Abstract
Helicobacter pylori infection results in the development of chronic gastritis, and CD4+ T cells are a major component of the gastric cellular infiltrate. To examine whether CD4+ T cells are important in initiating and maintaining H. pylori-induced gastritis, mice deficient in CD4+ T cells (B6.BM1.GK 1.5 mice [GK 1.5 mice]) were infected with H. pylori. We found that as in normal mice, H. pylori-specific antibodies, mostly of the immunoglobulin M isotype, developed in GK 1.5 mice but were unable to cure H. pylori infection. Further, while the stomachs of H. pylori-infected GK 1.5 mice were more heavily infiltrated with CD8+ T cells and B cells, mice deficient in both CD4+ and CD8+ T cells developed mild inflammation comparable to the level observed for C57BL/6 mice. These observations suggest that CD4+ T cells may play an important role in regulating or suppressing gastric CD8+ T cells which, in the absence of CD4+ T cells, may mediate more-severe disease. These studies have revealed a potentially important role for CD8+ T cells in the gastric disease resulting from H. pylori infection.
Helicobacter pylori colonizes approximately 50% of the world's population, resulting in persistent stomach inflammation in infected individuals (30). Chronic H. pylori gastritis can lead to the development of gastric and duodenal ulcers and, ultimately, gastric cancer (10, 14, 15). The growth of H. pylori is restricted to the stomach lumen and epithelium, a site poorly served by classical immune effectors, and the proximal duodenum, and successful attempts at bacterial eradication through therapeutic vaccination have not been achieved (29). Immunomodulation might provide an alternate means of reducing H. pylori-mediated disease through qualitative changes to the inflammation induced by chronic infection. Any rational attempt at immunomodulation will require a more complete understanding of the bacterial and host drivers of chronic inflammation and the cellular infiltrate which is characteristic of Helicobacter-associated gastritis.
CD4+ T cells are a major component of the gastric cellular infiltrate in both human and experimental animal models of H. pylori infection (5-7, 18, 27). Severe combined immunodeficiency (SCID) mice that receive adoptively transferred splenic CD4+ T cells develop gastritis after Helicobacter infection, but mice which received splenocytes depleted of CD4+ T cells do not (7, 20). Hence, CD4+ T cells are believed to be fundamental to the initiation and maintenance of gastric inflammation. We aimed to examine the role of CD4+ T cells with respect to both effector and regulatory function and to determine whether gastritis was dependent on infiltration by CD4+ T cells. In this study, CD4+ T-cell-deficient mice were infected with H. pylori and examined for the development of gastritis.
MATERIALS AND METHODS
Mice.
Female C57BL/6 mice were reared under specific-pathogen-free conditions at the animal facility of the Department of Microbiology and Immunology, The University of Melbourne. Female B6.BM1.GK 1.5 (GK 1.5) (33) and B6.BM1.GK/2.43 (GK/2.43) (31) mice were obtained from the Walter and Eliza Hall Institute (Melbourne, Australia) and housed at the animal facility of the Department of Microbiology and Immunology, The University of Melbourne. Mice were from 6 to 16 weeks old at the time of H. pylori infection, and age-matched naive mice were included in all experiments. All animal experiments were approved by The University of Melbourne Animal Ethics and Experimental Committee and complied with relevant legislation.
H. pylori infection, quantification from the stomach, and assessment of serum anti-H. pylori antibody titers.
The mouse-adapted H. pylori SS1 strain (16) was kindly provided by Hazel Mitchell (University of New South Wales, NSW, Australia). The methods used for laboratory bacterial culture, mouse infection, and isolation from mouse stomachs were as described previously (13). Total H. pylori-specific and H. pylori lipopolysaccharide (LPS)-specific antibody titers from mouse sera were determined by enzyme-linked immunosorbent assay (ELISA) as described before (13). Briefly, Maxisorp immunoplates (Nunc, A/S) were coated with 5 μg/ml H. pylori sonicate or purified LPS and the plates were blocked with skim milk and washed. Following incubation with serum samples, total H. pylori-specific antibodies were detected using sheep anti-mouse immunoglobulin (Ig) conjugated to horseradish peroxidase (Chemicon). Mouse sera were analyzed individually for H. pylori-specific ELISAs and pooled for LPS-specific ELISAs. IgG and IgM isotypes were determined in the same manner, except that rabbit anti-mouse IgG1 or IgG3 (ICN Biomedicals) was added after serum incubation followed by detection with goat anti-rabbit Ig conjugated to horseradish peroxidase (Sigma).
Purification of H. pylori SS1 for determination of anti-H. pylori LPS-specific antibody levels.
H. pylori LPS was kindly provided by Rebecca J. Gorell (Department of Microbiology and Immunology, University of Melbourne) and was purified from H. pylori SS1 based on the methods described by Hitchcock and Brown (12). Briefly, H. pylori cells were lysed with LPS cell lysis buffer (1 M Tris-HCl [pH 6.8], 10% [vol/vol] glycerol, 4% [vol/vol] β-mercaptoethanol, 2% [wt/vol] sodium dodecyl sulfate, 0.0002% [wt/vol] bromophenol blue) before heat denaturation by boiling for 10 min. Proteins were digested with 0.5 mg/ml proteinase K (Sigma) and LPS was extracted with hot phenol before dialysis with water.
Assessment of stomach inflammation by histology.
Mouse stomachs were dissected, fixed in formalin, embedded, sectioned, and stained with hematoxylin and eosin as described previously (13). Stomach sections were blindly graded by a pathologist using criteria defined elsewhere (28). Briefly, the severity of chronic inflammation was assessed and graded according to the density and distribution of lymphocytes. The inflammation scores used in the grading system were as follows: 0, none; 1, mild multifocal; 2, mild widespread or moderate multifocal; 3, mild widespread and moderate multifocal or severe multifocal; 4, moderate widespread; 5, moderate widespread and severe multifocal; and 6, severe widespread (28).
Analysis of gastric cellular infiltrate by flow cytometry.
