Abstract
Although it is capable of eliciting strong innate and adaptive immune responses, Borrelia burgdorferi often evades immune clearance through largely unknown mechanisms. Our previous studies determined that infected interlukin-10−/− (IL-10−/−) mice show significantly lower B. burgdorferi levels than wild-type (B6) mice and that IL-10 inhibits innate immune responses critical for controlling B. burgdorferi infection. To determine whether virulent B. burgdorferi preferentially enhances IL-10 production, we developed an in vitro coculture medium (RPMI.B) in which both B. burgdorferi and primary macrophages (Mφs) remain viable. B. burgdorferi grew at similar rates and was able to regulate expression of immunoreactive proteins with similar kinetics in RPMI.B and in traditional BSK medium; in contrast, B. burgdorferi cultured in conventional tissue culture medium (RPMI) rapidly lost viability. Coculture of viable B. burgdorferi in RPMI.B with Mφs resulted in more rapid and significant increases in IL-10 transcripts and secreted proteins than coculture with nonviable B. burgdorferi in RPMI, which corresponded with decreased production of proinflammatory cytokines. Addition of live B. burgdorferi to Mφs in RPMI.B also elicited substantially higher IL-10 levels than heat-killed bacteria elicited, confirming that increased IL-10 production was not inherent to coculture in RPMI.B. Transfer of supernatants from B. burgdorferi-stimulated Mφs into naïve Mφ cultures resulted in suppressed activation upon subsequent stimulation with different bacterial agonists, and this suppression was obviated by IL-10-specific antibody. In vivo analyses determined that murine skin samples exhibited substantial upregulation of IL-10 within 24 h of injection of B. burgdorferi. Together, these results suggest that viable B. burgdorferi can suppress early Mφ responses during infection by causing increased release of IL-10.
Infection of susceptible hosts with strains of Borrelia burgdorferi sensu lato causes Lyme disease, which accounts for the majority of vector-borne illness in the United States and most of the temperate regions of Europe and Asia (18, 41). Upon introduction into the host dermis by an infected tick, the spirochetal bacteria must quickly adapt to their vertebrate host, disseminate from the skin to various host tissues, and evade the developing immune responses. This evasion must continue until the bacteria receive appropriate signals that lead to reemergence from their immunoprivileged niche and subsequent migration to and infection of a feeding tick. Although this extended persistence within an immunocompetent host is essential for the existence of the bacteria, the mechanisms that they utilize to escape immune clearance are largely unknown.
Infection with B. burgdorferi triggers strong innate and adaptive immune responses that are largely directed against the ≥127 putative lipoproteins believed to be produced by this spirochete (8, 12, 37). All of these proteins possess a signal II peptidase sequence which can be used to predict their subsequent cleavage and the triacyl modification of the resultant N-terminal cysteine residue. The triacylated lipoproteins are highly immunogenic, as injection of prototypic lipoproteins elicits strong antibody responses and sera collected from infected individuals possess high levels of antibodies that are specific to varioous lipoproteins (31, 45). Additionally, these triacylated lipoproteins directly activate macrophages (Mφs) (30), dendritic cells (35, 42), neutrophils (28), endothelial cells (48, 49), mast cells (24), fibroblasts (11), and B lymphocytes (23) through signaling events mediated by Toll-like receptor 2 (TLR2) (16, 47, 50). Since B. burgdorferi either lacks many of the known bacterial agonists that have stimulatory activities (e.g., lipopolysaccharide [LPS] and lipoteichoic acid) or sequesters them so that they cannot be readily accessed by host immune mediators (e.g., endoflagella), it appears that borrelial lipoproteins are a major focus of both innate and adaptive immune responses to this spirochete. The importance of these interactions is reflected by the relative inability of Mφs derived from TLR2−/− mice to respond to B. burgdorferi and/or its lipoproteins, as well as the high levels of B. burgdorferi that persist in target tissues of infected TLR2−/− mice compared to wild-type mice (47). Interestingly, TLR2−/− mice produce a B. burgdorferi-specific antibody response that appears to be almost identical to that produced by wild-type mice (47, 52), and passive transfer of B. burgdorferi-elicited immune serum from TLR2−/− mice and passive transfer of B. burgdorferi-elicited immune serum from wild-type mice protect naïve mice from infection equally (52), suggesting that appropriate innate immune responses are the most critical responses for bacterial clearance.
The importance of the innate immune responses, particularly Mφs, for controlling B. burgdorferi infection is also reflected by the results of multiple studies assessing the immunosuppressive effects of interleukin-10 (IL-10) on the development of Lyme disease. IL-10 is a significant immunomodulatory cytokine, largely due to its broad but potent anti-inflammatory properties (27). Although IL-10 can be produced by and act upon many cell types, myeloid cells are reported to be the major source of IL-10 production (15). Many of the suppressive immune functions generally associated with IL-10 activities are also mediated through myeloid cells, either by direct downregulation of their production of proinflammatory mediators or by inhibition of their ability to mediate critical antigen-presenting functions to lymphocytes. Exposure of human and murine Mφs to B. burgdorferi elicits significant IL-10 production (7, 13, 14, 29), and addition of exogenous IL-10 to Mφs can suppress the production of proinflammatory mediators produced in response to B. burgdorferi (7). Infection studies have indicated that IL-10−/− mice are significantly better at clearing B. burgdorferi from target tissues than wild-type mice. Infected IL-10−/− mice produce higher levels of B. burgdorferi-specific antibodies than wild-type mice, but the differences are not directly responsible for the enhanced bacterial clearance that occurs (19). IL-10−/− mice also exhibit enhanced innate immune barriers to B. burgdorferi infection compared to wild-type mice, and IL-10−/− Mφs exposed to spirochetes in vitro produce higher levels of multiple proinflammatory mediators than wild-type Mφs (19). Based on these studies, evasion of early innate responses appears to be critical for efficient dissemination and persistence of B. burgdorferi, and suppression of these innate responses by IL-10 is detrimental to effective immune clearance.
Recently, several bacterial and viral pathogens have been shown to promote elevated IL-10 production, to suppress various host immune functions, and to subsequently evade immune clearance (26, 33), raising the possibility that virulent B. burgdorferi might also possess mechanisms that preferentially induce Mφs to upregulate IL-10 production. Because most in vitro analyses of mammalian immune cells are performed in tissue culture medium that does not permit the growth or survival of this fastidious spirochete, it is unlikely that B. burgdorferi could exhibit such a putative virulence mechanism using traditional mammal-based culture conditions. We therefore first developed an in vitro coculture medium that maintains the viability of this fastidious bacterium but does not inherently activate murine Mφs. Subsequent studies using this optimized coculture system indicated that viable B. burgdorferi does potently elicit rapid IL-10 induction by Mφs, and the IL-10 then acts in an autocrine loop to suppress appropriate inflammatory responses necessary to promote efficient immune clearance.
MATERIALS AND METHODS
B. burgdorferi growth and viability.
The clonal N40 isolate (3) of B. burgdorferi was generously provided by Steve Barthold (University of California, Davis) as a passage 2 culture after isolation from the urinary bladder of a Rag-1−/− mouse. For all experiments, a passage 4 culture was grown in BSK-II medium supplemented with 6% rabbit serum (Sigma Chemical, St. Louis, MO) (BSK) for 3 to 5 days at 33°C and directly enumerated using a Petroff-Hauser chamber and dark-field microscopy. For subsequent assays, B. burgdorferi was cultured in combinations of BSK and RPMI 1640 medium containing 10% fetal bovine serum (RPMI). For viability assays, B. burgdorferi was enumerated at the indicated times with a Petroff-Hauser chamber using dark-field microscopy, and only the bacteria exhibiting characteristic spiral movement were classified as viable. Viability was confirmed by transferring equal volumes of the B. burgdorferi cultures into BSK and then directly enumerating the bacteria after 24 h of subculture at 33°C. As an additional parameter, 2 × 107 B. burgdorferi cells were grown for 12 h or more in either RPMI, BSK, or a medium containing 75% RPMI and 25% BSK (RPMI.B) and stained to determine viability using a LIVE/DEAD BacLight kit (Molecular Probes) according to manufacturer's protocols. Stained bacteria were visualized using a fluorescence microscope and were scored live if they incorporated SYTOr9 green fluorescent nucleic acid stain and excluded the red fluorescent nucleic acid dye propidium iodide (PI). B. burgdorferi was grown in the media mentioned above in duplicate, and at least 100 B. burgdorferi cells from three different fields of view were examined to assess viability.
