Abstract
Previous studies have shown that Peyer's patches (PP) are not required for intestinal immunoglobulin A (IgA) responses to orally administered soluble protein. However, the roles of PP in regulation of mucosal immune responses against bacterial antigen remain to be clarified. In the present study, we generated several gut-associated lymphoreticular tissue-null mice by treatment with anti-interleukin-7 receptor antibody, the fusion protein of lymphotoxin β receptor and IgG Fc, and/or tumor necrosis factor receptor p55 and IgG Fc. These mice were then immunized with recombinant Salmonella expressing the C fragment of the tetanus toxin (rSalmonella-Tox C). Orally immunized PP-null mice as well as isolated lymphoid follicle (ILF)-null, PP/ILF-null, and PP/ILF/mesenteric lymph node-null mice induced identical levels of tetanus toxoid (TT)-specific systemic IgG responses to those of control mice. However, PP-null mice, but not ILF-null mice, failed to induce TT-specific intestinal IgA antibodies. Analysis of TT-specific CD4+ T-cell responses showed a reduction of gamma interferon (IFN-γ) synthesis in the intestinal lamina propriae of PP-null mice given oral rSalmonella-Tox C. In contrast, TT-specific IFN-γ responses in the spleen and delayed-type hypersensitivity responses were intact in those immunized mice. Interestingly, Salmonella lipopolysaccharide (LPS)-specific fecal IgA responses were not elicited in PP-null mice, while serum IgG anti-LPS antibodies were identical to those of control mice. These results suggest that while none of the gut-associated lymphoreticular tissues are required for the induction of systemic immune responses, PP are an essential lymphoid tissue for induction and regulation of intestinal IgA immunity against orally administered rSalmonella.
Secretory immunoglobulin A (S-IgA) antibodies (Abs) play a major role in preventing viral and bacterial pathogens from colonizing mucosal surfaces such as the gastrointestinal tract. The mechanisms of induction, homing, and regulation of intestinal S-IgA Abs are completely different from the mechanisms involved in systemic immunity, and gut-associated lymphoreticular tissues (GALT), including Peyer's patches (PP), isolated lymphoid follicles (ILF), and mesenteric lymph nodes (MLN), play critical roles (17, 23). PP have been thought to be the essential inductive sites for regulation of antigen (Ag)-specific IgA Ab responses following oral immunization (3, 11). However, oral immunization of mice that lack PP resulted in the generation of Ag-specific IgA Ab responses in the gastrointestinal tract when soluble proteins were administered orally or intestinally (18, 35, 36), suggesting the existence of a PP-independent pathway for mucosal IgA Ab responses.
ILF are recently identified lymphoid structures distributed throughout the murine intestines. ILF formation occurs postnatally and requires lymphotoxin β receptor (LTβR)-dependent events (8, 20, 22). The structure of these small lymphoid follicles resembles that of PP, as ILF consist of a large B-cell area, including a germinal center, and an epithelium containing M cells (8). Furthermore, mRNAs specific for activation-induced cytidine deaminase and Iμ-Cα transcripts were detected in lymphocytes isolated from ILF (30). These findings suggest that ILF may be a component of GALT that contributes to intestinal IgA immunity. However, we previously found that mice treated with the fusion protein of tumor necrosis factor receptor and IgG Fc (TNFR55-Ig) and with LTβR-Ig in utero, which lack PP and MLN but retain intact ILF, failed to induce Ag-specific IgA responses after oral immunization with protein Ag mixed with a mucosal adjuvant, i.e., cholera toxin (35).
On the other hand, recent studies elucidating the roles of PP during Helicobacter infection have demonstrated that CD4+ Th1 cells in PP play an important role in inducing local and systemic humoral and cellular immune responses, including the development of Helicobacter-induced gastritis (16, 24). Furthermore, PP are essential for inducing protective Th1 responses against Eimeria vermiformis (19), implying that PP play a critical role in the induction of immune responses against gastrointestinal infection.
The potential of live attenuated Salmonella strains to induce broad-based immune responses, including cell-mediated, humoral, and S-IgA Ab responses, has prompted their use as oral vaccine delivery vectors for recombinant proteins associated with virulence (1, 5, 6, 29). Genes from bacteria, viruses, parasites, and mammalian species have been expressed in attenuated Salmonella strains; however, few studies have elucidated the immunological mechanisms for induction of immune responses to the expressed Ag. In particular, the roles of GALT in the induction of mucosal and systemic immune responses after oral Salmonella vaccination remain to be clarified. In the present study, we assessed the importance of GALT for induction and regulation of intestinal IgA immunity to oral recombinant Salmonella (rSalmonella) by using GALT-compromised mice, which are generated by blocking signals mediated by the interleukin-7 receptor (IL-7R), TNFR55, and/or LTβR.
MATERIALS AND METHODS
Mice.
C57BL/6 mice were purchased from SLC Japan (Tokyo, Japan). These mice were maintained under pathogen-free conditions in the experimental facility of the Nihon University School of Dentistry at Matsudo (Chiba, Japan). Mice received sterile food and water, and they were 8 to 12 weeks old when used for these experiments. All animals were maintained and used in accordance with the Nihon University School of Dentistry at Matsudo guide for the care and use of laboratory animals.
Generation of GALT-null mice.