Lymphocytes were isolated from mouse stomachs by injecting 10 ml of phosphate-buffered saline (1.9 mM KH2PO4, 8.1 mM Na2HPO4, 150 mM NaCl, pH 7.4) into the mucosa as previously described (2). Cells were resuspended in fluorescence-activated cell sorter buffer (0.1% [wt/vol] bovine serum albumin, 0.1% [wt/vol] sodium azide in phosphate-buffered saline), counted by trypan blue dye exclusion, and stained with the appropriate antibodies. The antibodies used were anti-mouse CD3-fluorescein isothiocyanate (clone 145-2C11), anti-mouse CD4-Cy5 (clone H129.19), anti-mouse CD4-fluorescein isothiocyanate (clone RM4-4), anti-mouse CD8-allophycocyanin (clone 53-6.7), and anti-mouse CD19-phycoerythrin (clone 1D3) from BD Biosciences (San Diego, CA). Flow cytometry was performed using a BD FACSCalibur flow cytometer (BD Biosciences). Results were analyzed using the CELLQuest software (Becton Dickinson Immunocytometry Systems).
Statistics.
Statistical significance was assessed by the nonparametric Mann-Whitney U test, and two-tailed P values of less than 0.05 were considered statistically significant.
RESULTS
Progression of H. pylori-induced disease in CD4-deficient mice. (i) H. pylori colonization and serum antibody levels.
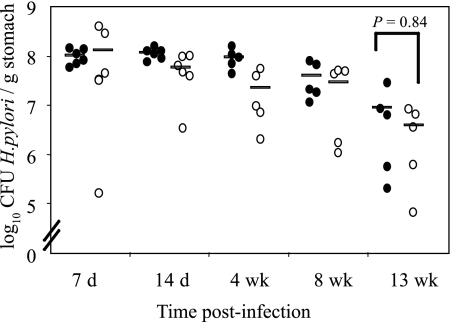
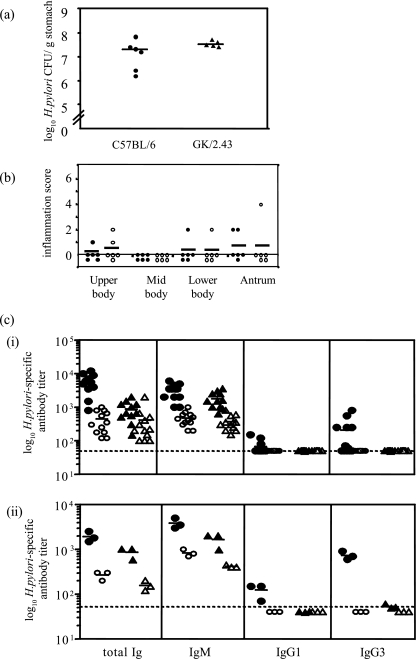
GK 1.5 transgenic mice constitutively produce the anti-CD4 GK 1.5 antibody, which results in the depletion of CD4+ cells (33). Flow cytometric analysis of splenocytes and lymph node cells by us (data not shown) and others (32, 33) has shown that the percentages of CD4+ cells in GK 1.5 mice are reduced to 0.4% and 0.1%, respectively. We infected GK 1.5 and C57BL/6 mice with H. pylori (five mice per group) and monitored the infection for up to 13 weeks. Throughout the experiment, H. pylori was isolated from the stomachs of all infected mice, and there was no statistical difference in colonization levels, measured by viable count, between the mouse strains at any time point during the experiment (Fig. 1). The circulating H. pylori-specific antibody responses indicated that the total anti-H. pylori antibody titers in both mouse strains increased progressively from 4 to 13 weeks after infection but, while the antibody levels detected in infected C57BL/6 mice were consistently higher than those in GK 1.5 mice, this difference was not statistically significant (P = 0.15, 0.31, and 0.06, comparing GK 1.5 mice and C57BL/6 mice at 4, 8, and 13 weeks, respectively [data not shown]).
FIG. 1.
Progression of H. pylori infection in GK 1.5 mice. H. pylori was isolated from the stomachs of C57BL/6 and GK 1.5 mice up to 13 weeks postinfection with H. pylori and enumerated by viable count. Represented are results from individual C57BL/6 (•) and GK 1.5 (○) mice, with the mean of each group represented by the bars. d, days; wk, weeks.
(ii) Assessment of gastric inflammation by histology.
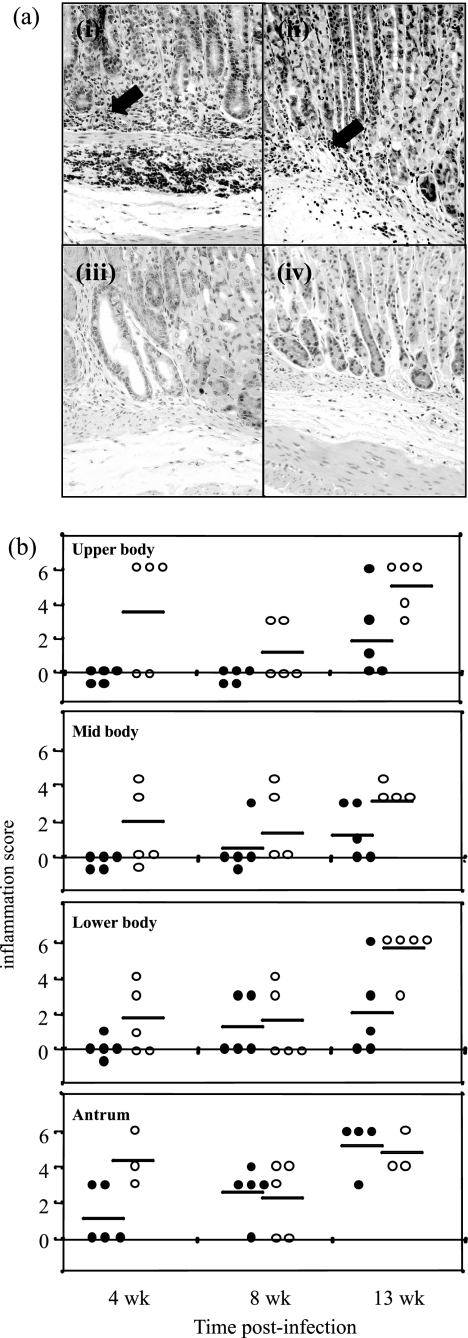
Gastritis was not detected in uninfected mice of either strain (data not shown), but H. pylori-infected C57BL/6 and GK 1.5 mice developed characteristic H. pylori-induced inflammation, with infiltration of mononuclear cells into the stomach mucosa (Fig. 2a). Inflammation scores increased during the 13-week period of H. pylori infection in C57BL/6 mice. The gastritis observed for infected GK 1.5 mice appeared to be more severe than that in C57BL/6 mice. For example, the average inflammation score for C57BL/6 mice at 13 weeks postinfection for the lower body was 2.0, whereas the average inflammation score for the corresponding GK 1.5 mice was 5.4 (Fig. 2b).
