/B. Using a similar approach, the neuronal cell receptor for BoNT/A was determined to be a luminal-loop peptide of synaptic vesicle protein 2 (13, 22). Subsequent studies observed high-affinity binding of BoNT/A and BoNT/B to a neuronal synaptic vesicle protein complex, which included synaptic vesicle protein 2, synaptotagmin, synaptophysin, vesicle-associated membrane protein 2, and the vacuolar proton pump. This implicated a physiological role for neuronal protein complexes as high-affinity BoNT receptors (3).
A Clostridium-derived, penta-serotype BoNT (BoNT/ABCDE) toxoid vaccine was manufactured by Parke, Davis and Co. and subsequently by the Michigan Department of Public Health (14). The vaccine was prepared by mixing the five serotypes of BoNT with 0.6% formalin (BoNT toxoid) and then with aluminum phosphate as an adjuvant and thimerosal as a preservative. The vaccine from the latter source is currently used to immunize personnel at risk, but it is in limited supply (31). The protective epitopes of the BoNTs are located in the HCR (11, 27, 29), and HCRs have been used for vaccine development. Relative to the Clostridium-derived vaccine immunogens, recombinant HCRs (rHCRs) can be produced in large scale without neurotoxin contamination. Middlebrook and coworkers (11) described an Escherichia coli-derived expression system for HCR serotype A (HCR/A) and showed that the HCR/A produced in E. coli was an effective immunogen. Subsequent studies utilized rHCRs that were expressed in the yeast Pichia pastoris as a heterologous host (6, 7, 31). HCRs expressed in P. pastoris are immunogenic, induce protective immunity in mice, and represent a first generation of vaccine development. However, expression of HCRs in P. pastoris can be a challenge with respect to genetic manipulation and ease of purification (28). Recently, our laboratory and others have expressed HCR/A in E. coli at levels sufficient for vaccine development, addressing strain and culture conditions to optimize HCR expression (4, 34). This promoted the current study to produce a hepta-serotype HCR vaccine to prevent botulism.
MATERIALS AND METHODS
Materials.
Chemicals and molecular reagents were from Sigma-Aldrich and New England Biolabs, respectively, unless otherwise stated.
Construction of rHCR expression vectors.
DNA encoding the indicated HCRs (Table 1) was synthesized (EZBiolab, Inc., Westfield, IN) with optimal codon usage for expression in E. coli. These DNA fragments were ligated into a TA cloning vector (pGEM-T; Promega), and the sequence was verified. DNA encoding each HCR from pGEM-HCR was subcloned into a modified pET28a (Novagen) expression vector that contained unique KpnI and PstI sites. pET28-HCR was transformed into E. coli BL-21(DE3) (Stratagene) and used for protein expression.
TABLE 1.
Source of BoNT HCR used for protein production
| Source strain(s)a | Serotype | HCR (residues)b |
|---|---|---|
| ATCC 3502 | A | 870-1295 |
| Eklund 17B, ATCC 25765 | B | 857-1290 |
| Phage type C-Stockholm | C | 864-1290 |
| Phage d-16 phi (1873) | D | 861-1276 |
| Beluga | E | 843-1250 |
| Langeland NCTC 1028 | F | 862-1274 |
| 113/30 | G | 860-1297 |
HCRs were derived from C. botulinum, except for serotype G, which was derived from C. argentinense.
Residues referenced from the N terminus of each BoNT.
HCR expression in E. coli.
