Abstract
In this study, we describe wosA, a Proteus mirabilis gene identified by its ability to increase swarming motility when overexpressed. At various times during the swarming cycle, the increased expression of wosA resulted in a 4- to 16-fold upregulation of the transcription of flhDC, encoding the master regulator of the flagellar cascade. In turn, the expression of flaA, encoding flagellin, was substantially increased in wosA-overexpressing strains. The overexpression of wosA also resulted in constitutive swarmer cell differentiation in liquid medium, a normally nonpermissive condition. However, in wosA-overexpressing strains, the onset of swarming was not altered. A null wosA allele resulted in a slight decrease in swarming motility. The expression of wosA was growth phase dependent during growth in liquid and on agar plates during swarmer cell differentiation. Increasing the viscosity of liquid medium by the addition of polyvinylpyrrolidone induced swarmer cell differentiation and resulted in a fourfold increase in wosA transcription. A fliL mutation that results in constitutive swarmer cell elongation also increased wosA transcription. In this study, we discuss the possible role of the wosA gene product in signal transduction from solid surfaces to induce swarmer cell differentiation, possibly via alterations in the motor switch complex. This study also suggests that despite constitutive swarmer cell differentiation in wosA-overexpressing strains, there are additional regulatory and/or environmental conditions that may control the onset of swarming migration.
Proteus mirabilis is a motile gram-negative bacterium that undergoes cellular differentiation and a coordinated social behavior termed swarming motility that results in movement over solid surfaces (reviewed in references 3, 15, and 36). During differentiation, short rod-shaped vegetative cells with few peritrichous flagella elongate 20- to 40-fold and increase the number of flagella by >50-fold to form swarmer cells. Swarming then occurs as cellular differentiation, migration away from a central inoculation point, and consolidation or a period of dedifferentiation to produce terraces in a characteristic pattern of growth on 1.5 to 2% agar (15, 37). The process of differentiation depends on certain environmental conditions, including a solid surface and the inhibition of flagellar rotation (1, 15, 37). In addition, although not well understood, extracellular signaling has been implicated in the process of swarmer cell differentiation (4, 43).
In Escherichia coli and Salmonella enterica serovar Typhimurium, the production of flagella requires the expression of more than 50 genes that are coordinately regulated in a regulon comprised of three temporally regulated hierarchical transcriptional classes: class I, class II, and class III. The heterotetrameric FlhD2C2 master regulator of the flagellar gene hierarchy is encoded by the class I operon flhDC. The transcription of all the class II and III genes of the flagellar cascade is dependent on the expression of flhDC (reviewed in reference 10). In E. coli and S. enterica serovar Typhimurium, the FlhD2C2 regulator acts to integrate a variety of regulatory inputs to control the cascade of flagellar gene expression. These inputs include catabolite repression (41, 46), osmolarity (40), heat shock (39), acetyl phosphate (40), and cell cycle regulation (33). The regulation of swarmer cell differentiation in P. mirabilis and other swarming bacteria has also been shown previously to act through FlhD2C2 (12, 14, 16, 17, 42, 44, 47). One hallmark of the switch to a swarmer cell from a vegetatively growing swimmer cell is a sharp transient increase in the expression of flhDC (11). The increased FlhDC levels then ultimately lead to a drastic increase in the expression of the class III flagellar gene flaA that encodes the monomer subunit of the flagellar filament, resulting in hyperflagellation of the swarmer cell (15). The artificial overexpression of flhDC in both P. mirabilis and another swarming bacterium, Serratia liquefaciens, causes increased elongation and hyperflagellation, with a corresponding increase in both the rapidity of onset and the velocity of swarming (12, 16). Moreover, E. coli FlhD has been shown previously to suppress cell division (35). Multiple P. mirabilis mutations that affect flhDC transcription and, therefore, swarming have been identified. Mutations of the global transcriptional regulator leucine-responsive protein (encoded by lrp) (21) and the four novel regulators encoded by umoA to umoD (11) that normally upregulate flhDC transcription prevent or cause defective swarmer cell differentiation. The RcsCDB phosphorelay protein, originally identified as a positive regulator of capsular polysaccharide synthesis in E. coli (reviewed in reference 28), has also been shown previously to repress the transcription of the flhDC operon in E. coli (14). The P. mirabilis homolog of RcsD is the RsbA protein (7, 27). Mutations in rsbA lead to a hyperswarming, or “precocious,” phenotype likely caused by the derepression of flhDC expression (7, 27).
Many bacteria, including Escherichia, Salmonella, Bacillus, Proteus, Serratia, and Vibrio species, undergo swarming or surface-induced motility (2, 20, 22, 45). Swarmer cell differentiation is initiated upon surface contact and typically involves the expression of peritrichous flagella and the elongation of a vegetatively growing cell. In Vibrio parahaemolyticus, the restriction of the rotation of the polar flagellum by the addition of anti-polar flagellum antiserum to the culture medium (8) or by an increase in the medium's viscosity (30), as well as plating onto a solid agar surface, results in the expression of the laf genes which encode components of the lateral flagella used for swarming motility. The polar flagellum therefore acts as a mechanosensor that senses a decrease in flagellar rotation and subsequently directs laf gene expression (26). In P. mirabilis, the molecular signals for swarming are still not well understood. However, the same conditions that inhibit flagellar rotation in V. parahaemolyticus to cause swarmer cell differentiation in normally noninducing liquid medium (with the addition of anti-flagellum antiserum and with high viscosity) also cause swarmer cell differentiation in P. mirabilis (9). Moreover, mutations in the P. mirabilis genes fliL, fliF, and fliG, which encode components of the flagellar basal body, confer a defective swarming phenotype yet result in swarmer cell differentiation in normally noninducing liquid medium and hyperelongated swarmer cells on a solid agar surface. This finding again implicates the rotating flagellar filament as being critical for signal sensing during the process of swarmer cell differentiation (9).
In this work, we describe the gene wosA, which causes a novel swarming phenotype when overexpressed in wild-type P. mirabilis. wosA overexpression caused constitutive swarmer cell differentiation under noninducing conditions and hyperswarming despite maintaining the same pattern of onset of swarming migration as that of the wild-type strain. The hyperswarming phenotype is likely due to (i) increased swarming velocity and (ii) the overexpression of flhDC throughout the swarm cycle. A null allele of wosA resulted in a slight decrease in swarming motility. Interestingly, the inhibition of flagellar rotation by an increase in the medium viscosity or by a mutation in the fliL gene led to the upregulation of wosA expression. Our results indicate that wosA is involved in the regulation of flhDC expression but also suggest that wosA may function in a signaling pathway from the flagellar motor switch complex. Moreover, our results suggest that additional regulation exists to control swarmer cell differentiation and the onset of swarming migration.
MATERIALS AND METHODS
Strains, plasmids, and media.
The bacterial strains and plasmids used in this work are listed in Table 1. Routine microbiological procedures and enzymatic manipulations of DNA were carried out by standard methods (38). Both E. coli and P. mirabilis strains were maintained in Luria-Bertani (LB) broth (10 g of tryptone, 5 g of yeast extract, 5 g of NaCl per liter of distilled water) or on LB with agar at a concentration of 1.5 to 2.0%. The concentration of antibiotics used for the selection of E. coli on LB agar and in culture was 25 μg/ml for both chloramphenicol and streptomycin. Antibiotic concentrations used for the selection of P. mirabilis on LB agar and in culture were 100 μg/ml for chloramphenicol, 35 μg/ml for streptomycin, and 15 μg/ml for tetracycline. X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) in N,N-dimethylformamide was used at a final concentration of 100 μg/ml in indicator agar.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Description and/or relevant genotype | Source or reference |
|---|---|---|
| E. coli strains | ||
| DH5α | F− φ80dlacZΔM15 Δ(lacZYA-argF′)U169 endA1 recA1 hsdR17(rK− mK−) deoR thi-1 supE44 λ−gyrA96 relA1 | Laboratory stock |
| SM10 λpir | thi thr leu tonA lacY supE recA RP4-2Tc::Mu Kmr λpir | 32 |
| P. mirabilis strains | ||
| PM7002 | Wild type; Tcr | ATCC |
| PM437 | speA::mini-Tn5lacZ1 Tcr | 43 |
| BB2000 | Wild type; Rfr Tcr | 6 |
| BB2204 | BB2000 fliL2204::mini-Tn5-Cm Cmr Rfr Tcr; non-wild-type elongation | 9 |
| PM7002 wosA::pKNG101 wosA | Smr Tcr | This study |
| PM7002 wosA::pKNG101 wosA-lacZ | Smr Tcr | This study |
| Plasmids | ||
| pBC SK− | High copy no.; Cmr | Stratagene |
| pKNG101 | R6K-derived suicide vector; Smr | 25 |
| pQF50 | Ampr | 13 |
| pSwarm7 | High copy no.; Cmr | This study |
| pORF1 | High copy no.; Cmr | This study |
| pYbhKPm | High copy no.; Cmr | This study |
| pKNG101 wosA | Smr | This study |
| pKNG101 wosA-lacZ | Smr | This study |
Strain construction.