Anti-IL-7Rα monoclonal Ab-producing hybridoma A7R34 and plasmid pMKIT expressing LTβR-Ig or TNFR55-Ig were kindly provided by Shin-ichi Nishikawa (Riken Center for Developmental Biology, Kobe, Japan). Timed pregnant C57BL/6 mice were injected intravenously with 600 μg of anti-IL-7R Ab on gestational day 14 for the generation of PP-null mice (18). Adult mice (7 weeks old) were injected intraperitoneally with 50 μg of LTβR-Ig at weekly intervals during the immunization period, from day −7 to day +21 relative to the day of immunization, to remove ILF (35). Mice lacking both PP and ILF were generated by treatment with 600 μg of anti-IL-7R Ab on gestational day 14 and with 50 μg of LTβR-Ig at weekly intervals during the immunization period, from day −7 to day +21 relative to the day of immunization. Mice that lack PP, MLN, and ILF were generated by treatment with 100 μg each of LTβR-Ig and TNFR55-Ig fusion proteins on gestational days 11, 14, and 17 and with 50 μg of LTβR-Ig at weekly intervals during the immunization period, from day −7 to day +21 relative to the day of immunization (35).
Evaluation of ILF characteristics.
Small intestinal segments were fixed in 4% paraformaldehyde, embedded in paraffin, and then stained with hematoxylin-eosin. For immunohistochemical analysis, cryostat sections were fixed in ice-cold acetone and preblocked with anti-CD16/CD32 Ab. Sections were then stained with fluorescein isothiocyanate (FITC)-conjugated anti-B220 Ab (BD Biosciences, SanDiego, CA) for B cells or biotinylated anti-CR1 Ab (BD Biosciences) followed by streptavidin-phycoerythrin (streptavidin-PE) for follicular dendritic cell (FDC) clusters. For flow cytometric analysis, mononuclear cells were isolated from ILF as previously described (8). Single-cell suspensions were preincubated with anti-CD16/CD32 Ab. Cells were then stained with FITC-conjugated anti-CD19 Ab and PE-conjugated anti-CD3 Ab. Analysis was performed using a FACSCalibur flow cytometer (BD Biosciences).
Immunization.
The recombinant Salmonella enterica serovar Typhimurium BRD 847 strain used in this study (rSalmonella-Tox C) is a double aroA aroD mutant that expresses the nontoxic, immunogenic 50-kDa fragment C of tetanus toxin from plasmid pTETnir15 under the control of the anaerobically inducible nirB promoter (2). Salmonella organisms were grown statically for 4 h at 37°C in L broth containing ampicillin (100 μg/ml) as previously described (32). Mice were deprived of food for 2 h, given a single oral dose of 5 × 109 CFU (in 200 μl phosphate-buffered saline [PBS]) by gastric intubation, and then killed on day 28. Blood and fecal extracts were collected at weekly intervals for detection of Ag-specific Ab isotype responses.
Detection of Ab responses.
Serum and fecal extracts were collected, and Ag-specific Ab titers were determined by enzyme-linked immunosorbent assay (ELISA) as previously described (12). Purified tetanus toxoid (TT) was kindly provided by the Biken Foundation, Osaka University (Osaka, Japan). Briefly, plates were coated with TT (2 μg/ml) and blocked with 1% bovine serum albumin in PBS. In some experiments, Salmonella lipopolysaccharide (LPS) was used to coat plates for examination of LPS-specific Ab responses. LPS was dissolved in the organic solvent, a chloroform-ethanol (1:9 [vol/vol]) mixture, and used to coat plates (10 μg/ml) (7). Serial twofold dilutions of serum or fecal extracts were added to wells in duplicate. Following 4 h of incubation, the plates were washed, and peroxidase-labeled goat anti-mouse γ, γ1, γ2c, γ2b, and α heavy chain-specific Abs (Southern Biotechnology Associated, Birmingham, AL) were added to the appropriate wells. Finally, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) with H2O2 (Moss, Inc., Pasadena, MD) was added for color development. End-point titers were expressed as the reciprocal log2 of the last dilution which gave an optical density at 415 nm of 0.1 greater than background after 15 min of incubation.
ELISPOT assay for assessment of AFCs.
Single-cell suspensions were obtained from the intestinal lamina propria (LP), PP, spleen, and MLN as previously described (12). The mononuclear cells from the LP and PP were obtained at the interface of the 40% and 75% layers of a discontinuous Percoll gradient (Amersham Pharmacia Biotech, Piscataway, NJ). To determine the number of Ag-specific Ab-forming cells (AFCs), an enzyme-linked immunospot (ELISPOT) assay was performed as previously described (4). Briefly, 96-well nitrocellulose plates (Millititer HA; Millipore, Bedford, MA) were coated with TT (5 μg/ml), incubated for 20 h at 4°C, and then washed extensively and blocked with complete RPMI 1640 containing 10% fetal calf serum (FCS), 50 μM 2-mercaptoethanol, 10 mM HEPES, 100 μU/ml penicillin, and 100 μg/ml streptomycin. The blocking solution was discarded, and lymphoid cell suspensions at various dilutions were added to wells and then incubated for 4 h at 37°C in 5% CO2 in moist air. The detection Abs consisted of horseradish peroxidase-conjugated goat anti-mouse γ or α heavy chain-specific Abs. Following overnight incubation, the plates were washed with PBS and developed by addition of 3-amino-9-ethylcarbazole dissolved in 0.1 M sodium acetate buffer containing H2O2 (Moss) to each well. Plates were incubated at room temperature for 25 min and washed with water, and AFCs were counted with the aid of a stereomicroscope (SZH-ILLB; Olympus, Tokyo, Japan).
Measurement of DTH responses.
Delayed-type hypersensitivity (DTH) responses were measured as previously described (32). Six weeks after immunization, 30 μg of TT in sterile PBS was injected into the left ear pinna and the other ear pinna received sterile PBS as a control. Ear swelling was measured 24 h later with a spring-loaded dial thickness gauge. The DTH response was expressed as the difference in ear swelling between the TT- and PBS-injected ears.
Measurement of IFN-γ production.