Infection of mice with B. burgdorferi.
Mice were housed in the Department of Lab Animal Medicine at the University of Toledo Health Sciences Campus according to National Institutes of Health guidelines for the care and use of laboratory animals. All usage protocols were reviewed and approved by the Institutional Animal Care and Usage Committee. C57BL/6NCr (B6) (National Cancer Institute) mice were shaved and depiliated (Nair) on both flanks and then allowed to recover for 2 days. For all infections, a passage 5 culture grown at 33°C was counted and resuspended in BSK such that the desired numbers of B. burgdorferi cells were present in 20 μl. Each mouse was then inoculated intradermally in the left flank with either 20 μl of BSK alone or 104 live B. burgdorferi cells, while the right flank received no injection (control). After 24 h, the animals were euthanized, and skin samples were removed from both sites and immediately flash frozen in liquid N2 until they were used for RNA extraction (see below).
SDS-PAGE and Western blotting.
B. burgdorferi cells grown in BSK were divided into parallel aliquots containing 1 × 107 bacteria, resuspended in 1 ml phosphate-buffered saline containing 25 μg/ml proteinase K (pK), and incubated for 1 h at 30°C. After addition of a protease inhibitor cocktail (Roche Applied Science) and washing, these bacteria were incubated in either RPMI, RPMI.B, or BSK for 1 h at 37°C. The cultures were then boiled in sample buffer under reducing conditions, and the bacterial proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 4 to 12% bis-Tris NuPAGE gel (Invitrogen). The gel contents were then either visualized by silver staining (Invitrogen) or transferred to a polyvinylidene difluoride membrane and probed with immune sera collected from mice 4 weeks after infection with 2 × 103 B. burgdorferi cells, as previously described (19). Antigen-antibody complexes were detected using goat anti-mouse immunoglobulin (Ig) conjugated to horseradish peroxidase (Southern Biotech) and were visualized by chemiluminescence. Multiple film exposure times were used for each blot to ensure that all protein bands were recorded irrespective of differences in concentration between samples.
In vitro Mφ analyses.
Femurs were harvested from naïve wild-type B6 and congenic B6.129P2-Il10tm1Cgn/J (Jackson Lab) mice that are unable to produce IL-10 (IL-10−/−), and the bone marrow was used to expand Mφs for in vitro analyses using our previously described techniques (7, 22). Briefly, dissociated marrow tissue was cultured for 6 days in RPMI supplemented with 30% L929-conditioned medium. Adherent Mφs were recovered from culture dishes by scraping in ice-cold phosphate-buffered saline, and trypan blue-excluding cells were enumerated using a hemacytometer before they were reseeded in 24-well culture dishes at a concentration of 2 × 105 Mφs/well in RPMI. The monolayers were incubated overnight at 37°C in 7.5% CO2, and nonadherent cells were aspirated prior to stimulation. For B. burgdorferi stimulation, live or heat-killed (55°C for 1 h) bacteria were added to Mφ cultures at the indicated multiplicity of infection (MOI), and the preparations were immediately centrifuged at 300 × g for 10 min to encourage bacterial contact with the monolayers before incubation at 37°C in 7.5% CO2. High-purity LPS from Salmonella enterica serovar Typhimurium (List Biological) was also used as indicated.
For experiments examining the suppressive properties of secreted Mφ components, supernatants collected from Mφ monolayers after 24 h of incubation with viable or killed B. burgdorferi were centrifuged to remove residual bacteria, diluted 1:5 or 1:10 in RPMI, and added to naïve Mφ monolayers. In some cases these supernatants were pretreated for 1 h with 1 μg/ml of either rat anti-mouse IL-10 monoclonal antibody (MAb) or an IgG1 MAb isotype control (BD Biosciences). These naïve cultures were subsequently stimulated with the indicated amounts of LPS, live B. burgdorferi, or sonicated B. burgdorferi. The suppressive effects of the transferred supernatants on cytokine production were assessed by an enzyme-linked immunosorbent assay (ELISA), as indicated below.
ELISA analyses.
Culture supernatants were collected at various times poststimulation and frozen at −20°C until analyses were performed as previously described (7). The cytokine content was assessed by a sandwich ELISA using paired MAbs specific to murine tumor necrosis factor alpha (TNF-α), IL-6, and IL-10 (BD Biosciences). Bound cytokines were visualized using biotinylated detection antibodies and avidin-horseradish peroxidase (Vector Laboratories). Cytokine levels were quantified by comparison to appropriate recombinant cytokine standards (BD Biosciences).
RNA preparation and reverse transcription-PCR analyses.
For in vitro Mφ samples, total RNA was harvested from duplicate Mφ monolayers and pooled under the indicated conditions using an RNeasy kit (Qiagen). Total RNA (1 μg) was reverse transcribed into cDNA using ImProm II reverse transcriptase (Promega) according to the manufacturer's specifications and was quantified using our previously described real-time quantitative PCR techniques and a LightCycler (Roche Diagnostics) rapid fluorescence temperature cycler (19). The values reported below reflect the cytokine transcript levels after normalization to β-actin values for the same sample. The following primers were used for PCR: TNF-α forward primer TTCTGTCTACTGAACTTCGGGGTGATCGGTCC, TNF-α reverse primer GTATGAGATAGCAAATCGGCTGACGGTGTGGG, IL-6 forward primer GTTCTCTGGGAAATCGTGGA, IL-6 reverse primer TGTACTCCAGGTAGCTATGG, IL-10 forward primer CGGGAAGACAATAACTG, IL-10 reverse primer CATTTCCGATAAGGCTTGG, β-actin forward primer TGGAATCCTGTGGCATCCATGAAAC, and β-actin reverse primer TAAAACGCAGCTCAGTAACAGTCCG.
For murine skin samples, flash-frozen tissues were ground using a mortar and pestle, resuspended in QIAzol lysis reagent, and sonicated to facilitate tissue dissociation, and total RNA was extracted using an RNeasy lipid tissue kit according to the manufacturer's instructions (Qiagen). Total RNA was then reverse transcribed, and the indicated cDNA levels were determined as indicated above.
Statistical analyses.
The statistical significance of the quantitative differences between the different sample groups was determined by application of Student's two-tailed t test. P values of ≤0.05 were considered statistically significant.
RESULTS
B. burgdorferi viability in different media.
Since the goal of this study was to determine if viable B. burgdorferi can manipulate Mφ activation and the subsequent production of IL-10, it was essential to utilize an in vitro system that best ensured the viability of both spirochetes and Mφs, especially since B. burgdorferi is known to have much more complex growth requirements than murine Mφs. Importantly, we needed to ensure that the coculture conditions would allow B. burgdorferi to remain viable and virulent during the time frame of our analyses, yet Mφs would not undergo background activation. Our initial approach was to try different mixtures of the optimal medium for B. burgdorferi (BSK) and the optimal medium for Mφs (RPMI). In these studies, equal numbers of B. burgdorferi cells were placed into culture tubes containing BSK and RPMI at different ratios, and after 24 h of incubation at 37°C the percentage of motile (viable) bacteria was determined for each culture by direct counting. The majority of the bacteria in cultures containing ≥25% BSK remained motile for at least 24 h (data not shown), and additional experiments were performed to further delineate the viability in RPMI containing between 25 and 75% BSK. These studies suggested that the levels of B. burgdorferi viability were quite similar in this BSK range (data not shown), and a medium containing 25% BSK and 75% RPMI (RPMI.B) was chosen for further analysis (J. J. Lazarus, Y. Chung, M. A. Kay, A. L. McCarter, and R. M. Wooten, submitted for publication).
As a measure of viability, the membrane integrity of B. burgdorferi grown in BSK, RPMI, or RPMI.B was assessed using the LIVE/DEAD BacLight stains. After 12 h of culture in RPMI, 46% of B. burgdorferi cells did not exclude PI (“dead”), compared to significantly lower values for B. burgdorferi grown in either RPMI.B (2% ± 0.9%) or BSK (6% ± 3.3%); no heat-killed B. burgdorferi cells excluded PI. These differences were magnified after 18 h of incubation in RPMI, at which time increases in membrane blebbing and few intact spirochetes were observed, while ≥90% of B. burgdorferi cells in BSK or RPMI.B cultures excluded PI (data not shown). These initial studies indicated that significant numbers of B. burgdorferi cells cultured in RPMI have compromised membranes, suggesting that they are dead or debilitated.
