Abstract
Serum factors, including mannose binding lectins (MBL), influence innate responses to microbes. Little is known about the effects of serum factors or MBL on the interaction of Blastomyces dermatitidis, a pulmonary fungal pathogen, with macrophages or on tumor necrosis factor alpha (TNF-α) production. Since macrophage production of TNF-α is an important innate immune response, we examined a mouse peritoneal macrophage (PM) cell line (RAW) and resident PM from CD-1 mice to study TNF-α production by PM stimulated with heat-killed (HK) or live B. dermatitidis yeast cells. Mouse serum and heat-inactivated mouse serum inhibited TNF-α production 94% when macrophages were stimulated by B. dermatitidis, whereas mouse immunoglobulin G (IgG) did not have this effect. HK B. dermatitidis incubated with serum and then washed also failed to stimulate significant TNF-α production by PM. By the sandwich immunofluorescent antibody (IFA) method with anti-mouse MBL (MBL-A or -C), we showed that serum MBL bound to B. dermatitidis. When serum was absorbed with HK B. dermatitidis or live B. dermatitidis, absorbed serum failed to significantly inhibit TNF-α production by RAW cells plus B. dermatitidis, and immunoblotting showed that absorbed serum was depleted of MBL-C. If serum was absorbed with live B. dermatitidis, unbound serum was eluted, and bound serum factor(s) (BS) was released with guanidine buffer, BS inhibited TNF-α production by PM plus B. dermatitidis in a concentration-dependent manner. BS contained MBL-C, which bound B. dermatitidis, as shown by IFA assay. 1,3-β-Glucan stimulated TNF-α production by PM, and this was inhibited by mouse serum. Treatment of B. dermatitidis with anti-1,3-β-glucan antibody inhibited TNF-α production by PM. With anti-1,3-β-glucan antibody, we showed by IFA assay that B. dermatitidis contained 1,3-β-glucan. In an IFA study with B. dermatitidis, serum with an anti-mouse IgG conjugate did not result in fluorescence, yet serum blocked IFA staining of B. dermatitidis by anti-1,3-β-glucan IgG antibody. This indicated that non-IgG serum factors binding to B. dermatitidis prevented access to 1,3-β-glucan by anti-1,3-β-glucan antibody. These results suggest that the mechanism of inhibition of the innate proinflammatory immune response of PM to B. dermatitidis is mediated by serum MBL binding to B. dermatitidis at 1,3-β-glucan sites or sterically masking 1,3-β-glucan sites, thus preventing 1,3-β-glucan stimulation of PM for TNF-α production.
Innate immune responses to certain microorganisms are affected, either positively (25) or negatively (17, 18), by mannose binding lectins (MBL) in serum. Interaction of the thermally dimorphic pulmonary fungal pathogen Blastomyces dermatitidis (28) with the first line of host defense, i.e., innate defenses, can critically influence the outcome of the infection. Innate production of proinflammatory cytokines and chemokines by stimulated macrophages promotes subsequent adaptive immune responses necessary for control of the infection (11, 21). Macrophages stimulated in vitro by yeast cells of B. dermatitidis produce proinflammatory cytokines, e.g., tumor necrosis factor alpha (TNF-α), part of the innate immune response necessary for resistance to infection (11, 21). A major fungal stimulus for macrophages is mediated by fungal 1,3-β-glucan binding to the macrophage receptor dectin-1 (2, 3).
The role of serum factors in macrophage interaction with yeast cells of B. dermatitidis in vitro, with respect to TNF-α production, has not been reported. We report that the presence of mouse serum (MS) in in vitro cultures inhibited B. dermatitidis stimulation of macrophages for TNF-α production in a concentration-dependent manner. We present evidence that serum MBL bind to B. dermatitidis, apparently at 1,3-β-glucan sites, and thus prevent access to a major macrophage stimulating ligand on B. dermatitidis.
MATERIALS AND METHODS
Reagents and media.
Mouse immunoglobulin G (IgG) (1 mg/ml), goat anti-mouse IgG-fluorescein isothiocyanate (FITC) conjugate (1.5 mg/ml), and rabbit anti-rat IgG-FITC conjugate (0.75 mg/ml) were obtained from Zymed Laboratory, South San Francisco, CA. Rat anti-MBL (MBL-A and -C) antibody (100 μg/ml) was purchased from Cell Sciences, Canton, MA. Mouse monoclonal anti-1,3-β-glucan IgG (1 mg/ml) was purchased from Biosupplies Australia, Parkville, Victoria, Australia. 1,3-β-Glucan from baker's yeast was purchased from Sigma Chemical Co., St. Louis, MO. Complete tissue culture medium (CTCM) consisted of RPMI 1640, 10% fetal bovine serum (FBS) treated at 56°C for 30 min, and penicillin-streptomycin (100 U and 100 μg/ml, respectively) (Sigma). Except where otherwise specified, reagent concentrations expressed as percentages are to be understood as expressed with CTCM as the diluent. Incubation buffer (phosphate-buffered saline without calcium or magnesium [PBS]-TEDTA) consisted of 1.46 g EDTA, 6.25 g pure Tris, 4.38 g NaCl, and 0.25 ml Tween 20 in 500 ml distilled water. Release buffer contained 5 M guanidine in PBS-TEDTA buffer (12). MS was obtained by bleeding CD-1 mice. Heat-inactivated (HI) MS refers to MS heated at 56°C for 30 min.
Mice.
C3H/HeN (Simonsen Laboratory, Gilroy, CA), C3H/HeJ (Jackson Laboratory, Bar Harbor, ME), and CD-1 (Charles River Laboratory, Hollister, CA) male mice of 8 to 12 weeks of age were used in these experiments. Mice were housed in sterilized cages and bedding and provided with acidified water and sterilized chow. All animal studies conformed to National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee of the California Institute for Medical Research.
Blastomyces dermatitidis.
B. dermatitidis ATCC 26199 (ATCC, Manassas, VA) (virulent [V]) and B. dermatitidis ATCC 60915 (attenuated [A]) were studied (31). Yeast cells were grown for 3 days at 35°C on blood agar plates, harvested, washed with saline, and counted in a hemocytometer. For some experiments, yeast cells were killed by heat in saline at 60°C for 1 h (HK B. dermatitidis). Aliquots of HK B. dermatitidis were stored at −80°C until needed. Where necessary, HK B. dermatitidis (A), HK B. dermatitidis (V), and live B. dermatitidis (V) are distinguished in the text.
Macrophages.