Levels of gamma interferon (IFN-γ) in culture supernatants of CD4+ T cells of LP were determined by an IFN-γ-specific ELISA (Endogen, Boston, MA). CD4+ T cells were purified with the Imag system (BD Biosciences, San Jose, CA). Briefly, mononuclear cells were mixed with anti-CD4 Ab and incubated at 4°C for 30 min. CD4+ T cells were then separated using a magnet. Uniform latex microspheres (Polysciences, Inc., Warrington, PA) were coated with TT by methods described elsewhere (34). CD4+ T cells (2 × 106 cells/ml) were cultured with T-cell-depleted, mitomycin-treated splenic feeder cells (2.5 × 106 cells/ml) from naive mice in complete medium. TT-coated beads were added for restimulation of Ag-specific CD4+ T cells. After being incubated for 6 days at 37°C in 5% CO2 in air, supernatants were analyzed by ELISA.
Bacterial burdens of organs.
Seven days after oral immunization with rSalmonella-Tox C, MLN and spleens were collected and homogenized in PBS. Serial dilutions of lysates were plated on LB agar plates containing ampicillin (2), and colonies were counted after overnight culture.
Statistics.
The data were expressed as means ± standard deviations (SD) and compared using unpaired Student's t test.
RESULTS
PP, ILF, and MLN are not required for induction of systemic Ag-specific Ab responses against rSalmonella-derived Ag.
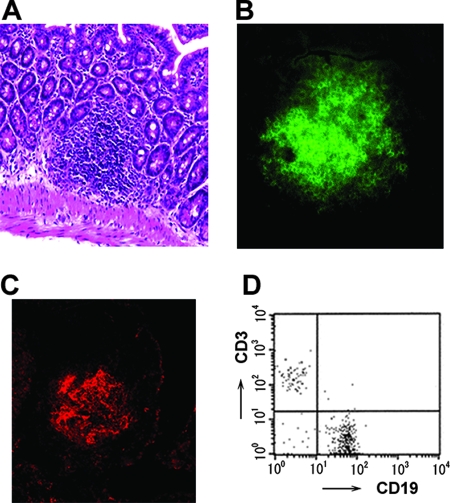
To assess the roles of GALT, including MLN, PP, and ILF, in the induction of immune responses against orally administered rSalmonella-Tox C, we first generated PP-null, ILF-null, PP/ILF-null, and PP/MLN/ILF-null mice by treatment with anti-IL-7R, TNFR55-Ig, and/or LTβR-Ig. The absence of PP and MLN was confirmed visually. Furthermore, small intestinal tissues were carefully examined with the aid of a stereomicroscope to confirm the absence of ILF. No evidence of MLN, PP, and/or ILF was seen in those GALT-null mice. On the other hand, anti-IL-7R Ab-treated PP-null mice showed ILF formation (Fig. 1A). Immunohistochemical analysis showed clearly defined B220+ B-cell accumulation and FDC clusters (Fig. 1B and C). Furthermore, lymphocytes isolated from the ILF of PP-null mice contained CD3+ T cells as well as CD19+ B cells (Fig. 1D). These results indicate that in utero anti-IL-7R Ab treatment does not affect the development of ILF and that PP-null mice possess mature ILF.
FIG. 1.
Characteristics of ILF in PP-null mice. (A) Hematoxylin-eosin staining. Immunohistochemical analyses of B cells (B) and FDC clusters (C) were performed by staining with FITC-anti-B220 Ab and PE-anti-CR1 Ab, respectively. Original magnification, ×100. Flow cytometric analysis shows the proportions of CD3+ T cells and CD19+ B cells (D).
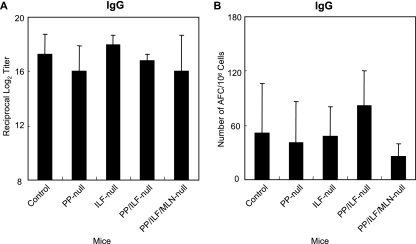
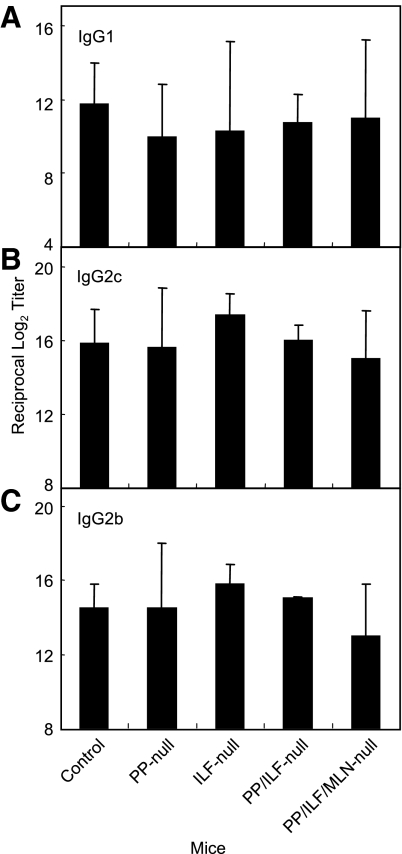
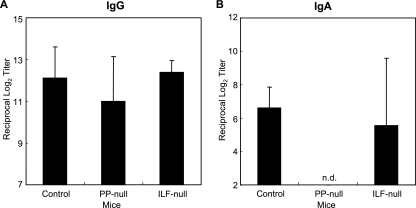
We evaluated the levels of systemic IgG Ab responses. PP-null, ILF-null, PP/ILF-null, and PP/MLN/ILF-null mice induced identical IgG Ab responses to those of control mice (Fig. 2A). Analysis of the Ab-forming cells supported the TT-specific IgG Ab titers and showed that significant numbers of TT-specific IgG AFCs were detected in splenic mononuclear cells isolated from all GALT-null mouse groups (Fig. 2B). Furthermore, similar patterns of TT-specific IgG1, IgG2c, and IgG2b Ab responses to those of control mice were detected in all GALT-null mice (Fig. 3). These findings indicate that GALT, including MLN, PP, and ILF, are not required for induction of systemic anti-TT IgG Ab responses when mice are immunized orally with rSalmonella-Tox C.
