Abstract
Staphylococcus aureus is a major cause of hospital-acquired pneumonia and is emerging as an important etiological agent of community-acquired pneumonia. Little is known about the specific host-pathogen interactions that occur when S. aureus first enters the airway. A shotgun proteomics approach was utilized to identify the airway proteins associated with S. aureus during the first 6 h of infection. Host proteins eluted from bacteria recovered from the airways of mice 30 min or 6 h following intranasal inoculation under anesthesia were subjected to liquid chromatography and tandem mass spectrometry. A total of 513 host proteins were associated with S. aureus 30 min and/or 6 h postinoculation. A majority of the identified proteins were host cytosolic proteins, suggesting that S. aureus was rapidly internalized by phagocytes in the airway and that significant host cell lysis occurred during early infection. In addition, extracellular matrix and secreted proteins, including fibronectin, antimicrobial peptides, and complement components, were associated with S. aureus at both time points. The interaction of 12 host proteins shown to bind to S. aureus in vitro was demonstrated in vivo for the first time. The association of hemoglobin, which is thought to be the primary staphylococcal iron source during infection, with S. aureus in the airway was validated by immunoblotting. Thus, we used our recently developed S. aureus pneumonia model and shotgun proteomics to validate previous in vitro findings and to identify nearly 500 other proteins that interact with S. aureus in vivo. The data presented here provide novel insights into the host-pathogen interactions that occur when S. aureus enters the airway.
Staphylococcus aureus is the leading bacterial cause of hospital-acquired pneumonia and is reemerging as an important cause of community-acquired pneumonia (1, 24). A steady increase in the frequency of isolation of methicillin-resistant strains of S. aureus from patients with hospital-acquired pneumonia and community-acquired pneumonia underscores the importance of identifying host and bacterial factors that facilitate the progression of staphylococcal pneumonia. A number of S. aureus microbial surface components recognizing adhesive matrix molecules have been shown in vitro to interact with proteins of the host extracellular matrix, including fibronectin, collagen, elastin, vitronectin, and laminin (for a review, see reference 4). IsdA is a multifunctional iron-regulated microbial surface component recognizing adhesive matrix molecules that has been shown to bind to fibronectin, fibrinogen, fetuin, hemoglobin, and transferrin (6, 31, 43). IsdB and IsdH/HarA have also been shown to bind hemoglobin and haptoglobin (6, 9, 31, 44). Cell wall-anchored protein A binds to the Fc fragment of immunoglobulin G (IgG) to inhibit opsonophagocytosis. In addition, protein A associates with von Willebrand factor and tumor necrosis factor alpha receptor 1 (TNFR1) (10, 12, 16, 25). Several S. aureus proteins have been shown to interact with fibrinogen and plasminogen, which are components of the coagulation cascade (32, 35, 37, 46).
While the interactions described above have been extensively characterized in vitro on a molecular level, the physical binding of any of the host proteins to their cognate bacterial receptor(s) in vivo during pneumonia has not been established. Virulence studies using rodent models of pneumonia have been performed to determine whether particular bacterial genes contribute to pneumonia. Strains containing mutations in the genes encoding sortase A, alpha-hemolysin, protein A, and the global virulence regulators SarA and AgrA exhibit attenuated virulence in mouse models of pneumonia (12, 18, 47). Sortase A catalyzes the anchoring of proteins, including protein A, into the cell wall peptidoglycan (30). Gomez et al. showed that protein A binds to TNFR1 to induce airway inflammatory responses during S. aureus infection (12). In addition, protein A expression increases in some Panton-Valentine leukocidin-expressing strains; increased levels of protein A may contribute to the overwhelming inflammatory response that occurs during necrotizing pneumonia caused by Panton-Valentine leukocidin-positive S. aureus (26). In contrast to the results of these studies, McElroy et al. demonstrated that a strain missing fibronectin binding proteins A and B was more virulent than the wild type in a rat model of intratracheal infection (33). The data from these virulence studies using isogenic S. aureus mutants defective in binding to host proteins clearly highlight the importance of determining the role of these molecular interactions in the pathogenesis of staphylococcal pneumonia.
To our knowledge, no data describing the specific interactions that occur between S. aureus and host proteins during early pneumonia are currently available. In addition, the roles of these specific interactions in allowing the bacterium to establish a niche in the airway and/or in providing the host with mechanisms promoting bacterial clearance are currently unknown. We provide evidence of a rapid and vigorous cytokine and neutrophil response to S. aureus in the airway during the first 6 h of infection by using our recently developed model of adult staphylococcal pneumonia (C. L. Ventura, R. Higdon, L. Hohmann, D. Martin, E. Kolker, H. D. Liggitt, S. J. Skerrett, and C. E. Rubens, submitted for publication). An analysis of the airway proteome in this model demonstrated a robust initial host response to infection. In this study, we used a shotgun proteomics approach to define the airway proteins that directly interact with S. aureus during the first 6 h after the bacterium enters the airway. We confidently identified 513 proteins that were associated with the bacterial cells during the initial stages of infection; in addition, we validated the use of shotgun proteomics to identify host proteins associated with bacteria during infection using immunoblotting techniques. The data presented here provide critical new information about the airway proteins associated with S. aureus upon the entry of the bacterium into the airway.
MATERIALS AND METHODS
Bacteria and growth conditions.
S. aureus strain JP1 was used in these studies. JP1 is a human blood isolate obtained from the microbiology laboratory of the Veterans Affairs Puget Sound Health Care System (41). S. aureus was grown in tryptic soy broth (TSB) for incubation in human bronchoalveolar lavage (BAL) fluid or in Luria-Bertani (LB) broth for mouse infections at 37°C under aerobic conditions. For incubation in human BAL fluid, S. aureus was grown in TSB (4:1 flask-to-medium volume ratio) for 16 to 18 h with shaking (200 rpm). For mouse infections, bacteria were grown from a frozen stock for 6 to 10 h in LB broth and then diluted 1/100 in fresh LB broth (4:1 flask volume-to-medium ratio) and grown for an additional 16 to 18 h with shaking (180 rpm). Stationary-phase bacteria were harvested by centrifugation at room temperature, washed twice with endotoxin-free phosphate-buffered saline (PBS; Mediatech, Herndon, VA), and resuspended in endotoxin-free PBS to the desired concentration as estimated by optical density and confirmed by quantitative plate counting.
Incubation of S. aureus in human BAL fluid.
BAL fluid was obtained from normal volunteers and frozen as described previously (38). The BAL fluid harvest protocol was approved by the Institutional Review Board of the University of Washington. S. aureus JP1 was grown overnight in TSB, harvested by centrifugation, and washed twice with PBS. Bacterial cells were resuspended in 1 ml of PBS or 1 ml of human BAL fluid and incubated for 2 h at 37°C. Triton X-100 was added to obtain a concentration of 0.1%, and the samples were incubated for 15 min at 37°C. Bacteria were pelleted by centrifugation and washed twice with PBS. Paired samples (one PBS sample and one BAL fluid sample) were incubated in Laemmli buffer (27) at 100°C for 10 min, Laemmli buffer at 37°C for 30 min, 0.5% sodium deoxycholate at 37°C for 30 min, 0.5% Triton X-100 at 37°C for 30 min, or 0.5% Tween 20 at 37°C for 30 min. Bacteria were pelleted by centrifugation for 5 min and discarded. Laemmli buffer containing bromophenol blue and glycerol was added to each supernatant prior to boiling for 5 min. Samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and stained with SYPRO Ruby (Bio-Rad, Hercules, CA). Proteins were detected using a gel documentation system (Bio-Rad).
Experimental design.
Two independent biological experiments were performed to identify the host proteins that associate with S. aureus during early airway infection. In both experiments, 20 mice were inoculated intranasally with 3 × 108 CFU of S. aureus JP1. Ten mice were sacrificed at 30 min and at 6 h postinoculation, and BAL was performed. In the first experiment (see SCX in Table S1 in the supplemental material), BAL fluid was frozen immediately, thawed, and processed as described below in “Identification of mouse airway proteins associated with S. aureus.” Recovered host proteins were digested with trypsin, separated by strong cation exchange (SCX) fractionation, and subjected to liquid chromatography-tandem mass spectrometry (LC-MS-MS) using an LCQ-DECA mass spectrometer. In the second experiment (see GEL in Table S1 in the supplemental material), BAL fluid was subjected to centrifugation at 300 × g for 10 min to remove eukaryotic cells. The BAL fluid was frozen and then thawed and processed as described below. Recovered host proteins were separated by one-dimensional (1D) SDS-PAGE and subjected to in-gel trypsin digestion, followed by LC-MS-MS using an LTQ mass spectrometer. Each biological sample was subjected to a single fractionation process and LC-MS-MS analysis.