The pSwarm7 plasmid was digested with HpaII, and a 5.1-kb fragment consisting of the pBC SK− vector and orf1 was recircularized to form the orf1 plasmid. This plasmid contains the full 274-bp sequence upstream of orf1 (wosA) that includes the putative wosA promoter and 500 bp downstream of the gene found in the original pSwarm7 plasmid. The P. mirabilis ybhK (ybhKPm) gene was isolated on a 1.1-kb DraI fragment and ligated into the EcoRV site of pBC SK−. Both plasmids were introduced into wild-type P. mirabilis PM7002 by electroporation using standard procedures. The transcription of ybhKPm results from the lac promoter found on the pBC SK− cloning vector.
The disruption of wosA was accomplished by homologous recombination between the chromosomal copy of wosA and a 400-bp internal fragment of wosA, PCR amplified using the 5′-ATGCTCTAGAACCAATGACTTTTACCAGTTAC-3′ and 5′-GGTACTCGAGCGACATCGGAAAATGTTTGATG-3′ primers incorporating XbaI and XhoI restriction sites and cloned into the XbaI- and SalI-digested suicide vector pKNG101 (25). Southern blot analysis confirmed the disruption of the wosA locus (data not shown).
T7 and T3 primers were used to amplify wosA from the pWosA plasmid. The 1,493-bp PCR product was then gel purified and digested with XbaI and HindIII to produce an 844-bp fragment containing 274 bp upstream of wosA and 570 bp of the wosA gene. This fragment was then ligated into the XbaI- and HindIII-digested promoter probe vector pQF50 (13) to give the pQF50wosA-lacZ promoter fusion plasmid. The wosA-lacZ fusion was then isolated on an XbaI-ScaI fragment from the pQF50wosA-lacZ plasmid and ligated into the XbaI- and SmaI-digested suicide vector pKNG101 (25). Single-copy wosA-lacZ fusions were constructed in wild-type and wosA::Kmr strains by homologous recombination between the chromosomal copy of wosA and the wosA-lacZ fusion ligated into pKNG101 (25). Southern blotting and PCR analysis confirmed the correct insertion at the wosA locus (data not shown).
Swarmer cell elongation and swarming migration assays.
Strains were grown overnight at 37°C in LB medium containing appropriate antibiotics with or without shaking. Cultures were diluted to the same optical density at 600 nm (OD600), 4 μl of each culture was spotted onto 1.5 or 2.0% LB agar, and the culture plates were incubated at 37°C. Swarmer cell elongation was determined by examining cells from the peripheries of the swarming colonies by phase-contrast microscopy. Because cell samples from the surface of an agar plate are often made up of mixed populations of both swimmer cells and swarmer cells, for this study, any cell with a length ≥5 times the mean length of a swimmer cell (1.96 ± 0.30 μm) was defined as a swarmer cell. At least 100 total cells were measured to determine the percentage of swarmer cells in the sample, and at least 50 swarmer cells were measured to determine the mean swarmer cell length, reported as the mean ± the standard deviation (SD). Swarming diameter was measured every 30 min to determine migration distance.
Northern blot analyses.
To assay flhDC, flaA, or wosA mRNA levels, relevant strains were grown overnight in LB medium containing appropriate antibiotics and used to inoculate fresh media, and cultures were grown with or without shaking at 37°C to a specified OD600. Two hundred-microliter aliquots of the cultures were then plated onto several LB agar plates in parallel to produce synchronously differentiating plate cultures. RNA was prepared from cells harvested from these plates in Tris-EDTA at 1-h intervals throughout the swarm cycle (1 h postinoculation of the plates [T1] to T6). One milliliter of a culture at a specified OD600 in liquid medium (LB) was also harvested for RNA extraction. Total RNA was prepared from each sample using the Masterpure RNA purification kit (Epicenter). Northern blot analysis was carried out with equivalent amounts of formamide-formaldehyde-denatured RNA electrophoretically separated through a 1.2% formaldehyde agarose gel and transferred onto a nylon membrane (Magnagraph; GE Osmonics) by downward capillary transfer. Blots were probed with a digoxigenin-labeled PCR-amplified segment of either the flhDC gene (5′-TCGGACGGGATGTAAAGAGA-3′ and 5′-CAGGATTGGCGGAAAGTTTA-3′), the flaA gene (5′-TATCTGGGGTGCCGATAAAC-3′ and 5′-ACGGTTTTGAATCGCACCTA-3′), or the wosA gene (5′-ATGCTCTAGAACCAATGACTTTTACCAGTTAC-3′ and 5′-GGTACTCGAGCGACATCGGAAAATGTTTGATG-3′) from P. mirabilis PM7002. Transcripts were visualized using the CDP-Star substrate according to the instructions of the manufacturer (Roche Applied Science).
Cell growth and measurement of wosA gene expression using a wosA-lacZ transcriptional fusion.
For samples from liquid culture, an overnight culture of the PM7002 wosA::wosA-lacZ strain in LB containing streptomycin was grown at room temperature without shaking to produce a low-density inoculum. The next morning, 30 ml of LB containing streptomycin was inoculated at a 1:1,000 dilution of the overnight culture. The cells were then incubated at 37°C with shaking, and 0.9-ml samples at various OD600s were collected in duplicate. The β-galactosidase activity of sodium dodecyl sulfate-chloroform-treated cells was assayed using the substrate ortho-nitrophenol-β-d-galactopyranoside by the method of Miller (31), and the results were reported as the means ± SDs.
To assay β-galactosidase activity from swarmer cells during a swarm cycle, an overnight culture of the PM7002 wosA::wosA-lacZ strain in LB containing streptomycin was grown at 37°C without shaking. The next morning, 30 ml of LB containing streptomycin was inoculated at a 1:200 dilution of the overnight culture. The cells were then incubated at 37°C with shaking to an OD600 of 0.4, and 200-μl samples were plated in parallel onto multiple plates to produce synchronously differentiated cells. Plates were harvested in phosphate-buffered saline, pH 7.4, at 1-h intervals, the OD600 at each time point was determined, and 0.9-ml samples of cells were collected in duplicate for analysis. β-galactosidase activity was assayed as described above for liquid culture samples.
For conditioned-medium preparations, 30-ml aliquots of LB broth cultures were used. Cells were grown to an OD600 of 1.2, and spent medium was collected by pelleting the cells at 4,300 × g for 10 min. Cell-free medium was prepared by adjusting the pH to 7.5 and filter sterilizing with a 0.22-μm-pore-size filter unit (Nalgene). The first 5 ml was discarded to wash off filter components. Individual cultures for the assay of β-galactosidase activity were grown in conditioned medium at 37°C with shaking, and 0.9-ml samples at various OD600s were collected in duplicate. β-galactosidase activity was assayed as described in the previous paragraphs.
Preparation of PVP and cell growth in PVP.
To increase the viscosity of LB medium, polyvinylpyrrolidone 360 (PVP; Sigma) was dissolved to a concentration of 30% in distilled water. This solution was dialyzed overnight against five changes of 100 volumes of distilled water. The volume of the PVP solution doubled upon dialysis, resulting in a concentration of 15% PVP. LB solids were added directly to the dialyzed PVP solution, and the solution was sterilized, resulting in LB medium containing 15% PVP. For growth in PVP, vegetative P. mirabilis cells were harvested by centrifugation of an overnight LB broth culture grown at 37°C without shaking and were resuspended in fresh broth to an OD600 of 0.6. The washed cells were inoculated at a dilution of 1:100 into both LB and LB medium containing 15% PVP and the appropriate antibiotics and grown at 37°C with shaking. After 2 h of growth, cells were harvested at 30-min intervals for the assay of β-galactosidase activity. β-galactosidase activity was assayed as described above.