FIG. 2.
TT-specific serum IgG Ab titers (A) and IgG AFCs in the spleen (B). Mice were treated with anti-IL-7R Ab in utero (PP-null), LTβR-Ig postnatally (ILF-null), both anti-IL-7R Ab in utero and LTβR-Ig postnatally (PP/ILF-null), or both LTβR-Ig and TNFR55-Ig in utero and LTβR-Ig postnatally (PP/ILF/MLN-null). The mice were then orally immunized with rSalmonella-Tox C. Four weeks after the immunization, serum samples were collected and examined for TT-specific Abs by ELISA. Mononuclear cells were isolated, and TT-specific IgG AFCs were examined by ELISPOT assay. The results are representative of three separate experiments. Each group consisted of three or four mice.
FIG. 3.
TT-specific serum IgG1 (A), IgG2c (B), and IgG2b (C) Ab titers. Groups of GALT-deficient mice (as described in the legend to Fig. 1) were orally immunized with rSalmonella-Tox C. Four weeks after the immunization, serum samples were collected and examined for TT-specific Abs by ELISA. The results are representative of three separate experiments. Each group consisted of three or four mice.
PP-null mice lack mucosal IgA responses following oral immunization with rSalmonella-Tox C.
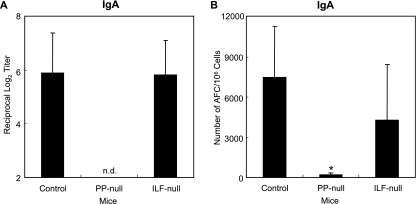
Next, we examined TT-specific intestinal IgA Ab responses in several groups of GALT-null mice after oral immunization with rSalmonella-Tox C. Interestingly, PP-null mice failed to induce TT-specific fecal IgA Ab responses. In contrast, identical levels of intestinal anti-TT IgA Ab titers were induced in ILF-null mice and control mice (Fig. 4A). Because the lack of PP may lead to different kinetics for mucosal IgA responses, we assessed TT-specific fecal IgA Ab responses for a longer period. Although we continued to examine IgA levels until day 56, responses were not detected in PP-null mice (data not shown).
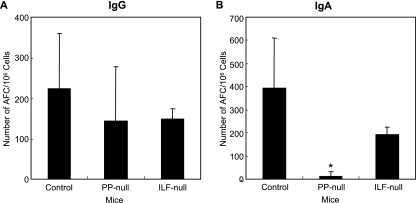
FIG. 4.
TT-specific fecal IgA Ab titers (A) and IgA AFCs in LP (B). PP-null mice or ILF-null mice were generated as described in the legend to Fig. 1. The mice were orally immunized with rSalmonella-Tox C. Four weeks after the immunization, fecal samples were collected and examined for TT-specific Abs by ELISA. Mononuclear cells were isolated from LP, and TT-specific IgA AFCs were examined by ELISPOT assay. The results are representative of three separate experiments. Each group consisted of three or four mice. *, P < 0.05 compared to control mice. n.d., not detectable.
The appearance of TT-specific IgA Abs correlated with the presence of Ag-specific IgA AFCs, because only a few AFCs were detected in LP of PP-null mice, while ILF-null mice possessed significant numbers of AFCs, similar to the control mice (Fig. 4B). In addition, the numbers of TT-specific IgA-expressing AFCs in MLN of PP-null mice, but not ILF-null mice, were significantly lower than those of control mice; however, large numbers of anti-TT IgG-expressing AFCs were found in both PP-null mice and ILF-null mice (Fig. 5A and B). These results clearly indicate that PP are important inductive tissues for mucosal IgA Ab responses against rSalmonella- Tox C.
FIG. 5.
TT-specific IgG AFCs (A) and IgA AFCs (B) in MLN. PP-null mice or ILF-null mice were generated as described in the legend to Fig. 1. The mice were orally immunized with rSalmonella-Tox C. Four weeks after the immunization, mononuclear cells were isolated from the MLN, and TT-specific IgG and IgA AFCs were examined by ELISPOT assay. The results are representative of three separate experiments. Each group consisted of three or four mice. *, P < 0.05 compared to control mice.
PP are critical for mucosal but not systemic Ab responses to Salmonella LPS.
In the next study, we examined Ab responses to Salmonella LPS after oral immunization with rSalmonella-Tox C. PP-null mice as well as ILF-null mice possessed significant levels of serum IgG anti-LPS responses, which were comparable to the responses of control mice (Fig. 6A). However, LPS-specific IgA Abs were not detected in the fecal extracts of PP-null mice, while ILF-null mice as well as control mice showed significant IgA Ab responses (Fig. 6B). These results suggest that organized PP are required for induction of either T-cell-dependent or T-cell-independent Ab responses in the intestinal tract after oral immunization with rSalmonella- Tox C.
FIG. 6.
Anti-Salmonella LPS-specific serum IgG Ab titers (A) and fecal IgA Ab titers (B). PP-null mice or ILF-null mice were generated as described in the legend to Fig. 1. The mice were orally immunized with rSalmonella-Tox C. Four weeks after the immunization, serum and fecal samples were collected and examined for anti-Salmonella LPS-specific Abs by ELISA. The results are representative of three separate experiments. Each group consisted of three or four mice. n.d., not detectable.
