Abstract
The Bacillus subtilis anti-TRAP protein regulates the ability of the tryptophan-activated TRAP protein to bind to trp operon leader RNA and promote transcription termination. AT synthesis is regulated both transcriptionally and translationally by uncharged tRNATrp. In this study, we examined the roles of AT synthesis and tRNATrp charging in mediating physiological responses to tryptophan starvation. Adding excess phenylalanine to wild-type cultures reduced the charged tRNATrp level from 80% to 40%; the charged level decreased further, to 25%, in an AT-deficient mutant. Adding tryptophan with phenylalanine increased the charged tRNATrp level, implying that phenylalanine, when added alone, reduces the availability of tryptophan for tRNATrp charging. Changes in the charged tRNATrp level observed during growth with added phenylalanine were associated with increased transcription of the genes of tryptophan metabolism. Nutritional shift experiments, from a medium containing tryptophan to a medium with phenylalanine and tyrosine, showed that wild-type cultures gradually reduced their charged tRNATrp level. When this shift was performed with an AT-deficient mutant, the charged tRNATrp level decreased even further. Growth rates for wild-type and mutant strains deficient in AT or TRAP or that overproduce AT were compared in various media. A lack of TRAP or overproduction of AT resulted in phenylalanine being required for growth. These findings reveal the importance of AT in maintaining a balance between the synthesis of tryptophan versus the synthesis of phenylalanine, with the level of charged tRNATrp acting as the crucial signal regulating AT production.
In Bacillus subtilis, free l-tryptophan (Trp) and charged and uncharged tRNATrp are sensed as regulatory signals in modulating the expression of the genes of Trp biosynthesis (14, 18). The expression of seven genes is required for Trp synthesis. Six of these are arranged in the trpEDCFBA suboperon (trp operon), a segment of the aromatic amino acid supraoperon (13). The seventh trp gene, trpG (pabA), is within the unlinked folate operon (13, 14). Figure 1 summarizes the regulatory signals, proteins, and processes currently known to influence trp operon transcription in this organism. The expression of all seven trp genes is regulated by TRAP (the Trp-activated RNA-binding attenuation protein) in response to the intracellular concentration of Trp (13, 14, 37). When the Trp concentration is high, TRAP is activated, and it binds to trp operon leader RNA, promoting transcription termination (5, 27). TRAP binds to a transcript segment containing 11 (G/U)AG trinucleotide repeats; these repeats are separated by 2 to 3 nonconserved nucleotides (2, 3). Six of these repeats are located within an RNA antiterminator structure. Thus, when TRAP is activated, it binds to these RNA repeats and prevents the formation of the antiterminator structure. A 5′ stem-loop structure located upstream of the triplet repeat region also interacts with TRAP, increasing the affinity of TRAP for the nascent trp leader transcript during the early stages of transcription (23). When TRAP is bound, the overlapping terminator structure therefore forms, promoting transcription termination in the trp operon leader region (4). Activated TRAP also inhibits translation initiation on the transcripts of four coding regions: trpE, in the trp operon (10, 24); trpG (pabA), in the folate operon (11, 37); trpP (yhaG), a gene believed to encode a Trp transport protein (29); and ycbK, a gene in the at operon that encodes a putative efflux protein (38, 39).
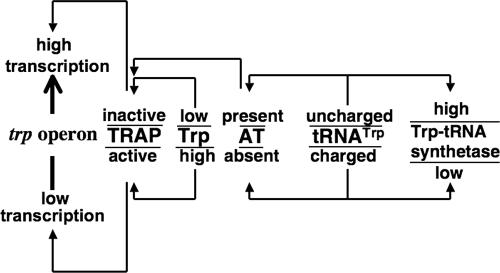
FIG. 1.
Signal molecules, regulatory proteins, and events influencing trp operon transcription in B. subtilis. The molecules presently known to participate in regulating the transcription of the trp operon are shown. The two signal molecules that play a regulatory role are Trp and tRNATrp. The two responding regulatory proteins are TRAP and AT. When Trp is plentiful, it activates the TRAP protein, which binds to trp operon leader RNA, promoting transcription termination. When cells are deficient in Trp, TRAP is inactive and transcription of the suboperon can proceed. When cells are deficient in charged tRNATrp, the AT protein is synthesized. AT binds to Trp-activated TRAP, reducing or preventing TRAP function. In addition, when there is a charged-tRNATrp deficiency, the synthesis of tryptophanyl-tRNA synthetase increases, improving the rate of tRNATrp charging.
Charged tRNATrp levels also influence the action of the TRAP protein of B. subtilis. Studies with a temperature-sensitive tryptophanyl-tRNA synthetase mutant, trpS1, that is defective in tRNATrp charging at elevated temperatures revealed that a reduction of the charged tRNATrp level leads to increased trp operon expression and Trp biosynthesis (30); this increase occurs despite the presence of Trp-activated TRAP (40). The gene responsible for sensing charged tRNATrp levels and mediating the increase in trp operon expression was identified as rtpA of the at operon (29, 33). The product of rtpA, a protein designated anti-TRAP (AT), can bind to Trp-activated TRAP (33). When AT is bound to Trp-activated TRAP, it reduces or prevents TRAP's ability to bind to its target RNAs (32, 33). Synthesis of the AT protein is regulated by transcriptional and translational regulatory processes, both designed to sense changes in the fraction of tRNATrp that is charged or uncharged (8, 9). The transcription of the structural gene region of the at operon is regulated by the T-box mechanism (17, 29). Uncharged tRNATrp is believed to pair with the at operon's T-box leader RNA and prevent the formation of a transcription terminator, thereby allowing the continuation of at operon transcription (17, 29). The leader region of the at operon also contains a 10-residue leader peptide-coding region, designated rtpLP, containing three consecutive Trp codons. The translation of these three Trp codons is believed to provide a second, translational, opportunity for the at operon to sense and respond to changes in the level of charged tRNATrp (9). The completion of the translation of this coding region inhibits AT synthesis, whereas incomplete translation, induced by the reduced availability of charged tRNATrp, increases AT production (9). Additionally, uncharged tRNATrp accumulation regulates the transcription of the trpS operon, encoding tryptophanyl-tRNATrp synthetase, the enzyme that charges Trp onto tRNATrp. Computational analyses have predicted that the trpS operon of B. subtilis contains a T-box element that is regulated by sensing uncharged tRNATrp (25). Microarray analyses performed with B. subtilis confirmed that trpS transcription is affected by the level of charged tRNATrp (6). The expression of rtpA (encoding AT) has also been shown to be dependent upon the ratio of uncharged versus charged tRNATrp (6). However, unlike the results of previous studies on trpS expression (31), it was not known how AT synthesis influences the level of charged tRNATrp or how it affects cell physiology.