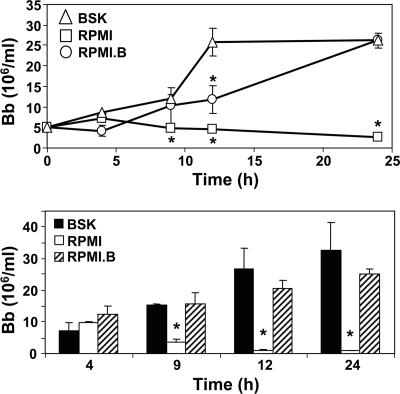
To assess the growth rates in the different media, equivalent numbers of B. burgdorferi cells were placed in culture tubes containing BSK, RPMI, or RPMI.B and cultured at 37°C for 24 h. Samples were removed at the indicated times, and the total numbers of bacteria were determined by direct microscopic counting. While the numbers of B. burgdorferi cells grown in BSK were higher at some times, the bacteria reached similar densities in BSK and RPMI.B (Fig. 1, top panel); this trend was observed in three separate experiments. However, at ≥4 h, the numbers of spirochetes cultured in RPMI were significantly lower than the numbers in the other media, and the numbers actually decreased after 4 h, suggesting that the bacteria were irreversibly damaged after ≥4 h of incubation in RPMI. To test this possibility, samples were collected from parallel (duplicate) cultures grown under all three culture conditions at the indicated times, transferred (subcultured) into fresh tubes containing traditional BSK, and allowed to grow for an additional 24 h at 37°C; these subcultures were then enumerated by direct counting. Samples collected from all three media at 4 h postinoculation grew to similar levels after subculture in fresh BSK (Fig. 1, bottom panel). At all subsequent times postinoculation, aliquots from the BSK and RPMI.B cultures grew to similar high levels, suggesting that B. burgdorferi cells grown in these two media maintained similar levels of viability. In contrast, in samples subcultured from RPMI after 4 h postinoculation the spirochete numbers were significantly reduced compared to samples subcultured from the other media (P ≤ 0.004), and the bacteria recovered after 12 h were almost undetectable and unable to replicate when they were subsequently placed in BSK. This confirms our observation that B. burgdorferi appears to undergo irreversible damage after ≥4 h of culture in RPMI.
FIG. 1.
Viability of B. burgdorferi grown in different media. (Top panel) B. burgdorferi growth in different media. Equal numbers of B. burgdorferi (Bb) cells were inoculated into the indicated media and incubated at 37°C. The total numbers of B. burgdorferi cells were determined at the indicated times postinoculation, irrespective of motility, by direct counting using a Petroff-Hauser chamber and dark-field microscopy. (Bottom panel) Recovery of viable B. burgdorferi cells after growth in different media. In parallel cultures, 100-μl aliquots were removed from the different cultures at the indicated times postinoculation and transferred into fresh tubes containing 400 μl of sterile BSK. The resulting subcultures were incubated at 37°C for an additional 24 h to allow outgrowth of viable B. burgdorferi before direct determination of the bacterial content. For both panels, the data are the means and standard errors for duplicate cultures and are representative of three separate experiments. An asterisk indicates a value that is statistically significantly (P ≤ 0.05) different than the value for bacteria cultured in BSK.
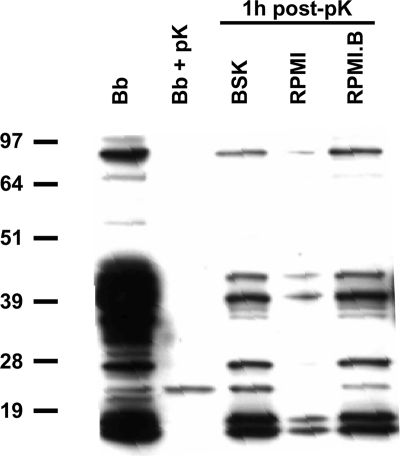
Although significant damage (as determined by growth) was not detected until ≥4 h postinoculation, it is possible that B. burgdorferi cells cultured in RPMI sustained other damage related to their viability or virulence at earlier times postinoculation, which would not have been detected by growth assays. Virulent B. burgdorferi strains display different tightly regulated lipoprotein expression patterns on their outer surfaces, depending on the environmental conditions and/or hosts that they encounter (20, 36, 43). The ability to rapidly express various lipoproteins on the surface may be essential for adaptation and the viability of these organisms within their different host reservoirs. To assess the effects of RPMI.B on lipoprotein expression, identical samples of B. burgdorferi were incubated with pK to remove surface proteins, and the stripped cells were then resuspended in either BSK, RPMI, or RPMI.B for 1 h before the synthesis of new surface protein in each culture was assessed by SDS-PAGE and immunoblotting. pK treatment removed all proteins recognizable by B. burgdorferi immune sera (except an unidentified 23-kDa protein) (Fig. 2) but did not result in significant loss of spirochete viability, as determined by direct counting of motile bacteria (data not shown). Within 1 h of subculture in RPMI.B, pK-treated spirochetes were able to restore surface immunoreactive proteins qualitatively and quantitatively like spirochetes subcultured in BSK (Fig. 2). However, the ability of pK-treated bacteria subcultured in RPMI to regenerate immunoreactive proteins was substantially impaired, and these bacteria even were unable to express the 23-kDa protein, suggesting that B. burgdorferi loses some functionality (e.g., protein synthesis or export or both) and/or gains a function that leads to diminished viability within 1 to 2 h after transfer to RPMI. This suggests that B. burgdorferi cocultured in RPMI might be unable to quickly adapt or express putative virulence mechanisms upon subsequent exposure to host cells. Thus, RPMI.B appeared to be a feasible choice for Mφ coculture analyses and could provide conditions that allow more accurate assessment of the host-pathogen adaptations that occur during infection.
FIG. 2.
B. burgdorferi expresses surface proteins at different rates under different medium conditions. Aliquots containing 1 × 107 cells of BSK-grown B. burgdorferi (Bb) were treated with 25 μg/ml pK (Bb + pK) and subsequently incubated in RPMI, RPMI.B, or BSK for 1 h. At that time, spirochetal proteins were separated on a 4 to 12% bis-Tris gel by SDS-PAGE, transferred to a polyvinylidene difluoride membrane, and probed with immune sera collected from mice at 4 weeks after infection with B. burgdorferi.
Stimulatory profiles of Mφs cultured in RPMI and RPMI.B.
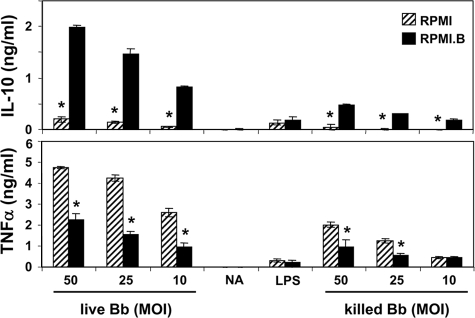
Because BSK is a rich medium (2), it was necessary to ensure that the added components do not adversely affect Mφ health or responsiveness to different immune stimuli in vitro. To assess the general viability, both wild-type and IL-10−/− Mφs were cultured in the presence and absence of optimal concentrations of LPS or B. burgdorferi at MOIs ranging from 1 to 100 in both RPMI and RPMI.B. Replicate monolayers were washed at 12 and 24 h poststimulation and tested to determine their abilities to exclude trypan blue. All control and stimulated Mφ cultures contained similar percentages of live and dead cells in RPMI and RPMI.B at each time assayed (P ≥ 0.25) (data not shown), suggesting that both media were able to sustain similar Mφ viabilities, at least during 24 h of in vitro culture. To assess the inherent stimulation potentials of the different media, supernatants were collected from parallel Mφ cultures incubated with or without LPS, and the cytokine content was assessed by an ELISA. In the absence of an exogenous agonist, the levels of secreted IL-10, IL-6, and TNF-α produced by either type of Mφs in both media were at or below the detection level of the assay system (Fig. 3 and data not shown), indicating that the 25% BSK present in RPMI.B did not directly activate the Mφ cultures (based on the three cytokines tested). Addition of LPS to these cultures elicited production of IL-10, IL-6, and TNF-α (data not shown and Fig. 3), and the levels of cytokines produced in RPMI and RPMI.B were comparable over the course of three separate experiments (P > 0.05). Together, these data suggest that RPMI.B is similar to RPMI in terms of the effects on Mφ function, while it has a vastly enhanced ability to support B. burgdorferi growth and viability and thus is useful for coculture analyses of spirochete-Mφ interactions.
