Abstract
Radiation and genetic attenuation of Plasmodium sporozoites are two approaches for whole-organism vaccines that protect against malaria. We evaluated chemical attenuation of sporozoites as an alternative vaccine strategy. Sporozoites were treated with the DNA sequence-specific alkylating agent centanamycin, a compound that significantly affects blood stage parasitemia and transmission of murine malaria and also inhibits Plasmodium falciparum growth in vitro. Here we show that treatment of Plasmodium berghei sporozoites with centanamycin impaired parasite function both in vitro and in vivo. The infection of hepatocytes by sporozoites in vitro was significantly reduced, and treated parasites showed arrested liver stage development. Inoculation of mice with sporozoites that were treated in vitro with centanamycin failed to produce blood stage infections. Furthermore, BALB/c and C57BL/6 mice vaccinated with treated sporozoites were protected against subsequent challenge with wild-type sporozoites. Our findings demonstrate that chemically attenuated sporozoites could be a viable alternative for the production of an effective liver stage vaccine for malaria.
The development of an effective vaccine is critical to curb the significant health, social, and economic impacts caused annually by malaria in countries where the disease is endemic (39). Malaria infection involves injection of Plasmodium sporozoites from a mosquito into humans. The sporozoites migrate to the liver, invade hepatocytes, and transform into exoerythrocytic forms (EEFs) that replicate to produce schizonts containing thousands of merozoites (35). These merozoites are released into the host bloodstream and invade erythrocytes. The blood stages of malaria are responsible for producing the symptoms of the disease. Many attempts have been made in recent years to develop effective subunit vaccines composed of recombinant Plasmodium antigens. Due to the complexity of Plasmodium, these vaccines have been only partially effective (2, 12, 14, 38).
Recently, there has been renewed interest in the attenuated whole-organism vaccine strategy (16, 22, 38, 53). The whole-organism approach has historically used radiation-attenuated sporozoites (RAS) to obtain sterile immunity experimentally in both mice and humans (16, 30). The RAS invade hepatocytes in a susceptible host and begin to develop into EEFs, but the majority of parasites fail to undergo nuclear division and do not progress to the merozoite form (44, 45). Using mice, RAS dosing regimens that generate protective immunity have varied, although most regimens require a prime-boost schedule (4, 11, 32, 50). A meta-analysis of 10 years of immunization of human volunteers using irradiated Plasmodium falciparum sporozoites showed a dose response in terms of the immunization dose required for protection (16, 22). One key issue with RAS has been the delivery of the correct irradiation dose to ensure adequate attenuation of the parasite (16, 23, 38, 41, 51). A strategy to overcome this issue has been to generate genetically attenuated sporozoites (GAS) in which genes essential to sporozoite function in parasite strains are deleted. Since the publication of the Plasmodium genome (13), there have been several studies using this strategy in rodent models of malaria. These studies have included deletion of the uis3 (28), uis4 (26), and P36p (50) genes and simultaneous deletion of the uis3 and uis4 (18) genes in Plasmodium berghei, as well as deletions of uis3 and uis4 (46) and simultaneous deletion of the P52 and P36 genes (19) in Plasmodium yoelii. These GAS resemble RAS in terms of invasion of host hepatocytes and arrested development, but GAS-infected hepatocytes disappear almost completely after 24 to 36 h in culture (26, 28, 46, 50), while RAS persist for longer times in the arrested form (20, 45). Like RAS, most GAS need to be delivered using a multiple-dose strategy in order to induce sterile immunity.
We have developed a new strategy to generate attenuated parasites based on the in vitro chemical treatment of sporozoites. We previously reported the antimalarial activity of AT-specific DNA binding agents that exploit the AT richness of the Plasmodium genome (56) and showed that the compound centanamycin has a significant effect both on blood stages and on transmission of malaria to mosquitoes (55). Here we used centanamycin to attenuate P. berghei sporozoites in vitro. Chemically attenuated sporozoites (CAS) showed a significant reduction in hepatocyte infection, and in the hepatocytes that were infected, the sizes of EEFs were greatly reduced. We showed that CAS do not generate blood stage infections in mice and that immunization of BALB/c and C57BL/6 mice with CAS produced sterile immunity against challenge with wild-type sporozoites.
MATERIALS AND METHODS
Treatment of sporozoites with centanamycin.
Anopheles stephensii mosquitoes were maintained at 70% humidity and 22°C and infected with P. berghei ANKA wild-type parasites as described previously (48). Infected mosquito salivary glands were dissected on or about day 18 postfeeding and kept on ice. Sporozoites were quantified by microscopic counting with a hemocytometer. Centanamycin (2 M) was prepared in a PET (polyethylene glycol 400, ethanol, Tween 80)-glucose solution (40). Each group of sporozoites was treated with 2 mM centanamycin diluted in Dulbecco modified Eagle medium (DMEM), while control groups received the same volume of vehicle. Incubation was performed at room temperature for 30, 60, or 90 min, and the sporozoites were centrifuged at 21,000 × g for 5 min at room temperature and resuspended in the appropriate medium for each assay.
Analysis of viability and infectivity of treated sporozoites in vitro.
Mouse hepatoma cells (Hepa 1-6) (25) were grown in DMEM with 10% fetal bovine serum with 1% PSG (penicillin, streptomycin, gentamicin) at 37°C and 5% CO2, and 2 × 105 cells were seeded on glass coverslips in 24-well plates 24 h prior to testing.
Membrane integrity.
Sporozoites were incubated with 10 μg/ml propidium iodide for 5 min at room temperature after incubation with the vehicle or centanamycin. Sporozoites were washed three times, resuspended in 10 μl DMEM, and then wet mounted on a microscope slide and covered with a glass coverslip. The number of fluorescent sporozoites was determined using a Nikon Eclipse E600 microscope. As a control, freshly dissected sporozoites were labeled and quantified soon after dissection to ensure that the dissected sporozoites were viable. Sporozoites that were heat killed at 65°C for 15 min served as a control. Incubation was performed in triplicate in two independent experiments, and 100 sporozoites were counted per well.
Gliding motility.