In a recent survey of completely sequenced genomes of gram-positive bacteria (16), it was revealed that only five species in addition to B. subtilis have a trp operon within an aro supraoperon. Of these, only B. subtilis and Bacillus licheniformis appear to have an at operon, presumably producing an AT protein. Most of the gram-positive species with sequenced genomes have an intact, discrete trp operon, containing trpG, regulated by a T box or tandem T boxes, each presumably responding to uncharged tRNATrp. In view of the different regulatory mechanisms known to influence trp operon expression in gram-positive bacteria, it was important to provide a more-thorough understanding of the physiological role of the AT protein and the influence of tRNATrp charging on trp operon expression in B. subtilis.
In this report, we describe the relationships between rtpA expression, the cellular levels of charged and uncharged tRNATrp, and the expression of the genes of Trp metabolism. We also examine and compare trp operon and at operon transcription in various mutants grown under different conditions. We also determine the levels of charged and uncharged tRNATrp in the wild type and in these mutants under these different growth conditions. The crucial strains examined were the wild type; mutant strains lacking mtrB or rtpA, the structural genes for TRAP and AT, respectively; and an additional strain, designated ↑AT, that has a mutated at operon leader region. This alteration eliminates the need for uncharged tRNATrp recognition in maximizing AT production (40). Cell growth-rate analyses revealed the physiological consequences of differences in gene expression, some of which resulted in phenylalanine (Phe) being required for growth.
MATERIALS AND METHODS
Bacterial strains used and determination of growth curves.
The strains examined in this study are listed in Table 1. Strain CYBS318 [Δ(rtpA-ycbK)::Spr] lacks the coding region for the AT protein and the 5′ end of the ycbK open reading frame; it does not produce either the AT or the YcbK protein. This strain was constructed by replacing the 614-bp chromosomal rtpA-ycbK region with a spectinomycin resistance gene (29). Strain CYBS223 (mtrBΩTcr) has a tetracycline resistance gene inserted into the mtrB coding region; this strain lacks the ability to synthesize a functional TRAP protein (24). Strain CYBS542 (↑AT) has the normal chromosomal rtpA-ycbK segment of the at operon replaced by a sparsomycin resistance gene; it also contains an inserted, modified at operon with a deletion removing much of its regulatory leader region preceding rtpA, followed by a ycbK-lacZ fusion; this strain overproduces the AT protein (↑AT). This modified operon was integrated into the chromosomal amyE locus (9). Strain BS166 trpE26 contains a trpE-inactivating mutation. This mutant was isolated following X-ray irradiation of B. subtilis spores (7). To prepare strain BS166 trpE26 ΔmtrB, cells of BS166 trpE26 were transformed with chromosomal DNA from strain CYBS223, a strain containing the tetracycline resistance gene inserted in the mtrB coding region. Transformants were selected on plates containing 10 μg/ml tetracycline.
TABLE 1.
Strains of B. subtilis used in this study
| Strain | Genotypea | Phenotype | Source |
|---|---|---|---|
| CYBS400 | Wild type | Control; prototroph | Our stock |
| CYBS318 | CYBS400 Δ(rtpA-ycbK)::Spr | No AT, no YcbK | 29 |
| CYBS223 | CYBS400 mtrBΩTcr | Phenylalanine bradytroph, no TRAP | 24 |
| CYBS542 | CYBS400 amyE::[P-ΔLR(ΔT) Δ(S/D and AUG for rtpLP-ΔrtpLP ycbK′-′lacZ] Cmr | Phenylalanine bradytroph, overproduction of AT, no YcbK | 8 |
| BS166 trpE26 | trpE26 | Tryptophan auxotroph | 7 |
| BS166 trpE26 ΔmtrB | BS166 trpE26 mtrBΩTcr | Tryptophan auxotroph, no TRAP | This work |
S/D, Shine-Dalgarno sequence; AUG, start codon.
The growth curves for each strain examined were determined by measuring the cell density in cultures grown in Vogel-Bonner minimal medium (35) supplemented with 0.5% glucose, trace elements (6), and other compounds, as indicated in the figures, at 37°C. The growth rates were determined by measuring cell density using a Klett-Summerson colorimeter with a 660-nm filter.
Determining charged and uncharged tRNATrp levels.
Total tRNA was extracted under acidic conditions as described previously (34). Twenty-milliliter cultures were grown under the conditions indicated in the figures to a density of 70 to 180 Klett units. Growth was stopped by adding 0.01% sodium azide, and the cultures were chilled by placing them in an ice-water bath. To measure accurately the fraction of tRNATrp that was charged in vivo, it was essential to stop bacterial growth very rapidly, under acidic conditions, as the pool of charged tRNA in the cell is low, and it turns over rapidly (12). After chilled cells were harvested by centrifugation, they were immediately resuspended in 300 μl of 0.3 M sodium acetate solution containing 10 mM EDTA at pH 5.2. Total tRNA was extracted by mixing the final suspension with the same volume of acidic phenol (pH 5.2) and sonicating the suspension. After separation of the phenol phase by centrifugation, the tRNA in the aqueous solution was precipitated by the addition of 2 volumes of ethanol. Equivalent amounts of total tRNA were run on a 6.5% acidic urea gel to separate uncharged tRNATrp from charged tRNATrp. Northern blotting was performed by using a 5′-end 32P-labeled detection oligonucleotide (5′-TCG AAC CCA CAC CGG AGG TTT TGG-3′). An aliquot of total tRNA from one preparation was deacylated by alkaline hydrolysis and run as a position marker for uncharged tRNA (not shown).
Quantitation of relative mRNA levels using real-time RT-PCR.
Cultures were grown to a density of 150 Klett units, and the cells in 10 ml were harvested by centrifugation. Cells were lysed by being resuspended in 500 μl of a solution containing 100 mM Tris-HCl, pH 7.5, 1 mM EDTA, and 0.1% sodium dodecyl sulfate and were incubated with 10 μg/ml proteinase K at 37°C for 20 min. Total RNA was extracted using phenol and precipitated with ethanol. To degrade contaminating DNA, the RNA extract was treated by adding DNase. The RNA was extracted again using phenol. cDNA synthesis was carried out using 1 μg of starting material (purified RNA) with a Bio-Rad kit. One-tenth of the cDNA total reaction mixture was used for PCR analyses. Gene-specific primers were designed to amplify 100- to 200-nucleotide fragments of target genes (Table 2). Each reaction was carried out in a 20-μl (total) volume containing 50% (vol/vol) Sybr green PCR master mixture (MJ Research), 20 pmol of each of the two primers, and 6 μl of each cDNA sample (1/100 dilution of cDNA mentioned above). PCR amplification was carried out using an ABI 7700 Thermocycler (PE Applied Biosystems) and the following thermal cycling conditions: 95°C for 3 min, followed by 40 cycles at 95°C for 15 s, 52°C for 15 s, and 72°C for 20 s. The relative mRNA levels for the genes analyzed were calculated using the 2−ΔΔCT threshold cycle method (22). rpoB gene expression was used as an internal control because it is considered an excellent chromosomal marker for real-time reverse transcription-PCR (RT-PCR) (28).