Mouse model of pneumonia and BAL.
Specific-pathogen-free male and female C57BL/6 mice, aged 8 to 12 weeks, were purchased from the Jackson Laboratory (Bar Harbor, ME). Animals were group housed in filtered, ventilated cages containing autoclaved bedding and were permitted ad libitum access to sterile food and water. Cage changes and animal handling occurred in a laminar flow hood. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Washington. For infection, mice were anesthetized with isoflurane, held vertically, and inoculated intranasally with 3 × 108 CFU of S. aureus JP1 in 50 μl of endotoxin-free PBS. At 30 min and at 6 h postinoculation in each of two experiments, 10 mice were euthanized and BAL was performed as described previously (41, 42). The BAL fluid samples were pooled by time point and processed as described above in “Experimental design.”
Quantitation of cell-associated bacteria.
Mice (10 per group) were inoculated intranasally as described above, and BAL fluid was harvested 30 min and 6 h postinoculation. An aliquot of BAL fluid from each animal was removed for quantitative culture and for the preparation of cytocentrifuge slides, which were stained by a modified Wright-Giemsa method using Diff-Quik (Dade-Behring, Dudingen, Switzerland) and scored for cell-associated bacteria, as described previously (41). Statistical analysis was performed using the Kruskal-Wallis test with Dunn's multiple-comparison posttest. A P value of <0.05 was considered significant.
Identification of mouse airway proteins associated with S. aureus.
Upon thawing of the BAL samples, Triton X-100 was added to each sample to obtain a final concentration of 0.1% and the samples were vortexed for 10 s and incubated at 37°C for 15 min. The bacteria were pelleted by centrifugation at 10,000 × g for 15 min at 4°C and washed twice with 1 ml of PBS. Following centrifugation, the bacterial pellet was resuspended in 30 μl of Laemmli buffer (27) and the suspension was incubated for 30 min at 37°C. The cell debris was pelleted, and the supernatant containing the host proteins was stored at −80°C until further analysis. Each sample contained less than 20 μg of total protein; therefore, only one fractionation procedure and LC-MS-MS analysis could be performed on each biological sample, as described below.
For SCX fractionation and LC-MS-MS, samples were thawed and the proteins were precipitated twice with acetone. The protein pellet was resuspended in a mixture of 200 mM Tris (pH 8.3), 5 mM EDTA, 0.05% SDS, and 6 M urea, reduced in 2 mM Tris(2-carboxyethyl)phosphine for 30 min at 37°C, and alkylated in 2 mM iodoacetamide for 1 h in the dark at room temperature. The sample was diluted threefold and digested with 3 μg of trypsin for 3 h. Peptides were separated into three fractions by SCX using polysulfolethyl A (PolyLC, Inc., Columbia, MD) and a KCl buffer system (10-min gradient from 100% buffer A [25% acetonitrile {ACN}, 5 mM KH2PO4] to 35% buffer B [25% ACN, 5 mM KH2PO4, 360 mM KCl]). Fractions were desalted with a microspin C18 column (Nest Group) and subjected to LC-MS-MS using a Thermo-Finnigan LCQ-DECA ion trap mass spectrometer with an in-house microelectrospray ionization source. Each cation exchange fraction was loaded onto a 75-μm-internal-diameter fused-silica column packed with 10 cm of 5-μm-particle-size spherical C18 resin (Magic C18aq; Michrom Bioresources) and further separated using a 60-min linear gradient from 5 to 35% ACN in 0.1% formic acid at a 300-nl/min flow rate with an Agilent 1100 binary pump. Three MS-MS scans per MS scan were collected in a data-dependent manner.
For 1D gel electrophoresis and LC-MS-MS, BAL proteins were precipitated twice with acetone and separated in a Novex NuPAGE 4 to 12% bis-Tris gel (Invitrogen) at 200 V for 60 min. The proteins were silver stained according to established protocols (36), excised from the gel (in eight slices representing the entire lane of the gel for each sample), and washed extensively with water. Proteins were destained twice with a 1:1 solution of 100 mM sodium thiosulfate-30 mM potassium ferricyanide and washed twice with water. The gel slices were equilibrated in 25 mM ammonium bicarbonate (ambic) and dehydrated twice in 25 mM ambic-50% ACN. Proteins were reduced in a solution of 25 mM dithiothreitol in 25 mM ambic for 30 min at 56°C and alkylated in a solution of 550 mM iodoacetamide in 25 mM ambic for 30 min at room temperature in the dark. The gel slices were washed with water, reequilibrated in 25 mM ambic for 10 min, and dehydrated twice for 10 min in 25 mM ambic-50% ACN. The proteins were digested in the gel with trypsin (20 ng/ml) in 25 mM ambic for 16 to 24 h at 37°C. Following digestion, the peptides in the solution were extracted with 1 volume of 0.1% trifluoroacetic acid by vortexing for 20 min at room temperature, followed by two additional 20-min extraction steps with 1 volume of 5% trifluoroacetic acid-70% ACN. All extraction products were combined in one tube and dried. Following reconstitution, fractions were subjected to LC-MS-MS using a Thermo-Finnigan LTQ mass spectrometer fitted with an in-house-designed microspray device. Each fraction was loaded onto a 75-μm-internal-diameter fused-silica column packed with 10 cm of 5-μm-particle-size spherical C18 resin (Magic C18aq; Michrom Bioresources) and further separated using a 60-min linear gradient from 5 to 35% ACN in 0.1% formic acid at a 200-nl/min flow rate with an Agilent 1100 binary pump. Three MS-MS scans per MS scan were collected in a data-dependent manner.
Proteomics data analysis.
The MS-MS scans from each LC-MS-MS run were converted from the .RAW file format into mzXML files using the program ReAdW.exe (Institute for Systems Biology, Seattle, WA). The database search program X!Tandem, included in the CPAS data analysis system, was used for peptide identification from the MS-MS spectra. The Comet scoring function was used in place of the default X!Tandem scoring function. The following parameters were used in the database search: trypsin enzyme specificity, a peptide mass tolerance of 2.5 Da, a fragment ion tolerance of 0.5 Da, monoisotopic molecular weights for both the peptide and the fragment ion masses, the b/y ion search, variable modification at M of +15.995, and static modification at C of +57.1. The data were compared against a combined database consisting of the mouse IPI version 3.22, S. aureus COL, and a list of contaminants. In addition, randomly reshuffled versions of each database were also appended.
A composite peptide identification score was generated from the X!Tandem output based on the combination of the Comet score, the degree of difference from the second best match, the expectation value, the percentage of matching ions, the charge state, the peptide length, and the difference between the observed and theoretical masses by using the logistic identification of peptide sequences model (19). Experiment-specific peptide identification probabilities were generated from the distribution of reshuffled peptide matches (20). A minimum 90% identification certainty was used to accept a peptide spectrum identification. This parameter resulted in an overall false-positive peptide identification rate of 0.5% (with a 95% upper bound of 0.7%) based on reshuffled database matches. Protein identification for each experimental condition was based on four levels of certainty (very high, high, medium, and low). All proteins identified by using two or more unique peptides were classified with a very high level of certainty. Single-hit proteins were classified in the remaining three certainty levels based on a model using the peptide identification probability and the peptide length (20). The estimated false-discovery rates for the four categories are 0, 1.4, 19, and 32% (with 95% upper bounds of 0.4, 2.5, 32, and 54%). Proteins identified with very high or high certainty were considered to be confidently identified and were described further.
Western immunoblotting.
