Abstract
In order to unravel the role of regulation on transcript level in central carbohydrate metabolism (CCM) of Thermoproteus tenax, a focused DNA microarray was constructed by using 85 open reading frames involved in CCM. A transcriptional analysis comparing heterotrophic growth on glucose versus autotrophic growth on CO2-H2 was performed.
The anaerobic, facultative heterotrophic crenarchaeum Thermoproteus tenax shows a sulfur-dependent energy metabolism and grows optimally at 86°C and pH 5.6. In addition to autotrophic growth of this organism on carbon dioxide and hydrogen (CO2-H2), heterotrophic growth on various carbohydrates, such as starch, glycogen, and glucose, was reported (39). A combination of genomics-based and biochemical approaches revealed that T. tenax is the only archaeum currently known that uses two different pathways for carbohydrate catabolism in parallel, a reversible Embden-Meyerhof-Parnas (EMP) pathway and the catabolic, so-called “branched” Entner-Doudoroff (ED) pathway, both of which represent modified versions of the known classical bacterial and eukaryotic pathways (1, 26, 28, 30). In T. tenax, the operation of a reversible citric acid cycle (CAC), which is involved in the oxidation of pyruvate to CO2 under heterotrophic growth conditions (24) and in CO2 fixation under autotrophic growth conditions (30), has been suggested. Compared to the available knowledge about the complexity of archaeal central carbohydrate metabolism (CCM) and its various modifications, information on the regulation of the CCM is rather scarce. As shown for the EMP pathway, the allosteric regulation at the protein level, which plays an important role in the classical pathway of bacteria and eucarya, seems to be of minor relevance in archaea. The classical control points at the beginning (hexokinase/glucose-6-phosphatase and ATP-phosphofructokinase/fructose-1,6-bisphosphatase) and at the end (pyruvate kinase [PK]/phosphoenolpyruvate [PEP] synthetase [PEPS]) of the pathway are absent in T. tenax and all archaea studied so far (31). The key reactions are catalyzed by nonallosteric enzymes in archaea. Due to the ability of T. tenax to grow under autotrophic as well as heterotrophic growth conditions, the organism represents an ideal object for the study of the regulation of the glycolytic/gluconeogenic switch of carbon flux.
A focused CCM microarray study including 90 sequences representing 85 genes encoding homologs engaged in the central carbohydrate pathways of T. tenax (Table 1; see Table S2 in the supplemental material) (30) was performed. Changes in transcript levels of the CCM-related genes of T. tenax in response to autotrophic growth conditions in comparison to expression levels under heterotrophic growth conditions were followed. Seven hybridization experiments with cDNA derived from 14 independent cultures of T. tenax (seven grown autotrophically on H2 and CO2 as the sole carbon source and seven grown heterotrophically on glucose) were performed. For the construction of the CCM DNA microarray, primers were designed by using the software PrimeArray0.82 (19; kindly provided by C. Dehio, University of Basel) and purchased from MWG Biotech. Preparation of the PCR products (≥100 ng/μl), printing (sixfold) on polylysine-coated slides (Poly-Prep; Sigma Diagnostics), and slide postprocessing were performed as reported previously (5, 7, 38). Mass cultures of T. tenax strain Kra 1 (DSM 2078) (10, 39) were grown, as described previously (4), in a medium as described by Brock et al. (2) with slight modifications. Concentrations of 5 g/liter dispersed elemental sulfur and 0.01 g/liter yeast extract were added. For heterotrophic growth, 2 g/liter glucose was added. Cultures were continuously gassed with 80% H2-20% CO2 (autotrophic growth) or 80% H2-20% N2 (heterotrophic growth) at a flow rate of 1 liter/min and stirred at 250 rpm. Cells were harvested in the exponential growth phase, quickly cooled down to 4°C by a capillary cooler, and pelleted by centrifugation (10,000 × g, 20 min, 4°C). Total RNA was prepared from the autotrophically and heterotrophically grown T. tenax cells by using TRIzol reagent and the RNeasy mini kit according to the instructions of the manufacturers (Life Technologies and Qiagen, respectively). On-column DNase I treatment was performed following the manufacturer's instructions (Qiagen). Afterwards, RNA samples were checked for DNA contamination by PCR (primer set of the hxk probe, 5′-TGGTGAGCAGAGATGGGCGAGT-3′ [sense] and 5′-ACTTCTTCAGAGTATCCGGCGGC-3′ [antisense]). The synthesis of labeled cDNA (using 15 μg of total RNA), sample treatment prior to hybridization, and hybridization (at 60°C, overnight) were performed as described previously (7, 38). Scanning and image processing were carried out by using a GenePix 4000a scanner and GenePixPro 3.0 software (Axon Instruments). Low-quality spots (<1,000 intensity units) were excluded from further analysis. The obtained signal intensities were normalized by using the method of internal standardization as reported previously (38). Briefly, the PCR product of rpoS, coding for the stationary-phase sigma factor of Escherichia coli, was printed eightfold and evenly distributed onto the arrays. Preparation of the internal standard was carried out as described previously (38), and identical amounts (40 ng) of in vitro-transcribed rpoS were added to each RNA preparation immediately after cell lysis. This normalization method compensates for methodical differences, e.g., due to differential dye incorporation. Statistical significance for the observed fluorescence signal ratios was calculated by paired t test analysis (significance level, P < 0.05) using GeneSpring software and Microsoft Excel. The fluorescence intensity changes and log2-transformed mean intensity ratios and their standard deviations are given in Table 1 and Table S2 (in the supplemental material). In order to check for reproducibility and underline the reliability of the microarray data, a control experiment was performed by using cDNA derived from two independent autotrophically grown cultures (Fig. 1A). As shown in Fig. 1B, the change in the offered carbon source has a significant effect on the transcript levels of the CCM genes. For data validation, Northern blot analyses were performed for six selected genes (pfp, gltA2, pps, frdB, gaa, and gpmA) by using radiolabeled antisense mRNA probes and total RNA derived from autotrophically and heterotrophically grown cells harvested in the exponential growth phase (see Fig. S1 and Table S1 in the supplemental material). Evaluation and quantitation of the signals were performed via phosphorimaging (Image Reader FLA 5000, version 2.1; Fuji film). For all genes, microarray data were confirmed, with consideration of method-based deviations (see Fig. S1 in the supplemental material).