TABLE 2.
Specific deoxyoligonucleotide primers used in analyzing the expression of the genes studied
| Gene | Deoxyoligonucleotide sequence, 5′→3′ (upstream; downstream) |
|---|---|
| mtrB | AGC ATT CAA GTG ATT TTG TC; TGA GCG ATG ATC ACT TCT CC |
| trpE | GTT TGC CGG ACA TTA ATT GC; AAT GAG ATT TTG AAG CTC C |
| rtpA | ATG GTC ATT GCA ACT GAT G; TCA GAA TAA CAC CTT TTC CG |
| ycbK | GCT GAG CCA GAT GCC GTG CTG C; AAC ACT CCC GCG CCA CAA GCC |
| trpG | CAC GGG AAA ACC TCG GAT ATC; GTG GCG AAT AGC CAT GAT TTC |
| trpS | AAG AGT TAG TCA TTA TGG C; CGC GGG AAC AGC AGA ATG CCC |
| rpoB | CGT GTT ATC GTT TCC CAG C (28); GTG TGC GAT CAA TGC GGA C |
RESULTS
Charged tRNATrp levels in mutant strains lacking TRAP or AT or overproducing AT under different growth conditions.
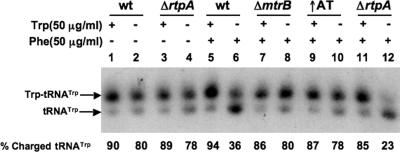
It has been shown that the level of charged tRNATrp in B. subtilis cultures is associated with differences in the expression of the genes of Trp metabolism (29). Changes in the level of charged tRNATrp affect the synthesis of both the AT protein (9, 29, 33) and tryptophanyl-tRNA synthetase (6); they also influence trp operon expression (9, 29). Despite these observations, it was not known how AT activity affects the cellular level of charged tRNATrp or how AT activity influences general cell physiology. To address these questions, we initially measured the levels of charged tRNATrp in the wild-type and mutant cultures by using Northern blot analyses (see Materials and Methods). The four B. subtilis strains examined were a wild-type strain; a mutant strain with a deletion removing the mtrB gene (encoding TRAP) and requiring phenylalanine for growth (20, 33); a mutant strain with a deletion of rtpA and lacking AT; and a mutant strain with a deletion in the at operon leader region that overproduces the AT protein (↑AT mutant). The latter strain also requires phenylalanine for growth (see below). To determine the effects of these mutations on tRNATrp charging, we measured the charged tRNATrp levels in mutant and wild-type cultures grown with different supplements. This was done because the ΔmtrB and ↑AT strains require phenylalanine for growth (see below). The results of these analyses are presented in Fig. 2. When the wild-type or the ΔrtpA culture was grown in minimal medium, we observed that the level of charged tRNATrp was approximately 80% (Fig. 2, lanes 2 and 4); it remained slightly higher, ca. 90%, in the presence of Trp (Fig. 2, lanes 1 and 3). Thus, deleting the rtpA gene did not affect the tRNATrp charging level significantly under these growth conditions (Fig. 2, compare lanes 1 and 2 with 3 and 4). During growth in minimal medium containing Phe, the level of charged tRNATrp was considerably lower in the wild-type strain (36%) and even lower in the ΔrtpA culture (23%) (Fig. 2, lanes 6 and 12). This low level of charged tRNATrp was reversed upon the addition of Trp to the medium (Fig. 2, compare lanes 6 and 12 with lanes 5 and 11, respectively). Thus, these data suggest that the presence of Phe in the growth medium reduces the concentration of Trp in the cell, affecting the charging of tRNATrp. These findings further indicate that AT is needed for maintaining a higher level of charged tRNATrp when cells are shifted to medium containing Phe and lacking Trp. Interestingly, we observed that the charged tRNATrp levels for the ΔmtrB and ↑AT strains were around 80 to 85% in growth medium with or without added Trp (Fig. 2, lanes 7 to 10). It is evident that the absence of TRAP activity, as well as growth with added Trp, reversed the reduction in the charged tRNATrp level resulting from the presence of high concentrations of Phe (Fig. 2, lanes 6 and 12).
FIG. 2.
Analysis of the tRNATrp charging levels in different strains grown under restricted conditions. Cultures of B. subtilis strains CYBS400 (wild type), CYBS223 (ΔmtrB), CYBS542 (↑AT), and CYBS318 (ΔrtpA) were grown in Vogel-Bonner minimal medium supplemented with 0.5% glucose and trace elements at 37°C. One hundred micrograms/ml Trp and/or 50 μg/ml Phe were added to selected cultures. Northern blot assays were performed using gel electrophoresis and a 32P-labeled deoxyoligonucleotide complementary to the tRNATrp sequence (see Materials and Methods). The percent charged tRNATrp was calculated by dividing the amount of charged tRNATrp by the sum of the amounts of charged and uncharged tRNATrp. wt, wild type.
Real-time RT-PCR measurements of mRNA levels for genes of Trp metabolism in TRAP and AT mutant strains under different growth conditions.
An appreciable reduction in the level of charged tRNATrp was observed in ΔrtpA cells when they were grown in minimal medium with Phe; this reduction was slightly greater than in the wild-type strain (Fig. 2). To determine if the additional reduction in the charged tRNATrp level observed in the ΔrtpA strain was correlated with increased transcription of the genes of Trp metabolism, their transcript levels were measured in several strains using real-time RT-PCR (Table 3; also see Materials and Methods). The relative mRNA concentrations determined by this method reflect changes in transcription and/or mRNA decay; most of the genes studied are transcriptionally regulated (see above or introduction). The genes analyzed in Table 3 were rtpA and ycbK, which are adjacent genes residing in the at operon (29); trpE, the first gene of the trp operon (14); trpG, located within the folate operon (41); trpS, the gene encoding tryptophanyl-tRNA synthetase, the enzyme that charges Trp onto tRNATrp (31); and mtrB, the structural gene for the TRAP protein (14).
TABLE 3.