Groups of five mice were inoculated as described above with JP1 or PBS, and BAL fluid was harvested 30 min and 6 h following inoculation, pooled, and frozen at −80°C until use. BAL fluids from PBS-inoculated animals were used as controls for insoluble airway protein in the processing of the samples and were treated exactly the same as the infected samples. Upon thawing of the samples at room temperature, Triton X-100 was added to obtain a concentration of 0.1% and the BAL fluids were vortexed for 15 s. Bacteria were harvested by centrifugation at 4,000 × g for 10 to 20 min and then resuspended in 1 ml of the same BAL fluid and harvested again at 16,100 × g for 5 min. The bacteria were resuspended in 30 μl of Laemmli buffer (27) and incubated for 30 min at 37°C. Following centrifugation at 16,100 × g for 5 min, the supernatants containing the airway proteins were separated by SDS-PAGE using 4 to 20% Criterion gradient gels (Bio-Rad). Following SDS-PAGE, proteins were transferred onto nitrocellulose membranes by using a Trans-Blot SD semidry electrophoretic transfer apparatus (Bio-Rad). Membranes were blocked and antibodies were diluted using Odyssey blocking buffer (Li-Cor Biosciences, Lincoln, NE) mixed 1:1 with PBS. The primary antibody was rabbit anti-mouse hemoglobin (MP Biomedicals, Aurora, OH), the secondary antibody was goat anti-rabbit Ig conjugated to horseradish peroxidase (Bio-Rad), and the tertiary antibody was donkey anti-goat Ig conjugated to Alexa Fluor 680 (Invitrogen, Carlsbad, CA). Hemoglobin was detected using an Odyssey infrared imaging system (Li-Cor Biosciences).
RESULTS
Isolation of airway proteins from the surface of S. aureus cells.
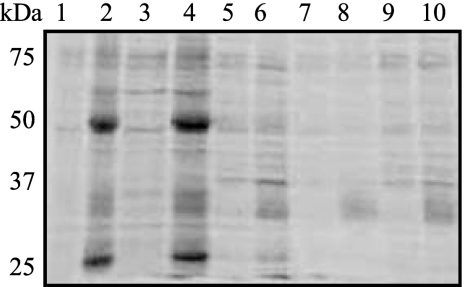
The purpose of this study was to identify the host proteins that associate with S. aureus during the early stages of pneumonia. We hypothesized that the protein-protein interactions between the host and the pathogen during the first 6 h of infection would dictate the course and severity of the disease. To determine the most efficient method for isolating host proteins from the surface of S. aureus cells following airway exposure, S. aureus JP1, a community-acquired methicillin-susceptible S. aureus strain isolated from a patient with bacteremia (41), was incubated with PBS or human BAL fluid as a physiologically relevant source of host proteins. The host proteins were recovered from the bacterial surface by incubation in Laemmli buffer (27) at 37 or 100°C or in the detergent 0.5% sodium deoxycholate, 0.5% Tween 20, or 0.5% Triton X-100. As shown in lanes 2 and 4 of Fig. 1, Laemmli buffer was the most effective reagent for the removal of host proteins from the bacterial surface without removing excess bacterial proteins (lanes 1 and 3). Because S. aureus cells were more susceptible to detergent-mediated lysis at high temperatures following exposure to the airway environment (our unpublished observations), the incubation of the harvested bacteria in Laemmli buffer at 37°C rather than 100°C was determined to be the better method of removing host proteins from the bacterial surface.
FIG. 1.
Laemmli buffer effectively removes airway proteins from the surfaces of S. aureus cells with minimal bacterial protein contamination. S. aureus JP1 was incubated with PBS or human BAL fluid and treated to remove the BAL proteins from the bacterial surface, and the BAL proteins were separated by SDS-PAGE and stained with SYPRO Ruby. Lanes 1, 3, 5, 7, and 9, JP1 incubated in PBS; lanes 2, 4, 6, 8, and 10, JP1 incubated in BAL fluid. BAL proteins were removed under the following conditions: lanes 1 and 2, Laemmli buffer at 100°C; lanes 3 and 4, Laemmli buffer at 37°C; lanes 5 and 6, 0.5% sodium deoxycholate at 37°C; lanes 7 and 8, 0.5% Tween 20 at 37°C; lanes 9 and 10, 0.5% Triton X-100.
Identification of airway proteins associated with S. aureus during early pneumonia.
A shotgun proteomics approach was used to identify the airway proteins that associated with S. aureus during the first 6 h of pneumonia. Groups of 10 mice were inoculated intranasally under anesthesia with JP1 by use of a recently developed model of S. aureus pneumonia (Ventura et al., submitted), and BAL fluid was harvested 30 min and 6 h postinoculation. Triton X-100 was added to the BAL fluid prior to bacterial recovery by centrifugation to solubilize host protein aggregates that formed during centrifugation. Following centrifugation, means ± standard errors of the means of 2.18 × 108 ± 1.06 × 108 CFU and 1.45 × 107 ± 6.03 × 106 CFU were recovered from the pooled BAL fluids of 10 mice 30 min and 6 h postinoculation, respectively. The supernatant from the incubation of the bacteria in Laemmli buffer was subjected to 1D SDS-PAGE and in-gel digestion with trypsin or to digestion with trypsin and SCX, followed by LC-MS-MS. Each host protein sample contained approximately 10 to 20 μg of protein, as determined empirically from visualizing stained SDS-PAGE gels (an exact measurement of the total protein by use of a biochemical assay was not performed because all of the protein was required for subsequent analyses).
A total of 513 host proteins were confidently identified as being associated with S. aureus 30 min and/or 6 h following inoculation (see Table S1 in the supplemental material), with 288 (56%) of those proteins identified with very high confidence (using at least two peptides). Fractionation by 1D SDS-PAGE yielded 386 protein identifications, while SCX fractionation resulted in the identification of 219 proteins; 92 proteins (18% of the total) were identified following both fractionation approaches. A larger number of proteins was associated with S. aureus at 6 h after inoculation than at 30 min (358 versus 295 proteins, respectively); of these, 197 and 162 proteins, respectively, were identified with very high confidence. Interestingly, 155 proteins were identified at 30 min and not at 6 h, while 218 proteins were identified only at 6 h. Of those proteins identified with very high confidence at only one time point (65 at 30 min and 104 at 6 h), 27% were identified by using at least five unique peptides and 42% were identified by using at least four peptides. Taken together, these data strongly suggest that the complement of associated host proteins changed significantly during early infection.
Phagocytic proteins rapidly associated with S. aureus in the airway.
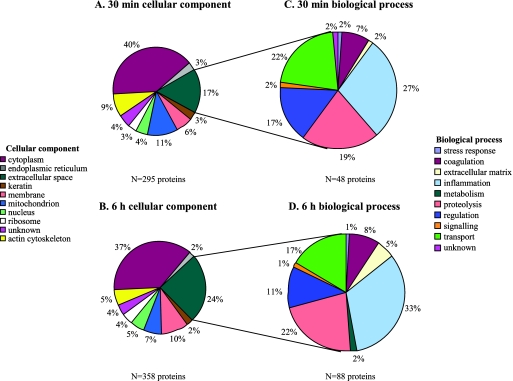
A cellular component Gene Ontology (GO) category was assigned to each identified protein (Fig. 2A and B). A majority of the proteins (83 and 76% of the total proteins identified at 30 min and 6 h, respectively) were characterized as being intracellular, suggesting that (i) S. aureus was internalized rapidly in the airway, (ii) a significant amount of host cell lysis occurred during infection, possibly as a result of apoptosis, necrosis, and/or S. aureus toxin-mediated cytolysis, and/or (iii) host cell lysis and subsequent centrifugation of intracellular proteins onto the bacteria occurred during sample processing. To determine if S. aureus was associated with alveolar macrophages (AMs) and polymorphonuclear leukocytes (PMNs) during the first 6 h following inoculation, the number of cell-associated bacteria in BAL specimens was determined (it is important to note that this method does not distinguish intracellular from surface-bound bacteria). By 30 min postinoculation, bacteria were associated with 65% ± 6% (mean ± standard error of the mean) of AMs; the percentage of AMs associated with S. aureus did not change significantly during the first 6 h of infection (60% ± 6% at 6 h), indicating that association with and/or uptake by AMs was avid and very rapid. PMNs were nearly undetectable in the airway 30 min following inoculation, but the proportion of PMNs associated with S. aureus at 6 h was 25% ± 4%. Because S. aureus was associated with and/or internalized by AMs and PMNs in the airway, we determined if any of the host proteins identified as being associated with S. aureus were phagosomal proteins. Recent proteomics studies have defined the proteins present in phagosomes resulting from the internalization of latex beads by AMs and PMNs (3, 11). Of the 513 total proteins associated with S. aureus, 105 have been shown to be present in the phagosome (Table 1) . At least 70 phagosomal proteins were associated with S. aureus 30 min after inoculation, with 78 phagosomal proteins associated after 6 h. Cytoskeletal proteins, including actin and actin binding proteins (alpha-actinin and F-actin capping protein), would be predicted to interact with S. aureus during actin rearrangement events that occur early during phagocytosis. Proteolytic and inflammatory proteins, including cathepsins D and G, calgranulin A, catalase, cathelicidin antimicrobial peptide, and myeloperoxidase, are present in late phagosomes and lysosomes and play an important role in killing intracellular bacteria. Interestingly, S. aureus is relatively resistant to phagocytic killing and survives within the phagocytic cell (15).