FIG. 2.
Gastric pathology in H. pylori-infected GK 1.5 mice. The gastric pathology of H. pylori-induced gastric inflammation in GK 1.5 and C57BL/6 mice was assessed using hematoxylin and eosin-stained stomach sections, which were blindly graded by a pathologist and to which inflammation scores from 0 to 6 (28) were assigned. (a) Representative hematoxylin and eosin-stained stomach sections from 13-week-infected GK 1.5 (i) and C57BL/6 (iii) mice and from naive GK 1.5 (iii) and C57BL/6 (iv) mice. Arrows indicate lymphocyte infiltration. Magnification, ×20. (b) Summary of inflammation scores for infected mice, with analysis of the various stomach regions. Represented here are individual C57BL/6 (•) and GK 1.5 (○) mice, with the mean of each group represented by bars. wk, weeks.
Examination of H. pylori-specific antibody responses in CD4+ T-cell-deficient mice.
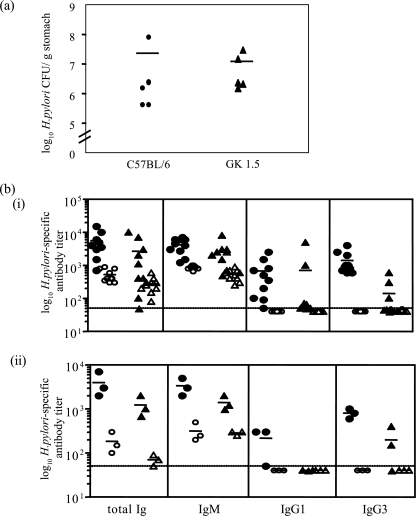
The H. pylori infection experiment was repeated to examine the immune response in C57BL/6 and GK 1.5 mice at 13 weeks after H. pylori infection. H. pylori colonization levels (Fig. 3a) and gastritis scores (data not shown) were consistent with the previous experiments. H. pylori- and LPS-specific antibody titers (total Ig, IgG1, IgG3, and IgM) were measured by ELISA and are shown in Fig. 3b. Anti-H. pylori antibody levels increased in both mouse strains following H. pylori infection (Fig. 3bi) (P < 0.001 and P = 0.06 for C57BL/6 and GK 1.5 mice, respectively, comparing infected to naive mice). However, C57BL/6 mice mounted a stronger antibody response when infected with H. pylori than did GK 1.5 mice (P = 0.053, comparing total Ig levels between infected C57BL/6 and GK 1.5 mice). The majority of the H. pylori-specific antibodies raised in GK 1.5 mice were of the IgM subtype, and minimal or no IgG responses were detected (Fig. 3bi). Since CD4+ T cells provide B-cell help for protein-specific antibody responses, H. pylori LPS-specific antibody responses were determined in this study as a measure of T-cell-independent antibody responses. Figure 3bii shows that in both strains of mice, the majority of anti-LPS antibodies was of the IgM subtype with low IgG3 titers, while only H. pylori-infected C57BL/6 mice raised LPS-specific IgG1.
FIG. 3.
H. pylori colonization and antibody responses in GK 1.5 and C57BL/6 mice 13 weeks after infection. (a) Viable counts of H. pylori isolated from mouse stomachs at 13 weeks postinfection were consistent with results from the previous experiment. H. pylori was not isolated from naive mice (data not shown). (b) Total H. pylori (i)- and H. pylori LPS (ii)-specific serum antibodies and their isotypes in C57BL/6 and GK 1.5 mice were measured by ELISA. Mouse sera were analyzed individually for H. pylori-specific ELISA and pooled for LPS-specific ELISA; each symbol represents a sample pooled from three mice. Represented are results from infected C57BL/6 (•) and GK 1.5 (▴), and naive C57BL/6 (○) and GK 1.5 (▵) mice, with the mean of each group represented by the bars. The dashed line indicates the level of detection of the assay.
Examination of gastric infiltration in CD4+ T-cell-deficient mice by flow cytometry.
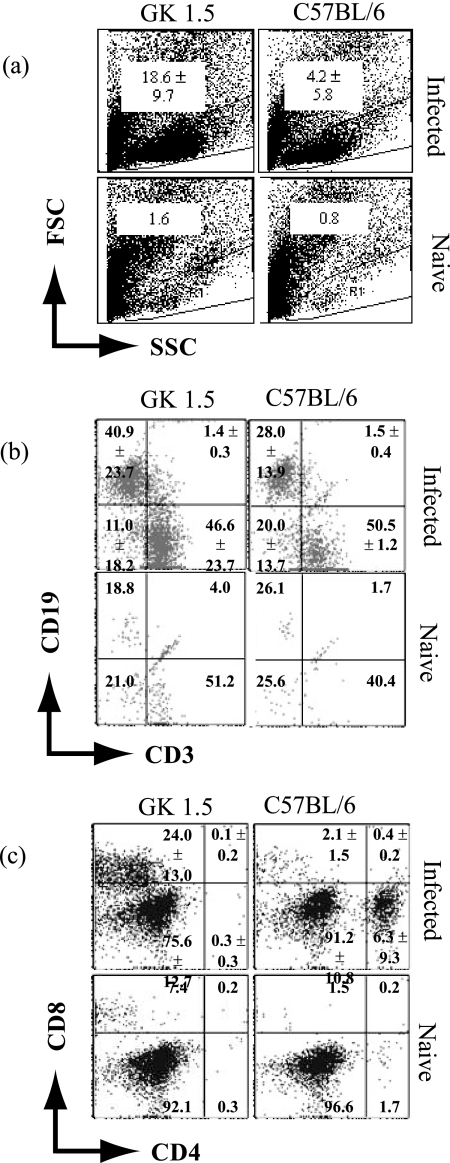
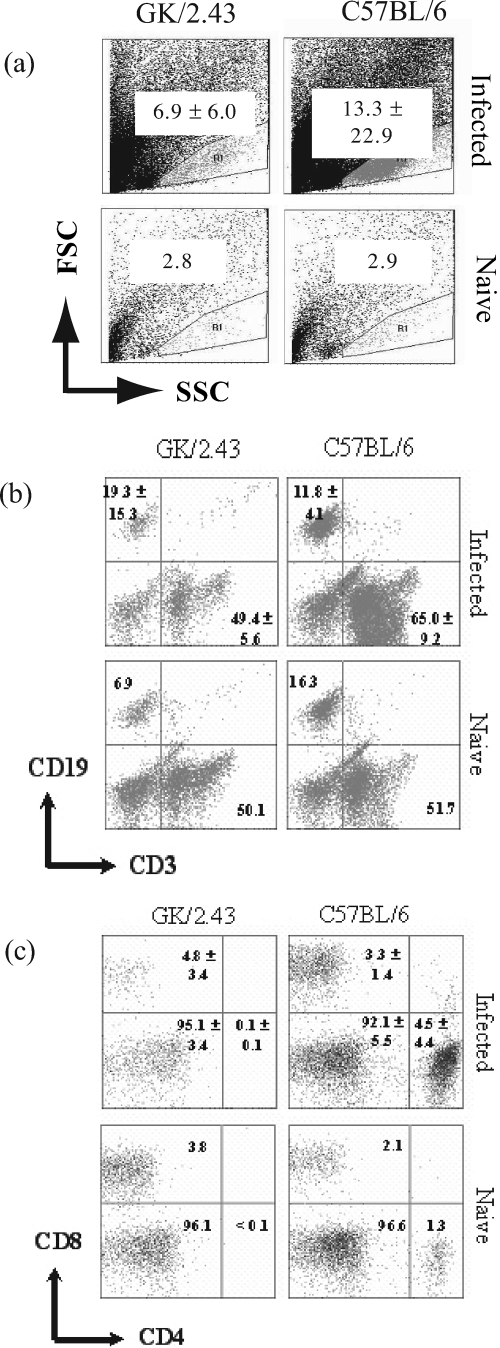
