Abstract
Neisseria meningitidis is a global cause of meningitis and septicemia. Immunity to N. meningitidis involves both innate and specific mechanisms with killing by serum bactericidal activity and phagocytic cells. C-reactive protein (CRP) is an acute-phase serum protein that has been shown to help protect the host from several bacterial pathogens, which it recognizes by binding to phosphorylcholine (PC) on their surfaces. Pathogenic Neisseria species can exhibit phase-variable PC modification on type 1 and 2 pili. We have shown that CRP can bind to piliated meningococci in a classical calcium-dependent manner. The binding of CRP to the meningococcus was concentration dependent, of low affinity, and specific for PC. CRP appears to act as an opsonin for N. meningitidis, as CRP-opsonized bacteria showed increased uptake by human macrophages and neutrophils. Further investigation into the downstream effects of CRP-bound N. meningitidis may lead us to a better understanding of meningococcal infection and help direct more effective therapeutic interventions.
Neisseria meningitidis is an important global pathogen which causes meningitis (with mortality rates of 4 to 6%) and septic shock (with mortality rates of up to 40%) (15). Successful capsule-based vaccines against serogroup A and C strains of N. meningitidis have been developed, but the serogroup B capsule is poorly immunogenic, and cross-reactivity of anti-serogroup B antibodies with host antigens has prevented the adoption of a similar approach for serogroup B disease. The production of a serogroup B vaccine has therefore become a priority in many developed countries. N. meningitidis is carried harmlessly in the nasopharynx by an estimated 10% of the world's population (36). In some individuals, however, virulent meningococci are capable of attaching to epithelial cells, entering the cells by phagocytosis, and accumulating within phagocytic vacuoles (32). Multiplication of meningococci within the blood and at secondary sites of infection, such as the meninges, pericardium, and joints, causes systemic release of proinflammatory cytokines, including interleukins 1 and 6 and tumor necrosis factor alpha.
Immunity to N. meningitidis involves both innate and specific mechanisms. Carriage of commensal strains of Neisseria can elicit antibody responses that are cross-protective against pathogenic meningococci. However, although bactericidal antibodies can be protective, complement deficiencies can render individuals more susceptible to meningococcal disease. For example, individuals lacking components of the terminal membrane attack complex are 1,000 to 10,000 times more likely to develop disseminated meningococcal disease in their lifetime than the general population (26). Moreover, the organism has evolved several mechanisms to avoid clearance by a competent complement system. These include the recruitment of factor H, a negative regulator of the alternative pathway (28), and the presentation of a polysaccharide capsule and sialylated lipooligosaccharide (LOS), which can down-regulate activation of the alternative pathway and inhibit formation of the membrane attach complex (21). Additionally, absence of serum bactericidal activity does not always correlate with susceptibility (35); thus, other mechanisms of protection, such as opsonization and killing by phagocytic cells, have recently been considered of increased importance, particularly with respect to serogroup B disease.
C-reactive protein (CRP) has long been recognized as a key player in the innate response to pathogenic infection and has been suggested to play a possible role in linking innate and acquired immune responses (41). This ancient member of the pentraxin family was discovered in 1930 as a serum protein that precipitated with the cell wall C polysaccharide of Streptococcus pneumoniae (34). CRP has since been shown to bind to several microorganisms within the respiratory tract and is thought to be involved in inflammatory diseases such as arthritis and atherosclerosis. CRP is a pentameric molecule with two faces: a recognition face, which binds to ligands on apoptotic cells, cell debris, and several microorganisms, and a reverse effector face, which facilitates interaction with host receptors, such as Fcγ receptors (31). These two functional faces allow CRP to recognize pathogens and damaged host cells and to facilitate their removal by recruiting the complement cascade and phagocytic cells. No CRP deficiency states have been identified in humans, suggesting that the protein is crucial to survival. However, at least 31 single-nucleotide polymorphisms have been identified for the human CRP gene (10). One recent study on three single-nucleotide polymorphisms within the CRP gene found polymorphisms that were associated with increased mortality in bacteremic patients (14). Allelic polymorphisms in the FcγRII CRP receptors also occur and may be associated with the severity of meningococcal disease (7). CRP may have a role in meningococcal infections, as higher serum CRP levels are found in patients admitted to hospitals with meningococcal disease than patients suffering from viral infections (6).
The principal ligand for CRP is phosphorylcholine (PC), which is found on the cell wall teichoic acids of S. pneumoniae (9), the LOS of Haemophilus influenzae, and the surface of Pseudomonas aeruginosa (17), Proteus morganii, and Aspergillus fumigatus (22). The expression of PC by pathogens appears to be critical for colonization of the human respiratory tract, and although it may help pathogens colonize the nasopharynx, it can also increase CRP-mediated serum killing of bacteria in human sera and increase antibody-mediated serum resistance in mice (8, 39). There have been no reports on the binding of CRP to pathogenic Neisseria species, although binding between CRP and PC motifs on the LOS of commensal species such as Neisseria lactamica and Neisseria subflava has been demonstrated (29). Unlike the commensal species, pathogenic Neisseria species do not express PC on their LOS, but the epitope is phase-variably expressed on both the class I and class II pili (38). In this report, we confirm the presence of PC on a piliated serogroup B strain of N. meningitidis and demonstrate that CRP binds to this strain in a concentration-dependent, calcium-dependent manner. We also explore the biological consequences of this binding in terms of the uptake of N. meningitidis by human professional phagocytes.
MATERIALS AND METHODS
CRP purification.