Ag-specific CD4+ T-cell responses.
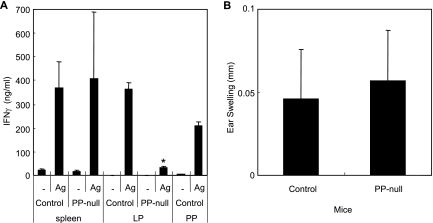
Since our results indicated that organized PP play a critical role in the induction of mucosal but not systemic Ab responses to rSalmonella-Tox C, it was important to analyze CD4+ T-cell responses. CD4+ T cells isolated from the spleens or LP of immunized PP-null mice were restimulated with TT in vitro. The results showed that splenic TT-specific CD4+ T cells possessed high levels of IFN-γ production, comparable to the IFN-γ production of control mice (Fig. 7A). Furthermore, identical TT-specific DTH responses were observed in PP-null mice and control mice (Fig. 7B). In contrast, the culture supernatant of CD4+ T cells from the LP of PP-null mice contained marginal levels of IFN-γ (Fig. 7A). These results indicate that Ag-specific CD4+ T cells and the subsequent IgA Ab responses in the intestinal tract are induced via the PP-dependent pathway following oral immunization with rSalmonella-Tox C.
FIG. 7.
IFN-γ levels in CD4+ T cells of PP-null mice (A) and TT-specific DTH responses (B). PP-null mice were generated as described in the legend to Fig. 1 and were orally immunized with rSalmonella-Tox C. Four weeks after the immunization, CD4+ T cells (2.0 × 106/ml) obtained from the spleen, LP, and PP were stimulated by TT-coated beads and cultured in the presence of T-cell-depleted splenic feeder cells (2.5 × 106/ml) and IL-2 (4 ng/ml) for 6 days. Culture supernatants were harvested, and the levels of secreted cytokines were assessed by cytokine-specific ELISA. Six weeks after the immunization, mice were injected with TT in the left ear and PBS in the right ear. After 24 h, ear swelling was measured. The results are expressed as means ± SD for three mice per group and a total of five experiments. *, P < 0.05 compared with control mice.
Distribution of rSalmonella organisms.
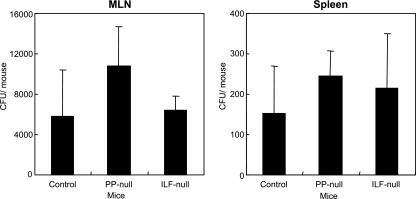
To assess the dissemination of rSalmonella-Tox C, MLN and spleens were collected from PP-null, ILF-null, and control mice 7 days after administration, and dilutions of organ homogenates were spread onto LB agar plates. Interestingly, rSalmonella-Tox C colonized the MLN and spleen of PP-null mice or ILF-null mice at levels comparable to those of control mice (Fig. 8A and B). These results indicate that transport of rSalmonella-Tox C from the intestinal tract to the MLN and spleen is intact even in the absence of PP.
FIG. 8.
Bacterial burdens in MLN and spleens of PP-null or ILF-null mice. MLN and spleens were collected from PP-null mice, ILF-null mice, or control mice, and bacterial counts were determined 7 days after oral administration of rSalmonella-Tox C. The results are expressed as means ± SD for six mice per group.
DISCUSSION
We assessed the roles of PP in the induction and regulation of intestinal IgA immune responses to rSalmonella. Our results revealed that mice lacking organized PP and treated in utero with anti-IL-7R Ab failed to induce Ag-specific CD4+ Th cells in intestinal LP with subsequent mucosal IgA Ab responses to orally administered rSalmonella-Tox C. In contrast, Ag-specific intestinal IgA Ab responses were intact in LTβR-Ig-treated ILF-null mice. There are several systems, including a different gene mutation, with which to generate PP-null mice, but we chose in utero anti-IL-7R Ab treatment because mice generated with this system develop completely intact spleen and lymphoid tissues, with the exception of PP (18, 37). In support of these findings, the results in this study showed that the PP-null mice formed mature ILF containing T and B cells as well as FDC clusters, indicating that in utero anti-IL-7R Ab treatment does not affect ILF development. Thus, failure of Ag-specific intestinal IgA responses was due to the lack of PP, not to dysfunction of other secondary lymphoid tissues.
M cells provide an entry site for Salmonella serovar Typhimurium. Orally administered Salmonella leads to the transport of bacterial Ag from the lumen of the intestinal tract into subjacent follicular lymphocytes by means of M cells for the initial priming of Ag-specific mucosal immune responses (15, 25, 26). Indeed, a previous study has shown that a Salmonella strain lacking the invasion genes of Salmonella pathogenicity island 1, which are necessary for bacterial entry into M cells, failed to induce Salmonella-specific intestinal mucosal IgA Ab responses (33). In this regard, recent studies have demonstrated that ILF possess a follicle-associated epithelium containing M cells (8, 20). Furthermore, the structure of this small lymphoid follicle resembles that of PP (8, 20). These findings imply that ILF could be an IgA inductive site for the induction of Ag-specific intestinal IgA Ab responses after oral immunization with rSalmonella-Tox C. However, despite the presence of ILF in PP-null mice (8, 9, 20, 22), the PP-null mice in the present study failed to induce Ag-specific mucosal IgA Ab responses following oral immunization with rSalmonella-Tox C. In addition, Salmonella LPS-specific IgA Ab responses were not detected in the intestines of PP-null mice, while ILF-null mice induced mucosal IgA anti-LPS Ab responses when rSalmonella-Tox C was given orally. These results suggest that ILF do not compensate for the function of PP in the induction of intestinal IgA Ab responses when rSalmonella-Tox C is given orally. In this regard, mature ILF were recently defined as well-organized lymphoid nodules, while immature ILF comprise loosely clustered B cells (8, 20). Since the ILF in the PP-null mice used in this study may not have been mature, they could not play a role in the induction of Ag-specific IgA Ab responses when rSalmonella-Tox C was administered orally. However, previous studies have shown that mature ILF are formed in the absence of PP (9, 20, 22). Furthermore, our results showed that in utero anti-IL-7R Ab-treated PP-null mice formed mature ILF. These studies, together with the present study, indicate that mature ILF are not an IgA inductive site, even in the absence of PP. In support of this finding, our separate study demonstrated that wild-type bone marrow-reconstituted LTα−/− mice lacking PP and MLN but possessing mature ILF failed to induce Ag-specific intestinal IgA Ab responses following oral administration of rSalmonella-Tox C (9). Thus, intestinal IgA Ab responses against orally administered rSalmonella are regulated solely by PP, and ILF do not compensate for the function of PP.
