Abstract
The conjugative plasmid pYI14 (61 kbp) was isolated from Enterococcus faecalis YI714, a clinical isolate. pYI14 conferred a pheromone response on its host and encoded bacteriocin 41 (bac41). Bacteriocin 41 (Bac41) only showed activity against E. faecalis. Physical mapping of pYI14 showed that it consisted of EcoRI fragments A to P. The clone pHT1100, containing EcoRI fragments A (12.6 kbp) and H (3.5 kbp), conferred the bacteriocin activity on E. faecalis strains. Genetic analysis showed that the determinant was located in a 6.6-kbp region within the EcoRI AH fragments. Six open reading frames (ORFs) were identified in this region and designated ORF7 (bacL1) ORF8 (bacL2), ORF9, ORF10, ORF11 (bacA), and ORF12 (bacI). They were aligned in this order and oriented in the same direction. ORFs bacL1, bacL2, bacA, and bacI were essential for expression of the bacteriocin in E. faecalis. Extracellular complementation of bacteriocin expression was possible for bacL1 and -L2 and bacA mutants. bacL1 and -L2 and bacA encoded bacteriocin component L and activator component A, respectively. The products of these genes are secreted into the culture medium and extracellularly complement bacteriocin expression. bacI encoded immunity, providing the host with resistance to its own bacteriocin activity. The bacL1-encoded protein had significant homology with lytic enzymes that attack the gram-positive bacterial cell wall. Sequence data for the deduced bacL1-encoded protein suggested that it has a domain structure consisting of an N-terminal signal peptide, a second domain with the enzymatic activity, and a third domain with a three-repeat structure directing the proenzyme to its cell surface receptor.
Bacteriocins are bacterial proteins or peptides which inhibit the growth of other bacteria that are closely related to the producer strain. They usually exhibit a relatively narrow spectrum of activity and are produced by a wide variety of gram-positive and gram-negative bacteria (27). Bacteriocin production is thought to provide the host strain with an ecological or other selective advantage over other strains.
Many Enterococcus faecalis clinical isolates produce a bacteriocin (3, 5), and the bacteriocin is frequently encoded on the E. faecalis pheromone-responding conjugative plasmid (6, 14, 21, 46). Several E. faecalis bacteriocins have been genetically and biochemically characterized (15, 35), including the β-hemolysin/bacteriocin (cytolysin) (6, 7, 18, 20, 22) and the peptide antibiotics AS-48 (33), bacteriocin 21 (47), and bacteriocin 31 (46), which are encoded by the E. faecalis conjugative plasmids pAD1 (58 kbp), pMB2 (58 kbp), pPD1 (59 kbp), and pYI17 (57.5 kbp), respectively.
A significant number of E. faecalis clinical isolates produce hemolysin/bacteriocin (10, 26), and more than 50% of the hemolytic clinical isolates carry transferable hemolysin/bacteriocin determinants (21, 26). The hemolysin/bacteriocin of pAD1 is associated with virulence in animal models (4, 25, 29), and this plasmid is considered to be a typical E. faecalis hemolysin/bacteriocin plasmid (21, 31). The mechanism of hemolysin/bacteriocin production in E. faecalis has been studied in detail with the hemolysin/bacteriocin determinant on this plasmid (16, 17, 18, 22, 39). The active hemolysin/bacteriocin is produced by extracellular complementation of the two CylL factors (i.e., CylLL and cylLS) and CylA.
Previously, we have shown that bacteriocins or bacteriocinogenic E. faecalis clinical isolates can be classified into five groups on the basis of their bacteriocin activity against E. faecalis FA2-2 and OG1-10, Enterococcus hirae 9790, Streptococcus pyogenes, Streptococcus agalactiae, Streptococcus sanguinis, Streptococcus pneumoniae, Staphylococcus aureus, and Staphylococcus epidermidis (46). E. faecalis FA-2-2 and OG1-10 and E. hirae have been chosen as representative enterococcal strains for the examination and classification of the bacteriocins produced by the clinical isolates in this study. Class 1 types produce the β-hemolysin/bacteriocin (cytolysin) and are active against a wide variety of gram-positive bacteria, including S. aureus (2, 15, 17, 24, 46). The β-hemolysin/bacteriocin (cytolysin) of pAD1 belongs to class 1. Class 2 is active against a broad spectrum of bacteria, including E. faecalis, other Streptococcus spp., and S. aureus. AS-48 and bacteriocin 21 belong to class 2. Class 3 is active against E. faecalis and E. hirae. Class 4 is active against E. faecalis, and class 5 is active against E. hirae. The YI717, YI718, and YI719 strains belong to class 3 and harbor plasmids pYI17 (57.5 kb), pYI18, and pYI19, respectively (46). These plasmids encode the same bacteriocin with respect to immunity to the bacteriocin activity. Bacteriocin 31 (Bac31), encoded on pYI17, is representative of the class 3 bacteriocins and is active against E. faecalis and E. hirae, as is the membrane-active class II bacteriocin of lactic acid bacteria (46). The Bac31 determinant consists of the structural gene bacA and the immunity gene bacB.
In this report, we describe the cloning and genetic analysis of the bacteriocin 41 determinant encoded on E. faecalis pheromone-responsive conjugative plasmid pYI14, which is a representative class 4 bacteriocin. We also describe the identification of the two functional domains that are required to produce the active bacteriocin by extracellular complementation of the two factors.
MATERIALS AND METHODS
Bacterial strains, plasmids, oligonucleotides, media, and reagents.
The bacterial strains, plasmids, and oligonucleotides used in this study are listed in Table 1. E. faecalis strains were grown in Todd-Hewitt broth (THB; Difco Laboratories) at 37°C, unless otherwise noted. Escherichia coli strains were grown in Luria-Bertani (LB) broth. The following antibiotic concentrations were used for the selection of E. faecalis: erythromycin, 12.5 μg ml−1; streptomycin, 250 μg ml−1; kanamycin, 250 μg ml−1; spectinomycin, 250 μg ml−1; chloramphenicol, 20 μg ml−1; rifampin, 25 μg ml−1; fusidic acid, 25 μg ml−1. The antibiotic concentrations used for the selection of E. coli were as follows: ampicillin, 100 μg ml−1; kanamycin, 40 μg ml−1; chloramphenicol 50 μg ml−1; spectinomycin, 50 μg ml−1. All antibiotics were obtained from Sigma Chemical Co. X-Gal (5-bromo-4-chloro-3 indolyl-β-d-galactopyranoside) was obtained from Wako Pure Chemical Industries, Ltd., and was used at 40 μg ml−1.
TABLE 1.