Purified (>97%) human CRP was purchased from The Binding Site and further purified by affinity column chromatography based on the method detailed by Volanakis et al. (37). The purified CRP was dialyzed repeatedly in phosphate-buffered saline (PBS) to remove traces of Tris-buffered saline and sodium azide, and purity was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions. Recombinant human CRP (Calbiochem) was also used in noncellular assays. Recombinant CRP was produced in Escherichia coli as a nonglycosylated polypeptide chain with a molecular mass of 115 kDa and the same amino acid sequence as native human CRP (40). The recombinant protein was purified by Calbiochem by PC affinity chromatography and was determined to be pure by SDS-PAGE analysis. The product contained 9 ng/ml LPS as determined by the Limulus assay.
Bacterial culture.
The serogroup B meningococcal strain C311 and a spontaneous nonpiliated mutant, C311p−, were kindly donated by Mumtaz Virji, University of Bristol. An isolate of H44/76 containing the green fluorescent protein (GFP)-expressing plasmid pEG2 was gratefully received from Myron Christodoulides, University of Southampton (11). This plasmid is a hybrid shuttle vector containing the red-shifted gfp gene under the control of a porA promoter and has been modified by replacing the ampicillin resistance cassette (bla) with an erythromycin resistance cassette (ermC), resulting in pEG2-Ery (M. Christodoulides, unpublished data). The GFP-containing plasmid was harvested from strain H44/76 using a standard kit (Qiagen) and transformed into strain C311. Strains were stored frozen in Mueller-Hinton broth (MHB; Oxoid) with 15% glycerol and subcultured onto Columbia blood agar plates (Columbia agar base [Oxoid] supplemented with 5% [vol/vol] defibrinated horse blood [TC Supplies]). Plates were incubated at 35°C with 5% CO2 for 18 to 24 h and then used to inoculate MHB at an optical density at 595 nm of 0.02. Cultures were shaken at 125 rpm at 35°C for 4 h for use in experiments (with the exception of time course studies).
Cell culture.
The premonocytic cell lines THP-1 and U937 were obtained from the European Collection of Cell Cultures and maintained in complete RPMI medium containing 100 units/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, and 10% heat-inactivated fetal bovine serum (all from Invitrogen). For differentiation into adherent macrophages, THP-1 cells were seeded at 4 × 105/ml (400 μl/well) in eight-well plastic chamber slides (Nunc) in medium containing 10 nM phorbol myristate acetate (PMA; Sigma-Aldrich) for 72 h prior to experiments. Similarly, U937 cells were seeded at 2 × 105/ml and were differentiated with 10 nM PMA for 72 h and 100 nM vitamin D3 and 100 nM retinoic acid (both from Sigma-Aldrich) for 24 h.
Preparation of human monocyte-derived macrophages and granulocytes.
Peripheral blood was extracted from healthy consenting volunteers in accordance with local research ethical committee guidelines. Mononuclear cells were separated using Histopaque 1077 as per the manufacturer's instructions (Sigma-Aldrich). Peripheral blood mononuclear cells (PBMCs) were seeded at 3 × 106/ml in eight-well plastic chamber slides in RPMI without serum and positively selected for by plastic adherence. Adherent cells were cultured for 7 days in complete RPMI, and macrophage-like morphology was examined by light microscopy. Granulocytes were separated from blood using Polymorphoprep as per the manufacturer's instructions (Axis-Shield). Granulocytes were seeded at 2 × 107/ml in chamber slides in RPMI without serum and positively selected for by plastic adherence.
CRP binding studies.
Bacteria were grown in MHB for 4 h, spun at 1,000 × g for 15 min, fixed in 4% paraformaldehyde (PFA) for 2 h, spun at 2,000 × g for 2 min, and resuspended in RPMI at an optical density at 595 nm of 5.00. Bacteria were used at an optical density of 5 because this density demonstrated the maximum differential for CRP binding between calcium-rich and calcium-depleted conditions during dose-response experiments (data not shown). A CRP binding assay was developed based on a method developed for Leishmania donovani (12). Briefly, 50-μl aliquots of culture were dispensed onto 96-well Nunc Maxisorp microtiter plates alongside RPMI-only controls. Plates were dried overnight in an incubator at 35°C, and the dried wells were blocked with 1% bovine serum albumin (Sigma-Aldrich) in PBS for 1 h. Wells were washed with PBS, and 25 μl of a given treatment was added to each well in triplicate. Treatments were prepared in PBS and included PBS only, 10 mM EDTA, and PC-calcium salt at various concentrations (Sigma-Aldrich). CRP was conjugated to alkaline phosphatase (AP) using glutaraldehyde in a standard one-stage procedure (1). CRP-AP was diluted to 20 μg/ml in PBS plus 1 mM CaCl2 and added in 25-μl volumes to wells. Plates were incubated in the dark for 2 h and then washed three times with PBS and once with 0.1 M bicarbonate buffer (pH 9.6). Plates were developed with 50 μl/well p-nitrophenyl phosphate (pNPP; Sigma-Aldrich) at 1 mg/ml in 0.1 M bicarbonate buffer (pH 9.6) containing 2 mM MgCl2. Plates were incubated in the dark for a further hour and read at 405 nm with a reference wavelength of 490 nm on a microplate reader (Biotek).
For time course binding studies, the CRP-binding assay used cultures harvested between 2 and 10 h incubation. For saturation experiments the assay was carried out with a range of concentrations of CRP-AP. Competitive-inhibition assays used the original concentration of CRP-AP with added PC-calcium salt (Sigma-Aldrich) diluted in PBS at a range of concentrations. Calcium dependency assays used bacteria dried on plates in bicarbonate buffer instead of RPMI to reduce background calcium levels, and CRP-AP was added with 10-fold dilutions of CaCl2 or no added calcium.
Analysis of PC expression.
