Abstract
The irradiated-sporozoite vaccine elicits sterile immunity against Plasmodium parasites in experimental rodent hosts and human volunteers. Based on rodent malaria models, it has been proposed that CD8+ T cells are the key protective effector mechanism required in sporozoite-induced immunity. To investigate the role of class II-restricted immunity in protective immunity, we immunized β2-microglobulin knockout (β2M−/−) mice with irradiated Plasmodium yoelii or P. berghei sporozoites. Sterile immunity was obtained in the CD8+-T-cell-deficient mice immunized with either P. berghei or P. yoelii sporozoites. β2M−/− mice with the BALB/c (H-2d) genetic background as well as those with the C57BL (H-2b) genetic background were protected. Effector mechanisms included CD4+ T cells, mediated in part through the production of gamma interferon, and neutralizing antibodies that targeted the extracellular sporozoites. We conclude that in the absence of class I-restricted CD8+ T cells, sporozoite-induced protective immunity can be effectively mediated by class II-restricted immune effector mechanisms. These results support efforts to develop subunit vaccines that effectively elicit high levels of antibody and CD4+ T cells to target Plasmodium preerythrocytic stages.
The irradiated sporozoite remains the “gold standard” for the development of malaria vaccines that target the preerythrocytic stages of Plasmodium parasites. Early studies demonstrated that experimental rodent hosts and human volunteers immunized with irradiated sporozoites developed antibodies that neutralized sporozoite infectivity (6, 34, 35, 37, 57). Recent studies have shown that these antibodies inhibit sporozoite motility, which is required for sporozoite entry into the circulation, migration to the liver, and invasion of host hepatic cells (47, 49, 52).
In addition to antibody, gamma interferon (IFN-γ) secreted by either CD8+ or Th1-type CD4+ T cells can block the development of intracellular hepatic-stage parasites by stimulating the upregulation of inducible nitric oxide synthase and the production of NO by the infected hepatocytes (14, 20, 44). In the murine malaria model, CD8+ T cells have been hypothesized to be essential for protection against sporozoite challenge following immunization with sporozoites or with subunit vaccines based on DNA and recombinant viral vectors (10, 55). In a previous study (56), β2-microglobulin knockout (β2M−/−) mice, which lack CD8+ T cells, were not protected following immunization with Plasmodium berghei sporozoites, leading to the conclusion that CD8+ T cells are essential for protective immunity and that redundant immune mechanisms are not elicited by attenuated sporozoites. These findings have led to significant effort in recent vaccine trials to elicit high levels of CD8+ T cells specific for circumsporozoite (CS) protein and other preerythrocytic-stage antigens (11, 15, 29).
In other infectious disease models, it has been shown that in the absence of CD8+ T cells, CD4+ T cells can mediate protective immunity (12, 13, 32). Moreover, malaria peptide subunit vaccines have been shown to effectively elicit CD4+-T-cell-mediated protective immunity against sporozoite challenge in the absence of CD8+-T-cell responses (5, 8, 28, 39, 53). Consistent with the results of these studies, we demonstrated that β2M−/− mice immunized with irradiated Plasmodium sporozoites could develop sterile immunity in the absence of CD8+ T cells, indicating that immune resistance can be mediated solely by class II-restricted effector mechanisms.
MATERIALS AND METHODS
Sporozoite immunization.
β2M−/− mice and wild-type (WT) controls were purchased from Jackson Labs, Bar Harbor, ME (21). The experiments utilized mice with the C57BL background, except for a limited number of experiments using β2M−/− mice with the BALB/c background. Mice were immunized at 2- to 3-week intervals by three to four intravenous (i.v.) injections of 104 to 105 P. berghei (ANKA 65) or P. yoeli (17XNL) irradiated sporozoites. Hyperimmunized mice were challenged by i.v. injection with 2,500 P. berghei or 200 P. yoelii sporozoites dissected from the salivary glands of infected Anopheles stephensi mosquitoes. The different challenge inocula reflect the differences in infectivity of the rodent malaria parasite species (2, 19).
Protective immunity.
Sterile immunity was assayed by Giemsa-stained blood smears obtained on days 3 to 14 post-sporozoite challenge. Mice that failed to develop patent blood-stage infection during this period of time were considered to have developed sterile immunity.
To measure the hepatic-stage parasite burden, naïve or immunized mice were injected i.v. with 0.2 × 105 to 5 × 105 viable sporozoites and livers were obtained 40 to 42 h postchallenge. Total RNA was extracted, and 1 μg was reverse transcribed using species-specific primers for P. yoelii or P. berghei 18S rRNA as previously described (2, 3). Amounts of parasite rRNA were quantified by competitive (2, 27) or real-time (3) PCR. Results are expressed as the numbers of rRNA copies determined based on an rRNA plasmid standard or the percent reduction of rRNA in livers of immunized mice versus those of naïve controls. Statistical analysis was carried out using Student's t test.
Cellular assays.
The role of cell-mediated immunity in the protection of sporozoite-immunized β2M−/− mice was determined by the depletion of CD4+ T cells prior to sporozoite challenge. Immunized mice were injected with three doses of 300 μg of anti-CD4 monoclonal antibody (MAb; GK 1.5) or anti-CD8 MAb (2.43) as a control, starting 3 days prior to challenge with viable sporozoites. The depletion of CD4+ T cells was confirmed by fluorescence-activated cell sorter (FACS) analysis to be <1% of those in untreated mice. To deplete NK cells, mice were treated with a single injection of anti-asialoglyprotein 1 antiserum (Wako Chemicals, Richmond, VA) 1 day prior to challenge (9). IFN-γ was depleted by i.v injection of 1 mg of anti-IFN-γ MAb (DB-1) on days 0 and 1 post-sporozoite challenge (45). Results of cell or cytokine depletion were determined by measuring parasitemia in Giemsa-stained blood smears or by assaying parasite levels in the liver by real-time PCR.
Serological assays.
Antibody levels were determined by an enzyme-linked immunosorbent assay using P. yoelii recombinant CS protein or by an indirect immunofluorescence assay using air-dried sporozoites. Antibody function was evaluated by an in vivo or in vitro sporozoite neutralization assay (SNA) (17, 34). P. yoelii sporozoites were incubated for 45 min with preimmune or immune sera obtained from P. yoelii sporozoite-immunized β2M−/− or WT mice, the incubated sporozoites were injected into naïve BALB/c mice, and levels of liver-stage parasites at 42 h postinjection were determined by reverse transcriptase PCR (RT-PCR) (3).