FIG. 3.
Viable B. burgdorferi elicits a muted inflammatory cytokine response compared to killed bacteria. B6 Mφ monolayers were placed in the indicated media and stimulated with either live or killed (heated at 55°C for 1 h) B. burgdorferi (Bb) at the indicated MOI. Secreted cytokines were assayed in supernatants at 24 h poststimulation by a sandwich ELISA. NA, medium control; LPS, 500 ng/ml LPS. An asterisk indicates a value that is statistically significantly (P ≤ 0.05) different than the value for Mφs cultured in RPMI.
Viable B. burgdorferi rapidly elicits IL-10 production and decreases proinflammatory cytokine production.
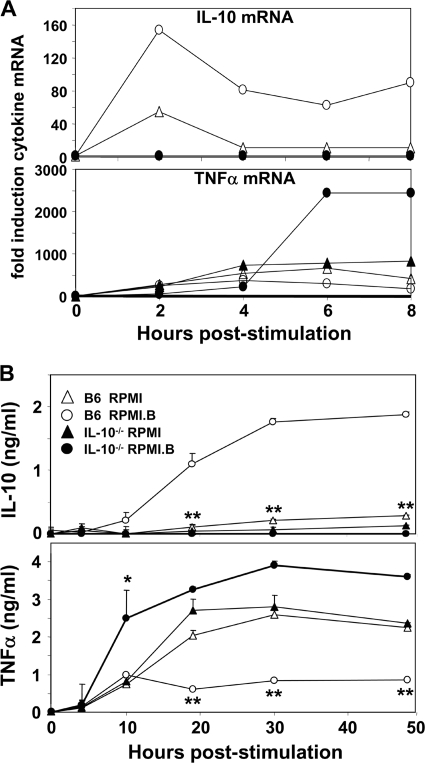
Because strong proinflammatory responses appear to be necessary to control the number of B. burgdorferi cells during infection (4, 47) and IL-10 suppresses proinflammatory responses, we performed kinetic studies to determine whether there was a negative correlation between the production of proinflammatory cytokines and the production of IL-10 by Mφs subsequent to infection. Activation of B6 Mφs resulted in a significant increase in IL-10 transcripts (relative to β-actin transcripts) by 2 h poststimulation (Fig. 4A) and an even more significant increase (fourfold) when activation occurred in RPMI.B (viable B. burgdorferi) rather than RPMI (nonviable B. burgdorferi). By 4 h poststimulation, substantial increases in the levels of a number of proinflammatory mediators, including TNF-α (Fig. 4A), IL-6, inducible nitric oxide synthase, and IL-12 (not shown), were noted in B6 Mφs, and the levels continued to rise over time at rates that were distinct for each mediator.
FIG. 4.
Kinetics of cytokine expression by wild-type and IL-10−/− Mφs in response to viable B. burgdorferi. (A) Duplicate B6 and IL-10−/− Mφ cultures were stimulated with live B. burgdorferi (MOI, 10) in either RPMI or RPMI.B, and total RNA was harvested from the pooled samples at the indicated times. Total RNA (1 μg) was reverse transcribed into cDNA, and quantitative PCR was performed using primers specific for TNF-α and IL-10. The cytokine values were normalized to the β-actin levels for each sample. (B) Wild-type and IL-10−/− Mφ cultures were stimulated with live B. burgdorferi in the indicated media, and the cytokine levels were determined in culture supernatants collected at the indicated times postinfection by ELISA. One asterisk indicates an individual value that is statistically significantly (P ≤ 0.05) different than the value for B6 Mφs in RPMI. Two asterisks indicate all other values that are statistically significantly (P ≤ 0.05) different than the value for B6 Mφs in RPMI.
To better address the role of IL-10 in these trends, parallel studies were performed using Mφs derived from IL-10−/− mice (Fig. 4A). Interestingly, IL-10−/− Mφs also showed increases in proinflammatory mediators by 4 h postinfection (Fig. 4A and data not shown), but the subsequent increases in these levels were significantly greater in IL-10−/− Mφs activated in RPMI.B (viable B. burgdorferi) than in either B6 Mφs grown in both media or IL-10−/− Mφs cultured in RPMI (nonviable B. burgdorferi). Again, the major differences in the proinflammatory mediators produced by B6 and IL-10−/− Mφs negatively correlated with the distinct differences in IL-10 production between these Mφs. These studies indicated that significant differences in immune mediators produced by Mφs in response to B. burgdorferi are apparent as early as 2 h poststimulation and are present for at least 8 h.
To determine whether these observed early differences in RNA transcript levels corresponded to differences in cytokine production, parallel kinetic studies were performed with Mφ culture supernatants using ELISA analyses. For wild-type (B6) Mφs, addition of B. burgdorferi in RPMI resulted in a strong proinflammatory response and an inversely low IL-10 response (Fig. 4B), which was likely influenced by the nonviable state of the spirochetes in this medium. However, addition of live spirochetes to B6 Mφs in RPMI.B resulted in a significantly reduced proinflammatory response that corresponded with the significantly increased IL-10 levels that were observed by ≥10 h poststimulation (Fig. 4B), suggesting that viable B. burgdorferi elicits a muted proinflammatory response that is mediated through the production of high levels of IL-10 by wild-type Mφs. These findings are bolstered by the fact that addition of both viable (RPMI.B) and nonviable (RPMI) spirochetes to IL-10−/− Mφs resulted in similarly high levels of proinflammatory cytokines (Fig. 4B); in this case the inordinately high proinflammatory response to viable B. burgdorferi cells was likely due to their inability to elicit IL-10 from IL-10−/− mouse-derived Mφs. It is unlikely that the significant differences in IL-10 production between RPMI.B and RPMI cocultures were directly due to enhanced bacterial proliferation in RPMI.B because (i) the large majority of the centrifuged bacteria were ingested by the Mφs, (ii) the few remaining bacteria either remained stuck to the plate or were free in the supernatant and thus had limited contact with the Mφs, and (iii) >10-fold differences in IL-10 levels were observed at times postinoculation when the differences in the few remaining bacterial numbers would be minimal (Fig. 4B and data not shown). Together, these data suggest that the ability of virulent B. burgdorferi strains to elicit high levels of IL-10 could potentially dysregulate host inflammatory responses and adversely affect Mφ functions in vivo.
Viable B. burgdorferi elicits increased IL-10 levels and a muted proinflammatory cytokine response compared to killed bacteria.
Using our defined B. burgdorferi-Mφ coculture system, we next wanted to determine whether live spirochetes elicited a distinct cytokine response from killed bacteria, which presumably could not adapt upon encountering Mφs in vitro. B6 Mφs in RPMI or RPMI.B were cultured with either live or heat-killed B. burgdorferi added at different MOIs for 24 h before the cytokine content was assessed by ELISA. Again, Mφs cultured in either RPMI or RPMI.B in the absence of any exogenous agonist (medium) did not produce any detectable cytokine (Fig. 3). Addition of live but damaged B. burgdorferi cells in classic Mφ medium (RPMI) (Fig. 1 and 2) led to substantial levels of IL-10 at all MOIs tested (Fig. 3). However, addition of live and healthy B. burgdorferi cells in RPMI.B resulted in an increase in IL-10 much larger than the increase observed with the RPMI cultures (P ≤ 4 × 10−5). Addition of heat-killed B. burgdorferi in RPMI.B resulted in a significantly decreased IL-10 response (P ≤ 1 × 10−4) compared to addition of an equal number of viable bacteria in RPMI.B, again indicating that viable bacteria elicit increased IL-10 levels. In contrast, addition of heat-killed B. burgdorferi in RPMI resulted in levels of IL-10 similar to those observed with an equal number of “live” (but nonviable) bacteria in RPMI, also confirming that the bacteria need to be viable to mediate the increased IL-10 secretion. All of the differences in IL-10 production noted above also appear to inversely correspond with the production of TNF-α in each culture condition(Fig. 3, top panel), suggesting that the observed differences in IL-10 production can translate into suppressed production of proinflammatory mediators.