TABLE 1.
CCM-related ORFs that show differential transcript levels in response to CO2 and to glucose
| ORF function and no. | Gene | Gene product | Accession no. | EC no. | COG no.a | Fluorescence intensity fold change (mean intensity ratio [log2] ± SD)b | No. in Fig. 2c |
|---|---|---|---|---|---|---|---|
| Reversible EMP pathway | |||||||
| 1277 | pfp | PPi-PFK | Y14655 | 2.7.1.90 | 0205 | 8.2 (−3.04 ± 0.9) | 3 |
| 1278 | fba | FBPA, archaeal-type class I | AJ310483 | 4.1.2.13 | 1830 | 9.2 (−3.2 ± 0.6) | 5 |
| 1534 | gap | Phosphorylating GAPDH | Y10126 | 1.2.1.13 | 0057 | 3.8 (2.12 ± 0.5) | 8 |
| 2037 | gor | Fd-dependent GAPOR | AJ621330 | 1.2.7.6 | 2414 | 3.9 (−1.96 ± 0.1) | 11 |
| 1535 | pgk | PGK | Y10126 | 2.7.2.3 | 0126 | 5.4 (2.42 ± 0.5) | 9 |
| 0910 | pps | PEPS | AJ515537 | 2.7.9.2 | 0574 | 11.5 (3.8 ± 0.7) | 15 |
| ED pathway | |||||||
| 0788 | garK | GK | AJ621354 | 2.7.1.31 | 2379 | 2.2 (1.14 ± 0.7) | 23 |
| 0789 | ilvD/edd | DHAD/6-phoshogluconate dehydratase | AM884391 | 4.2.1.9/4.2.1.12 | 0129 | 2.3 (1.2 ± 0.4) | |
| Citric acid cycle | |||||||
| 1513 | gltA2 | CS2 | AJ621309 | 2.3.3.1 | 0372 | 2.4 (−1.23 ± 0.2) | 26 |
| 0493 | acn | ACN | AJ515539 | 4.2.1.3 | 1048 | 2.5 (−1.3 ± 0.4) | 28 |
| 1489 | idhA | IDH | AJ621308 | 1.1.1.42 | 0538 | 2.6 (−1.4 ± 1.1) | 29 |
| 0209 | oorA | Fd-dependent OOR, candidate 2, α subunit | AJ621339 | 1.2.7.- | 0674 | 4.7 (2.24 ± 0.7) | 30/24 |
| 0210 | oorB | Fd-dependent OOR, candidate 2, β subunit | AJ621340 | 1.2.7.- | 1013 | 5.0 (2.33 ± 0.7) | 30/24 |
| 0208 | oorCD | Fd-dependent OOR, candidate 2, γ/δ subunit | AJ621338 | 1.2.7.- | 1014 | 3.5 (1.82 ± 0.3) | 30/24 |
| 0864 | sdhA (frdA) | Succinate dehydrogenase (fumarate reductase), candidate 1, α subunit | AJ621269 | 1.3.99.1 | 1053 | 7.5 (−2.9 ± 0.7) | 32 |
| 0863 | sdhB (frdB) | Succinate dehydrogenase (fumarate reductase), candidate 1, β subunit | AJ621268 | 1.3.99.1 | 0479 | 4.7 (−2.23 ± 0.7) | 32 |
| 0862 | sdhC (frdC) | Succinate dehydrogenase (fumarate reductase), candidate 1, γ subunit | AJ621267 | 1.3.99.1 | 1053 | 3.0 (−1.57 ± 0.6) | 32 |
| 0861 | sdhD (frdD) | Succinate dehydrogenase (fumarate reductase), candidate 1, δ subunit | AJ621266 | 1.3.99.1 | 3.2 (−1.69 ± 0.7) | 32 | |
| 1104 | frdA | Fumarate reductase (succinate dehydrogenase), candidate 2, α subunit | AJ621278 | 1.3.99.1 | 1053 | 12.5 (3.64 ± 0.9) | 33 |
| 1105 | frdB | Fumarate reductase (succinate dehydrogenase), candidate 2, β subunit | AJ621279 | 1.3.99.1 | 0479 | 4.1 (2.04 ± 0.6) | 33 |
| 1106 | adh | Zn2+-dependent alcohol dehydrogenase class III | AJ621280 | 1.1.1.1 | 1062 | 10.6 (3.4 ± 1.8) | |
| Fd-dependent oxidoreductase | |||||||
| 1785 | oorA | Pyruvate-Fd OR, 2-oxoacid Fd-OR, candidate 3, α subunit | AJ621319 | 1.2.7.- | 0674 | 3.0 (−1.57 ± 0.3) | |
| 1786 | oorB | Pyruvate-Fd OR, 2-oxoacid Fd-OR, candidate 3, β subunit | AJ621320 | 1.2.7.- | 1013 | 2.3 (−1.21 ± 0.7) |
COG, clusters of orthologous groups of proteins.
The changes and log2-transformed mean intensity ratios as well as the standard deviations (SD) are given. ORFs that show >2-fold increased regulation are listed. For all ORFs, signals are statistically significant (P < 0.05).
Numbering of the ORFs corresponds to the numbers given in Fig. 2.
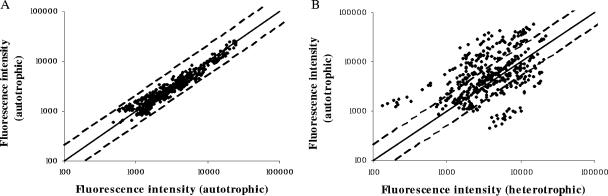
FIG. 1.