For the in vitro SNA, 2 × 104 P. berghei sporozoites were preincubated on ice for 40 min with immune sera obtained from β2M−/− mice at various time points after immunization with irradiated P. berghei sporozoites. The preincubated sporozoites were added to confluent cultures of human HepG2 cells (16), and the development of intracellular hepatic-stage parasites was assayed at 40 h by real-time PCR (22).
RESULTS
Irradiated sporozoites elicit sterile immunity in the absence of CD8+ T cells.
In four separate experiments, 75 to 100% of β2M−/− mice primed with 105 irradiated P. yoelii sporozoites and given booster immunizations with three injections of 104 irradiated sporozoites developed sterile immunity against challenge with viable P. yoelii sporozoites (Table 1). No blood-stage parasites were detectable in Giemsa-stained blood smears obtained from the protected immunized β2M−/− mice. The single β2M−/− P. yoelii-immunized mouse that became infected had a delayed prepatent period of 5.0 days, compared to 4.2 days for naïve β2M−/− mice.
TABLE 1.
Immunization with irradiated sporozoites elicits sterile immunity in β2M−/− mice
| Mouse strain | Immunization inoculuma | No. protected/total no. of miceb | % Protected | Prepatent period (days) |
|---|---|---|---|---|
| β2M−/− | Irradiated P. yoelii sporozoites | 20/21 | 94 | 5.0 |
| None | 0/16 | 0 | 4.2 | |
| WT | Irradiated P. yoelii sporozoites | 9/9 | 100 | |
| None | 0/9 | 0 | 4.4 | |
| β2M−/− | Irradiated P. berghei sporozoites | 11/12 | 92 | 6.0 |
| None | 0/12 | 0 | 4.8 |
Pooled results from four independent P. yoelii experiments and two independent P. berghei experiments are shown.
Mice were challenged i.v. with 200 P. yoelii sporozoites or 2,500 P. berghei sporozoites, and patent parasitemia was determined by Giemsa staining of blood smears.
The level of sterile immunity in the P. yoelii-immunized β2M−/− mice was comparable to the level of protection obtained in WT mice with CD8+ T cells (Table 1). All of the WT mice immunized with P. yoelii sporozoites were protected against challenge. The prepatent periods in naïve WT and β2M−/− mice were similar (4.4 and 4.2 days, respectively), suggesting that nonspecific resistance to sporozoites was not increased in the β2M−/− mice (9, 42).
Irradiated P. berghei sporozoites also elicited sterile immunity in the β2M−/− mice (Table 1). In two independent experiments, 75% (three of four) and 100% (eight of eight) of P. berghei sporozoite-immunized β2M−/− mice were protected against challenge with viable P. berghei sporozoites. A delayed prepatent period of 6.0 days in the single P. berghei-immunized infected β2M−/− mouse was observed, compared to 4.8 days in the naïve β2M−/− controls.
Reduction of liver parasite burden in sporozoite-immunized β2M−/− mice.
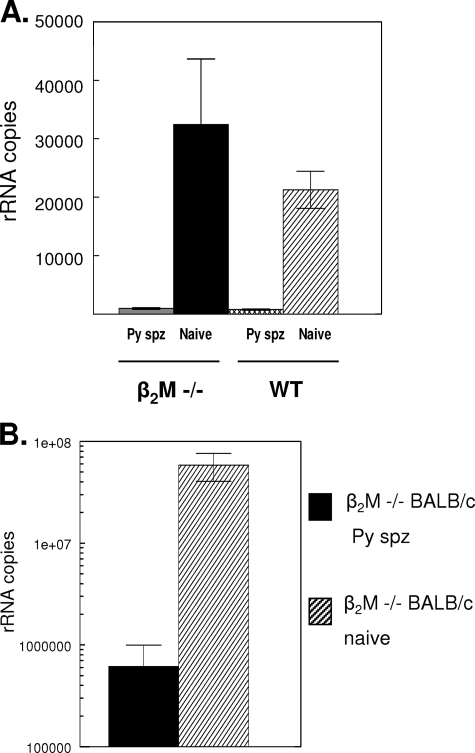
Protection in the sporozoite-immunized β2M−/− mice was also reflected in the reduction of hepatic-stage parasites. Following P. yoelii sporozoite challenge, levels of parasite rRNA in the livers of P. yoelii-immunized β2M−/− mice were reduced 97% compared to those in the livers of nonimmune β2M−/− mice (Fig. 1A). The level of inhibition in the β2M−/− mice was comparable to that observed in sporozoite-immunized WT mice with normal levels of CD8+ T cells, in which the total level of parasite rRNA was reduced 96% compared to that in naïve WT controls.
FIG. 1.
Immunization with irradiated P. yoelii sporozoites reduces the liver-stage parasite burden in β2M−/− mice. (A) Immune (P. yoelii sporozoite-immunized [Py spz]) or naïve β2M−/− mice with a C57BL background and WT controls were challenged with 5 × 105 viable P. yoelii sporozoites, and levels of parasite 18S rRNA in the livers were determined 40 h postchallenge by a PCR competition assay (2). (B) Immune or naïve β2M−/− mice with a BALB/c background were challenged with 2 × 104 P. yoelii sporozoites, and levels of liver-stage parasites were measured by RT-PCR (3). Results are shown as the mean numbers of parasite 18S rRNA copies ± the standard deviations (SD).
The β2M−/− mice were originally derived on the B6/129 background (21), and both the C57BL (H-2b) and 129 (H-2a) murine strains are less dependent than the B6/129 strain on CD8+-T-cell-mediated immune mechanisms following sporozoite immunization (10). Since CD8+ T cells are suggested to be the primary effector mechanism in H-2d strains (10, 41, 43, 55), we immunized β2M−/− BALB/c mice with P. yoelii sporozoites to examine the role of the genetic background on class II-mediated immune resistance.
CD8+-T-cell-independent sporozoite-induced immunity was not restricted by the genetic background. Levels of parasite rRNA in the livers of β2M−/− BALB/c mice challenged after three immunizations with irradiated P. yoelii sporozoites were reduced 99% compared to those in controls (Fig. 1B). In the five immunized β2M−/− BALB/c mice, mean rRNA copy numbers were reduced 2 logs compared to those in unimmunized β2M−/− BALB/c controls, with 0.0614 × 107 rRNA copies compared to 5.93 × 107 rRNA copies, respectively. Therefore, even in strains in which the CD8+ T cells are believed to be the primary effector mechanism, significant protective immunity was elicited in the absence of CD8+ T cells.
