Abstract
Homeostatic regulatory mechanisms maintain the constant ratios between different lymphocyte subsets in the secondary lymphoid organs. How this dynamic equilibrium is achieved, in particular following the clonal expansion and subsequent contraction of different cells after infection, remains poorly understood. Expression of the proteasome immunosubunits has been shown to influence not only major histocompatibility complex class I (MHC-I) antigen processing and thereby T-cell responses, but also the CD4/CD8 T-cell ratios in lymphoid organs. We examined the relationships between these different immunosubunit-mediated effects in mice of various proteasome subunit compositions during infection with Listeria monocytogenes. Mice that lacked the immunosubunit multicatalytic endopeptidase complex-like 1 (MECL-1) maintained enhanced CD4/CD8 T-cell ratios during infection, while MHC-I surface levels resembled those in wild-type (wt) mice. LMP7 gene-deficient mice, on the other hand, showed reduced MHC-I expression, while their splenic CD4/CD8 ratios were similar to those in wt mice. Remarkably, analysis of bone marrow-chimeric immunosubunit gene-deficient mice, reconstituted with a mixture of wt and LMP7- plus MECL-1-deficient bone marrow, revealed that the LMP7- plus MECL-1-deficient T-cell population maintained a higher CD4/CD8 T-cell ratio than the wt T-cell population before, during, and after infection and T-cell memory formation. Since in these mice the immunosubunit-positive and immunosubunit-negative T-cell populations were selected in the same thymus and expanded in the same lymphoid environments, our findings indicate that MECL-1 influences the homeostatic equilibrium between T-cell subsets, not through indirect extracellular signals, such as MHC-I expression or the cytokine milieu, but through direct effects on T-cell-intrinsic processes.
In secondary lymphoid organs, the ratios between different types of leukocytes are remarkably stable. Even during most infections, when lymphoid organs expand and then retract, this homeostatic balance remains well preserved. The mechanisms leading to this balance are poorly understood.
Analyses of gene-deficient mice lacking the expression of different proteins involved in the major histocompatibility complex class I (MHC-I) antigen-processing pathway, such as the proteasome immunosubunits, have indicated that this pathway influences the ratio between CD4 and CD8 T cells.
The proteasome is an abundant cellular protease that degrades both short- and long-lived intracellular proteins and thereby regulates many cellular processes (reviewed in reference 15). One function of proteasomes involves the generation of peptides that bind to MHC-I molecules, which is effected through the degradation of self- and foreign proteins. In order to optimize the processing of antigenic peptides and thereby the induction of CD8 T-cell responses, 20S proteasomes in infected tissues are equipped with three cytokine-inducible proteasome subunits (i.e., the immunosubunits LMP2/iβ1, multicatalytic endopeptidase complex-like 1 (MECL-1)/iβ2, and LMP7/iβ5), which replace their constitutive homologues (β1, β2, and β5) in the cellular proteasome population (8, 17). The same three inducible subunits are constitutively expressed in both immature and mature dendritic cells (10, 11).
Over recent years, different functions have been ascribed to the proteasome immunosubunits. Their incorporation into the proteasome complex modifies the 20S cleavage specificity and thereby the repertoire of peptides generated (9, 16, 18-20), MHC-I cell surface expression levels (6), and the specificity of CD8 T-cell responses (3, 5). In addition, the proteasome immunosubunits have been reported to influence T-cell repertoire selection (1, 3) and CD4/CD8 T-cell ratios (2, 6).
In particular, the question of how the expression of the three proteasome immunosubunits influences the relative frequencies of CD4 and CD8 T cells remains enigmatic. Since the expression of the immunosubunits influences the liberation of MHC-I ligands and thereby the repertoire of MHC-I-presented peptides on the cell surface, one could argue that the expression of the immunosubunits in the thymus during T-cell maturation could influence the CD4/CD8 T-cell ratios in the secondary lymphoid organs mainly through effects on peptide processing. Such a notion is supported by a recent publication describing a thymus-specific proteasome subunit (tβ5) that prevents the incorporation of LMP7/iβ5 into proteasomes in the cortical tissue of the thymus (12). Mice gene deficient for this thymus-specific proteasome subunit showed dramatic alterations in the positive selection of CD8 T cells, which strongly influenced the CD4/CD8 T-cell ratio in the secondary lymphoid organs as well. However, since this proteasome subunit replaces an immunosubunit, this finding also suggests that the immunosubunits are less likely to influence the CD4/CD8 T-cell ratios on the level of the positive selection of CD8 T cells in the thymus.