Intensity scatter plots of Cy5 versus Cy3 intensities of two independent autotrophically grown cultures (control) (A) and an autotrophically grown culture (CO2-H2) versus a heterotrophically grown culture (glucose) (B). The solid line indicates no differential transcript level and the dashed lines indicate twofold up-regulation under autotrophic or heterotrophic growth conditions.
Overall, 75 of the 85 open reading frames (ORFs) turned out to be transcribed in T. tenax under the chosen growth conditions, glucose and CO2-H2. For 10 ORFs, no signal could be detected, suggesting that these ORFs are not transcribed under the chosen growth conditions (see Table S2 in the supplemental material). However, for these genes, alternative candidates were shown to be transcribed (e.g., genes encoding AOR3 [Fd-dependent aldehyde oxidoreductase, candidate 3], ALDH1 [aldehyde dehydrogenase, candidate 1], fumarate hydratase class II, sugar nucleotidyl transferase 1, and pyruvate-ferrdeoxin [Fd] oxidoreductase 3) (see Table S2 in the supplemental material) or the genes seemed to be involved in alternative pathways (e.g., oxoglutarate dehydrogenase [E3 subunit], malate synthase, dTDP-glucose-4,6-dehydratase) (see Table S2 in the supplemental material). A total of 25 ORFs showed transcript level changes more than twofold. Among them, 14 genes are up-regulated in response to the offered carbon source glucose and 11 genes were up-regulated under growth on CO2-H2 (Table 1; Fig. 2).
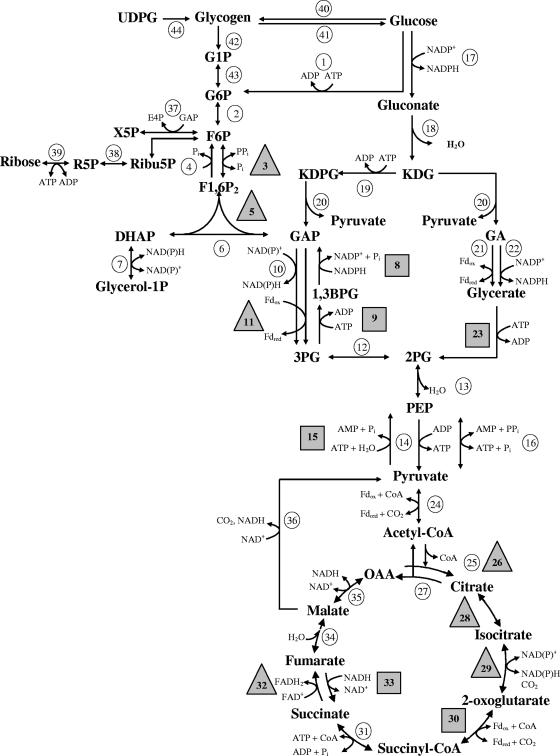
FIG. 2.
Overview of the CCM of T. tenax. Enzymes are given by numbers which correspond to those listed in Table 1 and Table S2 (in the supplemental material). Circles indicate enzymes for which the encoding genes are not up-regulated in response to the offered carbon source glucose versus CO2-H2, triangles indicate up-regulated genes under heterotrophic growth conditions, and boxes indicate up-regulated genes under autotrophic growth conditions. Abbreviations: UDPG, UDP-glucose; G1P, glucose 1-phosphate; G6P, glucose 6-phosphate; E4P, erythrose 4-phosphate; X5P, xylulose 5-phosphate; Ribu5P, ribulose 5-phosphate; R5P, ribose 5-phosphate; AA, acetaldehyde; F1,6P, fructose 1,6-bisphosphate; DHAP, dihydroxyacetonephosphate; GA(P), glyceraldehyde(-3-phosphate); KD(P)G, 2-keto-3-deoxy-(6-phosphate)-gluconate; 1,3 BPG, 1,3-bisphosphoglycerate; 3PG, 3-phosphoglycerate; 2PG, 2-phosphoglycerate; OAA, oxaloacetic acid; Fdred, reduced ferredoxin; Fdox, oxidized ferredoxin; CoA, coenzyme A.
The reversible EMP pathway.
A total of 6 genes of the 16 genes (encoding 15 enzymes) involved in the modified reversible T. tenax EMP pathway show differential transcript levels in response to the offered carbon source, glucose or CO2 (Table 1). The genes encoding reversible pyrophosphate-dependent phosphofructokinase (PPi-PFK; pfp, no. 3 in Fig. 2) and fructose-1,6-bisphosphate aldolase (FBPA; fba, no. 5 in Fig. 2), which form an operon (fba-pfp), as well as the catabolic Fd-dependent glyceraldehyde-3-phosphate (GAP) oxidoreductase (GAPOR; gor, no. 11 in Fig. 2), are up-regulated in response to growth on glucose. In contrast, the genes encoding anabolic PEPS (pps, no. 15 in Fig. 2) and phosphoglycerate kinase (PGK; pgk, no. 9 in Fig. 2), as well as the classical gene encoding NADP+-dependent GAP dehydrogenase (GAPDH; gap, no. 8 in Fig. 2) (the latter two form an operon) are up-regulated in response to growth on CO2-H2.
The first site of carbon flux control of the T. tenax EMP variant is localized at the beginning of the pathway (at fructose 6-phosphate/fructosebisphosphate [F6P/FBP] conversion) and exclusively executed at the gene level. The EMP variant of T. tenax is characterized by the enzymes ATP-dependent hexokinase (ATP-HK; hxk, no. 1 in Fig. 2), which shows very low regulatory properties (6), and the reversible PPi-PFK, which is without regulatory potential (29). The increased transcript level of the fba-pfp operon, encoding PPi-PFK and FBPA, corresponds nicely to the determined increase in enzyme activity in heterotrophically grown cells (27). These data suggest higher catabolic fluxes under heterotrophic growth conditions. In addition to the reversible PPi-PFK, a gene encoding a homolog of an archaeal-type fructose-1,6-bisphosphatase (FBPase, class V) has been identified in the genome of T. tenax. The encoding gene shows no differential transcript levels in response to the different growth conditions. However, so far, no biochemical data are available.