Lymphocytes were isolated from five mice per group and analyzed by flow cytometry. Results from the analysis were expressed in terms of the percentages of specific cells detected (Fig. 4), which were subsequently extrapolated to absolute numbers (Table 1). It may be technically difficult to extract lymphocytes from nonlymphoid organs like the stomach for immunological assays, but gastric lymphocytes from infected animals could clearly be detected using the employed “ballooning” method, whereby the stomach mucosa is injected with medium (2) (Fig. 4a). Indeed, the lymphocyte population in the gastric cell preparation was identified using the forward- and side-scatter profiles of spleen cells obtained from uninfected C57BL/6 mice (Fig. 4a). Many cells were present in the lymphocyte gate in the gastric cell preparations from GK 1.5 mice compared to what was seen for C57BL/6 mice (Table 1). Analysis of cell surface marker expression, indicated in Fig. 4b and c, revealed that infected GK 1.5 mice had sixfold as many gastric B cells, defined by CD19 expression, and fourfold as many T cells, defined by CD3 expression, as C57BL/6 mice, although these differences were not statistically significant (Table 1). Analysis of the T-cell subsets showed that CD8+ T cells comprised a significant proportion of CD3+ cells within the GK 1.5 mouse stomach and were 30-fold more numerous than in C57BL/6 stomachs (P = 0.032). In contrast, the gastric mucosae of C57BL/6 mice were infiltrated with more CD4+ T cells than CD8+ T cells. In both mouse strains, the majority of the CD3+ cells in the stomach were double-negative (DN) cells (CD4− CD8−). The CD4 deficiency in GK 1.5 mice was confirmed using the RM4-4 anti-CD4 antibody clone, which recognizes an epitope different from that recognized by the GK 1.5 and H129.19 antibodies. Although the DN T-cell population in infected GK 1.5 stomachs was also substantial, the dominant cellular infiltrate accounting for the increase in lymphocyte numbers in the GK 1.5 mouse stomachs appeared to be CD8+ T cells.
FIG. 4.
Flow cytometric analysis of gastric lymphocytes from H. pylori-infected GK 1.5 mice. Flow cytometric analysis of gastric lymphocyte populations in GK 1.5 and C57BL/6 mice at 13 weeks after H. pylori infection (n = 5). Numbers within plots refer to mean percentages of cells ± standard deviations (SD) for infected animals, and proportions of cells in pooled naive animals, within the gate or quadrant. (a) Forward- and side-scatter (FSC and SSC) profiles of stomach cell preparations with lymphocytes within the gate. (b) B (CD19+) and T (CD3+) lymphocytes in the stomachs of infected and naive GK 1.5 and C57BL/6 mice. (c) CD4+ and CD8+ cell populations within CD3+ lymphocytes from infected and naive animals.
TABLE 1.
Analysis of cells by flow cytometry in stomachs of GK 1.5 and C57BL/6 mice during H. pylori infectiona
| Mouse strain and infection status | No. of cells | No. of lymphocytes | No. of indicated lymphocyte:
|
No. of indicated CD3+ cell type:
|
|||
|---|---|---|---|---|---|---|---|
| CD19+ | CD3+ | CD8+ | CD4+ | CD4− CD8− | |||
| GK 1.5 | |||||||
| Infectedb | 44.9 ± 19.6 | 9.4 ± 6.1 | 4.7 ± 3.2 | 4.2 ± 3.1 | 1.2 ± 0.9 | 0.006 ± 0.004 | 3.0 ± 2.2 |
| Naivec | 5.0 | 0.08 | 0.02 | 0.04 | 0.003 | <0.001 | 0.04 |
| C57BL/6 | |||||||
| Infected | 34.2 ± 28.4 | 1.9 ± 3.4 | 0.8 ± 1.5 | 1.0 ± 1.7 | 0.04 ± 0.08 | 0.2 ± 0.4 | 0.7 ± 1.2 |
| Naive | 3.3 | 0.03 | 0.007 | 0.01 | <0.001 | <0.001 | 0.01 |
Results shown are per 104 cells.
H. pylori-infected mice (13 weeks), mean ± SD (n = 5 per group).
Pooled results for age-matched naive mice (n = 5 per group).
Examination of H. pylori-induced disease in mice deficient in both CD4+ and CD8+ T cells. (i) H. pylori colonization, serum antibody levels, and gastric inflammation by histology.