Thus, in order to unravel whether the different effects of the proteasome immunosubunits on T-cell regulation can be explained solely by the altered liberation of MHC-I ligands, in particular in the thymus, or whether proteasome functions unrelated to antigen processing are involved in this process, we analyzed the T-cell compartments of uninfected and Listeria monocytogenes-infected mixed bone marrow (BM)-chimeric and gene-deficient mice that differed with respect to proteasome subunit composition. Our studies show that the observed effects of immunosubunit expression on CD4/CD8 T-cell ratios can neither be explained by altered MHC-I antigen processing and peptide presentation in the thymus nor by alterations in T-cell-proliferative capacity. Instead, they are a direct result of MECL-1 expression in the T-cell subsets.
MATERIALS AND METHODS
Mice and infections.
B6 and B6.SJL (CD45.1) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). LMP7 plus MECL-1 double-gene-deficient B6 mice (2) were maintained by in-house breeding under standard conditions. F2 offspring were generated by crossing of LMP7 plus MECL-1 double-gene-deficient B6 mice with wild-type (wt) B6 mice. The L. monocytogenes strain rLM-E1 was grown in brain heart infusion medium (BD Biosciences, Franklin Lakes, NJ) supplemented with 250 μg/ml spectinomycin and harvested while in log phase. For primary infection, 6- to 12-week-old female mice were inoculated intravenously in the tail vein with 0.1 50% lethal dose (5 × 103 rLM-E1 bacteria) in 100 μl phosphate-buffered saline (PBS). All experiments involving animals were approved by the Institutional Committee on Animal Resources of the University of Rochester Medical Center (Rochester, NY) or the Committee on Animal Experiments of the University of Utrecht Veterinary School.
Construction of BM-chimeric mice.
BM cells, flushed from the femurs of donor mice, were depleted of mature T lymphocytes by incubation with anti-CD4 monoclonal antibody (clone GK1.5) and anti-CD8 monoclonal antibody (clone 3-55) and subsequently with Guinea pig complement (Invitrogen) added at a concentration of 4.5 μg/ml for 30 min. Recipient mice were irradiated with 7 Gy as a single dose from an X-ray irradiator, reconstituted via the tail vein with 107 1-to-1-mixed BM cells from CD45.1pos B6.SJL and CD45.2pos LMP7- plus MECL-1-deficient donor mice (mixed BM-chimeric mice) or with 107 BM cells from CD45.1pos B6.SJL or CD45.2pos LMP7- plus MECL-1-deficient donors (single-chimeric mice) and then allowed to reconstitute for 28 days until additional experiments were performed.
Western blot analysis.
Splenocytes (20 × 106 to 40 × 106) were washed twice with PBS and lysed in 100 μl of lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA, pH 8.0, 0.5% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 6 μg/ml aprotinin, 7 μM pepstatin A, 10 μM leupeptin) for 20 min on ice, followed by three cycles of freezing and thawing. The lysates were cleared by centrifugation (15 min at 14,000 rpm) at 4°C and quantified by determining the optical density at 280 nm. Aliquots of 100 μg were electrophoresed on 12% sodium dodecyl sulfate-polyacrylamide gels and blotted onto nitrocellulose membranes, and proteins were visualized by Ponceau red staining, using standard procedures. The blots were blocked for 1 h in blocking buffer (PBS with 10% horse serum, 5% [wt/vol] dry milk, and 0.4% Tween 20) at room temperature, incubated overnight at 4°C in a 500-fold dilution of anti-mouse MECL-1 or a 1:1,000 dilution of anti-mouse LMP7 rabbit antiserum in PBS with 2% dry milk and 0.1% Tween 20, and developed with horseradish peroxidase-conjugated goat-anti-rabbit immunoglobulin G and enhanced chemiluminescence according to the manufacturer's instructions (Roche, Indianapolis, IN).
Antibodies and flow cytometry.
The monoclonal antibodies used in this study included fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-Cy5- and allophycocyanin (APC)-conjugated anti-mouse CD8α (clone 53-6.7), PE- and APC-conjugated anti-mouse CD4 (clone GK1.5), FITC-conjugated anti-mouse TCRβ (clone H57-597), PE-conjugated anti-mouse CD19 (clone MB19-2), PE-conjugated anti-mouse gamma interferon (IFN-γ) (clone XMG1.2), PE-Cy5-conjugated anti-mouse CD45.1 (clone A20), FITC- and PE-conjugated anti mouse CD45.2 (clone 104), biotin-conjugated anti-B220 (clone RA3-6B2), biotin-conjugated anti-H-2Kb (clone AF6-88.5), and PE- and APC-conjugated streptavidin. All these reagents were purchased from eBioscience, San Diego, CA. To analyze spleen cell subsets, mice were sacrificed at the time points indicated in the figure legends, and their spleens were collected and pressed through a cell strainer to prepare single-cell suspensions. Samples of 1 × 106 to 5 × 106 spleen cells were resuspended in ice-cold PBS with 1% bovine serum albumin and 0.02% NaN3 (PBA buffer), incubated with anti-mouse CD16/CD32 (clone 2.4G2) to block Fc receptors for 10 min, then with the appropriate fluorochrome-conjugated antibodies or with biotin-conjugated anti-class I antibody for 30 min, and then with streptavidin-PE or -APC for 30 to 60 min on ice. The cells were analyzed on a FACScalibur (BD Biosciences, Franklin Lakes, NJ), using Cellquest software.
