Abstract
For the human polyomaviruses JC virus (JCV) and BK virus (BKV), the first step to a successful infection involves binding to sialic acid moieties located on the surfaces of host cells. By stripping and then reconstituting specific sialic acid linkages on host cells, we show that JCV uses both α(2,3)-linked and α(2,6)-linked sialic acids on N-linked glycoproteins to infect cells. For both JCV and BKV, the sialic acid linkages required for cell surface binding directly correlate with the linkages required for infection. In addition to sialic acid linkage data, these data suggest that the third sugar from the carbohydrate chain terminus is important for virus binding and infection.
JC virus (JCV) and BK virus (BKV) are common viruses that are believed to infect 70% of the world's population (13, 14, 22). A healthy immune system keeps these lifelong infections subclinical; however, immunosuppression can lead to increased viral replication, viruria, viremia, and disease. Kidney transplantation followed by chemotherapy is the major cofactor for BKV-induced disease. In approximately 5% of renal transplant patients, a condition known as polyomavirus-associated nephropathy causes the lytic destruction of renal tubules and other epithelial cells, leading to kidney dysfunction (9, 23). Patients with polyomavirus-associated nephropathy disease often require an additional kidney transplant. JCV also resides in a slow-growing, persistent state within the epithelial cells of the kidney. During human immunodeficiency virus-induced immunosuppression, JCV can cause lytic destruction of myelin-producing oligodendrocytes within the brain (20). How JCV translocates to the central nervous system is currently unknown. This fatal demyelinating disease, known as progressive multifocal leukoencephalopathy, caused by JCV reactivation occurs in approximately 5% of advanced human immunodeficiency virus-positive individuals (4, 10).
Many polyomaviruses as well as some rotaviruses, orthomyxoviruses, and adenoviruses use cellular sialic acid molecules attached to proteins or glycolipids in order to bind to the surfaces of host cells (1, 11, 24, 28). The simian polyomavirus simian virus 40 is independent of sialic acid and instead uses the ganglioside GM1 as well as major histocompatibility complex class I proteins to attach to host cells (2, 31). Mouse polyomavirus (mPyV) requires α(2,3)-linked sialic acid; however, some strains of mPyV can bind both α(2,3) and α(2,6) linkages (6). Previous observations have suggested that JCV binds to α(2,6)-linked sialic acid exclusively and that BKV requires α(2,3) linkages for a productive infection (7, 18). The role of another linkage, α(2,8)-linked sialic acid, has not been investigated, as α(2,8)-specific neuraminidases (NAs) and sialyltransferases (STs) are not commercially available; however, gangliosides with α(2,8)-linked sialic acid moieties have been shown to play a role in human polyomavirus infection (15, 19).
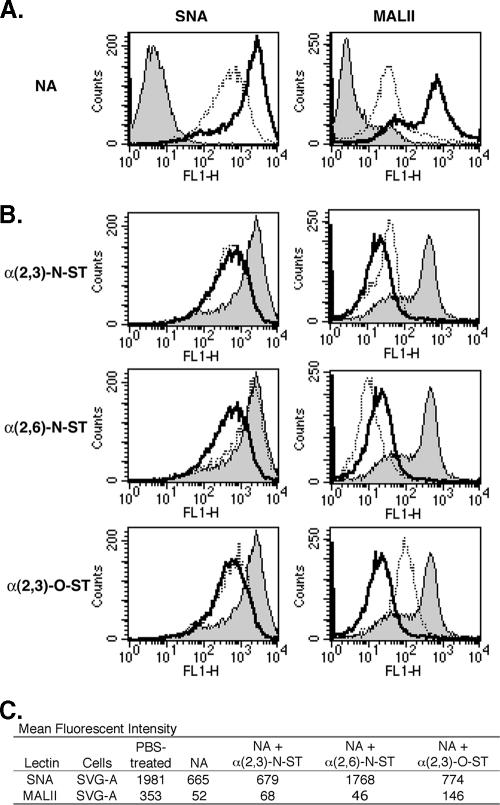