We next infected GK/2.43 mice with H. pylori together with infecting C57BL/6 mice as controls. GK/2.43 mice are transgenic for the GK 1.5 and GK/2.43 antibodies that deplete CD4+ and CD8+ cells, respectively; hence, these mice are deficient in both CD4+ and CD8+ T cells (reference 31 and data not shown). Both GK/2.43 and C57BL/6 mice were colonized by H. pylori at 13 weeks postinfection (n = 6) (Fig. 5a), and there was no significant difference between the H. pylori burdens of the mouse strains (P = 0.082).
FIG. 5.
H. pylori infection in GK/2.43 mice at 13 weeks postinfection. (a) H. pylori populations were enumerated in the stomachs of C57BL/6 and GK/2.43 mice by viable count at 13 weeks after infection. (b) Summary of gastric inflammation scores of H. pylori-infected GK/2.43 and C57BL/6 mice examined by histology, using a grading scale from 0 to 6 as described previously (28). No inflammation was noted for naive mice (data not shown). Represented are individual inflammation scores for C57BL/6 (•) and GK/2.43 (○) mice, with the mean of each group represented by the bars. (c) Total H. pylori (i)- and H. pylori LPS (ii)-specific serum antibodies and their isotypes in C57BL/6 and GK/2.43 mice were measured by ELISA. Mouse sera were analyzed individually for H. pylori-specific ELISA and pooled for LPS-specific ELISA; each symbol represents a sample pooled from three mice. Represented are results of infected C57BL/6 (•) and GK/2.43 (▴) mice and naive C57BL/6 (○) and GK/2.43 (▵) mice, with the mean of each group represented by the bars. The dashed line indicates the level of detection of the assay.
Assessment of gastric inflammation by histology revealed that naive mice did not develop gastritis (data not shown) and, although both C57BL/6 and GK/2.43 mice demonstrated mild inflammation at 13 weeks after infection, there was no apparent difference in the severities of inflammation seen for the mouse strains (Fig. 5b).
Helicobacter-specific antibody levels were significantly increased after H. pylori infection (P = 0.001 and 0.013 for C57BL/6 and GK/2.43 mice, respectively, comparing infected to naive mice), and the total Ig and IgG antibody levels in infected C57BL/6 mice were significantly high compared to those for infected GK/2.43 mice (P ≤ 0.0003) (Fig. 5ci). As with GK 1.5 mice, the H. pylori-specific antibodies elicited following H. pylori infection in GK/2.43 mice were mostly of the IgM subtype, and no H. pylori-specific IgG1 or IgG3 was detectable. Further analysis of H. pylori-specific antibody responses revealed that in contrast to infected C57BL/6 mice, GK/2.43 mice elicited only LPS-specific IgM (Fig. 5cii).
(ii) Examination of gastric infiltration in GK/2.43 mice by flow cytometry.
The gastric cellular infiltrate of infected GK/2.43 mice was also analyzed by flow cytometry and compared with that of infected C57BL/6 mice (n = 6 each). The results from the flow cytometric analyses are expressed in terms of percentages of specific cells detected (Fig. 6) and as absolute numbers in Table 2. The results indicated that there were approximately three times as many lymphocytes in the infected C57BL/6 mouse stomach as in GK/2.43 mice, whereas only few cells were isolated from naive mice from both strains. There were also more T cells than B cells in the infected stomachs in both strains of mice. There was a distinct population of CD4+ T cells in infected C57BL/6 mice but not in GK/2.43 mice. Most of the CD3+ cells isolated from the stomachs of both GK/2.43 and C57BL/6 mice were DN cells (95.1% and 92.1%, respectively). Unlike the GK 1.5 mouse, where transgenic expression of the GK 1.5 antibody resulted in the almost complete removal of CD4+ T cells, consistent with previous reports (32, 33), the mice expressing the anti-CD8 antibody (i.e., GK/2.43 mice) were not depleted of all CD8+ cells.
FIG. 6.
Flow cytometric analysis of H. pylori-infected GK/2.43 mice. Flow cytometry was used to analyze the gastric lymphocyte populations in GK/2.43 and C57BL/6 mice 13 weeks after H. pylori infection. (a) Forward- and side-scatter (FSC and SSC) profiles of stomach cell preparations with lymphocytes within the gate (n = 6 for each group). Numbers within plots refer to mean percentages of cells ± SD for infected animals, and percentages of cells in pooled naive animals, within the gate or quadrant. (b) B (CD19+) and T (CD3+) lymphocytes in infected and naive GK/2.43 and C57BL/6 mice. (c) CD4+ and CD8+ cell populations within CD3+ lymphocytes in infected and naive animals.
TABLE 2.
Analysis of cells by flow cytometry in stomachs of GK/2.43 and C57BL/6 mice during H. pylori infectiona
| Mouse strain and infection status | No. of cells | No. of lymphocytes | No. of indicated lymphocyte:
|
No. of indicated CD3+ cell type:
|
|||
|---|---|---|---|---|---|---|---|
| CD19+ | CD3+ | CD8+ | CD4+ | CD4− CD8− | |||
| GK/2.43 | |||||||
| Infectedb | 7.2 ± 6.5 | 0.8 ± 1.4 | 0.28 ± 0.6 | 0.34 ± 0.5 | 0.03 ± 0.05 | <0.001 | 0.3 ± 0.5 |
| Naivec | 1.8 | 0.05 | 0.003 | 0.03 | 0.001 | <0.001 | 0.02 |
| C57BL/6 | |||||||
| Infected | 12.3 ± 1.3 | 1.8 ± 3.2 | 0.16 ± 0.3 | 1.28 ± 2.4 | 0.06 ± 0.1 | 0.1 ± 0.2 | 1.1 ± 2.1 |
| Naive | 1.2 | 0.04 | 0.006 | 0.02 | <0.001 | <0.001 | 0.02 |
Results shown are per 104 cells.
H. pylori-infected mice (13 weeks), mean ± SD (n = 6 per group).
Pooled results for age-matched naive mice (n = 6 per group).