Cultures of piliated C311 and the nonpiliated mutant C311p− were grown in MHB, washed, and resuspended in sample buffer every 2 h over a 10-h time course. Cell lysates were subjected to SDS-PAGE through a 5% stacking gel and 12% resolving gel. Gels were blotted onto polyvinylidene fluoride membranes (Roche) and probed with anti-PC antibody (TEPC-15; Sigma-Aldrich). Equal loading of samples was confirmed by Coomassie-R staining of replicate gels. After washing, blots were incubated with peroxidase-conjugated anti-mouse immunoglobulin A (Sigma-Aldrich). The blots were developed on Kodak film using the Luminex system (Roche) and photographed using a Gene Genius bioimaging system, and densitometry of bands was performed using GeneTools software (Synoptics).
Incubation of phagocytes with N. meningitidis.
Bacteria were grown for 4 h in MHB, fixed for 2 h in 4% PFA, washed, and resuspended in PBS containing 10 mM CaCl2 to a concentration of approximately 8 × 108 bacteria per ml. Bacteria were then treated for 30 min with equal volumes of heat-inactivated pooled normal human serum (Sigma-Aldrich), 50 μg/ml purified CRP, or PBS. Treated bacteria were then added to phagocytes at a concentration equivalent to a multiplicity of infection of 200 bacteria per cell and incubated at 37°C and 5% CO2. Macrophages were incubated with bacteria for 2 h, and granulocytes were incubated with bacteria for 20 min only. Wells were washed four times with PBS and fixed with 1% PFA, and slides were mounted with Vectashield soft-set mounting medium containing propidium iodide (Vecta Labs) for examination using a confocal fluorescent microscope (Zeiss LSM 510). At least 100 cells from 10 randomly selected fields of view were analyzed for enumeration of phagocytes colocalized with at least one bacterium and counts of total bacteria and total phagocyte numbers per field of view. The presence of internalized bacteria was confirmed by staining macrophages postinfection with a lipophilic membrane dye (FM 4-64FX; Molecular Probes) as per the manufacturer's instructions and mounting in Vectashield soft-set mounting medium without propidium iodide. This allowed a z stack of images to be taken through the depth of the cell.
Statistical analyses.
All experiments were performed in duplicate or triplicate and repeated at least three times. Data analysis was performed by Student's paired t test using GraphPad Prism 4.1 software. A P value of <0.05 was considered significant.
RESULTS
CRP binds to piliated N. meningitidis during early log-phase growth.
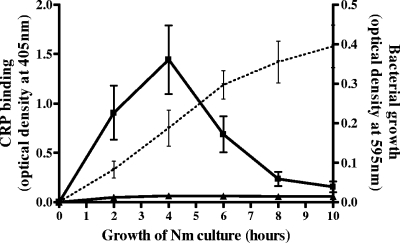
CRP binding to N. meningitidis strain C311 was measured over a 10-h time course. CRP binding was observed in the presence of calcium after 2 h and peaked at 4 h of growth. Binding then rapidly decreased to negligible levels by 8 h and remained low up to the final 10-h time point (Fig. 1).
FIG. 1.
Time course of CRP binding to piliated N. meningitidis C311. Piliated C311 cells were grown in MHB, dried on microtiter plates, and probed with CRP-AP in the presence of calcium (squares) or EDTA (triangles). Plates were washed thoroughly, and the remaining CRP-AP was detected by adding pNPP substrate and measuring the optical density at 405 nm with a reference wavelength of 490 nm. The dashed line is the growth curve of the cultures measured by optical density. Data are the means from four replicate experiments ± standard deviations.
The maximum CRP binding at the 4-h time point corresponds to early log phase in the growth curve of N. meningitidis C311 in MHB. At 6 h, growth of N. meningitidis was still exponential but CRP binding was almost halved. No CRP binding was observed in the absence of calcium.
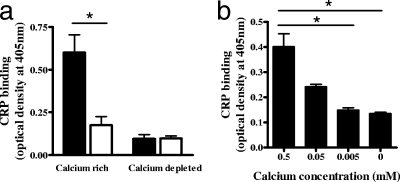
CRP binding to N. meningitidis requires the presence of pili and calcium.
As optimum CRP binding to piliated N. meningitidis was observed after 4 h growth in MHB, the binding assay was repeated at this time point, and the result was compared to CRP binding to a spontaneous nonpiliated mutant of the same strain (C311p−). Negligible CRP binding of either strain was observed in the absence of calcium (Fig. 2a), whereas in the presence of calcium, the piliated strain bound significantly more CRP than the nonpiliated mutant (P = 0.011). There was no significant difference in the binding of CRP to nonpiliated C311p− in the presence or absence of available calcium. To confirm that CRP binding to the piliated strain was dependent on the presence of calcium rather than the absence of EDTA, CRP was incubated with the meningococcus with no added CaCl2 or increasing concentrations of CaCl2 up to the normal 0.5 mM concentration (Fig. 2b). CRP binding increased with calcium concentration and was significantly higher with the standard 0.5 mM CaCl2 than either no CaCl2 or 0.005 mM CaCl2 (P = 0.029 and P = 0.031, respectively).
FIG. 2.
CRP binding to piliated and nonpiliated N. meningitidis in the presence or absence of calcium. (a) Cells of the piliated strain C311 (solid bars) and nonpiliated mutant C311p− (open bars) were dried on microtiter plates and incubated with CRP-AP with 0.5 mM CaCl2 (calcium rich) or 0.5 mM CaCl2 with 10 mM EDTA (calcium depleted). Plates were washed thoroughly, and bound CRP-AP was detected by adding pNPP substrate and measuring the optical density at 405 nm with a reference wavelength of 490 nm. (b) The calcium dependency of CRP binding to the piliated strain was confirmed by repeating the experiment without added calcium alongside 10-fold dilutions of the standard 0.5 mM CaCl2. Data are expressed as the mean absorbances from six replicate experiments plus standard errors of the mean (*, P < 0.05).