Glass, eight-chamber Lab-Tek chamber slides (Nalgene) were coated with 5 μg/ml 3D11, a monoclonal antibody directed against the repeat region of P. berghei circumsporozoite protein (57), in phosphate-buffered saline (PBS) overnight at room temperature. The 3D11 antibody was used to capture shed circumsporozoite protein. The wells were washed three times with PBS. For each well 2 × 104 sporozoites were treated as described above. Sporozoites were centrifuged, the medium was replaced with DMEM containing 3% bovine serum albumin (BSA), and the cultures were incubated at 37°C in 5% CO2 for 1 h, after which the sporozoites were fixed with 4% paraformaldehyde at 4°C overnight. Each well was washed with PBS and blocked with 1% BSA in PBS. Biotinylated 3D11 monoclonal antibody (9) was added, followed by addition of streptavidin-fluorescein isothiocyanate (Sigma) and incubation for 1 h at 37°C. The percentage of gliding motility was determined by counting both the number of sporozoites with trails and the number of circles that each trail contained using a Nikon Eclipse E600 microscope. Incubation was performed in triplicate in two independent experiments, and 100 sporozoites were counted per chamber.
Invasion of hepatoma cell line in vitro.
A total of 5 × 104 sporozoites were treated as described above, resuspended in cell medium, and added to wells containing semiconfluent Hepa 1-6 cells. Plates were incubated for 1 h at 37°C, fixed in 4% paraformaldehyde at 4°C, and stained using 3D11 and a double staining technique (36). Intracellular and extracellular sporozoites were differentially stained and counted using a Nikon Eclipse E600 microscope. Incubation was performed in triplicate in two independent experiments, and 100 sporozoites were counted per well.
Liver stage development in vitro.
A total of 5 × 104 sporozoites were treated as described above, resuspended in cell medium, and added to wells containing semiconfluent Hepa 1-6 cells. Plates were incubated for 42 h at 37°C to allow EEF development and then fixed in 4% paraformaldehyde at 4°C overnight and washed with PBS. Each coverslip was blocked and permeabilized in a solution containing 10% goat serum, 1% BSA, 100 mM glycine, 0.05% NaN3 (pH 7), and 0.2% saponin for 30 min at room temperature. The coverslips were then incubated with 2E6 (a monoclonal antibody that recognizes Plasmodium HSP70) (47) for 1 h at room temperature, washed with PBS, and then incubated with anti-mouse fluorescein isothiocyanate-conjugated antibodies (Sigma) for 1 h. Coverslips were washed with PBS and mounted on microscope slides, and the number of EEFs was counted using a Nikon Eclipse E600 microscope. Images were taken with a Leica TCS SP2 AOBS confocal microscope using Leica LCS software (version 5). Incubation was performed in triplicate in two independent experiments, and 50 random fields were counted per coverslip.
Liver stage development in vivo.
Procedures for animal experiments were approved by New York University School of Medicine Institutional Animal Care and Use Committee. Eight-week-old female C57BL/6 mice were inoculated intravenously (i.v.) with 2 × 104 sporozoites treated with centanamycin for 30 min and resuspended in DMEM. Livers were harvested from infected mice 40 h later, as well as from one uninfected mouse. Total RNA was isolated using TRIzol (Invitrogen), and cDNA was synthesized according to the manufacturer's instructions (Applied Biosystems). Malaria infection was quantified using quantitative real-time PCR with primers specific for P. berghei 18S rRNA (5, 6). Tenfold dilutions of a plasmid construct containing the 18S rRNA sequence were used to create a standard curve. Two independent experiments were performed, and each sample was analyzed in triplicate with four mice per treatment group.
Blood stage development from treated sporozoites.
Eight-week-old female BALB/c and C57BL/6 mice were inoculated i.v. with 5 × 104 or 2 × 104 CAS which had been treated with centanamycin for 30 min and resuspended in DMEM. Parasitemia was evaluated from day 3 postinfection (p.i.) onward by using Giemsa-stained thin blood smears. The percentage of parasitemia was calculated by using 1,000 cells per slide. Animals were evaluated for 7, 10, or 21 days after injection of treated sporozoites as indicated below.
Challenge of mice with wild-type sporozoites.
The same groups of mice that were inoculated with CAS to determine blood stage development were challenged by i.v. inoculation using 5 × 103 untreated, wild-type P. berghei ANKA sporozoites (for the BALB/c mice) and 1 × 103 or 1 × 104 untreated wild-type sporozoites (for the C57BL/6 mice) 21, 14, or 10 days after the immunization regimen. Age-matched, naïve mice were inoculated with the same number of sporozoites as infection controls to assess the infectivity of the untreated sporozoites. Parasitemia was evaluated from day 3 p.i. onward by using Giemsa-stained thin blood smears. The percentage of parasitemia was calculated by using 1,000 cells per slide. Animals were evaluated for at least 30 days postchallenge.
Statistical analyses.
All statistical analyses were completed using Prism (version 4.0a). When differences in toxicity, gliding motility, invasion, EEF formation, and quantitative PCR were assessed, normality was tested using the Kolmogorov-Smirnov goodness-of-fit test. Data with a P value of >0.10 were considered normal. The differences were then tested using an analysis of variance (ANOVA) with Tukey's multiple comparison post hoc test. Only the assays showing significant differences were noted.
RESULTS
Chemical attenuation does not result in sporozoite death.
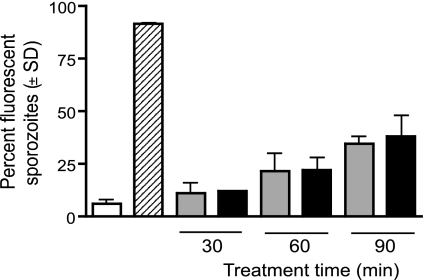
Whole-organism malaria vaccine strategies depend on the parasite being replication deficient, yet still capable of invading host cells and producing antigens to induce an immune response. Incubation of sporozoites for 30, 60, and 90 min with centanamycin in vitro did not result in decreased viability of sporozoites compared to controls (Fig. 1). Propidium iodide, which is membrane impermeable, is frequently used to stain viable cells in a population. Similar labeling with propidium iodide was observed in control and centanamycin-treated sporozoites, suggesting that centanamycin did not affect membrane integrity. As incubation times increased from 30 to 90 min, the number of nonviable sporozoites increased similarly in the drug-treated population compared with controls. A decrease in the viability of sporozoites is expected after dissection from mosquito salivary glands (34).
FIG. 1.
Treatment of sporozoites with centanamycin in vitro does not affect sporozoite membrane integrity. P. berghei sporozoites were incubated with vehicle (gray bars) or 2 mM centanamycin (black bars) for 30, 60, or 90 min before addition of propidium iodide. Control sporozoites were either tested immediately following dissection (open bar) or heat killed (striped bar) for 15 min at 65°C before counting. For each sample, 100 sporozoites were counted in two separate experiments, and the average percentage of staining with propidium iodide is shown.
Gliding motility and invasion of hepatocytes by drug-treated sporozoites.