DISCUSSION
In this study, we examined the immune and inflammatory responses induced by H. pylori infection in mice lacking either CD4+ T cells or CD4+ and CD8+ T cells. As CD4+ T cells had been implicated in H. pylori-induced gastritis in several independent reports (7, 20), we hypothesized that CD4+ T-cell-deficient mice infected with H. pylori would not develop stomach inflammation. However, our results suggested that the absence of CD4+ T cells was linked to increased gastritis, dominated by an influx of CD8+ T cells, and further supported existing data that CD4+ T cells provide an important regulatory function in Helicobacter-induced gastritis.
GK 1.5 mice were used in our study as an in vivo model of CD4 deficiency. The immunological characteristics of GK 1.5 mice have been examined and summarized by Zhan et al. (32). Briefly, the absence of CD4+ cells in the periphery was confirmed by flow cytometry, using an anti-CD4 antibody that recognized an epitope different from that by the GK 1.5 antibody, and the percentage of CD4+ T cells present in the spleen and lymph nodes was less than 0.1% (32). This is in accordance with our evaluation of GK 1.5 mice, where the percentage of splenic CD4+ T cells detected in uninfected GK 1.5 mice was 0.4% (n = 5) (data not shown). With regard to other immune cells, GK 1.5 mice were found to have comparable populations of CD8+ T cells, α/β and γ/δ T-cell-receptor-positive cells, B cells (B220+), and NK T cells but reduced numbers of dendritic cells (due to the depletion of CD4+ dendritic cells) compared to wild-type mice (32). Unlike CD4−/− and major histocompatibility complex (MHC) class II−/− mice, aberrant helper populations were absent in GK 1.5 mice, which raised only a low IgG response toward influenza A viral antigens and no detectable IgG responses toward simple antigens (32).
In this study, CD4+ T-cell deficiency had no apparent effect on H. pylori colonization, suggesting either that H. pylori colonization was independent of CD4+ T-cell activity or that another cell type (e.g., CD8+ T cells) could compensate for the lack of CD4+ T cells in controlling the growth of the bacterium. The former conclusion, if proven, would have important consequences for the development of vaccines which aim to reduce bacterial burden in gastric Helicobacter disease.
Circulating H. pylori-specific antibodies, although present in H. pylori-infected humans, are generally believed to play a minor role in protection against bacterial colonization. Clinical studies have revealed that, despite the development of a serological response, H. pylori infection is typically lifelong unless eradicated through antibiotic therapy. It has been shown that murine H. pylori disease and vaccine-mediated protection can occur in the absence of conventional B cells and their antibodies (9, 25). In our study, both infected GK 1.5 and C57BL/6 mice developed anti-H. pylori circulating antibodies, an unexpected result, as GK 1.5 mice lacked CD4+ T-cell help provided to B cells for the production of antigen-specific antibodies. A further analysis of the isotypes of H. pylori-specific antibodies revealed that most H. pylori-specific antibodies were of the IgM subtype. H. pylori-infected C57BL/6 mice also developed strong IgG (IgG1 and IgG3) responses, consistent with other studies (4, 26). In contrast, most GK 1.5 mice had barely detectable H. pylori-specific IgG antibodies. To further analyze the H. pylori-specific antibody response, we used H. pylori LPS as a CD4+ T-cell-independent antigen and demonstrated that many of the H. pylori-specific antibodies, mostly IgM, appeared to be LPS specific. In infected GK 1.5 mice, only LPS-specific IgM and IgG3 were detected. Taken together, these results suggest that the GK 1.5 mice have no residual CD4+ helper responses. Further, our results imply that both T-cell-dependent and -independent H. pylori-specific antibodies may be ineffective in reducing bacterial colonization.
The gastritis that developed in CD4+ T-cell-deficient mice after H. pylori infection appeared to be more severe than that seen for immunocompetent C57BL/6 mice, suggesting both that there were other immune cells capable of infiltrating the stomach in large numbers and that the CD4+ T cells, which normally infiltrate the stomachs of H. pylori-infected mice, might be negative regulators of gastric inflammation. Upon closer examination, we found that the predominant cells in the gastric infiltrate in infected GK 1.5 mice were CD8+ T cells, which were present in infected GK 1.5 mice at significantly high levels compared to C57BL/6 mice. Indeed, mice deficient in both CD4+ and CD8+ T cells developed only mild gastritis after H. pylori infection at 13 weeks, which was comparable to the inflammation that developed in immunocompetent mice. Hence, our results appear to contradict those reported by Eaton et al. (7), where no gastric inflammation was observed for SCID mouse recipients of splenocytes depleted of CD4+ T cells. While the discrepancy between our results and those reported by Eaton et al. might be explained by the presence of a subset of CD4 helper cells in the GK 1.5 mice, this is highly unlikely, since extensive studies by Zhan et al. have demonstrated that GK 1.5 mice lack residual aberrant helper cells (32, 33). Further, our results show that GK 1.5 mice developed an IgM anti-Helicobacter antibody response. Therefore, the difference between this study and the work of Eaton et al. may also be explained by the use of different experimental systems. In contrast to SCID mice, the GK 1.5 mice have, except for the absence of CD4+ cells, a normal immune repertoire (32, 33). Nevertheless, our results agree with those of Pappo et al. (21), who noted that CD8+ T cells were the major infiltrating cellular group in the stomachs of immunized MHC class II-deficient mice which were subsequently challenged with H. pylori. Although a predominant role of gastric CD4+ T cells has been reported in many studies of H. pylori-induced inflammation (4, 7, 20), Hatz et al. compared the cellular constituents between lamina propria lymphocytes and intraepithelial lymphocytes in patients with H. pylori-associated gastritis and found that, while CD4+ T-cell numbers exceeded CD8+ T cells among lamina propria lymphocytes, the reverse was observed for intraepithelial lymphocytes, where CD8+ T-cell levels were higher than those for CD4+ T cells (11). Hence, the severe inflammation seen for humans and animals after H. pylori infection might be caused not only by MHC class II-restricted antigen-specific CD4+ T cells but also by CD8+ T cells which are activated by H. pylori antigens presented on MHC class I molecules that are expressed on virtually all cell types. Mice that were deficient in both CD4+ and CD8+ T cells developed a relatively mild gastritis compared with what was seen for GK 1.5 mice, suggesting that in the absence of CD4+ T cells, CD8+ T cells might be directly contributing to the inflammation typical of Helicobacter-associated gastritis.