Viable B. burgdorferi suppresses Mφ responses to subsequent stimuli.
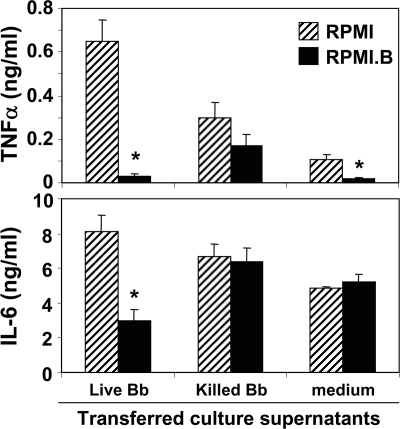
To directly test whether Mφ interactions with viable B. burgdorferi can dysregulate subsequent Mφ functions via soluble mediators, supernatants were collected from B6 Mφs cocultured with live or heat-killed spirochetes in either RPMI or RPMI.B and then diluted into fresh medium. These supernatants were then transferred onto naïve B6 Mφs to assess their effects on subsequent Mφ activation by LPS. Mφs that received supernatants from unstimulated medium controls showed little or no notable change in their ability to be activated by LPS, as shown by IL-6 and TNF-α secretion, respectively (Fig. 5). The responses to LPS of Mφs that received supernatants from Mφs initially activated by heat-killed B. burgdorferi also were essentially unchanged, regardless of the medium in which the initial stimulation occurred. However, Mφs that received supernatants from Mφs initially activated by live B. burgdorferi in RPMI.B (viable B. burgdorferi) showed a significantly decreased response to subsequent LPS stimulation, as shown by reduced proinflammatory cytokine production (Fig. 5). This was quite different from the results for Mφs that received supernatants from Mφs initially activated by live but damaged spirochetes in RPMI (Fig. 1 and 2), which showed increased proinflammatory cytokine production upon exposure to LPS. These findings suggest that Mφ interaction with viable B. burgdorferi leads to the production of soluble mediators that suppress subsequent Mφ immune functions, such as production of inflammatory mediators.
FIG. 5.
Viable B. burgdorferi suppresses Mφ responses to subsequent stimuli. Supernatants were collected from B6 Mφs cultured with equal numbers (MOI, 10) of either live or heat-killed B. burgdorferi (Bb) for 24 h in either RPMI or RPMI.B. These supernatants were centrifuged to remove residual bacteria, diluted 1:5 into fresh medium, and transferred (subcultured) onto naïve B6 Mφ cultures in the presence of LPS (500 ng/ml). After an additional 24 h of incubation, supernatants were harvested, and cytokine contents were quantified by an ELISA. An asterisk indicates a value that is statistically significantly (P ≤ 0.05) different than the value for Mφs that received supernatants cultured in RPMI.
IL-10 is directly responsible for the suppressive activities in B. burgdorferi-elicited Mφ supernatants.
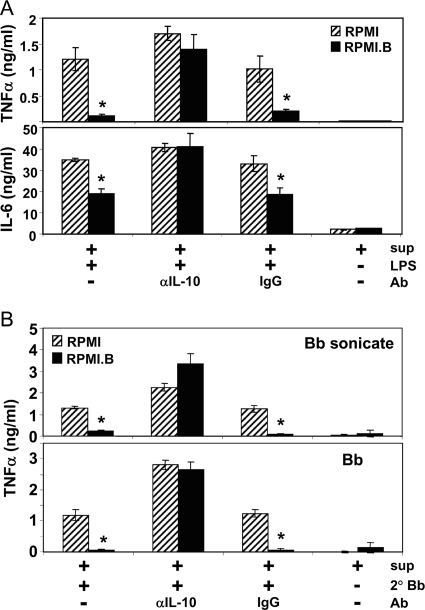
Based on the experiments described above, it is feasible that the suppressive properties of the B. burgdorferi-Mφ coculture supernatants are mediated via IL-10. To address this possibility, an experiment like the experiment whose results are shown in Fig. 5 was performed, except that the supernatants collected from Mφ cultures stimulated with B. burgdorferi in either RPMI (nonviable B. burgdorferi) or RPMI.B (viable B. burgdorferi) were preincubated with either an antibody that specifically neutralizes IL-10 or an IgG1 isotype control before they were transferred to naïve Mφ cultures (Fig. 6). In these experiments, as described above, the primary Mφ supernatants produced in response to nonviable bacteria (RPMI) had no suppressive effect when they were added to naïve Mφs that were subsequently stimulated with LPS; however, the supernatants produced by Mφs in response to viable bacteria (RPMI.B) did have a suppressive effect on naïve Mφs (Fig. 6A), as shown by the suppressed production of TNF-α (Fig. 6A, top panel) and IL-6 (bottom panel) in response to subsequent LPS stimulation. Notably, when both of these primary supernatants were preincubated with an IL-10-neutralizing antibody prior to transfer and LPS stimulation, the suppressive properties of the RPMI.B supernatants were lost and appeared to be similar to those of RPMI supernatants, indicating that the suppressive properties depend on the IL-10 content. When the supernatants were preincubated with the control antibody, the trend was the same as the trend observed if no antibody was added, confirming that the antibodies must be IL-10 specific to neutralize the suppressive effects. As a final control, no differences in the inherent abilities of the two transferred supernatants to directly stimulate cytokine production from the naïve Mφ cultures were seen. A similar overall trend was also observed for IL-6 production (Fig. 6A, bottom panel).
FIG. 6.
IL-10 is directly responsible for the suppressive activities in B. burgdorferi-elicited Mφ supernatants. B6 Mφ cultures were stimulated with B. burgdorferi (Bb) (MOI, 10) in either RPMI.B (viable B. burgdorferi) or RPMI (nonviable B. burgdorferi). Supernatants were collected after 24 h, spun to remove any remaining bacteria, and then treated with either no antibody (Ab), anti-mouse IL-10 (αIL-10) (5 μg/ml), or control IgG (5 μg/ml) for 1 h. These conditioned supernatants were then diluted 1:10 in fresh RPMI and added to naïve B6 Mφ monolayers. The naïve Mφs (containing the treated conditioned supernatants) were then stimulated with either (A) LPS (500 ng/ml), (B) sonicated B. burgdorferi (1 μg/ml), (B) live B. burgdorferi (MOI, 10), or (A and B) no agonist and cultured for an additional 24 h before the supernatants were collected and the cytokine contents were quantified by ELISA. sup, supernatants from Mφs stimulated with B. burgdorferi in either RPMI.B or RPMI; LPS, LPS from S. enterica serovar Typhimurium; 2°Bb, secondary B. burgdorferi. An asterisk indicates a value that is statistically significantly (P ≤ 0.05) different than the value for Mφs subcultured with RPMI-grown B. burgdorferi supernatants.
To directly address whether the observed IL-10-mediated suppression might also affect subsequent Mφ interactions with B. burgdorferi, experiments identical to those whose results are shown in Fig. 6A were performed, except that the secondary stimulus was either sonicated B. burgdorferi cultures or live B. burgdorferi. These experiments showed that the trend was exactly the same as the trend observed for the LPS-stimulated subcultures (Fig. 6A), where primary Mφ supernatants produced against viable B. burgdorferi suppressed the responses of naïve Mφs to both sonicated and live B. burgdorferi cultures, whereas preincubation with an IL-10-specific antibody eliminated the suppressive effects from the transferred supernatants (Fig. 6B). Together with the results shown in Fig. 3 and 5, these findings indicate that Mφs interacting with viable B. burgdorferi secrete enhanced levels of IL-10 that can subsequently suppress the ability of other Mφs to produce an efficient inflammatory response.