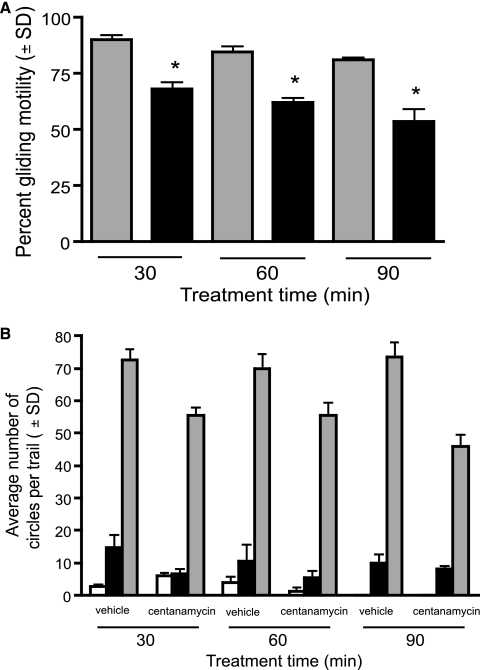
Gliding motility is a feature of Plasmodium sporozoites and is required for invasion of hepatocytes (42). Treatment of sporozoites for 30, 60, or 90 min with centanamycin reduced the motility of sporozoites in a time-dependent manner (Fig. 2A). While the motility of vehicle-treated sporozoites also decreased, the motility of centanamycin-treated sporozoites was approximately 24% less than that of vehicle-treated sporozoites throughout the time course. The sporozoites that remained motile after treatment with centanamycin produced trails whose quality was similar to that of vehicle-treated sporozoite trails (Fig. 2B). The gliding motility of RAS and all GAS reported so far is not different from that of wild-type sporozoites (19, 26, 28, 49, 50).
FIG. 2.
Gliding motility of sporozoites treated with centanamycin in vitro. P. berghei sporozoites were incubated with vehicle or 2 mM centanamycin for 30, 60, or 90 min and then incubated for 1 h at 37°C to allow parasites to move, and then gliding motility was assessed. (A) A significant reduction in the percentage of centanamycin-treated sporozoites (black bars) that exhibited gliding motility was observed at all time points compared to controls (gray bars) (P < 0.0001, ANOVA; n = 100). (B) Quality of the trails, indicated by the number of circles that each sporozoite generated (open bars, 1 trail; black bars, 2 to 10 trails; gray bars, >10 trails). Incubation was performed in triplicate in two independent experiments, and 100 sporozoites were counted per well.
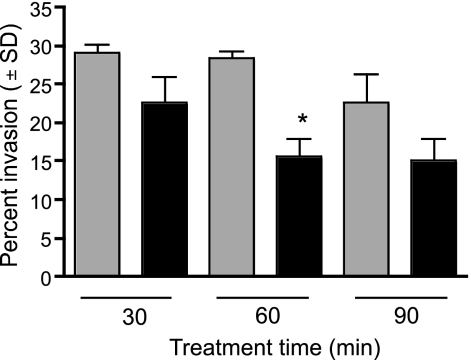
RAS are able to invade hepatocytes with the same efficiency as wild-type sporozoites, even if their development is later impaired (26, 43, 44). We therefore analyzed the ability of centanamycin-treated sporozoites to invade hepatocytes (Hepa 1-6) in vitro. Treated sporozoites invaded hepatocytes efficiently (Fig. 3), although there was an apparent mean reduction in invasion. However, the difference was significant only at the 60-min treatment time when the average for all three experiments was considered. The cause of the reduced invasion rates is not known, but since sporozoite motility is required for invasion (42), the reduced invasion could be a consequence of the decreased motility observed with CAS.
FIG. 3.
Invasion of hepatoma cells in vitro is not significantly reduced after treatment with centanamycin. P. berghei sporozoites were incubated with vehicle (gray bars) or 2 mM centanamycin (black bars) for 30, 60, or 90 min. Sporozoites were stained with 3D11, followed by secondary antibodies, both before and after permeabilization to determine the number of sporozoites that invaded the cells. A significant reduction in invasion was observed only after the 60-min treatment (P = 0.0065, ANOVA; n = 100). Incubation was performed in triplicate in two independent experiments, and 100 sporozoites were counted per well.
EEF development is impaired after drug treatment.
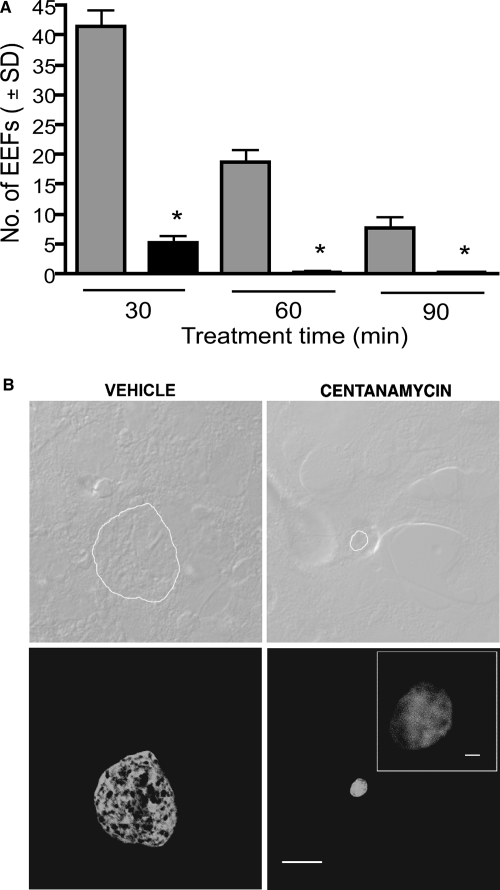
EEF formation is a critical phase in the Plasmodium life cycle and is responsible for the generation of thousands of merozoites that infect erythrocytes. Hepatocytes infected with wild-type sporozoites develop EEFs whose size increases, reaching approximately 10 μm after 48 h of culture (25). There was a decrease in EEF formation by control sporozoites after 60 and 90 min of incubation in vitro, which occurs when sporozoites are not immediately used for infection assays, since sporozoite infectivity is progressively lost after dissection from salivary glands (7). Treatment with centanamycin for 30, 60, and 90 min significantly reduced the number of EEFs formed in vitro after 42 h (Fig. 4A), although a low number of EEFs could still be observed even after the 90-min treatment. This is in sharp contrast to GAS, which, in general, do not produce EEFs persisting beyond 24 to 36 h (19, 26, 28, 50), and it is more similar to RAS, which produce EEFs that persist at least until 48 h (20, 41, 45). While the sizes of vehicle-treated EEFs were homogeneous, centanamycin-treated EEFs were always smaller (Fig. 4B). These results show that centanamycin treatment significantly affects EEF formation in vitro.