In preliminary experiments, lungs of C3H/HeN and C3H/HeJ mice were lavaged with 10 ml bronchoalveolar lavage fluid (PBS, 10% FBS, 1% EDTA)/mouse, cells were pelleted (400 × g, 10 min), and pelleted cells were pooled, washed in CTCM, and counted in a hemocytometer. Bronchoalveolar macrophages (BAM) were obtained by plating lavaged cells as 0.1 ml/microtest plate (Corning 3696; Corning, NY) well at 106 cells/ml CTCM, with incubation at 37°C in 5% CO2 plus 95% air for 90 min and removal of nonadherent cells. BAM monolayers contained approximately 9 × 104 BAM/well. Two experiments were performed, with effector-to-target (E/T) ratios of 2:1 and 1:1.
Peritoneal cavities of CD-1 mice were lavaged with 10 ml of RPMI 1640/mouse, peritoneal cells were pelleted (400 × g, 10 min), and pelleted cells were pooled, washed in CTCM, and counted in a hemocytometer. Peritoneal macrophages (PM) were obtained by adherence of peritoneal cells after plating of 2 × 106/ml CTCM at 0.2 ml/microtest plate (Corning) well, with incubation at 37°C in 5% CO2 plus 95% air for 90 min. After incubation, nonadherent cells were aspirated and monolayers of PM consisted of approximately 106 PM/well.
RAW 264.7 (RAW) cells, a mouse (BALB/c) monocyte-macrophage cell line (ATCC TI B71), were purchased from ATCC and maintained in Dulbecco's modified Eagle's medium (ATCC) plus 10% FBS and penicillin-streptomycin (RAW-CTCM) in tissue culture flasks (Falcon 35381; Becton Dickinson, Franklin Lake, NJ) at 37°C in 5% CO2 plus 95% air. RAW cells were collected, counted, plated, allowed to adhere, and tested in RAW-CTCM in the same manner as that for PM. Since in preliminary experiments we found RAW cells to give similar results to those for CD-1 PM, RAW cells gave us a ready source of macrophages, enabling some initial experiments, in which a number of variables were defined, to be independent of mouse supply. However, in later experiments assessing mechanisms, we believed that we should study ex vivo PM rather than a cell line.
Enzyme-linked immunosorbent assay.
TNF-α concentrations in supernatants were measured using enzyme-linked immunosorbent assay kits purchased from Endogen, Woburn, MA. Measurements were done with the TNF-α standards and reagents supplied and were performed according to the supplier's instructions.
Absorption of serum.
MS (1 or 10%) in CTCM (1 ml) was incubated with 108 HK B. dermatitidis or live B. dermatitidis cells in microcentrifuge tubes for 1 h at room temperature. B. dermatitidis was pelleted by centrifugation, and supernatants were removed (1× absorbed serum). This serum was incubated with new (108) HK B. dermatitidis or live B. dermatitidis for 1 h as described above. B. dermatitidis was pelleted by centrifugation as before, and supernatants were removed (2× absorbed serum). Portions of 2× absorbed serum were stored at −80°C until needed for testing.
Electrophoresis and immunoblotting.
Samples were electrophoresed in 10% Tris-glycine, 1-mm-thick precast sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels in an X-Cell sure-lock chamber (Novex; Invitrogen, Carlsbad, CA), using reagents and instructions supplied by the vendor. Protein bands in SDS-PAGE gels were visualized by staining with simple blue stain (Invitrogen). Stained gels were photographed with a digital camera (Bio-Image, Ann Arbor, MI). Proteins in SDS-PAGE gels were blotted onto polyvinylidene difluoride membranes by using an X-Cell II blot module (Novex), using reagents and instructions provided by the vendor. Blotted polyvinylidene difluoride membranes were air dried and stored at 4°C until needed.
Western immunoblotting of membranes was done with reagents and according to instructions supplied with a Super Signal West Pico chemiluminescent substrate kit (Pierce, Rockford, IL). After blotting and blockade of unblotted areas with skim milk protein, the membranes were probed for 1 h with the primary antibody, rat-anti-mouse MBL-C plus MBL-A (1:100), in primary antibody buffer. After being washed, the membranes were treated with rabbit anti-rat IgG-horseradish peroxidase conjugate (1:1,000) in secondary antibody buffer for 1 h. Following washing of the membranes, the substrate (H2O2 plus luminol) was added for 5 min. After draining of the substrate, the membrane was exposed to X-ray film (CL-X Posure film; 5 × 7 in.) (Pierce). X-ray film was developed, and images were digitized (Bio-Image).
BS and UBS.
One milliliter of 1% MS in incubation buffer was incubated with 2 × 108 HK B. dermatitidis cells or 10 ml of 10% MS in incubation buffer was incubated with 2 × 109 live B. dermatitidis cells in columns at room temperature for 1 h. Unbound serum factors (UBS) were eluted from columns with 1 ml or 10 ml of incubation buffer, respectively. Bound serum factors (BS) were released by elution of columns with 1 ml or 10 ml of guanidine release buffer, respectively. UBS and BS were concentrated by lyophilization, followed by dialysis (membrane exclusion, molecular weight of 6,000 to 8,000) against saline. Protein concentrations of UBS and BS were determined using a BCA protein assay kit (Pierce).
Inhibition of TNF-α production by BS factors.
Duplicate 0.2-ml cultures of PM were stimulated with B. dermatitidis (5 × 104), B. dermatitidis plus 10% MS, or B. dermatitidis plus increasing concentrations of BS ranging from 6.25 to 100 μg/ml in CTCM. After overnight incubation at 37°C in 5% CO2 plus 95% air, cell-free supernatants were collected and stored at −80°C.
IFA staining.
Immunofluorescent antibody (IFA) staining of treated live B. dermatitidis was done in 1-ml microtest centrifuge tubes at room temperature. Live B. dermatitidis (5 × 104) in 0.2 ml of 10% MS or BS in CTCM was incubated for 1 h, centrifuged, and decanted, and pelleted cells were washed by centrifugation with CTCM. Washed cells were incubated with rat anti-MBL-A or -C (1:10 in CTCM) for 30 min and then centrifuged and washed with CTCM. Next, treated live B. dermatitidis cells were incubated with rabbit anti-rat IgG-FITC (1:10 in CTCM) for 30 min. Cells were pelleted by centrifugation, and fluorescence on cells was visualized with a Zeiss I-F microscope equipped with transmission (490 to 650 nm) and barrier (500 to 510 nm) filters for FITC microscopy.
IFA staining for 1,3-β-glucan.
B. dermatitidis (5 × 104) in 0.2 ml was incubated with mouse anti-1,3-β-glucan IgG antibody (1:10) in CTCM, with 10% MS, or with CTCM for 1 h at room temperature in 1-ml microtest centrifuge tubes. B. dermatitidis cells pelleted by centrifugation were washed with 0.2 ml of CTCM and treated with goat anti-mouse IgG-FITC conjugate (1:10) in CTCM for 30 min. B. dermatitidis cells pelleted by centrifugation and suspended in 0.02 ml CTCM were examined with an I-F microscope.