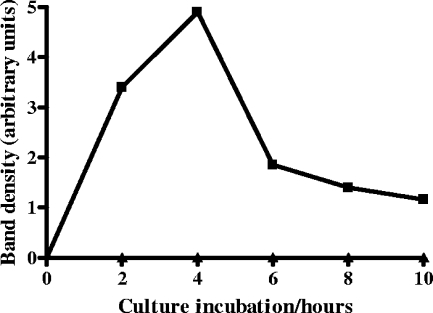
CRP binding to N. meningitidis correlates with PC expression and is inhibited by free PC.
Probing for PC expression in a Western blot of whole-cell lysates from N. meningitidis grown from 2 to 10 h showed that PC was not expressed in the nonpiliated strain at any point during this time course, whereas PC was detected in the piliated strain at all time points tested (Fig. 3). PC expression was compared semiquantitatively by densitometry and showed maximum expression after 4 h of growth, the time point that gave maximum CRP binding. PC expression dropped by a factor of more than 2.5 between 4 and 6 h of growth and remained low for the remaining time points.
FIG. 3.
PC expression by piliated N. meningitidis with time. N. meningitidis C311 (squares) and C311p− (triangles) were grown in broth culture and harvested at 2-h intervals for detection of PC by PAGE and immunoblotting for PC expression with monoclonal antibody TEPC-15. The blot was captured digitally, and densitometry was performed to quantify relative expression of PC. Data are from one representative experiment.
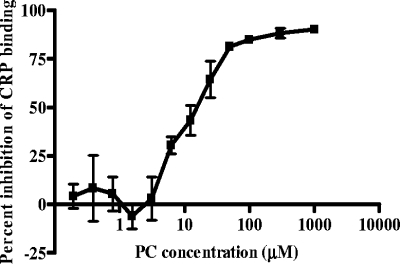
The CRP binding assay was used with increasing concentrations of soluble PC to assay for competitive inhibition. PC concentrations below 1 μM did not inhibit binding of CRP-AP to piliated meningococci, but concentration-dependent inhibition was demonstrated above 5 μM (Fig. 4). At a PC concentration of 20 μM, 50% inhibition was observed, and the inhibitory effect was saturated at PC concentrations above 100 μM.
FIG. 4.
PC inhibition of CRP binding to N. meningitidis. Piliated meningococci were dried on enzyme-linked immunosorbent assay plates and incubated with CRP-AP at a constant concentration of 10 μg/ml with soluble PC at a range of concentrations. Data are means from three replicate experiments ± standard deviations.
CRP binding to N. meningitidis is concentration dependent and of low affinity.
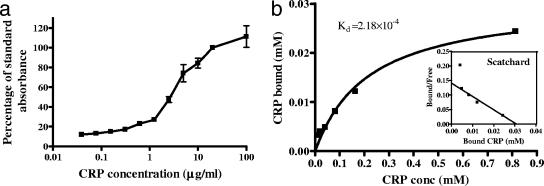
The affinity of binding between CRP and piliated N. meningitidis was investigated by saturation binding experiments. CRP-AP was added at increasing concentrations to microtiter plates coated with a constant number of meningococci, and the binding was measured (expressed as a percentage of the value recorded for 10 μg/ml CRP-AP to overcome interplate and interday variation of the assay). As can be seen from Fig. 5a, CRP binding to N. meningitidis was concentration dependent and began to plateau at 20 μg/ml, whereas CRP concentrations below 1 μg/ml did not show detectable binding to immobilized meningococci. Scatchard analysis of CRP saturation binding allowed the dissociation constant (Kd) to be estimated as 2.18 × 10−4 M (Fig. 5b).
FIG. 5.
Concentration dependency and affinity of CRP binding to N. meningitidis. Piliated meningococci were dried on microtiter plates and incubated with CRP-AP at a range of concentrations. (a) CRP binding was calculated as a percentage of standard absorbance at 405 nm. Data are means from three replicate experiments ± standard deviations. (b) Saturation binding data were used to plot a binding curve of specific binding versus CRP concentration and a Scatchard plot (inset). Nonlinear regression of the binding curve was performed using GraphPad Prism 4.1, and Kd was calculated from the graph as 2.18 × 10−4 M. Data are means of triplicate data from one representative experiment.
CRP opsonization of N. meningitidis increases uptake of meningococci by a macrophage-like cell line and peripheral blood-derived macrophages and granulocytes.
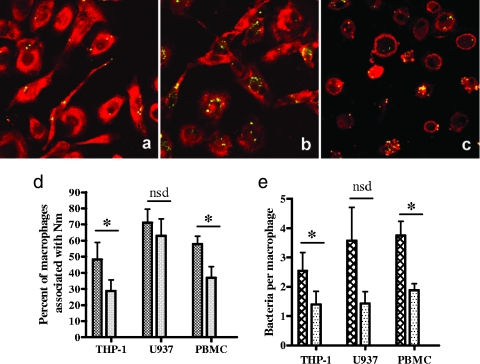
The piliated N. meningitidis strain C311, which expresses GFP, was used to monitor uptake of N. meningitidis by the PMA-differentiated monocytic cell lines THP-1 and U937 and by human peripheral blood-derived macrophages. Confocal fluorescence microscopy revealed an increased association of CRP-opsonized N. meningitidis with peripheral blood-derived macrophages compared to unopsonized bacteria (Fig. 6a and b). Subsequent z-stack analysis of membrane-stained macrophages confirmed that bacteria had been internalized by macrophages, as green meningococci could be seen within the cell wall boundary (Fig. 6c). Increased association and uptake of CRP-opsonized meningococci were also observed for the THP-1 macrophages, although not for U937 cells.