FIG. 4.
Treatment of sporozoites with centanamycin decreases the formation of EEFs in hepatoma cells. P. berghei sporozoites were incubated with vehicle (gray bars) or 2 mM centanamycin (black bars) for 30, 60, or 90 min. Sporozoites were added to Hepa 1-6 cells for 42 h at 37°C. (A) The number of EEFs was significantly reduced in all treatment groups (asterisks, P < 0.0001, ANOVA). (B) Phase-contrast images (upper images) and fluorescent images (lower images) of representative EEFs from vehicle-treated (left images) and centanamycin-treated (right images) sporozoites. The outline of the EEF is shown in the upper images. Scale bars = 10 μm. Scale bar in inset = 1 μm.
Centanamycin-treated sporozoites fail to establish infection in mice.
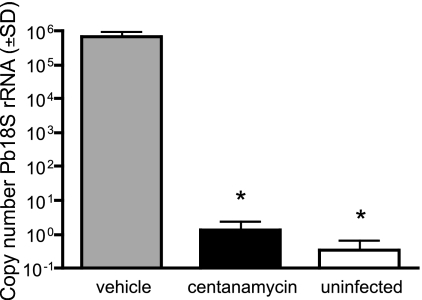
Since the number of EEFs formed in culture was significantly reduced when sporozoites were treated with centanamycin, we analyzed the ability of sporozoites treated for 30 min to establish a liver stage infection in C57BL/6 mice. A total of 2 × 104 treated sporozoites were inoculated into mice i.v., and parasites were allowed to develop for 40 h. Using real-time PCR to quantify the level of infection in the liver, mice inoculated with vehicle-treated sporozoites produced nearly 60 times more copies of P. berghei 18S rRNA than the mice inoculated with centanamycin-treated sporozoites (Fig. 5). The number of copies in mice that received the drug-treated sporozoites was not significantly different than the number of copies in an uninfected control mouse.
FIG. 5.
Sporozoites treated with centanamycin for 30 min do not establish a significant liver stage infection. P. berghei sporozoites were incubated with vehicle or 2 mM centanamycin for 30 min, washed, and then inoculated i.v. into C57BL/6 mice. Forty hours later, mice were sacrificed and total liver RNA was extracted. An uninfected mouse served as a negative control. Malaria infection was determined by quantitative reverse transcription-PCR. Infection is expressed as the number of copies of P. berghei 18S rRNA (Pb18S rRNA). Treatment of sporozoites with centanamycin resulted in significant reduction (asterisks, P < 0.0001, ANOVA) in 18S rRNA levels compared with the vehicle-treated controls. Shown are the results of one of two independent experiments with four mice per treatment group.
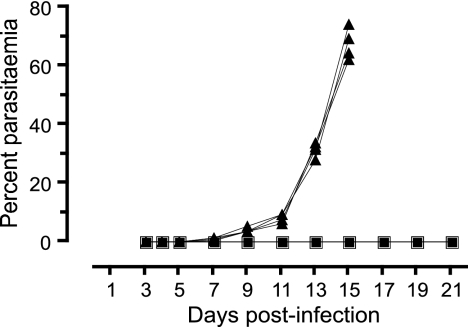
Since the CAS reduced the extent of liver stage infection, we tested the ability of centanamycin-treated sporozoites to block a blood stage infection in both BALB/c and highly susceptible C57BL/6 mice. Mice were infected with 2 × 104 or 5 × 104 sporozoites that were treated with vehicle or centanamycin for only 30 min. BALB/c mice that received the vehicle-treated sporozoites developed hyperparasitemia and were euthanized by day 15 p.i. In contrast, mice that received the centanamycin-treated sporozoites never developed a patent parasitemia during the 21 days of observation (Fig. 6). C57BL/6 mice that received the vehicle-treated sporozoites were euthanized after developing symptoms of cerebral malaria by day 8 p.i., while the mice that received the centanamycin-treated sporozoites never developed patent parasitemia during the 14 days of observation (data not shown). This is in agreement with the in vitro data suggesting that EEFs derived from centanamycin-treated sporozoites do not differentiate into infectious merozoites.
FIG. 6.
Treatment of sporozoites with centanamycin for 30 min in vitro before injection of mice prevents blood stage infection. P. berghei sporozoites were incubated with vehicle (▴) or 2 mM centanamycin (▪) for 30 min before injection of washed sporozoites into BALB/c mice. Development of detectable blood stage parasites was followed for 21 days p.i. Mice that received vehicle-treated sporozoites developed parasites on day 4 p.i. and were euthanized by day 15 p.i. Experiments were performed twice with four mice per group. The results of one representative experiment are shown.
Immunization with treated sporozoites protects mice against subsequent infection.
Some RAS and GAS immunization schemes generate sterile protection in mice against subsequent challenge with wild-type, nonattenuated sporozoites. We used mice that received one dose of the noninfectious CAS described above and challenged them with 5 × 103 or 1 × 103 untreated sporozoites to evaluate the efficacy of CAS as a vaccine. BALB/c mice that received the CAS did not develop any detectable parasitemia over 30 days of observation, while naïve mice inoculated with the same challenge doses developed high levels of parasitemia and were euthanized by day 16 p.i. (Table 1). In C57BL/6 mice given a single immunization there was a 2-day delay in the development of detectable parasitemia with the challenge dose compared to naïve mice. A multiple immunization schedule consisting of 5 × 104 CAS and two doses of 2 × 104 CAS 7 days apart was used to generate sterile immunity in C57BL/6 mice with a challenge dose of 104 untreated sporozoites. This dosing schedule also produced sterile immunity using RAS from the same mosquito batches (Table 1). Most GAS and RAS vaccine schedules require some combination of multiple-dose periods in order to consistently obtain complete protection. CAS fully protected BALB/c mice in two independent experiments using a single-dose schedule. Interestingly, mice that were challenged again with 5 × 103 sporozoites 30 days after the initial challenge did not develop detectable blood stage parasitemia (data not shown).
TABLE 1.