Relative real-time RT-PCR gene expression (mRNA) levels for different strains grown with or without Phe or Trp
| Row | Strain and culture conditionsa | Expression level of gene (Trp residues/totalb)
|
% Trp-tRNATrpe | |||||
|---|---|---|---|---|---|---|---|---|
| rtpA (0/53) | ycbK (5/312) | trpE (2/515) | trpG (0/194) | trpS (1/330) | mtrB (0/75) | |||
| −Phe | ||||||||
| Wild type | ||||||||
| 1 | −Trp | 1c | 1 | 1 | 1 | 1 | 1 | 85 |
| 2 | +Trp | 1 | 1 | 1 | 1 | 1 | 0.9 | 88 |
| ΔrtpA | ||||||||
| 3 | −Trp | NAd | NA | 1 | 1 | 1 | 0.9 | 80 |
| 4 | +Trp | NA | NA | 1 | 1 | 1 | 0.8 | 87 |
| +Phe | ||||||||
| Wild type | ||||||||
| 5 | −Trp | 2.5 | 2.5 | 10 | 1 | 2 | 1 | 47 |
| 6 | +Trp | 1 | 1.5 | 2 | 0.7 | 0.6 | 0.7 | 89 |
| ΔrtpA | ||||||||
| 7 | −Trp | NA | NA | 5 | 0.4 | 2.8 | 0.8 | 28 |
| 8 | +Trp | NA | NA | 2 | 0.5 | 0.4 | 0.8 | 89 |
| ΔmtrB | ||||||||
| 9 | −Trp | 0.5 | 2.3 | 110 | 2 | 0.4 | NA | 87 |
| 10 | +Trp | 0.5 | 1.5 | 90 | 2 | 0.4 | NA | 92 |
| ↑AT | ||||||||
| 11 | −Trp | 5 | NA | 120 | 2 | 0.4 | 0.7 | 88 |
| 12 | +Trp | 3.8 | NA | 70 | 2 | 0.4 | 0.7 | 94 |
RNA was extracted from cultures grown in minimal medium with the supplements indicated (see Materials and Methods). +, present; −, absent.
Number of Trp residues relative to the total number of residues in each polypeptide.
As a reference, we used the relative mRNA concentration calculated from wild-type cultures grown in the presence of Phe (see Materials and Methods). trpP, a Trp transporter (27) was not included in these analyses. Three parallel experiments were performed, and the averaged results are presented. The values obtained in the repeated analyses varied by less than 15 percent.
NA, not applicable because these genes have been deleted from the chromosome of the corresponding strain.
The percent Trp-tRNATrp corresponds to the amount of Trp-charged tRNATrp in an extract divided by the total amount of tRNATrp. The amounts of charged and uncharged tRNATrp in each sample were determined by using Northern blot analyses (see Materials and Methods).
As a general reference in calculating the relative mRNA levels, the level of each mRNA from each culture was related to the value calculated for the wild-type culture grown in minimal medium without added amino acids; this reference value was set at 1 for each mRNA (Table 3, row 1). Using the same cultures, the levels of charged tRNATrp, as well as the mRNA level for each gene, were measured. As also shown in Fig. 2, no appreciable differences in the charged tRNATrp levels were observed when wild-type and ΔrtpA strains were grown in the presence or absence of Trp (Table 3, seventh column, compare rows 1 through 4). The expression of the trp genes was analyzed in wild-type and ΔrtpA cultures grown in the absence or presence of Trp (Table 3, rows 1 through 4). In addition to their charged tRNATrp levels, no appreciable differences in transcript abundance were observed in these strains under any of these growth conditions (Table 3, first to sixth columns, compare rows 1 through 4). Since the addition of Trp under these growth conditions did not reduce the trp gene transcript levels, we assume that there was sufficient Trp synthesized by the bacterium to totally charge its tRNATrp, leading to the appropriate regulatory response.
When Phe was added to wild-type cultures, the levels of charged tRNATrp were reduced (Table 3, seventh column, compare row 5 with row 1; also see Fig. 2). This reduction in the charged tRNATrp level was accompanied by increases in the transcript levels for rtpA (2.5-fold), ycbK (2.5-fold), trpS (2-fold), and trpE (a 10-fold increase) mRNA (Table 3, first to fifth columns, compare row 1 with row 5). When Trp was added, a 2.5-fold reduction was observed in the rtpA mRNA level in the wild-type strain (Table 3, first column, compare row 5 with row 6), and the trpE and trpS mRNA levels were reduced 5-fold and 3-fold, respectively, in the same cultures (Table 3, third and fifth columns, compare row 5 with row 6). Intermediate reductions in ycbK (1.7-fold), trpG (1.4-fold), and mtrB (1.4-fold) mRNA were also observed (Table 3, second, fourth, and sixth columns, compare row 5 with row 6). These results indicate that the presence of Phe in the growth medium reduces the cell's ability to produce Trp, increasing the need for overexpression of the genes responsible for Trp synthesis.
The relative mRNA levels were also calculated for the ΔrtpA strain grown in the presence of Phe with and without Trp (Table 3: rows 7 and 8). In the cultures grown with Phe and without Trp, the trpE and trpG mRNA levels were half the levels observed in the wild-type cultures (Table 3, third or fourth column, compare rows 5 and 7). In the absence of added Trp, there was a slight increase in the trpS mRNA level (Table 3, fifth column, compare rows 5 and 7), and mtrB gene transcription did not change (Table 3, sixth column, compare rows 5 and 7). These differences in mRNA levels were associated with an almost-twofold difference in the charged tRNATrp level between the wild-type culture (47%) and the ΔrtpA culture (28%). Insignificant differences in transcript levels for these genes were observed between wild-type and ΔrtpA cultures when they were both grown in minimal medium plus Phe and Trp (Table 3, all columns, compare row 6 with row 8). These findings indicate that the presence of the rtpA gene leads to a twofold increase in trpE and trpG transcript levels in cultures grown with Phe in the absence of added Trp. Therefore, AT's partial inactivation of TRAP must compensate somewhat for the reduced Trp biosynthesis resulting from the added Phe, leading to a slight increase in Trp synthesis and an increase in the level of charged tRNATrp.