Synthetic peptides.
Synthetic peptides corresponding to the adenovirus type 5-derived epitopes E1A234-243 and E1B192-200 and the L. monocytogenes-derived epitopes LLO296-304 and LLO189-201 were purchased from Invitrogen, Carlsbad, CA.
Analysis of T-cell responses.
At the time points specified in the figure legends, mice were sacrificed and their spleens were collected. To quantify the percentages of splenic CD4 and CD8 T cells specific for the different rLM-E1-derived epitopes, approximately 10 × 106 erythrocyte-depleted splenocytes were incubated for 6 h with or without 500 nM synthetic peptide in 1 ml RPMI medium (RPMI with 10% fetal calf serum [HyClone Laboratories, Logan, UT], 2 mM l-glutamine, 30 μM 2-mercaptoethanol, and penicillin/streptinomycin) containing 50 μg/ml gentamicin and 9 μM monensin (eBioscience, San Diego, CA). Thereafter, the cells were stained with FITC-conjugated anti-mouse CD8 or APC-conjugated anti-mouse CD4 antibody in the presence of anti-CD16/CD32 (clone 2.4G2), fixed with 2% paraformaldehyde, and then stained with XMG1.2-PE in the presence of 0.5% saponin to detect intracellular IFN-γ. The cells were analyzed on a FACSCalibur.
RESULTS
Differential roles of the individual proteasome immunosubunits in MHC ligand generation and T-cell expansion are maintained during infection.
To explore the respective effects of LMP7 and MECL-1 on antigen processing and CD4/CD8 T-cell frequencies during an ongoing immune response, when the immunosubunits are highly expressed and immunoproteasome function is expected to be needed, we crossed LMP7 plus MECL-1 gene-deficient B6 mice with control (wt) B6 mice. The F2 offspring (5 LMP7pos plus MECL-1neg, 6 LMP7neg plus MECL-1pos, and 25 LMP7pos plus MECL-1pos mice, as determined by Western blotting [data not shown]) were infected with a sublethal dose of the L. monocytogenes strain rLM-E1 (5).
Analysis of spleen cell populations of uninfected and infected mice at the peak of the CD8 T-cell response to Listeria infection showed that the cell surface levels of MHC-I molecules were reduced on splenic lymphocytes of both infected LMP7 gene-deficient and uninfected and infected LMP7 plus MECL-1 double-gene-deficient mice (Fig. 1A and data not shown). In contrast, no reduction of MHC-I expression was observed on cells of infected MECL-1-deficient mice (Fig. 1A). Thus, from our data, we infer that LMP7 plays a predominant role in the generation of high-affinity class I ligands during infection, whereas MECL-1 has a relatively moderate effect on the repertoire of class I-presented peptides.
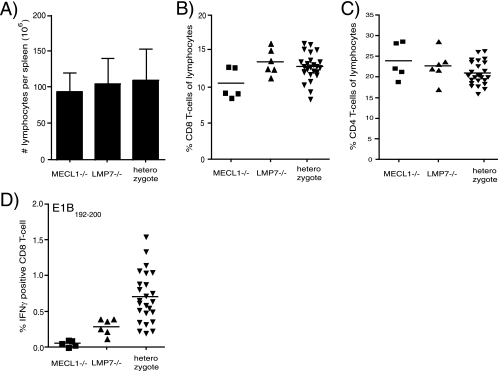
FIG. 1.
Effects of LMP7 and MECL-1 on MHC-I cell surface expression (A) and CD4/CD8 ratios (B) in uninfected and L. monocytogenes-infected mice. Splenocytes of age-matched B6 wt, MECL-1−/−, LMP7−/−, and LMP7−/− plus MECL-1−/− mice were stained with anti-CD19-APC, biotin-conjugated anti-H-2Kb, and SAV-PE (uninfected mice; n = 4); anti-CD19-PE, biotin-conjugated anti-H-2Kb, and SAV-APC (infected mice) (A); or anti-CD4-FITC and anti-CD8α-APC antibodies (B) and analyzed by flow cytometry. Mean fluorescence channels of the CD19+ populations (A) and CD4/CD8 T-cell ratios (means plus standard deviations; n = 5 or 6) (B) are depicted.