Protection of mice immunized with CAS against challenge with wild-type sporozoites
| Group | Mouse strain | Immunization with RAS or CAS (103 sporozoites)a | Challenge (sporozoites)b | Day of challenge | Sterile immunity | Prepatent period (days) | No. of mice protected (no. challenged)
|
||
|---|---|---|---|---|---|---|---|---|---|
| Controlc | CAS | RAS | |||||||
| 1 | BALB/c | 20 | 5,000 | 21 | Yes | NAd | 0 (4) | 4 (4) | NIe |
| 2 | BALB/c | 20 | 5,000 | 21 | Yes | NA | 0 (4) | 4 (4) | NI |
| 3 | C57BL/6 | 20 | 1,000 | 14 | No | 5f | 0 (4) | 0 (4) | NI |
| 4 | C57BL/6 | 50/20/20 | 10,000 | 10 | Yes | NA | 0 (8) | 4 (4) | 4 (4) |
Groups of mice were immunized with P. berghei ANKA control (vehicle-treated) sporozoites, CAS, or RAS as indicated, using sporozoites isolated from the same mosquito batches; in group 4, mice were immunized three times with CAS or RAS with 7-day intervals between doses.
Groups of mice were challenged with P. berghei ANKA wild-type sporozoites isolated from the same mosquito batches.
Naïve, age-matched mice were used at the time of all immunizations and challenges.
NA, not applicable.
NI, the treatment group was not included.
Naïve, age-matched mice developed patent parasitemia 3 days p.i.
DISCUSSION
Our results show that P. berghei CAS are completely arrested at the liver stage, fail to produce blood stage parasites in mice, and induce sterile protection in BALB/c and C57BL/6 mice following vaccination. A previous study attempted a chemical attenuation strategy by treating P. berghei NK65 sporozoites with high doses (0.8 mg/ml) of chloroquine (33). It was found that using five immunizing doses of 2.5 × 104 sporozoites treated with 0.8 mg/ml of chloroquine for 60 min produced 78.6% protection in mice, although the viability of the sporozoites was not reported. This malaria vaccine strategy, as well as the RAS vaccine approach, was then abandoned in favor of the subunit vaccine approach (22).
With the renewed interest in whole-organism vaccines (22, 38, 53), we evaluated the chemical attenuation of parasites using the DNA sequence-specific alkylating agent centanamycin and characterized its effects on sporozoites both in vitro and in vivo. Centanamycin has been shown to block P. falciparum blood stage growth in vitro, to inhibit blood stage infections with Plasmodium chabaudi adami and P. berghei in mice, and to significantly reduce the transmission potential of P. berghei, with a 99% reduction in sporozoite production (55). Our studies show that treatment of P. berghei ANKA sporozoites with centanamycin for 30, 60, or 90 min in vitro does not affect membrane integrity. We found that there was a moderate decrease in gliding motility of treated sporozoites that probably caused the small decrease observed in hepatocyte invasion in vitro. However, this small decrease in hepatocyte invasion did not seem to affect the capacity of treated sporozoites to induce protective responses in mice.
Hepatocyte invasion by sporozoites is an important step in eliciting an immune response to the parasite. Inactivated sporozoites that are not able to infect hepatocytes have consistently failed to induce protective immune responses (1, 23, 31), although they can efficiently prime the immune system (15). Conversely, malaria-infected hepatocytes and their extracts induce significant protection when they are injected into rats or mice (37, 41). Previous experiments suggested that when animals are immunized with RAS, the protection against a challenge dose of sporozoites is dependent upon the persistence of irradiated sporozoites in the liver (23, 41). However, more recent data indicate that GAS do not require persistence in the liver to induce protective immunity (26, 28, 46, 50). Our study shows that CAS do produce liver stages in vitro, albeit at significantly lower levels (>85% reduction) than control sporozoites, and these liver stages were much smaller than those of the controls. Whereas RAS and GAS both invade liver cells and transform into the rounded trophozoite stage, they generally do not enter schizogony (51). GAS-infected hepatocytes normally do not persist longer than 24 to 36 h (26, 28, 46, 50), compared to RAS-infected hepatocytes, which persist much longer (20, 41, 45). Our study showed that CAS persist in cultured hepatocytes for at least 42 h. Taken together, these results suggest that the CAS strategy is an effective attenuation strategy that can produce the infective liver stages needed to elicit an immune response.
A dose of 2 × 104 CAS in BALB/c and C57BL/6 mice failed to establish a blood stage infection, and the CAS-vaccinated BALB/c mice exhibited protective immunity when they were challenged with 5 × 103 untreated, wild-type sporozoites. A multiple-dose regimen was employed to produce sterile immunity in C57BL/6 mice. The genetic restriction observed, where sterile immunization requires more doses of attenuated sporozoites in C57BL/6 mice than in BALB/c mice, has been reported previously (10, 11, 26, 50). This is probably a consequence of the fact that vaccination with attenuated sporozoites induces different mechanisms of protection in these two mouse strains (10). The highly susceptible C57BL/6 mice (24) require booster doses of vaccine to induce fully effective immune responses. In general, both RAS and GAS require higher initial doses of P. berghei ANKA sporozoites in BALB/c mice (at least 2 × 104 sporozoites) to provide complete protection against lower or similar challenge doses of wild-type parasites (1 × 103 sporozoites) (8, 11, 50). This suggests that the CAS approach could be an efficient approach for producing a whole-organism malaria vaccine.
The immune responses against both RAS and GAS are complex and involve both cell-mediated and humoral immunity (10, 18, 27, 29, 37, 46). In addition, some RAS and GAS seem to induce long-lasting, cross-species protection (11, 31, 32). Attenuation of irradiated sporozoites presumably occurs due to double-strand breaks in the DNA that lead to a block in liver stage development. Each sporozoite would contain a number of strand breaks randomly distributed in its DNA. In the case of CAS treated with centanamycin, the attenuated sporozoites would contain a set of adducts covalently bound to adenine nucleotides (40). This compound, like other AT-specific binding compounds, recognizes selective DNA sequences, and the potential number of adducts can be defined bioinformatically (52, 54). Given that both the CAS and RAS approaches disrupt the integrity of the parasite DNA, it is possible that the immune responses generated by RAS and CAS would be similar, but further studies are necessary to confirm this.