CD4+ T cells elicited by sporozoite immunization protect β2M−/− mice.
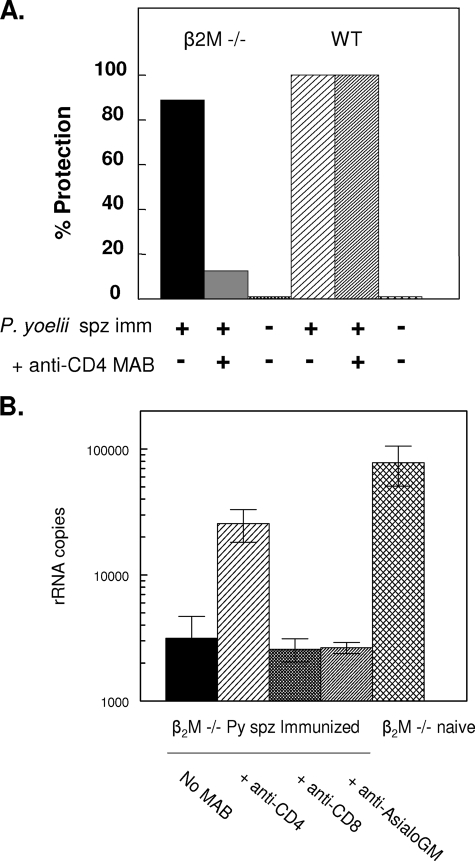
To examine the role of CD4+ T cells in protective immunity, P. yoelii-immunized β2M−/− mice with the C57BL background were depleted of CD4+ T cells prior to challenge. The appearance of patent blood-stage infection following the in vivo depletion of CD4+ T cells demonstrated that CD4+ T cells were required for the protection of the sporozoite-immunized β2M−/− mice (Fig. 2A). Only 12.5% of the immunized β2M−/− mice treated with anti-CD4 were protected against challenge. In contrast, protection was obtained in 89% (eight of nine) of the immunized β2M−/− mice not depleted of CD4+ T cells.
FIG. 2.
The depletion of CD4+ T cells inhibits immune protection in β2M−/− mice. P. yoelii (Py) sporozoite-immunized (spz imm) β2M−/− mice, with or without depleted T cells, were challenged. Protection was determined by measuring (A) parasitemia in daily blood smears or (B) levels of liver-stage parasites by RT-PCR. Pooled results from two independent experiments are shown (n, 8 to 9 mice total). Following treatment with anti-CD4 MAb GK 1.5, FACS analysis confirmed the depletion of CD4+ T cells, with 0.22% CD4+ T cells in treated versus 26% in untreated β2M−/− mice and 0.09% versus 21.7% in treated versus untreated WT mice, respectively. CD8+-T-cell levels in β2M−/− mice were 0.41%, versus 19.8% in WT mice. Anti-AsialoGM, anti-asialoglyprotein 1 antiserum.
Consistent with the loss of sterile immunity, levels of parasite rRNA in livers of β2M−/− mice depleted of CD4+ T cells prior to challenge were increased compared to those in mice with normal levels of CD4+ T cells (Fig. 2B). NK1.1-positive cells did not play a role in immune resistance, as immunized β2M−/− mice treated with anti-asialoglyprotein 1 antiserum antibody had levels of protection similar to those of untreated immunized mice or mice treated with the negative control anti-CD8 MAb.
In contrast to that in β2M−/− mice, the depletion of CD4+ T cells in sporozoite-immunized WT mice with functioning CD8+ T cells did not abolish sporozoite-induced immunity (Fig. 2A). All of the sporozoite-immunized WT mice (nine of nine) treated with anti-CD4 MAb were protected, as were 100% of the untreated immunized WT mice (seven of seven). The prepatent periods for naïve WT and β2M−/− mice were similar, with means of 4.1 and 4.4 days, respectively. These findings indicate that, in the absence of the CD8+-T-cell-mediated immunity found in WT mice (10), CD4+ effector T cells play a dominant role in protective immunity.
Resistance to secondary challenge.
CD4+ T cells also played a critical role in resistance to secondary challenge in P. yoelii sporozoite-immunized β2M−/− mice. One month after resisting primary P. yoelii sporozoite challenge (Table 1), the protected β2M−/− mice were rechallenged, with or without T-cell depletion. The immunized β2M−/− mice that had not been depleted of CD4+ T cells were fully protected against rechallenge and did not develop patent infections (Table 2). In contrast, the depletion of CD4+ T cells prior to rechallenge resulted in patent parasitemia similar to that in naïve controls (with a prepatent period of 4.75 days, versus 4.0 days in controls). Therefore, in B2M−/− mice, P. yoelii sporozoite-induced protection against rechallenge was CD4+-T-cell dependent, as found for resistance to the primary challenge (Fig. 2). In contrast, P. berghei-immunized β2M−/− mice were not protected following rechallenge, either with or without CD4+-T-cell depletion. These findings suggest that P. berghei sporozoites elicit a more transient immune response in the β2M−/− mice than P. yoelii sporozoites.
TABLE 2.
Results of rechallenge of β2M−/− mice immunized with P. yoelii or P. berghei sporozoites
| Rechallenge organism | Immunization inoculum | Anti-CD4 treatmenta | No. protected/total no. of miceb | % Protected | Prepatent period (days) |
|---|---|---|---|---|---|
| P. yoelii | Irradiated P. yoelii sporozoites | − | 4/4 | 100 | |
| Irradiated P. yoelii sporozoites | + | 0/4 | 0 | 4.8 | |
| None | − | 0/3 | 0 | 4.0 | |
| P. berghei | Irradiated P. berghei sporozoites | − | 1/4 | 25 | 6.0 |
| Irradiated P. berghei sporozoites | + | 0/4 | 0 | 4.8 | |
| None | − | 0/3 | 0 | 4.0 |
Sporozoite-immunized β2M−/− mice protected against a primary challenge were depleted of CD4+ T cells prior to rechallenge. β2M−/− mice treated with anti-CD4 MAb had CD4+ T cell levels of 0.36 to 0.38%, versus 20.1 to 17.0% in the untreated immune β2M−/− mice, as determined by FACS analysis. +, treated with anti-CD4; −, not treated with anti-CD4.
Protection was assayed by determining parasitemia in Giemsa-stained blood smears.