The procedure for expression and purification of the rHCRs is as follows: E. coli BL-21(DE3)(pET28-HCR) was grown overnight on LB agar plates with 50 μg per ml of kanamycin. Cells from one plate were inoculated into LB medium (0.4 liter) containing kanamycin and grown at 30°C at 250 rpm for 2 h to an optical density at 600 nm of ∼0.6, and then 0.25 mM of IPTG (isopropyl-β-d-thiogalactopyranoside) was added, followed by overnight culturing at 16°C. Cells (2.4 liters) were harvested and broken with a French press (two to three times) in 40 ml ice-cold buffer (20 mM Tris-HCl [pH 7.9], 0.5 M NaCl, 1 mM dithiothreitol, and 10 mM imidazole) containing an EDTA-free protease inhibitor cocktail (Sigma), 2.5 μg/ml DNase I, and 2.5 μg/ml RNase A. The lysate was clarified by centrifugation at 20,000 × g for 30 min at 4°C and passed through a 0.45-μm filter. The filtered lysate was subjected to Ni2+-nitrilotriacetic acid affinity chromatography (5-ml bed volume; Qiagen). The resin was washed with 40 ml of buffer, and the His-fusion protein was eluted with buffer containing 0.25 M imidazole. Peak fractions were pooled and subjected to gel filtration (Sephacryl S200 high-resolution filtration) (300 ml resin equilibrated at room temperature [RT] in 20 mM Tris-HCl [pH 7.9], 1 mM EDTA, 0.2 M NaCl, and 0.1% Triton X-100). Peak fractions of HCR were pooled and concentrated with a second passage over Ni2+-nitrilotriacetic acid resin. Purified HCRs were dialyzed overnight in 10 mM Tris-HCl (pH 7.9), 200 mM NaCl, and 40% (vol/vol) glycerol. The yields of the HCRs varied from ∼5 to 20 mg per batch culture.
Immunoreactivity and vaccine challenge with the rHCRs (ELISA).
Individual rHCRs (0.1 μg per well) were added in 100 μl of coating buffer (50 mM Na2CO3, pH 9.6) to enzyme-linked immunosorbent assay (ELISA) plates (Corning enzyme immunoassay/radioimmunoassay high-binding plate) and incubated overnight at 4°C. The plates were then washed four times with 200 μl of phosphate-buffered saline (PBS) and blocked for 1 h at 37°C with 200 μl per well 2% (wt/vol) bovine serum albumin (BSA) in a coating buffer. The plates were incubated for 1 h at 37°C with serial dilutions of sera (100 μl) from mice immunized with the hepta-serotype HCR vaccine in binding buffer (PBS, 1% [wt/vol] BSA). Controls included wells without antigen or primary antibody or wells with preimmune sera. Following a washing step, the plates were incubated for 1 h at 37°C with goat anti-mouse immunoglobulin G-horseradish peroxidase (IgG-HRP, 1:12,000; Pierce) in binding buffer. Plates were washed four times with 200 μl PBS and then incubated with 100 μl per well tetramethyl benzidine (TMB; Pierce Slow TMB) as the substrate. Reactions were terminated after 30 min with 100 μl of 0.1 M H2SO4, and absorbance was read at 450 nm.
Vaccine challenge.
Female ICR mice (18 to 22 g) were immunized intraperitoneally with a pool containing 1.0 μg of each HCR of serotypes A through G (hepta-serotype HCR vaccine) mixed with an equal volume of Alhydrogel as an adjuvant. The mice were vaccinated at 0, 14, 28, and 42 days. Ten days after the final boost, the mice were challenged with 1,000 50% lethal doses (LD50) and monitored for survival; after 3 days, survivors were challenged with 10,000 LD50 and monitored for 4 days for survival. In this experiment, five of five mice immunized with the adjuvant alone did not survive the challenge with 100 LD50 of BoNT/A. Homologous BoNT serotypes were used in the challenge, except for BoNT/E, for which the Alaska subtype was used to challenge mice immunized with the subtype Beluga. Potency of the BoNT serotypes (A to G) was verified with unimmunized mice. These experiments were approved by the animal care and use committee at the University of Wisconsin—Madison.
Solid-phase binding of BoNT HCRs to GT1b.