Bacterial strains, plasmids, and oligonucleotides used in this study
| Strain, plasmid, or oligonucleotide | Relevant features or sequence (5′-3′)a | Reference(s), source, or generated plasmid(s) |
|---|---|---|
| Strains | ||
| E. faecalis | ||
| FA2-2 | rif fus | 7 |
| JH2SS | spc str | 44 |
| OG1-10 | str; derivative of OG1 | 12 |
| OG1X | str; protease-negative derivative of OG1-10 | 23 |
| YI712 | pYI12(Bac) | This study |
| YI714 | pYI14(Bac), pYI141 (48 kb); clinical isolate | This study |
| YI715 | pYI15(Bac), pYI151 (48 kb); clinical isolate | This study |
| E. coli DH5α | endA1 recA1 gyrA96 thi-1 hsdR17 supE44 relA1 Δ(argE-lacZYA)U169 | Bethesda Research Laboratories |
| Plasmids | ||
| pAM401 | E. coli-E. faecalis shuttle plasmid; cat tet | 50 |
| pLZ12-Km | E. coli-Streptococcus shuttle plasmid; aphA | 19 |
| pBlueScript SKII(+) | E. coli cloning vector; Ampr | Stratagene |
| pPD1 | Bac21, 59-kb conjugative plasmid from strain 39-5 | 14, 47 |
| pMG326 | pMW119 containing a 16.7-kbp EcoRI-SalI fragment of pPD1; pheromone-regulatory region | 14, 41 |
| pYI12 | Bac41, 72-kb conjugation plasmid from YI712 | This study |
| pYI14 | Bac41, 61-kb conjugative plasmid from YI714 | This study |
| pYI15 | Bac41, 61-kb conjugative plasmid from YI715 | This study |
| pHT1100 | EcoRI-relational clone of pYI14; pAM401 containing EcoRI fragments A and H | This study |
| pHT1101 | EcoRI-relational clone of pYI14; pAM401 containing EcoRI fragment A | This study |
| pHT1102 | EcoRI-relational clone of pYI14; pAM401 containing EcoRI fragments H and M | This study |
| pMG1103 | Derivative of pHT1100 with BamHI E fragment deleted | This study |
| pMG1104 | Derivative of pHT1100 with BamHI F fragment deleted | This study |
| pMG1106 | Derivative of pHT1100; BamHI site at 4.1 kbp blunted with Klenow enzyme | This study |
| pMG1108 | Derivative of pHT1100; BamHI site at 6.3 kbp blunted with Klenow enzyme | This study |
| pMG1109 | Derivative of pHT1100; KpnI site at 4.6 kbp blunted with DNA-blunting kit (Takara) | This study |
| pMG1105-n | Tn5 insertional derivatives of pHT1100 | This study |
| pMG1107-n | Mini-Tn7 insertional derivatives of pHT1100 created with GPS kit (New England BioLabs) | This study |
| pMG1110 | bacL1 and bacL2; pAM401 containing 2,932-bp EcoRI fragment amplified by PCR | This study |
| pMG1111 | bacA; pAM401 containing 2,836-bp SalI fragment amplified by PCR | This study |
| pMG1112 | bacI; pAM401 containing 777-bp BamHI fragment amplified by PCR | This study |
| pMG1113 | bacI and ORF13; pAM401 containing 1,513-bp BamHI fragment amplified by PCR | This study |
| pMG1114 | pLZ12-Km containing 10-kbp BglII fragment mapped from 1.7 kbp to 11.7 kbp | This study |
| pMG1115 | Derivative of pMG1114; EcoRI fragment (8.5 kbp to vector region) deleted | This study |
| pMG1116 | Derivative of pMG1114; three HindIII fragments (4.6- to 6.6-kbp region) deleted | This study |
| Oligonucleotides | ||
| B9P2842F | ccg gaa tTC TAG CAA CCG AAA ACC ACG TTG G | pMG1110 |
| B9P5773R | gcg gaa tTC ATT GCG CAG CAA ATC ATT GC | pMG1110 |
| B9P6180F | aac gcg tcg ACA GGA ATT GAG ACA TAC GCT | pMG1111 |
| B9P9015R | aac gcg tcg acT TCG TCA AAT CCA TTT CCC CTA | pMG1111 |
| B9P8823F | ggc gga tcc GCA GCA GAA TTA GCA GGA GCG | pMG1112, pMG1113 |
| B9P9599R | gcc gga tcc CAA AAG TCA TAC ATG ACC TCC | pMG1112 |
| B9P10335R | gcc gga tcc CTG TAT AAA TCC ATA CTA CAC | pMG1113 |
Underlining indicates the following restriction endonuclease recognition sequences: GAATTC; EcoRI, GTCGAC; SalI, GGATCC; BamHI. Lowercase letters indicate incorporated tag sequences.
Conjugation experiments.
Broth mating and solid-surface mating were performed as previously described (48, 49), with a donor/recipient ratio of 1:10. Broth matings (in THB) were carried out for 4 h, and solid-surface matings (on THB agar plates) were carried out overnight (16 h) at 37°C. Transfer frequencies were calculated as the number of transconjugants per donor cell (at the end of mating). Pheromone induction and detection of cell aggregation were performed as previously described (11, 12).
Soft-agar assay for bacteriocin production and immunity.
The bacteriocin production assay was performed as described previously (22). The test for immunity to the bacteriocin was performed essentially as described previously (22).
Plasmid DNA methodology.
Recombinant plasmids were generated in E. coli DH5α. Transformation of bacterial cells with plasmid DNA was achieved by electrotransformation as described previously (13). Plasmid DNA was purified from E. coli (38) or from E. faecalis as previously described (14). DNA fragments were purified from an agarose gel after electrophoresis with a Gene Clean II kit (Bio 101, Inc.). Recombinant DNA methodology, analyses of plasmid DNA with restriction enzymes, and agarose gel electrophoresis were carried out by standard methods (38). Restriction enzymes were purchased from New England BioLabs, Roche, Nippon Gene, and Takara Co., and reactions were carried out under the conditions recommended by the manufacturers. DNA ligations were performed with a DNA ligation kit from Takara. To end fill the endonuclease-digested DNA fragment for ligation, a DNA-blunting kit and Klenow enzyme were obtained from Takara and used according to the manufacturer's protocol (45).
Determination of the pYI14 restriction map.
pYI14 plasmid DNA was digested with EcoRI, BamHI, KpnI, SphI, or XbaI or double digested with a combination of two of these restriction enzymes. Agarose gel electrophoresis analysis of the digested DNAs was performed to determine the cleavage sites within the plasmid. To determine the order of the EcoRI fragments of pYI14, a relational clone set was constructed as previously described (14, 46). After agarose gel electrophoresis of plasmid pYI14 DNA partially digested with EcoRI, fragments greater than 7 kb in size were eluted and used for cloning. The cloning vectors used were pBluescript-SK(+) and pAM401, and the host strain was E. coli DH5α.
DNA sequence analysis.
Nucleotide sequence analysis was carried out as previously described (14). A deletion kit (Nippon Gene) was used. BamHI-E, BamHI-F, EcoRI-H, and the 2.1-kb fragment between BamHI-F and EcoRI-H were individually cloned into the pBluescript vector. The clones were used to construct a series of deletional clones. The resulting constructs were sequenced in both orientations with the Taq Dye primer and the Taq Big Dye terminator cycle sequencing kit (Applied Biosystems), a model 377 DNA sequencer, and a 310 gene analyzer (ABI Prism). A database search was performed with the BLASTn and tBLASTx programs of the National Center for Biotechnology Information, Bethesda, MD (1).
Generation of transposon (Tn5, mini-Tn7) insertion mutants.
Insertion of Tn5 (Kmr) into the cloned plasmid DNA was performed as described elsewhere (47). Target plasmid pHT1100(pAM401 containing EcoRI fragments A and H) was introduced into E. coli K-12 TH688 (with Tn5 in the thr locus) (42) by electrotransformation. Transformants were spread onto selective plates containing kanamycin and chloramphenicol, and the plates were left at room temperature for 10 days. The bacteria that grew on the selective plates were pooled, and the plasmid DNA was then isolated and used to transform E. coli DH5α. The transformants were selected on plates containing kanamycin and chloramphenicol for the selection of Tn5-borne kanamycin resistance and plasmid-borne chloramphenicol resistance, respectively. The transformants were purified and examined to determine the location of Tn5 within the plasmid. The precise locations of Tn5 insertions were determined by DNA sequence analysis with a synthetic primer that hybridized to the end of Tn5. A GPS kit (NEB) was used to generate mini-Tn7 insertion mutants with plasmid pHT1100 according to the manufacturer's instructions.
PCR amplification and primers.
PCR amplification was performed with the thermostable DNA polymerase Takara Taq (Takara Bio Inc.) and a Perkin-Elmer 9600 thermal cycler. PCR conditions varied according to the primers used and the size of the anticipated product. The custom primers used in this study were obtained from Invitrogen (Tokyo, Japan) and are listed in Table 1. Each of the amplified PCR products was trimmed by the appropriate restriction enzyme, purified with a QIAquick-spin column (Qiagen), and cloned into plasmid pAM401.
Nucleotide sequence accession number.
The nucleotide sequence reported in this article is available from the DDBJ, EMBL, and GenBank nucleotide sequence databases under accession number AB271686.
RESULTS
Bacteriocinogenic E. faecalis strain and the pheromone-responsive bacteriocin plasmid.
Four strains that were active against E. faecalis and were classified as class 4 bacteriocinogenic strains were isolated from clinical urine samples and were designated YI712, YI714, YI715, and YI716. YI712 harbored plasmid pYI12 (72 kb). YI714 and YI715 harbored plasmids pYI14 (61 kb) and pYI141 (48 kb) and plasmids pYI15 (61 kb) and pYI151 (48 kb), respectively. YI716 did not carry any plasmid. Each strain was used as a donor in mating experiments with plasmid-free recipient strain E. faecalis FA2-2 (Rifr Fusr) to determine whether these plasmids conferred bacteriocinogenic activity on the host. After incubating the broth mating cultures for 4 h, appropriately diluted mixtures were plated on an agar plate containing rifampin (25 μg/ml) and fusidic acid (25 μg/ml) to select for the recipient strains. After overnight incubation of the plates, a total of approximately 500 E. faecalis FA2-2 colonies were obtained from each mating and examined for bacteriocin production. Approximately 1 in 500 cells obtained from the mating experiments with each of the strains described above expressed bacteriocin activity against E. faecalis FA2-2. The bacteriocinogenic transconjugants of YI712, YI714, and YI715 harbored pYI12 (72 kb), pYI14 (61 kb), and pYI15 (61 kb), respectively. The same EcoRI restriction profiles were obtained for pYI14 and pYI15, implying that the two plasmids were identical. Each plasmid transferred between E. faecalis FA2-2 and E. faecalis OG1-10 at a frequency of about 10−3 per donor cell by broth mating. E. faecalis FA2-2(pYI12), FA2-2(pYI14), and FA2-2(pYI15) did not exhibit bacteriocin activity against E. faecalis OG1-10(pYI12), OG1-10(pYI14), or OG1-10(pYI15). These results imply that plasmids pYI12, pYI14, and pYI15 encoded the same bacteriocin with respect to the immunity characteristic. E. faecalis FA2-2 strains carrying pYI12, pYI14, or pYI15 were tested for bacteriocin production against the indicator strains S. aureus FDA209P, E. faecalis FA2-2 and OG1-10, Enterococcus faecium BM4105RF, E. hirae ATCC 9790, Enterococcus durans ATCC 49135, Enterococcus raffinosus JCM8733, Enterococcus gallinarum BM4174, S. agalactiae, S. pyogenes, Listeria monocytogenes, and Listeria denitrificans. Each of the three bacteriocinogenic strains only showed bacteriocin activity against E. faecalis. Plasmid pYI14, isolated from strain YI714, was used as the representative plasmid encoding the bacteriocin.