Cellular effector mechanisms in sporozoite-immunized β2M−/− mice.
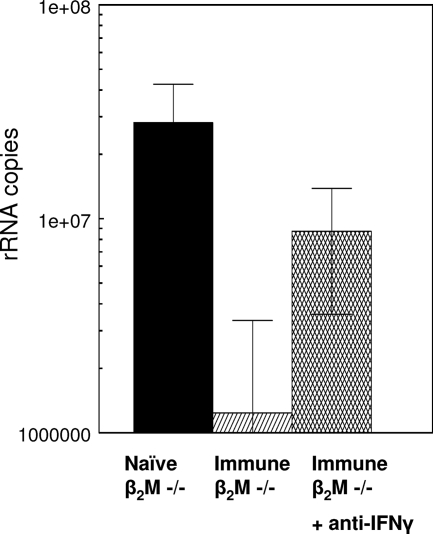
The cytokine IFN-γ, which is produced by both CD4+ and CD8+ T cells, plays a critical role in the inhibition of hepatic-stage parasites (10, 14, 25, 44). To assess the role of IFN-γ, P. yoelii sporozoite-immunized β2M−/− mice were treated with anti-murine IFN-γ MAb prior to challenge. While >95% inhibition of hepatic-stage parasites occurred in the untreated immunized mice, protection in the mice treated with anti-IFN-γ MAb was reduced to 69% compared to that in controls (Fig. 3). The mean number of rRNA copies in the anti-IFN-γ-treated mice (0.87 × 107) was significantly higher than that in the untreated immunized β2M−/− mice (0.124 × 107; P = 0.04).
FIG. 3.
Treatment with anti-IFN-γ antibody reduces protective immunity in immunized β2M−/− mice. P. yoelii sporozoite-immunized β2M−/− mice were depleted of IFN-γ by i.v. injection of 1 mg of anti-IFN-γ MAb (MAb DB-1) on the day of sporozoite challenge and 24 h post-sporozoite challenge (45). Levels of rRNA in the livers were measured by RT-PCR at 40 h postchallenge. Results are shown as mean numbers of parasite 18S rRNA copies ± SD.
Sporozoite-neutralizing antibodies in sera of immunized β2M−/− mice.
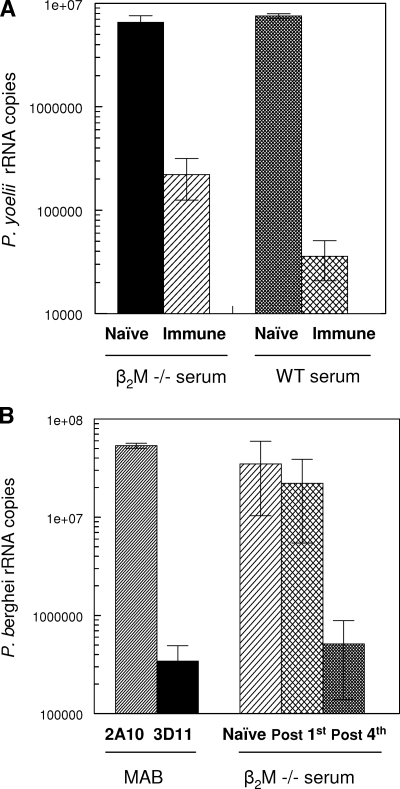
In addition to immune cells, antisporozoite antibodies play a critical role in protective immunity (47, 49, 52). Similar antibody titers were elicited in the sporozoite-immunized β2M−/− mice and in WT mice (indirect immunofluorescence assay titer, >104). These antisporozoite antibodies were biologically active, as determined by both in vivo and in vitro SNAs. Preincubation of sporozoites in sera from P. yoelii sporozoite-immunized β2M−/− mice prior to injection into naïve recipients reduced P. yoelii sporozoite infectivity in the liver by 97% (Fig. 4A). A mean of 0.221 × 106 parasite rRNA copies was detected in the livers of mice injected with sporozoites preincubated in β2M−/− mouse immune sera, compared to 6.56 × 106 parasite rRNA copies in the livers of mice receiving sporozoites incubated in β2M−/− mouse preimmune sera. The neutralizing activity in sera from β2M−/− mice was comparable to that in immune sera from WT C57BL mice, which reduced sporozoite infectivity 99.5% compared to that of sporozoites incubated with preimmune sera (0.036 × 106 rRNA copies compared to 7.55 × 106 rRNA copies, respectively).
FIG. 4.
Sporozoite-neutralizing antibodies in sera of β2M−/− mice immunized with P. yoelii or P. berghei irradiated sporozoites. (A) Viable P. yoelii sporozoites were incubated for 45 min at 37°C with sera from P. yoelii sporozoite-immunized β2M−/− mice prior to injection into naïve recipients (2 × 104 sporozoites/mouse). Livers were obtained 40 h postchallenge, and levels of parasite rRNA were measured by RT-PCR. (B) Viable P. berghei sporozoites were incubated with sera obtained from eight β2M−/− mice following the first or fourth immunization with irradiated P. berghei sporozoites. The sporozoites incubated with sera (2 × 104) were added to HepG2 hepatoma cell cultures, and levels of P. berghei 18S rRNA were measured by RT-PCR after 48 h of incubation. Means ± SD are shown for the seven of eight anti-P. berghei serum samples that had positive SNA activity (>80% inhibition). Controls included P. berghei sporozoites incubated with 25 μg/ml of MAb 3D11, specific for P. berghei CS protein repeats, or negative control MAb 2A10, specific for P. falciparum CS protein repeats (33, 57).
Consistent with the results obtained with anti-P. yoelii β2M−/− mouse immune sera (Fig. 4A), immune sera of P. berghei sporozoite-immunized β2M−/− mice had high levels of sporozoite-neutralizing activity when tested in vitro (Fig. 4B). The majority of the immune sera from P. berghei-immunized β2M−/− mice (seven of eight) reduced sporozoite infectivity 98.5% compared to the infectivity of sporozoites incubated in naïve β2M−/− mouse sera. Hepatoma cell cultures inoculated with P. berghei sporozoites preincubated in immune sera developed a mean of 0.5 × 106 rRNA copies, compared to 34.9 × 106 rRNA copies in cultures inoculated with sporozoites incubated in normal sera. This level of inhibition was equivalent to that observed when sporozoites were incubated with protective MAb 3D11, specific for P. berghei CS protein repeats (mean number of rRNA copies, 0.342 × 106). The development of sporozoite-neutralizing activity required booster immunizations, as sera obtained from β2M−/− mice after the first immunization did not reduce P. berghei parasite rRNA levels (22.2 × 106 rRNA copies).