Analysis of the ratios of CD4 to CD8 T cells showed that these were significantly enhanced in MECL-1-deficient mice compared to LMP7-deficient and control (LMP7pos plus MECL-1pos) mice (P = 0.0043; two-tailed Mann-Whitney test), both when infected (day 7) and in the absence of infection (Fig. 1B). Thus, consistent with previous observations (1, 2), our data indicate that the absence of LMP7 leads to reduced MHC-I expression, whereas the expression of MECL-1 determines the CD4/CD8 T-cell ratios found in the secondary lymphoid organs. This difference in T-cell ratios between MECL-1−/− and wt mice is maintained even during an ongoing T-cell response. The use of F2 littermates in these experiments excluded the possibility that observed differences between gene-deficient and control B6 mice were due to genetic differences.
The predominant role of LMP7 in MHC-I ligand production is most likely explained by the fact that LMP7-containing proteasomes preferentially cleave after hydrophobic residues (20), leading to enhanced generation of MHC-I binding peptides. The effects of MECL-1 on CD4/CD8 T-cell ratios are more difficult to explain but apparently do not result from effects of MECL-1 on MHC-I cell surface levels.
Expression of MECL-1 is necessary for the priming of E1B192-200-specific immune responses.
Remarkably, in contrast to observations in uninfected immunosubunit-deficient mice (6), the spleen sizes of the infected F2 littermates failed to correlate with the absence or presence of either of the immunosubunits (Fig. 2A). Also, although the CD4/CD8 ratios were consistently enhanced in MECL-1−/− mice (Fig. 1D), the absolute percentages of splenic CD4 and CD8 T cells (Fig. 2B and C) fluctuated in all mouse groups. Thus, although there was a slight tendency toward lower CD8 T-cell frequencies in MECL1−/− mice, we failed to find any statistically significant differences between the sizes of the CD8 T-cell populations of the different mouse groups (Fig. 2B). Therefore, the increase in the CD4/CD8 ratio in the absence of MECL-1 is not explained simply by a reduced positive selection of CD8 T cells in these mice.
FIG. 2.
Effects of LMP7 and MECL-1 in Listeria-infected mice. F2 offspring of B6 wt × LMP7−/− plus MECL-1−/− mice were infected with 5 × 103 rLM-E1 cells intravenously. The spleens were collected at day 7 after infection and analyzed for the presence of LMP7 and/or MECL-1 by immunoblot analysis, using specific rabbit antisera (x axis). (A) Total numbers of splenocytes per spleen (means plus standard deviations). (B and C) Cells were stained with anti-CD8α-APC and anti-CD4-FITC to determine the relative frequencies of CD8 and CD4 T cells, respectively. (D) Cells were incubated with 500 nM synthetic E1B192-200 for 6 h in the presence of monensin, and the frequencies of E1B192-200-specific CD8 T cells were determined by staining for CD8 (anti-CD8α-FITC) and intracellular IFN-γ (IFN-γ-PE). Background, detected in samples incubated without peptide, was subtracted.
We previously generated a recombinant Listeria strain, rLM-E1, that secretes a modified form of Listeria p60 encompassing two adenovirus-derived CD8 T-cell epitopes (5). One of these two epitopes, E1B192-200, is generated in an immunoproteasome-dependent fashion (5). To examine whether the absence of LMP7 and/or MECL-1 affects the CD8 T-cell response to rLM-E1-derived E1B192-200, the infected F2 littermates were analyzed for the presence of E1B192-200 T cells by IFN-γ intracellular staining and flow cytometry. These analyses revealed CD8 T-cell responses to E1B192-200 in both control and LMP7-deficient, but not MECL-1-deficient, mice (Fig. 2D). Thus, MECL-1 appears to be essential for the processing of this epitope. Caudill and coworkers (2) previously showed a partial deficit in the maturation of MECL-1-containing proteasomes in LMP7 gene-deficient mice. Consistent with this finding, as well as with our own observation that the spleens of LMP7neg plus MECL-1pos F2 mice contained relatively little MECL-1 (data not shown), the levels of E1B-specific responses in LMP7-deficient mice were significantly lower than those in control mice (P = 0.0081) (Fig. 2D).