For photography of anti-1,3-β-glucan IFA staining of B. dermatitidis, the same procedure as that described above was used, except that goat anti-mouse IgG conjugated to Alexa Fluor 594 (Molecular Probes, Eugene, OR) was used instead of the goat anti-mouse-FITC conjugate (optimal concentrations were 1:10 for the monoclonal antibody and 1:50 for the conjugate).
Stimulation of PM with 1,3-β-glucan.
Duplicate 0.2-ml cultures of PM were stimulated with increasing concentrations of 1,3-β-glucan, ranging from 62 to 250 μg/ml, in CTCM, with 10% MS in CTCM, or with CTCM. After incubation overnight at 37°C in 5% CO2 plus 95% air, cell-free supernatants were collected and stored at −80°C.
Coating of B. dermatitidis with anti-1,3-β-glucan antibody.
The following methods were used to coat B. dermatitidis with anti-1,3-β-glucan antibody. Briefly, B. dermatitidis (105) was incubated in 0.1 ml CTCM containing mouse anti-1,3-β-glucan IgG (200 μg/ml), with 10% MS, or with CTCM for 1 h. B. dermatitidis was pelleted by centrifugation, washed with CTCM, and suspended to 0.1 ml in CTCM, and 0.01 ml was used to stimulate duplicate 0.2-ml PM cultures. After overnight incubation at 37°C in 5% CO2 plus 95% air, cell-free supernatants were collected and stored at −80°C.
Statistics.
Student's t test was used for statistical analysis of data, and significance was set at P values of <0.05. The GB-STAT program (Microsoft, Richmond, VA) was used, and Bonferroni's adjustment to the t test was applied where appropriate.
RESULTS
Preliminary experiments.
In preliminary experiments with C3H/HeN and C3H/HeJ mice, we showed that macrophages from both strains produced TNF-α after incubation with HK B. dermatitidis (V) (C3H/HeJ mice produced significantly more [n = 2 experiments]), whereas lipopolysaccharide (LPS) could only stimulate TNF-α from C3H/HeN mice (data not shown). In the presence of 10% MS, B. dermatitidis-induced TNF-α production was inhibited equally with macrophages of both strains (79 to 81%; P < 0.01). These preliminary studies showed that LPS is not responsible for the stimulation in our in vitro setting of B. dermatitidis-induced TNF-α production from macrophages and also that Toll-like receptor 4 is not involved, as C3H/HeJ mice are insensitive to LPS and have a mutated, nonfunctional receptor (16).
RAW cell responses to HK B. dermatitidis.
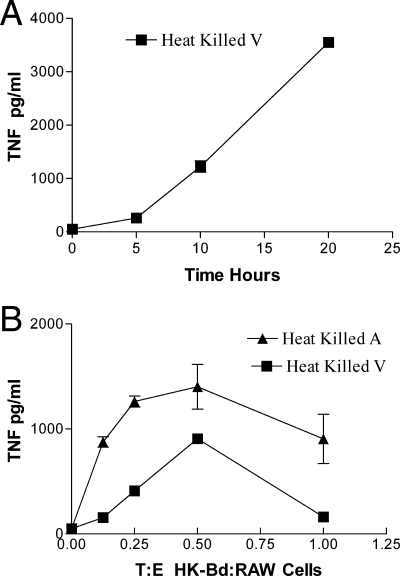
RAW cells stimulated with HK B. dermatitidis secreted TNF-α in a time-dependent manner (Fig. 1A). Production of TNF-α reached high levels by 20 h in RAW cells plus HK B. dermatitidis (V), and consequently this sufficiently convenient time point was used in future experiments. Further preliminary experiments showed that HK B. dermatitidis and live B. dermatitidis gave indistinguishable results with respect to stimulating TNF production by RAW cells.
FIG. 1.
(A) Effect of time on TNF-α production by RAW cells plus HK B. dermatitidis (V). The E/T ratio was 2:1. TNF-α production is shown as the mean ± standard deviation (SD) for duplicate determinations. (B) Effect of E/T ratio on TNF-α production by RAW cells plus HK B. dermatitidis. TNF-α production by RAW cells stimulated with HK B. dermatitidis (A) and HK B. dermatitidis (V) is shown. TNF-α production is given as the mean ± SD for triplicate determinations.
The effect of the T/E ratio on TNF-α production by RAW cells infected with HK B. dermatitidis is shown in Fig. 1B. A ratio of two RAW cells to one HK B. dermatitidis cell in 20-h cultures was optimal for TNF-α production. It can be noted that in the absence of B. dermatitidis (zero T/E point on graph), RAW cell production of TNF was at the level of detection of the assay (and was equal to that of CTCM alone, without RAW cells). It can also be noted that HK B. dermatitidis (A) was more stimulatory (P < 0.01 at all time points) than HK B. dermatitidis (V) (Fig. 1B).
Effect of serum on TNF-α production by RAW cells.
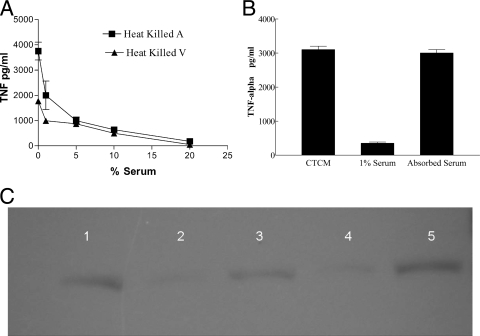
RAW cell responses to HK B. dermatitidis in CTCM resulted in robust production of TNF-α (Fig. 2A). However, TNF-α production was inhibited in a concentration-dependent manner by MS. As little as 1% MS in CTCM caused >50% inhibition of TNF-α production by RAW cells plus HK B. dermatitidis. Since 10% MS in CTCM caused near-maximal inhibition of TNF-α production by RAW cells plus HK B. dermatitidis, 10% MS in CTCM was used in future experiments.
FIG. 2.
(A) Effect of serum concentration on TNF-α production by RAW cells plus HK B. dermatitidis. Inhibition by 1, 5, 10, 15, or 20% MS of TNF-α production by RAW cells plus HK B. dermatitidis (A) or HK B. dermatitidis (V) is shown as the mean ± SD for triplicate determinations. (B) Effect of absorbing serum with live B. dermatitidis. PM were stimulated with live B. dermatitidis without MS, with 1% MS, or with 1% MS absorbed with live B. dermatitidis. TNF-α production is shown as the mean ± SD for triplicate determinations. (C) Immunoblot with anti-MBL-C of MBL-C in MS compared to MBL-C in MS absorbed by live B. dermatitidis. MBL-C in 1% MS is shown in lanes 1, 3, and 5, and 1% MS absorbed with live B. dermatitidis (V) is shown in lanes 2 and 4.