Nucleotide sequence accession number.
The orf1 (wosA) nucleotide sequence determined in this study has been deposited in GenBank under accession no. EU164546.
RESULTS
Identification of pSwarm7, a multicopy suppressor of a swarming-defective speA mutant.
Previous work identified a putrescine biosynthetic pathway mutant, PM437 (speA::mini-Tn5lacZ1), that was defective in swarmer cell differentiation and, thus, swarming motility (43). The addition of exogenous putrescine restored the swarming motility of this mutant to wild-type levels. In an attempt to identify multicopy suppressors of the defect in the putrescine signaling pathway, PM437 was electroporated with a pBC SK− library of partial Sau3AI-digested chromosomal DNA from the PM437 strain. pSwarm7 was identified as a plasmid that restored the swarming phenotype to PM437 (Fig. 1A). However, the restoration of the swarming phenotype appeared to be independent of the speA mutation, as wild-type P. mirabilis PM7002 transformed with the pSwarm7 plasmid also exhibited increased swarming (Fig. 1A).
FIG. 1.
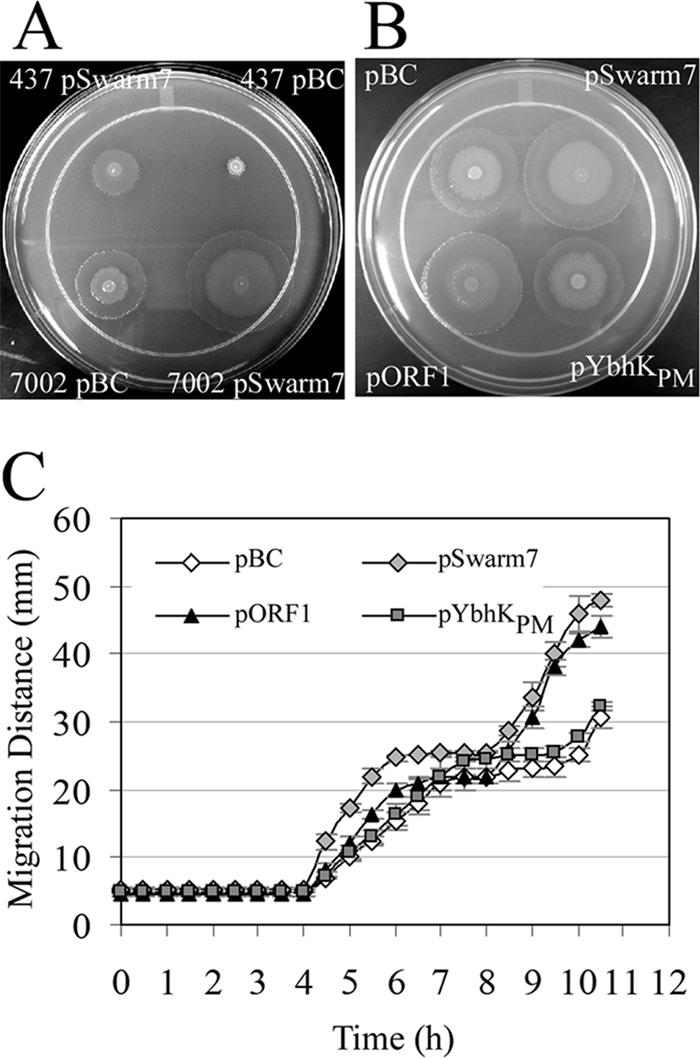
WosA overexpression increases the swarming motility of P. mirabilis. (A) Swarming behavior of wild-type P. mirabilis PM7002 and PM437 (speA::mini-Tn5lacZ1) carrying the cloning vector pBC SK− or the pSwarm7 plasmid and grown on 2% LB agar at 37°C for 16 h. (B) Comparison of the swarming behavior of wild-type P. mirabilis PM7002 carrying cloning vector pBC SK− or the pSwarm7 plasmid with that of PM7002 containing the pORF1 or pYbhKPm plasmid. Strains were grown on 1.5% LB agar at 37°C for 10 h. (C) Swarming migration of wild-type P. mirabilis 7002 carrying either the pBC vector alone or the pSwarm7, pORF1, or pYbhKPm plasmid. Strains were grown overnight at 37°C without shaking in 2.5 ml of LB supplemented with 100 μg of chloramphenicol/ml. Cultures were diluted to the same OD600 (0.4), 4 μl of each culture was spotted onto 1.5% LB agar with appropriate antibiotics, and swarming diameter was measured every 30 min over a 10.5-h period. Values are the means of results from three independent experiments. Error bars, SDs.
The sequencing of the pSwarm7 clone revealed the presence of two divergently transcribed open reading frames (ORFs) in the cloned insert. Neither of the two ORFs encoded products of known function, as determined by comparing the ORF sequences to sequences in GenBank. orf1 encodes a putative 321-residue protein with an unknown function and no significant similarity to other proteins. Interestingly, although the organization of the genes in the genome surrounding orf1 is conserved between P. mirabilis and E. coli, a homolog of orf1 is not present in the E. coli genome. The second ORF, a homolog of the E. coli ybhK gene, encodes a putative 311-residue protein that belongs to an uncharacterized protein family, UPF0052 (29); we designated this gene ybhKPm.
To determine which gene was responsible for the hyperswarming phenotype, a library of random Tn7 Kan insertions in pSwarm7 was generated in vitro using the pGPS5 vector from the GPS-LS linker-scanning system (New England Biolabs). Plasmids unable to restore the swarming phenotype to PM437 were sequenced, and it was shown that all insertions that prevented the enhanced swarming phenotype were within orf1. Moreover, it was unlikely that polar effects on the ybhKPm gene were responsible for the lack of enhanced swarming in these strains, as the ybhKPm gene and orf1 are transcribed divergently.
To independently verify that orf1 was the gene responsible for the phenotype observed in the pSwarm7 overexpression strain, both orf1 and ybhKPm were individually subcloned into the pBC SK− multicopy cloning vector. The swarming phenotype and the migration of the strains containing each of the genes were evaluated in comparison to those of wild-type PM7002 containing vector alone and PM7002 containing the original pSwarm7 plasmid (Fig. 1B and C). All four strains examined initiated swarming at 4 h after plating. Both PM7002(pSwarm7) and PM7002(pORF1) displayed a hyperswarming phenotype relative to wild-type PM7002(pBC SK−), with swarming velocities of approximately 10, 8, and 6 mm/h, respectively. PM7002(pYbhKPm), however, did not show enhanced swarming relative to the wild-type strain. Interestingly, although the patterns of the onset of swarming were the same for all four strains, the pSwarm7 and pORF1 strains appeared to have a shorter swarming period of 2 h (hours 4 to 6 postplating). Wild-type and pYbhKPm strains continued swarming for an additional hour. Moreover, the pSwarm7 and pORF1 strains remained in consolidation for only 2 h, while the wild-type and pYbhKPm strains were in consolidation for 2.5 h. Indeed, if we define consolidation by a change of less than 10% in migration distance during a 1/2-h period, then the four strains showed significantly different consolidation times (analysis of variance [ANOVA]: F3,8 = 22.22; P = 0.0003). We used Tukey's honestly significantly different test (34) to determine which consolidation times differed significantly (P < 0.05) from each other and found that the consolidation times of approximately 2 h for the pSwarm7 and pORF1 strains did not differ significantly from each other but did differ significantly from those for the wild-type and pYbhKPm strains, which were in consolidation for ∼2.5 h. This pattern of swarming migration of the pSwarm7 and pORF1 strains resulted in larger swarming diameters than those for the wild-type or pYbhKPm strain and was reproducible when examined upon multiple occasions. Similar effects on swimming-area diameters for the strains on 0.3% LB agar were observed when swimming motility was examined (data not shown). These results suggested that the overexpression of orf1 confers the swarming phenotype observed for the pSwarm7 overexpression strain. Thus, this ORF was designated wosA (wild-type onset with superswarming) and the pORF1 plasmid was designated pWosA.
wosA is a gene predicted to encode a membrane-associated protein.