Taking the data together, while the expression of MECL-1 does not influence the overall quantity of cellular peptides presented by MHC-I molecules or the absolute frequencies of CD8 T cells in infected mice, its expression can have a significant influence on the presentation of specific pathogen-derived epitopes and therefore on the CD8 T-cell response to infection.
Differences in homeostatic CD4 and CD8 T-cell expansion between immunosubunit-deficient and -expressing T cells in mixed BM-chimeric mice.
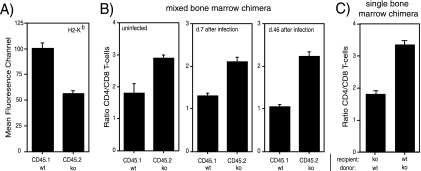
To further examine whether expression of MECL-1 in T cells themselves or in their cellular interaction partners (such as thymic or peripheral professional antigen-presenting cells [pAPC]) influences homeostatic T-cell expansion, we reconstituted irradiated LMP7- plus MECL-1-deficient mice with a mix of BM derived from wt B6/SJL (CD45.1-positive [CD45.1+]) and from LMP7- plus MECL-1-deficient (CD45.2+) mice. In these mixed BM-chimeric mice, T cells that derive from either source of BM will mature and differentiate in the same thymic and lymphoid environments. Analysis of the different CD45.1+ and CD45.2+ lymphocyte subsets in the spleens of mixed BM chimeric mice by flow cytometry 28 days after reconstitution showed the expected reduction of MHC-I expression on CD45.2+ (LMP7 plus MECL-1−/−) cells compared to CD45.1+ (wt) cells (Fig. 3A). Remarkably, these experiments further revealed enhanced ratios between CD4 and CD8 T cells of CD45.2+ (immunosubunit-deficient) origin in comparison to ratios between CD4 and CD8 T cells of CD45.1+ (wt) origin (Fig. 3B). Thus, the differences in ratios between T-cell subsets as detected in gene-deficient versus wt mice (Fig. 1) are maintained when those T cells mature, differentiate, and expand in the same thymic and peripheral lymphoid environments. Most interestingly, at both day 7 and day 46 after infection of these BM-chimeric mice with L. monocytogenes, the CD4/CD8 T-cell ratios remained significantly higher in the CD45.2pos population than in the CD45.1pos population (Fig. 3B). An enhanced ratio between CD4/CD8 T cells was also observed in wt recipients transplanted with immunosubunit-deficient BM (Fig. 3C), further indicating that the recipient background does not influence the T-cell distribution. Taken together, these findings strongly suggest that MECL-1 influences the CD4/CD8 T-cell ratios directly through T-cell-intrinsic regulatory mechanisms and not via other cells that interact with these T cells, either in the thymus or in the secondary lymphoid organs.
FIG. 3.
MHC-I cell surface levels and CD4/CD8 T-cell ratios of LMP7pos plus MECL-1pos and LMP7neg plus MECL-1neg T-cell subsets in mixed BM-chimeric mice. Lethally irradiated LMP7−/− plus MECL-1−/− mice were reconstituted with a mixture of BM from B6.SJL (wt; CD45.1pos) and LMP7−/− plus MECL-1−/− (ko; CD45.2pos) mice and 28 days later were infected with rLM-E1. (A) Splenocytes of mixed BM-chimeric mice were stained at day 28 after BM injection with fluorochrome-conjugated anti-CD45.1, anti-CD45.2, anti-CD19, and biotin-conjugated anti-H-2Kb and SAV-APC to determine the H-2Kb expression levels on the CD45.1 and CD45.2 B-cell subsets. Mean fluorescence channels are depicted (means plus standard deviations [SD]; n = 2). (B) Relative frequencies of CD45.1pos and CD45.2pos CD4 and CD8 T cells in the spleens of mixed BM-chimeric mice were determined at day 28 after BM injection and at days 7 and 46 following Listeria infection by staining with fluorochrome-conjugated anti-CD45.1, anti-CD45.2, anti-TCRβ, anti-CD4, and anti-CD8α antibodies. CD4/CD8 T-cell ratios are depicted (means plus SD; n = 2 to 5). The data are representative of two independent experiments. (C) Relative frequencies of CD4 and CD8 T cells in the spleens of CD45.1pos wt recipients reconstituted with CD45.2pos LMP7−/− plus MECL-1−/− BM and LMP7−/− plus MECL-1−/− recipients reconstituted with wt BM (means plus SD; n = 4).
Immunosubunit-deficient and -expressing T cells exert similar antigen-specific proliferation in mixed BM-chimeric mice.