Porcine brain gangliosides (Avanti Polar Lipids) were dissolved in methanol and applied to high-affinity 96-well plates (Costar 9018; Corning) (0.1 μg mixed gangliosides in 100 μl/well). The solvent was evaporated at RT, and the wells were washed with PBS. Nonspecific binding sites were blocked by incubation at RT for 1 h in sodium carbonate buffer, pH 9.6, supplemented with 1% (wt/vol) BSA. Binding assays were performed in binding buffer (10 mM Tris-HCl, 150 mM NaCl, 1% [wt/vol] BSA, pH 7.6; 100 μl/well) for 1 h at RT containing wild-type or mutated HCR/A domains (150 nM final concentration) which had been preincubated for 30 min at 37°C with either preimmune mouse serum or sera from mice immunized with the hepta-serotype HCR vaccine. Unbound protein was removed by washing each well three times with 400 μl of PBS each time. Bound HCRs were detected by incubation with anti-FLAG M2 monoclonal antibody-HRP for 15 min at 4°C. TMB-Ultra served as the substrate for HRP. The reaction was terminated by the addition of 0.2 M H2SO4, and the absorbance at 450 nm was determined using an ELISA plate reader (Victor 3V, PerkinElmer). Controls included wells without antigen (GT1b) or primary antibody or with preimmune sera. The binding of GT1b to the plates was confirmed by probing coated wells with anti-GT1b antibody.
RESULTS
Expression and purification of the BoNT HCRs.
Synthetic genes encoding E. coli codon-optimized HCR/A to HCR/G were synthesized from the indicated sources of Clostridium (Table 1). The strain selection was based upon known serological properties and the ability to produce the BoNT from clostridia. Protein expression of each HCR serotype in E. coli BL-21(DE3) cells was optimized by titration of induction temperature and IPTG concentration. Purification protocols were developed for each serotype by adjusting the purification temperature and ionic strength of the buffers in the chromatography steps. While the overall expression levels of the seven serotypes of HCRs in E. coli were similar, differences in final yields were due primarily to the differential solubility of the HCRs when extracted from the cell lysates (>90% for HCR/A and HCR/D to ∼20% for HCR/B and HCR/C [data not shown]). The two-step purification, utilizing affinity chromatography and gel filtration, was sufficient to yield purifications of each HCR to >90% purity (Fig. 1C). The final yield of soluble HCRs ranged from ∼5 to 20 mg in batch culture.
rHCRs elicit an immune response in mice.
Mice were immunized with the hepta-serotype HCR vaccine in an aluminum hydroxide adjuvant (Alhydrogel) as the primary immunogen and then with the vaccine alone as a boost immunogen. Pooled antisera from mice immunized with the heptavalent HCR (serotypes A to G) vaccine demonstrated IgG titers to each of the individual HCRs based on ELISA (Fig. 2). HCR/C, HCR/D, HCR/E, and HCR/F elicited the strongest titer by ELISA, while HCR/B and HCR/A elicited an intermediate titer and HCR/G elicited the lowest titer. Preimmune sera did not show reactivity above that of a nonserum control at the lowest dilution used in the assay (1:50). Immunization with the hepta-serotype HCR vaccine did not elicit distress in the mice.
FIG. 2.
Immunoreactivity of mouse anti-hepta-serotype HCR vaccine in mice. An ELISA was performed, using 100 ng each of the individual rHCRs (serotypes A to G). The plates were incubated for 1 h at 37°C with serial dilutions of sera from mice immunized with the hepta-serotype HCR vaccine obtained prior to BoNT challenge or with preimmunization sera. The plates were then incubated for 1 h at 37°C with goat anti-mouse IgG-HRP (1:12,000; Pierce), washed with PBS, and incubated with TMB as the substrate. Reactions were terminated with 0.2 M H2SO4, and absorbance at 450 nm (A450) was read. The serum titer represents the inverse of the serum dilution used in the analysis. Data presented are representative of two independent immunization challenge experiments.
Hepta-serotype HCR vaccine protects against challenge by the seven serotypes of BoNT.
BoNT serotypes A to F were purified from C. botulinum, while BoNT/G was purified from Clostridium argentinense. The potency of each neurotoxin (serotypes A to G) was determined using the standard mouse bioassay in accordance with CDC protocols and varied from ∼1 × 107 to 10 × 107 mouse LD50 per mg of toxin. Mice immunized with the hepta-serotype HCR vaccine were resistant to challenge with each of the homologous BoNT serotypes (A to G) (Table 2) at either a 1,000-LD50 challenge or a 10,000-LD50 challenge. This is the first demonstration of a single vaccine protecting against the seven serotypes of BoNT.