As deduced from previous biochemical studies, the major control point of the T. tenax EMP variant is found at the level of GAP conversion, which is characterized by three different enzymes: reversible, classical GAPDH (4), irreversible, nonphosphorylating GAPDH (GAPN; gapN, no. 10 in Fig. 2) (3, 4), and GAPOR. GAPOR catalyzes, like GAPN, the irreversible, nonphosphorylating oxidation of GAP, yielding 3-phosphoglycerate; however, GAPOR uses Fd instead of pyridine nucleotides as a cosubstrate. Whereas the GAPN of T. tenax is well characterized and was shown to exhibit allosteric properties (4, 16), no biochemical information was available for the GAPOR of T. tenax. However, the GAPOR of the closely related Pyrobaculum aerophilum (59% sequence identity) was characterized recently, and higher levels of activity in cells grown on maltose were reported (20). Furthermore, the enzyme of Pyrococcus furiosus (37% sequence identity) was shown to exhibit no allosteric properties, and a significant up-regulation of transcript amounts in response to heterotrophic growth conditions was reported (17, 23, 35). In T. tenax, GAPN is regulated by the energy charge of the cell (i.e., activation by ADP and AMP and inhibition by ATP) and early intermediates of the EMP pathway (F6P) as well as intermediates of the glycogen metabolism (glucose 1-phosphate) (both activators). No regulation of the transcript level was observed (4). The up-regulation of the gor transcript levels under growth on glucose seems to enhance the catabolic carbon flow through the pathway. In the anabolic (gluconeogenic) direction, the enzyme couple GAPDH and PGK substitutes for the unidirectionally catabolic enzymes GAPN and GAPOR in T. tenax. Biochemical and transcriptional analyses of the classical reversible phosphorylating GAPDH in T. tenax and P. furiosus, as well as a mutational approach with Thermococcus kodakaraensis (T. kodakaraensis and P. furiosus also harbor the three GAP-converting enzymes), revealed a true anabolic role in these organisms (4, 21, 23; K. Matsubara, H. Atomi, and T. Imanaka, presented at the Sixth International Conference on Extremophiles, Brest, France). The significant up-regulation of the PGK- and GAPDH-encoding genes (gap-pgk operon) under autotrophic growth conditions confirms the proposed anabolic function of the GAPDH-PGK couple in T. tenax. Therefore, the second and main control point in the EMP variant of T. tenax is executed at the protein level by an allosterically regulated GAPN and, additionally, at the transcript level by inversely regulated genes encoding the catabolic GAPOR and the anabolic GAPDH-PGK enzyme couple.
The third control point is also characterized by three enzymes involved in PEP/pyruvate conversion: catabolic PK (encoded by pyk, no. 14 in Fig. 2) (22), anabolic PEPS (33), and reversible pyruvate phosphate dikinase (PPDK; ppdk, no. 16 in Fig. 2) (33). The catabolic PK shows only very low levels of regulatory properties and exhibits positive cooperativity with PEP and metal ions but is not regulated by common PK effectors (22). For PK, the microarray experiments showed no statistically significant results, but previous Northern analysis revealed an up-regulation of the encoding pyk gene under heterotrophic growth on glucose, although this occurred in a later growth phase (22). The PEPS of T. tenax has been characterized in detail and was shown to catalyze the unidirectional ATP-dependent conversion of pyruvate to PEP, AMP, and Pi (33), thus representing a true anabolic enzyme. In accordance with its solely anabolic function, the encoding gene (pps) is up-regulated in response to growth on CO2-H2 (Table 1) (33). In addition, the enzyme exhibited regulatory properties and was significantly inhibited in the presence of 2-oxoglutarate, AMP, and ADP, suggesting reduced activity at a low-energy charge of the cell and under ammonia limitation. Additionally, the reversible PPDK was strongly inhibited at elevated ATP concentrations, and no regulation at the gene level was observed (see Table S2 in the supplemental material) (33).
In summary, up-regulation of the genes encoding PPi-PFK, FBPA, GAPOR, and PK in cells grown on glucose might allow the enhancement of the carbon flux under heterotrophic growth conditions, whereas up-regulation of the genes coding for GAPDH and PGK as well as PEPS drives the flux in the opposite, anabolic direction. Therefore, the reversible EMP pathway of T. tenax is characterized by one control point being exclusively executed at the gene level (F6P/FBP conversion) and by two control points exhibiting combined regulation at the protein and gene levels (GAP and PEP/pyruvate conversion).
The catabolic, branched ED pathway.