The transcript levels for genes of Trp metabolism were also examined in the ΔmtrB and ↑AT strains (Table 3, rows 9 to 12). Both strains require the addition of Phe for growth (see below). The transcript level for rtpA in ΔmtrB cells was reduced relative to its level in wild-type cells; this reduction was most apparent in the absence of Trp (Table 3, first column, compare rows 5 and 6 with rows 9 and 10, respectively). Interestingly, in both the ΔmtrB strain and the ↑AT strain, the transcription of trpE mRNA in the absence of added Trp was increased substantially, ca. 12-fold, relative to the increase in the wild-type strain (Table 3, third column, compare row 5 with rows 9 and 11) and ca. 40-fold in the presence of Trp (Table 3, third column, compare row 6 with rows 10 and 12). Not only did the trpE mRNA level increase, but the level of trpG mRNA also increased, to double the wild-type levels, in both mutant strains (Table 3, fourth column, compare row 5 with rows 9 and 11 and row 6 with rows 10 and 12). However, the level of trpS mRNA was reduced to one-fifth in both strains in the absence of added Trp (Table 3, fifth column, rows 9 and 11). This reduction in trpS transcript levels was more tempered in the presence of Trp (Table 3, fifth column, rows 10 and 12). The level of charged tRNATrp in strains ΔmtrB and ↑AT was maintained at around 90% under all conditions tested, despite the presence of Phe (Table 3, see seventh column). These findings reveal that, in the absence of TRAP or with reduced TRAP activity caused by elevated AT levels, the concentration of Trp in the cell appears to be elevated, allowing the production of sufficient charged tRNATrp in the absence of an external source of Trp. As a consequence of tRNATrp charging, the transcription of the trpS gene is invariably reduced (6, 25).
In summary, the data in Table 3 demonstrate that (i) the presence of Phe in the growth medium reduces the level of charged tRNATrp produced, resulting in an increase in the level of trp operon mRNA, an increase that was reduced when the rtpA gene was removed; and (ii) the reduction in TRAP activity resulting from the overproduction of the AT protein led to an increase in the level of trp operon mRNA, as well as the level of charged tRNATrp.
Analysis of the kinetics of charged tRNATrp depletion following a shift from a Trp-rich medium to a medium with Phe and Tyr.
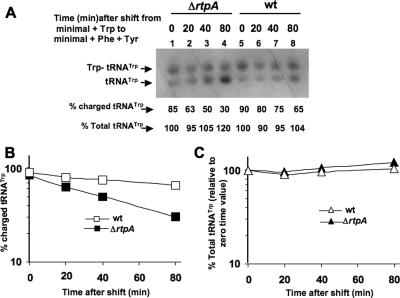
It was previously shown in nutritional shift experiments that switching cells to a medium lacking Trp and containing excess Phe and tyrosine (Tyr) partially starved the cells of Trp, leading to increases in the levels of both AT and anthranilate synthase (35). The extent of the increase in anthranilate synthase activity (TrpE and TrpG polypeptides) was influenced by the presence of the AT protein (40). As shown in Fig. 2, the level of charged tRNATrp in the cell is also influenced by the presence of Phe in the growth medium. These observations suggest that AT synthesis and function may play a role in maintaining a higher cellular level of charged tRNATrp when Trp synthesis is reduced. On the basis of these considerations, nutritional shift experiments were performed and the effects on tRNATrp charging were determined at different times following a shift from a medium with Trp to a medium with Phe and Tyr (Fig. 3). It can be seen that, with wild-type cells, this shift led to a moderate reduction in the charged tRNATrp level; there was no effect on the total level of tRNATrp during the period examined (Fig. 3A, lanes 5 to 8). When the ΔrtpA mutant was subjected to the same shift, there was a greater reduction in the charged tRNATrp level (Fig. 3A, lanes 1 to 4). After the shift, the rate of reduction of the charged tRNATrp level in the ΔrtpA mutant was twofold (40.5% per hour) faster than the rate of reduction (20.1% per hour) in the wild-type culture (Fig. 3B). Since the addition of Phe to minimal medium reduces the synthesis of chorismate, the precursor of Phe, Tyr, and Trp (26), the concentration of Trp in the cell, as well as the level of charged tRNATrp, must be temporarily reduced by the presence of Phe and Tyr. The results shown in Fig. 3 suggest that, following a shift to a Phe- and Tyr-containing medium, the AT protein is essential for maintaining a higher level of Trp in the cell. These data are consistent with the data presented in Table 3 in which the presence of Phe without Trp resulted in elevated trpE transcript levels (Table 3, third column, rows 1 and 5). Presumably the AT protein partially inactivates some of the TRAP molecules present, thereby leading to increased Trp synthesis and, hence, charging of tRNATrp. Significant changes in the total tRNATrp level were not observed for either of the strains examined (Fig. 3C).
FIG. 3.
Analyses of the levels of charged and total tRNATrp following a growth shift from minimal medium plus Trp to minimal medium plus Phe and Tyr. (A) Cells of the wild-type strain (CYBS400) or the ΔrtpA mutant (CYBS318) grown to 120 Klett units in Vogel-Bonner minimal medium with 0.5% glucose, trace elements, and 100 μg/ml Trp at 37°C were harvested, washed with minimal medium, and then shifted to minimal medium with 0.5% glucose, trace elements, and 100 μg/ml Phe plus 100 μg/ml Tyr at 37°C. Following the shift, samples were taken at the times indicated and were assayed. Northern blot analyses were performed, and the percent charged tRNATrp was calculated as described in the legend to Fig. 2. The percent total tRNATrp was calculated by dividing the sum of the charged and uncharged tRNATrp bands for each lane relative to the level obtained in the sample from time 0. (B) Plot of the changes in the two cultures in percent charged tRNATrp over time. (C) Plot of changes in the percent total tRNATrp over time. wt, wild type.
Growing mutant strains under different growth conditions.
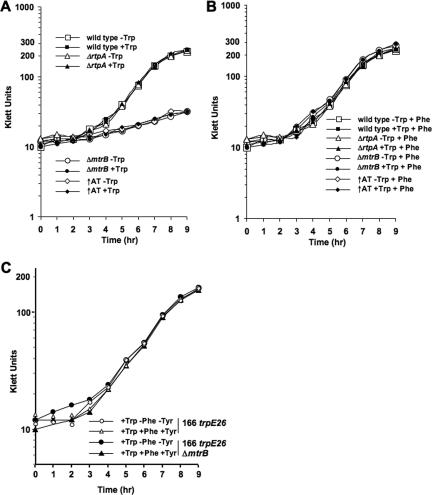
Since AT action affects tryptophan-activated TRAP function (33) and the presence of Phe affects the level of charged tRNATrp (Fig. 2), we examined the growth characteristics of strains with and without (ΔrtpA) AT in the presence and absence of Trp and/or Phe (Fig. 4). Despite the differences observed in the levels of charged tRNATrp (Fig. 2), it can be seen in the results shown in Fig. 4A and B that the growth of the ΔrtpA mutant strain was identical to that of the wild type under any conditions tested, in minimal medium or in this medium plus Trp or Phe or both Trp and Phe (Fig. 4A and B). Regardless of the reduction of the level of charged tRNAtrp induced by the presence of Phe or the absence of the rtpA gene (Fig. 2), the results shown in Fig. 4 suggest that the level of charged tRNATrp observed under these conditions was sufficient for the cells to maintain their growth rates.