TABLE 2.
Hepta-serotype HCR vaccine protects against challenge by the seven serotypes of BoNTa
| Serotype | No. of surviving mice/total no. challenged with serotype at:
|
|
|---|---|---|
| 1,000 LD50 | 10,000 LD50 | |
| A | 5/5 | 5/5 |
| B | 5/5 | 5/5 |
| C | 4/5 | 4/4 |
| D | 5/5 | 5/5 |
| E | 5/5 | 5/5 |
| F | 5/5 | 5/5 |
| G | 5/5 | 5/5 |
Mice were immunized as follows: day 0, 1 μg of each HCR (serotypes A to G) with Alhydrogel; day 14, 1 μg of each HCR with Alhydrogel; day 28, 1 μg of each HCR with Alhydrogel; day 42, 1 μg of each HCR alone. On day 52, mice were challenged individually with 1,000 LD50 of the indicated serotype of BoNT. After 3 days, survival was scored, and survivors were challenged with 10,000 LD50 of the indicated BoNT serotype and scored for survival at 4 days. Data presented are representative of several vaccine trial experiments performed with this immunogen. Control experiments showed that five of five mice vaccinated with the adjuvant alone did not survive challenge with 100 LD50 of BoNT/A.
Anti-hepta-serotype HCR antibody blocks HCR binding to ganglioside GT1b.
Montecucco and colleagues (24) proposed that BoNTs bind neurons through coreceptor interactions. Recent studies have implicated gangliosides as the coreceptor for the BoNTs, except for BoNT/D, which appears to utilize another class of small molecules as the coreceptor (35). To address the molecular basis for the neutralizing capacity of HCR immunization, a solid-phase assay was developed. This allowed the assessment of the ability of anti-hepta-serotype HCR antibody to block HCR binding to ganglioside GT1b.
Preliminary studies observed that among the seven serotypes, HCR/F showed the highest affinity for GT1b (data not shown), while BoNT/D did not show any significant binding under the conditions tested (data not shown). Further analysis of ganglioside binding was carried out using HCR/A and HCR/B due to the significant association of these serotypes with human disease and the availability of structural data (10, 15). Controls using anti-GT1b antibody confirmed the binding of GT1b to the ELISA plate and established conditions in which neither HCR/A nor HCR/B bound to non-GT1b-coated plates (data not shown). Preimmune mouse sera did not interfere with the binding of either HCR/A or HCR/B to GT1b, while the anti-hepta-serotype HCR sera showed dose-dependent inhibition of HCR/A and HCR/B binding to GT1b (Fig. 3). We previously reported the ability of HCR/A alone to generate a serotype-specific neutralizing antibody response (4). Sera from mice immunized with HCR/A alone inhibited the binding of HCR/A, but not HCR/B, to GT1b (data not shown). This indicates that anti-hepta-serotype HCR antibody neutralizes the first step in BoNT intoxication of neurons, the binding of BoNT to gangliosides.
FIG. 3.
Anti-hepta-serotype HCR blocks the binding of HCR/A and HCR/B to GT1b. Gangliosides (0.1 μg in 100 μl methanol) were added to individual wells of an ELISA and incubated overnight at RT. The plates were washed with PBS and blocked for 1 h at RT with 2% (wt/vol) BSA in binding buffer. The plates were then washed and incubated for 1 h at 37°C with the indicated HCR alone (▪), with preimmune sera (□), or with mouse anti-hepta-serotype HCR sera ( ) in 1% (wt/vol) BSA in Tris-buffered saline. Following a washing step, the plates were incubated with an anti-3 FLAG M2 monoclonal IgG-HRP conjugate (1:12,000) in binding buffer. The plates were washed and then incubated with TMB as the substrate. Reactions were terminated with 0.2 M H2SO4. Absorbance at 450 nm (A450) was read on a Victor 3V plate reader.