Most genes coding for the six enzymes utilized in the branched ED pathway and not shared by the reversible EMP pathway show no change in transcript amount in response to the different carbon sources, CO2 and glucose (see Table S2 in the supplemental material). Only the genes encoding glycerate kinase (GK; garK, no. 23 in Fig. 2) and dihydroxy-acid dehydratase (DHAD; ilvD), which are supposed to form an operon, show an up-regulation in response to autotrophic growth conditions (Table 1). The presence of the branched ED pathway with a nonphosphorylative ED branch as well as a semiphosphorylative ED branch in T. tenax was deduced from comparative genome context analysis and biochemical studies (1). In vivo labeling studies with 1-13C-glucose revealed that the EMP pathway and the ED pathway represent alternative routes for glucose degradation in T. tenax. However, the EMP pathway seems to be preferred under the condition of heterotrophic growth on glucose (28). Three enzymes, (i) the bifunctional 2-keto-3-deoxy-(6-phospho)gluconate aldolase [KD(P)GA; kdgA, no. 20 in Fig. 2] using KDG and KDPG as the substrate, (ii) KDG kinase (KDGK; kdgK, no. 19 in Fig. 2; semiphosphorylative ED branch), and (iii) the GK (nonphosphorylative ED branch) catalyzing the phosphorylation of KDG or glycerate, respectively, represent the key enzymes of the pathway. So far, not much is known about regulation of the ED enzymes of T. tenax at the protein level. Only for GK are studies available, and a significant inhibition by ADP is observed, suggesting feedback inhibition by a low energy charge of the cell (12). Up-regulation of the GK-encoding gene (garK) under autotrophic growth conditions is surprising, given the fact that the branched ED pathway is generally regarded as a pathway for glucose degradation in archaea. The DHAD from Sulfolobus solfataricus was characterized recently, and significant activity on dihydroxyisovalarate and gluconate was demonstrated (14). Therefore, a function in the biosynthesis of branched chain amino acids as well as in the branched ED pathway was predicted for this organism. However, the up-regulation of the garK gene of T. tenax under autotrophic growth conditions remains unclear.
The reversible CAC.
The complete set of required candidate genes coding for CAC enzymes, with the sole exception of the 2-oxoglutarate dehydrogenase gene (genes encoding the E1 and E2 components are missing), were identified in the T. tenax genome, although some candidate genes could not be assigned unequivocally (Table 1; see Table S2 in the supplement material) (30). Most of the CAC enzymes catalyze reversible reactions, whereas the enzyme couples citrate synthase (CS) and citrate lyase, 2-oxoglutarate dehydrogenase and 2-oxoglutarate oxidoreductase (OOR), and succinate dehydrogenase (SDH) and fumarate reductase (FRD) are supposed to work in the opposite direction to control the flux through the pathway. Transcript levels of the genes encoding the key enzymes of the oxidative direction CS2 (one of the two CSs; gltA2, no. 26 in Fig. 2) and the predicted genes encoding SDH (the sdhD-sdhC-sdhB-sdhA operon, no. 32 in Fig. 2) are up-regulated in glucose-grown cells (Table 1). Also, the genes encoding aconitase (ACN; acn, no. 28 in Fig. 2) and isocitrate dehydrogenase (IDH; idhA, no. 29 in Fig. 2), which catalyze reversible reactions, show an up-regulation under heterotrophic growth conditions (Table 1). The genes predicted to code for FRD (frdA and frdB, no. 33 in Fig. 2) are up-regulated in CO2-H2-grown cells. In addition, significant changes in transcript levels are found for the genes encoding one of the two identified candidates for OOR (the oorA-oorB-oorCD operon, no. 30 in Fig. 2) under autotrophic conditions (Table 1). OOR is suggested to operate in both directions in T. tenax (30) and to substitute for the lack of 2-oxoglutarate dehydrogenase. Therefore, it can be concluded that the CAC enzymes CS2 and SDH as well as ACN and IDH function in a coordinated manner under heterotrophic growth conditions (oxidative CAC), whereas FRD and the reversible OOR support the carbon flux under autotrophic growth (reductive direction). However, respective enzyme activities and their functions in the reversible CAC remain to be shown.
In summary, the results of our transcriptional analysis of the CCM genes of T. tenax reflect a highly coordinated transcription of the genes involved in the reversible EMP pathway and the reversible CAC for controlling the catabolic and anabolic carbon flux. In contrast, the genes of the catabolic, branched ED pathway exhibit no strong regulation at the gene level under the chosen growth conditions (glucose and CO2-H2).
Regulation of CCM in archaea: evidence for common principles?
With the present microarray analysis of T. tenax, comparing growth on CO2-H2 versus glucose, and respective studies of glycolytic and proteolytic growth of two other hyperthermohilic archaea, the aerobic crenarchaeum S. solfataricus (32) and the anaerobic euryarchaeum P. furiosus (23), as well as the moderate halophile Haloferax volcanii (38), data sets of four archaea are available. The comparison of the four different systems revealed common features in the regulation of CCM pathways (EMP and ED pathways).
S. solfataricus uses the branched ED pathway for carbohydrate catabolism (1), which is promiscuous for glucose and galactose (15), whereas the upper part of the EMP pathway seems to be exclusively responsible for gluconeogenesis. Glucose is completely oxidized to CO2 via an oxidative CAC with oxygen as the terminal electron acceptor (25). In S. solfataricus, a combined transcriptomic/proteomic approach was performed (32) to monitor expression differences during growth on yeast extract and tryptone versus growth on glucose. The analyses revealed no strong regulation of the CCM genes on transcript level in S. solfataricus. However, the proteome data revealed that the allosteric, catabolic GAPN (8) is induced during growth on glucose. PGK and PEPS are induced in yeast extract-tryptone-grown cells and, therefore, seem to represent truly anabolic enzymes (Fig. 3) (32).
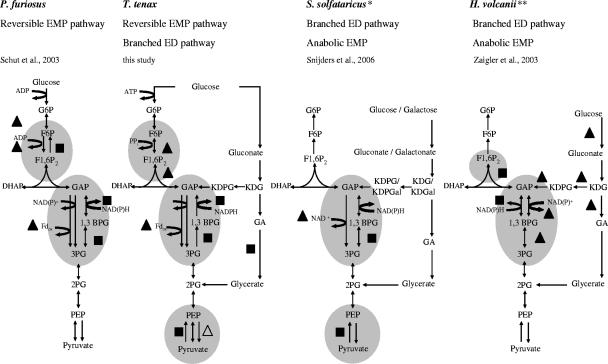
FIG. 3.