FIG. 4.
Growth of various strains under specific culture conditions. Cultures of B. subtilis strains CYBS400 (wild type), CYBS223 (ΔmtrB), CYBS542 (↑AT), and CYBS318 (ΔrtpA) were grown in Vogel-Bonner minimal medium with the various supplements indicated plus 0.5% glucose and trace elements at 37°C. Cell density was determined hourly by using a Klett-Summerson colorimeter. Klett units were plotted versus time to obtain the growth curves shown. (A) Growth with or without 100 μg/ml Trp. (B) Growth with 50 μg/ml Phe with or without 100 μg/ml Trp. (C) The mtrB gene was deleted from the genome of the B. subtilis strain BS166 trpE26. Growth rates were determined for cultures of strains BS166 trpE26 and BS166 trpE26 ΔmtrB, as shown in panel A. Cultures were grown with the supplements indicated in the figure: 100 μg/ml Trp with or without 50 μg/ml Phe and 50 μg/ml Tyr.
Mutants lacking TRAP are known to require Phe for growth; their trp operon is overexpressed, and they secrete appreciable amounts of tryptophan (20, 33). TRAP mutants presumably use the available chorismate for Trp synthesis, reducing its availability for Phe and Tyr synthesis. To test this interpretation, the ΔmtrB and ↑AT strains were also compared with the wild type in the presence and absence of Trp and/or Phe (Fig. 4). Unlike the results for the wild-type strain, in the absence of Phe, a reduced growth rate was observed for strains containing the ΔmtrB or the ↑AT alteration (Fig. 4A). However, the addition of Phe to cultures containing strains with the ΔmtrB mutation and ↑AT strains resulted in faster growth, as it did with the wild-type strain (Fig. 4B). We did not observe a significant increase in the growth rate for the ΔmtrB and ↑AT strains when Tyr was added to the cultures instead of Phe (not shown). The loss or inactivation of TRAP might be expected to lead to depletion of the chorismate available for Phe synthesis, since it would be preferentially used for Trp synthesis by the action of anthranilate synthase activity (TrpE and TrpG polypeptides). To test this interpretation, we introduced a trpE26 mutation into the ΔmtrB strain. This double mutant required Trp for growth but was able to grow in the absence of Phe (Fig. 4C). Thus, the ΔmtrB deletion strain's requirement of Phe for growth must be due to the depletion of chorismate, primarily for Trp synthesis, since the levels of all the Trp pathway enzymes are elevated in this strain. The addition of Trp did not increase the growth rate of the ΔmtrB or ↑AT strains, implying that the extent of feedback inhibition of anthranilate synthase activity by Trp was insufficient to allow these cells to synthesize enough Phe to support their growth. These findings establish that the AT inhibition of TRAP activity can affect the rate of synthesis of Phe, as well as the rate of synthesis of Trp.
DISCUSSION
To obtain a better understanding of the role of the AT protein in cell physiology and to learn how B. subtilis benefits from having an at operon in its genome, we analyzed the roles of AT synthesis and tRNATrp charging in the transcription of the trp operon and the need of Phe for growth. The charging of tRNATrp was measured in mutant strains lacking specific proteins normally involved in regulating Trp synthesis in this bacterium (Fig. 2). The data obtained show that the cellular level of charged tRNATrp depends on the levels of Trp and Phe in the cell, as well as on the activity of the TRAP and AT proteins (Fig. 2). The addition of Phe to the growth medium reduced the level of charged tRNATrp in wild-type cells; this reduction was accentuated in AT-deficient cells (Fig. 2). The addition of Phe to the growth medium also increased the trpE mRNA level, as well as the rtpA and trpS mRNA levels (Table 3). Since TRAP affects the transcription of the trp operon, these findings suggest that added Phe reduces the cellular level of Trp, and hence, the genes responsible for increasing Trp production are transcriptionally activated by reducing the TRAP-mediated control. Nutritional-shift experiments from a medium lacking Phe to a medium containing Phe plus Tyr led to a gradual reduction in the charged tRNATrp level in the wild-type strain; this effect was more pronounced in rtpA-deficient cells (Fig. 3). These findings establish that the AT protein is essential for maintaining adequate levels of charged tRNATrp for growth when Trp synthesis is reduced by changes in nutrient availability. Once the Trp concentration in the cell reaches a level high enough to charge its tRNATrp, the expression of AT is reduced.
When is AT most important or essential for cell growth? In B. subtilis, as in other organisms, the level of charged tRNATrp depends on the cellular concentration of Trp and the cellular level of tryptophanyl-tRNATrp synthetase. We observed that tRNATrp was almost totally charged in cultures grown in minimal medium, indicating that Trp availability and tryptophanyl-tRNA synthetase activity are sufficient to charge this tRNA fully under these growth conditions. However, when the growth conditions in our experiments were changed by adding Phe plus Tyr, which reduced the rate of Trp synthesis (21), increased formation of the Trp biosynthetic enzymes was essential. Obviously, the rate of synthesis of charged tRNAtrp must also be adjusted, and thus, trpS expression is increased when the tRNATrp is mostly uncharged (Table 3). Our results show that AT action is essential for this adjustment. When cells lacking AT are grown in the presence of added Phe, the charged tRNATrp level drops to around 25% (Fig. 2; Table 3). However, in a strain with AT, the charged tRNATrp level is 40 to 50% (Fig. 2; Table 3). This increase in the charged tRNATrp level produced by the presence of AT correlates with an increase in the transcription of trpE and, presumably, the entire trp operon (Table 3: third column, compare row 1 with row 3). The quantification of the number of molecules of AT protein and TRAP protein present per cell in cultures grown in the presence of Phe revealed that the ratio of AT (trimers) to TRAP (11-mers) is less than 0.39 (40). Nevertheless, despite this low AT/TRAP ratio, the presence of AT results in a significant increase in the expression of the trp operon (Table 3) (40) and an increase in the level of charged tRNATrp (Fig. 2; Table 3). It is difficult to predict the precise inhibitory action of AT on TRAP on the basis of their molar ratios because the level of TRAP activity depends on how many of its 11 Trp binding sites are activated by bound Trp. It is likely, therefore, that AT functions as a supplementary, fine-tuning factor. Its presence enhances the response to events that reduce the level of charged tRNATrp in the cell. Low levels of AT appear to affect the expression of the trp operon significantly (this work; 40). The cell growth rate was not affected by the absence of AT from the strain examined (Fig. 4). A plausible explanation for this may be the low number of Trp residues present in most proteins (1). However, since AT action can establish a higher level of charged tRNATrp in the cell, AT may play a role in maintaining the synthesis of highly expressed proteins containing multiple Trp residues.