On the other hand, a previous study demonstrated that oral infection of LTα+/+ bone marrow-reconstituted LTα−/− mice with wild-type S. enterica serovar Typhimurium induced Salmonella-specific fecal IgA Ab responses. Furthermore, ILF cells from in utero LTβR-Ig-treated mice induced Ag-specific IgA Ab responses after oral immunization with sheep red blood cells (21). The basis for this discrepancy is unknown. A possible explanation would be that since in utero LTβR-Ig-treated mice possess MLN in addition to ILF, MLN may contribute to the regulation of intestinal IgA Ab responses. Several studies have suggested that MLN may act as an inductive site for Ag-specific intestinal IgA Ab responses (10, 14, 28). Intestinal LP dendritic cells (DCs), which phagocytose apoptotic epithelial cells, directly migrate into MLN, with mediation by CCR7 and CCL21 (14). Furthermore, CD11c+ LP cells expressing Toll-like receptor 5 transport pathogenic Salmonella organisms to MLN for systemic infection (31). These studies imply that Ags taken up by LP DCs are transported into MLN for Ag stimulation. Indeed, our previous study showed that Ag-specific CD4+ T cells are induced in the MLN, with intestinal IgA Ab responses in the absence of PP following oral immunization with soluble protein mixed with the mucosal adjuvant cholera toxin (36). Thus, MLN could be an inductive site for Ag-specific intestinal IgA immunity when soluble protein is given orally. The results presented in this study support this idea and show that rSalmonella-Tox C colonizes the MLN of PP-null mice at levels comparable to those of ILF-null mice and control mice. However, the lack of PP failed to induce intestinal IgA Ab responses against rSalmonella-Tox C even in the presence of intact MLN. Antigen-specific CD4+ T-cell responses supported the Ab responses and showed that significantly lower TT-specific IFN-γ responses were detected in the LP of PP-null mice than in those of control mice. These results suggest that although PP-independent pathways exist for soluble protein and MLN may act as an IgA inductive site, PP are essential for the induction of intestinal IgA Ab responses when rSalmonella is administered orally.
Our results indicate that PP-null mice, as well as ILF-null mice, PP/ILF-null mice, and PP/MLN/ILF-null mice, induce identical levels of TT-specific serum IgG Ab responses to those of control mice. Furthermore, the patterns of IgG subclass responses were identical among the GALT-null mice. In addition, identical levels of IFN-γ responses in splenic CD4+ T cells as well as DTH responses were detected in PP-null mice that were orally immunized with rSalmonella-Tox C compared with control mice. These results indicate that GALT is not required for induction of immune responses in the systemic compartment following oral immunization with rSalmonella-Tox C. Our previous study demonstrated that non-follicle-associated M cells reside in the intestinal villous epithelium. These M cells take up several bacteria, including Salmonella (13). Furthermore, as described above, DCs in the intestinal LP offer another possible uptake site (14, 27, 28). Other studies have demonstrated that Salmonella pathogenicity island 1-deficient Salmonella can be disseminated from the intestinal epithelium to the systemic compartment by means of the CD18-dependent pathway (33). Indeed, our results demonstrated that the numbers of bacteria in the spleens of PP-null mice were similar to those of ILF-null mice and control mice when rSalmonella-Tox C was given orally. Thus, it is likely that several cell types are involved in permitting the penetration of Salmonella and the transport of bacterial antigen from the intestinal tract into the spleen for induction of systemic IgG Ab responses.
In summary, the present study demonstrates that mice lacking PP fail to induce intestinal IgA Ab responses against rSalmonella-Tox C in both T-cell-dependent and T-cell-independent manners. Furthermore, the lack of PP function was not compensated for by mature ILF. In contrast, none of the GALT types (PP, ILF, and MLN) was required for the induction of systemic immune responses. These results suggest that PP, but not ILF, control the generation of intestinal mucosal IgA Ab responses against rSalmonella-Tox C, whereas bacteria transported into LP regions contribute to the initiation of systemic immune responses.
Acknowledgments
This work was supported by grants-in-aid for scientific research (18592270 and 19791624) from the Japan Society for the Promotion of Science “Academic Frontier” Project for Private Universities, matching fund subsidy from the Ministry of Education, Culture, Sports, Science and Technology (2007-2011); by a Nihon University multidisciplinary research grant (2006, 2007); and by a Nihon University research grant for assistants and young researchers (2007).
Editor: B. A. McCormick
Footnotes
Published ahead of print on 17 December 2007.