Many issues have been raised concerning the feasibility of both GAS and RAS as whole-organism sporozoite vaccines, including mass production of sterile parasites, proper storage to maintain viability, and the safety of a mosquito-derived vaccine (3, 16, 22, 38). Both types of attenuation have individual inherent weaknesses. In the case of RAS, the overattenuation of sporozoites has been shown to block liver stage development at the trophozoite stage (23, 44) and generate poor protection (23), suggesting that the dose of irradiation is pivotal to the success of each lot of RAS. Uniform exposure of parasites to the radiation source is essential to prevent the escape of sporozoites that could generate a malaria infection following vaccination (16, 38, 51). In contrast, the risk of “breakthrough infections” with GAS is low due to the gene knockout strategy employed (17, 26, 50). Yet the widespread distrust of genetically modified products, especially for a vaccine that would be inoculated into humans and invade host cells, may complicate efforts to utilize GAS in the field. Our proposed CAS vaccine has the advantage that the chemical attenuation process can be strictly controlled, leading to a vaccine that is reproducibly attenuated. Given that centanamycin shows covalent DNA sequence specificity similar to that of adozelesin and that the frequency of binding sites for adozelesin has been estimated to be 440 sites per kb of genomic Plasmodium DNA (52), treatment of sporozoites with centanamycin could potentially saturate these binding sites to obtain a maximal effect on the parasite. Since the generation of viable, cryopreserved sporozoites is currently being optimized (22), chemical attenuation using centanamycin could be considered an additional strategy for the production of whole-organism vaccines against malaria. Although the potential toxicity of residual centanamycin in humans is a concern, the risk may be minimal since free drug can be washed from the parasites before vaccine delivery and the drug that is present in treated sporozoites is covalently bound to parasite DNA and thus not available to modify host DNA. Nevertheless, the risk of toxicity needs to be addressed by in-depth pharmacokinetic and mutagenicity studies. More generally, our results suggest that chemical attenuation with drugs such as centanamycin may be a feasible approach for generating live attenuated vaccines for other major parasites with AT-rich DNA, such as Theileria (21, 54).
Acknowledgments
We thank J. Noonon, S. Gonzalez, and A. Coppi for assistance with mosquito experiments and E. Bettiol for assistance with confocal microscopy. We thank A. Waters, who kindly provided the P. berghei ANKA strain. We thank Spirogen Ltd. and Centana Pharmaceuticals for use of centanamycin.
This work was supported by a Canada Graduate Scholarship from the Natural Sciences and Engineering Research Council of Canada (to L.P.), by Canadian Foundation for Innovation grant 201221 (to T.S.), by Le fonds québécois de la recherche sur la nature et les technologies (FQRNT) Centre for Host-Parasite Interactions grant 87902 (to T.S.), by Canada Research Chair in Immunoparasitology grant 201221 (to T.S.), and by NIH grant RO1 AI 053698 (to A.R.).
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 3 January 2008.
REFERENCES
- 1.Alger, N. E., and J. Harant. 1976. Plasmodium berghei: heat-treated sporozoite vaccination of mice. Exp. Parasitol. 40261-268. [DOI] [PubMed] [Google Scholar]
- 2.Alonso, P. L., J. Sacarlal, J. J. Aponte, A. Leach, E. Macete, J. Milman, I. Mandomando, B. Spiessens, C. Guinovart, M. Espasa, Q. Bassat, P. Aide, O. Ofori-Anyinam, M. M. Navia, S. Corachan, M. Ceuppens, M. C. Dubois, M. A. Demoitie, F. Dubovsky, C. Menendez, N. Tornieporth, W. R. Ballou, R. Thompson, and J. Cohen. 2004. Efficacy of the RTS, S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet 3641411-1420. [DOI] [PubMed] [Google Scholar]
- 3.Ballou, W. R. 2005. Malaria vaccines in development. Expert Opin. Emerg. Drugs 10489-503. [DOI] [PubMed] [Google Scholar]
- 4.Belnoue, E., F. T. Costa, T. Frankenberg, A. M. Vigario, T. Voza, N. Leroy, M. M. Rodrigues, I. Landau, G. Snounou, and L. Renia. 2004. Protective T cell immunity against malaria liver stage after vaccination with live sporozoites under chloroquine treatment. J. Immunol. 1722487-2495. [DOI] [PubMed] [Google Scholar]
- 5.Bhanot, P., K. Schauer, I. Coppens, and V. Nussenzweig. 2005. A surface phospholipase is involved in the migration of Plasmodium sporozoites through cells. J. Biol. Chem. 2806752-6760. [DOI] [PubMed] [Google Scholar]
- 6.Bruna-Romero, O., J. C. Hafalla, G. Gonzalez-Aseguinolaza, G. Sano, M. Tsuji, and F. Zavala. 2001. Detection of malaria liver-stages in mice infected through the bite of a single Anopheles mosquito using a highly sensitive real-time PCR. Int. J. Parasitol. 311499-1502. [DOI] [PubMed] [Google Scholar]
- 7.Carrolo, M., S. Giordano, L. Cabrita-Santos, S. Corso, A. M. Vigario, S. Silva, P. Leiriao, D. Carapau, R. Armas-Portela, P. M. Comoglio, A. Rodriguez, and M. M. Mota. 2003. Hepatocyte growth factor and its receptor are required for malaria infection. Nat. Med. 91363-1369. [DOI] [PubMed] [Google Scholar]
- 8.Chatterjee, S., E. Ngonseu, C. Van Overmeir, A. Correwyn, P. Druilhe, and M. Wery. 2001. Rodent malaria in the natural host—irradiated sporozoites of Plasmodium berghei induce liver-stage specific immune responses in the natural host Grammomys surdaster and protect immunized Grammomys against P. berghei sporozoite challenge. Afr. J. Med. Med. Sci. 30(Suppl.)25-33. [PubMed] [Google Scholar]
- 9.Coppi, A., M. Cabinian, D. Mirelman, and P. Sinnis. 2006. Antimalarial activity of allicin, a biologically active compound from garlic cloves. Antimicrob. Agents Chemother. 501731-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doolan, D. L., and S. L. Hoffman. 2000. The complexity of protective immunity against liver-stage malaria. J. Immunol. 1651453-1462. [DOI] [PubMed] [Google Scholar]