DISCUSSION
β2M-deficient mice, lacking class I molecules, can develop strong protective immunity following immunization with irradiated P. yoelii or P. berghei sporozoites. Following challenge with viable sporozoites, the majority (>90%) of the sporozoite-immunized β2M−/− mice developed sterile immunity; that is, no blood-stage infection was detected following challenge. Protective immunity could also be measured as a significant reduction in liver-stage parasites, as determined by PCR using probes specific for parasite rRNA. This high level of immune resistance was obtained in the absence of functional CD8+ T cells, which have been proposed to be an essential effector mechanism in sporozoite-induced immunity.
Immune protection was observed in β2M−/− mice with the C57BL (H-2b) background, as well as those with the BALB/c (H-2d) genetic background, in which CD8+ T cells are a primary effector mechanism for WT mice (10). Therefore, class II-mediated mechanisms can function in different genetic backgrounds to protect against sporozoite challenge. This finding is consistent with the presence of redundant immune mechanisms that can protect the host against Plasmodium when one effector mechanism is absent or defective, as found previously for other pathogens (12, 13, 32).
In the absence of CD8+ T cells, CD4+ T cells play a critical role in protection against sporozoite challenge. The depletion of CD4+ T cells prior to the challenge of sporozoite-immunized β2M−/− mice abolished immune resistance, as measured either by the development of patent blood-stage parasitemia or by increased liver-stage parasite burdens (Fig. 2). In contrast to that in β2M−/− mice, however, the depletion of CD4+ T cells in WT mice did not inhibit immunity (Fig. 2A), suggesting that CD4+-T-cell effector mechanisms provide a default protective mechanism that is functional when CD8+ T cells are lacking. These findings are consistent with results from studies demonstrating that protective immunity can be elicited with malaria peptide immunogens that lack CD8+-T-cell epitopes (5, 28, 53, 54), showing that protection against sporozoite challenge can be obtained when only CD4+ T cells are elicited.
CD4+ T cells can potentially function by directly targeting liver-stage parasites via cytotoxic mechanisms or cytokine production. While the majority of cytotoxic cells isolated from sporozoite-immunized mice have been CD8+ (41, 43), a protective cytotoxic CD4+-T-cell clone in the P. berghei malaria model has been characterized (51). Cytolytic CD4+-T-cell clones have also been isolated from human volunteers immunized with irradiated P. falciparum sporozoites or P. falciparum CS peptide vaccines (4, 30). Increased numbers of cytotoxic CD4+ T cells have been found in virus-infected β2M−/− mice (26). However, preliminary studies did not detect lytic activity with spleen cells derived from the sporozoite-immunized β2M−/− mice (data not shown).
Nonlytic CD4+ T cells derived from peptide-immunized mice can also protect against sporozoite challenge by IFN-γ production in some, but not all, cases (5, 8, 28, 39, 53). IFN-γ is a potent cytokine inhibitor of intracellular hepatic-stage parasites (14, 44). Human volunteers immunized with P. falciparum sporozoites or CS peptides develop predominantly Th1-type CD4+-T-cell clones that produce high levels of IFN-γ (4, 31). The presence of CS-specific CD4+ T cells producing IFN-γ has been correlated with protection against P. falciparum in volunteers immunized with RTS,S vaccine and in individuals with naturally acquired immunity (24, 38, 50). In the present study, treatment of the P. yoelii-immunized β2M−/− mice with anti-IFN-γ significantly reduced, but did not completely abolish, protective immunity (Fig. 3). Increased concentrations and/or more frequent treatment with anti-IFN-γ MAb may be required if high levels of IFN-γ are produced by the murine CD4+ T cells following sporozoite challenge.
Alternatively, a combination of T cells and antibody may mediate resistance in the sporozoite-immunized β2M−/− mice. Antibody titers in immunized β2M−/− and WT mice were similar, and high levels of sporozoite-neutralizing activity were present in the immune sera (Fig. 4). A role for antibodies, in addition to CD4+ T cells, in the sporozoite-immunized β2M−/− mice was suggested by the reduced levels of liver-stage parasites in the immunized β2M−/− mice treated with anti-CD4 MAb compared to those in naïve controls (Fig. 2B). The passive transfer of anti-sporozoite antibody and immune cells has been shown to protect naïve WT recipients against sporozoite challenge more effectively than the transfer of either serum or cells alone (40, 45). The IFN-γ produced by CD4+ T cells may activate macrophages to enhance the clearance of antibody-immobilized or opsonized sporozoites (7, 36). In recent studies, the presence of CS-specific opsonizing antibodies in the sera of RTS,S-immunized volunteers correlated with protection against P. falciparum sporozoite challenge (46). Antibody may function synergistically with T cells by significantly reducing the number of sporozoites that reach the liver, thereby facilitating CD4+ T-cell clearance of a smaller number of hepatic exoerythrocytic forms, either by direct cytotoxicity or by production of inhibitory cytokines.
Regardless of the immune mechanism, the high levels of sterile immunity obtained in the sporozoite-immunized β2M−/− mice demonstrate that strong immune resistance can be obtained in the absence of CD8+ T cells. These findings are in contrast to those of a previous study using P. berghei, in which CD8+ T cells were proposed to be the sole effector mechanism functioning in sporozoite-induced immunity based on the failure of immunization with P. berghei sporozoites to protect β2M−/− mice (56). The present studies used a different immunization regime, with higher priming doses of P. berghei sporozoites, more frequent booster doses, and a longer interval between sporozoite immunizations, which most likely increased protective immunity. The rechallenge study results (Table 2), however, suggest that class II-mediated immunity elicited by P. berghei sporozoites was less robust than that elicited by P. yoelii sporozoites. The lower infectivity of P. berghei sporozoites and the nonspecific inflammation elicited by P. berghei but not P. yoelii sporozoites may modulate adaptive immune responses in this rodent malaria model (2, 19).
P. yoelii is believed to provide a more accurate model for human malaria, with infectious-sporozoite inocula that are logs lower than that required for P. berghei (2, 19). The high levels of sterile immunity obtained in P. yoelii sporozoite-immunized β2M−/− mice indicate that class II-mediated immunity effectively protects against highly infective sporozoite challenge. Recent studies with transgenic mice expressing the CS protein suggest that this protein is the immunodominant antigen in sporozoite-induced protective immunity (23). That CS-specific class II-mediated immune resistance may function in humans is suggested by results from phase II trials of the CS-based RTS,S vaccine. Protection in the RTS,S-immunized volunteers was positively correlated with anti-repeat antibody and strong CD4+ Th1 responses in the absence of vigorous CD8+-T-cell responses (1, 18, 24, 46, 48).