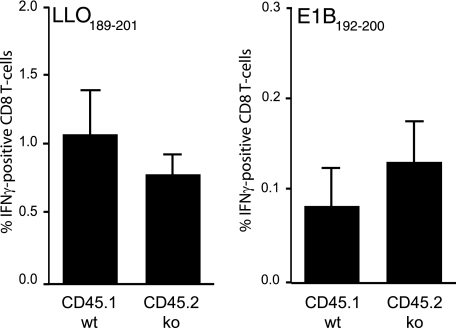
Although LMP7- plus MECL-1-deficient lymphocytes were found to hyperproliferate following stimulation with polyclonal mitogens in vitro (2), hyperproliferation was not detected in the sole absence of MECL-1 and therefore is unlikely to explain the enhanced CD4/CD8 T-cell ratios detected in mice lacking MECL-1 (1, 2). Nevertheless, enhanced T-cell expansion may bias the CD4/CD8 T-cell ratios during an ongoing immune response. To explore whether the absence of LMP7 and MECL-1 influences T-cell proliferative capacity under physiological conditions as well, we infected mixed BM-chimeric, LMP7 plus MECL-1 gene-deficient mice with rLM-E1. Quantifications of T cells specific for the rLM-E1-derived CD8 T-cell epitope E1B192-200 and CD4 T-cell epitope LLO189-201 at day 7 after infection showed that the CD45.1pos and CD45.2pos T-cell subsets responded in largely similar fashions (Fig. 4). Thus, after antigen-specific activation, LMP7- plus MECL-1-deficient (CD45.2pos) and control (CD45.1pos) T cells expanded at comparable rates. Of note, due to inefficient processing in the absence of MECL-1, rLM-E1-infected LMP7- plus MECL-1-deficient mice were not able to respond to the p60E1-derived E1B192-200 epitope (Fig. 2D) (5). Therefore, in the chimeras, all E1B192-200-specific CD8 T cells of wt and of knockout origin must have been primed by antigen-presenting CD45.1pos (wt) pAPC. Consequently, due to the presence of both LMP7- plus MECL-1-deficient (CD45.2pos) and control (CD45.1pos) pAPC, E1B192-200 responses in mixed BM-chimeric mice were smaller (Fig. 4) than those observed in wt mice (Fig. 2D).
FIG. 4.
T-cell responses in rLM-E1-infected mixed BM-chimeric mice. Mixed BM chimeras were infected with rLM-E1, and the relative frequencies of CD45.1pos and CD45.2pos T cells in the spleens specific for the rLM-E1-derived CD8 T-cell epitope E1B192-200 and CD4 T-cell epitope LLO189-201, were determined by intracellular IFN-γ staining (means plus standard deviations; n = 5). Background, detected in samples incubated without peptide, was subtracted.
DISCUSSION
The initial finding that proteasomes in cells with antigen-processing function exchange their catalytic machinery to incorporate three cytokine-inducible subunits strongly suggested an important function for the immunoproteasome formed in this way in antigenic-peptide generation and CD8 T-cell activation. However, although immunoproteasomes play an essential role in the processing of multiple pathogen-derived, immunodominant antigenic peptides and determine the fine specificity of T-cell responses (3, 5), recent studies have indicated that the immunosubunits may regulate CD8 T-cell immunity at multiple levels, possibly through diverse mechanisms (2, 3). Analyses of immunosubunit-deficient mouse strains have revealed that the absence of either subunit can modify the T-cell repertoire, presumably by effects on positive and negative selection of T cells in the thymus (12, 14), which was also seen as an explanation for the enhanced CD4/CD8 T-cell ratios detected in mice that lack MECL-1 expression (1-3, 14). In contrast to this assumption, we showed here that MECL-1 affects the T-cell compartment (i.e., CD4/CD8 T-cell ratios) through a mechanism that operates independently of the epitope-processing function of proteasomes or of thymic selection processes.
Importantly, we found that the CD4/CD8 T-cell ratios in the MECL-1-deficient T-cell population were enhanced, not only in uninfected, but also in infected mice during an ongoing T-cell response. This effect was observed in both MECL-1-deficient and mixed BM-chimeric mice after infection with different Listeria strains (Fig. 1B and 3B and data not shown) and selectively affected the MECL-1-deficient T-cell population (Fig. 3B). Unlike previously reported observations in uninfected mice (1, 2), we found that the absolute numbers of CD8 T cells or CD8 percentages were neither consistently nor significantly reduced in spleens of infected MECL-1-deficient mice compared to MECL-1pos mice (Fig. 2B and data not shown), implying that the enhanced CD4/CD8 T-cell ratios in the former mice cannot be explained by differences in relative expansion of the CD4 and CD8 T-cell subset.