Conserved regulation sites of the CCM pathways in the hyperthermophiles T. tenax, S. solfataricus, and P. furiosus as well as the mesophilic halophile H. volcanii. Regulation sites of the respective pathways are indicated by gray shading. Filled triangles indicate genes up-regulated under heterotrophic growth conditions, and filled boxes indicate genes up-regulated under autotrophic/proteolytic growth conditions. The open triangle indicates the previously reported up-regulation of the T. tenax pyk gene encoding PK when cells are grown on glucose (22). The GAPDHs from P. furiosus and H. volcanii are not characterized so far, and the cosubstrate specificities are unknown. For S. solfataricus (*), proteomic data are shown, since no transcript level changes have been observed (32). The pair of asterisks for H. volcanii indicates a carbon source shift from Casamino Acids-based growth to glucose-based growth (38). For abbreviations, see the legend to Fig. 2.
The fermentative P. furiosus utilizes a reversible modification of the EMP pathway, and glucose is finally converted to acetate as a main fermentation product (via ADP-forming acetyl coenzyme A synthetase) (18). The EMP variant of P. furiosus is characterized by ADP-dependent kinases (glucokinase and phosphofructokinase) (13, 34) and, as in T. tenax, three GAP-converting enzymes (GAPN, GAPOR, and anabolic GAPDH) (17, 21, 35). At the level of PEP/pyruvate conversion, the PEPS of P. furiosus seems to have an unusual catabolic function in carbohydrate degradation (for a discussion, see references 11 and 36). A whole-genome microarray analysis of P. furiosus was performed with cultures grown on either peptides or maltose as a carbon source (23). In maltose-grown cells, the genes coding for glucose-6-phosphate isomerase (PGI) (37), ADP-PFK, GAPOR, and triosephosphate isomerase are up-regulated. In cells grown on peptides, an up-regulation of the FBPase-, PGK-, and GAPDH-encoding genes was observed (Fig. 3).
The facultative anaerobic H. volcanii, a moderate halophile, uses a branched ED pathway (12), as found for S. solfataricus, and glucose is finally oxidized to CO2 with either oxygen or nitrate as the terminal electron acceptor. The EMP pathway is active only in the gluconeogenic direction, due to missing PFK. Transcriptome analyses using a onefold coverage shotgun DNA microarray were performed (38), and after transition of the organism from Casamino Acids-based medium to glucose-based medium, the ED genes encoding glucose dehydrogenase, KDG kinase, KD(P)G aldolase, GAPDH-2, and PGK were found to be up-regulated. In contrast, the genes encoding GAPDH-1 as well as FBPA were repressed as a result of the shift in the carbon source (Fig. 3). The differential transcript levels of the genes encoding the two GAPDHs suggest an anabolic (GAPDH-1) and catabolic (GAPDH-2) function as described for the two GAPDHs of Bacillus subtilis (9). Therefore, also in halophiles, the conversion of GAP seems to play an important role in the regulation of carbohydrate metabolism. The repression of the FBPA gene in response to glucose might selectively reduce gluconeogenesis under conditions in which glucose is degraded via the branched ED pathway, thereby indicating an important regulation site at the C6 level in halophiles as well.
From the comparison of CCM regulation in the three hyperthermophiles and one mesophilic halophile, central control points can be depicted (Fig. 3). (i) In the archaea, regulation is executed at the hexosephosphate/triosephosphate transition within the EMP pathway, which is only executed on the transcriptional level and seems to be less conserved. (ii) The main control point with the highest conservation in all archaea studied so far is localized at the level of GAP. It is characterized by different combinations of the three GAP-converting enzymes (GAPDH, GAPN, and GAPOR). In (hyper)thermophiles, regulation of the carbon flux is executed at the gene level as well as at the protein level in a synergistic way. This control point is not common to most members of the Bacteria or Eucarya analyzed so far. (iii) Control at the level of PEP/pyruvate that is common in bacteria and eucarya is also executed by archaea. However, it represents a less conserved site of regulation which is performed at the gene and protein levels.
In general, the current understanding of the archaeal CCM pathways reveals that the utilization of modifications of the classical pathways known from bacteria and eucarya also requires new, alternative modes of regulation (executed at the gene and protein levels), which seem to be established only within the archaeal domain.
Microarray data accession number.
The microarray data have been submitted to the ArrayExpress database (http://www.ebi.ac.uk/arrayexpress) and received the accession number E-MEXP-1376.
Supplementary Material
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft through the grants He1238/16 and SO264/10 (SPP1112).
We thank Christian Lange (Biocenter Niederursel, Goethe-University Frankfurt, Germany) and Günter Raddatz (Max Planck Institute for Biological Cybernetics, Tübingen, Germany) for support in bioinformatics and Christoph Dehio (Biocenter, University of Basel, Basel, Switzerland) for kindly providing PrimeArray software.