FIG. 2.
A total of 513 airway proteins were associated with S. aureus in the first 6 h of infection (see Table S1 in the supplemental material for a complete list of proteins). The proteins identified at 30 min (A) and 6 h (B) were divided into GO cellular component categories. The extracellular proteins identified at 30 min (C) and 6 h (D) were further categorized according to the corresponding GO biological processes.
TABLE 1.
Proteins previously identified in phagosomes associate with S. aureus in the airway 30 min and/or 6 h postinoculation
| Biological process(es) | Host protein | Phagosome source(s)a | Level of confidence in IDb at:
|
|
|---|---|---|---|---|
| 30 min | 6 h | |||
| Basic cellular processes | Acid ceramidase | Mφ | Very high | |
| Aldehyde dehydrogenase Ahd-2-like protein | PMNs | Very high | Very high | |
| ATPase H+-transporting V1 subunit B | PMNs, Mφ | Very high | High | |
| ATP synthase subunit α | PMNs, Mφ | Very high | Very high | |
| ATP synthase subunit β | PMNs | High | ||
| Carbohydrate sulfotransferase 12 | PMNs | High | ||
| Cytochrome P450 2A5 | Mφ | High | ||
| Electron transfer flavoprotein subunit α | PMNs | High | Very high | |
| Elongation factor 1-alpha 1 | Mφ | High | Very high | |
| Endoplasmin | Mφ | Very high | ||
| Glutathione S-transferase Mu 1 | PMNs | Very high | ||
| Glyceraldehyde-3-phosphate dehydrogenase | PMNs, Mφ | Very high | Very high | |
| Histone H2A family member J | PMNs | High | Very high | |
| Histone H2B type 1-A | PMNs | High | ||
| Histone 4 protein | PMNs | Very high | High | |
| Hydroxyacyl coenzyme A dehydrogenase/3-ketoacyl coenzyme A thiolase/enoyl coenzyme A hydratase | PMNs | High | ||
| Isocitrate dehydrogenase 1 (NADP+) | PMNs | Very high | ||
| Lamin A/C isoform 1 | Mφ | Very high | ||
| Malate dehydrogenase | PMNs | High | ||
| NADH dehydrogenase iron-sulfur protein 3 | PMNs | Very high | ||
| Phosphoglycerate kinase 1 | PMNs | Very high | ||
| Phosphoglycerate mutase 1 | PMNs, Mφ | High | ||
| Prohibitin-2 | PMNs, Mφ | High | ||
| Prolyl 4-hydroxylase | PMNs | Very high | High | |
| Protein disulfide isomerase-associated 3 | PMNs, Mφ | Very high | High | |
| Pyruvate kinase | PMNs | Very high | High | |
| T-complex protein 1 subunit α | Mφ | Very high | ||
| T-complex protein 1 subunit β | Mφ | Very high | High | |
| T-complex protein 1 subunit ε | PMNs, Mφ | High | ||
| Transketolase | PMNs | Very high | ||
| Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein | PMNs | High | Very high | |
| Ubiquinol-cytochrome c reductase complex core protein 2 | PMNs | Very high | ||
| Vesicle-associated membrane protein-associated A | Mφ | High | ||
| Coagulation | Fibrinogen | PMNs | Very high | Very high |
| Cytoskeleton | Alpha-actin | PMNs | Very high | Very high |
| Cytoplasmic actin | Mφ | Very high | Very high | |
| Actin-related protein 2 | PMNs | Very high | ||
| Adenylyl cyclase-associated protein 1 | PMNs, Mφ | Very high | Very high | |
| Alpha-actinin 1 | Mφ | High | ||
| Calreticulin | Mφ | High | ||
| Cofilin-2 | PMNs | High | ||
| Coronin-1B | Mφ | High | High | |
| Desmoplakin | PMNs | Very high | Very high | |
| Dynein intermediate chain 1 | PMNs | Very high | ||
| F-actin capping protein | Mφ | Very high | ||
| Gelsolin | PMNs | Very high | Very high | |
| Moesin | PMNs | Very high | Very high | |
| Myosin heavy chain | Mφ | Very high | Very high | |
| Regulatory myosin light chain | PMNs | Very high | High | |
| Myosin light chain 6B | PMNs | Very high | Very high | |
| Plastin-2 | PMNs | Very high | Very high | |
| Profilin-1 | PMNs | Very high | ||
| Syntenin-1 | PMNs, Mφ | High | ||
| Tubulin alpha | PMNs | Very high | Very high | |
| Tubulin beta-5 | Mφ | High | ||
| Vimentin | PMNs, Mφ | Very high | Very high | |
| Inflammation | Calgizzarin/endothelial monocyte-activating protein polypeptide/protein S100-A11 | PMNs | High | |
| Calgranulin A/migration inhibitory factor-related protein 8/protein S100-A8 | PMNs | High | ||
| Catalase | PMNs | Very high | ||
| Cathelicidin antimicrobial peptide | PMNs | Very high | ||
| Igsc | PMNs | Very high | High | |
| Lysozyme | PMNs, Mφ | Very high | Very high | |
| Major histocompatibility complex | PMNs | Very high | ||
| Myeloperoxidase | PMNs | Very high | Very high | |
| Neutrophil collagenase/matrix metalloproteinase 8 | PMNs | Very high | ||
| Neutrophil gelatinase-associated lipocalin | PMNs | Very high | ||
| Proteolysis | Cathepsin D | Mφ | Very high | |
| Cathepsin G | PMNs | High | ||
| Dipeptidase 1 | PMNs | High | ||
| Napsin-A | Mφ | Very high | Very high | |
| Proteasome subunit alpha type 6 | PMNs | High | ||
| Serine (or cysteine) proteinase inhibitor, clade B, member 1a | PMNs | High | High | |
| Whey acidic protein 4-disulfide core domain protein 2 | PMNs | Very high | Very high | |
| Regulation | Annexin A11 | PMNs | Very high | |
| Annexin A3 | PMNs | High | ||
| Annexin A5 | PMNs, Mφ | Very high | Very high | |
| Stomatin | PMNs, Mφ | Very high | ||
| Signaling | 14-3-3 protein | Mφ | High | |
| Annexin A1 | PMNs | Very high | Very high | |
| Guanine nucleotide binding protein α-2 | Mφ | Very high | ||
| Guanine nucleotide binding protein β-1 | Mφ | High | ||
| Guanine nucleotide binding protein β-2 | Mφ | High | ||
| Mitogen-activated protein kinase 1 | PMNs | High | ||
| Rab7 (Ras-related protein) | Mφ | Very high | ||
| Rab11B (Ras-related protein) | PMNs, Mφ | High | ||
| Rho GDP dissociation inhibitor 1 | Mφ | Very high | ||
| Rho GDP dissociation inhibitor 2 | PMNs | Very high | ||
| Stress response | Heat shock protein 1 (60 kDa) | PMNs | Very high | |
| Heat shock protein 1β | PMNs | Very high | Very high | |
| Heat shock protein 70 | Mφ | Very high | ||
| Heat shock protein 70/8 | PMNs | Very high | Very high | |
| Heat shock 70-kDa protein 5 | PMNs | Very high | Very high | |
| Heat shock protein 90-alpha | Mφ | Very high | Very high | |
| Peroxiredoxin 2 | PMNs | Very high | ||
| Peroxiredoxin 5 | PMNs | Very high | ||
| Peroxiredoxin 6 | PMNs | Very high | High | |
| Transport | Chloride intracellular channel protein 1 | PMNs | Very high | |
| Ferritin | PMNs, Mφ | Very high | ||
| Haptoglobin | PMNs | Very high | Very high | |
| Hemoglobin | PMNs | Very high | Very high | |
| Selenium binding protein 1 | PMNs | Very high | Very high | |
| Serotransferrin | PMNs | Very high | Very high | |
| Serum albumin | PMNs | Very high | Very high | |
| Voltage-dependent anion-selective channel protein 1 | Mφ | High | ||
The phagosome source was determined according to the results of Garin et al. (for macrophages [Mφ]) (11) and Burlak et al. (for PMNs) (3).