The donor E. faecalis OG1-10(pYI14) and recipient E. faecalis FA2-2 formed a mating aggregate in the mating mixture. When OG1-10(pYI14) cells were exposed to E. faecalis FA2-2 culture filtrate (pheromone) for 4 h at 37°C, the OG1-10(pYI14) cells showed aggregation. Agarose gel electrophoresis of the EcoRI restriction fragments of pYI14 DNA was carried out, and the DNA was transferred to a membrane for Southern hybridization. The membrane was hybridized with a DNA probe containing the pheromone response genes of the pheromone-responsive plasmid pPD1 or plasmid pMG326, which contains the putative surface exclusion protein gene and the N-terminal region of the aggregation substance gene of pPD1 (14, 41). Each probe hybridized to specific pYI14 EcoRI fragments (data not shown). These results indicated that plasmid pYI14 was a pheromone-responsive plasmid.
Restriction map of pYI14.
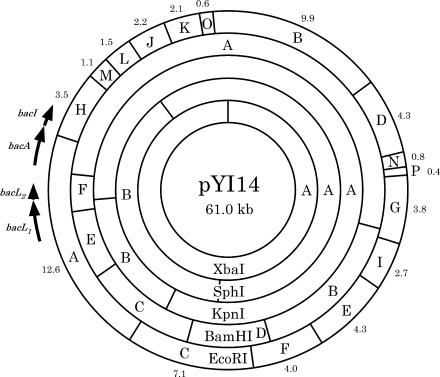
To determine the order of the EcoRI fragments, a relational clone set was obtained. The order of EcoRI fragments was determined to be A-H-M-L-J-K-O-B-D-N-P-G-I-E-F-C (Fig. 1). Each clone was digested with BamHI, KpnI, SphI, and XbaI, and the cleavage sites were determined (Fig. 1). Restriction sites within the EcoRI A and H fragments were also confirmed by sequencing (see the supplemental material).
FIG. 1.
Physical map of pYI14 showing the locations of bacteriocin 41 determinants bacL1, bacL2, bacA, and bacI. Each value is the size of the fragment in kilobases.
Bacteriocin activity of the cloned DNA fragment.
To examine the bacteriocin activity of the relational clones, each clone was introduced into E. faecalis OG1-10 and the resulting transformant was examined for bacteriocin activity. E. faecalis OG1-10 carrying plasmid pHT1100, which contained the EcoRI A and H fragments (16.1 kb), exhibited the bacteriocin activity (Fig. 2). E. faecalis OG1-10 carrying either the EcoRI A (12.6 kb) or HM (4.6 kb) fragments (plasmids pHT1101 and pHT1102, respectively) did not exhibit bacteriocin activity (Fig. 2). E. faecalis OG1-10 carrying the EcoRI HM fragments showed resistance to the bacteriocin activity of E. faecalis OG1-10(pYI14). These results indicated that the bacteriocin determinant of pYI14 is located on the EcoRI A and H (AH) fragments and the immunity gene (i.e., the gene for resistance to its own bacteriocin) is located on the EcoRI H fragment.
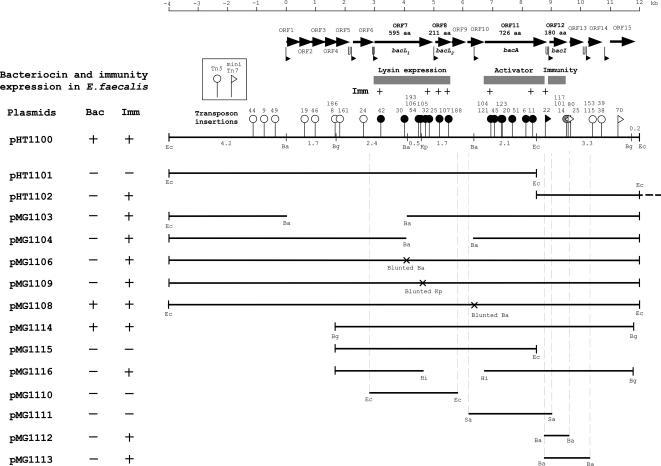
FIG. 2.
Physical maps of the 16.1-kb region containing EcoRI fragments A (12.6 kb) and H (3.5 kb) in pYI14 (which is carried on pHT1100), transposon insertions, and subclones. The zero position of the numerical scale (top horizontal line) indicates the BamHI endonuclease recognition site located between the BamHI C and E fragments, and it runs in a clockwise direction on the physical map of Fig. 1. Thick horizontal arrows indicate the predicted ORFs and the direction of ORF transcription. The flags and hairpins below the ORFs indicate the potential promoter regions and inverted repeat sequences. The horizontal lines under the map represent the cloned pYI14 DNA fragments in the derivative plasmids listed on the left. Small vertical bars at ends of the lines represent the endonuclease recognition sites for cloning. The dotted vertical lines represent the ends of the amplified PCR fragment of pYI14 DNA used to clone the bacteriocin determinant. The endonuclease recognition sites incorporated for the cloning of the PCR products are indicated. Abbreviations of the endonuclease recognition sites: Eco, EcoRI; Ba, BamHI; Kp, KpnI; Bg, BglII; Hi, HindIII; Sa, SalI. Bac +, normal bacteriocin expression; Bac −, no bacteriocin expression; Imm +, resistance to bacteriocin 41; Imm −, sensitive to bacteriocin 41. The vertical lines with circular or triangular heads on the pHT1100 map show the points of transposon insertion. The circular heads indicate Tn5, and the triangular heads indicate mini-Tn7 and its orientations. The heads represent the levels of bacteriocin 41 expression in E. faecalis strains as follows: open heads, normal bacteriocin expression; black heads, no bacteriocin expression; gray heads, weak bacteriocin expression (Fig. 3A). The values on the insertions indicate the numbers of insertions and correspond to those shown in Table 3 (see also Fig. S1 in the supplemental material). The cross marks on the clones indicate the mutated endonuclease recognition sites (a four-base insertion or deletion). aa, amino acids.
DNA sequence analysis.
The EcoRI AH fragments were sequenced, and computer analysis was used to identify open reading frames (ORFs) within the sequence. Fifteen ORFs (ORF1 to ORF15) were located in the region spanning map positions 0 to 12 kbp, as indicated by the numerical scale shown in Fig. 2, where position 0 is the BamHI site located between BamHI fragments E and C and position 12 kbp is the EcoRI site located between the EcoRI H and M fragments. (Fig. 1 and 2 and Table 2; see Fig. S1 in the supplemental material). Figure 2 shows the ORFs that have a deduced ribosome-binding site in the 20-base region upstream of the predicted start codon and the potential promoters for initiation of transcription.
TABLE 2.