To determine if NAs and STs could deplete and restore, respectively, specific linkages of sialic acids to the cell surface, we tested the binding of biotinylated lectins to treated and untreated cells. Human-glial-cell-derived SVG-A cells in suspension were treated with phosphate-buffered saline (PBS) or 0.25 U/ml NA from Vibrio cholerae for 1 h at 37°C. This NA enzyme is reported to preferentially cleave α(2,3)-linked sialic acid from glycoproteins and glycolipids but also to have activity on α(2,6) and α(2,8) linkages (26, 33). After NA treatment, the cells were washed in PBS twice and then incubated with 4 μg/ml biotinylated Sambucus nigra (SNA) or 10 μg/ml Maackia amurensis lectin II (MALII) for 30 min on ice. SNA is a lectin that recognizes α(2,6)-linked sialic acids, while MALII binds α(2,3)-linked sialic acids (12, 25). After two washes, cells were incubated with 3 μg/ml AlexaFluor-488-labeled streptavidin, and fluorescent intensity was determined using flow cytometry. NA treatment of SVG-A cells reduced α(2,3) and α(2,6) linkages as the mean fluorescent intensities (MFI) of MALII and SNA decreased 66% and 85%, respectively (Fig. 1A).
FIG. 1.
Enzymatic treatments of SVG-A cells can deplete and restore cell surface sialic acids. (A) SVG-A cells were treated with 0.25 U/ml NA in PBS buffer (dotted lines) or with PBS alone (bold lines) in suspension at 37°C for 1 h. Cells were incubated with 4 μg/ml biotinylated SNA or 10 μg/ml biotinylated MALII at 4°C for 30 min. To detect lectin binding, the cells were incubated with 3 μg/ml streptavidin-labeled AlexaFluor-488 at 4°C for 30 min. Fluorescence intensity was evaluated using flow cytometry. The shaded histograms represent PBS-treated cells incubated with streptavidin-labeled AlexaFluor-488 only. (B) SVG-A cells were treated with PBS (shaded histograms), 0.25 U/ml NA and then 1 mM NeuAc-CMP (bold lines), or NA followed by NeuAc-CMP and one of the ST enzymes (dotted lines) as follows: 75 mU/ml α(2,3)-N-ST (top row), 25 mU/ml α(2,6)-N-ST (middle row), or 100 mU/ml α(2,3)-O-ST (bottom row). The SVG-A cells were washed and then incubated with biotinylated SNA or biotinylated MALII and streptavidin-labeled AlexaFluor-488 as described above. (C) MFI of SVG-A cells with the treatments described above. FL1-H, fluorescence 1 height.
To assess whether STs could restore specific sialic acid linkages, NA-treated cells were incubated with one of three STs in the presence of 1 mM N-acetylneuraminic acid sodium salt (NeuAc)-CMP or NeuAc-CMP alone for 1 h at 37°C (Fig. 1B). NeuAc-CMP acts as a sialic acid donor, while the ST attaches this neuraminic acid molecule to a specific oligosaccharide chain in a particular orientation. This orientation or linkage is defined by which carbon of galactose binds to sialic acid. The restoration of sialic acid linkages to cells was determined by lectin binding as described above. The α(2,3)-N-ST enzyme, which attaches sialic acid in an α(2,3) orientation to a chain that consists of galactose attached to the N-acetylglucosamine [NeuAc-(α2,3)-β-d-galactosyl-β1,3/4-N-acetyl-β-d-glucosamine] found on N-linked glycoproteins, increased the MFI of MALII 20% compared to that resulting from NA treatment, indicating that α(2,3) linkages had been partially restored (34). This was specific, as SNA binding was not elevated in α(2,3)-N-ST-treated cells. Similar results were also observed for α(2,3)-O-ST, an enzyme that attaches sialic acid in an α(2,3) orientation to a galactose molecule attached to N-acetylgalactosamine [NeuAc-(α2,3)-Gal-GalNAc] (16). The NeuAc-(α2,3)-Gal-GalNAc fragment is the terminal portion of carbohydrate chains found on O-linked proteins and some glycolipids, such as gangliosides. In contrast, α(2,6)-N-ST, which catalyzes the transfer of sialic acid to β-d-galactosyl-β1,4-N-acetyl-β-d-glucosamine, could only restore SNA binding and, interestingly, reduced α(2,3)-linked sialic acid content on the cell surface more significantly than NA alone (Fig. 1B). Very similar results were found for Vero cells in a previous study (7).