The protective CD4+ T cells and sporozoite-neutralizing antibodies induced by sporozoite immunization of β2M−/− mice suggest that subunit vaccines eliciting strong class II-mediated immunity may provide effective preerythrocytic vaccines for human malaria.
Acknowledgments
We thank Diana Barrios Rodrigues and Rita Altszuler for expert technical assistance.
These studies were supported by NIH AI025085 and AI045138.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 26 December 2007.
REFERENCES
- 1.Bojang, K. A., P. J. Milligan, M. Pinder, L. Vigneron, A. Alloueche, K. E. Kester, W. R. Ballou, D. J. Conway, W. H. Reece, P. Gothard, L. Yamuah, M. Delchambre, G. Voss, B. M. Greenwood, A. Hill, K. P. McAdam, N. Tornieporth, J. D. Cohen, and T. Doherty. 2001. Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: a randomised trial. Lancet 3581927-1934. [DOI] [PubMed] [Google Scholar]
- 2.Briones, M. R., M. Tsuji, and V. Nussenzweig. 1996. The large difference in infectivity for mice of Plasmodium berghei and Plasmodium yoelii sporozoites cannot be correlated with their ability to enter into hepatocytes. Mol. Biochem. Parasitol. 777-17. [DOI] [PubMed] [Google Scholar]
- 3.Bruna-Romero, O., J. C. Hafalla, G. Gonzalez-Aseguinolaza, G. Sano, M. Tsuji, and F. Zavala. 2001. Detection of malaria liver-stages in mice infected through the bite of a single Anopheles mosquito using a highly sensitive real-time PCR. Int. J. Parasitol. 311499-1502. [DOI] [PubMed] [Google Scholar]
- 4.Calvo-Calle, J. M., G. A. Oliveira, and E. H. Nardin. 2005. Human CD4+ T cells induced by synthetic peptide malaria vaccine are comparable to cells elicited by attenuated Plasmodium falciparum sporozoites. J. Immunol. 1757575-7585. [DOI] [PubMed] [Google Scholar]
- 5.Charoenvit, Y., V. F. Majam, G. Corradin, J. B. Sacci, Jr., R. Wang, D. L. Doolan, T. R. Jones, E. Abot, M. E. Patarroyo, F. Guzman, and S. L. Hoffman. 1999. CD4(+) T-cell- and gamma interferon-dependent protection against murine malaria by immunization with linear synthetic peptides from a Plasmodium yoelii 17-kilodalton hepatocyte erythrocyte protein. Infect. Immun. 675604-5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clyde, D. F. 1975. Immunization of man against falciparum and vivax malaria by use of attenuated sporozoites. Am. J. Trop. Med. Hyg. 24397-401. [DOI] [PubMed] [Google Scholar]
- 7.Danforth, H. D., M. Aikawa, A. H. Cochrane, and R. S. Nussenzweig. 1980. Sporozoites of mammalian malaria: attachment to, interiorization and fate within macrophages. J. Protozool. 27193-202. [DOI] [PubMed] [Google Scholar]
- 8.Del Giudice, G., D. Grillot, L. Renia, I. Muller, G. Corradin, J. A. Louis, D. Mazier, and P. H. Lambert. 1990. Peptide-primed CD4+ cells and malaria sporozoites. Immunol. Lett. 2559-63. [DOI] [PubMed] [Google Scholar]
- 9.Denkers, E. Y., R. T. Gazzinelli, D. Martin, and A. Sher. 1993. Emergence of NK1.1+ cells as effectors of IFN-gamma dependent immunity to Toxoplasma gondii in MHC class I-deficient mice. J. Exp. Med. 1781465-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doolan, D. L., and S. L. Hoffman. 2000. The complexity of protective immunity against liver-stage malaria. J. Immunol. 1651453-1462. [DOI] [PubMed] [Google Scholar]
- 11.Doolan, D. L., and S. L. Hoffman. 2001. DNA-based vaccines against malaria: status and promise of the multi-stage malaria DNA vaccine operation. Int. J. Parasitol. 31753-762. [DOI] [PubMed] [Google Scholar]
- 12.Eichelberger, M., W. Allan, M. Zijlstra, R. Jaenisch, and P. C. Doherty. 1991. Clearance of influenza virus respiratory infection in mice lacking class I major histocompatibility complex-restricted CD8+ T cells. J. Exp. Med. 174875-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Epstein, S. L., J. A. Misplon, C. M. Lawson, E. K. Subbarao, M. Connors, and B. R. Murphy. 1993. Beta 2-microglobulin-deficient mice can be protected against influenza A infection by vaccination with vaccinia-influenza recombinants expressing hemagglutinin and neuraminidase. J. Immunol. 1505484-5493. [PubMed] [Google Scholar]
- 14.Ferreira, A., L. Schofield, V. Enea, H. Schellekens, P. van der Meide, W. E. Collins, R. S. Nussenzweig, and V. Nussenzweig. 1986. Inhibition of development of exoerythrocytic forms of malaria parasites by gamma-interferon. Science 232881-884. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman, S. L., and D. L. Doolan. 2000. Malaria vaccines—targeting infected hepatocytes. Nat. Med. 61218-1219. [DOI] [PubMed] [Google Scholar]
- 16.Hollingdale, M. R., P. Leland, and A. L. Schwartz. 1983. In vitro cultivation of the exoerythrocytic stage of Plasmodium berghei in a hepatoma cell line. Am. J. Trop. Med. Hyg. 32682-684. [DOI] [PubMed] [Google Scholar]
- 17.Hollingdale, M. R., E. H. Nardin, S. Tharavanij, A. L. Schwartz, and R. S. Nussenzweig. 1984. Inhibition of entry of Plasmodium falciparum and P. vivax sporozoites into cultured cells: an in vitro assay of protective antibodies. J. Immunol. 132909-913. [PubMed] [Google Scholar]
- 18.Kester, K. E., D. A. McKinney, N. Tornieporth, C. F. Ockenhouse, D. G. Heppner, T. Hall, U. Krzych, M. Delchambre, G. Voss, M. G. Dowler, J. Palensky, J. Wittes, J. Cohen, and W. R. Ballou. 2001. Efficacy of recombinant circumsporozoite protein vaccine regimens against experimental Plasmodium falciparum malaria. J. Infect. Dis. 183640-647. [DOI] [PubMed] [Google Scholar]