) in 1% (wt/vol) BSA in Tris-buffered saline. Following a washing step, the plates were incubated with an anti-3 FLAG M2 monoclonal IgG-HRP conjugate (1:12,000) in binding buffer. The plates were washed and then incubated with TMB as the substrate. Reactions were terminated with 0.2 M H2SO4. Absorbance at 450 nm (A450) was read on a Victor 3V plate reader.
DISCUSSION
BoNTs are one of the most useful proteins for therapeutic intervention of neurological diseases (30). However, BoNTs are also potential toxic agents for malicious application. Thus, there is a need for improved vaccines and therapies to prevent and neutralize intoxication. Middlebrook and coworkers (11) initially showed the utility of E. coli-derived HCR/A as an effective immunogen for protecting mice from BoNT intoxication. Recently, Popoff and coworkers (34) showed that the C terminus of the HCR was primarily responsible for eliciting a protective immune response against BoNT/A, while Lee et al. (18) showed that the C terminus of the HCR yielded an efficient vaccine against challenge by BoNT/C and BoNT/D. Smith and coworkers have developed a Pichia pastoris yeast expression system for HCR- derived vaccines (31). Subsequent studies showed that immunization of rhesus monkeys with HCR/B purified from P. pastoris yielded a protective response to aerosol challenge and that these animals had detectable neutralizing antibody titers 24 months postvaccination (5), which showed the feasibility of HCR vaccination in a nonhuman primate. BoNT HCs have also been used as immunogens in multiagent vaccines. Smith and coworkers (19) used a Venezuelan equine encephalitis virus replicon system to elicit an immune response in mice that protected against the Marburg virus, anthrax toxin, and BoNT. The current study extends these findings to show the feasibility of generating a multiserotype vaccine that protects against the seven serotypes of BoNT.
There are two approved therapies against botulism: human botulinum immune globulin (Big-IV), which is used to treat infant botulism in California (2), and horse-derived BoNT antitoxin. Current therapies against botulism are focused on antibody or small-molecule inhibitors of catalysis. Casadevall (8) proposed a strategy for stockpiling antibodies to protect against biological threats such as botulism. Botulism is treated with horse polyclonal antibodies that are associated with a high incidence of systemic reactions. Human monoclonal antibodies are being developed as a safer therapy (23), but these will inevitably be serotype specific and thus a long-term project to produce monoclonal antibodies against each serotype. In addition, subserotype neutralization may also have to be considered in monoclonal antibody therapy. Identifying neutralizing epitopes on BoNTs is also an important step in generating neutralizing monoclonal antibodies and has implications for vaccine development. The development of a hepta-serotype BoNT HCR vaccine provides a mechanism for producing a polyclonal response that will have broad serotype neutralization against BoNTs.
While each HCR serotype elicited a detectable immune response by ELISA, the titer did not correlate with neutralization capacity. This is similar to a recent report by Takeda et al. (33), who observed that serum ELISA titers did not correspond with the neutralization titers of immunized ducks. The study concluded that the neutralizing titer was more accurate than the ELISA titer for establishing levels of BoNT protection. In contrast, Lee et al. (20) reported that the level of protection against BoNT intoxication elicited by immunization with HCR/A in a Venezuelan equine encephalitis virus replicon vector directly correlated with serum ELISA titers against BoNT/A. In addition, using a high-level BoNT/A challenge, Smith and coworkers (7) observed a correlation between the number of doses of vaccine with serum neutralization titers and ELISA titers, while Steinman et al. (32) reported a correlation between protection status and ELISA titers with a BoNT/D challenge. At this stage, there is a need to establish the molecular basis for protection to allow a more accurate interpretation of the neutralization versus ELISA titer data.