ORFs encoded on the BamHI/EcoRI 11,952-bp-spanning region
| ORF | Gene | Map location (bp) | Gene/protein size (base pairs/amino acids) | Homology | % Identity/similarity (amino acids) | Function |
|---|---|---|---|---|---|---|
| 1 | 136-588 | 453/150 | pcfS (E. faecalis pCF10) | 98/100 | Ssba | |
| 2 | 602-754 | 153/50 | EFB0044 (E. faecalis V583 pTEF2) | 100/100 | ||
| 3 | 766-1344 | 579/192 | pcfT (E. faecalis pCF10) | 89/90 | Thermonuclease | |
| 4 | 1350-1670 | 321/106 | pcfU (E. faecalis pCF10) | 93/97 | ||
| 5 | 1827-2009 | 183/60 | Efae03001107 (E. faecium) | 50/67 | ||
| 6 | 2204-2920 | 717/238 | Lipoprotein (E. faecalis V583) | 31/45 | ||
| 7 | bacL1 | 3058-4845 | 1,788/595 | Lysozyme (B. subtilis bacteriophage B103) | 37/52 (1-151) | Lysin (bacteriocin 41) |
| Lysin (S. agalactiae prophage lambda Sa1) | 46/63 (160-309) | |||||
| Muramidase (L. plantarum WCFS1) | 24/41 (318-577) | |||||
| 8 | bacL2 | 5031-5666 | 636/211 | Lysin expression | ||
| 9 | 5689-6120 | 432/143 | ORF50 (S. pneumoniae bacteriophage MM1) | 31/51 | Holin | |
| 10 | 6123-6650 | 528/175 | EF0637 (E. faecalis V583) | 27/45 | ||
| 11 | bacA | 6693-8873 | 2,181/726 | ybfG (B. subtilis) | 41/56 | Lysin activator |
| ykuG (B. subtilis) | 40/55 | |||||
| 12 | bacI | 8981-9523 | 543/180 | Immunity | ||
| 13 | 9590-10165 | 576/191 | ||||
| 14 | 10308-10640 | 333/110 | EFB0057 (E. faecalis V583 pTEF2) | 98/100 | ||
| 15 | 11080-11781 | 702/233 |
Ssb, single-stranded binding protein.
Generation of Tn5 or mini-Tn7 insertion mutants.
To examine the location of the bacteriocin determinant, mutants with altered bacteriocin expression were generated by Tn5 or mini-Tn7 insertion into pHT1100. The precise locations of Tn5 or mini-Tn7 insertions in the ORFs were determined by DNA sequence analysis (see Fig. S1 in the supplemental material), and the results are shown in Fig. 2 and Table 3. Tn5 insertions into ORF7, ORF8, and ORF11 resulted in defective bacteriocin activity in E. faecalis OG1-10. Insertion of mini-Tn7 into the C-terminal region of ORF11 also resulted in defective bacteriocin activity in E. faecalis OG1-10. E. faecalis OG1S carrying each of the insertion mutants showed resistance to the bacteriocin activity of E. faecalis OG1-10(pYI14), indicating that the mutant plasmids retained immunity to the bacteriocin.
TABLE 3.
Transposon insertion mutants of pHT1100 and bacteriocin expression
| Insertion no. in Fig. 2 | Plasmid(s)a | Transposon | Map position (kb)b | Insertion location | Bacc | Immd |
|---|---|---|---|---|---|---|
| pYI14 | ++ | + | ||||
| pHT1100 | ++ | + | ||||
| 44 | pMG1105-44 | Tn5 | −1.2 | Upstream of ORF1 | ++ | + |
| 9 | pMG1105-9 | Tn5 | −0.8 | Upstream of ORF1 | ++ | + |
| 49 | pMG1105-49 | Tn5 | −0.4 | Upstream of ORF1 | ++ | + |
| 19 | pMG1105-19 | Tn5 | 0.7 | ORF2 | ++ | + |
| 46 | pMG1105-46 | Tn5 | 1.0 | ORF3 | ++ | + |
| 8, 186 | pMG1105-8, -186 | Tn5 | 1.7 | Between ORF4 and ORF5 | ++ | + |
| 161 | pMG1105-161 | Tn5 | 1.9 | ORF5 | ++ | + |
| 24 | pMG1105-24 | Tn5 | 2.6 | ORF6 | ++ | + |
| 42 | pMG1105-42 | Tn5 | 3.2 | ORF7 (bacL1) | − | + |
| 30 | pMG1105-30 | Tn5 | 4.0 | ORF7 (bacL1) | − | + |
| 54, 105, 193 | pMG1105-54, -105, -193 | Tn5 | 4.5 | ORF7 (bacL1) | − | + |
| 105 | pMG1105-105 | Tn5 | 4.6 | ORF7 (bacL1) | − | + |
| 32 | pMG1105-32 | Tn5 | 4.7 | ORF7 (bacL1) | − | + |
| 25 | pMG1105-25 | Tn5 | 4.8 | ORF7 (bacL1) | − | + |
| 107 | pMG1105-107 | Tn5 | 5.2 | ORF8 (bacL2) | − | + |
| 188 | pMG1105-188 | Tn5 | 5.5 | ORF8 (bacL2) | − | + |
| 104, 121 | pMG1105-104, -121 | Tn5 | 6.9 | ORF11 (bacA) | − | + |
| 45 | pMG1105-45 | Tn5 | 7.1 | ORF11 (bacA) | − | + |
| 123 | pMG1105-123 | Tn5 | 7.3 | ORF11 (bacA) | − | + |
| 20 | pMG1105-20 | Tn5 | 7.3 | ORF11 (bacA) | − | + |
| 51 | pMG1105-51 | Tn5 | 7.7 | ORF11 (bacA) | − | + |
| 6 | pMG1105-6 | Tn5 | 8.1 | ORF11 (bacA) | − | + |
| 11 | pMG1105-11 | Tn5 | 8.4 | ORF11 (bacA) | − | + |
| 22 | pMG1107-22 | Mini-Tn7 | 8.7 | ORF11 (bacA) | − | + |
| 14, 101, 117 | pMG1105-14, -101, -117 | Tn5 | 9.5 | ORF12 (bacI) | + | ± |
| 80 | pMG1107-80 | Mini-Tn7 | 9.5 | Between ORF12 and ORF13 | ++ | + |
| 25 | pMG1107-25 | Mini-Tn7 | 9.6 | ORF13 | ++ | + |
| 115, 153 | pMG1105-115, -153 | Tn5 | 10.4 | ORF14 | ++ | + |
| 38, 39, 163 | pMG1105-38, -39, -163 | Tn5 | 10.7 | Between ORF14 and ORF15 | ++ | + |
| 87 | pMG1107-87 | Mini-Tn7 | 11 | Between ORF14 and ORF15 | ++ | + |
| 74 | pMG1107-74 | Mini-Tn7 | 11 | Between ORF14 and ORF15 | ++ | + |
| 76 | pMG1107-76 | Mini-Tn7 | 11.1 | Between ORF14 and ORF15 | ++ | + |
| 69 | pMG1107-69 | Mini-Tn7 | 11.1 | ORF15 | ++ | + |
| 59 | pMG1107-59 | Mini-Tn7 | 11.2 | ORF15 | ++ | + |
| 70 | pMG1107-70 | Mini-Tn7 | 11.3 | ORF15 | ++ | + |
| 40 | pMG1107-40 | Mini-Tn7 | 11.4 | ORF15 | ++ | + |
| 80 | pMG1107-80 | Mini-Tn7 | 11.4 | ORF15 | ++ | + |
| 83 | pMG1107-83 | Mini-Tn7 | 11.5 | ORF15 | ++ | + |
The host strain of the derivative was E. faecalis OG1S (OG1-10).
The map position is the distance from the junction between EcoRI fragments A and H. Minus values indicate the opposite direction.
Bac, bacteriocin expression. Symbols: ++, normal bacteriocin expression; +, weak bacteriocin expression (Fig. 3A); −, no bacteriocin expression.
Imm, immunity expression. Symbols: +, positive expression; −, no expression; ±, weak expression.
Generation of deletion mutants by end filling after cleavage with a restriction enzyme.
Mutant pHT1100 plasmids with BamHI fragment deletions within were also generated to examine the location of the bacteriocin determinant as described in Materials and Methods (Fig. 2) (47). Deletion mutant plasmids pMG1103 and pMG1104 possessed deletions of the 4.1-kbp BamHI E fragment between map positions 0 kb and 4.1 kb and the 2.2-kbp BamHI F fragment between map positions 4.1 kb and 6.3 kb, respectively. Plasmid pMG1103, which had a deletion in the amino-terminal region of ORF7 and had lost the six ORFs located upstream of ORF7, did not exhibit bacteriocin activity but retained immunity to the bacteriocin. Plasmid pMG1104, which had deletions within the carboxyl-terminal region of ORF7, ORF8, and ORF9 and the amino-terminal region of ORF10, did not exhibit bacteriocin activity but retained immunity to the bacteriocin. These results implied that the gene for immunity is located downstream of ORF11.
Generation of four-nucleotide insertion (deletion) mutants.