A high level of confidence in the identification (ID) indicates that the protein was confidently identified using one peptide, and a very high level of confidence in the identification indicates that the protein was confidently identified using two or more peptides.
A total of 11 different Ig proteins were identified in the analysis (see Table S1 in the supplemental material). The 11 different Igs were combined in this table for simplicity.
Extracellular proteins associate with S. aureus in the airway.
Extracellular proteins, which constituted 19% of the total associated proteome, were further categorized by GO biological process assignments. A total of 17% (48) and 24% (88) of the proteins associated with S. aureus 30 min and 6 h postinoculation, respectively, were classified as extracellular proteins. A majority of the proteins associated at each time point were involved in inflammation, proteolysis, regulation, and transport (Fig. 2C and D and Table 2 ). More proteins involved in inflammation, coagulation, extracellular matrix, and proteolysis were associated with S. aureus at 6 h than at 30 min.
TABLE 2.
Extracellular host proteins associate with S. aureus in the airway 30 min and/or 6 h postinoculation
| Biological process(es) | Host protein(s) | Level of confidence in IDa at:
|
|
|---|---|---|---|
| 30 min | 6 h | ||
| Coagulation | Alpha-2-antiplasmin | High | Very high |
| Antithrombin III | Very high | ||
| Coagulation factor XIII | Very high | ||
| Fibrinogen | Very high | Very high | |
| Heparin cofactor 2 | Very high | ||
| Hyaluronan binding protein 2 | High | ||
| Kininogen 1 | Very high | Very high | |
| Plasminogen | Very high | ||
| Prothrombin | Very high | ||
| Extracellular matrix | Fibronectin | Very high | |
| Nephronectin | High | ||
| Neutrophil collagenase/matrix metalloproteinase 8 | Very high | ||
| Neutrophil gelatinase-associated lipocalin | Very high | ||
| Vitronectin | High | Very high | |
| Inflammation | Alpha-1-microglobulin/bikunin | High | Very high |
| Calgranulin A/protein S100-A8 | High | ||
| Cathelicidin antimicrobial peptide | Very high | ||
| Chitinase 3-like protein 1 | Very high | High | |
| Chitinase 3-like protein 3 | Very high | Very high | |
| Complement C3 | Very high | Very high | |
| Complement C4b | Very high | ||
| Complement C5 | Very high | Very high | |
| Complement C7 | High | ||
| Complement C8 | Very high | High | |
| Complement C9 | Very high | High | |
| Complement factor B | Very high | ||
| Complement factor H | High | ||
| Granulin/epithelin | High | ||
| Igs (11 identifications)b | Very high | Very high | |
| Long palate, lung, and nasal epithelium carcinoma-associated protein 1 | Very high | ||
| Long palate, lung, and nasal epithelium carcinoma-associated protein 3 | High | ||
| Lysozyme | Very high | Very high | |
| Myeloid bactenecin | Very high | ||
| Palate, lung, and nasal epithelium clone | High | Very high | |
| Serum amyloid A-1 | Very high | ||
| Serum amyloid A-2 | High | ||
| Serum amyloid A-4 | Very high | ||
| Serum amyloid P component | Very high | ||
| Metabolism | Serum paraoxonase/arylesterase 1 | Very high | |
| Proteolysis | Alpha-1-antitrypsins 1-2 | Very high | Very high |
| Alpha-1-antitrypsins 1-4 | Very high | Very high | |
| Alpha-1-antitrypsins 1-5 | Very high | Very high | |
| Alpha-1-antitrypsins 1-6 | Very high | Very high | |
| Alpha-2-macroglobulin | Very high | ||
| Histidine-rich glycoprotein | Very high | ||
| Inter-alpha trypsin inhibitor heavy chain 4 | Very high | ||
| Inter-alpha trypsin inhibitor heavy chain H2 | High | ||
| Murinoglobulin-1 | Very high | ||
| Myeloblastin | High | ||
| Napsin-A | Very high | Very high | |
| Plasma protease C1 inhibitor | High | ||
| Serine proteinase inhibitor B1a | High | High | |
| Serine proteinase inhibitor B6 | Very high | ||
| Serine proteinase inhibitor B6b | Very high | ||
| Serine proteinase inhibitor B9 | Very high | ||
| Serine protease inhibitor A3C | High | ||
| Serine protease inhibitor A3K | Very high | Very high | |
| Serine protease inhibitor A3M | High | Very high | |
| Serine protease inhibitor A3N | Very high | Very high | |
| Whey acidic protein 4-disulfide core domain protein 2 | Very high | Very high | |
| Regulation | α2-HS-glycoprotein/fetuin | Very high | Very high |
| Protein-glutamine gamma-glutamyltransferase 2 | High | ||
| Pulmonary surfactant-associated protein B | Very high | Very high | |
| Pulmonary surfactant-associated protein D | Very high | Very high | |
| Regulation and transport | Apolipoprotein A-I | Very high | Very high |
| Apolipoprotein A-II | High | ||
| Apolipoprotein A-IV | Very high | Very high | |
| Apolipoprotein C-I | High | ||
| Apolipoprotein C-III | High | Very high | |
| Apolipoprotein E | Very high | ||
| Apolipoprotein H/beta-2-glycoprotein I | High | ||
| Signaling | Uteroglobin | Very high | Very high |
| Stress response and regulation | Clusterin | Very high | Very high |
| Transport | Apolipoprotein B | Very high | |
| Ceruloplasmin | Very high | ||
| Haptoglobin | Very high | Very high | |
| Hemopexin | Very high | Very high | |
| Mucin 5B | Very high | ||
| Phospholipid transfer protein | Very high | High | |
| Pulmonary surfactant-associated protein A | Very high | Very high | |
| Serotransferrin | Very high | Very high | |
| Serum albumin | Very high | Very high | |
| Transthyretin | High | ||
| Vitamin D binding protein | Very high | ||
| Transport and metabolism | Apolipoprotein M | High | |
| Unknown | Leubrin | High | |
A high level of confidence in the identification (ID) indicates that the protein was confidently identified using one peptide, and a very high level of confidence in the identification indicates that the protein was confidently identified using two or more peptides.
A total of 11 different Ig proteins were identified in the analysis (see Table S1 in the supplemental material). The 11 different Igs were combined in this table for simplicity.
At least 16 host proteins have previously been shown to interact with S. aureus in vitro (for a review, see reference 4). As shown in Table 3, 8 of these proteins were associated with S. aureus at 30 min and 12 were associated with the bacterium at 6 h postinoculation. Complement C3, Ig, and pulmonary surfactant protein A are opsonins that bind to S. aureus to promote phagocytosis by macrophages and neutrophils. As mentioned above, S. aureus rapidly associated with and/or was internalized by both AMs and PMNs during the early stages of infection. Fibronectin and vitronectin are components of the extracellular matrix; the binding of S. aureus to fibronectin promotes internalization by alveolar epithelial cells (33). Taken together, these data demonstrate that interactions that have been described using in vitro experiments also occur in the context of an infection and therefore may be important for the establishment of disease. In addition, the data show that previously uncharacterized host-pathogen interactions occur during the early stages of pneumonia.