FIG. 6.
Uptake of CRP-opsonized and nonopsonized N. meningitidis by macrophages. Representative microscope images of PBMC-derived macrophages incubated with unopsonized GFP-expressing meningococci (a) and CRP-opsonized GFP-expressing meningococci (b) are shown. Macrophages were stained red with propidium iodide postinfection (a and b), or macrophage membranes were stained with a fluorescent lipophilic dye postinfection to enable z stacking through the macrophage to reveal internalized bacteria (c). (d and e) Enumeration of the association of bacteria with macrophages was carried out on at least 100 cells from 10 randomly selected fields of view for CRP-opsonized bacteria (cross-hatched bars) and unopsonized bacteria (stippled bars) colocalized with THP-1, U937, and peripheral blood-derived macrophages. The percentage of macrophages associated with bacteria was calculated (d) as well as the mean number of adherent or intracellular bacteria per macrophage (e). Data are means from at least three replicate experiments plus standard deviations (*, P < 0.05; nsd, no statistical difference).
Enumeration of bacterium-containing macrophages revealed that significantly more differentiated THP-1 macrophages and donor-derived macrophages were associated with meningococci when the bacteria were opsonized with CRP (P = 0.045 and P = 0.019, respectively) (Fig. 6d). However, no significant increase in the number of differentiated U937 macrophages associated with meningococci was observed with CRP opsonization (P = 0.485) (Fig. 6d). The mean number of bacteria per macrophage was significantly higher for CRP-opsonized bacteria than unopsonized bacteria incubated with THP-1 and donor-derived macrophages (P = 0.014 and P = 0.013, respectively). For U937 cells, the increase in bacteria per macrophage seen with CRP opsonization was not statistically significant (P = 0.112).
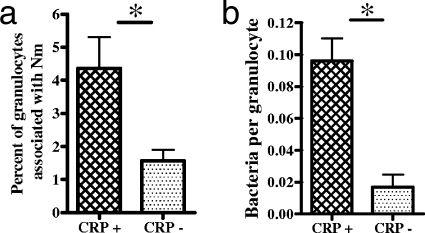
Human peripheral blood-derived granulocytes were incubated with the GFP-expressing piliated N. meningitidis strain C311 with or without CRP opsonization. Opsonization with CRP significantly increased the uptake of N. meningitidis by granulocytes in terms of both the percentage of cells associated with bacteria and the mean number of bacteria per cell (Fig. 7a and b) (P = 0.0478 and P = 0.013).
FIG. 7.
Uptake of N. meningitidis by granulocytes. GFP-expressing C311 cells were grown, fixed in PFA, washed, and opsonized with CRP (cross-hatched bars) or incubated with PBS (stippled bars) before incubation with granulocytes adhered to plastic chamber slides. Slides were washed, fixed, and stained with propidium iodide, and at least 100 cells from 10 randomly selected fields of view were counted for enumeration of the percentage of granulocytes associated with bacteria (a) and the mean number of adherent or intracellular bacteria per granulocyte (b). Data are means from three replicate experiments plus standard deviations (*, P < 0.05).
DISCUSSION
Our data demonstrate that CRP binds to N. meningitidis and that this binding is dependent on the presence of calcium and is likely to be dependent on the expression of PC by the pili of the bacterium. The affinity of binding between CRP and the meningococcus was estimated at 2.18 × 10−4 M. This binding was found to have biological relevance, as the opsonization of PC-expressing N. meningitidis with CRP resulted in increased uptake of the bacteria by macrophages and neutrophils.
Purified human CRP bound to immobilized piliated meningococci of strain C311 but did not bind to the nonpiliated mutant of the same strain, suggesting that the interaction is pilus dependent. This was supported by Western blot analysis of whole-meningococcal-cell lysates, in which CRP was found to bind exclusively to a single band of protein, which was the only band that could be labeled with the SM-1 antipilus antibody (data not shown). These data, therefore, strongly suggest that the pilus is critical for CRP binding to N. meningitidis. Further confirmation of this could be achieved by examining CRP binding to meningococcal mutants with deliberately inactivated pilin genes.
CRP did not bind to the meningococcus in the absence of calcium, and binding increased with the concentration of added calcium, suggesting that the binding was calcium dependent, as is typically seen between CRP and PC. In subsequent 10-h growth curve experiments, PC expression correlated with the level of CRP binding, strongly suggesting PC as the target for CRP on the meningococcus. As described above, the negative control strain for PC expression used in these assays was a natural nonpiliated mutant, making it possible that the failure of this isolate to bind CRP might be due to non-pilus-associated (uncharacterized) changes to the surface of the bacterium. This is, however, highly unlikely, since CRP binding to the meningococcus was shown to be PC-dependent (in our inhibition studies) and Western blotting of N. meningitidis with an anti-PC antibody detected binding only to a single pilus-associated protein (38). In addition, a panel of 64 pathogenic Neisseria strains were screened for the presence of the PC epitope, and only the pilin subunits of class I- and class II-piliated strains were positive for PC expression (29). It is, therefore, highly likely that the nonpiliated strain was unable to bind CRP because it lacked pili and thereby the PC modification of pilus.