Mutants with changes in ORF7 and ORF10 were generated to obtain mutants with in-frame changes in the determinant by blunt ending the recessed 3′ terminus of the BamHI site or the prominent 3′ terminus of the KpnI cleavage site within pHT1100 DNA that had been partially digested with these enzymes prior to ligation (Fig. 2) (45). Blunt ending the BamHI and KpnI sites resulted in the insertion of four nucleotides (5′-GATC-3′) with the Klenow enzyme in the case of the BamHI site and the deletion of four nucleotides (5′-GTAC-3′) with the T4 DNA polymerase DNA-blunting kit (Takara) in the case of the KpnI site. The pMG1106 and pMG1109 mutants that resulted from the blunt ending of the BamHI site and KpnI sites in ORF7 did not exhibit bacteriocin activity but retained the immunity activity, indicating that ORF7 is essential for bacteriocin expression. The pMG1108 mutant, which resulted from the end filling of the BamHI site in ORF10, expressed both bacteriocin and immunity activity, suggesting that ORF10 is not essential for bacteriocin expression.
Subcloning of the bacteriocin determinant and generation of the derivative mutants.
The 10.0-kb BglII fragment that is located between 1.7 kb and 11.7 kb on the map was cloned into shuttle vector pLZ12-Km (19) (Fig. 2), and the cloned plasmid was designated pMG1114. pMG1114 expressed both bacteriocin activity and immunity, indicating that the bacteriocin determinant was located within the 10.0-kb BglII fragment. Deletion mutants pMG1115 and pMG1116 were generated from pMG1114. pMG1115 had a deletion of the 3.3-kbp EcoRI/BglII fragment between 8.4 kb and 11.7 kb on the map, which contains the C-terminal region of ORF11. pMG1115 did not express either the bacteriocin or immunity, indicating that ORF11 is necessary for bacteriocin activity. pMG1116 had a deletion of two HindIII fragments totaling 1.4 kb that were located between 5.2 kb and 6.6 kb on the map and contains the C-terminal region of ORF7 and all of ORF8, ORF9, and ORF10. pMG1116 did not express the bacteriocin but expressed immunity. Analysis of the insertion mutants and deletion mutants showed that ORF7, ORF8, ORF11, and ORF12 are necessary for bacteriocin expression.
Extracellular complementation of nonbacteriocinogenic mutants.
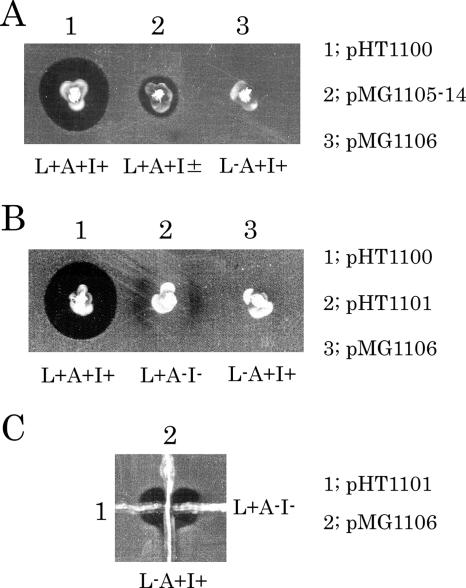
Extracellular complementation experiments to express bacteriocin activity were performed with ORF7 or ORF8 and ORF11 mutant strains on soft agar plates containing the indicator strain. OG1-10(pMG1106) and OG1-10(pMG1109), which were ORF7 mutants prepared by blunt ending, were streaked in proximity to streaks of either OG1-10 carrying the pHT1100 derivatives of the Tn5 insertion mutants in ORF11 or OG1-10(pHT1101) with a deletion in ORF11. This experiment showed that there was complementation of the bacteriocin activity at the streak junction. When OG1-10(pHT1101) was streaked in proximity to streaks of OG1-10 carrying the pHT1100 derivatives of the Tn5 insertion mutants in ORF7 or ORF8 and the end-filled mutants of ORF7, complementation of the bacteriocin activity was observed at the streak junction. These results indicated that the mutants fell into one of two complementation groups. Representative results are shown in Fig. 3 and Table 4. The OG1-10(pHT1100), OG1-10(pHT1101), and OG1-10(pMG1106) strains were inoculated in proximity in soft agar containing the indicator strain (Fig. 3B). Bacteriolysis was observed around the wild-type strain and also between OG1-10(pHT1101) and the wild-type strain or OG1-10(pMG1106), respectively. Bacteriolysis was also observed surrounding OG1-10(pHT1101). Figure 3C shows the complementation activity that resulted from cross-streaking of OG1-10(pMG1106) and OG1-10(pHT1101) on soft agar containing the indicator strain. Bacteriolysis was observed at the junction of the two strains. Based on these observations, the two complementation substances were tentatively designated L (lysin) and A (activator). OG1-10(pHT1101) and the ORF11 mutants were presumed to be defective in bacteriocin component A synthesis and tentatively assigned an L+ A− phenotype. The ORF11 gene was designated bacA. OG1-10(pMG1106) and the ORF7 and ORF8 mutants were presumed to be defective in bacteriocin component L synthesis and tentatively assigned an L− A+ phenotype. The ORF7 and ORF8 genes were designated bacL1 and bacL2, respectively.
FIG. 3.
Bacteriocin expression assay by the soft-agar method with E. faecalis OG1-10 carrying the representative pYI14 bacteriocin derivatives (A) and complementation assays (B and C). The indicator strain was E. faecalis OG1-10. The strains used are shown in Fig. 2 and Table 3. (A) 1, OG1-10(pHT1100) wild type; 2, OG1-10(pMG1105-14, a transposant of pHT1100::Tn5) (Tn5 inserted in the C-terminal region of ORF12); 3, OG1-10(pMG1106) in-frame bacL1 mutant. (B) 1, OG1-10(pHT1100); 2, OG1-10(pHT1101) bacA and bacI deletion mutant; 3, OG1-10(pMG1106). (C) 1; OG1-10(pHT1101), 2; OG1-10(pMG1106). L, bacL1 and bacL2 expression; A, bacA expression; I, immunity expression; +, positive expression; −, no expression; ±, weak expression.
TABLE 4.
Extracellular trans-complementation analysis of bacteriocin 41 activitya
| Plasmid(s) | Genotype | Phenotypeb | pMG1105-42, pMG1105-25 | pMG1106, pMG1109 | pMG1105-107, pMG1105-188 | pMG1105-104, pMG1105-11 | pHT1101 | pMG1110 | pMG1111 |
|---|---|---|---|---|---|---|---|---|---|
| pMG1105-42, pMG1105-25 | bacL1 L2+A+I+ | L− A+ I+ | NT | ||||||
| pMG1106, pMG1109 | bacL1 L2+A+I+ | L− A+ I+ | C− | NT | |||||
| pMG1105-107, MG1105-188 | bacL1+L2 A+I+ | L− A+ I+ | C− | C− | NT | ||||
| pMG1105-104, pMG1105-11 | bacL1+L2+A I+ | L+ A− I+ | C+ | C+ | C+ | NT | |||
| pHT1101 | bacL1+L2+A I | L+ A− I− | C+ | C+ | C+ | C− | NT | ||
| pMG1110 | bacL1+L2+A I | L+ A− I− | C+ | C+ | C+ | C− | C− | NT | |
| pMG1111 | bacL1 L2 A+I | L− A+ I− | C− | C− | C− | C+ | C+ | C+ | NT |
C+; bacteriocin activity was detected at the intersection of the two strains by the soft-agar assay, C−; no bacteriocin activity, NT; not tested.
L, expression of lytic protein(s); A, activator expression; I, immunity expression.
Cloning of component L, component A, and the immunity genes.
The PCR product of each ORF was cloned to analyze its function in bacteriocin expression (Fig. 2). Cloned pMG1110, pMG1111, pMG1112, and pMG1113 contained ORF7/8 (bacL1 and -L2), ORF11 (bacA), ORF12, and ORF12/13, respectively (Fig. 2). Each of the individually cloned fragments did not express bacteriocin activity. pMG1112 and pMG1113, which contained ORF12 and ORF12/13, expressed immunity to the bacteriocin activity, indicating that ORF12 was the immunity gene, and it was designated bacI.
Extracellular complementation between cloned L and A components.
Cross streaks of strains carrying the two cloned fragments were made on bacteriocin assay plates. When OG1-10(pMG1110), which contained ORF7 (bacL1) and ORF8 (bacL2), was streaked across a preexisting streak of OG1S (pMG1111), which contained ORF11 (bacA), a large area of bacteriolysis was observed around the two crossed strains (Table 4). Growth of the two strains was markedly inhibited. These data indicated that the product of each strain complemented to produce an active bacteriocin, but the two strains have no immunity to the bacteriocin; therefore, growth of the strains was inhibited by the bacteriocin.
DNA sequence analysis of ORFs located in the region containing the bacteriocin 41 determinant.