The wosA gene encodes a putative protein consisting of 321 residues with a calculated molecular mass of 36.91 kDa and an isoelectric point (pI) of 4.44. The wosA gene is immediately adjacent to the uvrB and ybhKPm genes on the P. mirabilis chromosome. Upstream of the uvrB gene is the biotin biosynthesis operon, and downstream of the ybhKPm gene is the molybdopterin biosynthesis operon. This genome organization corresponds to that found in E. coli except for the absence of the wosA gene from the E. coli genome. The wosA gene is not part of an operon, and the WosA protein has no significant identity to any proteins of known function but shows 27% identity over 185 residues to a hypothetical protein, HCH 06118, from the marine bacterium Hahella chejuensis KCTC 2396 (24). The WosA protein is predicted to have a single transmembrane domain and is oriented such that the N-terminal 6 to 29 residues span the cell membrane while the remainder of the protein is found in the cytoplasm (23). No other protein motifs were identified.
The overexpression of wosA causes swarmer cell differentiation under noninducing conditions.
In cells containing pWosA, the level of wosA overexpression was >50-fold above that in cells containing vector only as determined by Northern analyses (data not shown). Swarming migration assays with the PM7002(pWosA) strain [PM7002(pORF1)] indicated that the onset of swarming was the same as that for wild-type PM7002 (Fig. 1C). Interestingly, PM7002(pWosA) exhibited what appeared to be constitutive swarmer cell differentiation during growth in liquid culture and at 2 h after plating onto LB agar (T2), during the early stages of synchronous growth (Fig. 2). The overexpression of the ybhKPm gene did not induce swarmer cell differentiation under the same conditions (data not shown). At 5 h after plating (Fig. 2, row T5), both wild-type and pWosA strains showed the presence of swarmer cells; however, the swarmer cells of the pWosA strain were significantly longer and more abundant than those of the wild-type strain. Forty percent of the cells of the wild-type strain carrying pBC differentiated into swarmer cells, with a mean cell length of 13.83 ± 3.53 μm, and ∼70% of the cells of the strain carrying pWosA were swarmer cells, with a mean cell length of 23.6 ± 15.58 μm (ANOVA: F1,194 = 19.14; P = 0.00002). In addition, swarmer cells of the strains carrying pWosA varied greatly in size and could reach >100 μm in length. The effect of overexpressing wosA was specific to P. mirabilis, as the introduction of the pWosA plasmid into E. coli did not induce swarmer cell differentiation in E. coli (data not shown).
FIG. 2.
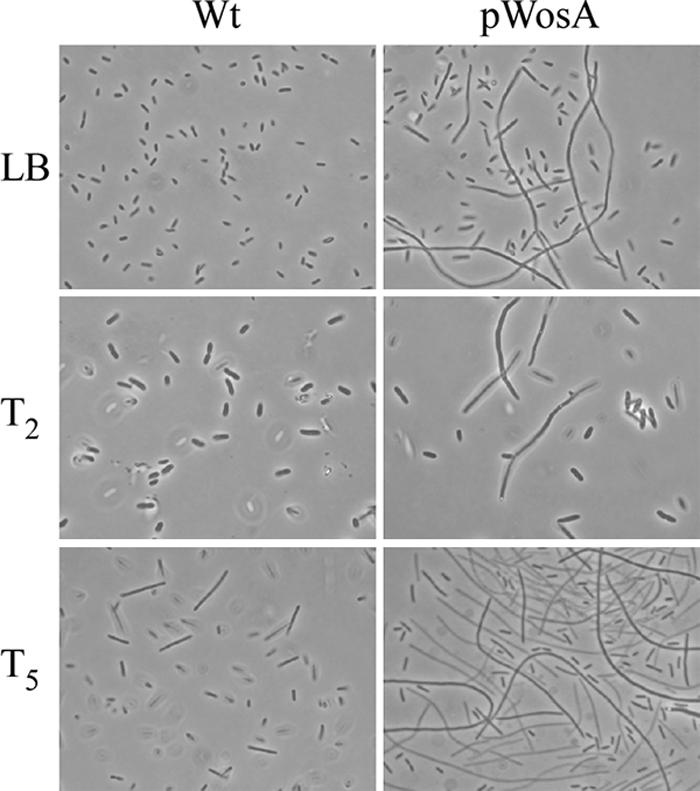
Effect of wosA overexpression on the differentiation of wild-type P. mirabilis 7002 cells. (Top row) Strains were grown in LB medium at 37°C with shaking, harvested at an OD600 of 1.2, and examined by phase-contrast microscopy. (Middle and bottom rows) Overnight cultures of wild-type (Wt) and pWosA strains were adjusted to the same OD and seeded onto LB agar plates, and the plates were incubated at 37°C to produce synchronous cultures. At time points of 2 h (T2) and 5 h (T5) after seeding, the cells were washed from agar plates and examined by phase-contrast microscopy.
The overexpression of wosA causes the upregulation of flhDC and flaA transcript levels.
In P. mirabilis, the master operon flhDC of the flagellar gene hierarchy is strongly induced during swarmer cell differentiation and the artificial overexpression of flhDC increases elongation and hyperflagellation, resulting in the early onset of swarming and greater swarming velocity (16). To determine if the overexpression of wosA resulted in the upregulation of genes in the flagellar hierarchy, levels of flhDC and flaA transcripts were assayed by Northern blotting (Fig. 3). In both PM7002 containing the vector control and PM7002 containing pWosA, the levels of flhDC transcripts began to increase at 3 h after plating (T3), with a greater increase at T4 (Fig. 3, top panel). However, in PM7002 containing pWosA, the levels of flhDC at T4 were approximately fourfold higher than those in cells containing the vector only, as determined by comparisons of hybridization intensities of diluted RNA samples (data not shown). At T5 and T6, when cells were in consolidation, flhDC mRNA levels in cells with pWosA were 8- and 16-fold, respectively, above the levels in cells with the vector only (Fig. 3). The levels of flhDC transcripts in samples from cultures in liquid medium (LB) taken at the mid-log phase of growth did not appear to differ significantly between wild-type PM7002 carrying the pBC SK− vector and PM7002 carrying the pWosA plasmid. The flaA mRNA levels, however, suggest that there may have been a subtle increase in flhDC transcript levels, as we saw a clear and significant 2.2-fold increase in flaA mRNA levels in the pWosA culture sample relative to the levels in the wild-type culture (in LB) (Fig. 3, middle panel). Western blot analysis of flagellin in liquid-grown cells with pWosA indicated that the levels of flagellin were at least threefold higher than those in vector-containing cells (data not shown). These results indicate that the elongated cells observed in liquid culture were likely to be differentiated swarmer cells. Moreover, the effect of increased flhDC expression due to the overexpression of wosA was specific to P. mirabilis. The introduction of the pWosA plasmid into the E. coli strain FDCF7789 carrying a flhDC-lacZ translational fusion produced no increase in flhDC expression, as demonstrated when cells were harvested from fronts of swarming E. coli cells on Eiken agar and compared to cells of the same strain carrying the pBC vector (data not shown).
FIG. 3.
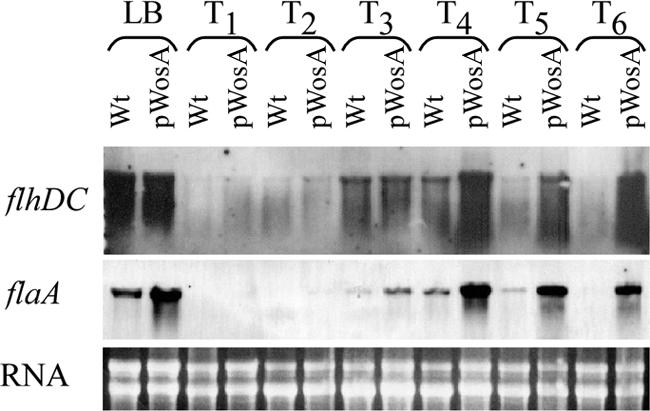
Effect of wosA overexpression on flhDC and flaA transcription. Northern hybridization of flhDC (top panel) and flaA (middle panel) probes to total RNA from wild-type (Wt) PM7002 carrying the pBC SK− vector alone and PM7002 carrying the pWosA plasmid. Strain samples were taken from the liquid inoculum at an OD600 of 0.6 (LB) and from synchronous plate cultures at 1-h intervals over a swarmer cell differentiation cycle (T1 to T6). For each Northern blot, the same preparation of RNA was used to allow for the direct comparison of different transcripts during the swarm cycle. A representative ethidium bromide-stained gel containing the same amount of RNA used in each Northern blot is shown in the bottom panel.