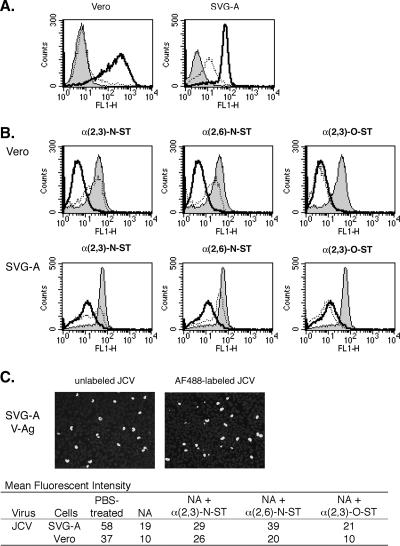
Once we established that NA could reduce α(2,3)- and α(2,6)-linked sialic acids and that STs could selectively restore individual sialic acid linkages, we analyzed the binding of JCV after these enzymatic treatments. A cesium chloride-purified Mad-1/SVEΔ strain of JCV was prepared, incubated with 20 μg/ml AlexaFluor-488 succinimidyl ester dye, and dialyzed to remove any unbound dye (32). JCV was shown to be fully infectious after labeling (Fig. 2C). Host cells were treated with NA and ST as described above and then incubated with 4.8 focus-forming units (FFU)/μl AlexaFluor-488-labeled JCV for 30 min at 4°C. Flow cytometry determined that NA treatment of Vero and SVG-A cells reduced JCV binding by 73% and 67%, respectively (Fig. 2A). We also found that the reduced binding of labeled JCV seen after NA treatment could be reversed if cells were incubated with either α(2,3)-N-ST or α(2,6)-N-ST. Interestingly, α(2,3)-O-ST did not restore JCV binding (Fig. 2B). These data indicate that JCV can use either an α(2,3)- or α(2,6)-sialic acid linkage as long as it is attached to the N-linked oligosaccharide fragment Gal-GlcNAc. This also indicates that the third sugar from the end is important for binding.
FIG. 2.
JCV binding to SVG-A and Vero cells after NA and ST treatments. (A) SVG-A cells were either treated with 0.25 U/ml NA (dotted lines) or PBS (bold lines and shaded histograms) at 37°C for 1 h. After two PBS washes, the cells were mixed with 10 FFU/μl JCV-labeled AlexaFluor-488 at 4°C for 30 min (dotted and bold lines). Cells were then washed with PBS to remove unbound virus and analyzed using flow cytometry. The shaded histograms represent PBS-treated cells that were not incubated with the labeled virus. (B) Vero and SVG-A cells were PBS treated (shaded histograms), NA and then NeuAc-CMP (bold lines), or NA followed by NeuAc-CMP and STs (dotted lines) as described in the legend to Fig. 1B. The cells were washed and incubated with 10 FFU/μl AlexaFluor-488-labeled JCV at 4°C for 30 min and analyzed using flow cytometry. (C) To determine if labeling JCV inactivated the virus, we infected SVG-A cells plated on a coverslip with 4.8 FFU/μl of unlabeled JCV or the same volume of AlexaFluor-488-labeled JCV at 37°C for 2 h. The virus medium was replaced with fresh medium, and the cells were incubated for 3 days. Cells were fixed, permeabilized, and stained with PAB597. A secondary AlexaFluor-488-labeled goat anti-mouse antibody allowed us to visualize the cells productively infected with JCV. Representative images of the SVG-A cells infected with JCV before and after labeling are shown (magnification, ×100). The bright spots are cells positive for VP1 expression. FL1-H, fluorescence 1 height.