Footnotes
Published ahead of print on 4 January 2008.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Ahmed, H., T. J. Ettema, B. Tjaden, A. C. Geerling, J. van der Oost, and B. Siebers. 2005. The semi-phosphorylative Entner-Doudoroff pathway in hyperthermophilic Archaea: a re-evaluation. Biochem. J. 390529-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brock, T. D., K. M. Brock, R. T. Belley, and R. L. Weiss. 1972. Sulfolobus: a new genus of sulphur-oxidizing bacteria living at low pH and high temperature. Arch. Microbiol. 8454-68. [DOI] [PubMed] [Google Scholar]
- 3.Brunner, N. A., H. Brinkmann, B. Siebers, and R. Hensel. 1998. NAD+-dependent glyceraldehyde-3-phosphate dehydrogenase from Thermoproteus tenax: the first identified archaeal member of the aldehyde dehydrogenase superfamily is a glycolytic enzyme with unusual regulatory properties. J. Biol. Chem. 2736149-6156. [DOI] [PubMed] [Google Scholar]
- 4.Brunner, N. A., B. Siebers, and R. Hensel. 2001. Role of two different glyceraldehyde-3-phosphate dehydrogenases in controlling the reversible Embden-Meyerhof-Parnas pathway in Thermoproteus tenax: regulation on protein and transcript level. Extremophiles 5101-109. [DOI] [PubMed] [Google Scholar]
- 5.Diehl, F., S. Grahlmann, M. Beier, and J. D. Hoheisel. 2001. Manufacturing DNA microarrays of high spot homogeneity and reduced background signal. Nucleic Acids Res. 29E38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dörr, C., M. Zaparty, B. Tjaden, H. Brinkmann, and B. Siebers. 2003. The hexokinase of the hyperthermophile Thermoproteus tenax: ATP-dependent hexokinases and ADP-dependent glucokinases, two alternatives for glucose phosphorylation in Archaea. J. Biol. Chem. 27818744-18753. [DOI] [PubMed] [Google Scholar]
- 7.Eisen, M. B., and P. O. Brown. 1999. DNA arrays for the analysis of gene expression. Methods Enzymol. 303179-205. [DOI] [PubMed] [Google Scholar]
- 8.Ettema, T. J. G., H. Ahmed, A. C. M. Geerling, J. van der Oost, and B. Siebers. 2007. The non-phosphorylating glyceraldehyde-3-phosphate dehydrogenase (GAPN) of Sulfolobus solfataricus: a key-enzyme of the semi-phosphorylative branch of the Entner-Doudoroff pathway. Extremophiles [Epub ahead of print.] doi: 10.1007/s00792-007-0082-1. [DOI] [PubMed]
- 9.Fillinger, S., S. Boschi-Muller, S. Azza, E. Dervyn, G. Branlant, and S. Aymerich. 1999. Two glyceraldehyde-3-phosphate dehydrogenases with opposite physiological roles in a nonphotosynthetic bacterium. J. Biol. Chem. 27514031-14037. [DOI] [PubMed] [Google Scholar]
- 10.Fischer, F., W. Zillig, K. O. Stetter, and G. Schreiber. 1983. Chemolithoautotrophic metabolism of anaerobic extremely thermophilic archaebacteria. Nature 301511-513. [DOI] [PubMed] [Google Scholar]
- 11.Imanaka, H., A. Yamatsu, T. Fukui, H. Atomi, and T. Imanaka. 2006. Phosphoenolpyruvate synthase plays an essential role for glycolysis in the modified Embden-Meyerhof pathway in Thermococcus kodakaraensis. Mol. Microbiol. 61898-909. [DOI] [PubMed] [Google Scholar]
- 12.Kehrer, D., H. Ahmed, H. Brinkman, and B. Siebers. 2007. The glycerate kinase of the hyperthermophilic archaeon T. tenax: new insights into phylogenetic distribution of physiological role of members of the three different families. BMC Genomics doi: 10.1186/1471-2164-8-301. [DOI] [PMC free article] [PubMed]
- 13.Kengen, S. W. M., J. E. Tuininga, F. A. De Bok, A. J. Stams, and W. M. De Vos. 1995. Purification and characterization of a novel ADP-dependent glucokinase from the hyperthermophilic archaeon Pyrococcus furiosus. J. Biol. Chem. 27030453-30457. [DOI] [PubMed] [Google Scholar]
- 14.Kim, S., and S. B. Lee. 2006. Catalytic promiscuity in dihydroxy-acid dehydratase from the thermoacidophilic archaeon Sulfolobus solfataricus. J. Biochem. 139591-596. [DOI] [PubMed] [Google Scholar]
- 15.Lamble, H. J., N. I. Heyer, S. D. Bull, D. W. Hough, and M. J. Danson. 2003. Metabolic pathway promiscuity in the archaeon Sulfolobus solfataricus revealed by studies on glucose dehydrogenase and 2-keto-3-deoxygluconate aldolase. J. Biol. Chem. 27834066-34072. [DOI] [PubMed] [Google Scholar]
- 16.Lorentzen, E., R. Hensel, T. Knura, H. Ahmed, and E. Pohl. 2004. Structural basis of allosteric regulation and substrate specificity of the non-phosphorylating glyceraldehyde-3-phosphate dehydrogenase from Thermoproteus tenax. J. Mol. Biol. 341815-828. [DOI] [PubMed] [Google Scholar]
- 17.Mukund, S., and M. W. W. Adams. 1995. Glyceraldehyde-3-phosphate ferredoxin oxidoreductase, a novel tungsten-containing enzyme with a potential glycolytic role in the hyperthermophilic archaeon Pyrococcus furiosus. J. Biol. Chem. 2708389-8392. [DOI] [PubMed] [Google Scholar]
- 18.Musfeld, M., M. Selig, and P. Schönheit. 1999. Acetyl coenzyme A synthetase (ADP-forming) from the hyperthermophilic archaeon Pyrococcus furiosus: identification, cloning, separate expression of the encoding genes, acdAI and acdBI, in Escherichia coli, and in vitro reconstitution of the active heterotetrameric enzyme from its recombinant subunits. J. Bacteriol. 1815885-5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raddatz, G., M. Dehio, T. F. Meyer, and C. Dehio. 2001. PrimeArray: genome-scale primer design for DNA-microarray construction. Bioinformatics 1798-99. [DOI] [PubMed] [Google Scholar]
- 20.Reher, M., S. Gebhard, and P. Schönheit. 2007. Glyceraldehyde-3-phosphate ferredoxin oxidoreductase (GAPOR) and non-phosphorylating glyceraldehyde-3-phosphate dehydrogenase (GAPN), key enzymes of the respective modified Embden-Meyerhof pathways in the hyperthermophilic crenarchaeota Pyrobaculum aerophilum and Aeropyrum pernix. FEMS Microbiol. Lett. 273196-205. [DOI] [PubMed] [Google Scholar]
- 21.Schäfer, T., and P. Schönheit. 1993. Gluconeogenesis from pyruvate in the hyperthermophilic archaeon Pyrococcus furiosus: involvement of reactions of the Embden-Meyerhof-Parnas pathway. Arch. Microbiol. 159354-363. [Google Scholar]
- 22.Schramm, A., B. Siebers, B. Tjaden, H. Brinkmann, and R. Hensel. 2000. Pyruvate kinase of the hyperthermophilic crenarchaeote Thermoproteus tenax: physiological role and phylogenetic aspects. J. Bacteriol. 1822001-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schut, G. J., S. D. Brehm, S. Datta, and M. W. W. Adams. 2003. Whole-genome DNA microarray analysis of a hyperthermophile and an archaeon: Pyrococcus furiosus grown on carbohydrates or peptides. J. Bacteriol. 1853935-3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selig, M., and P. Schönheit. 1994. Oxidation of organic-compounds to CO2 with sulfur or thiosulfate as electron-acceptor in the anaerobic hyperthermophilic archaea Thermoproteus tenax and Pyrobaculum islandicum proceeds via the citric-acid cycle. Arch. Microbiol. 162286-294. [Google Scholar]
- 25.Selig, M., K. B. Xavier, H. Santos, and P. Schönheit. 1997. Comparative analysis of Embden-Meyerhof and Entner-Doudoroff glycolytic pathways in hyperthermophilic Archaea and the bacterium Thermotoga. Arch. Microbiol. 167217-232. [DOI] [PubMed] [Google Scholar]
- 26.Siebers, B., and R. Hensel. 1993. Glucose catabolism of the hyperthermophilic archaeum Thermoproteus tenax. FEMS Microbiol. Lett. 1111-8. [Google Scholar]
- 27.Siebers, B. 1995. Ph.D. thesis. University of Essen, Essen, Germany.