Essentially three strategies/mechanisms are now known to be used in regulating the transcription of the trp operon in gram-positive bacteria: (i) the T-box mechanism, responding to uncharged tRNATrp; (ii) TRAP action, responding to Trp; and (iii) AT inhibition of TRAP function, responding to changes in the levels of charged tRNATrp and Trp (4, 15, 18, 33, 41). Gram-positive species with a trp operon employing the T-box regulatory mechanism presumably recognize changes in tRNATrp charging, which is an indirect means of sensing the cellular Trp concentration. How Trp is specifically recognized in these species, if it is recognized, is not yet known. Similarly, those identified species that produce TRAP but lack AT (16) may only recognize changes in the concentration of Trp or may use a different mechanism of sensing uncharged tRNATrp. However, since trpS of these species is regulated by uncharged tRNATrp by the T-box mechanism, conceivably some relationship between trpS expression and TRAP function is used in responding to a charged tRNATrp deficiency. Finally, only one identified gram-positive species, B. licheniformis (16), in addition to B. subtilis is known to produce an AT protein as well as TRAP. These two species presumably respond to changes in the level of either Trp or charged tRNATrp. Our results also suggest that TRAP and AT action may be very sensitive to slight changes in the intracellular concentrations of the three aromatic amino acids, as well as their common precursor, chorismate.
Our findings also demonstrate that AT action affects Phe synthesis, as well as Trp synthesis (Fig. 4). Both of these amino acids are synthesized from the common aromatic precursor, chorismic acid (26). Hoch and collaborators (20) have shown that the inactivation of mtrB of B. subtilis can increase Trp synthesis and reduce Phe synthesis. On the other hand, in the B. subtilis Marburg strain, the enzyme catalyzing the first step in the chorismate pathway, 3-deoxy-d-arabino-heptulosonate-7-phosphate synthase, is principally feedback inhibited by prephenate, an intermediate in the Phe and Tyr biosynthetic pathways (19, 36), as well as by Phe and Tyr (26), resulting in reduced production of Trp (26). Prephenate also regulates its own synthesis from chorismate by feedback inhibition (19). Since AT can reduce TRAP action, which regulates the trp operon, AT presumably can also influence the fate of chorismate, regulating its use for the synthesis of Trp or Phe. This suggests that AT may not only contribute to regulation of Trp synthesis, it may also play a role in maintaining the Phe level in the cell. We do not have sufficient data to explain why the presence of Tyr did not affect the growth of the TRAP-deficient strain. Additional experiments must be performed to examine this potential relationship.
Acknowledgments
We are grateful to Wen-Jen Yang and Anastasia Levitin for helpful discussions concerning the experiments described in this paper. We also thank Paul Babitzke for his valuable suggestions on the manuscript.
This work was supported by National Science Foundation grants MCB-0093023 and MCB-0615390 to C.Y.
Footnotes
Published ahead of print on 4 January 2008.
REFERENCES
- 1.Akashi, H., and T. Gojobori. 2002. Metabolic efficiency and amino acid composition in the proteomes of Escherichia coli and Bacillus subtilis. Proc. Natl. Acad. Sci. USA 993695-3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antson, A. A., E. J. Dodson, G. Dodson, R. B. Greaves, X. Chen, and P. Gollnick. 1999. Structure of the trp RNA-binding attenuation protein, TRAP, bound to RNA. Nature 401235-242. [DOI] [PubMed] [Google Scholar]
- 3.Babitzke, P., J. T. Stults, S. J. Shire, and C. Yanofsky. 1994. TRAP, the trp RNA-binding attenuation protein of Bacillus subtilis, is a multisubunit complex that appears to recognize G/UAG repeats in the trpEDCFBA and trpG transcripts. J. Biol. Chem. 26916597-16604. [PubMed] [Google Scholar]
- 4.Babitzke, P., and C. Yanofsky. 1993. Reconstitution of Bacillus subtilis trp attenuation in vitro with TRAP, the trp RNA-binding attenuation protein. Proc. Natl. Acad. Sci. USA 90133-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Babitzke, P., and C. Yanofsky. 1995. Structural features of L-tryptophan required for activation of TRAP, the trp RNA-binding attenuation protein of Bacillus subtilis. J. Biol. Chem. 27012452-12456. [DOI] [PubMed] [Google Scholar]
- 6.Berka, R. M., X. Cui, and C. Yanofsky. 2003. Genomewide transcriptional changes associated with genetic alterations and nutritional supplementation affecting tryptophan metabolism in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 1005682-5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burkholder, P. R., and N. H. Giles. 1947. Induced biochemical mutations in Bacillus subtilis. Am. J. Bot. 34345-348. [PubMed] [Google Scholar]
- 8.Chen, G., and C. Yanofsky. 2004. Features of a leader peptide coding region that regulate translation initiation for the anti-TRAP protein of B. subtilis. Mol. Cell 13703-711. [DOI] [PubMed] [Google Scholar]
- 9.Chen, G., and C. Yanofsky. 2003. Tandem transcription and translation regulatory sensing of uncharged tryptophan tRNA. Science 301211-213. [DOI] [PubMed] [Google Scholar]
- 10.Du, H., and P. Babitzke. 1998. trp RNA-binding attenuation protein-mediated long distance RNA refolding regulates translation of trpE in Bacillus subtilis. J. Biol. Chem. 27320494-20503. [DOI] [PubMed] [Google Scholar]
- 11.Du, H., R. Tarpey, and P. Babitzke. 1997. The trp RNA-binding attenuation protein regulates TrpG synthesis by binding to the trpG ribosome binding site of Bacillus subtilis. J. Bacteriol. 1792582-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Folk, W. R., and P. Berg. 1970. Characterization of altered forms of glycyl transfer ribonucleic acid synthetase and the effects of such alterations on aminoacyl transfer ribonucleic acid synthesis in vivo. J. Bacteriol. 102204-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gollnick, P., and P. Babitzke. 2002. Transcription attenuation. Biochim. Biophys. Acta 1577240-250. [DOI] [PubMed] [Google Scholar]
- 14.Gollnick, P., P. Babitzke, A. Antson, and C. Yanofsky. 2005. Complexity in regulation of tryptophan biosynthesis in Bacillus subtilis. Annu. Rev. Genet. 3947-68. [DOI] [PubMed] [Google Scholar]
- 15.Grundy, F. J., W. C. Winkler, and T. M. Henkin. 2002. tRNA-mediated transcription antitermination in vitro: codon-anticodon pairing independent of the ribosome. Proc. Natl. Acad. Sci. USA 9911121-11126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gutierrez-Preciado, A., C. Yanofsky, and E. Merino. 2007. Comparison of tryptophan biosynthetic operon regulation in different Gram-positive bacterial species. Trends Genet. 23422-426. [DOI] [PubMed] [Google Scholar]
- 17.Henkin, T. M. 2000. Transcription termination control in bacteria. Curr. Opin. Microbiol. 3149-153. [DOI] [PubMed] [Google Scholar]
- 18.Henkin, T. M., and C. Yanofsky. 2002. Regulation by transcription attenuation in bacteria: how RNA provides instructions for transcription termination/antitermination decisions. Bioessays 24700-707. [DOI] [PubMed] [Google Scholar]
- 19.Henner, D., and Yanofsky, C. 1993. Biosynthesis of aromatic amino acids, p. 269-280. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. American Society for Microbiology, Washington, DC.