- 19.Khan, Z. M., and J. P. Vanderberg. 1991. Role of host cellular response in differential susceptibility of nonimmunized BALB/c mice to Plasmodium berghei and Plasmodium yoelii sporozoites. Infect. Immun. 592529-2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klotz, F. W., L. F. Scheller, M. C. Seguin, N. Kumar, M. A. Marletta, S. J. Green, and A. F. Azad. 1995. Co-localization of inducible-nitric oxide synthase and Plasmodium berghei in hepatocytes from rats immunized with irradiated sporozoites. J. Immunol. 1543391-3395. [PubMed] [Google Scholar]
- 21.Koller, B. H., P. Marrack, J. W. Kappler, and O. Smithies. 1990. Normal development of mice deficient in beta 2M, MHC class I proteins, and CD8+ T cells. Science 2481227-1230. [DOI] [PubMed] [Google Scholar]
- 22.Kumar, K. A., G. A. Oliveira, R. Edelman, E. Nardin, and V. Nussenzweig. 2004. Quantitative Plasmodium sporozoite neutralization assay (TSNA). J. Immunol. Methods 292157-164. [DOI] [PubMed] [Google Scholar]
- 23.Kumar, K. A., G. Sano, S. Boscardin, R. S. Nussenzweig, M. C. Nussenzweig, F. Zavala, and V. Nussenzweig. 2006. The circumsporozoite protein is an immunodominant protective antigen in irradiated sporozoites. Nature 444937-940. [DOI] [PubMed] [Google Scholar]
- 24.Lalvani, A., P. Moris, G. Voss, A. A. Pathan, K. E. Kester, R. Brookes, E. Lee, M. Koutsoukos, M. Plebanski, M. Delchambre, K. L. Flanagan, C. Carton, M. Slaoui, C. Van Hoecke, W. R. Ballou, A. V. Hill, and J. Cohen. 1999. Potent induction of focused Th1-type cellular and humoral immune responses by RTS,S/SBAS2, a recombinant Plasmodium falciparum malaria vaccine. J. Infect. Dis. 1801656-1664. [DOI] [PubMed] [Google Scholar]
- 25.Maheshwari, R. K., C. W. Czarniecki, G. P. Dutta, S. K. Puri, B. N. Dhawan, and R. M. Friedman. 1986. Recombinant human gamma interferon inhibits simian malaria. Infect. Immun. 53628-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marusic-Galesic, S., K. Udaka, and P. Walden. 1993. Increased number of cytotoxic T cells within CD4+8− T cells in beta 2-microglobulin, major histocompatibility complex class I-deficient mice. Eur. J. Immunol. 233115-3119. [DOI] [PubMed] [Google Scholar]
- 27.McKenna, K. C., and M. R. Briones. 2002. Quantitation of liver-stage parasites by competitive RT-PCR. Methods Mol. Med. 72141-148. [DOI] [PubMed] [Google Scholar]
- 28.Migliorini, P., B. Betschart, and G. Corradin. 1993. Malaria vaccine: immunization of mice with a synthetic T cell helper epitope alone leads to protective immunity. Eur. J. Immunol. 23582-585. [DOI] [PubMed] [Google Scholar]
- 29.Moorthy, V. S., E. B. Imoukhuede, P. Milligan, K. Bojang, S. Keating, P. Kaye, M. Pinder, S. C. Gilbert, G. Walraven, B. M. Greenwood, and A. S. Hill. 2004. A randomised, double-blind, controlled vaccine efficacy trial of DNA/MVA ME-TRAP against malaria infection in Gambian adults. PLoS Med. 1e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moreno, A., P. Clavijo, R. Edelman, J. Davis, M. Sztein, D. Herrington, and E. Nardin. 1991. Cytotoxic CD4+ T cells from a sporozoite-immunized volunteer recognize the Plasmodium falciparum CS protein. Int. Immunol. 3997-1003. [DOI] [PubMed] [Google Scholar]
- 31.Moreno, A., P. Clavijo, R. Edelman, J. Davis, M. Sztein, F. Sinigaglia, and E. Nardin. 1993. CD4+ T cell clones obtained from Plasmodium falciparum sporozoite-immunized volunteers recognize polymorphic sequences of the circumsporozoite protein. J. Immunol. 151489-499. [PubMed] [Google Scholar]
- 32.Muller, D., B. H. Koller, J. L. Whitton, K. E. LaPan, K. K. Brigman, and J. A. Frelinger. 1992. LCMV-specific, class II-restricted cytotoxic T cells in beta 2-microglobulin-deficient mice. Science 2551576-1578. [DOI] [PubMed] [Google Scholar]
- 33.Nardin, E. H., V. Nussenzweig, R. S. Nussenzweig, W. E. Collins, K. T. Harinasuta, P. Tapchaisri, and Y. Chomcharn. 1982. Circumsporozoite proteins of human malaria parasites Plasmodium falciparum and Plasmodium vivax. J. Exp. Med. 15620-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nussenzweig, R., J. Vanderberg, and H. Most. 1969. Protective immunity produced by the injection of x-irradiated sporozoites of Plasmodium berghei. IV. Dose response, specificity and humoral immunity. Mil. Med. 1341176-1182. [PubMed] [Google Scholar]
- 35.Nussenzweig, R. S., J. Vanderberg, H. Most, and C. Orton. 1967. Protective immunity produced by the injection of x-irradiated sporozoites of Plasmodium berghei. Nature 216160-162. [DOI] [PubMed] [Google Scholar]
- 36.Nussenzweig, R. S., J. P. Vanderberg, Y. Sanabria, and H. Most. 1972. Plasmodium berghei: accelerated clearance of sporozoites from blood as part of immune-mechanism in mice. Exp. Parasitol. 3188-97. [DOI] [PubMed] [Google Scholar]
- 37.Nussenzweig, V., and R. S. Nussenzweig. 1989. Rationale for the development of an engineered sporozoite malaria vaccine. Adv. Immunol. 45283-334. [DOI] [PubMed] [Google Scholar]
- 38.Reece, W. H., M. Pinder, P. K. Gothard, P. Milligan, K. Bojang, T. Doherty, M. Plebanski, P. Akinwunmi, S. Everaere, K. R. Watkins, G. Voss, N. Tornieporth, A. Alloueche, B. M. Greenwood, K. E. Kester, K. P. McAdam, J. Cohen, and A. V. Hill. 2004. A CD4(+) T-cell immune response to a conserved epitope in the circumsporozoite protein correlates with protection from natural Plasmodium falciparum infection and disease. Nat. Med. 10406-410. [DOI] [PubMed] [Google Scholar]