In other experiments, we found that 10% HI MS inhibited TNF-α production by RAW cells plus HK B. dermatitidis (V) to a level that was equivalent to that in 10% normal MS (both at ≤25 pg/ml), where 825 ± 7 pg/ml was produced in the absence of MS (P < 0.01 for no-MS group versus either HI MS or MS group). Similarly, TNF-α production by RAW cells plus HK B. dermatitidis (A) (962 ± 229 pg/ml) was inhibited (P < 0.01) by either 10% HI MS (167 ± 10 pg/ml) or 10% normal MS (162 ± 3 pg/ml). In preliminary experiments, commercial IgG was found to contain endotoxin that was completely neutralized by 50 μg/ml of polymyxin B, and consequently, sufficient polymyxin was used in the following experiments to test IgG. When RAW cells plus HK B. dermatitidis (V) were cultured in CTCM plus IgG (250 μg/ml), we found that TNF-α production (775 ± 10 pg/ml) was equivalent to that in cultures without IgG (807 ± 152 pg/ml). Taken together, these results indicate that the inhibitory activity of serum factors is heat stable and not due to IgG.
Absorption of serum inhibitory factors by B. dermatitidis (V).
In preliminary experiments, it was difficult to remove inhibitory activity from 1 ml of 10% MS by incubation with 2 × 108 HK B. dermatitidis (A) or HK B. dermatitidis (V) cells. However, when 1% MS was absorbed twice with HK B. dermatitidis (V) or live B. dermatitidis (V), absorbed serum failed (P < 0.01) to inhibit TNF-α production by PM plus live B. dermatitidis (V) (8% and 1% for absorbed serum versus 80% and 87% for unabsorbed 1% MS, respectively) (Fig. 2B). Moreover, electrophoresis of absorbed serum followed by immunoblotting for MBL showed that absorption by live B. dermatitidis (V) removed MBL from MS (Fig. 2C). This suggested that the presence of MBL in MS was necessary for inhibition of TNF-α production by PM plus B. dermatitidis.
Binding of serum inhibitory factors by B. dermatitidis (V).
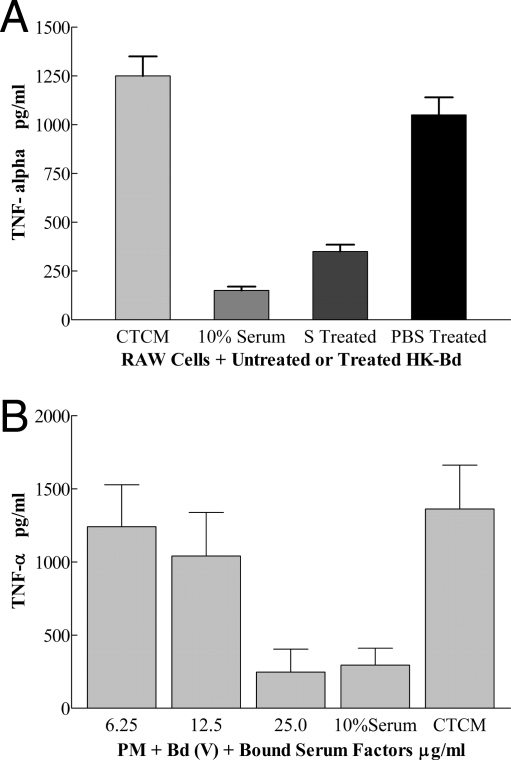
When HK B. dermatitidis (V) was incubated in 10% MS or PBS for 1 h at room temperature and then washed, PM TNF-α production was inhibited (P < 0.01) by MS treatment compared to that with PBS treatment (Fig. 3A, right two bars). This finding indicated that serum factors bound to B. dermatitidis and inhibited stimulation of RAW cell TNF-α production. Similar results were found in another experiment which studied live B. dermatitidis (V) incubated with MS or PBS and PM, where TNF-α production was inhibited 90% (P < 0.01) by serum-treated live B. dermatitidis (V) (data not shown). These findings suggested the possibility of capturing MS inhibitory factors on a B. dermatitidis column and releasing bound factors for study of inhibitory activity.
FIG. 3.
(A) Effect of incubating HK B. dermatitidis (V) with MS on HK B. dermatitidis stimulation of RAW cells for TNF-α production. TNF-α production by RAW cells plus HK B. dermatitidis in CTCM, CTCM plus 10% MS, CTCM plus MS (S)-treated HK B. dermatitidis, or CTCM plus PBS-treated HK B. dermatitidis is given as the mean ± SD for triplicate determinations. The left two bars are a control done concurrently for purposes of comparison. (B) Effect of serum factors bound to B. dermatitidis (V) on TNF-α production by PM. Concentration-dependent B. dermatitidis-bound MS factor (numbers on the x axis are μg protein/ml) inhibition of TNF-α production by PM plus B. dermatitidis is shown. TNF-α levels in supernatants are given as means ± SD for triplicate determinations.
Quantitation of serum factors that bind B. dermatitidis.
SDS-PAGE of BS and UBS showed the absence of bands for UBS compared to released BS, indicating that binding removed serum proteins (data not shown). Serum factors that bound to live B. dermatitidis (V) in two experiments were tested for inhibition of TNF-α production by PM plus B. dermatitidis (V). In preliminary tests, BS at 100 μg/ml, once released, inhibited (P < 0.01) TNF-α production 98%, which was more than inhibition by 10% MS (86%). In another test of this type, BS at 50 μg/ml inhibited (P < 0.01) TNF-α production 90%. Finally, BS inhibited TNF-α production by PM plus B. dermatitidis (V) in a concentration-dependent manner (Fig. 3B), and it can be noted that 25 μg/ml of BS in CTCM was as inhibitory (P < 0.01) (81%) as 10% MS in CTCM (78%).
Identification of B. dermatitidis-binding serum factor as MBL.
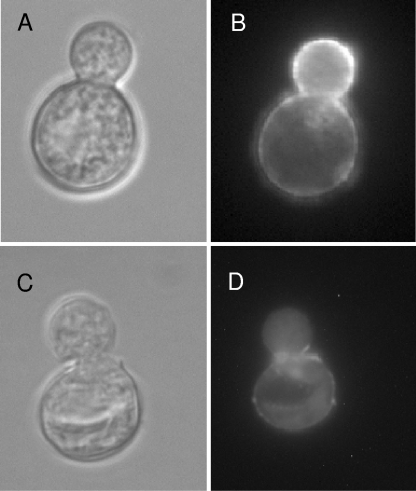
When live B. dermatitidis (V) was incubated with 10% MS or BS (100 μg/ml), we found that it bound MBL from serum and BS. Binding was detected with antibody to MBL and the IFA system (Fig. 4). The results from three experiments are shown in Table 1. B. dermatitidis (V) stained green, and staining was most intense at the broad base of budding yeast cells when 10% MS plus anti-MBL-C was used. Cell walls of yeast cells incubated with either CTCM, CTCM with another 10% FBS, or 10% mouse IgG and stained with anti-MLB-C appeared bright red under these conditions, whereas yeast cells incubated with MS or BS and stained with anti-MBL-C appeared green.