- 11.Douradinha, B., M. R. van Dijk, R. Ataide, G. J. van Gemert, J. Thompson, J. F. Franetich, D. Mazier, A. J. Luty, R. Sauerwein, C. J. Janse, A. P. Waters, and M. M. Mota. 2007. Genetically attenuated P36p-deficient Plasmodium berghei sporozoites confer long-lasting and partial cross-species protection. Int. J. Parasitol. 371511-1519. [DOI] [PubMed] [Google Scholar]
- 12.Epstein, J. E., B. Giersing, G. Mullen, V. Moorthy, and T. L. Richie. 2007. Malaria vaccines: are we getting closer? Curr. Opin. Mol. Ther. 912-24. [PubMed] [Google Scholar]
- 13.Gardner, M. J., N. Hall, E. Fung, O. White, M. Berriman, R. W. Hyman, J. M. Carlton, A. Pain, K. E. Nelson, S. Bowman, I. T. Paulsen, K. James, J. A. Eisen, K. Rutherford, S. L. Salzberg, A. Craig, S. Kyes, M. S. Chan, V. Nene, S. J. Shallom, B. Suh, J. Peterson, S. Angiuoli, M. Pertea, J. Allen, J. Selengut, D. Haft, M. W. Mather, A. B. Vaidya, D. M. Martin, A. H. Fairlamb, M. J. Fraunholz, D. S. Roos, S. A. Ralph, G. I. McFadden, L. M. Cummings, G. M. Subramanian, C. Mungall, J. C. Venter, D. J. Carucci, S. L. Hoffman, C. Newbold, R. W. Davis, C. M. Fraser, and B. Barrell. 2002. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419498-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Genton, B., I. Betuela, I. Felger, F. Al-Yaman, R. F. Anders, A. Saul, L. Rare, M. Baisor, K. Lorry, G. V. Brown, D. Pye, D. O. Irving, T. A. Smith, H. P. Beck, and M. P. Alpers. 2002. A recombinant blood-stage malaria vaccine reduces Plasmodium falciparum density and exerts selective pressure on parasite populations in a phase 1-2b trial in Papua New Guinea. J. Infect. Dis. 185820-827. [DOI] [PubMed] [Google Scholar]
- 15.Hafalla, J. C., U. Rai, A. Morrot, D. Bernal-Rubio, F. Zavala, and A. Rodriguez. 2006. Priming of CD8+ T cell responses following immunization with heat-killed Plasmodium sporozoites. Eur. J. Immunol. 361179-1186. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman, S. L., L. M. Goh, T. C. Luke, I. Schneider, T. P. Le, D. L. Doolan, J. Sacci, P. de la Vega, M. Dowler, C. Paul, D. M. Gordon, J. A. Stoute, L. W. Church, M. Sedegah, D. G. Heppner, W. R. Ballou, and T. L. Richie. 2002. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J. Infect. Dis. 1851155-1164. [DOI] [PubMed] [Google Scholar]
- 17.Janse, C. J., J. Ramesar, and A. P. Waters. 2006. High-efficiency transfection and drug selection of genetically transformed blood stages of the rodent malaria parasite Plasmodium berghei. Nat. Protoc. 1346-356. [DOI] [PubMed] [Google Scholar]
- 18.Jobe, O., J. Lumsden, A. K. Mueller, J. Williams, H. Silva-Rivera, S. H. Kappe, R. J. Schwenk, K. Matuschewski, and U. Krzych. 2007. Genetically attenuated Plasmodium berghei liver stages induce sterile protracted protection that is mediated by major histocompatibility complex class I-dependent interferon-gamma-producing CD8+ T cells. J. Infect. Dis. 196599-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Labaied, M., A. Harupa, R. F. Dumpit, I. Coppens, S. A. Mikolajczak, and S. H. Kappe. 2007. Plasmodium yoelii sporozoites with simultaneous deletion of P52 and P36 are completely attenuated and confer sterile immunity against infection. Infect. Immun. 753758-3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leiriao, P., S. S. Albuquerque, S. Corso, G. J. van Gemert, R. W. Sauerwein, A. Rodriguez, S. Giordano, and M. M. Mota. 2005. HGF/MET signalling protects Plasmodium-infected host cells from apoptosis. Cell. Microbiol. 7603-609. [DOI] [PubMed] [Google Scholar]
- 21.Lightowlers, M. W. 1994. Vaccination against animal parasites. Vet. Parasitol. 54177-204. [DOI] [PubMed] [Google Scholar]
- 22.Luke, T. C., and S. L. Hoffman. 2003. Rationale and plans for developing a non-replicating, metabolically active, radiation-attenuated Plasmodium falciparum sporozoite vaccine. J. Exp. Biol. 2063803-3808. [DOI] [PubMed] [Google Scholar]
- 23.Mellouk, S., F. Lunel, M. Sedegah, R. L. Beaudoin, and P. Druilhe. 1990. Protection against malaria induced by irradiated sporozoites. Lancet 335721. [DOI] [PubMed] [Google Scholar]
- 24.Most, H., R. S. Nussenzweig, J. Vanderberg, R. Herman, and M. Yoeli. 1966. Susceptibility of genetically standardized (JAX) mouse strains to sporozoite- and blood-induced Plasmodium berghei infections. Mil. Med. 131(Suppl.)915-918. [PubMed] [Google Scholar]
- 25.Mota, M. M., and A. Rodriguez. 2000. Plasmodium yoelii: efficient in vitro invasion and complete development of sporozoites in mouse hepatic cell lines. Exp. Parasitol. 96257-259. [DOI] [PubMed] [Google Scholar]
- 26.Mueller, A. K., N. Camargo, K. Kaiser, C. Andorfer, U. Frevert, K. Matuschewski, and S. H. Kappe. 2005. Plasmodium liver stage developmental arrest by depletion of a protein at the parasite-host interface. Proc. Natl. Acad. Sci. USA 1023022-3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mueller, A. K., M. Deckert, K. Heiss, K. Goetz, K. Matuschewski, and D. Schluter. 2007. Genetically attenuated Plasmodium berghei liver stages persist and elicit sterile protection primarily via CD8 T cells. Am. J. Pathol. 171107-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mueller, A. K., M. Labaied, S. H. Kappe, and K. Matuschewski. 2005. Genetically modified Plasmodium parasites as a protective experimental malaria vaccine. Nature 433164-167. [DOI] [PubMed] [Google Scholar]
- 29.Nussenzweig, R. S., and C. A. Long. 1994. Malaria vaccines: multiple targets. Science 2651381-1383. [DOI] [PubMed] [Google Scholar]
- 30.Nussenzweig, R. S., J. Vanderberg, H. Most, and C. Orton. 1967. Protective immunity produced by the injection of X-irradiated sporozoites of Plasmodium berghei. Nature 216160-162. [DOI] [PubMed] [Google Scholar]
- 31.Nussenzweig, R. S., J. Vanderberg, G. L. Spitalny, C. I. Rivera, C. Orton, and H. Most. 1972. Sporozoite-induced immunity in mammalian malaria. A review. Am. J. Trop. Med. Hyg. 21722-728. [DOI] [PubMed] [Google Scholar]