The expression of flaA during the swarming cycle in cells containing vector only and in cells containing pWosA was also examined (Fig. 3). Although not apparent from the exposure shown, flaA transcripts were present in the pWosA strain throughout the early stages of the swarm cycle (T1 to T2), albeit at much lower levels than in later stages (T3 to T6). No flaA transcript was observed in the wild-type strain at T1 and T2, even after extended film exposure (data not shown). From T3 to T6 in the swarm cycle, cells with pWosA exhibited large differences in flaA expression compared to wild-type cells. The increase in flaA transcripts in the wild-type strain at T3 and the subsequent decrease at T5 correspond to the pattern expected of a swarm cycle. However, in cells with pWosA, flaA expression was maintained at high levels during the consolidation period (T5 to T6).
The disruption of the wild-type chromosomal copy of the wosA gene causes a slight decrease in swarming.
To determine the role of the wosA gene product in swarming, a null allele was constructed by disrupting the wild-type chromosomal copy by homologous recombination with an internal fragment of wosA cloned into the suicide vector pKNG101 (25). The swarming migration of the wild-type PM7002 and that of the isogenic wosA null mutant are shown in Fig. 4. The PM7002 wosA::pKNG101 strain exhibited a mild but reproducible swarming defect relative to the swarming of the wild type. After 10 h of growth, the wosA mutant strain had migrated a significantly smaller distance than the wild-type PM7002 strain (ANOVA: F1,6 = 19.02; P = 0.0047). The PM7002 wosA::pKNG101 strain also exhibited a slight swimming defect in 0.3% soft agar, in which migration was decreased by 11% relative to that of the wild type (data not shown).
FIG. 4.
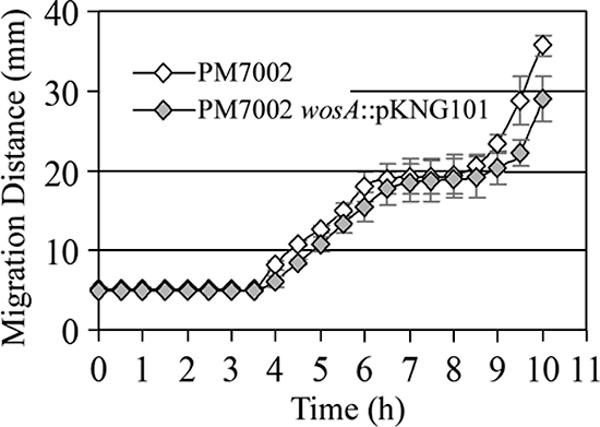
Swarming migration of a wosA mutant. Wild-type P. mirabilis PM7002 and PM7002 carrying a chromosomal disruption of wosA (PM7002 wosA::pKNG101) were grown overnight with shaking at 37°C in LB or LB supplemented with streptomycin at 35 μg/ml, respectively. Cultures were adjusted to the same OD600, 4 μl of each culture was spotted onto a 1.5% LB agar plate, and swarming diameters were measured every 30 min over a 10-h period. Values represent the means of results from four independent experiments. Error bars, SDs.
wosA gene expression is regulated in a growth phase-dependent manner.
To investigate the expression of wosA, β-galactosidase from a wosA-lacZ single-copy transcriptional fusion was measured during growth. This fusion was constructed at the native wosA locus and maintains a wild-type copy of wosA. Initial assays of samples plated to establish synchronously differentiated swarmer cells demonstrated relatively high levels of wosA expression at early time points after plating (data not shown). To determine if the activity observed was due to the expression of wosA in liquid culture, a low-density culture of the PM7002 wosA::wosA-lacZ strain was used to inoculate LB containing streptomycin and β-galactosidase activity from cells taken at various culture densities was determined. The results are shown in Fig. 5. The level of transcription of wosA remained low (∼3 Miller units [MU]) until the culture reached an OD600of 0.8, and it rapidly increased up to a value of 14.5 ± 1.8 MU in stationary phase. The resulting increase in transcription from early log phase to stationary phase was approximately 4.8-fold. However, no extracellular factors that had an effect on wosA expression were identified through the use of cell-free spent medium and the wosA-lacZ fusion strain (data not shown).
FIG. 5.
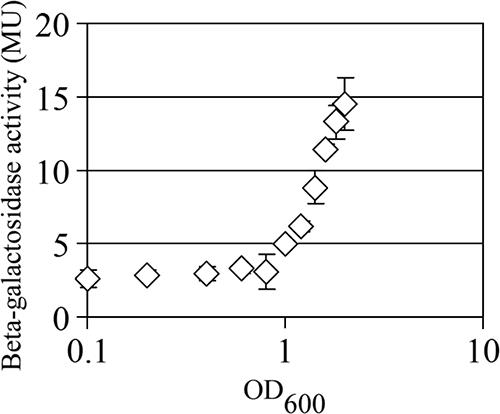
Growth phase-dependent expression of wosA in liquid culture. Wild-type P. mirabilis PM7002 carrying a chromosomal insertion of a wosA-lacZ transcriptional fusion (PM7002 wosA::wosA-lacZ) was grown with shaking at 37°C in LB supplemented with streptomycin at 35 μg/ml. The culture was sampled in duplicate at various OD600s and assayed for β-galactosidase activity. Values are the means of results for duplicate samples from three independent experiments. Error bars, SDs.
wosA gene expression during growth on plates.
To minimize carryover from the growth phase-dependent expression of wosA in the liquid inoculum, P. mirabilis strain PM7002 wosA::wosA-lacZ was plated onto several plates in parallel at a relatively low density (OD600 = 0.4) to produce synchronously differentiated cells. Cells taken at 1-h intervals after plating were assayed for β-galactosidase activity, and the results are shown in Fig. 6. At 1 h postplating (T1), wosA expression was at its lowest level, with 3.01 ± 0.11 MU. β-galactosidase activity increased approximately 1.5-fold during hours 2 to 6 and reached 6.31 ± 0.72 MU by 8 h, resulting in a total increase of 2-fold. Therefore, despite considerable variation from one time point to the next, there was a significant linear increase in wosA expression over the entire course of the experiment (β = 0.344; t25 = 6.76; P < 0.00001). In addition, wosA expression is unlikely to be dependent on a feedback mechanism, as there was no significant difference in β-galactosidase activity from single-copy wosA-lacZ fusions in a wild-type background and in a wosA::Kmr background after 6 h of growth on plates (2.6 ± 0.1 and 2.5 ± 0.1 MU, respectively) or in liquid medium (4.3 ± 0.2 and 4.1 ± 0.03 MU, respectively) (data not shown).
FIG. 6.
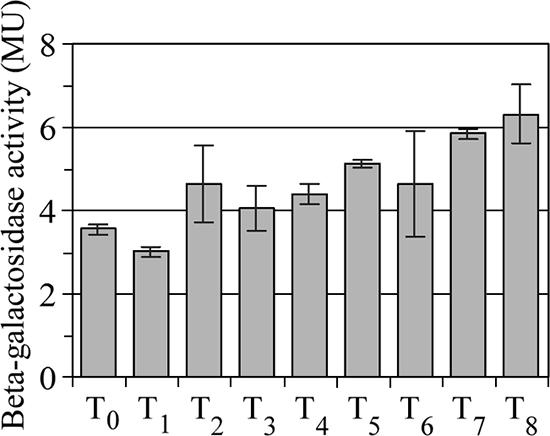
Expression of wosA during the swarm cycle. Wild-type P. mirabilis PM7002 carrying a chromosomal insertion of a wosA-lacZ transcriptional fusion (PM7002 wosA::wosA-lacZ) was grown with shaking at 37°C in LB supplemented with streptomycin at 35 μg/ml. At an OD600 of 0.4, 1-ml samples of duplicate cultures were harvested for assaying β-galactosidase activity (T0), and 200-μl aliquots of the cultures were plated onto several LB agar plates in parallel to produce synchronously differentiating cells. Plates were harvested in phosphate-buffered saline at 1-h intervals, the OD600 at each time point (T1 to T6) was determined, and cultures were assayed for β-galactosidase activity. Values are the means of results for duplicate samples from three independent experiments. Error bars, SDs.
wosA gene expression is independent of the putrescine signaling pathway.