FIG. 4.
IFA staining of MBL bound to HK B. dermatitidis. A phase-contrast picture (A) of HK B. dermatitidis is compared to IFA staining of MBL in MS bound to HK B. dermatitidis (B). A phase-contrast picture (C) of HK B. dermatitidis is compared to a negative IFA control (FBS substituted for MS) (D).
TABLE 1.
IFA staining of B. dermatitidis for serum binding factors
| Primary treatment of B. dermatitidisa | IFA stainingb
|
Appearance of B. dermatitidis | |
|---|---|---|---|
| Anti-MBL-A | Anti-MBL-C | ||
| CTCM | Yes | Yes | Bright red cell wall |
| CTCM plus another 10% FBS | Yes | Yes | Bright red cell wall |
| 10% mouse IgG | Yes | Yes | Bright red cell wall |
| 10% MS | Yes | No | Green spots on B. dermatitidis |
| No | Yes | Solid green staining of B. dermatitidis | |
| BS | No | Yes | Solid green staining of B. dermatitidis |
Primary treatment of B. dermatitidis, as described in Materials and Methods.
Secondary treatment of B. dermatitidis with rat anti-MBL and then rabbit anti-rat IgG-FITC conjugate.
Effect of serum on 1,3-β-glucan stimulation of PM for TNF-α production.
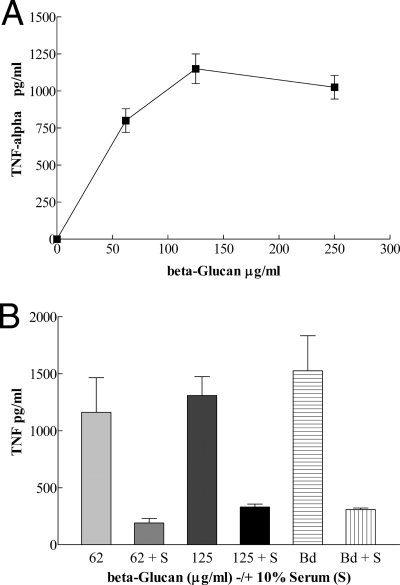
PM responded to 1,3-β-glucan in a concentration-dependent manner for TNF-α production (Fig. 5A). The addition of polymyxin to bind possible endotoxin contamination of the glucan preparation did not affect these results. 1,3-β-Glucan at 125 μg/ml appeared to be an optimal concentration for TNF-α production under these conditions; increasing the concentration to 250 μg/ml did not significantly increase TNF-α secretion. As shown in Fig. 5B, TNF-α production by 1,3-β-glucan-stimulated PM was significantly inhibited by 10% MS. These results indicated that serum factors interacted with 1,3-β-glucan and inhibited 1,3-β-glucan stimulation of PM for TNF-α production.
FIG. 5.
(A) Effect of 1,3-β-glucan concentration on stimulation of PM for TNF-α production. The PM response to increasing concentrations of 1,3-β-glucan (μg protein/ml) is shown as increasing levels of TNF-α production. Means ± SD for triplicate determinations are shown. (B) Effect of MS on 1,3-β-glucan stimulation of PM for TNF-α production. Serum (S; 10%) inhibition of 1,3-β-glucan (62 and 125 μg/ml) stimulation of PM for TNF-α production is shown. TNF-α production by PM plus B. dermatitidis, with or without 10% MS, is also shown. TNF-α production is given as the mean ± SD for triplicate determinations.
Inhibition of B. dermatitidis serum binding factors with anti-1,3-β-glucan antibody.
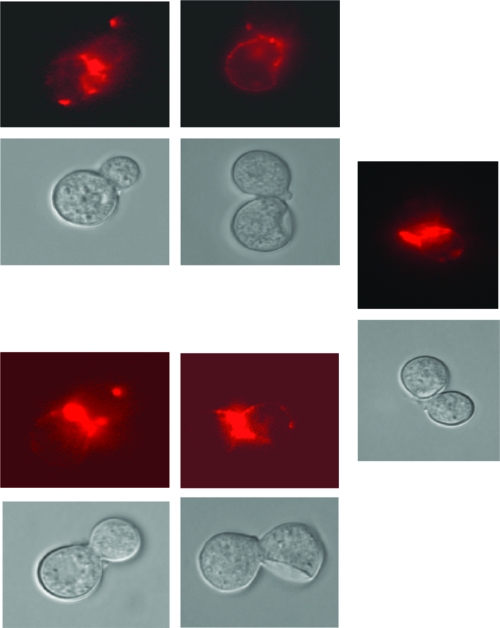
When B. dermatitidis (V) was incubated with mouse anti-1,3-β-glucan and then a goat anti-mouse IgG-FITC conjugate, in two experiments B. dermatitidis (V) stained green by fluorescence microscopy. If CTCM or 10% MS was substituted for the mouse monoclonal antibody, there was no fluorescence with the conjugate. With the same protocol, but using goat anti-mouse IgG conjugated to Alexa Fluor 594, staining of 1,3-β-glucan on B. dermatitidis is shown in photomicrographs (Fig. 6). These studies confirm many reports of 1,3-β-glucan in the cell wall of B. dermatitidis and show that non-IgG factors bind to the glucan.
FIG. 6.
IFA staining of 1,3-β-glucan on HK B. dermatitidis. Phase-contrast pictures of HK B. dermatitidis are shown on the bottom of each pair of pictures. Corresponding IFA-stained (mouse monoclonal anti-1,3-β-glucan IgG plus goat anti-mouse IgG conjugated to Alexa Fluor 594) HK B. dermatitidis pictures are shown on the top of each pair of pictures. There was no fluorescent staining with the conjugate in the absence of the anti-glucan antibody.
If B. dermatitidis was first incubated with MS or eluted BS and then with anti-mouse 1,3-β-glucan plus rabbit anti-mouse-FITC, staining of B. dermatitidis was completely inhibited. This indicated that factors in MS or BS interacted with B. dermatitidis and prevented anti-1,3-β-glucan antibody staining of B. dermatitidis, suggesting that serum binding factors or BS made 1,3-β-glucan on B. dermatitidis inaccessible to anti-1,3-β-glucan staining.
Antibody to 1,3-β-glucan inhibits B. dermatitidis stimulation of PM for TNF-α production.