We previously found that LMP7- plus MECL-deficient mice are incapable of mounting CD8 T-cell responses to the rLM-E1-derived E1B192-200 epitope due to delayed presentation of this antigenic peptide on immunoproteasome-deficient pAPC (5). Our present studies (Fig. 2D) ascribe this failure to the absence of MECL-1. Despite earlier observations that MECL-1 and LMP2 incorporate in preproteasome complexes in a codependent fashion (7, 13), LMP2 incorporation is at best slightly reduced in both MECL-1- and LMP7- plus MECL-1-deficient mice (2). Thus, LMP2 cannot be responsible for defects in epitope processing or CD4/CD8 ratios, as detected in MECL-1 gene-deficient mice. In contrast, the absence of LMP7 diminishes the efficiency of MECL-1 incorporation dramatically (4). Still, LMP7-deficient mice raise significant responses to the E1B epitope, and moreover, ratios between CD4 and CD8 T cells are normal in these mice. Thus, these data indicate that even a relatively inefficient incorporation of single immunosubunits, such as MECL-1, into the cellular proteasome population already has pronounced effects.
Our analyses of mixed BM-chimeric mice showed that differences in ratios between MECL-1-deficient CD4 and CD8 T cells and MECL-1-expressing CD4 and CD8 T cells were maintained when these T-cell populations developed within the same mixed BM-chimeric mouse. This finding excludes the possibility that an altered repertoire of self-peptides presented in the thymus/secondary lymphoid organs or other external differences between MECL-1-deficient and MECL-1pos mice may explain the altered regulation of T-cell expansion in the former mice. Taking the data together, this leaves the alternative explanation that MECL-1, which is constitutively expressed in T cells (2), directly influences homeostatic expansion, perhaps by effects on the degradation of CD4 or CD8 T-cell-specific transcription or (anti)apoptotic factors.
In summary, we show here that the immunosubunit MECL-1 regulates T-cell immunity in different ways. On one hand, MECL-1 is essential for the processing and presentation of selected MHC-I-presented peptides, which has effects both on induction of CD8 T-cell responses (Fig. 2D) and on selection of the CD8 T-cell repertoire (1). On the other hand, we have provided evidence that the effects of MECL-1 on CD4 or CD8 T-cell expansion are entirely unrelated to its role in antigen processing. Our findings suggest that MECL-1 influences the homeostatic regulatory processes that maintain the relative proportions of both T-cell subsets through a T-cell-intrinsic mechanism independent of thymic or lymphoid interaction partners.
Acknowledgments
This work was supported by NIH grant AI064576 (to A.J.A.M.S.) and the MEERVOUD program of The Netherlands Organization for Scientific Research (NWO).
LMP7 plus MECL-1 gene-deficient mice were a kind gift of J. Monaco of the University of Cincinnati, Cincinnati, OH.
Editor: F. C. Fang
Footnotes
Published ahead of print on 26 December 2007.
REFERENCES
- 1.Basler, M., J. Moebius, L. Elenich, M. Groettrup, and J. J. Monaco. 2006. An altered T cell repertoire in MECL-1-deficient mice. J. Immunol. 1766665-6672. [DOI] [PubMed] [Google Scholar]
- 2.Caudill, C. M., K. Jayarapu, L. Elenich, J. J. Monaco, R. A. Colbert, and T. A. Griffin. 2006. T cells lacking immunoproteasome subunits MECL-1 and LMP7 hyperproliferate in response to polyclonal mitogens. J. Immunol. 1764075-4082. [DOI] [PubMed] [Google Scholar]
- 3.Chen, W., C. C. Norbury, Y. Cho, J. W. Yewdell, and J. R. Bennink. 2001. Immunoproteasomes shape immunodominance hierarchies of antiviral CD8+ T cells at the levels of T cell repertoire and presentation of viral antigens. J. Exp. Med. 1931319-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De, M., K. Jayarapu, L. Elenich, J. J. Monaco, R. A. Colbert, and T. A. Griffin. 2003. Beta 2 subunit propeptides influence cooperative proteasome assembly. J. Biol. Chem. 2786153-6159. [DOI] [PubMed] [Google Scholar]