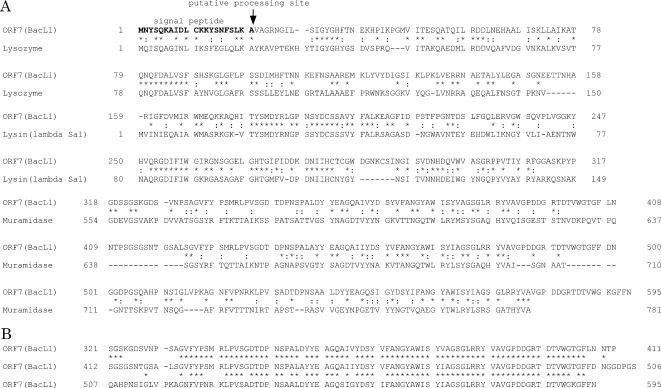
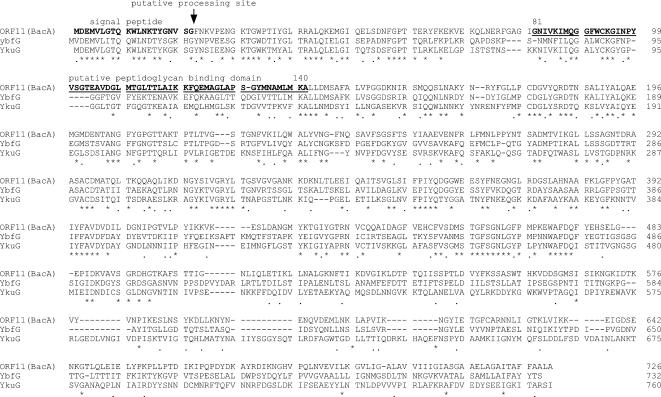
A homology search of the 15 ORFs contained in the 12-kbp region was performed by BLAST against the protein databases, and the results are shown in Table 2 (1). ORF7 (bacL1), ORF8 (bacL2), ORF11 (bacA), and ORF12 (bacI) were essential for the expression of bacteriocin 41. bacL1 encoded a 595-amino-acid protein. Computer analysis suggested that the deduced bacL1-encoded protein had a signal peptide sequence and that a potential signal peptidase processing site corresponding to the L-K-A sequence was located at positions 19 to 21 (Fig. 4A). Comparison of the primary structure of the deduced amino acid sequence of the BacL1 protein showed significant homology with the cell wall lytic enzymes found in gram-positive bacteria (Fig. 4A) (32). Of the 595 amino acid residues of the BacL1 protein, the N-terminal 151 amino acid residues showed a high level of homology with the lysozyme encoded on Bacillus subtilis bacteriophage B103 (accession number Q37896) (37). The 150-amino-acid sequence from residue 160 to residue 309, which is located in the center of the bacL1-encoded protein, showed a high level of homology with the N-terminal amino acid residues of the lysin encoded on the S. agalactiae prophage lambda Sa1 (accession number NP 687631) (43), and the C-terminal 260 amino acid residues showed a high level of homology with the C-terminal amino acid residues of the muramidase of Lactobacillus plantarum WCFS1 (accession number CAD64901) (30). The bacL1-encoded protein harbored a three-repeat structure of an almost identical amino acid sequence (Fig. 4B). The three-repeat structure located at the C terminus of the bacL1-encoded protein corresponded to the homologous C-terminal region of the L. plantarum WCS1 muramidase, which is thought to be a choline-binding region (28, 51). The repeat structure was composed of three copies of an almost identical 74-amino-acid sequence. The first copy was located between amino acid residues 333 and 406, the second copy was located between amino acid residues 424 and 497, and the third copy was located between amino acid residues 520 and 593. bacL2 encoded a 211-amino-acid protein and did not show any significant homology with other reported proteins. There was no obvious leader peptide with hydrophobic residues at the N-terminal peptide of the deduced bacL2-encoded protein. bacA encoded a 726-amino-acid protein and showed a significant degree of homology with ybfG and ykuG of B. subtilis, but the function of these proteins is unknown (Fig. 5) (accession numbers CAB12014 for YbfG and CAA10870 for YkuG, respectively). The bacA protein had a putative signal peptide sequence, and a potential signal peptidase processing site corresponding to the V-S-G sequence was located at positions 19 to 21 (Fig. 5). The bacA protein contained a 60-amino-acid sequence corresponding to the putative peptidoglycan-binding domain, which was located between amino acids 81 and 140 in the bacA-encoded protein, suggesting that the BacA protein could be directed to the bacterial cell surface.
FIG. 4.
Comparison of the amino acid sequence of the predicted BacL1 protein (ORF7) of bacteriocin 41 with the amino acid sequence of the cell wall lytic enzymes of gram-positive bacteria (A) and the repeat sequences found in the BacL1 protein (B). Lysozyme, B. subtilis bacteriophage B103 (accession number Q37896); lysin, S. agalactiae prophage lambda Sa1 (accession number NP 687631); muramidase, L. plantarum WCFS1 (accession number CAD64901).
FIG. 5.
Comparison of the amino acid sequence of the predicted BacA protein (ORF11) of bacteriocin 41 with those of the predicted proteins encoded by the genomic DNA of B. subtilis. The accession numbers are CAB12014 for YbfG and CAA10870 for YkuG, respectively.
DISCUSSION
Bacteriocin 41 of strain YI714 was encoded on E. faecalis pheromone-responsive plasmid pYI14 (61 kbp) and was only active against E. faecalis. The EcoRI AH fragments of pYI14, which conferred the bacteriocin activity, were cloned and used for genetic analysis of the bacteriocin determinant. Transposon insertion and deletion mutant analysis of the EcoRI AH fragments and further subcloning of the bacteriocin determinant showed that a 6.6-kb fragment of pYI14 was the minimum-size fragment required for bacteriocin expression. The 6.6-kb region contained six ORFs, which were designated bacL1, bacL2, ORF9, ORF10, bacA, and bacI. All of the ORFs were oriented in the same direction and in that order. The insertion mutants were classified into one of two complementation classes for component L and component A. Each class showed extracellular complementation to produce the active bacteriocin. A series of PCR products containing the L-encoding region for component L, the A-encoding region for component A, and the immunity-encoding region for resistance to the bacteriocin were subcloned into E. faecalis OG1-10. The subclones for the L-encoding, A-encoding, and immunity-encoding regions contained bacL1 and bacL2, bacA, and bacI, respectively. The subclone containing bacL1 and bacL2 produced an L component capable of extracellular complementation with the A component for expression of bacteriocin activity, indicating that bacL1 and bacL2 were required for component L. These results indicated that of the ORFs within the 6.6-kb region, bacL1, bacL2, and bacA are essential for the production of the active bacteriocin, and bacI is the immunity gene for resistance to the bacteriocin that is produced.
Tn5 insertions into bacL1 or bacL2 of the bacteriocin determinant did not result in a detectable polar effect on the expression of the downstream bacA or bacI gene, and insertion into bacA also did not result in a polar effect on the expression of bacI. Both component determinants and bacI were expressed when each of the determinants was cloned into vector plasmid pAM401 in either orientation within an E. faecalis OG1-10 background. These results suggested that a significant amount of transcription of the bacL1 and bacL2, bacA, and bacI genes can occur from different promoters.
In the complementation experiment between the L+ A− and L− A+ strains, bacteriocin activity was observed around the L+ A− strain. When the wild-type L+ A+ and mutant L+ A− strains were inoculated in proximity to the bacteriocin assay, bacteriolysis was observed around the L+ A− strain. The complementation experiment between the wild-type L+ A+ and mutant L− A+ strains did not show any bacteriocin activity. These results suggested that the activator of component A modified component L, that the activated component L possessed the bacteriocin activity, and also that an excess of component A existed in the extracellular medium.
The β-hemolysin/bacteriocin (cytolysin) determinant encoded on pAD1 consists of the eight genes cylR2, cylR1, cylLL, cylLS, cylM, cylB, cylA, and cylI (2, 8, 9, 17, 18, 39). CylLL and CylLS are the cytolysin structural subunits. The CylLL and CylLS proteins are modified posttranslation by CylLM (2), and the modified CylLL and CylLS proteins are secreted via CylLB, which is the ATP-binding exporter (16). The extracellular cytolysin precursors CylLL and CylLS are converted to the active cytolysin by CylA (2, 22). In an early study of the β-hemolysin/bacteriocin (cytolysin) determinant (22), two functional domains within the operon were identified and it was found that one region encodes the toxin precursor L component, which is now known to be encoded byCylL1, CylL2, CylM, and CylB, and the other region encodes an activator A component, which is now known to be encoded by CylA and CylI (2, 8, 9, 17, 18, 39). In the complementation experiment between the A component-producing strain or the wild-type strain and the L component-producing strain on blood agar plates, the β-hemolysis zone occurred around or along the L component-producing strain (22), indicating that the A component activates the L component extracellularly and that the activated L component possesses the β-hemolysin/bacteriocin activity and an excess of extracellular A component is present in the culture medium of the wild-type strain (24). These observations are similar to the extracellular complementation observed between the L component-producing strain and the A component-producing strain for bacteriocin 41.