TABLE 3.
Airway proteins implicated in host-pathogen interactions associate with S. aureus during airway infection
| Host protein | S. aureus receptor(s) | Associated with S. aureus at:
|
Reference(s) | |
|---|---|---|---|---|
| 30 min | 6 h | |||
| Cathelin-related antimicrobial peptide | Sak | + | 2 | |
| Complement C3 | Efb | + | + | 7, 29 |
| Fetuin/α2-HS-glycoprotein | IsdA | + | + | 4 |
| Fibrinogen | ClfA, ClfB, Emp, Map, FnbA, FnbB, IsdA | + | + | 6, 32, 37, 46 |
| Fibronectin | FnbPA, FnbPB, IsdA, Ebh, Emp, Atl, Aaa | + | 4-6, 14, 17, 21, 22, 39 | |
| Haptoglobin | IsdH/HarA | + | + | 9 |
| Hemoglobin | IsdB, IsdH/HarA | + | + | 6, 31, 44 |
| Ig | Spa | + | + | 10, 25 |
| Plasminogen | Eno | + | 35 | |
| Pulmonary surfactant protein A | Unknown | + | + | 34 |
| (Sero)transferrin | IsdA | + | + | 6, 43 |
| Vitronectin | Emp, Aaa | + | 17, 21 | |
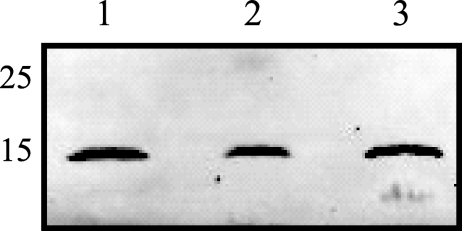
Hemoglobin associates with S. aureus during early airway infection.
Iron is essential to bacterial growth, and hemoglobin is the preferred iron source for S. aureus (40). Torres et al. demonstrated that an S. aureus strain lacking receptors for hemoglobin exhibited attenuated virulence in a mouse model of staphylococcal systemic abscess formation (44). In our proteomics analysis, 3.1% (30 min) and 7.0% (6 h) of the total spectra were identified as peptides of hemoglobin, suggesting that hemoglobin may be one of the more abundant host proteins associated with S. aureus in the airway. To validate the identification of hemoglobin associated with S. aureus in the airway, bacteria were recovered from mice 30 min and 6 h postinoculation and the associated host proteins were isolated as described above. Western blot analysis of the host proteins associated with S. aureus in the airway clearly confirmed that hemoglobin was associated 30 min and 6 h postinoculation (Fig. 3), thus demonstrating that shotgun proteomics of host proteins recovered from the surface of a small number of bacteria is sufficiently powerful to identify proteins involved in the host-pathogen interaction.
FIG. 3.
Hemoglobin associates with S. aureus 30 min and 6 h postinoculation into the airway. Bacteria were recovered from the BAL fluids of mice inoculated with S. aureus, and host proteins were harvested as described in Materials and Methods. The host proteins were separated by SDS-PAGE, transferred onto nitrocellulose membranes, and probed with antibodies against mouse hemoglobin. Lanes: 1, BAL fluid containing hemoglobin (control); 2, sample from 30 min postinoculation; 3, sample from 6 h postinoculation. Numbers on the left indicate molecular sizes in kilodaltons.
DISCUSSION
The goal of this study was to identify the host proteins that associate with S. aureus during the first 6 h of airway infection. We confidently identified 513 proteins associated with S. aureus 30 min and/or 6 h postinoculation using shotgun proteomics, despite recovering only 10 to 20 μg of host protein from the surfaces of S. aureus cells harvested from the airways of 10 mice per time point. In an attempt to be as comprehensive as possible, we utilized two different fractionation methods prior to LC-MS-MS. One experiment utilized 1D SDS-PAGE fractionation and in-gel digestion with LC-MS-MS performed using an LTQ mass spectrometer, while another experiment used SCX fractionation of digested peptides with subsequent LCQ-DECA analysis. Only 92 of the 513 proteins (18%) were identified in both experiments, highlighting both the limitations of MS-MS and the importance of using different techniques to generate a more comprehensive analysis of a highly complex protein sample in which each protein is present in very low abundance. In order to determine whether one technique is better than the other in a study such as this, several experiments, each requiring 20 mice and using each method of fractionation and LC-MS-MS, would be necessary. While we acknowledge that the use of mass spectrometry in the manner described in this report is novel, we believe that the true significance of this study lies in the biology rather than the technology.
We did not attempt to separate intracellular and extracellular bacteria but rather sampled the entire population in the airway to identify as many associated host proteins as possible. Therefore, the subcellular source of the proteins we detected included every compartment of the host cell, as well as the extracellular milieu. Nearly 80% of the proteins we identified were intracellular proteins, suggesting that S. aureus was rapidly internalized in the airway and that S. aureus infection caused rapid host cell lysis, presumably as a result of apoptosis, necrosis, or S. aureus toxin activity. S. aureus has previously been shown to be phagocytosed by AMs as rapidly as 30 min postinoculation in an aerosolization model (13, 23, 28); thus, it is not surprising that we detected a large number of phagosomal proteins associated with S. aureus 30 min and 6 h postinoculation. In fact, of the 404 intracellular proteins, 105 of them have previously been shown to be components of the phagosomal proteome of macrophages and/or PMNs (3, 11). It is important to point out that the association of these host proteins with the phagosome does not imply that they are found solely in the phagosome. In fact, nearly all of the proteins identified as being phagosomal play other roles in the cell that are unrelated to phagocytosis specifically. We identified proteins specific to different stages of phagosomal maturation at both time points. Actin, coronin, myosin, T-complex protein 1, and Rho GDP dissociation inhibitors are critical to the actin rearrangement process that occurs to facilitate the extension of pseudopodia, an early step in the phagocytic process (8, 45). The identification of proteins such as Rab7, Rab11B, and syntenin suggests that S. aureus is present in late endosomes; these three proteins are specific to late phagosomes that are preparing to fuse with lysosomes in the phagosome maturation process (8, 45). These data, combined with our data showing that S. aureus is rapidly associated with or internalized by AMs and PMNs in the airway, provide additional evidence to support the findings of previous studies demonstrating that the phagocytosis of S. aureus occurs quickly (13, 23, 28) and is followed by the survival of the bacteria within the phagocytes (15).
While we believe that the majority of the intracellular proteins that were identified were associated with S. aureus as a result of phagocytosis or host cell lysis in vivo, we acknowledge that the manner in which the samples were processed may have contributed to the identification of these proteins. BAL samples from infected mice were frozen, and Triton X-100 was added to thawed samples in order to solubilize aggregated host proteins in the BAL fluid. During the subsequent high-speed centrifugation to harvest the bacteria, intracellular host proteins that were present in the BAL fluid as a result of host cell lysis (in vivo as a result of infection or ex vivo during processing) may have become associated with S. aureus. The bacterial pellets were thoroughly washed twice with PBS following the initial harvest in an attempt to disrupt any nonspecific interactions between the bacteria and irrelevant host proteins. The following arguments support the conclusion that the host proteins identified as being associated with S. aureus in the airway represent biologically relevant interactions. (i) Two different separation and mass spectrometric approaches were utilized to define the host proteins, resulting in an overlap in protein identification of nearly 20% between unique biological samples. (ii) The complement of host proteins associated with S. aureus changed during the course of early infection; if sample processing had contributed significantly to protein identification, one would expect that the same proteins would have been identified in samples from both time points, when in fact 53 and 61% of the proteins identified 30 min and 6 h postinoculation, respectively, were unique to that time point. (iii) Twelve of 16 host proteins known to interact with S. aureus in vitro were identified in our in vivo analysis, and 8 of these 12 proteins were identified in biological samples from both time points. (iv) Immunoblot analysis allowed us to confirm the presence of hemoglobin on the surfaces of S. aureus cells recovered from the airway. Taken together, these arguments provide strong support for the conclusion that the majority of the host proteins that were associated with S. aureus during early infection represent potentially biologically relevant phenomena that should be characterized further.