- 5.Deol, P., D. M. Zaiss, J. J. Monaco, and A. J. Sijts. 2007. Rates of processing determine the immunogenicity of immunoproteasome-generated epitopes. J. Immunol. 1787557-7562. [DOI] [PubMed] [Google Scholar]
- 6.Fehling, H. J., W. Swat, C. Laplace, R. Kuhn, K. Rajewsky, U. Muller, and H. von Boehmer. 1994. MHC class I expression in mice lacking the proteasome subunit LMP-7. Science 2651234-1237. [DOI] [PubMed] [Google Scholar]
- 7.Groettrup, M., S. Standera, R. Stohwasser, and P. M. Kloetzel. 1997. The subunits MECL-1 and LMP2 are mutually required for incorporation into the 20S proteasome. Proc. Natl. Acad. Sci. USA 948970-8975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan, S., M. van den Broek, K. Schwarz, R. de Giuli, P. A. Diener, and M. Groettrup. 2001. Immunoproteasomes largely replace constitutive proteasomes during an antiviral and antibacterial immune response in the liver. J. Immunol. 1676859-6868. [DOI] [PubMed] [Google Scholar]
- 9.Kloetzel, P. M. 2001. Antigen processing by the proteasome. Nat. Rev. Mol. Cell Biol. 2179-187. [DOI] [PubMed] [Google Scholar]
- 10.Li, J., B. Schuler-Thurner, G. Schuler, C. Huber, and B. Seliger. 2001. Bipartite regulation of different components of the MHC class I antigen-processing machinery during dendritic cell maturation. Int. Immunol. 131515-1523. [DOI] [PubMed] [Google Scholar]
- 11.Macagno, A., M. Gilliet, F. Sallusto, A. Lanzavecchia, F. O. Nestle, and M. Groettrup. 1999. Dendritic cells up-regulate immunoproteasomes and the proteasome regulator PA28 during maturation. Eur. J. Immunol. 294037-4042. [DOI] [PubMed] [Google Scholar]
- 12.Murata, S., K. Sasaki, T. Kishimoto, S. Niwa, H. Hayashi, Y. Takahama, and K. Tanaka. 2007. Regulation of CD8+ T cell development by thymus-specific proteasomes. Science 3161349-1353. [DOI] [PubMed] [Google Scholar]
- 13.Nandi, D., E. Woodward, D. B. Ginsburg, and J. J. Monaco. 1997. Intermediates in the formation of mouse 20S proteasomes: implications for the assembly of precursor beta subunits. EMBO J. 165363-5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osterloh, P., K. Linkemann, S. Tenzer, H. G. Rammensee, M. P. Radsak, D. H. Busch, and H. Schild. 2006. Proteasomes shape the repertoire of T cells participating in antigen-specific immune responses. Proc. Natl. Acad. Sci. USA 1035042-5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rock, K. L., and A. L. Goldberg. 1999. Degradation of cell proteins and the generation of MHC class I-presented peptides. Annu. Rev. Immunol. 17739-779. [DOI] [PubMed] [Google Scholar]
- 16.Schwarz, K., M. van Den Broek, S. Kostka, R. Kraft, A. Soza, G. Schmidtke, P. M. Kloetzel, and M. Groettrup. 2000. Overexpression of the proteasome subunits LMP2, LMP7, and MECL-1, but not PA28 alpha/beta, enhances the presentation of an immunodominant lymphocytic choriomeningitis virus T cell epitope. J. Immunol. 165768-778. [DOI] [PubMed] [Google Scholar]
- 17.Shin, E. C., U. Seifert, T. Kato, C. M. Rice, S. M. Feinstone, P. M. Kloetzel, and B. Rehermann. 2006. Virus-induced type I IFN stimulates generation of immunoproteasomes at the site of infection. J. Clin. Investig. 1163006-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sijts, A., D. Zaiss, and P. M. Kloetzel. 2001. The role of the ubiquitin-proteasome pathway in MHC class I antigen processing: implications for vaccine design. Curr. Mol. Med. 1665-676. [DOI] [PubMed] [Google Scholar]
- 19.Sijts, A. J., S. Standera, R. E. Toes, T. Ruppert, N. J. Beekman, P. A. van Veelen, F. A. Ossendorp, C. J. Melief, and P. M. Kloetzel. 2000. MHC class I antigen processing of an adenovirus CTL epitope is linked to the levels of immunoproteasomes in infected cells. J. Immunol. 1644500-4506. [DOI] [PubMed] [Google Scholar]
- 20.Toes, R. E., A. K. Nussbaum, S. Degermann, M. Schirle, N. P. Emmerich, M. Kraft, C. Laplace, A. Zwinderman, T. P. Dick, J. Muller, B. Schonfisch, C. Schmid, H. J. Fehling, S. Stevanovic, H. G. Rammensee, and H. Schild. 2001. Discrete cleavage motifs of constitutive and immunoproteasomes revealed by quantitative analysis of cleavage products. J. Exp. Med. 1941-12. [DOI] [PMC free article] [PubMed] [Google Scholar]