- 28.Siebers, B., V. F. Wendisch, and R. Hensel. 1997. Carbohydrate metabolism in Thermoproteus tenax: in vivo utilization of the non-phosphorylative Entner-Doudoroff pathway and characterization of its first enzyme, glucose dehydrogenase. Arch. Microbiol. 168120-127. [DOI] [PubMed] [Google Scholar]
- 29.Siebers, B., H. P. Klenk, and R. Hensel. 1998. PPi-dependent phosphofructokinase from Thermoproteus tenax, an archaeal descendant of an ancient line in phosphofructokinase evolution. J. Bacteriol. 1802137-2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siebers, B., B. Tjaden, K. Michalke, C. Dörr, H. Ahmed, M. Zaparty, P. Gordon, C. Sensen, A. Zibat, H. P. Klenk, S. C. Schuster, and R. Hensel. 2004. Reconstruction of the central carbohydrate metabolism of Thermoproteus tenax by use of genomic and biochemical data. J. Bacteriol. 1862179-2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siebers, B., and P. Schönheit. 2005. Unusual pathways and enzymes of central carbohydrate metabolism. Curr. Opin. Microbiol. 8695-705. [DOI] [PubMed] [Google Scholar]
- 32.Snijders, A. P. L., J. Walther, S. Peter, I. Kinnman, M. G. J. de Vos, H. J. G. van de Werken, S. J. J. Brouns, J. van der Oost, and P. C. Wright. 2006. Reconstruction of central carbon metabolism in Sulfolobus solafataricus using a two-dimensional gel electrophoresis map, stable isotope labelling and DNA microarray analysis. Proteomics 61518-1529. [DOI] [PubMed] [Google Scholar]
- 33.Tjaden, B., A. Plagens, C. Dörr, B. Siebers, and R. Hensel. 2006. Phosphoenolpyruvate synthetase and pyruvate phosphate dikinase of Thermoproteus tenax: key pieces in the puzzle of archaeal carbohydrate metabolism. Mol. Microbiol. 60287-298. [DOI] [PubMed] [Google Scholar]
- 34.Tuininga, J. E., C. H. Verhees, J. van der Oost, S. W. Kengen, A. J. Stams, and W. M. de Vos. 1999. Molecular and biochemical characterization of the ADP-dependent phosphofructokinase from the hyperthermophilic archaeon Pyrococcus furiosus. J. Biol. Chem. 27421023-21028. [DOI] [PubMed] [Google Scholar]
- 35.Van der Oost, J., G. Schut, S. W. M. Kengen, W. R. Hagen, M. Thomm, and W. M. de Vos. 1998. The ferredoxin-dependent conversion of glyceraldehyde-3-phosphate in the hyperthermophilic archaeon Pyrococcus furiosus represents a novel site of glycolytic regulation. J. Biol. Chem. 27328149-28154. [DOI] [PubMed] [Google Scholar]
- 36.Van der Oost, J., and B. Siebers. 2007. The glycolytic pathways of Archaea: evolution by tinkering, p. 247-260. In R. A. Garrett and H.-P. Klenk (ed.), Archaea: evolution, physiology and molecular biology. 1st ed. Blackwell Publishing, Malden, MA.
- 37.Verhees, C. H., M. Huynen, D. Ward, E. Schiltz, W. M. de Vos, and J. van der Oost. 2001. The phosphoglucose isomerase from the hyperthermophilic archaeon Pyrococcus furiosus is a unique enzyme that belongs to the cupin superfamily. J. Biol. Chem. 27640926-40932. [DOI] [PubMed] [Google Scholar]
- 38.Zaigler, A., S. Schuster, and J. Soppa. 2003. Construction and usage of a one fold-coverage shotgun DNA microarray to characterise the metabolism of the archaeon Haloferax volcanii. Mol. Microbiol. 481089-1105. [DOI] [PubMed] [Google Scholar]
- 39.Zillig, W., K. O. Stetter, W. Schäfer, D. Janekovic, S. Wunderl, I. Holz, and P. Palm. 1981. Thermoproteales: a novel type of extremely thermoacidophilic anaerobic archaebacteria isolated from Icelandic solfatares. Zentralbl. Bakteriol. Hyg. Abt. 1 Org. C 2205-227. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.