- 32.Nussenzweig, R. S., J. P. Vanderberg, H. Most, and C. Orton. 1969. Specificity of protective immunity produced by X-irradiated Plasmodium berghei sporozoites. Nature 222488-489. [DOI] [PubMed] [Google Scholar]
- 33.Orjih, A. U., A. H. Cochrane, and R. S. Nussenzweig. 1982. Comparative studies on the immunogenicity of infective and attenuated sporozoites of Plasmodium berghei. Trans. R. Soc. Trop. Med. Hyg. 7657-61. [DOI] [PubMed] [Google Scholar]
- 34.Porter, R. J., R. L. Laird, and E. M. Dusseau. 1952. Studies on malarial sporozoites. I. Effect of various environmental conditions. Exp. Parasitol. 1229-244. [DOI] [PubMed] [Google Scholar]
- 35.Prudencio, M., A. Rodriguez, and M. M. Mota. 2006. The silent path to thousands of merozoites: the Plasmodium liver stage. Nat. Rev. Microbiol. 4849-856. [DOI] [PubMed] [Google Scholar]
- 36.Renia, L., F. Miltgen, Y. Charoenvit, T. Ponnudurai, J. P. Verhave, W. E. Collins, and D. Mazier. 1988. Malaria sporozoite penetration. A new approach by double staining. J. Immunol. Methods 112201-205. [DOI] [PubMed] [Google Scholar]
- 37.Renia, L., M. M. Rodrigues, and V. Nussenzweig. 1994. Intrasplenic immunization with infected hepatocytes: a mouse model for studying protective immunity against malaria pre-erythrocytic stage. Immunology 82164-168. [PMC free article] [PubMed] [Google Scholar]
- 38.Richie, T. 2006. High road, low road? Choices and challenges on the pathway to a malaria vaccine. Parasitology 133S113-S144. [DOI] [PubMed] [Google Scholar]
- 39.Sachs, J., and P. Malaney. 2002. The economic and social burden of malaria. Nature 415680-685. [DOI] [PubMed] [Google Scholar]
- 40.Sato, A., L. McNulty, K. Cox, S. Kim, A. Scott, K. Daniell, K. Summerville, C. Price, S. Hudson, K. Kiakos, J. A. Hartley, T. Asao, and M. Lee. 2005. A novel class of in vivo active anticancer agents: achiral seco-amino- and seco-hydroxycyclopropylbenz[e]indolone (seco-CBI) analogues of the duocarmycins and CC-1065. J. Med. Chem. 483903-3918. [DOI] [PubMed] [Google Scholar]
- 41.Scheller, L. F., and A. F. Azad. 1995. Maintenance of protective immunity against malaria by persistent hepatic parasites derived from irradiated sporozoites. Proc. Natl. Acad. Sci. USA 924066-4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sibley, L. D. 2004. Intracellular parasite invasion strategies. Science 304248-253. [DOI] [PubMed] [Google Scholar]
- 43.Sigler, C. I., P. Leland, and M. R. Hollingdale. 1984. In vitro infectivity of irradiated Plasmodium berghei sporozoites to cultured hepatoma cells. Am. J. Trop. Med. Hyg. 33544-547. [DOI] [PubMed] [Google Scholar]
- 44.Silvie, O., J. P. Semblat, J. F. Franetich, L. Hannoun, W. Eling, and D. Mazier. 2002. Effects of irradiation on Plasmodium falciparum sporozoite hepatic development: implications for the design of pre-erythrocytic malaria vaccines. Parasite Immunol. 24221-223. [DOI] [PubMed] [Google Scholar]
- 45.Suhrbier, A., L. A. Winger, E. Castellano, and R. E. Sinden. 1990. Survival and antigenic profile of irradiated malarial sporozoites in infected liver cells. Infect. Immun. 582834-2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tarun, A. S., R. F. Dumpit, N. Camargo, M. Labaied, P. Liu, A. Takagi, R. Wang, and S. H. Kappe. 2007. Protracted sterile protection with Plasmodium yoelii pre-erythrocytic genetically attenuated parasite malaria vaccines is independent of significant liver-stage persistence and is mediated by CD8+ T cells. J. Infect. Dis. 196608-616. [DOI] [PubMed] [Google Scholar]
- 47.Tsuji, M., D. Mattei, R. S. Nussenzweig, D. Eichinger, and F. Zavala. 1994. Demonstration of heat-shock protein 70 in the sporozoite stage of malaria parasites. Parasitol. Res. 8016-21. [DOI] [PubMed] [Google Scholar]
- 48.Vanderberg, J. 1980. The transmission by mosquitoes of plasmodia in the laboratory, p. 154-218. In J. Krier (ed.), Malaria: pathology, vector studies and culture. Academic Press, New York, NY.
- 49.Vanderberg, J. P. 1974. Studies on the motility of Plasmodium sporozoites. J. Protozool. 21527-537. [DOI] [PubMed] [Google Scholar]
- 50.van Dijk, M. R., B. Douradinha, B. Franke-Fayard, V. Heussler, M. W. van Dooren, B. van Schaijk, G. J. van Gemert, R. W. Sauerwein, M. M. Mota, A. P. Waters, and C. J. Janse. 2005. Genetically attenuated, P36p-deficient malarial sporozoites induce protective immunity and apoptosis of infected liver cells. Proc. Natl. Acad. Sci. USA 10212194-12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Waters, A. P., M. M. Mota, M. R. van Dijk, and C. J. Janse. 2005. Parasitology. Malaria vaccines: back to the future? Science 307528-530. [DOI] [PubMed] [Google Scholar]
- 52.Woynarowski, J. M., M. Krugliak, and H. Ginsburg. 2007. Pharmacogenomic analyses of targeting the AT-rich malaria parasite genome with AT-specific alkylating drugs. Mol. Biochem. Parasitol. 15470-81. [DOI] [PubMed] [Google Scholar]
- 53.Wykes, M., and M. F. Good. 2007. A case for whole-parasite malaria vaccines. Int. J. Parasitol. 37705-712. [DOI] [PubMed] [Google Scholar]
- 54.Yanow, S. K., L. A. Purcell, M. Lee, and T. W. Spithill. 2007. Genomics-based drug design targets the AT-rich malaria parasite: implications for antiparasite chemotherapy. Pharmacogenomics 81267-1272. [DOI] [PubMed] [Google Scholar]
- 55.Yanow, S. K., L. A. Purcell, G. Pradel, A. Sato, A. Rodriguez, M. Lee, and T. W. Spithill. Potent antimalarial and transmission-blocking activities of a novel DNA binding agent. J. Infect. Dis., in press. [DOI] [PubMed]
- 56.Yanow, S. K., L. A. Purcell, and T. W. Spithill. 2006. The A/T-specific DNA alkylating agent adozelesin inhibits Plasmodium falciparum growth in vitro and protects mice against Plasmodium chabaudi adami infection. Mol. Biochem. Parasitol. 14852-59. [DOI] [PubMed] [Google Scholar]
- 57.Yoshida, N., R. S. Nussenzweig, P. Potocnjak, V. Nussenzweig, and M. Aikawa. 1980. Hybridoma produces protective antibodies directed against the sporozoite stage of malaria parasite. Science 20771-73. [DOI] [PubMed] [Google Scholar]