The wosA gene was identified as a gene that when overexpressed restored the swarming phenotype to a mutant, PM437 (speA::mini-Tn5lacZ1), defective in the production of the extracellular signaling molecule putrescine and therefore delayed in swarmer cell differentiation and defective in swarming (43). The overexpression of the wosA gene was also able to eliminate the defect in swarming in a speB mutant, PM439 (speB::Sm) (data not shown). To determine if putrescine is involved in regulating the expression of wosA, cells plated onto LB agar containing 50 μM putrescine were assayed for β-galactosidase activity as described in the previous paragraph. The addition of 50 μM putrescine was previously shown to eliminate the swarming defect of the PM437 (speA::mini-Tn5lacZ1) and PM439 (speB::Sm) mutants (43). No significant difference (ANOVA: F1,2 = 4.73; P = 0.162) in β-galactosidase activity in cells in the absence and those in the presence of 50 μM putrescine was observed after 7 h of growth on plates (5.85 ± 0.13 and 4.66 ± 0.79 MU, respectively). In addition, wosA overexpression did not alter the levels of the extracellular signal putrescine, as assayed by the ability of cell-free spent medium from PM7002 with the pBC vector and PM7002 with the pWosA plasmid to repress putrescine expression in the mutant PM437 (speA::mini-Tn5lacZ1) background. No significant difference (ANOVA: F1,4 = 0.052; P = 0.831) between these two strains was observed when the strains were assayed for putrescine expression (145.29 ± 27.24 and 140.91 ± 19.01 MU, respectively), indicating that WosA functions by bypassing the putrescine signaling pathway.
Increasing medium viscosity activates wosA transcription.
In an attempt to better understand how WosA might be functioning, we assayed other P. mirabilis mutant strains known to induce swarmer cell differentiation under normally noninducing conditions. Since wosA overexpression leads to an increase in flhDC transcription, it was possible that WosA functions by decreasing RsbA or RcsC levels. The mutation of rsbA or rcsC in P. mirabilis leads to a precocious swarming phenotype (7). However, no difference in β-galactosidase activity between an rsbA::mini-Tn5lacZ1 strain and an rcsC::mini-Tn5lacZ1 strain carrying either the pBC vector or the pWosA plasmid was observed (data not shown).
Belas and Suvanasuthi (9) showed that conditions that inhibit flagellar rotation, such as high viscosity and the tethering of flagella, trigger swarmer cell differentiation in P. mirabilis. Since wosA overexpression leads to constitutive swarmer cell differentiation, we examined the effect of increasing the viscosity of the growth medium on wosA expression. The P. mirabilis strain PM7002 wosA::wosA-lacZ was grown in both LB and LB medium containing 15% PVP, and β-galactosidase activity from cells collected at specified time points was assayed. Cell numbers were too low at 1 h postinoculation for an accurate assay, so the first samples were taken at T2. As described previously (9), vegetative cells continued to swim in LB medium and ceased swimming in LB medium containing 15% PVP. The expression of wosA-lacZ was upregulated approximately fourfold after the cells were exposed to LB-15% PVP compared to the expression during growth in LB (T2 and T2.5) (Fig. 7). Moreover, wosA expression remained elevated throughout the entire period of growth in LB-15% PVP. Swarmer cells were observed from T3 to T4.5 and were also unable to move in 15% PVP, despite the presence of flagella (data not shown). It should also be noted that the level of wosA expression is likely underestimated, as some cell loss was unavoidable when cells were pelleted from LB containing PVP. In LB medium alone, wosA expression increased with increasing cell density, as was shown above.
FIG. 7.
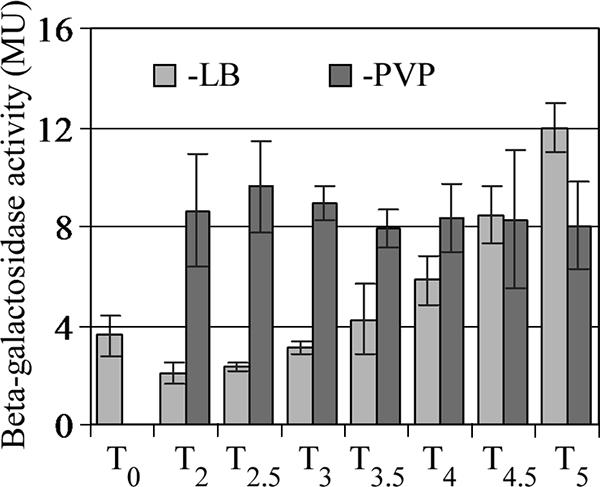
Effect of increasing medium viscosity on wosA gene expression. Wild-type P. mirabilis PM7002 carrying a chromosomal insertion of a wosA-lacZ transcriptional fusion (PM7002 wosA::wosA-lacZ) was incubated with shaking at 37°C in LB medium and LB medium containing 15% PVP (wt/vol). From 2 h postinoculation (T2), cells were harvested every 30 min and assayed for β-galactosidase activity. T0 corresponds to wosA-lacZ expression in the inoculum. Values are the means of results for duplicate samples from three independent experiments. Error bars, SDs.
To determine whether the effect of medium viscosity on wosA-lacZ expression required the presence of flagella, expression in a flaA::Cmr mutant that does not produce a flagellar filament was examined. In two independent experiments, the activation of wosA-lacZ by increased medium viscosity was reduced in a flaA::Cmr background, relative to that in the wild-type background, by an average of 31% (2.5-fold versus 3.3-fold activation, respectively). These results indicate that although a flagellar filament is required for the optimal induction of wosA by increased viscosity, there is an additional pathway that contributes to wosA activation.
Belas and Suvanasuthi (9) also examined P. mirabilis swarming mutants with defects in their ability to sense and respond to surface signals. The fliL mutation resulted in an exaggerated swarmer cell elongation phenotype similar to the phenotype observed when wosA was overexpressed. One explanation for the similar phenotypes is that a mutation in fliL results in the upregulation of wosA expression. Therefore, we assayed wosA transcript levels in wild-type P. mirabilis BB2000 and strain BB2204 (fliL::Tn5 Cm) by Northern blot analysis of RNA prepared from synchronously differentiating cells. The presence of the fliL mutation resulted in increased wosA transcript accumulation at all time points (Fig. 8, top panel). In addition, we observed a slight increase in flhDC transcript levels in the fliL strain compared to those in the wild type at all time points (data not shown). This increase may be the result of increased transcription of wosA, as we know that increased wosA levels lead to increased flhDC levels (Fig. 3).
FIG. 8.
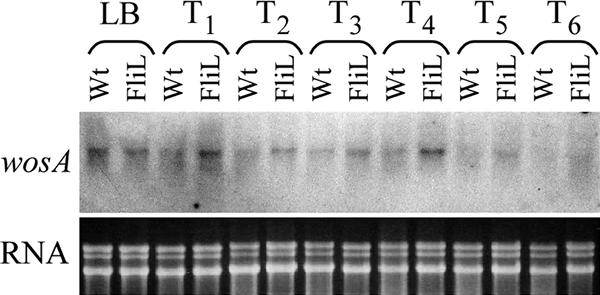
Effect of a fliL mutation on wosA transcription. Northern hybridization of a wosA probe to total RNA from wild-type P. mirabilis BB2000 (Wt) and BB2204 (FliL) (top panel). Strain samples were taken from the liquid inoculum at an OD600 of 0.4 (LB) and from synchronous plate cultures at 1-h intervals over a swarmer cell differentiation cycle (T1 to T6). A representative ethidium bromide-stained gel containing one-fifth of the RNA used in the wosA Northern blot is shown in the bottom panel. Similar results were obtained from RNAs isolated from a second set of independently grown cells (data not shown).