B. burgdorferi induces IL-10 in vivo.
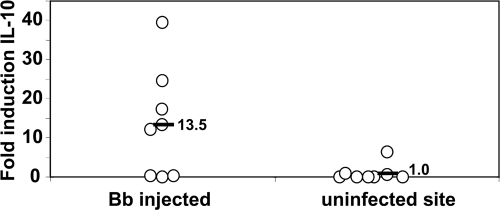
Although the in vitro analyses provided strong evidence that B. burgdorferi elicits IL-10 from Mφs, they did not show that significant levels of IL-10 are locally produced in response to infection in vivo. To address this possibility, groups of mice were inoculated intradermally with B. burgdorferi, and the relative levels of IL-10 transcripts at infected and noninfected (control) skin sites were compared. Sites inoculated with BSK alone contained very low levels of IL-10 transcripts at 24 h postinfection, which were quite similar to the baseline levels for uninoculated control sites on the same animals (data not shown). However, sites that received live B. burgdorferi showed substantially increased levels of IL-10 transcripts compared to the levels present at control sites on the same animals (Fig. 7), with the increases ranging from 11- to 35-fold between individual animals. These findings indicate that B. burgdorferi rapidly elicits transcription of the IL-10 gene at infection foci, which could subsequently lead to localized suppression of host inflammatory responses and inhibition of efficient immune clearance.
FIG. 7.
B. burgdorferi elicits IL-10 production in skin tissues. B6 mice were inoculated intradermally with 104 B. burgdorferi cells or BSK alone, and skin tissues were harvested after 24 h from both inoculated and control sites on each animal. RNA prepared from these tissues were reverse transcribed, and IL-10 levels were assessed by quantitative PCR. The values are the fold increases in IL-10 transcript levels compared to BSK-inoculated or uninoculated (control) skin samples from parallel groups of mice. The symbols indicate the infected and control site values for individual animals.
DISCUSSION
Previous reports have established that strong proinflammatory responses, largely directed against bacterial lipoproteins and mediated via TLR2-associated (16, 47) and MyD88-associated (4, 21) pathways, are necessary for effective clearance of B. burgdorferi from host tissues; the proinflammatory mediators suggested to be important include TNF-α (40, 54), IL-12 (35, 40), and the murine chemokine KC (6, 51). Our previous studies showed that the anti-inflammatory properties of IL-10 can be detrimental to immune clearance of B. burgdorferi (7) and that the effects appear to be largely mediated through deficiencies in the early innate immune responses (19). We and others have reported that Mφs produce IL-10 when they are exposed to B. burgdorferi and/or its lipoproteins (7, 13, 14), raising the possibility that B. burgdorferi could increase its virulence by enhancing IL-10 production from resident Mφs and subsequently dysregulating a productive inflammatory response. To address this possibility, we proposed that in vitro assays should be performed to assess Mφ responses to viable and nonviable B. burgdorferi.
An initial concern was determining if our in vitro system was optimal for assessing B. burgdorferi-Mφ interactions. Historically, the majority of studies (including our studies) assessing primary Mφ functions in vitro were performed using RPMI. However, B. burgdorferi is a fastidious organism, and the only known medium in which it can replicate and remain viable in vitro is BSK, which is a rich and complex medium that contains several poorly defined components (2). Since many of our proposed studies are designed to assess how Mφs interact with or are manipulated by virulent B. burgdorferi, it is essential to utilize an in vitro system that ensures the viability of both spirochetes and Mφs. This is particularly important because (i) we must ensure that any spirochete killing is due to Mφ-mediated effects rather than a stress-induced response because the bacteria lack essential nutrients and (ii) the bacteria need to be viable in order to rapidly respond to host-induced signals and display any virulence mechanisms that may be important for evading or dysregulating Mφ immune functions. Therefore, preliminary studies were performed to ensure that our in vitro coculture system met these conditions. Mixing studies using different combinations of RPMI and BSK showed that B. burgdorferi cultured in a medium consisting of 25% BSK and 75% RPMI (RPMI.B) allowed B. burgdorferi to attain similar growth densities, maintain identical viability characteristics, and regenerate immunoreactive proteins associated with in vivo growth and persistence at rates similar to BSK-cultured spirochetes (9). In contrast, B. burgdorferi displayed significantly reduced viability when it was cultured in traditional RPMI for as little as 1 to 4 h. Mφs also performed well in RPMI.B; they maintained viability similar to that in RPMI but were not inherently stimulated (background activation) by BSK components present in RPMI.B. Also, Mφs were similarly activated by the prototypical bacterial agonist LPS in both RPMI and RPMI.B at all times assayed, as measured by cytokine production. These data suggest that RPMI.B provides a more appropriate medium for coculture analyses to assess host immune cell responses in vitro to viable B. burgdorferi than traditional RPMI, although it obviously cannot completely replicate the true complexity of host-pathogen interactions that occur in vivo.
Studies using the optimized coculture system were able to address whether viable B. burgdorferi can significantly enhance IL-10 production. Mφs cocultured with live B. burgdorferi cells in RPMI.B showed rapid and significant upregulation of IL-10 compared to Mφs cocultured with similar numbers of killed B. burgdorferi cells in the same medium. A similar trend was also seen for “live” B. burgdorferi cocultured in RPMI.B and “live” B. burgdorferi cocultured in RPMI, a nonoptimal medium in which the bacteria are known to rapidly lose viability. In each of these coculture conditions, the relative levels of IL-10 elicited in response to B. burgdorferi appeared to correlate inversely with the relative levels of different proinflammatory cytokines produced by the Mφs. These IL-10 levels appeared to be biologically significant, since transfer of supernatants from Mφs cultured with viable B. burgdorferi could suppress the ability of naïve Mφs to become activated by a range of bacterial agonists, including LPS, sonicated B. burgdorferi cultures, and live B. burgdorferi. Preincubation of these supernatants with different antibodies confirmed that the suppressive properties were directly linked to the presence of IL-10. These in vitro biological effects for IL-10 are consistent with our previously published findings, which showed that Mφs derived from IL-10−/− mice responded more vigorously to B. burgdorferi agonists than wild-type Mφs and that IL-10−/− mice were much more efficient at clearing B. burgdorferi from multiple target tissues (7, 19).
Because of the potent and broadly immunosuppressive properties reported for IL-10, it is not surprising that a number of pathogens have developed mechanisms for exploiting host-produced IL-10 to promote their virulence mechanisms. Increased IL-10 production has been associated with increased host susceptibility to a number of intracellular and extracellular bacterial species, as well as to various viral pathogens (26, 33), although the mechanisms that they utilize vary. Some bacteria are known to produce a specific molecule that skews the normal kinetics of the host inflammatory response, promoting a rapid increase in the IL-10 level and concomitant early suppression of productive innate and adaptive immune responses (25, 38, 39). Certain pathogens that are known to persist after primary infection and produce a chronic disease state, such as Mycobacterium tuberculosis and human immunodeficiency virus, are associated with enhanced IL-10 production at infection foci by responding T lymphocytes, many of which possess functional characteristics of regulatory T cells (34, 44, 46). The importance of IL-10-mediated immunosuppression for persistent lymphocytic choriomeningitis virus infection of rodents was recently documented; prophylactic administration of an IL-10 receptor-blocking antibody restored suppressed T-cell activities, leading to complete viral clearance (5). Interestingly, two different viral pathogens have separately but convergently evolved to encode a viral IL-10 homolog that exhibits many of the immunosuppressive functions associated with host IL-10 but lacks many of the reported immunostimulatory properties that might be beneficial to the mammalian host (17, 32, 53). The fact that such a large range of pathogens have gone to such lengths to manipulate IL-10 levels and/or mimic the IL-10 immunosuppressive properties attests to the central role of IL-10 in downregulating host immune responses.