Previous studies have identified at least 16 host proteins that bind to S. aureus via either a receptor ligand or a nonspecific interaction (Table 3). In this study, we demonstrated that at least 12 of these proteins were present on the surfaces of S. aureus cells 30 min and/or 6 h following the inoculation of the bacteria into the airway. Complement C3, Igs, and pulmonary surfactant protein A are capable of opsonizing S. aureus via both nonspecific (complement C3 and pulmonary surfactant protein A) and specific (Igs) mechanisms. Interestingly the S. aureus receptors for complement C3 and Igs, Efb, and protein A, respectively, have antiopsonic properties. The extracellular fibrinogen binding protein Efb inhibits opsonophagocytosis by binding complement C3, thus preventing complement C3 from opsonizing the bacteria (29). Protein A is the most abundant protein on the surfaces of S. aureus cells and has been shown to be critical for the survival of S. aureus in the airway (12, 26, 47). Protein A binds the Fc portion of IgG molecules and renders the IgG molecules inaccessible to the Fc receptor on the surfaces of phagocytic cells, thus providing S. aureus with a mechanism for preventing antibody-mediated phagocytosis. In addition, previous studies in our laboratory have demonstrated that cathelicidin is capable of binding to staphylokinase to enhance the ability of staphylokinase to activate plasminogen and promote fibrinolysis, thus providing the organism with a mechanism for disseminating systemically (2). Thus, the pathogen has several mechanisms for evading host innate immune factors through direct binding to host proteins.
The four proteins previously shown to interact with S. aureus that we did not identify in our analysis are elastin, laminin, TNFR1, and von Willebrand factor (4, 12). Potential reasons for our inability to detect these proteins include the following: (i) the proteins were not present in the airway at the time points that we analyzed; (ii) the shotgun proteomics approach is not sufficiently sensitive to detect all of the proteins present in a complex mixture, particularly those present in low abundance; (iii) the proteins were present but did not bind to S. aureus in the airway; and (iv) the fractionation techniques (1D SDS-PAGE, SCX, and LC) may have eliminated proteins with extreme charges or molecular weights. The first explanation is supported by the results of our analysis of the airway proteome 30 min and 6 h postinoculation with JP1; we did not identify peptides corresponding to any of these four proteins in the airway at either time point (Ventura et al., submitted), which suggests that these proteins may not be present in the airway. Alternatively, our inability to detect these proteins in the airway may be a reflection of the fact that we sampled the proteins present in the lavage fluid rather than those present in the entire lung, which may preclude the identification of membrane or intracellular proteins. Further studies using later time points and/or different animal models are necessary to determine when the interactions between S. aureus and any of these four proteins are important for virulence.
Shotgun proteomics proved to be an extremely useful tool for identifying airway proteins that associate with S. aureus during the early stages of pneumonia. To our surprise, over 500 host proteins were found to interact with S. aureus 30 min and/or 6 h postinoculation. We were able to confirm the binding of one of these proteins, hemoglobin, to S. aureus at both time points by using an alternative method. The data presented here provide critical information regarding known as well as previously undescribed host-pathogen interactions that occur between S. aureus and the host during early airway infection. Further, these data form the foundation for future studies of the specific interactions between the host and the pathogen during the initial steps in the pathogenesis of pneumonia.
Supplementary Material
Acknowledgments
We thank Eric Skaar for helpful discussions about hemoglobin binding to S. aureus, Jeannette Crisostomo, Destry Taylor, and Michele Timko for expert technical assistance, and Amanda Jones for critical reading of the manuscript. Fractionation and LC-MS-MS were performed by Sam Donohoe and Laura Hohmann at the Institute for Systems Biology, Seattle, WA.
This work was supported by grant HL073996 from the National Institutes of Health (to C.E.R.).
Editor: B. A. McCormick
Footnotes
Published ahead of print on 14 January 2008.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Bradley, S. F. 2006. Staphylococcus aureus pneumonia: emergence of MRSA in the community. Semin. Respir. Crit. Care Med. 26643-649. [DOI] [PubMed] [Google Scholar]
- 2.Braff, M. H., A. L. Jones, S. J. Skerrett, and C. E. Rubens. 2007. Staphylococcus aureus exploits cathelicidin antimicrobial peptides produced during early pneumonia to promote staphylokinase-dependent fibrinolysis. J. Infect. Dis. 1951365-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burlak, C., A. R. Whitney, D. J. Mead, T. Hackstadt, and F. R. Deleo. 2006. Maturation of human neutrophil phagosomes includes incorporation of molecular chaperones and endoplasmic reticulum quality control machinery. Mol. Cell. Proteomics 5620-634. [DOI] [PubMed] [Google Scholar]
- 4.Clarke, S. R., and S. J. Foster. 2006. Surface adhesins of Staphylococcus aureus. Adv. Microb. Physiol. 51187-224. [DOI] [PubMed] [Google Scholar]
- 5.Clarke, S. R., L. G. Harris, R. G. Richards, and S. J. Foster. 2002. Analysis of Ebh, a 1.1-megadalton cell wall-associated fibronectin-binding protein of Staphylococcus aureus. Infect. Immun. 706680-6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke, S. R., M. D. Wiltshire, and S. J. Foster. 2004. IsdA of Staphylococcus aureus is a broad spectrum, iron-regulated adhesin. Mol. Microbiol. 511509-1519. [DOI] [PubMed] [Google Scholar]
- 7.Cunnion, K. M., J. C. Lee, and M. M. Frank. 2001. Capsule production and growth phase influence binding of complement to Staphylococcus aureus. Infect. Immun. 696796-6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desjardins, M. 2003. ER-mediated phagocytosis: a new membrane for new functions. Nat. Rev. Immunol. 3280-291. [DOI] [PubMed] [Google Scholar]
- 9.Dryla, A., D. Gelbmann, A. von Gabain, and E. Nagy. 2003. Identification of a novel iron regulated staphylococcal surface protein with haptoglobin-haemoglobin binding activity. Mol. Microbiol. 4937-53. [DOI] [PubMed] [Google Scholar]
- 10.Forsgren, A., and J. Sjoquist. 1966. “Protein A” from S. aureus. I. Pseudo-immune reaction with human gamma-globulin. J. Immunol. 97822-827. [PubMed] [Google Scholar]
- 11.Garin, J., R. Diez, S. Kieffer, J. F. Dermine, S. Duclos, E. Gagnon, R. Sadoul, C. Rondeau, and M. Desjardins. 2001. The phagosome proteome: insight into phagosome functions. J. Cell Biol. 152165-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomez, M. I., A. Lee, B. Reddy, A. Muir, G. Soong, A. Pitt, A. Cheung, and A. Prince. 2004. Staphylococcus aureus protein A induces airway epithelial inflammatory responses by activating TNFR1. Nat. Med. 10842-848. [DOI] [PubMed] [Google Scholar]
- 13.Green, G. M., and E. H. Kass. 1964. The role of the alveolar macrophage in the clearance of bacteria from the lung. J. Exp. Med. 119167-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greene, C., D. McDevitt, P. Francois, P. E. Vaudaux, D. P. Lew, and T. J. Foster. 1995. Adhesion properties of mutants of Staphylococcus aureus defective in fibronectin-binding proteins and studies on the expression of fnb genes. Mol. Microbiol. 171143-1152. [DOI] [PubMed] [Google Scholar]
- 15.Gresham, H. D., J. H. Lowrance, T. E. Caver, B. S. Wilson, A. L. Cheung, and F. P. Lindberg. 2000. Survival of Staphylococcus aureus inside neutrophils contributes to infection. J. Immunol. 1643713-3722. [DOI] [PubMed] [Google Scholar]