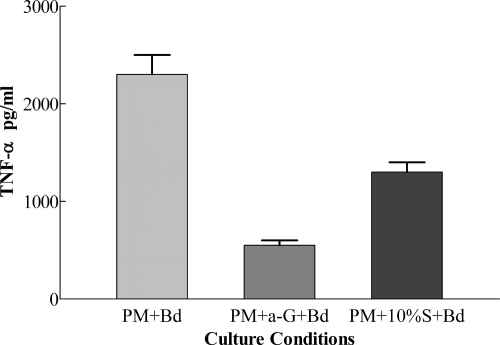
If B. dermatitidis was incubated with mouse anti-1,3-β-glucan IgG (200 μg/ml), washed, and used to stimulate PM, the coated B. dermatitidis cells had a deficient (P < 0.01) capacity to stimulate PM for TNF-α production (550 ± 50 pg/ml) compared to uncoated B. dermatitidis (2,300 ± 200 pg/ml) (77% reduction) (Fig. 7). Similar results were found in a second experiment where coated B. dermatitidis stimulation of PM for TNF-α production (850 ± 50 pg/ml) was 66% (P < 0.01) less than that with uncoated B. dermatitidis (2,500 ± 50 pg/ml). These findings, taken together with IFA staining of 1,3-β-glucan on B. dermatitidis, show that 1,3-β-glucan is a major ligand on B. dermatitidis for stimulation of PM for TNF-α production.
FIG. 7.
Effect of coating B. dermatitidis with anti-1,3-β-glucan antibody (a-G) on TNF-α production by PM. TNF-α production by PM plus B. dermatitidis, PM plus antibody-coated B. dermatitidis, or PM plus MS (S)-coated B. dermatitidis is given as the mean ± SD for triplicate determinations.
DISCUSSION
B. dermatitidis initiates disease as a pulmonary infection and frequently disseminates to sites such as the skin, thus the name B. dermatitidis (28). Hence, innate immunity in the pulmonary compartment must play an important role in the first line of defense against B. dermatitidis. It was previously demonstrated that a collectin, surfactant protein D (SP-D), can modulate the bronchoalveolar macrophage response to B. dermatitidis, down-regulating TNF-α production, likely by binding to B. dermatitidis 1,3-β-glucan (21). However, little is known about the roles of factors in the blood and at tissue sites of dissemination in innate immunity against B. dermatitidis. In studies of fungicidal activity of macrophages and neutrophils against B. dermatitidis yeast cells, fresh MS was routinely included in assay systems (5, 6, 23, 32). On the other hand, in most studies of lymphokine or cytokine production by lymphocytes or macrophages stimulated with B. dermatitidis, MS was not present in the culture system (4, 11). In retrospect, it is possible that the results from the latter experiments would have been different if MS was present in the system. Our studies indicate that FBS does not have the properties of MS in interaction with B. dermatitidis, mouse cells, or their combination.
Here we determined the optimal conditions for TNF-α production by RAW cells plus B. dermatitidis in CTCM and then measured the effect of MS in CTCM. It was noted that an attenuated strain (31) of B. dermatitidis (ATCC 60915) stimulated greater production of TNF-α by RAW cells than did the parent virulent strain (ATCC 26199) (Fig. 1B and 2A). Others have speculated that greater stimulation of TNF-α production of macrophages by strains of B. dermatitidis could be related to greater host resistance (11) and that differences in cell wall composition of strains could be responsible for these effects. One of the cell wall differences postulated to correlate with virulence relates to a higher α-1,3-glucan/1,3-β-glucan ratio in virulent isolates (15). It is possible that the lower 1,3-β-glucan content in the virulent isolate could be used to explain the differences in TNF production that we observed and that the lessened inflammatory response could explain the greater virulence. On the other hand, we show that the presence of serum can largely override differences in the capacity of B. dermatitidis strains to stimulate TNF-α production by RAW cells. For example, 5% serum in the assay system (Fig. 2A) could equally inhibit TNF-α production by RAW cells plus different B. dermatitidis strains.
The finding that B. dermatitidis incubated with MS and then washed had a significantly lower capacity to stimulate TNF-α production by RAW cells suggested that an MS factor(s) bound to B. dermatitidis and interfered with stimulation of macrophages. One possible mechanism by which coated B. dermatitidis down-regulated TNF-α production by macrophages is blockade. For example, a serum factor(s) that binds stimulatory structures on B. dermatitidis would shield these structures from macrophage receptors involved in signaling for TNF-α production.
MBL, which are other members of the family called collectins, bind to microorganisms, activate the complement system, act as opsonins to enhance phagocytosis, and may have activity against some microbes mediated by enzymes linked to the binding protein (8). By immunofluorescence staining with anti-MBL antibody, MS factors that bound B. dermatitidis were identified as MBL-A and MBL-C. MBL-A is an acute-phase protein, also known as C-reactive protein, whereas MBL-C is not. MBL-C is sixfold more abundant than MBL-A in normal serum (27) and showed more intense staining in the immunofluorescence assays. Although MBL-A can inhibit TNF-α production by LPS-stimulated human alveolar macrophages (7), we are not aware of published reports about MBL-C inhibition of TNF-α production by stimulated murine macrophages. Although MBL can initiate the complement cascade, the lack of difference we show between HI and normal MS in inhibiting TNF-α production suggests that complement activation is not required for this MBL function.
Our studies, which involved several mouse strains, indicate that serum effects on B. dermatitidis-macrophage interactions are not mouse strain specific. Variations in levels of MBL in sera from 10 inbred mouse strains have been reported (22). The MBL-C level in sera from DBA/1J mice was high (60 μg/ml) compared to that of MBL-C (20 μg/ml) in sera from BALB/c mice. Assuming, conservatively, that the MBL-C level in sera from outbred CD-1 mice is 60 μg/ml, we estimate that MBL-C at 6 μg/ml (10% serum) or 0.6 μg/ml (1% serum) strongly inhibited B. dermatitidis stimulation of PM for TNF-α production. Moreover, we showed that MBL-C in 1% MS resulted in strong MBL-C bands on immunoblots with anti-MBL-C (Fig. 2C).