DISCUSSION
We have identified a P. mirabilis gene, wosA, that when overexpressed results in constitutive swarmer cell differentiation and a hyperswarming phenotype. The hyperswarming phenotype associated with multicopy wosA did not result from decreasing the lag in swarming initiation, as has been shown for precocious mutants with alterations in the RcsC/RcsD(RsbA)/RcsB signal transduction pathway (7). Nor is the swarming phenotype due to both decreasing the lag and increasing swarming velocity, as was reported previously for flhDC overexpression (16). Rather, the pWosA strain exhibits a hyperswarming phenotype by increasing swarming velocity while still maintaining temporal control of the onset of swarmer cell migration. Increasing the expression of WosA resulted in a corresponding increase in flhDC mRNA accumulation (Fig. 3). The increase in swarming velocity is therefore likely to be due to increased levels of FlhDC. The increased flhDC expression in the multicopy pWosA-containing strain at time points during the swarm cycle (Fig. 3) also likely accounts for the shorter consolidation period. It is not clear why this strain also exhibited increased swimming motility (data not shown). It has been reported that in P. mirabilis and other swarming bacteria, including S. marcescens, Salmonella serovar Typhimurium, and E. coli, swarmer cells show prolonged smooth swimming when suspended in liquid (7, 19). This reasoning may explain the increase in swimming velocity; however, we cannot rule out an increase in flagellar rotation as the cause for the increased swimming velocity observed.
Despite the increased FlhDC expression and constitutive swarmer cell differentiation resulting from increased WosA expression, swarming in the WosA-overexpressing strain was initiated at the same time as that in the wild-type strain. In P. mirabilis strains that specifically overexpressed FlhDC, swarming was initiated earlier than that in wild-type strains; however, it was not accompanied by constitutive swarmer cell differentiation. Therefore, while the WosA-overexpressing strain was insensitive to differentiation controls under noninducing conditions, it still responded appropriately on solid surfaces to initiate swarming. This result provides evidence that in addition to the signals required to initiate differentiation, a signal is required for the initiation of swarming. In S. liquefaciens, two separate regulatory systems are proposed to control swarming (17). Swarmer cell differentiation is controlled by the flhDC operon, while swarming migration is controlled via the production of a biosurfactant that is facilitated by the SwrI-dependent autoinducer. It is possible that the WosA-overexpressing strain acts in a similar manner and requires the accumulation of a biosurfactant, such as colony migration factor (Cmf) (18), before swarming can be initiated. Although the wosA gene was identified by its ability at high copy numbers to suppress a mutation in the putrescine signaling pathway, our data indicate that WosA acts independently of this pathway to increase swarming migration.
In P. mirabilis, the inhibition of flagellar rotation is one requirement for swarmer cell differentiation (1, 15, 37). Medium conditions that restrict flagellar rotation, including the addition of anti-flagellar antiserum to the growth medium or an increase in the medium viscosity, result in the induction of swarmer cell differentiation in normally noninducing liquid medium (9). Mutations in the fliL, fliG, and fliF genes that encode the components of the flagellar basal body also result in mutant strains that produce abnormally long swarmer cells or produce swarmer cells under noninducing conditions, similar to the mutant phenotype observed for the Caulobacter crescentus fliL mutant strain (9). These mutations associated with the flagellar basal body have been proposed to cause defects in surface sensing. Moreover, the fliL mutation was shown to upregulate two known virulence genes of P. mirabilis, zapA and hpmB. These genes encode a major extracellular zinc metalloprotease and a hemolysin, respectively, and are known to be upregulated only in the differentiated swarmer cell (5), implying a role for FliL in the regulation of virulence genes (9).
Although the wosA gene was isolated based on the swarming phenotypes associated with overexpression, our studies suggest that wosA may be important in the process of swarmer cell differentiation. First, the expression of wosA increases during swarmer cell differentiation (Fig. 6). Second, the overexpression of wosA leads to increased flhDC expression, implying that WosA either directly or indirectly regulates flhDC expression and leads to swarmer cell differentiation in noninducing liquid medium. Finally, growth in high-viscosity medium (15% PVP) or a mutation of the fliL gene increased wosA expression (Fig. 7 and 8). These conditions are predicted to inhibit flagellar rotation or function, and both result in swarmer cell differentiation (1, 15, 37). Bioinformatic analyses of the deduced primary sequence of the WosA protein suggest that the protein contains a single transmembrane domain at the N-terminal end and give a high probability that the protein would be oriented in the out-to-in orientation. As no DNA binding domain has been identified, it is likely that wosA indirectly regulates flhDC expression. However, if FliL is found in the flagellar basal body, as hypothesized by Belas and Suvanasuthi (9), the putative location and orientation of the WosA protein may potentially allow an interaction between WosA and FliL. Although speculative, our data suggest that WosA may be involved in a signal transduction pathway that senses solid surfaces, possibly via interacting with FliL either directly or indirectly, and couple this to increased FlhDC expression.
The use of a flaA mutant demonstrated that although an intact flagellum is needed for the full induction of wosA-lacZ in high-viscosity media, a flagellum-independent pathway for wosA activation also exists under these conditions. There are at least two possibilities for this second pathway. First, although we could find no evidence for a role of extracellular signals in wosA expression, a highly unstable, wosA-activating signal may diffuse away from cells more slowly in viscous media. Second, viscous media may also elicit cell surface changes that are independent of the inhibition of flagellar rotation and trigger a second surface-sensing mechanism. Unpublished studies from our lab have uncovered evidence for this second mechanism, where mutants defective in O-antigen are unable to differentiate on solid surfaces yet swim normally in liquid, indicating that flagellar function is not impaired. Therefore, highly viscous media may also activate wosA via cell surface changes sensed by O-antigen, and experiments are in progress to test this possibility.
A null allele of wosA resulted in a slight decrease in swarming motility (Fig. 4). While this effect was subtle, it may reflect the presence of more than one protein that functions in a manner similar to that of WosA. A common theme in bacterial differentiation is the presence of multiple checkpoints that control each step leading to a differentiated cell. In P. mirabilis, the process of swarmer cell differentiation is controlled by multiple signals and regulatory checkpoints, many of which act through the flhDC flagellar master operon that controls the flagellar gene hierarchy and swarmer cell differentiation. WosA may act in one of these checkpoint pathways. In summary, although bioinformatics analyses do not provide many clues regarding the actual function of WosA, this study indicates that it regulates FlhDC expression, possibly through the sensing of environmental signals that result when cells encounter a solid surface.
Acknowledgments
This work was supported by a merit review award from the Department of Veterans Affairs. Philip N. Rather is the recipient of a research career development award from the Department of Veterans Affairs.
We are grateful to Robert Belas for providing the BB2000 and BB2204 strains. We are grateful to Katy Clemmer for conducting β-galactosidase assays.
Footnotes
Published ahead of print on 11 January 2008.