While the HCR appears to be the primary domain to elicit a neutralizing immune response against BoNT intoxication, there is limited information on the molecular mechanism for BoNT neutralization by BoNT antisera. Recently, a monoclonal antibody that was specific for the translocation domain of BoNT/B was demonstrated to neutralize the toxin (36). This observation, in conjunction with the data presented in the current study, suggests that numerous steps in the intoxication process can act as targets for neutralization. Levy et al. (21) utilized yeast display of the LCs, HCTs, and HCRs to map the binding sites of neutralizing BoNT/A monoclonal antibodies and reported monoclonal antibodies that bound to the C terminus of the HC, including one neutralizing monoclonal antibody that bound near the ganglioside binding site. In the current study, the neutralizing, anti-hepta-serotype HCR sera blocked the binding of HCR/A and HCR/B to ganglioside GT1b. In addition, Kubota et al. (17) reported the identification of a monoclonal antibody that blocked BoNT/E binding to cells that localized to the C terminus of the toxin. The current study is the first functional determination of how neutralizing antibodies block BoNT intoxication by interfering with ganglioside binding, the initial step in the BoNT intoxication process. Future studies will address whether or not subsequent steps in the intoxication process are neutralized by BoNT-neutralizing antibodies.
The immunogenic potencies of E. coli-derived rHCRs observed in the current study represent tools that allow genetic manipulation to develop the next generation of vaccines and immune therapies against botulism and also represent a malleable platform for responding to the malicious use of these toxins.
Acknowledgments
This work was sponsored by the NIH/NIAID Regional Center of Excellence for Biodefense and Emerging Infectious Diseases Research (RCE) Program. We acknowledge membership within and support from the Region V “Great Lakes” RCE (NIH award 1-U54-AI-057153). M.R.B. is supported by NIH/NINDS grant 1-K99-NS061763.
Editor: S. R. Blanke
Footnotes
Published ahead of print on 10 December 2007.
REFERENCES
- 1.Arnon, S. S., R. Schechter, T. V. Inglesby, D. A. Henderson, J. G. Bartlett, M. S. Ascher, E. Eitzen, A. D. Fine, J. Hauer, M. Layton, S. Lillibridge, M. T. Osterholm, T. O'Toole, G. Parker, T. M. Perl, P. K. Russell, D. L. Swerdlow, and K. Tonat. 2001. Botulinum toxin as a biological weapon: medical and public health management. JAMA 2851059-1070. [DOI] [PubMed] [Google Scholar]
- 2.Arnon, S. S., R. Schechter, S. E. Maslanka, N. P. Jewell, and C. L. Hatheway. 2006. Human botulism immune globulin for the treatment of infant botulism. N. Engl. J. Med. 354462-471. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin, M. R., and J. T. Barbieri. 2007. Association of botulinum neurotoxin serotypes A and B with synaptic vesicle protein complexes. Biochemistry 463200-3210. [DOI] [PubMed] [Google Scholar]
- 4.Baldwin, M. R., W. H. Tepp, C. L. Pier, M. Bradshaw, M. Ho, B. A. Wilson, R. B. Fritz, E. A. Johnson, and J. T. Barbieri. 2005. Characterization of the antibody response to the receptor binding domain of botulinum neurotoxin serotypes A and E. Infect. Immun. 736998-7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boles, J., M. West, V. Montgomery, R. Tammariello, M. L. Pitt, P. Gibbs, L. Smith, and R. D. LeClaire. 2006. Recombinant C fragment of botulinum neurotoxin B serotype (rBoNTB (HC)) immune response and protection in the rhesus monkey. Toxicon 47877-884. [DOI] [PubMed] [Google Scholar]
- 6.Byrne, M. P., and L. A. Smith. 2000. Development of vaccines for prevention of botulism. Biochimie 82955-966. [DOI] [PubMed] [Google Scholar]
- 7.Byrne, M. P., T. J. Smith, V. A. Montgomery, and L. A. Smith. 1998. Purification, potency, and efficacy of the botulinum neurotoxin type A binding domain from Pichia pastoris as a recombinant vaccine candidate. Infect. Immun. 664817-4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casadevall, A. 2002. Passive antibody administration (immediate immunity) as a specific defense against biological weapons. Emerg. Infect. Dis. 8833-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.CDC. 1998. Laboratory registration and select agent transfer tracking system. 42 CFR 72. Appendix A: CDC guidance and information on microorganisms, A-1-A-45. U.S. Department of Health and Human Services, Washington, DC.