Based on our previous studies and current findings, we propose the following role for IL-10 in B. burgdorferi infection. B. burgdorferi is deposited into the host dermis by its tick vector and soon comes in contact with resident and/or infiltrating myeloid cells. While B. burgdorferi stimulates these immune cells largely through TLR2-mediated events, these inflammatory responses are dysregulated such that IL-10 is rapidly and potently upregulated. The IL-10 levels suppress not only the normal immune functions of the resident host cells but also those of immune cells that subsequently migrate into the infection foci. This provides these versatile spirochetes with the additional time that they need to appropriately adapt to their new host and/or subsequently migrate to some immunoprivileged niche, where they are largely protected from the naturally developing B. burgdorferi-specific adaptive immune responses. In support of this hypothesis, a recent paper indicated that inflammatory recruitment of neutrophils is dysregulated in B. burgdorferi-associated tissues containing resident Mφs and that treatments that extend neutrophil residency in those tissues significantly enhance the clearance of resident spirochetes (51). Although our current studies clearly demonstrated that there is rapid upregulation of IL-10 by Mφs in vitro and in the skin of infected mice, the mechanism underlying this dysregulated inflammatory response is not yet known. B. burgdorferi rapidly changes the pattern of lipoproteins on its surface during murine infection, and many of these proteins have no known function. Several of the proteins involved in manipulation of IL-10 production by other pathogens are lipoproteins; however, none of these proteins have obvious sequence similarity to any putative B. burgdorferi lipoproteins. Other workers have proposed that TLR2-specific ligands are specialized to produce IL-10 as part of a “default” pathway (1, 39). Since the vast majority of the stimulatory properties associated with B. burgdorferi appear to be mediated via TLR2- and MyD88-mediated pathways, this mechanism deserves further investigation. A previous study by other investigators suggested that B. burgdorferi could desensitize human blood monocytes to subsequent stimulation through TLR2-mediated events (10); however, more vigorous investigation is necessary to pinpoint the specific mechanism(s). Further studies are also needed to delineate which host cell types are responsible for producing IL-10 during B. burgdorferi infection, as well as which types of immune cells are adversely affected by the IL-10 levels and which important immune mechanisms are dysregulated. A better understanding of these events could lead to treatments to help clear persistent cases of Lyme disease.
Acknowledgments
We thank Robert Blumenthal, Eric Lafontaine, and Akira Takashima for helpful discussions.
This work was supported by Scientist Development Grant 0335148N from the American Heart Association (R.M.W.) and by start-up funds from the University of Toledo College of Medicine (R.M.W.).
Editor: B. A. McCormick
Footnotes
Published ahead of print on 17 December 2007.
REFERENCES
- 1.Agrawal, S., A. Agrawal, B. Doughty, A. Gerwitz, J. Blenis, T. Van Dyke, and B. Pulendran. 2003. Cutting edge: different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J. Immunol. 1714984-4989. [DOI] [PubMed] [Google Scholar]
- 2.Barbour, A. G. 1984. Isolation and cultivation of Lyme disease spirochetes. Yale J. Biol Med. 57521-525. [PMC free article] [PubMed] [Google Scholar]
- 3.Barthold, S. W., D. S. Beck, G. M. Hansen, G. A. Terwilliger, and K. D. Moody. 1990. Lyme borreliosis in selected strains and ages of laboratory mice. J. Infect. Dis. 162133-138. [DOI] [PubMed] [Google Scholar]
- 4.Bolz, D. D., R. S. Sundsbak, Y. Ma, S. Akira, C. J. Kirschning, J. F. Zachary, J. H. Weis, and J. J. Weis. 2004. MyD88 plays a unique role in host defense but not arthritis development in Lyme disease. J. Immunol. 1732003-2010. [DOI] [PubMed] [Google Scholar]
- 5.Brooks, D. G., M. J. Trifilo, K. H. Edelmann, L. Teyton, D. B. McGavern, and M. B. Oldstone. 2006. Interleukin-10 determines viral clearance or persistence in vivo. Nat. Med. 121301-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, C. R., V. A. Blaho, and C. M. Loiacono. 2004. Treatment of mice with the neutrophil-depleting antibody RB6-8C5 results in early development of experimental Lyme arthritis via the recruitment of Gr-1− polymorphonuclear leukocyte-like cells. Infect. Immun. 724956-4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown, J. P., J. F. Zachary, C. Teuscher, J. J. Weis, and R. M. Wooten. 1999. Dual role of interleukin-10 in murine Lyme disease: regulation of arthritis severity and host defense. Infect. Immun. 675142-5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casjens, S., N. Palmer, R. van Vugt, W. M. Huang, B. Stevenson, P. Rosa, R. Lathigra, G. Sutton, J. Peterson, R. J. Dodson, D. Haft, E. Hickey, M. Gwinn, O. White, and C. M. Fraser. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35490-516. [DOI] [PubMed] [Google Scholar]
- 9.Cullen, P. A., D. A. Haake, and B. Adler. 2004. Outer membrane proteins of pathogenic spirochetes. FEMS Microbiol. Rev. 28291-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diterich, I., C. Rauter, C. J. Kirschning, and T. Hartung. 2003. Borrelia burgdorferi-induced tolerance as a model of persistence via immunosuppression. Infect. Immun. 713979-3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ebnet, K., K. D. Brown, U. K. Siebenlist, M. M. Simon, and S. Shaw. 1997. Borrelia burgdorferi activates nuclear factor-kappa B and is a potent inducer of chemokine and adhesion molecule gene expression in endothelial cells and fibroblasts. J. Immunol. 1583285-3292. [PubMed] [Google Scholar]
- 12.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J. F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, and J. C. Venter. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390580-586. [DOI] [PubMed] [Google Scholar]
- 13.Giambartolomei, G. H., V. A. Dennis, B. L. Lasater, P. K. Murthy, and M. T. Philipp. 2002. Autocrine and exocrine regulation of interleukin-10 production in THP-1 cells stimulated with Borrelia burgdorferi lipoproteins. Infect. Immun. 701881-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giambartolomei, G. H., V. A. Dennis, and M. T. Philipp. 1998. Borrelia burgdorferi stimulates the production of interleukin-10 in peripheral blood mononuclear cells from uninfected humans and rhesus monkeys. Infect. Immun. 662691-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grutz, G. 2005. New insights into the molecular mechanism of interleukin-10-mediated immunosuppression. J. Leukoc. Biol. 773-15. [DOI] [PubMed] [Google Scholar]
- 16.Hirschfeld, M., C. J. Kirschning, R. Schwandner, H. Wesche, J. H. Weis, R. M. Wooten, and J. J. Weis. 1999. Cutting edge: inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by Toll-like receptor 2. J. Immunol. 1632382-2386. [PubMed] [Google Scholar]
- 17.Jones, B. C., N. J. Logsdon, K. Josephson, J. Cook, P. A. Barry, and M. R. Walter. 2002. Crystal structure of human cytomegalovirus IL-10 bound to soluble human IL-10R1. Proc. Natl. Acad. Sci. USA 999404-9409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurtenbach, K., K. Hanincova, J. I. Tsao, G. Margos, D. Fish, and N. H. Ogden. 2006. Fundamental processes in the evolutionary ecology of Lyme borreliosis. Nat. Rev. Microbiol. 4660-669. [DOI] [PubMed] [Google Scholar]
- 19.Lazarus, J. J., M. J. Meadows, R. E. Lintner, and R. M. Wooten. 2006. IL-10 deficiency promotes increased Borrelia burgdorferi clearance predominantly through enhanced innate immune responses. J. Immunol. 1777076-7085. [DOI] [PubMed] [Google Scholar]