Both PC expression and CRP binding peaked at 4 h, coinciding with the early log phase of growth, but decreased thereafter, dropping to negligible levels in late log phase. This pattern of PC expression could be due to direct changes in PC expression or changes in the degree of piliation of the bacteria and is the focus of further investigation. In addition, PC expression can vary with microenvironments within the host. Weiser et al. tested 31 isolates from blood, cerebrospinal fluid, and throat cultures and found that PC expression was lower in N. meningitidis isolates taken from blood than isolates from other sites (38). The cause of these differences in PC expression is unknown, but growth rate-related variation of PC expression may be important in immune responses to N. meningitidis, as growth rates may vary in different niches within the host. Further evidence that CRP binding was PC specific was provided by competition experiments, in which the addition of soluble PC inhibited CRP binding to meningococci in a dose-dependent fashion, with 50% inhibition observed at 20 μM PC.
Several studies have highlighted an increase in circulating CRP levels during infectious disease, with CRP levels in bacteremic patients reported as 10 to 100 μg/ml, compared to less than 10 μg/ml in noninfected controls (16, 24). Our saturation binding assay revealed that CRP binding to N. meningitidis was concentration dependent, with binding being negligible below 1 μg/ml and starting to plateau at 100 μg/ml, well within the clinical range expected during a bacterial infection. Saturation binding experiments were used to estimate the binding affinity of CRP for N. meningitidis (Kd = 2.18 × 10−4 M). This affinity was low compared to the affinity of CRP for PC itself (estimated as 1.8 × 10−7 M by capillary electrophoresis [20]) and for other organisms, such as L. donovani (which has a Kd of 10−11 M [13]). L. donovani, however, is an intracellular parasite which has evolved to utilize the opsonic properties of CRP to increase uptake into human macrophages (13) without increasing activation or killing ability of the cells (4). In contrast, N. meningitidis is an extracellular pathogen and would not be expected to benefit from increased uptake into macrophages, where phagocytosis classically results in clearance of the bacteria. For this reason, a lower affinity of binding is not unexpected.
Our experiments indicated that CRP-opsonized N. meningitidis cells were more readily taken up by macrophages than unopsonized bacteria. This was the case for the cell line THP-1 and for peripheral blood monocyte-derived macrophages from human donors and resulted in an increase in the percent of macrophages associated with bacteria and the mean number of bacteria per macrophage. Due to the limits of the experimental procedure used, bacteria cannot be confirmed as truly intracellular; thus, the phagocytic index was not stated in these experiments. CRP has been shown to increase uptake of opsonized particles via FcγRI, FcγRIIa, and FcγRIIb (2, 3, 5), and work is currently under way to see if the enhanced uptake observed in our experiments is due to Fcγ receptor binding. The finding that the immortalized cell line U937 did not show increased association with CRP-opsonized meningococci was possibly due to differences in the state of activation or expression of cell surface receptors. The cells lines U937 and THP-1 were selected for these experiments because they have both been reported to express FcγRI and FcγRII receptors, but phenotypic differences obviously exist between different cell lines. For example, the expression profile of known or putative NF-κB target genes in response to LPS stimulation in U937 cells is much less similar than THP-1 cells to PBMC-derived macrophages (30).
CRP opsonization of piliated N. meningitidis resulted in a small but significant increase in uptake by human granulocytes. This increase was much smaller than the effect of CRP opsonization on uptake by human macrophages. This difference is likely to be due to the much shorter incubation time used for granulocytes but may also be influenced by the scarcity of FcγRI receptors on neutrophils, which constitutively express FcγRII and FcγRIII but require activation for the upregulation of FcγRI expression. Additionally, CRP binding to FcγRIIa has been shown to be dependent upon clustering of CRP molecules upon the surface of particles such as erythrocytes or S. pneumoniae (K. Bodman-Smith, unpublished data). It is likely, therefore, that scantily opsonized particles such as N. meningitidis may show poor uptake via the FcγRIIa of granulocytes.
CRP binding could be a help or a hindrance to meningococcal pathogenesis, and other CRP-binding organisms provide interesting comparisons when this question is considered. CRP binding helps L. donovani to gain access to macrophages without increasing killing of the organism (4) but has been shown to protect mice from fatal infection with S. pneumoniae (25). Murine studies of CRP function are, however, of limited use due to incompatibilities between human CRP and some mouse complement components (33) and the fact that CRP is not a prototypic acute-phase protein in mice. The closely related pentraxin serum amyloid P is CRP's counterpart in the mouse and has been shown to bind to N. meningitidis in vitro (27). Interestingly, rather than acting as an opsonin to increase phagocyte uptake of the organism, serum amyloid P binding of N. meningitidis actually decreases phagocytosis by neutrophils, enhancing virulence of the organism.
CRP may also play a role in mucosal immunity, since it is present in human nasal secretions and since CRP gene expression has been detected in epithelial cells from nasal polyps and in pharyngeal cell lines (19). Mucosal CRP can bind to PC on the surface of H. influenzae and S. pneumoniae and prevent PC-mediated attachment to epithelial cells via the platelet-activating factor receptor (18). It would be interesting to see what effect CRP has on meningococcal attachment to respiratory epithelial cells. We hypothesize that CRP bound to the pilus of the meningococcus could mask the pilus tip adhesin PilC and thereby diminish binding to epithelial cells via the putative pilus receptor CD46 (23).
In conclusion, we propose a potential role for CRP in the immune response to meningococcal infection. Exploring the downstream immunological consequences of CRP binding to the meningococcus will enable us to better understand meningococcal pathogenesis and may give cause to evaluate the potential use of CRP in therapeutic interventions to meningococcal disease.
Acknowledgments
We are grateful to John Raynes for providing the recombinant CRP, to Mumtaz Virji for providing the C311 strains, and to Myron Christodoulides for providing the GFP-expressing plasmid vector.
This work was funded by a scholarship from the Faculty of Health and Medical Sciences at the University of Surrey.
Editor: V. J. DiRita
Footnotes
Published ahead of print on 14 January 2008.