- 10.Chai, Q., J. W. Arndt, M. Dong, W. H. Tepp, E. A. Johnson, E. R. Chapman, and R. C. Stevens. 2006. Structural basis of cell surface receptor recognition by botulinum neurotoxin B. Nature 4441096-1100. [DOI] [PubMed] [Google Scholar]
- 11.Clayton, M. A., J. M. Clayton, D. R. Brown, and J. L. Middlebrook. 1995. Protective vaccination with a recombinant fragment of Clostridium botulinum neurotoxin serotype A expressed from a synthetic gene in Escherichia coli. Infect. Immun. 632738-2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong, M., D. A. Richards, M. C. Goodnough, W. H. Tepp, E. A. Johnson, and E. R. Chapman. 2003. Synaptotagmins I and II mediate entry of botulinum neurotoxin B into cells. J. Cell Biol. 1621293-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong, M., F. Yeh, W. H. Tepp, C. Dean, E. A. Johnson, R. Janz, and E. R. Chapman. 2006. SV2 is the protein receptor for botulinum neurotoxin A. Science 312592-596. [DOI] [PubMed] [Google Scholar]
- 14.Fiock, M. A., M. A. Cardella, and N. F. Gearinger. 1963. Studies on immunity to toxins of Clostridium botulinum. IX. Immunologic response of man to purified pentavalent ABCDE botulinum toxoid. J. Immunol. 90697-702. [PubMed] [Google Scholar]
- 15.Jin, R., A. Rummel, T. Binz, and A. T. Brunger. 2006. Botulinum neurotoxin B recognizes its protein receptor with high affinity and specificity. Nature 4441092-1095. [DOI] [PubMed] [Google Scholar]
- 16.Kozaki, S., Y. Kamata, S. Watarai, T. Nishiki, and S. Mochida. 1998. Ganglioside GT1b as a complementary receptor component for Clostridium botulinum neurotoxins. Microb. Pathog. 2591-99. [DOI] [PubMed] [Google Scholar]
- 17.Kubota, T., T. Watanabe, N. Yokosawa, K. Tsuzuki, T. Indoh, K. Moriishi, K. Sanda, Y. Maki, K. Inoue, and N. Fujii. 1997. Epitope regions in the heavy chain of Clostridium botulinum type E neurotoxin recognized by monoclonal antibodies. Appl. Environ. Microbiol. 631214-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee, J. C., H. J. Hwang, Y. Sakaguchi, Y. Yamamoto, H. Arimitsu, T. Tsuji, T. Watanabe, T. Ohyama, T. Tsuchiya, and K. Oguma. 2007. C terminal half fragment (50 kDa) of heavy chain components of Clostridium botulinum type C and D neurotoxins can be used as an effective vaccine. Microbiol. Immunol. 51445-455. [DOI] [PubMed] [Google Scholar]
- 19.Lee, J. S., J. L. Groebner, A. G. Hadjipanayis, D. L. Negley, A. L. Schmaljohn, S. L. Welkos, L. A. Smith, and J. F. Smith. 2006. Multiagent vaccines vectored by Venezuelan equine encephalitis virus replicon elicits immune responses to Marburg virus and protection against anthrax and botulinum neurotoxin in mice. Vaccine 246886-6892. [DOI] [PubMed] [Google Scholar]
- 20.Lee, J. S., P. Pushko, M. D. Parker, M. T. Dertzbaugh, L. A. Smith, and J. F. Smith. 2001. Candidate vaccine against botulinum neurotoxin serotype A derived from a Venezuelan equine encephalitis virus vector system. Infect. Immun. 695709-5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levy, R., C. M. Forsyth, S. L. LaPorte, I. N. Geren, L. A. Smith, and J. D. Marks. 2007. Fine and domain-level epitope mapping of botulinum neurotoxin type A neutralizing antibodies by yeast surface display. J. Mol. Biol. 365196-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahrhold, S., A. Rummel, H. Bigalke, B. Davletov, and T. Binz. 2006. The synaptic vesicle protein 2C mediates the uptake of botulinum neurotoxin A into phrenic nerves. FEBS Lett. 5802011-2014. [DOI] [PubMed] [Google Scholar]