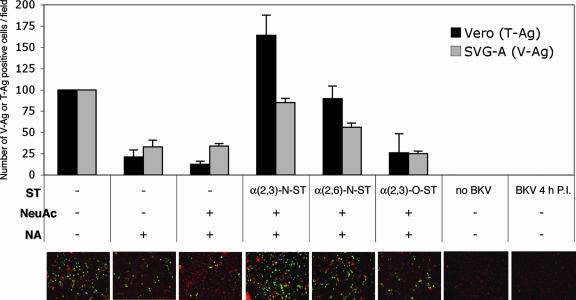
Viruses can often bind cell surface receptors nonspecifically. For example, an mPyV small-plaque strain can bind to α(2,6)-linked sialic acid as well as to α(2,3)-linked sialic acid. The binding of mPyV to α(2,6)-linked sialic acid is not required for infection; instead, this pseudoreceptor sequesters the virus and delays its entry into the cell, causing the virus to be less pathogenic (3, 6). The binding of α(2,6) linkages is attributed to a single amino acid change in virion protein 1 (VP1) (3, 6). To determine if the receptors for binding and infection are consistent, we incubated unlabeled JCV with PBS, NA, or NA followed by ST-treated Vero or SVG-A cells for 2 h at 37°C, washed away the unbound virus, and plated the cells onto medium-containing cell culture wells. After 3 days, the cells were fixed with 1% paraformaldehyde, permeabilized with Triton X-100, and stained for VP1 using PAB597, a hybridoma antibody directed against simian virus 40 that cross-reacts with JCV and BKV. To determine the effect of the input VP1 signal, the cells were stained for V antigen 4 h postinfection and shown to have some faint punctate cytoplasmic signal and no nuclear staining. This indicates that the VP1 staining conducted 3 days postinfection is a product of de novo protein synthesis. Both cell lines showed a reduction in infection after NA treatment that could be reversed if cells were treated with α(2,3)-N-ST or α(2,6)-N-ST. Neither α(2,3)-O-ST nor NeuAc-CMP without ST restored infection (Fig. 3). The binding of JCV seems to directly correlate with infectivity (Fig. 2 and 3). As branched chains containing α(2,6)-linked sialic acid are not normally found in N-linked glycoproteins, we believe that N-linked carbohydrate chains containing terminal α(2,6)- and α(2,3)-linked sialic acid are sufficient to mediate virus uptake (3, 5, 21).
FIG. 3.
Effect of NA and ST treatments on JCV infection of Vero and SVG-A cells. In suspension, Vero and SVG-A cells were treated with PBS or 0.80 U/ml NA at 37°C for 1 h. The cells were washed and then incubated with Dulbecco's modified Eagle's medium, 1 mM NeuAc-CMP, or NeuAc-CMP and one of the following: 75 mU/ml α(2,3)-N-ST, 25 mU/ml α(2,6)-N-ST, or 100 mU/ml α(2,3)-O-ST at 37°C for 1 h. Cells were infected with 4.8 FFU/μl unlabeled JCV at 37°C for 2 h, washed to remove the unbound virus, and plated onto cell culture dishes. Three days later, the cells were fixed, permeabilized, stained (Vero cells were stained for T antigen [T-Ag]; SVG-A cells were stained for VP1), and scored. To test if the V antigen (V-Ag)-positive SVG-A cells detected the input virus or newly synthesized VP1, the cells were stained for VP1 at 4 h postinfection. Representative images of JCV-infected SVG-A cells after various treatments are shown (magnification, ×100). The green spots are the cells positive for VP1 expression. The red spots indicate Evans blue cytoplasmic counterstaining. P.I., postinfection.