REFERENCES
- 1.Avrameas, S. 1969. Coupling of enzymes to proteins with glutaraldehyde. Use of the conjugates for the detection of antigens and antibodies. Immunochemistry 643-52. [DOI] [PubMed] [Google Scholar]
- 2.Bodman-Smith, K. B., R. E. Gregory, P. T. Harrison, and J. G. Raynes. 2003. C-reactive protein binds human FcγRIIa and FcγRIIB and is able to influence phagocytosis of erythrocytes by FcãR1 transfected cells. Immunology 11028. [Google Scholar]
- 3.Bodman-Smith, K. B., R. E. Gregory, P. T. Harrison, and J. G. Raynes. 2004. FcγRIIa expression with FcγRI results in C-reactive protein- and IgG-mediated phagocytosis. J. Leukoc. Biol. 751029-1035. [DOI] [PubMed] [Google Scholar]
- 4.Bodman-Smith, K. B., M. Mbuchi, F. J. Culley, P. A. Bates, and J. G. Raynes. 2002. C-reactive protein-mediated phagocytosis of Leishmania donovani promastigotes does not alter parasite survival or macrophage responses. Parasite Immunol. 24447-454. [DOI] [PubMed] [Google Scholar]
- 5.Bodman-Smith, K. B., A. J. Melendez, I. Campbell, P. T. Harrison, J. M. Allen, and J. G. Raynes. 2002. C-reactive protein-mediated phagocytosis and phospholipase D signalling through the high-affinity receptor for immunoglobulin G (FcγRI). Immunology 107252-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borschsenius, F., J. N. Bruun, T. E. Michaelsen, and T. Tonjun. 1986. Serum C-reactive protein in systemic infections due to Neisseria meningitidis. NIPH Ann. 915-21. [PubMed] [Google Scholar]
- 7.Bredius, R. G., B. H. Derkx, C. A. Fijen, T. P. de Wit, M. De Haas, R. S. Weening, J. G. van de Winkel, and T. A. Out. 1994. Fc gamma receptor IIa (CD32) polymorphism in fulminant meningococcal septic shock in children. J. Infect. Dis. 170848-853. [DOI] [PubMed] [Google Scholar]
- 8.Briles, D. E., M. Nahm, K. Schroer, J. Davie, P. Baker, J. Kearney, and R. Barletta. 1981. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 streptococcus pneumoniae. J. Exp. Med. 153694-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brundish, D. E., and J. Baddiley. 1968. Pneumococcal C-substance, a ribitol teichoic acid containing choline phosphate. Biochem. J. 110573-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlson, C. S., S. F. Aldred, P. K. Lee, R. P. Tracy, S. M. Schwartz, M. Rieder, K. Liu, O. D. Williams, C. Iribarren, E. C. Lewis, M. Fornage, E. Boerwinkle, M. Gross, C. Jaquish, D. A. Nickerson, R. M. Myers, D. S. Siscovick, and A. P. Reiner. 2005. Polymorphisms within the C-reactive protein (CRP) promoter region are associated with plasma CRP levels. Am. J. Hum. Genet. 7764-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christodoulides, M., J. S. Everson, B. L. Liu, P. R. Lambden, P. J. Watt, E. J. Thomas, and J. E. Heckels. 2000. Interaction of primary human endometrial cells with Neisseria gonorrhoeae expressing green fluorescent protein. Mol. Microbiol. 3532-43. [DOI] [PubMed] [Google Scholar]
- 12.Culley, F. J., K. B. Bodman-Smith, M. A. Ferguson, A. V. Nikolaev, N. Shantilal, and J. G. Raynes. 2000. C-reactive protein binds to phosphorylated carbohydrates. Glycobiology 1059-65. [DOI] [PubMed] [Google Scholar]
- 13.Culley, F. J., R. A. Harris, P. M. Kaye, K. P. McAdam, and J. G. Raynes. 1996. C-reactive protein binds to a novel ligand on Leishmania donovani and increases uptake into human macrophages. J. Immunol. 1564691-4696. [PubMed] [Google Scholar]
- 14.Eklund, C., R. Huttunen, J. Syrjanen, J. Laine, R. Vuento, and M. Hurme. 2006. Polymorphism of the C-reactive protein gene is associated with mortality in bacteraemia. Scand. J. Infect. Dis. 381069-1073. [DOI] [PubMed] [Google Scholar]
- 15.Emonts, M., J. A. Hazelzet, R. de Groot, and P. W. Hermans. 2003. Host genetic determinants of Neisseria meningitidis infections. Lancet Infect. Dis. 3565-577. [DOI] [PubMed] [Google Scholar]
- 16.Gill, C. W., W. S. Bush, W. M. Burleigh, and C. L. Fischer. 1981. An evaluation of a C-reactive protein assay using a rate immunonephelometric procedure. Am. J. Clin. Pathol. 7550-55. [DOI] [PubMed] [Google Scholar]
- 17.Gillespie, S. H., S. Ainscough, A. Dickens, and J. Lewin. 1996. Phosphorylcholine-containing antigens in bacteria from the mouth and respiratory tract. J. Med. Microbiol. 4435-40. [DOI] [PubMed] [Google Scholar]
- 18.Gould, J. M., and J. N. Weiser. 2002. The inhibitory effect of C-reactive protein on bacterial phosphorylcholine platelet-activating factor receptor-mediated adherence is blocked by surfactant. J. Infect. Dis. 186361-371. [DOI] [PubMed] [Google Scholar]
- 19.Gould, J. M., and J. N. Weiser. 2001. Expression of C-reactive protein in the human respiratory tract. Infect. Immun. 691747-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heegaard, N. H., and F. A. Robey. 1993. A capillary electrophoresis-based assay for the binding of Ca2+ and phosphorylcholine to human C-reactive protein. J. Immunol. Methods. 166103-110. [DOI] [PubMed] [Google Scholar]