- 16.Hartleib, J., N. Kohler, R. B. Dickinson, G. S. Chhatwal, J. J. Sixma, O. M. Hartford, T. J. Foster, G. Peters, B. E. Kehrel, and M. Herrmann. 2000. Protein A is the von Willebrand factor binding protein on Staphylococcus aureus. Blood 962149-2156. [PubMed] [Google Scholar]
- 17.Heilmann, C., J. Hartleib, M. S. Hussain, and G. Peters. 2005. The multifunctional Staphylococcus aureus autolysin aaa mediates adherence to immobilized fibrinogen and fibronectin. Infect. Immun. 734793-4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heyer, G., S. Saba, R. Adamo, W. Rush, G. Soong, A. Cheung, and A. Prince. 2002. Staphylococcus aureus agr and sarA functions are required for invasive infections but not inflammatory responses in the lung. Infect. Immun. 70127-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higdon, R., J. M. Hogan, G. Van Belle, and E. Kolker. 2005. Randomized sequence databases for tandem mass spectrometry peptide and protein identification. Omics 9364-379. [DOI] [PubMed] [Google Scholar]
- 20.Higdon, R., and E. Kolker. 2007. A predictive model for identifying proteins by a single peptide match. Bioinformatics 23277-280. [DOI] [PubMed] [Google Scholar]
- 21.Hussain, M., K. Becker, C. von Eiff, J. Schrenzel, G. Peters, and M. Herrmann. 2001. Identification and characterization of a novel 38.5-kilodalton cell surface protein of Staphylococcus aureus with extended-spectrum binding activity for extracellular matrix and plasma proteins. J. Bacteriol. 1836778-6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jonsson, K., C. Signas, H. P. Muller, and M. Lindberg. 1991. Two different genes encode fibronectin binding proteins in Staphylococcus aureus. The complete nucleotide sequence and characterization of the second gene. Eur. J. Biochem. 2021041-1048. [DOI] [PubMed] [Google Scholar]
- 23.Kim, M., E. Goldstein, J. P. Lewis, W. Lippert, and D. Warshauer. 1976. Murine pulmonary alveolar macrophages: rates of bacterial ingestion, inactivation, and destruction. J. Infect. Dis. 133310-320. [DOI] [PubMed] [Google Scholar]
- 24.Kollef, M. H., A. Shorr, Y. P. Tabak, V. Gupta, L. Z. Liu, and R. S. Johannes. 2005. Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest 1283854-3862. [DOI] [PubMed] [Google Scholar]
- 25.Kronvall, G., H. M. Grey, and R. C. Williams, Jr. 1970. Protein A reactivity with mouse immunoglobulins. Structural relationship between some mouse and human immunoglobulins. J. Immunol. 1051116-1123. [PubMed] [Google Scholar]
- 26.Labandeira-Rey, M., F. Couzon, S. Boisset, E. L. Brown, M. Bes, Y. Benito, E. M. Barbu, V. Vazquez, M. Hook, J. Etienne, F. Vandenesch, and M. G. Bowden. 2007. Staphylococcus aureus Panton-Valentine leukocidin causes necrotizing pneumonia. Science 3151130-1133. [DOI] [PubMed] [Google Scholar]
- 27.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227680-685. [DOI] [PubMed] [Google Scholar]
- 28.LaForce, F. M., W. J. Kelly, and G. L. Huber. 1973. Inactivation of staphylococci by alveolar macrophages with preliminary observations on the importance of alveolar lining material. Am. Rev. Respir. Dis. 108784-790. [DOI] [PubMed] [Google Scholar]
- 29.Lee, L. Y., M. Hook, D. Haviland, R. A. Wetsel, E. O. Yonter, P. Syribeys, J. Vernachio, and E. L. Brown. 2004. Inhibition of complement activation by a secreted Staphylococcus aureus protein. J. Infect. Dis. 190571-579. [DOI] [PubMed] [Google Scholar]
- 30.Mazmanian, S. K., G. Liu, H. Ton-That, and O. Schneewind. 1999. Staphylococcus aureus sortase, an enzyme that anchors surface proteins to the cell wall. Science 285760-763. [DOI] [PubMed] [Google Scholar]
- 31.Mazmanian, S. K., E. P. Skaar, A. H. Gaspar, M. Humayun, P. Gornicki, J. Jelenska, A. Joachmiak, D. M. Missiakas, and O. Schneewind. 2003. Passage of heme-iron across the envelope of Staphylococcus aureus. Science 299906-909. [DOI] [PubMed] [Google Scholar]
- 32.McDevitt, D., P. Francois, P. Vaudaux, and T. J. Foster. 1994. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol. Microbiol. 11237-248. [DOI] [PubMed] [Google Scholar]
- 33.McElroy, M. C., D. J. Cain, C. Tyrrell, T. J. Foster, and C. Haslett. 2002. Increased virulence of a fibronectin-binding protein mutant of Staphylococcus aureus in a rat model of pneumonia. Infect. Immun. 703865-3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McNeely, T. B., and J. D. Coonrod. 1993. Comparison of the opsonic activity of human surfactant protein A for Staphylococcus aureus and Streptococcus pneumoniae with rabbit and human macrophages. J. Infect. Dis. 16791-97. [DOI] [PubMed] [Google Scholar]
- 35.Molkanen, T., J. Tyynela, J. Helin, N. Kalkkinen, and P. Kuusela. 2002. Enhanced activation of bound plasminogen on Staphylococcus aureus by staphylokinase. FEBS Lett. 51772-78. [DOI] [PubMed] [Google Scholar]
- 36.Nesvizhskii, A. I., A. Keller, E. Kolker, and R. Aebersold. 2003. A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 754646-4658. [DOI] [PubMed] [Google Scholar]
- 37.Ni Eidhin, D., S. Perkins, P. Francois, P. Vaudaux, M. Hook, and T. J. Foster. 1998. Clumping factor B (ClfB), a new surface-located fibrinogen-binding adhesin of Staphylococcus aureus. Mol. Microbiol. 30245-257. [DOI] [PubMed] [Google Scholar]
- 38.Park, D. R., and S. J. Skerrett. 1996. IL-10 enhances the growth of Legionella pneumophila in human mononuclear phagocytes and reverses the protective effect of IFN-gamma: differential responses of blood monocytes and alveolar macrophages. J. Immunol. 1572528-2538. [PubMed] [Google Scholar]
- 39.Signas, C., G. Raucci, K. Jonsson, P. E. Lindgren, G. M. Anantharamaiah, M. Hook, and M. Lindberg. 1989. Nucleotide sequence of the gene for a fibronectin-binding protein from Staphylococcus aureus: use of this peptide sequence in the synthesis of biologically active peptides. Proc. Natl. Acad. Sci. USA 86699-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skaar, E. P., M. Humayun, T. Bae, K. L. DeBord, and O. Schneewind. 2004. Iron-source preference of Staphylococcus aureus infections. Science 3051626-1628. [DOI] [PubMed] [Google Scholar]
- 41.Skerrett, S. J., H. D. Liggitt, A. M. Hajjar, and C. B. Wilson. 2004. Cutting edge: myeloid differentiation factor 88 is essential for pulmonary host defense against Pseudomonas aeruginosa but not Staphylococcus aureus. J. Immunol. 1723377-3381. [DOI] [PubMed] [Google Scholar]
- 42.Skerrett, S. J., T. R. Martin, E. Y. Chi, J. J. Peschon, K. M. Mohler, and C. B. Wilson. 1999. Role of the type 1 TNF receptor in lung inflammation after inhalation of endotoxin or Pseudomonas aeruginosa. Am. J. Physiol. 276L715-L727. [DOI] [PubMed] [Google Scholar]
- 43.Taylor, J. M., and D. E. Heinrichs. 2002. Transferrin binding in Staphylococcus aureus: involvement of a cell wall-anchored protein. Mol. Microbiol. 431603-1614. [DOI] [PubMed] [Google Scholar]
- 44.Torres, V. J., G. Pishchany, M. Humayun, O. Schneewind, and E. P. Skaar. 2006. Staphylococcus aureus IsdB is a hemoglobin receptor required for heme iron utilization. J. Bacteriol. 1888421-8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vieira, O. V., R. J. Botelho, and S. Grinstein. 2002. Phagosome maturation: aging gracefully. Biochem. J. 366689-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wann, E. R., S. Gurusiddappa, and M. Hook. 2000. The fibronectin-binding MSCRAMM FnbpA of Staphylococcus aureus is a bifunctional protein that also binds to fibrinogen. J. Biol. Chem. 27513863-13871. [DOI] [PubMed] [Google Scholar]
- 47.Wardenburg, J. B., R. J. Patel, and O. Schneewind. 2007. Surface proteins and exotoxins are required for the pathogenesis of Staphylococcus aureus pneumonia. Infect. Immun. 751040-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.