REFERENCES
- 1.Cardenas, L., and J. D. Clements. 1992. Oral immunization using live attenuated Salmonella spp. as carriers of foreign antigens. Clin. Microbiol. Rev. 5328-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chatfield, S. N., I. G. Charles, A. J. Makoff, M. D. Oxer, G. Dougan, D. Pickard, D. Slater, and N. F. Fairweather. 1992. Use of the nirB promoter to direct the stable expression of heterologous antigens in Salmonella oral vaccine strains: development of a single-dose oral tetanus vaccine. Biotechnology 10888-892. [DOI] [PubMed] [Google Scholar]
- 3.Craig, S. W., and J. J. Cebra. 1971. Peyer's patches: an enriched source of precursors for IgA-producing immunocytes in the rabbit. J. Exp. Med. 134188-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Czerkinsky, C. C., L. A. Nilsson, H. Nygren, O. Ouchterlony, and A. Tarkowski. 1983. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J. Immunol. Methods 65109-121. [DOI] [PubMed] [Google Scholar]
- 5.Dougan, G. 1994. The molecular basis for the virulence of bacterial pathogens: implications for oral vaccine development. Microbiology 140215-224. [DOI] [PubMed] [Google Scholar]
- 6.Dougan, G., C. E. Hormaeche, and D. J. Maskell. 1987. Live oral Salmonella vaccines: potential use of attenuated strains as carriers of heterologous antigens to the immune system. Parasite Immunol. 9151-160. [DOI] [PubMed] [Google Scholar]
- 7.Freudenberg, M. A., A. Fomsgaard, I. Mitov, and C. Galanos. 1989. ELISA for antibodies to lipid A, lipopolysaccharides and other hydrophobic antigens. Infection 17322-328. [DOI] [PubMed] [Google Scholar]
- 8.Hamada, H., T. Hiroi, Y. Nishiyama, H. Takahashi, Y. Masunaga, S. Hachimura, S. Kaminogawa, H. Takahashi-Iwanaga, T. Iwanaga, H. Kiyono, H. Yamamoto, and H. Ishikawa. 2002. Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine. J. Immunol. 16857-64. [DOI] [PubMed] [Google Scholar]
- 9.Hashizume, T., F. Momoi, T. Kurita-Ochiai, S. Kaminogawa, A. Hosono, K. Kataoka, N. Shinozaki-Kuwahara, M. N. Kweon, and M. Yamamoto. 2007. Isolated lymphoid follicles are not IgA inductive sites for recombinant Salmonella. Biochem. Biophys. Res. Commun. 360388-393. [DOI] [PubMed] [Google Scholar]
- 10.Huang, F. P., N. Platt, M. Wykes, J. R. Major, T. J. Powell, C. D. Jenkins, and G. G. MacPherson. 2000. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J. Exp. Med. 191435-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Husband, A. J., and J. L. Gowans. 1978. The origin and antigen-dependent distribution of IgA-containing cells in the intestine. J. Exp. Med. 1481146-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson, R. J., K. Fujihashi, J. Xu-Amano, H. Kiyono, C. O. Elson, and J. R. McGhee. 1993. Optimizing oral vaccines: induction of systemic and mucosal B-cell and antibody responses to tetanus toxoid by use of cholera toxin as an adjuvant. Infect. Immun. 614272-4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jang, M. H., M. N. Kweon, K. Iwatani, M. Yamamoto, K. Terahara, C. Sasakawa, T. Suzuki, T. Nochi, Y. Yokota, P. D. Rennert, T. Hiroi, H. Tamagawa, H. Iijima, J. Kunisawa, Y. Yuki, and H. Kiyono. 2004. Intestinal villous M cells: an antigen entry site in the mucosal epithelium. Proc. Natl. Acad. Sci. USA 1016110-6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jang, M. H., N. Sougawa, T. Tanaka, T. Hirata, T. Hiroi, K. Tohya, Z. Guo, E. Umemoto, Y. Ebisuno, B. G. Yang, J. Y. Seoh, M. Lipp, H. Kiyono, and M. Miyasaka. 2006. CCR7 is critically important for migration of dendritic cells in intestinal lamina propria to mesenteric lymph nodes. J. Immunol. 176803-810. [DOI] [PubMed] [Google Scholar]
- 15.Jones, B. D., N. Ghori, and S. Falkow. 1994. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer's patches. J. Exp. Med. 18015-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiriya, K., N. Watanabe, A. Nishio, K. Okazaki, M. Kido, K. Saga, J. Tanaka, T. Akamatsu, S. Ohashi, M. Asada, T. Fukui, and T. Chiba. 2007. Essential role of Peyer's patches in the development of Helicobacter-induced gastritis. Int. Immunol. 19435-446. [DOI] [PubMed] [Google Scholar]
- 17.Kiyono, H., J. Bienenstock, J. R. McGhee, and P. B. Ernst. 1992. The mucosal immune system: features of inductive and effector sites to consider in mucosal immunization and vaccine development. Reg. Immunol. 454-62. [PubMed] [Google Scholar]
- 18.Kunisawa, J., I. Takahashi, A. Okudaira, T. Hiroi, K. Katayama, T. Ariyama, Y. Tsutsumi, S. Nakagawa, H. Kiyono, and T. Mayumi. 2002. Lack of antigen-specific immune responses in anti-IL-7 receptor α chain antibody-treated Peyer's patch-null mice following intestinal immunization with microencapsulated antigen. Eur. J. Immunol. 322347-2355. [DOI] [PubMed] [Google Scholar]
- 19.Kwa, S. F., P. Beverley, and A. L. Smith. 2006. Peyer's patches are required for the induction of rapid Th1 responses in the gut and mesenteric lymph nodes during an enteric infection. J. Immunol. 1767533-7541. [DOI] [PubMed] [Google Scholar]