- 21.Jarvis, G. A., and N. A. Vedros. 1987. Sialic acid of group B Neisseria meningitidis regulates alternative complement pathway activation. Infect. Immun. 55174-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jensen, T. D., H. Schonheyder, P. Andersen, and A. Stenderup. 1986. Binding of C-reactive protein to Aspergillus fumigatus fractions. J. Med. Microbiol. 21173-177. [DOI] [PubMed] [Google Scholar]
- 23.Kallstrom, H., M. K. Liszewski, J. P. Atkinson, and A. B. Jonsson. 1997. Membrane cofactor protein (MCP or CD46) is a cellular pilus receptor for pathogenic Neisseria. Mol. Microbiol. 25639-647. [DOI] [PubMed] [Google Scholar]
- 24.McCabe, R. E., and J. S. Remington. 1984. C-reactive protein in patients with bacteremia. J. Clin. Microbiol. 20317-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mold, C., S. Nakayama, T. J. Holzer, H. Gewurz, and T. W. Du Clos. 1981. C-reactive protein is protective against Streptococcus pneumoniae infection in mice. J. Exp. Med. 1541703-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgan, B. P., and M. J. Walport. 1991. Complement deficiency and disease. Immunol. Today. 12301-306. [DOI] [PubMed] [Google Scholar]
- 27.Noursadeghi, M., M. C. Bickerstaff, J. R. Gallimore, J. Herbert, J. Cohen, and M. B. Pepys. 2000. Role of serum amyloid P component in bacterial infection: protection of the host or protection of the pathogen. Proc. Natl. Acad. Sci. USA 9714584-14589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schneider, M. C., R. M. Exley, H. Chan, I. Feavers, Y. H. Kang, R. B. Sim, and C. M. Tang. 2006. Functional significance of factor H binding to Neisseria meningitidis. J. Immunol. 1767566-7575. [DOI] [PubMed] [Google Scholar]
- 29.Serino, L., and M. Virji. 2000. Phosphorylcholine decoration of lipopolysaccharide differentiates commensal neisseriae from pathogenic strains: identification of licA-type genes in commensal neisseriae. Mol. Microbiol. 351550-1559. [DOI] [PubMed] [Google Scholar]
- 30.Sharif, O., V. N. Bolshakov, S. Raines, P. Newham, and N. D. Perkins. 2007. Transcriptional profiling of the LPS induced NF-κB response in macrophages. BMC Immunol. 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shrive, A. K., G. M. T. Cheetham, D. Holden, D. A. A. Myles, W. G. Turnell, J. E. Volanakis, M. B. Pepys, A. C. Bloomer, and T. L. Greenhough. 1996. Three dimensional structure of human C-reactive protein. Nat. Struct. Biol. 3346-354. [DOI] [PubMed] [Google Scholar]
- 32.Stephens, D. S., L. H. Hoffman, and Z. A. McGee. 1983. Interaction of Neisseria meningitidis with human nasopharyngeal mucosa: attachment and entry into columnar epithelial cells. J. Infect. Dis. 148369-376. [DOI] [PubMed] [Google Scholar]
- 33.Szalai, A. J., J. L. VanCott, J. R. McGhee, J. E. Volanakis, and W. H. Benjamin, Jr. 2000. Human C-reactive protein is protective against fatal Salmonella enterica serovar Typhimurium infection in transgenic mice. Infect. Immun. 685652-5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tillet, W. S. F. T., Jr. 1930. Serological reactions in pneumonia with a non-protein fraction of pneumococcus. J. Exp. Med. 52561-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toropainen, M., L. Saarinen, E. Wedege, K. Bolstad, T. E. Michaelsen, A. Aase, and H. Kayhty. 2005. Protection by natural human immunoglobulin M antibody to meningococcal serogroup B capsular polysaccharide in the infant rat protection assay is independent of complement-mediated bacterial lysis. Infect. Immun. 734694-4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tzeng, Y. L., and D. S. Stephens. 2000. Epidemiology and pathogenesis of Neisseria meningitidis. Microbes Infect. 2687-700. [DOI] [PubMed] [Google Scholar]
- 37.Volanakis, J. E., W. L. Clements, and R. E. Schrohenloher. 1978. C-reactive protein: purification by affinity chromatography and physiochemical characterisation. J. Immunol. Methods 23285-295. [Google Scholar]
- 38.Weiser, J. N., J. B. Goldberg, N. Pan, L. Wilson, and M. Virji. 1998. The phosphorylcholine epitope undergoes phase variation on a 43-kilodalton protein in Pseudomonas aeruginosa and on pili of Neisseria meningitidis and Neisseria gonorrhoeae. Infect. Immun. 664263-4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiser, J. N., N. Pan, K. L. McGowan, D. Musher, A. Martin, and J. Richards. 1998. Phosphorylcholine on the lipopolysaccharide of Haemophilus influenzae contributes to persistence in the respiratory tract and sensitivity to serum killing mediated by C-reactive protein. J. Exp. Med. 187631-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woo, P., J. R. Korenberg, and A. S. Whitehead. 1985. Characterization of genomic and complementary DNA sequence of human C-reactive protein, and comparison with the complementary DNA sequence of serum amyloid P component. J. Biol. Chem. 26013384-13388. [PubMed] [Google Scholar]
- 41.Zhang, R., L. Becnel, M. Li, C. Chen, and Q. Yao. 2006. C-reactive protein impairs human CD14+ monocyte-derived dendritic cell differentiation, maturation and function. Eur. J. Immunol. 362993-3006. [DOI] [PubMed] [Google Scholar]