Since the cell wall of B. dermatitidis contains β-1,3-glucan (10, 24) and α-1,3-glucan (15), these glucans are more likely to be ligands or components of ligand sites for the carbohydrate-binding domain of MBL than is surface protein antigen A (36), also known as WI-1 (19). The importance of 1,3-β-glucan on B. dermatitidis and the interaction with serum factors has been reported. For example, naturally occurring factors in human serum that bind B. dermatitidis for complement activation could be inhibited by 1,3-β-glucan but not by α-glucan (37). We and others (20, 21) have shown that 1,3-β-glucan stimulates prominent production of TNF-α by macrophages. Here we report that TNF-α production by macrophages plus 1,3-β-glucan is inhibited in the presence of MS. It has been reported that production of TNF-α by elicited peritoneal macrophages in response to B. dermatitidis is affected by expression of the protein adhesin antigen WI-1 or antigen A (11). These experiments were done in the absence of fresh MS, and consequently, the effect of serum MBL on such results is not known. With other fungi, MBL have been shown to enhance or to decrease TNF-α release by human mononuclear cells (9, 13). The present studies suggest that B. dermatitidis may subvert the innate immune response in the blood or at local tissue sites by interacting with MBL to blunt the inflammatory response.
Despite their name, MBL are able to interact with the horizontal 3- and 4-hydroxyl groups of various sugars, such as mannose and glucose (34). Glucan is a polymer of repeating 1,3-linked glucose molecules, and fungal cell walls are comprised of mannan, glucan, and chitin components, all covalently cross-linked in a network (14, 29). It has been suggested mannose-protein complexes may protect underlying glucan sites (38). Our finding that anti-1,3-β-glucan antibody significantly blocks B. dermatitidis stimulation of macrophages for TNF-α production indicates that 1,3-β-glucan is a major ligand on B. dermatitidis for signaling macrophages for TNF-α production. Receptors on macrophages that bind 1,3-β-glucan have been identified as dectin-1 (2, 3) and characterized (1, 26). Dectin-1 expression and function (35) and its distinction from the mannose receptor on macrophages (33) have been reported.
In summary, the results of our studies demonstrating that MS incubated with B. dermatitidis resulted in inhibition of TNF-α production, whereas incubation with IgG did not, and of immunofluorescence studies showing that B. dermatitidis sites were stained by mouse anti-1,3-β-glucan plus anti-mouse IgG, whereas they were not stained by serum plus anti-mouse IgG, suggest that the serum factors that result in binding to 1,3-β-glucan and TNF inhibition are not IgGs. We provide, in several experiments, substantial, different pieces of evidence that the binding serum factors(s) that results in TNF inhibition is an MBL and also show that a specific antibody to 1,3-β-glucan inhibits TNF, and we thus infer that MBL blocks TNF production by binding at 1,3-β-glucan sites or binding at B. dermatitidis sites that mask surface 1,3-β-glucan. We cannot exclude the possibility that there could be additional serum factors that also bind B. dermatitidis, possibly at 1,3-β-glucan sites, and affect the interaction with macrophages, but they are not present in IgG, and MBL potency is sufficient to explain most or all of the inhibitory serum activity. SP-D, for example, is present at lower concentrations in human serum than in lung lavage fluid (30). If the concentration in the mouse approximates that in human serum, then 1% MS (a concentration we show is effective in blocking TNF production) would contain approximately 0.009 μg/ml, a level estimated to be >60-fold lower than the serum MBL-C concentration and >2,000-fold less than the SP-D concentration shown to be effective with BAM (21).
Editor: A. Casadevall
Footnotes
Published ahead of print on 10 December 2007.
REFERENCES
- 1.Adachi, Y., T. Ishii, Y. Ikeda, A. Hoshino, H. Tamura, J. Aketagawa, S. Tanaka, and N. Ohno. 2004. Characterization of beta-glucan recognition site on C-type lectin, dectin-1. Infect. Immun. 724159-4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown, G. D., and S. Gordon. 2001. Immune recognition. A new receptor for beta-glucan. Nature 41336-37. [DOI] [PubMed] [Google Scholar]
- 3.Brown, G. D., P. R. Taylor, D. M. Reid, J. A. Willment, D. L. Williams, L. Martinez-Pomares, S. Y. Wong, and S. Gordon. 2002. Dectin-1 is a major beta-glucan receptor on macrophages. J. Exp. Med. 196407-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brummer, E., L. H. Hanson, and D. A. Stevens. 1993. IL-4, IgE, and interferon-γ production in pulmonary blastomycosis: comparison in mice untreated, immunized, or treated with an antifungal (SCH 39304). Cell. Immunol. 149258-267. [DOI] [PubMed] [Google Scholar]
- 5.Brummer, E., C. J. Morrison, and D. A. Stevens. 1985. Recombinant and natural gamma interferon activation of macrophages in vitro: different dose requirements for induction of killing activity against phagocytizable and nonphagocytizable fungi. Infect. Immun. 49724-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brummer, E., and D. A. Stevens. 1987. Activation of pulmonary macrophages for fungicidal activity by gamma-interferon or lymphokines. Clin. Exp. Immunol. 70520-528. [PMC free article] [PubMed] [Google Scholar]
- 7.Casals, C., J. Arias-Diaz, F. Valino, A. Saenz, C. Garcia, J. L. Balibrea, and E. Vara. 2003. Surfactant strengthens the inhibitory effect of C-reactive protein on human lung macrophage cytokine release. Am. J. Physiol. Lung Cell. Mol. Physiol. 284466-472. [DOI] [PubMed] [Google Scholar]
- 8.Casanova, J., and L. Abel. 2004. Human mannose-binding lectin in immunity: friend, foe, or both? J. Exp. Med. 1991295-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaka, W., A. F. Verheul, V. V. Vaishnev, R. Cherniak, J. Scharringa, J. Verhoef, H. Snippe, and A. I. Hoepelman. 1997. Induction of TNF-alpha in human peripheral blood mononuclear cells by the mannoprotein of Cryptococcus neoformans involves human mannose binding protein. J. Immunol. 1592979-2985. [PubMed] [Google Scholar]
- 10.Cox, R. A., and G. K. Best. 1972. Cell wall composition of two strains of Blastomyces dermatitidis exhibiting differences in virulence for mice. Infect. Immun. 5449-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finkel-Jimenez, B., M. Wuthrich, T. Brandhorst, and B. S. Klein. 2001. The WI-1 adhesin blocks phagocyte TNF-α production imparting pathogenicity on Blastomyces dermatitidis. J. Immunol. 1662665-2673. [DOI] [PubMed] [Google Scholar]
- 12.Garvey, J. S., N. E. Cremer, and D. H. Sussdorf (ed.). 1977. Methods in immunology, 3rd ed., p. 252-254. W. A. Benjamin, Inc., Reading, MA.