REFERENCES
- 1.Alavi, M., and R. Belas. 2001. Surface sensing, swarmer cell differentiation, and biofilm development. Methods Enzymol. 33629-40. [DOI] [PubMed] [Google Scholar]
- 2.Alberti, L., and R. M. Harshey. 1990. Differentiation of Serratia marcescens 274 into swimmer and swarmer cells. J. Bacteriol. 1724322-4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allison, C., and C. Hughes. 1991. Bacterial swarming: an example of prokaryotic differentiation and multicellular behaviour. Sci. Prog. 75403-422. [PubMed] [Google Scholar]
- 4.Allison, C., H. C. Lai, D. Gygi, and C. Hughes. 1993. Cell differentiation of Proteus mirabilis is initiated by glutamine, a specific chemoattractant for swarming cells. Mol. Microbiol. 853-60. [DOI] [PubMed] [Google Scholar]
- 5.Allison, C., H. C. Lai, and C. Hughes. 1992. Co-ordinate expression of virulence genes during swarm-cell differentiation and population migration of Proteus mirabilis. Mol. Microbiol. 61583-1591. [DOI] [PubMed] [Google Scholar]
- 6.Belas, R., D. Erskine, and D. Flaherty. 1991. Transposon mutagenesis in Proteus mirabilis. J. Bacteriol. 1736289-6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belas, R., R. Schneider, and M. Melch. 1998. Characterization of Proteus mirabilis precocious swarming mutants: identification of rsbA, encoding a regulator of swarming behavior. J. Bacteriol. 1806126-6139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belas, R., M. Simon, and M. Silverman. 1986. Regulation of lateral flagella gene transcription in Vibrio parahaemolyticus. J. Bacteriol. 167210-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belas, R., and R. Suvanasuthi. 2005. The ability of Proteus mirabilis to sense surfaces and regulate virulence gene expression involves FliL, a flagellar basal body protein. J. Bacteriol. 1876789-6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chilcott, G. S., and K. T. Hughes. 2000. Coupling of flagellar gene expression to flagellar assembly in Salmonella enterica serovar Typhimurium and Escherichia coli. Microbiol. Mol. Biol. Rev. 64694-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dufour, A., R. B. Furness, and C. Hughes. 1998. Novel genes that upregulate the Proteus mirabilis flhDC master operon controlling flagellar biogenesis and swarming. Mol. Microbiol. 29741-751. [DOI] [PubMed] [Google Scholar]
- 12.Eberl, L., G. Christiansen, S. Molin, and M. Givskov. 1996. Differentiation of Serratia liquefaciens into swarm cells is controlled by the expression of the flhD master operon. J. Bacteriol. 178554-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farinha, M. A., and A. M. Kropinski. 1990. Construction of broad-host-range plasmid vectors for easy visible selection and analysis of promoters. J. Bacteriol. 1723496-3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francez-Charlot, A., B. Laugel, A. Van Gemert, N. Dubarry, F. Wiorowski, M. P. Castanie-Cornet, C. Gutierrez, and K. Cam. 2003. RcsCDB His-Asp phosphorelay system negatively regulates the flhDC operon in Escherichia coli. Mol. Microbiol. 49823-832. [DOI] [PubMed] [Google Scholar]
- 15.Fraser, G. M., and C. Hughes. 1999. Swarming motility. Curr. Opin. Microbiol. 2630-635. [DOI] [PubMed] [Google Scholar]
- 16.Furness, R. B., G. M. Fraser, N. A. Hay, and C. Hughes. 1997. Negative feedback from a Proteus class II flagellum export defect to the flhDC master operon controlling cell division and flagellum assembly. J. Bacteriol. 1795585-5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Givskov, M., J. Ostling, L. Eberl, P. W. Lindum, A. B. Christensen, G. Christiansen, S. Molin, and S. Kjelleberg. 1998. Two separate regulatory systems participate in control of swarming motility of Serratia liquefaciens MG1. J. Bacteriol. 180742-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gygi, D., M. M. Rahman, H. C. Lai, R. Carlson, J. Guard-Petter, and C. Hughes. 1995. A cell-surface polysaccharide that facilitates rapid population migration by differentiated swarm cells of Proteus mirabilis. Mol. Microbiol. 171167-1175. [DOI] [PubMed] [Google Scholar]
- 19.Harshey, R. M. 2003. Bacterial motility on a surface: many ways to a common goal. Annu. Rev. Microbiol. 57249-273. [DOI] [PubMed] [Google Scholar]
- 20.Harshey, R. M., and T. Matsuyama. 1994. Dimorphic transition in Escherichia coli and Salmonella typhimurium: surface-induced differentiation into hyperflagellate swarmer cells. Proc. Natl. Acad. Sci. USA 918631-8635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hay, N. A., D. J. Tipper, D. Gygi, and C. Hughes. 1997. A nonswarming mutant of Proteus mirabilis lacks the Lrp global transcriptional regulator. J. Bacteriol. 1794741-4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henrichsen, J. 1972. Bacterial surface translocation: a survey and a classification. Bacteriol. Rev. 36478-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hofmann, K., and W. Stoffel. 1993. TMbase—a database of membrane spanning protein segments. Biol. Chem. Hoppe-Seyler 374166. [Google Scholar]
- 24.Jeong, H., J. H. Yim, C. Lee, S. H. Choi, Y. K. Park, S. H. Yoon, C. G. Hur, H. Y. Kang, D. Kim, H. H. Lee, K. H. Park, S. H. Park, H. S. Park, H. K. Lee, T. K. Oh, and J. F. Kim. 2005. Genomic blueprint of Hahella chejuensis, a marine microbe producing an algicidal agent. Nucleic Acids Res. 337066-7073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaniga, K., I. Delor, and G. R. Cornelis. 1991. A wide-host-range suicide vector for improving reverse genetics in gram-negative bacteria: inactivation of the blaA gene of Yersinia enterocolitica. Gene 109137-141. [DOI] [PubMed] [Google Scholar]
- 26.Kawagishi, I., M. Imagawa, Y. Imae, L. McCarter, and M. Homma. 1996. The sodium-driven polar flagellar motor of marine Vibrio as the mechanosensor that regulates lateral flagellar expression. Mol. Microbiol. 20693-699. [DOI] [PubMed] [Google Scholar]
- 27.Liaw, S. J., H. C. Lai, S. W. Ho, K. T. Luh, and W. B. Wang. 2001. Characterisation of p-nitrophenylglycerol-resistant Proteus mirabilis super-swarming mutants. J. Med. Microbiol. 501039-1048. [DOI] [PubMed] [Google Scholar]
- 28.Majdalani, N., and S. Gottesman. 2005. The Rcs phosphorelay: a complex signal transduction system. Annu. Rev. Microbiol. 59379-405. [DOI] [PubMed] [Google Scholar]
- 29.Marchler-Bauer, A., and S. H. Bryant. 2004. CD-Search: protein domain annotations on the fly. Nucleic Acids Res. 32W327-W331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCarter, L., M. Hilmen, and M. Silverman. 1988. Flagellar dynamometer controls swarmer cell differentiation of V. parahaemolyticus. Cell 54345-351. [DOI] [PubMed] [Google Scholar]
- 31.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 32.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 1702575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishimura, A., and Y. Hirota. 1989. A cell division regulatory mechanism controls the flagellar regulon in Escherichia coli. Mol. Gen. Genet. 216340-346. [DOI] [PubMed] [Google Scholar]
- 34.Ott, L. 1988. An introduction to statistical methods and data analysis, 3rd ed. PWS-Kent Publishing Co., Boston, MA.
- 35.Pruss, B. M., and P. Matsumura. 1996. A regulator of the flagellar regulon of Escherichia coli, flhD, also affects cell division. J. Bacteriol. 178668-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rather, P. N. 2005. Swarmer cell differentiation in Proteus mirabilis. Environ. Microbiol. 71065-1073. [DOI] [PubMed] [Google Scholar]
- 37.Rauprich, O., M. Matsushita, C. J. Weijer, F. Siegert, S. E. Esipov, and J. A. Shapiro. 1996. Periodic phenomena in Proteus mirabilis swarm colony development. J. Bacteriol. 1786525-6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 39.Shi, W., Y. Zhou, J. Wild, J. Adler, and C. A. Gross. 1992. DnaK, DnaJ, and GrpE are required for flagellum synthesis in Escherichia coli. J. Bacteriol. 1746256-6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shin, S., and C. Park. 1995. Modulation of flagellar expression in Escherichia coli by acetyl phosphate and the osmoregulator OmpR. J. Bacteriol. 1774696-4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silverman, M., and M. Simon. 1974. Characterization of Escherichia coli flagellar mutants that are insensitive to catabolite repression. J. Bacteriol. 1201196-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stevenson, L. G., and P. N. Rather. 2006. A novel gene involved in regulating the flagellar gene cascade in Proteus mirabilis. J. Bacteriol. 1887830-7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sturgill, G., and P. N. Rather. 2004. Evidence that putrescine acts as an extracellular signal required for swarming in Proteus mirabilis. Mol. Microbiol. 51437-446. [DOI] [PubMed] [Google Scholar]
- 44.Takeda, S., Y. Fujisawa, M. Matsubara, H. Aiba, and T. Mizuno. 2001. A novel feature of the multistep phosphorelay in Escherichia coli: a revised model of the RcsC → YojN → RcsB signalling pathway implicated in capsular synthesis and swarming behaviour. Mol. Microbiol. 40440-450. [DOI] [PubMed] [Google Scholar]
- 45.Ulitzur, S. 1974. Induction of swarming in Vibrio parahaemolyticus. Arch. Microbiol. 101357-363. [DOI] [PubMed] [Google Scholar]
- 46.Yokota, T., and J. S. Gots. 1970. Requirement of adenosine 3′,5′-cyclic phosphate for flagella formation in Escherichia coli and Salmonella typhimurium. J. Bacteriol. 103513-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Young, G. M., M. J. Smith, S. A. Minnich, and V. L. Miller. 1999. The Yersinia enterocolitica motility master regulatory operon, flhDC, is required for flagellin production, swimming motility, and swarming motility. J. Bacteriol. 1812823-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]