The deduced amino acid sequence encoded by bacL1 showed a high degree of homology with the cell wall lytic enzymes and murein hydrolases of lysozyme, lysine, and the muramidase of gram-positive bacteria (32). These enzymes cleave glycan strains either between the N-acetylmuramic acid and N-acetylglucosamine or at the alternative acetylglucosamine-muramic acid glycoside linkage (34). Sequence alignments of the murein hydrolases of the gram-positive bacteria show that most of these enzymes display a domain structure. In general, these enzymes harbor an N-terminal signal peptide, followed by a second domain containing the enzymatic activity. In addition, these proteins harbor repeat structures or cell wall-targeting structures that flank either the N- or C-terminal side of the enzymatic domain (40). The repeated domains direct the murein hydrolase to its receptor on the cell surface of gram-positive bacteria (51). Murein hydrolase is usually synthesized as a preproenzyme, and after cleavage of the N-terminal signal peptide, the soluble proenzyme is secreted into the extracellular environment. The repeated domains or cell wall-targeting domains direct the proenzyme to its receptor on the bacterial cell surface. Proteolytic cleavage or activation of the proenzyme generates the mature enzyme (32).
Although the mechanism of activation or the precise mode of action of the bacL1-encoded protein is not known, analysis of the deduced amino acid sequence of the bacL1-encoded protein suggests that the protein exhibits a domain structure. The domain structure is composed of an N-terminal signal peptide followed by a second domain containing the enzymatic activity and a third domain with the three amino acid sequence repeat structures. The bacL1-encoded protein might be synthesized as a preproenzyme, and after signal peptide cleavage, the soluble proprotein encoded by bacL1 would be secreted into the extracellular environment. The repeat domains might function to direct the proprotein encoded by bacL1 to its receptor on the bacterial cell surface, and the proprotein encoded by bacL1 might be activated by the bacA protein, resulting in the generation of the mature BacL1 protein. As described above, bacL2 was also essential for the expression of the L component. The deduced bacL2-encoded protein was a 211-amino-acid protein with no leader peptide. The sequence data implied that the bacL2-encoded protein might modify the bacL1-encoded protein inside the bacterial cell. Bacteriocin 41 only showed bacteriocin activity against E. faecalis, which suggested that the bacL1-encoded protein of bacteriocin 41 was highly specific for the glycan strand of the E. faecalis cell wall.
Recently, another group reported the discovery of a novel cell wall-degrading bacteriocin, which has been named enterolysin A (EnlA), in an E. faecalis strain isolated from fish (36). The bacteriocin gene enlA encodes a 343-amino-acid preprotein with a sec-dependent signal peptide of 27 amino acids. The mature EnlA protein consists of 316 amino acids and is homologous to the catalytic domains of a variety of cell wall-degrading proteins. It might be that bacteriocin 41 belongs to the same group of enterococcal cell wall-degrading bacteriocins as EnlA, although the details of the mechanism of expression of EnlA, including the immunity factor, have not been clearly elucidated (15). However, our results imply that the mechanism of bacteriocin 41 expression is more complex than the EnlA expression system and that they are divergent systems.
Supplementary Material
Acknowledgments
This work was supported by grants from the Japanese Ministry of Education, Culture, Sport, Science and Technology [Tokutei-ryoiki (Matrix of Infection Phenomena), Kiban (B), Kiban (C)] and the Japanese Ministry of Health, Labor and Welfare (H15-Shinko-9, H18-Shinko-11).
We thank Nobuhiko Okada for providing plasmid pLZ12-Km. We thank Koichi Tanimoto, Shuhei Fujimoto, and Takako Inoue for helpful advice and discussions.
Footnotes
Published ahead of print on 18 January 2008.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215403-410. [DOI] [PubMed] [Google Scholar]
- 2.Booth, M. C., C. P. Bogie, H.-G. Sahl, R. J. Siezen, K. L. Hatter, and M. S. Gilmore. 1996. Structural analysis and proteolytic activation of Enterococcus faecalis cytolysin, a novel lantibiotic. Mol. Microbiol. 211175-1184. [DOI] [PubMed] [Google Scholar]
- 3.Brock, T. D., B. Peacher, and D. Pierson. 1963. Survey of the bacteriocins of enterococci. J. Bacteriol. 86702-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chow, J. W., L. A. Thal, M. B. Perri, J. A. Vazquez, S. M. Donabedian, D. B. Clewell, and M. J. Zervos. 1993. Plasmid-associated hemolysin and aggregation substance production contribute to virulence in experimental enterococcal endocarditis. Antimicrob. Agents Chemother. 372474-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clewell, D. B. 1981. Plasmids, drug resistance, and gene transfer in the genus Streptococcus. Microbiol. Rev. 45409-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clewell, D. B., and G. M. Dunny. 2002. Conjugation and genetic exchange in enterococci, p. 265-300. In M. S. Gilmore, D. B. Clewell, P. Courvalin, G. M. Dunne, B. E. Murray, and L. B. Rice. (ed.), The enterococci: pathogenesis, molecular biology and antibiotic resistance. ASM Press, Washington, DC.
- 7.Clewell, D. B., P. K. Tomich, M. C. Gawron-Burke, A. E. Franke, Y. Yagi, and F. Y. An. 1982. Mapping of Streptococcus faecalis plasmids pAD1 and pAD2 and studies relating to transposition of Tn917. J. Bacteriol. 1521220-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coburn, P. S., L. E. Hancock, M. C. Booth, and M. S. Gilmore. 1999. A novel means of self-protection, unrelated to toxin activation, confers immunity to the bactericidal effects of the Enterococcus faecalis cytolysin. Infect. Immun. 673339-3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coburn, P. S., C. M. Pillar, B. D. Jett, W. Haas, and M. S. Gilmore. 2004. Enterococcus faecalis senses target cells and in response expresses cytolysin. Science 3062270-2272. [DOI] [PubMed] [Google Scholar]
- 10.Coque, T. M., J. E. Patterson, J. M. Steckelberg, and B. E. Murray. 1995. Incidence of hemolysin, gelatinase, and aggregation substance among enterococci isolated from patients with endocarditis and other infections and from feces of hospitalized and community-based persons. J. Infect. Dis. 1711223-1229. [DOI] [PubMed] [Google Scholar]
- 11.Dunny, G. M., B. L. Brown, and D. B. Clewell. 1978. Induced cell aggregation and mating in Streptococcus faecalis: evidence for a bacterial sex pheromone. Proc. Natl. Acad. Sci. USA 753479-3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunny, G. M., R. A. Craig, R. L. Carron, and D. B. Clewell. 1979. Plasmid transfer in Streptococcus faecalis: production of multiple sex pheromones by recipients. Plasmid 2454-465. [DOI] [PubMed] [Google Scholar]
- 13.Fujimoto, S., H. Hashimoto, and Y. Ike. 1991. Low cost device for electrotransformation and its application to the highly efficient transformation of Escherichia coli and Enterococcus faecalis. Plasmid 26131-135. [DOI] [PubMed] [Google Scholar]
- 14.Fujimoto, S., H. Tomita, E. Wakamatsu, K. Tanimoto, and Y. Ike. 1995. Physical mapping of the conjugative bacteriocin plasmid pPD1 of Enterococcus faecalis and identification of the determinant related to the pheromone response. J. Bacteriol. 1775574-5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franz, C. M. A. P., M. J. van Belkum, W. H. Holzapfel, H. Abriouel, and A. Galvez. 2007. Diversity of enterococcal bacteriocins and their grouping in a new classification scheme. FEMS Microbiol. Rev. 31293-310. [DOI] [PubMed] [Google Scholar]
- 16.Gilmore, M. S., R. A. Segarra, and M. C. Booth. 1990. An HlyB-type function is required for expression of the Enterococcus faecalis hemolysin/bacteriocin. Infect. Immun. 583914-3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilmore, M. S., R. A. Segarra, M. C. Booth, C. P. Bogie, L. R. Hall, and D. B. Clewell. 1994. Genetic structure of the Enterococcus faecalis plasmid pAD1-encoded cytolytic toxin system and its relationship to lantibiotic determinants. J. Bacteriol. 1767335-7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haas, W., B. D. Shepard, and M. S. Gilmore. 2002. Two-component regulator of Enterococcus faecalis cytolysin responds to quorum-sensing autoinduction. Nature 41584-87. [DOI] [PubMed] [Google Scholar]