- 20.Liang, F. T., F. K. Nelson, and E. Fikrig. 2002. Molecular adaptation of Borrelia burgdorferi in the murine host. J. Exp. Med. 196275-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu, N., R. R. Montgomery, S. W. Barthold, and L. K. Bockenstedt. 2004. Myeloid differentiation antigen 88 deficiency impairs pathogen clearance but does not alter inflammation in Borrelia burgdorferi-infected mice. Infect. Immun. 723195-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma, Y., K. P. Seiler, K. F. Tai, L. Yang, M. Woods, and J. J. Weis. 1994. Outer surface lipoproteins of Borrelia burgdorferi stimulate nitric oxide production by the cytokine-inducible pathway. Infect. Immun. 623663-3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma, Y., and J. J. Weis. 1993. Borrelia burgdorferi outer surface lipoproteins OspA and OspB possess B-cell mitogenic and cytokine-stimulatory properties. Infect. Immun. 613843-3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marietta, E. V., J. J. Weis, and J. H. Weis. 1997. CD28 expression by mouse mast cells is modulated by lipopolysaccharide and outer surface protein A lipoprotein from Borrelia burgdorferi. J. Immunol. 1592840-2848. [PubMed] [Google Scholar]
- 25.McGuirk, P., and K. H. Mills. 2000. Direct anti-inflammatory effect of a bacterial virulence factor: IL-10-dependent suppression of IL-12 production by filamentous hemagglutinin from Bordetella pertussis. Eur. J. Immunol. 30415-422. [DOI] [PubMed] [Google Scholar]
- 26.Mege, J. L., S. Meghari, A. Honstettre, C. Capo, and D. Raoult. 2006. The two faces of interleukin 10 in human infectious diseases. Lancet Infect. Dis. 6557-569. [DOI] [PubMed] [Google Scholar]
- 27.Moore, K. W., R. de Waal Malefyt, R. L. Coffman, and A. O'Garra. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19683-765. [DOI] [PubMed] [Google Scholar]
- 28.Morrison, T. B., J. H. Weis, and J. J. Weis. 1997. Borrelia burgdorferi outer surface protein A (OspA) activates and primes human neutrophils. J. Immunol. 1584838-4845. [PubMed] [Google Scholar]
- 29.Murthy, P. K., V. A. Dennis, B. L. Lasater, and M. T. Philipp. 2000. Interleukin-10 modulates proinflammatory cytokines in the human monocytic cell line THP-1 stimulated with Borrelia burgdorferi lipoproteins. Infect. Immun. 686663-6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Norgard, M. V., L. L. Arndt, D. R. Akins, L. L. Curetty, D. A. Harrich, and J. D. Radolf. 1996. Activation of human monocytic cells by Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides proceeds via a pathway distinct from that of lipopolysaccharide but involves transcriptional activator NF-κB. Infect. Immun. 643845-3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nowalk, A. J., R. D. Gilmore, Jr., and J. A. Carroll. 2006. Serologic proteome analysis of Borrelia burgdorferi membrane-associated proteins. Infect. Immun. 743864-3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raftery, M. J., D. Wieland, S. Gronewald, A. A. Kraus, T. Giese, and G. Schonrich. 2004. Shaping phenotype, function, and survival of dendritic cells by cytomegalovirus-encoded IL-10. J. Immunol. 1733383-3391. [DOI] [PubMed] [Google Scholar]
- 33.Redpath, S., P. Ghazal, and N. R. Gascoigne. 2001. Hijacking and exploitation of IL-10 by intracellular pathogens. Trends Microbiol. 986-92. [DOI] [PubMed] [Google Scholar]
- 34.Ribeiro-Rodrigues, R., T. Resende Co, R. Rojas, Z. Toossi, R. Dietze, W. H. Boom, E. Maciel, and C. S. Hirsch. 2006. A role for CD4+ CD25+ T cells in regulation of the immune response during human tuberculosis. Clin. Exp. Immunol. 14425-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salazar, J. C., C. D. Pope, M. W. Moore, J. Pope, T. G. Kiely, and J. D. Radolf. 2005. Lipoprotein-dependent and -independent immune responses to spirochetal infection. Clin. Diagn. Lab Immunol. 12949-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwan, T. G., J. Piesman, W. T. Golde, M. C. Dolan, and P. A. Rosa. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. USA 922909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Setubal, J. C., M. Reis, J. Matsunaga, and D. A. Haake. 2006. Lipoprotein computational prediction in spirochaetal genomes. Microbiology 152113-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sing, A., A. Roggenkamp, A. M. Geiger, and J. Heesemann. 2002. Yersinia enterocolitica evasion of the host innate immune response by V antigen-induced IL-10 production of macrophages is abrogated in IL-10-deficient mice. J. Immunol. 1681315-1321. [DOI] [PubMed] [Google Scholar]
- 39.Sing, A., D. Rost, N. Tvardovskaia, A. Roggenkamp, A. Wiedemann, C. J. Kirschning, M. Aepfelbacher, and J. Heesemann. 2002. Yersinia V-antigen exploits Toll-like receptor 2 and CD14 for interleukin 10-mediated immunosuppression. J. Exp. Med. 1961017-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sjowall, J., A. Carlsson, O. Vaarala, S. Bergstrom, J. Ernerudh, P. Forsberg, and C. Ekerfelt. 2005. Innate immune responses in Lyme borreliosis: enhanced tumour necrosis factor-alpha and interleukin-12 in asymptomatic individuals in response to live spirochetes. Clin. Exp. Immunol. 14189-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steere, A. C., J. Coburn, and L. Glickstein. 2004. The emergence of Lyme disease. J. Clin. Invest. 1131093-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thoma-Uszynski, S., S. M. Kiertscher, M. T. Ochoa, D. A. Bouis, M. V. Norgard, K. Miyake, P. J. Godowski, M. D. Roth, and R. L. Modlin. 2000. Activation of Toll-like receptor 2 on human dendritic cells triggers induction of IL-12, but not IL-10. J. Immunol. 1653804-3810. [DOI] [PubMed] [Google Scholar]
- 43.Tokarz, R., J. M. Anderton, L. I. Katona, and J. L. Benach. 2004. Combined effects of blood and temperature shift on Borrelia burgdorferi gene expression as determined by whole genome DNA array. Infect. Immun. 725419-5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turner, J., M. Gonzalez-Juarrero, D. L. Ellis, R. J. Basaraba, A. Kipnis, I. M. Orme, and A. M. Cooper. 2002. In vivo IL-10 production reactivates chronic pulmonary tuberculosis in C57BL/6 mice. J. Immunol. 1696343-6351. [DOI] [PubMed] [Google Scholar]
- 45.Weis, J. J., Y. Ma, and L. F. Erdile. 1994. Biological activities of native and recombinant Borrelia burgdorferi outer surface protein A: dependence on lipid modification. Infect. Immun. 624632-4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weiss, L., V. Donkova-Petrini, L. Caccavelli, M. Balbo, C. Carbonneil, and Y. Levy. 2004. Human immunodeficiency virus-driven expansion of CD4+ CD25+ regulatory T cells, which suppress HIV-specific CD4 T-cell responses in HIV-infected patients. Blood 1043249-3256. [DOI] [PubMed] [Google Scholar]
- 47.Wooten, R. M., Y. Ma, R. A. Yoder, J. P. Brown, J. H. Weis, J. F. Zachary, C. J. Kirschning, and J. J. Weis. 2002. Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. J. Immunol. 168348-355. [DOI] [PubMed] [Google Scholar]
- 48.Wooten, R. M., V. R. Modur, T. M. McIntyre, and J. J. Weis. 1996. Borrelia burgdorferi outer membrane protein A induces nuclear translocation of nuclear factor-kappa B and inflammatory activation in human endothelial cells. J. Immunol. 1574584-4590. [PubMed] [Google Scholar]
- 49.Wooten, R. M., T. B. Morrison, J. H. Weis, S. D. Wright, R. Thieringer, and J. J. Weis. 1998. The role of CD14 in signaling mediated by outer membrane lipoproteins of Borrelia burgdorferi. J. Immunol. 1605485-5492. [PubMed] [Google Scholar]
- 50.Wooten, R. M., and J. J. Weis. 2001. Host-pathogen interactions promoting inflammatory Lyme arthritis: use of mouse models for dissection of disease processes. Curr. Opin. Microbiol. 4274-279. [DOI] [PubMed] [Google Scholar]
- 51.Xu, Q., S. V. Seemanapalli, K. E. Reif, C. R. Brown, and F. T. Liang. 2007. Increasing the recruitment of neutrophils to the site of infection dramatically attenuates Borrelia burgdorferi infectivity. J. Immunol. 1785109-5115. [DOI] [PubMed] [Google Scholar]
- 52.Yoder, A., X. Wang, Y. Ma, M. T. Philipp, M. Heilbrun, J. H. Weis, C. J. Kirschning, R. M. Wooten, and J. J. Weis. 2003. Tripalmitoyl-S-glyceryl-cysteine-dependent OspA vaccination of Toll-like receptor 2-deficient mice results in effective protection from Borrelia burgdorferi challenge. Infect. Immun. 713894-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoon, S. I., B. C. Jones, N. J. Logsdon, and M. R. Walter. 2005. Same structure, different function crystal structure of the Epstein-Barr virus IL-10 bound to the soluble IL-10R1 chain. Structure 13551-564. [DOI] [PubMed] [Google Scholar]
- 54.Yrjanainen, H., J. Hytonen, X. Y. Song, J. Oksi, K. Hartiala, and M. K. Viljanen. 2007. Anti-tumor necrosis factor-alpha treatment activates Borrelia burgdorferi spirochetes 4 weeks after ceftriaxone treatment in C3H/He mice. J. Infect. Dis. 1951489-1496. [DOI] [PubMed] [Google Scholar]