- 20.Hoch, S. O., C. W. Roth, I. P. Crawford, and E. W. Nester. 1971. Control of tryptophan biosynthesis by the methyltryptophan resistance gene in Bacillus subtilis. J. Bacteriol. 10538-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen, R. A., and E. W. Nester. 1965. The regulatory significance of intermediary metabolites: control of aromatic acid biosynthesis by feedback inhibition in Bacillus subtilis. J. Mol. Biol. 12468-481. [DOI] [PubMed] [Google Scholar]
- 22.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)) Method. Methods 25402-408. [DOI] [PubMed] [Google Scholar]
- 23.McGraw, A. P., P. C. Bevilacqua, and P. Babitzke. 2007. TRAP-5′ stem loop interaction increases the efficiency of transcription termination in the Bacillus subtilis trpEDCFBA operon leader region. RNA 132020-2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merino, E., P. Babitzke, and C. Yanofsky. 1995. trp RNA-binding attenuation protein (TRAP)-trp leader RNA interactions mediate translational as well as transcriptional regulation of the Bacillus subtilis trp operon. J. Bacteriol. 1776362-6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merino, E., and C. Yanofsky. 2005. Transcription attenuation: a highly conserved regulatory strategy used by bacteria. Trends Genet. 21260-264. [DOI] [PubMed] [Google Scholar]
- 26.Nester, E. W., and R. A. Jensen. 1966. Control of aromatic acid biosynthesis in Bacillus subtilis: sequential feedback inhibition. J. Bacteriol. 911594-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Otridge, J., and P. Gollnick. 1993. MtrB from Bacillus subtilis binds specifically to trp leader RNA in a tryptophan-dependent manner. Proc. Natl. Acad. Sci. USA 90128-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qi, Y., G. Patra, X. Liang, L. E. Williams, S. Rose, R. J. Redkar, and V. G. DelVecchio. 2001. Utilization of the rpoB gene as a specific chromosomal marker for real-time PCR detection of Bacillus anthracis. Appl. Environ. Microbiol. 673720-3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarsero, J. P., E. Merino, and C. Yanofsky. 2000. A Bacillus subtilis gene of previously unknown function, yhaG, is translationally regulated by tryptophan-activated TRAP and appears to be involved in tryptophan transport. J. Bacteriol. 1822329-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steinberg, W. 1974. Temperature-induced derepression of tryptophan biosynthesis in a tryptophanyl-transfer ribonucleic acid synthetase mutant of Bacillus subtilis. J. Bacteriol. 1171023-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steinberg, W., and C. Anagnostopoulos. 1971. Biochemical and genetic characterization of a temperature-sensitive, tryptophanyl-transfer ribonucleic acid synthetase mutant of Bacillus subtilis. J. Bacteriol. 1056-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valbuzzi, A., P. Gollnick, P. Babitzke, and C. Yanofsky. 2002. The anti-trp RNA-binding attenuation protein (Anti-TRAP), AT, recognizes the tryptophan-activated RNA binding domain of the TRAP regulatory protein. J. Biol. Chem. 27710608-10613. [DOI] [PubMed] [Google Scholar]
- 33.Valbuzzi, A., and C. Yanofsky. 2001. Inhibition of the B. subtilis regulatory protein TRAP by the TRAP-inhibitory protein, AT. Science 2932057-2059. [DOI] [PubMed] [Google Scholar]
- 34.Varshney, U., C. P. Lee, and U. L. RajBhandary. 1991. Direct analysis of aminoacylation levels of tRNAs in vivo. Application to studying recognition of Escherichia coli initiator tRNA mutants by glutaminyl-tRNA synthetase. J. Biol. Chem. 26624712-24718. [PubMed] [Google Scholar]
- 35.Vogel, H. J., and D. M. Bonner. 1956. Acetylornithinase of Escherichia coli: partial purification and some properties. J. Biol. Chem. 21897-106. [PubMed] [Google Scholar]
- 36.Wu, J., G. Y. Sheflyan, and R. W. Woodard. 2005. Bacillus subtilis 3-deoxy-D-arabino-heptulosonate 7-phosphate synthase revisited: resolution of two long-standing enigmas. Biochem. J. 390583-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yakhnin, H., A. V. Yakhnin, and P. Babitzke. 2007. Translation control of trpG from transcripts originating from the folate operon promoter of Bacillus subtilis is influenced by translation-mediated displacement of bound TRAP, while translation control of transcripts originating from a newly identified trpG promoter is not. J. Bacteriol. 189872-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yakhnin, H., A. V. Yakhnin, and P. Babitzke. 2006. The trp RNA-binding attenuation protein (TRAP) of Bacillus subtilis regulates translation initiation of ycbK, a gene encoding a putative efflux protein, by blocking ribosome binding. Mol. Microbiol. 611252-1266. [DOI] [PubMed] [Google Scholar]
- 39.Yakhnin, H., H. Zhang, A. V. Yakhnin, and P. Babitzke. 2004. The trp RNA-binding attenuation protein of Bacillus subtilis regulates translation of the tryptophan transport gene trpP (yhaG) by blocking ribosome binding. J. Bacteriol. 186278-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang, W.-J., and C. Yanofsky. 2005. Effects of tryptophan starvation on levels of the trp RNA-binding attenuation protein (TRAP) and anti-TRAP regulatory protein and their influence on trp operon expression in Bacillus subtilis. J. Bacteriol. 1871884-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yanofsky, C. 2007. RNA-based regulation of genes of tryptophan synthesis and degradation, in bacteria. RNA 131141-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]