- 19.Hanski, E., P. A. Horvitz, and M. G. Caparon. 1992. Expression of protein F, the fibronectin-binding protein of Streptococcus pyogenes JRS4, in heterologous streptococcal and enterococcal strains promotes their adherence to respiratory epithelial cells. Infect. Immun. 605119-5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ike, Y., and D. B. Clewell. 1984. Genetic analysis of the pAD1 pheromone response in Streptococcus faecalis, using transposon Tn917 as an insertional mutagen. J. Bacteriol. 158777-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ike, Y., and D. B. Clewell. 1992. Evidence that the hemolysin/bacteriocin phenotype of Enterococcus faecalis subsp. zymogenes can be determined by plasmids in different incompatibility groups as well as by the chromosome. J. Bacteriol. 1748172-8177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ike, Y., D. B. Clewell, R. A. Segarra, and M. S. Gilmore. 1990. Genetic analysis of the pAD1 hemolysin/bacteriocin determinant in Enterococcus faecalis: Tn917 insertional mutagenesis and cloning. J. Bacteriol. 172155-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ike, Y., R. A. Craig, B. A. White, Y. Yagi, and D. B. Clewell. 1983. Modification of Streptococcus faecalis sex pheromones after acquisition of plasmid DNA. Proc. Natl. Acad. Sci. USA 805369-5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ike, Y., S. E. Flannagan, and D. B. Clewell. 1992. Hyperhemolytic phenomena associated with insertions of Tn916 into the hemolysin determinant of Enterococcus faecalis plasmid pAD1. J. Bacteriol. 1741801-1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ike, Y., H. Hashimoto, and D. B. Clewell. 1984. Hemolysin of Streptococcus faecalis subspecies zymogenes contributes to virulence in mice. Infect. Immun. 45528-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ike, Y., H. Hashimoto, and D. B. Clewell. 1987. High incidence of hemolysin production by Enterococcus (Streptococcus) faecalis strains associated with human parenteral infection. J. Clin. Microbiol. 251524-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jack, R. W., J. R. Tagg, and B. Ray. 1995. Bacteriocins of gram-positive bacteria. Microbiol. Rev. 59171-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jedrzejas, M. K. 2001. Pneumococcal virulence factors: structure and function. Microbiol. Mol. Biol. Rev. 65187-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jett, B. D., H. G. Jensen, R. E. Nordquist, and M. S. Gilmore. 1992. Contribution of the pAD1-encoded cytolysin to the severity of experimental Enterococcus faecalis endophthalmitis. Infect. Immun. 602445-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kleerebezem, M., J. Boekhorst, R. van Kranenburg, D. Molenaar, O. P. Kuipers, R. Leer, R. Tarchini, S. A. Peters, H. M. Sandbrink, M. W. E. J. Fiers, W. Stiekema, R. M. Klein Lankhorst, P. A. Bron, S. M. Hoffer, M. N. Nierop Groot, R. Kerkhoven, M. de Vries, B. Ursing, W. M. de Vos, and R. J. Siezen. 2003. Complete genome sequence of Lactobacillus plantarum WCFS1. Proc. Natl. Acad. Sci. USA 1001990-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LeBlanc, D. J., L. N. Lee, D. B. Clewell, and D. Behnke. 1983. Broad geographical distribution of a cytotoxin gene mediating beta-hemolysis and bacteriocin activity among Streptococcus faecalis strains. Infect. Immun. 401015-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lópes, R., and E. Garcia. 2004. Recent trends on the molecular biology of pneumococcal capsules, lytic enzymes, and bacteriophage. FEMS Microbiol. Rev. 28553-580. [DOI] [PubMed] [Google Scholar]
- 33.Martínez-Bueno, M., M. Maqueda, A. Galvez, B. Samyn, J. V. Beeumen, J. Coyette, and E. Valdivia. 1994. Determination of the gene sequence and the molecular structure of the enterococcus peptide antibiotic AS-48. J. Bacteriol. 1766334-6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Navarre, W. W., and O. Schneewind. 1999. Surface proteins of gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol. Mol. Biol. Rev. 63174-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nes, I. F., D. B. Diep, and H. Holo. 2007. Bacteriocin diversity in Streptococcus and Enterococcus. J. Bacteriol. 1891189-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nilsen, T., I. F. Nes, and H. Holo. 2003. Enterolysin A, a cell wall-degrading bacteriocin from Enterococcus faecalis LMG 2333. Appl. Environ. Microbiol. 692975-2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pecenková, T., V. Benes, J. Paces, C. Vlcek, and V. Paces. 1997. Bacteriophage B103: complete DNA sequence of its genome and relationship to other Bacillus phages. Gene 199157-163. [DOI] [PubMed] [Google Scholar]
- 38.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 39.Segarra, R. A., M. C. Booth, D. A. Morales, M. M. Huycke, and M. S. Gilmore. 1991. Molecular characterization of the Enterococcus faecalis cytolysin activator. Infect. Immun. 591239-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheehan, M. M., J. L. García, R. López, and P. García. 1997. The lytic enzyme of the pneumococcal phage Dp-1: a chimeric lysin of intergeneric origin. Mol. Microbiol. 25717-725. [DOI] [PubMed] [Google Scholar]
- 41.Shiojima, M., H. Tomita, K. Tanimoto, S. Fujimoto, and Y. Ike. 1997. High-level plasmid-mediated gentamicin resistance and pheromone response of plasmids present in clinical isolates of Enterococcus faecalis. Antimicrob. Agents Chemother. 41702-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanimoto, K., and T. Iino. 1985. Additional genes essential for replication of the mini-F plasmid from origin I. Mol. Gen. Genet. 198358-359. [DOI] [PubMed] [Google Scholar]
- 43.Tettelin, H., V. Masignani, M. J. Cieslewicz, J. A. Eisen, S. Peterson, M. R. Wessels, I. T. Paulsen, K. E. Nelson, I. Margarit, T. D. Read, L. C. Madoff, A. M. Wolf, M. J. Beanan, L. M. Brinkac, S. C. Daugherty, R. T. DeBoy, A. S. Durkin, J. F. Kolonay, R. Madupu, M. R. Lewis, D. Radune, N. B. Fedorova, D. Scanlan, H. Khouri, S. Mulligan, H. A. Carty, R. T. Cline, S. E. Van Aken, J. Gill, M. Scarselli, M. Mora, E. T. Iacobini, C. Brettoni, G. Galli, M. Mariani, F. Vegni, D. Maione, D. Rinaudo, R. Rappuoli, J. L. Telford, D. L. Kasper, G. Grandi, and C. M. Fraser. 2002. Complete genome sequence and comparative genomic analysis of an emerging human pathogen, serotype V Streptococcus agalactiae. Proc. Natl. Acad. Sci. USA 9912391-12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomich, P. K., F. Y. An, and D. B. Clewell. 1980. Properties of erythromycin-inducible transposon Tn917 in Streptococcus faecalis. J. Bacteriol. 1411366-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tomita, H., and D. B. Clewell. 2000. A pAD1-encoded small RNA molecule, mD, negatively regulates Enterococcus faecalis pheromone response by enhancing transcription termination. J. Bacteriol. 1821062-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tomita, H., S. Fujimoto, K. Tanimoto, and Y. Ike. 1996. Cloning and genetic organization of the bacteriocin 31 determinant encoded on the Enterococcus faecalis pheromone-responsive conjugative plasmid pYI17. J. Bacteriol. 1783585-3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tomita, H., S. Fujimoto, K. Tanimoto, and Y. Ike. 1997. Cloning and genetic and sequence analysis of the bacteriocin 21 determinant encoded on the Enterococcus faecalis pheromone-responsive conjugative plasmid pPD1. J. Bacteriol. 1797843-7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tomita, H., and Y. Ike. 2005. Genetic analysis of transfer-related regions of the vancomycin resistance Enterococcus conjugative pHTβ: identification of oriT and a putative relaxase gene. J. Bacteriol. 1877727-7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tomita, H., C. Pierson, S. K. Lim, D. B. Clewell, and Y. Ike. 2002. Possible connection between a widely disseminated conjugative gentamicin resistance(pMG1-like) plasmid and the emergence of vancomycin resistance in Enterococcus faecium. J. Clin. Microbiol. 403326-3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wirth, R., F. Y. An, and D. B. Clewell. 1986. Highly efficient protoplast transformation system for Streptococcus faecalis and a new Escherichia coli-S. faecalis shuttle vector. J. Bacteriol. 165831-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wren, B. W. 1991. A family of clostridial and streptococcal ligand-binding proteins with conserved C-terminal repeat sequences. Mol. Microbiol. 5797-803. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.