- 13.Ghezzi, M. C., G. Raponi, S. Angeletti, and C. Mancini. 1998. Serum mediated enhancement of TNF-alpha release by human monocytes stimulated with the yeast form of Candida albicans. J. Infect. Dis. 1781743-1749. [DOI] [PubMed] [Google Scholar]
- 14.Hector, R. F. 1993. Compounds active against cell walls of medically important fungi. 1993. Clin. Microbiol. Rev. 61-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hogan, L. H., and B. S. Klein. 1994. Altered expression of surface α-1,3-glucan in genetically related strains of Blastomyces dermatitidis that differ in virulence. Infect. Immun. 623543-3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoshino, K., O. Takeuchi, T. Kawai, H. Sanjo, T. Ogawa, Y. Takeda, K. Takeda, and S. Akira. 1999. Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 1623749-3752. [PubMed] [Google Scholar]
- 17.Jack, D. J., R. C. Read, A. J. Turner, M. Forsch, M. W. Turner, and N. J. Klein. 2001. Mannose-binding lectin regulates the inflammatory response of human professional phagocytes to Neisseria meningitidis serogroup B. J. Infect. Dis. 1841152-1162. [DOI] [PubMed] [Google Scholar]
- 18.Kitz, D. J., P. D. Stahl, and J. R. Little. 1992. The effect of a mannose binding protein on macrophage interaction with Candida albicans. Cell. Mol. Biol. 34407-412. [PubMed] [Google Scholar]
- 19.Klein, B. S., P. M. Sondel, and J. M. Jones. 1992. WI-1, a novel 120-kilodalton surface protein on Blastomyces dermatitidis yeast cells, is a target of cell-mediated immunity in human blastomycosis. Infect. Immun. 604291-4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee, D. Y., I. H. Ji, H. I. Chang, and C. W. Kim. 2002. High-level TNF-α secretion and macrophage activity with soluble β-glucan from Saccharomyces cerevisiae. Biosci. Biotechnol. Biochem. 66233-238. [DOI] [PubMed] [Google Scholar]
- 21.Lekkala, M., A. M. LeVine, M. J. Linke, E. C. Crouch, B. Linders, E. Brummer, and D. A. Stevens. 2006. Effect of lung surfactant collectins on bronchoalveolar macrophage interaction with Blastomyces dermatitidis: inhibition of tumor necrosis factor alpha production by surfactant protein D. Infect. Immun. 744549-4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu, H., L. Jensen, S. Hansen, S. V. Petersen, K. Takahashi, A. B. Ezekowitz, F. D. Hansen, J. C. Jensenius, and S. Thiel. 2001. Characterization and quantitation of mouse mannan-binding lectins (MBL-A and MBL-C) and study of acute phase responses. Scand. J. Immunol. 53489-497. [DOI] [PubMed] [Google Scholar]
- 23.Morrison, C. J., E. Brummer, R. A. Isenberg, and D. A. Stevens. 1987. Activation of murine polymorphonuclear neutrophils for fungicidal activity by recombinant gamma-interferon. J. Leukoc. Biol. 41434-440. [DOI] [PubMed] [Google Scholar]
- 24.Nemecek, J. C., M. Wuthrich, and B. S. Klein. 2006. Global control of dimorphism and virulence in fungi. Science 312583-588. [DOI] [PubMed] [Google Scholar]
- 25.Neth, O., D. L. Jack, A. W. Dodds, H. Holzel, N. J. Klein, and M. W. Turner. 2000. Mannose binding lectin binds a range of clinically relevant microorganisms and promotes complement deposition. Infect. Immun. 68688-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reid, D. M., M. Montoya, P. R. Taylor, P. Barrow, S. Gordon, G. D. Brown, and S. Y. C. Wong. 2004. Expression of the β-glucan receptor, dectin-1, on murine leukocytes in situ correlates with function in pathogen recognition and reveals potential roles in leukocyte interactions. J. Leukoc. Biol. 7686-94. [DOI] [PubMed] [Google Scholar]
- 27.Sastry, K., K. Zahedi, J. M. Lelias, A. S. Whitehead, and R. A. Ezekowitz. 1991. Molecular characterization of the mouse mannose-binding proteins. The mannose-binding protein A but not C is an acute phase reactant. J. Immunol. 147692-697. [PubMed] [Google Scholar]
- 28.Schwarz, J., and G. L. Baum. 1959. Blastomycosis. Am. J. Clin. Pathol. 21999-1029. [DOI] [PubMed] [Google Scholar]
- 29.Selitrennikoff, C. P., and G. R. Ostroff. 1999. Emerging therapeutic cell wall targets in fungal infections. Emerg. Ther. Targets 353-72. [Google Scholar]
- 30.Sorensen, G. L., J. V. B. Hjelmborg, K. O. Kyvik, M. Fenger, A. Hoj, C. Bendixen, T. I. A. Sorensen, and U. Holmskov. 2006. Genetic and environmental influences of surfactant protein D serum levels. Am. J. Physiol. Lung Cell. Mol. Physiol. 290L1010-L1017. [DOI] [PubMed] [Google Scholar]
- 31.Stevens, D. A., E. Brummer, A. F. DiSalvo, and A. Ganer. 1997. Virulent isolates and mutants of Blastomyces in mice; a legacy for studies of pathogenesis. Semin. Respir. Infect. 12189-195. [PubMed] [Google Scholar]
- 32.Sugar, A. M., E. Brummer, and D. A. Stevens. 1986. Fungicidal activity of murine broncho-alveolar macrophages against Blastomyces dermatitidis. J. Med. Microbiol. 217-11. [DOI] [PubMed] [Google Scholar]
- 33.Taylor, P. R., G. D. Brown, J. Here, D. L. Williams, J. A. Willment, and S. Gordon. 2004. The role of SIGNR and β-glucan receptor dectin-1 nonopsonic recognition of yeast by specific macrophages. J. Immunol. 1721157-1162. [DOI] [PubMed] [Google Scholar]
- 34.Weis, W. I., D. Drickamer, and W. A. Hendrickson. 1992. Structure of a C-type mannose-binding protein complexed with an oligosaccharide. Nature 360127-134. [DOI] [PubMed] [Google Scholar]
- 35.Willment, J. A., H. Lin, D. M. Reid, P. R. Taylor, D. L. Williams, S. Y. Wong, S. Gordon, and G. D. Brown. 2003. Dectin-1 expression and function are enhanced on alternatively activated and GM-CSF treated macrophages and are negatively regulated by IL-10, dexamethasone, and lipopolysaccharide. J. Immunol. 1714569-4573. [DOI] [PubMed] [Google Scholar]
- 36.Young, K. D., and H. W. Larsh. 1981. Identification of the active preciptin components in a purified preparation of the A-antigen of Blastomyces dermatitidis. Infect. Immun. 33171-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang, M. X., and B. S. Klein. 1997. Activation, binding, and processing of complement component 3 (C3) by Blastomyces dermatitidis. Infect. Immun. 651849-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zlotnick, H., M. P. Fernandez, B. Bowers, and E. Cabib. 1984. Saccharomyces cerevisiae mannoproteins from an external cell wall layer that determines wall porosity. J. Bacteriol. 1591018-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]