- 23.Marks, J. D. 2004. Deciphering antibody properties that lead to potent botulinum neurotoxin neutralization. Mov. Disord. 19(Suppl. 8)S101-S108. [DOI] [PubMed] [Google Scholar]
- 24.Montecucco, C., O. Rossetto, and G. Schiavo. 2004. Presynaptic receptor arrays for clostridial neurotoxins. Trends Microbiol. 12442-446. [DOI] [PubMed] [Google Scholar]
- 25.Montecucco, C., and G. Schiavo. 1995. Structure and function of tetanus and botulinum neurotoxins. Q. Rev. Biophys. 28423-472. [DOI] [PubMed] [Google Scholar]
- 26.Montecucco, C., and G. Schiavo. 1993. Tetanus and botulism neurotoxins: a new group of zinc proteases. Trends Biochem. Sci. 18324-327. [DOI] [PubMed] [Google Scholar]
- 27.Nowakowski, A., C. Wang, D. B. Powers, P. Amersdorfer, T. J. Smith, V. A. Montgomery, R. Sheridan, R. Blake, L. A. Smith, and J. D. Marks. 2002. Potent neutralization of botulinum neurotoxin by recombinant oligoclonal antibody. Proc. Natl. Acad. Sci. USA 9911346-11350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Potter, K. J., W. Zhang, L. A. Smith, and M. M. Meagher. 2000. Production and purification of the heavy chain fragment C of botulinum neurotoxin, serotype A, expressed in the methylotrophic yeast Pichia pastoris. Protein Expr. Purif. 19393-402. [DOI] [PubMed] [Google Scholar]
- 29.Ravichandran, E., F. H. Al-Saleem, D. M. Ancharski, M. D. Elias, A. K. Singh, M. Shamim, Y. Gong, and L. L. Simpson. 2007. Trivalent vaccine against botulinum toxin serotypes A, B, and E that can be administered by the mucosal route. Infect. Immun. 753043-3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scott, A. B. 2004. Development of botulinum toxin therapy. Dermatol. Clin. 22131-133. [DOI] [PubMed] [Google Scholar]
- 31.Smith, L. A., M. J. Jensen, V. A. Montgomery, D. R. Brown, S. A. Ahmed, and T. J. Smith. 2004. Roads from vaccines to therapies. Mov. Disord. 19(Suppl. 8)S48-S52. [DOI] [PubMed] [Google Scholar]
- 32.Steinman, A., M. Chaffer, D. Elad, and N. Y. Shpigel. 2006. Quantitative analysis of levels of serum immunoglobulin G against botulinum neurotoxin type D and association with protection in natural outbreaks of cattle botulism. Clin. Vaccine Immunol. 13862-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takeda, M., H. Kasai, Y. Torii, M. Mukamoto, T. Kohda, K. Tsukamoto, and S. Kozaki. 2006. Protective effect of botulinum C/D mosaic toxoid against avian botulism. J. Vet. Med. Sci. 68325-330. [DOI] [PubMed] [Google Scholar]
- 34.Tavallaie, M., A. Chenal, D. Gillet, Y. Pereira, M. Manich, M. Gibert, S. Raffestin, M. R. Popoff, and J. C. Marvaud. 2004. Interaction between the two subdomains of the C-terminal part of the botulinum neurotoxin A is essential for the generation of protective antibodies. FEBS Lett. 572299-306. [DOI] [PubMed] [Google Scholar]
- 35.Tsukamoto, K., T. Kohda, M. Mukamoto, K. Takeuchi, H. Ihara, M. Saito, and S. Kozaki. 2005. Binding of Clostridium botulinum type C and D neurotoxins to ganglioside and phospholipid. Novel insights into the receptor for clostridial neurotoxins. J. Biol. Chem. 28035164-35171. [DOI] [PubMed] [Google Scholar]
- 36.Yang, G. H., K. S. Kim, H. W. Kim, S. T. Jeong, G. H. Huh, J. C. Kim, and H. H. Jung. 2004. Isolation and characterization of a neutralizing antibody specific to internalization domain of Clostridium botulinum neurotoxin type B. Toxicon 4419-25. [DOI] [PubMed] [Google Scholar]