- 39.Renia, L., M. S. Marussig, D. Grillot, S. Pied, G. Corradin, F. Miltgen, G. Del Giudice, and D. Mazier. 1991. In vitro activity of CD4+ and CD8+ T lymphocytes from mice immunized with a synthetic malaria peptide. Proc. Natl. Acad. Sci. USA 887963-7967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodrigues, M., R. S. Nussenzweig, and F. Zavala. 1993. The relative contribution of antibodies, CD4+ and CD8+ T cells to sporozoite-induced protection against malaria. Immunology 801-5. [PMC free article] [PubMed] [Google Scholar]
- 41.Rodrigues, M. M., A. S. Cordey, G. Arreaza, G. Corradin, P. Romero, J. L. Maryanski, R. S. Nussenzweig, and F. Zavala. 1991. CD8+ cytolytic T cell clones derived against the Plasmodium yoelii circumsporozoite protein protect against malaria. Int. Immunol. 3579-585. [DOI] [PubMed] [Google Scholar]
- 42.Roland, J., V. Soulard, C. Sellier, A. M. Drapier, J. P. Di Santo, P. A. Cazenave, and S. Pied. 2006. NK cell responses to Plasmodium infection and control of intrahepatic parasite development. J. Immunol. 1771229-1239. [DOI] [PubMed] [Google Scholar]
- 43.Romero, P., J. L. Maryanski, G. Corradin, R. S. Nussenzweig, V. Nussenzweig, and F. Zavala. 1989. Cloned cytotoxic T cells recognize an epitope in the circumsporozoite protein and protect against malaria. Nature 341323-326. [DOI] [PubMed] [Google Scholar]
- 44.Schofield, L., A. Ferreira, R. Altszuler, V. Nussenzweig, and R. S. Nussenzweig. 1987. Interferon-gamma inhibits the intrahepatocytic development of malaria parasites in vitro. J. Immunol. 1392020-2025. [PubMed] [Google Scholar]
- 45.Schofield, L., J. Villaquiran, A. Ferreira, H. Schellekens, R. Nussenzweig, and V. Nussenzweig. 1987. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature 330664-666. [DOI] [PubMed] [Google Scholar]
- 46.Schwenk, R., L. V. Asher, I. Chalom, D. Lanar, P. Sun, K. White, D. Keil, K. E. Kester, J. Stoute, D. G. Heppner, and U. Krzych. 2003. Opsonization by antigen-specific antibodies as a mechanism of protective immunity induced by Plasmodium falciparum circumsporozoite protein-based vaccine. Parasite Immunol. 2517-25. [DOI] [PubMed] [Google Scholar]
- 47.Stewart, M. J., R. J. Nawrot, S. Schulman, and J. P. Vanderberg. 1986. Plasmodium berghei sporozoite invasion is blocked in vitro by sporozoite-immobilizing antibodies. Infect. Immun. 51859-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stoute, J. A., M. Slaoui, D. G. Heppner, P. Momin, K. E. Kester, P. Desmons, B. T. Wellde, N. Garcon, U. Krzych, M. Marchand, W. R. Ballou, and J. D. Cohen for the RTS,S Malaria Vaccine Evaluation Group. 1997. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. N. Engl. J. Med. 33686-91. [DOI] [PubMed] [Google Scholar]
- 49.Sultan, A. A., V. Thathy, U. Frevert, K. J. Robson, A. Crisanti, V. Nussenzweig, R. S. Nussenzweig, and R. Menard. 1997. TRAP is necessary for gliding motility and infectivity of plasmodium sporozoites. Cell 90511-522. [DOI] [PubMed] [Google Scholar]
- 50.Sun, P., R. Schwenk, K. White, J. A. Stoute, J. Cohen, W. R. Ballou, G. Voss, K. E. Kester, D. G. Heppner, and U. Krzych. 2003. Protective immunity induced with malaria vaccine, RTS,S, is linked to Plasmodium falciparum circumsporozoite protein-specific CD4(+) and CD8(+) T cells producing IFN-gamma. J. Immunol. 1716961-6967. [DOI] [PubMed] [Google Scholar]
- 51.Tsuji, M., P. Romero, R. S. Nussenzweig, and F. Zavala. 1990. CD4+ cytolytic T cell clone confers protection against murine malaria. J. Exp. Med. 1721353-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vanderberg, J. P., and U. Frevert. 2004. Intravital microscopy demonstrating antibody-mediated immobilisation of Plasmodium berghei sporozoites injected into skin by mosquitoes. Int. J. Parasitol. 34991-996. [DOI] [PubMed] [Google Scholar]
- 53.Wang, R., Y. Charoenvit, G. Corradin, P. De La Vega, E. D. Franke, and S. L. Hoffman. 1996. Protection against malaria by Plasmodium yoelii sporozoite surface protein 2 linear peptide induction of CD4+ T cell- and IFN-gamma-dependent elimination of infected hepatocytes. J. Immunol. 1574061-4067. [PubMed] [Google Scholar]
- 54.Wang, R., Y. Charoenvit, T. M. Daly, C. A. Long, G. Corradin, and S. L. Hoffman. 1996. Protective efficacy against malaria of a combination sporozoite and erythrocytic stage vaccine. Immunol. Lett. 5383-93. [DOI] [PubMed] [Google Scholar]
- 55.Weiss, W. R., M. Sedegah, R. L. Beaudoin, L. H. Miller, and M. F. Good. 1988. CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc. Natl. Acad. Sci. USA 85573-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.White, K. L., H. L. Snyder, and U. Krzych. 1996. MHC class I-dependent presentation of exoerythrocytic antigens to CD8+ T lymphocytes is required for protective immunity against Plasmodium berghei. J. Immunol. 1563374-3381. [PubMed] [Google Scholar]
- 57.Yoshida, N., R. S. Nussenzweig, P. Potocnjak, V. Nussenzweig, and M. Aikawa. 1980. Hybridoma produces protective antibodies directed against the sporozoite stage of malaria parasite. Science 20771-73. [DOI] [PubMed] [Google Scholar]