The role of regulatory CD25+ CD4+ T cells (Tregs) in H. pylori infection has now been extensively analyzed, where Tregs reportedly suppress the immune response after H. pylori infection (8, 17, 24). Our results support these findings inasmuch as exacerbated inflammation was observed for mice that lacked classical Tregs (i.e., CD25+ CD4+ T cells). It has also been suggested that Tregs may modulate the gamma interferon (IFN-γ)-producing CD4+ T cells in the gastric mucosa (23). In their analysis of cytokine production by CD4+ and CD8+ T cells isolated from human gastric biopsy samples, Quiding-Jarbrink et al. reported that CD8+ T cells produced more IFN-γ on a per-cell basis than did CD4+ T cells and that IFN-γ was produced preferentially by CD8+ T cells (22). Hence, our findings together with those from Quiding-Jarbrink et al. suggest that the regulatory action of CD4+ T cells may be directed toward the IFN-γ-producing CD8+ T cells, which were found to infiltrate the infected GK 1.5 stomach in large numbers, rather than to other (proinflammatory) CD4+ T cells.
There have been relatively few studies that examined the role of CD8+ T cells in H. pylori infection. CD8+ T-cell numbers reportedly increase in the stomachs of individuals infected with H. pylori (1), and the majority of human gastric CD8+ T cells had a memory phenotype (3). It is possible that CD8+ T cells contribute to H. pylori-induced pathology, as CD8 expression in H. pylori-infected children correlated positively with the severity of antral gastritis, and H. pylori-reactive CD8+ T cells from blood can be activated in vitro by H. pylori antigens (3, 19). Hence, it would be interesting (i) to examine the gastric CD8+ T cells found in GK 1.5 mice after H. pylori infection for antigen-specific activation and cytokine production, (ii) to investigate whether gastritis could be initiated after the adoptive transfer of CD8+ T cells into lymphopenic hosts such as RAG−/− mice, and (iii) to examine the progression of H. pylori infection in mice deficient in CD8+ T cells.
In summary, we confirmed results from earlier studies showing that anti-H. pylori antibodies, whether generated via T-cell-dependent mechanisms or not, cannot eliminate H. pylori colonization. We also showed that, in the absence of CD4+ T cells, CD8+ T cells were capable of initiating and maintaining gastric inflammation following murine H. pylori infection and that the severity of gastric inflammation was significantly increased. These results imply that CD4+ T cells, usually present in large numbers in the stomachs of H. pylori-infected hosts, are likely to have a dominant role in regulating or suppressing the local inflammation caused by H. pylori, and the target cells of such regulation may be CD8+ T cells.
Acknowledgments
This work was financially supported by the Australian Government's Cooperative Research Centre Program and an NHMRC program grant. O.L.C.W. is a recipient of an NHMRC R. D. Wright fellowship.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 19 November 2007.
REFERENCES
- 1.Agnihotri, N., D. K. Bhasin, H. Vohra, P. Ray, K. Singh, and N. K. Ganguly. 1998. Characterization of lymphocytic subsets and cytokine production in gastric biopsy samples from Helicobacter pylori patients. Scand. J. Gastroenterol. 33704-709. [DOI] [PubMed] [Google Scholar]
- 2.Alderuccio, F., B. H. Toh, P. A. Gleeson, and I. R. van Driel. 1995. A novel method for isolating mononuclear cells from the stomachs of mice with experimental autoimmune gastritis. Autoimmunity 21215-221. [DOI] [PubMed] [Google Scholar]
- 3.Azem, J., A. M. Svennerholm, and B. S. Lundin. 2006. B cells pulsed with Helicobacter pylori antigen efficiently activate memory CD8+ T cells from H. pylori-infected individuals. Clin. Immunol. 118284-291. [DOI] [PubMed] [Google Scholar]
- 4.Bamford, K. B., X. Fan, S. E. Crowe, J. F. Leary, W. K. Gourley, G. K. Luthra, E. G. Brooks, D. Y. Graham, V. E. Reyes, and P. B. Ernst. 1998. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology 114482-492. [DOI] [PubMed] [Google Scholar]
- 5.D'Elios, M. M., M. Manghetti, M. De Carli, F. Costa, C. T. Baldari, D. Burroni, J. L. Telford, S. Romagnani, and G. Del Prete. 1997. T helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. J. Immunol. 158962-967. [PubMed] [Google Scholar]
- 6.Di Tommaso, A., Z. Xiang, M. Bugnoli, P. Pileri, N. Figura, P. F. Bayeli, R. Rappuoli, S. Abrignani, and M. T. De Magistris. 1995. Helicobacter pylori-specific CD4+ T-cell clones from peripheral blood and gastric biopsies. Infect. Immun. 631102-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eaton, K. A., M. Mefford, and T. Thevenot. 2001. The role of T cell subsets and cytokines in the pathogenesis of Helicobacter pylori gastritis in mice. J. Immunol. 1667456-7461. [DOI] [PubMed] [Google Scholar]
- 8.Enarsson, K., A. Lundgren, B. Kindlund, M. Hermansson, G. Roncador, A. H. Banham, B. S. Lundin, and M. Quiding-Jarbrink. 2006. Function and recruitment of mucosal regulatory T cells in human chronic Helicobacter pylori infection and gastric adenocarcinoma. Clin. Immunol. 121358-368. [DOI] [PubMed] [Google Scholar]
- 9.Ermak, T. H., P. J. Giannasca, R. Nichols, G. A. Myers, J. Nedrud, R. Weltzin, C. K. Lee, H. Kleanthous, and T. P. Monath. 1998. Immunization of mice with urease vaccine affords protection against Helicobacter pylori infection in the absence of antibodies and is mediated by MHC class II-restricted responses. J. Exp. Med. 1882277-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ernst, P. B., and B. D. Gold. 2000. The disease spectrum of Helicobacter pylori: the immunopathogenesis of gastroduodenal ulcer and gastric cancer. Annu. Rev. Microbiol. 54615-640. [DOI] [PubMed] [Google Scholar]