- 20.Lorenz, R. G., D. D. Chaplin, K. G. McDonald, J. S. McDonough, and R. D. Newberry. 2003. Isolated lymphoid follicle formation is inducible and dependent upon lymphotoxin-sufficient B lymphocytes, lymphotoxin β receptor, and TNF receptor I function. J. Immunol. 1705475-5482. [DOI] [PubMed] [Google Scholar]
- 21.Lorenz, R. G., and R. D. Newberry. 2004. Isolated lymphoid follicles can function as sites for induction of mucosal immune responses. Ann. N. Y. Acad. Sci. 102944-57. [DOI] [PubMed] [Google Scholar]
- 22.McDonald, K. G., J. S. McDonough, and R. D. Newberry. 2005. Adaptive immune responses are dispensable for isolated lymphoid follicle formation: antigen-naive, lymphotoxin-sufficient B lymphocytes drive the formation of mature isolated lymphoid follicles. J. Immunol. 1745720-5728. [DOI] [PubMed] [Google Scholar]
- 23.Mestecky, J., R. S. Blumberg, H. Kiyono, and J. R. McGhee. 2003. The mucosal immune system, p. 965-1020. In W. E. Paul (ed.), Fundamental immunology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 24.Nagai, S., H. Mimuro, T. Yamada, Y. Baba, K. Moro, T. Nochi, H. Kiyono, T. Suzuki, C. Sasakawa, and S. Koyasu. 2007. Role of Peyer's patches in the induction of Helicobacter pylori-induced gastritis. Proc. Natl. Acad. Sci. USA 1048971-8976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neutra, M. R. 1998. Role of M cells in transepithelial transport of antigens and pathogens to the mucosal immune system. Am. J. Physiol. 274G785-G791. [DOI] [PubMed] [Google Scholar]
- 26.Neutra, M. R., A. Frey, and J. P. Kraehenbuhl. 1996. Epithelial M cells: gateways for mucosal infection and immunization. Cell 86345-348. [DOI] [PubMed] [Google Scholar]
- 27.Niess, J. H., S. Brand, X. Gu, L. Landsman, S. Jung, B. A. McCormick, J. M. Vyas, M. Boes, H. L. Ploegh, J. G. Fox, D. R. Littman, and H. C. Reinecker. 2005. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 307254-258. [DOI] [PubMed] [Google Scholar]
- 28.Rescigno, M., M. Urbano, B. Valzasina, M. Francolini, G. Rotta, R. Bonasio, F. Granucci, J. P. Kraehenbuhl, and P. Ricciardi-Castagnoli. 2001. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2361-367. [DOI] [PubMed] [Google Scholar]
- 29.Schodel, F., and R. Curtiss III. 1995. Salmonellae as oral vaccine carriers. Dev. Biol. Stand. 84245-253. [PubMed] [Google Scholar]
- 30.Shikina, T., T. Hiroi, K. Iwatani, M. H. Jang, S. Fukuyama, M. Tamura, T. Kubo, H. Ishikawa, and H. Kiyono. 2004. IgA class switch occurs in the organized nasopharynx- and gut-associated lymphoid tissue, but not in the diffuse lamina propria of airways and gut. J. Immunol. 1726259-6264. [DOI] [PubMed] [Google Scholar]
- 31.Uematsu, S., M. H. Jang, N. Chevrier, Z. Guo, Y. Kumagai, M. Yamamoto, H. Kato, N. Sougawa, H. Matsui, H. Kuwata, H. Hemmi, C. Coban, T. Kawai, K. J. Ishii, O. Takeuchi, M. Miyasaka, K. Takeda, and S. Akira. 2006. Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nat. Immunol. 7868-874. [DOI] [PubMed] [Google Scholar]
- 32.VanCott, J. L., H. F. Staats, D. W. Pascual, M. Roberts, S. N. Chatfield, M. Yamamoto, M. Coste, P. B. Carter, H. Kiyono, and J. R. McGhee. 1996. Regulation of mucosal and systemic antibody responses by T helper cell subsets, macrophages, and derived cytokines following oral immunization with live recombinant Salmonella. J. Immunol. 1561504-1514. [PubMed] [Google Scholar]
- 33.Vazquez-Torres, A., J. Jones-Carson, A. J. Baumler, S. Falkow, R. Valdivia, W. Brown, M. Le, R. Berggren, W. T. Parks, and F. C. Fang. 1999. Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes. Nature 401804-808. [DOI] [PubMed] [Google Scholar]
- 34.Xu-Amano, J., H. Kiyono, R. J. Jackson, H. F. Staats, K. Fujihashi, P. D. Burrows, C. O. Elson, S. Pillai, and J. R. McGhee. 1993. Helper T cell subsets for immunoglobulin A responses: oral immunization with tetanus toxoid and cholera toxin as adjuvant selectively induces Th2 cells in mucosa associated tissues. J. Exp. Med. 1781309-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamoto, M., M. N. Kweon, P. D. Rennert, T. Hiroi, K. Fujihashi, J. R. McGhee, and H. Kiyono. 2004. Role of gut-associated lymphoreticular tissues in antigen-specific intestinal IgA immunity. J. Immunol. 173762-769. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto, M., P. Rennert, J. R. McGhee, M. N. Kweon, S. Yamamoto, T. Dohi, S. Otake, H. Bluethmann, K. Fujihashi, and H. Kiyono. 2000. Alternate mucosal immune system: organized Peyer's patches are not required for IgA responses in the gastrointestinal tract. J. Immunol. 1645184-5191. [DOI] [PubMed] [Google Scholar]
- 37.Yoshida, H., K. Honda, R. Shinkura, S. Adachi, S. Nishikawa, K. Maki, K. Ikuta, and S. I. Nishikawa. 1999. IL-7 receptor alpha+ CD3(−) cells in the embryonic intestine induce the organizing center of Peyer's patches. Int. Immunol. 11643-655. [DOI] [PubMed] [Google Scholar]