- 11.Hatz, R. A., G. Meimarakis, E. Bayerdorffer, M. Stolte, T. Kirchner, and G. Enders. 1996. Characterization of lymphocytic infiltrates in Helicobacter pylori-associated gastritis. Scand. J. Gastroenterol. 31222-228. [DOI] [PubMed] [Google Scholar]
- 12.Hitchcock, P. J., and T. M. Brown. 1983. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J. Bacteriol. 154269-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaparakis, M., K. L. Laurie, O. Wijburg, J. Pedersen, M. Pearse, I. R. van Driel, P. A. Gleeson, and R. A. Strugnell. 2006. CD4+ CD25+ regulatory T cells modulate the T-cell and antibody responses in Helicobacter-infected BALB/c mice. Infect. Immun. 743519-3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuipers, E. J. 1999. Exploring the link between Helicobacter pylori and gastric cancer. Aliment. Pharmacol. Ther. 13(Suppl. 1)3-11. [DOI] [PubMed] [Google Scholar]
- 15.Kuipers, E. J., J. C. Thijs, and H. P. Festen. 1995. The prevalence of Helicobacter pylori in peptic ulcer disease. Aliment. Pharmacol. Ther. 9(Suppl. 2)59-69. [PubMed] [Google Scholar]
- 16.Lee, A., J. O'Rourke, M. Corazon de Ungria, B. Robertson, G. Daskalopoulos, and M. F. Dixon. 1997. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology 1121386-1397. [DOI] [PubMed] [Google Scholar]
- 17.Lundgren, A., E. Stromberg, A. Sjoling, C. Lindholm, K. Enarsson, A. Edebo, E. Johnsson, E. Suri-Payer, P. Larsson, A. Rudin, A.-M. Svennerholm, and B. S. Lundin. 2005. Mucosal FOXP3-expressing CD4+ CD25high regulatory T cells in Helicobacter pylori-infected patients. Infect. Immun. 73523-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lundgren, A., C. Trollmo, A. Edebo, A. M. Svennerholm, and B. S. Lundin. 2005. Helicobacter pylori-specific CD4+ T cells home to and accumulate in the human Helicobacter pylori-infected gastric mucosa. Infect. Immun. 735612-5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maciorkowska, E., I. Kasacka, K. Kondej-Muszynska, M. Kaczmarski, and A. Kemona. 2004. Cytotoxic lymphocytes (CD8+) in the antrum mucosa in children with chronic Helicobacter pylori-related inflammation before and after bacteria eradication. Rocz. Akad. Med. Bialymst. 49216-218. (In Polish.) [PubMed] [Google Scholar]
- 20.Mohammadi, M., J. Nedrud, R. Redline, N. Lycke, and S. J. Czinn. 1997. Murine CD4 T-cell response to Helicobacter infection: Th1 cells enhance gastritis and Th2 cells reduce bacterial load. Gastroenterology 1131848-1857. [DOI] [PubMed] [Google Scholar]
- 21.Pappo, J., D. Torrey, L. Castriotta, A. Savinainen, Z. Kabok, and A. Ibraghimov. 1999. Helicobacter pylori infection in immunized mice lacking major histocompatibility complex class I and class II functions. Infect. Immun. 67337-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quiding-Jarbrink, M., B. S. Lundin, H. Lonroth, and A. M. Svennerholm. 2001. CD4+ and CD8+ T cell responses in Helicobacter pylori-infected individuals. Clin. Exp. Immunol. 12381-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raghavan, S., M. Fredriksson, A. M. Svennerholm, J. Holmgren, and E. Suri-Payer. 2003. Absence of CD4+CD25+ regulatory T cells is associated with a loss of regulation leading to increased pathology in Helicobacter pylori-infected mice. Clin. Exp. Immunol. 132393-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raghavan, S., E. Suri-Payer, and J. Holmgren. 2004. Antigen-specific in vitro suppression of murine Helicobacter pylori-reactive immunopathological T cells by CD4+CD25+ regulatory T cells. Scand. J. Immunol. 6082-88. [DOI] [PubMed] [Google Scholar]
- 25.Roth, K. A., S. B. Kapadia, S. M. Martin, and R. G. Lorenz. 1999. Cellular immune responses are essential for the development of Helicobacter felis-associated gastric pathology. J. Immunol. 1631490-1497. [PubMed] [Google Scholar]
- 26.Smythies, L. E., K. B. Waites, J. R. Lindsey, P. R. Harris, P. Ghiara, and P. D. Smith. 2000. Helicobacter pylori-induced mucosal inflammation is Th1 mediated and exacerbated in IL-4, but not IFN-γ, gene-deficient mice. J. Immunol. 1651022-1029. [DOI] [PubMed] [Google Scholar]
- 27.Sommer, F., G. Faller, P. Konturek, T. Kirchner, E. G. Hahn, J. Zeus, M. Rollinghoff, and M. Lohoff. 1998. Antrum- and corpus mucosa-infiltrating CD4+ lymphocytes in Helicobacter pylori gastritis display a Th1 phenotype. Infect. Immun. 665543-5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sutton, P., S. J. Danon, M. Walker, L. J. Thompson, J. Wilson, T. Kosaka, and A. Lee. 2001. Post-immunisation gastritis and Helicobacter infection in the mouse: a long term study. Gut 49467-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sutton, P., J. Wilson, and A. Lee. 2000. Further development of the Helicobacter pylori mouse vaccination model. Vaccine 182677-2685. [DOI] [PubMed] [Google Scholar]
- 30.Taylor, D. N., and M. J. Blaser. 1991. The epidemiology of Helicobacter pylori infection. Epidemiol. Rev. 1342-59. [DOI] [PubMed] [Google Scholar]
- 31.Thien, C. B., F. D. Blystad, Y. Zhan, A. M. Lew, V. Voigt, C. E. Andoniou, and W. Y. Langdon. 2005. Loss of c-Cbl RING finger function results in high-intensity TCR signaling and thymic deletion. EMBO J. 243807-3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhan, Y., L. E. Brown, G. Deliyannis, S. Seah, O. L. Wijburg, J. Price, R. A. Strugnell, P. J. O'Connell, and A. M. Lew. 2004. Responses against complex antigens in various models of CD4 T-cell deficiency: surprises from an anti-CD4 antibody transgenic mouse. Immunol. Res. 301-14. [DOI] [PubMed] [Google Scholar]
- 33.Zhan, Y., A. J. Corbett, J. L. Brady, R. M. Sutherland, and A. M. Lew. 2000. CD4 help-independent induction of cytotoxic CD8 cells to allogenic P815 tumor cells is absolutely dependent on costimulation. J. Immunol. 1653612-3619. [DOI] [PubMed] [Google Scholar]