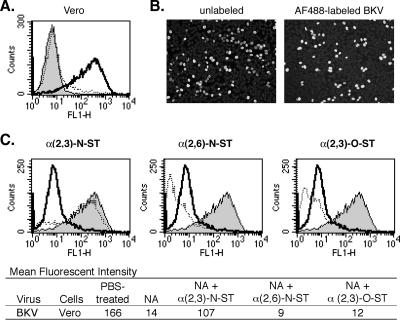
The sialic acid requirements necessary for BKV infection involve α(2,3)-linked sialic acid attached to an N-linked glycoprotein (7). To determine whether the receptors for BKV binding directly correlate with receptors necessary for infection, we observed AlexaFluor-488-labeled BKV binding after sialic acids were removed and then selectively restored to the surfaces of Vero cells (Fig. 4). BKV attachment, as determined by MFI analysis, was reduced by over 90% after NA treatment and could be restored only when cells were treated with α(2,3)-N-ST. These observations indicate that BKV requires α(2,3) linkage of sialic acid for binding and infection (7).
FIG. 4.
BKV binding to NA- and ST-treated Vero cells. (A) Vero cells were treated with PBS (bold line and shaded histogram) or 0.25 U/ml NA (dotted line) at 37°C for 1 h. Cells were then incubated with 10 FFU/μl AlexaFluor-488-labeled BKV at 4°C for 30 min (bold and dotted lines). The shaded histogram represents cells that were not treated with labeled virus. (B) To determine if labeling BKV reduced the viability of the virus, Vero cells were infected with 8.9 FFU/μl BKV before label was added or with the same volume of AlexaFluor-488-labeled BKV at 37°C for 2 h. After unbound virus was washed away, the cells were incubated for 3 days, fixed, and stained exactly as JCV was, as described in the legend for Fig. 2. Representative images of BKV before and after labeling are shown (magnification, ×100). The bright spots are cells positive for VP1 expression. (C) Vero cells were treated with PBS (shaded histograms), NA followed by NeuAc-CMP (bold lines), or NA followed by NeuAc-CMP and ST (dotted lines), as described for JCV in the legend for Fig. 2. After the treatments, the Vero cells were washed and incubated with 10 FFU/μl AlexaFluor-488-labeled BKV at 4°C for 30 min. BKV binding was evaluated using flow cytometry. FL1-H, fluorescence 1 height.
These data show that the third sugar from the terminus of the carbohydrate chain is important for JCV and BKV binding to host cells, suggesting that these two viruses bind their host receptors differently than mPyV. Crystallization studies show that the large-plaque strain of mPyV interacts with only the two most terminal sugar motifs, sialic acid and galactose (30).
Gangliosides have a role in human polyomavirus cell attachment and infection (19, 29, 31). Gangliosides are one type of glycosphingolipid and are composed of ceramide and oligosaccharide chains that contain one or more sialic acid molecules positioned in an α(2,3)- or α(2,8)-linked orientation. Complex gangliosides consist of the basic structure, Gal-GalNAc-Gal-Glc-ceramide, in which sialic acids attach to either of the Gal residues (17). Because α(2,3)-O-ST, which transfers neuraminic acid to Gal-GalNAc motifs of glycolipids and glycoproteins, cannot restore JCV or BKV binding or infection, virus attachment to the α(2,3)-linked sialic acid of the more terminal Gal molecule is unlikely. We cannot exclude the possibility that α(2,3)-N-ST restores α(2,3)-sialic acid linkages to the internal galactose motif of the gangliosides; however, these types of gangliosides, such as GM1, have not been shown to play a role in JCV or BKV infection. From this, we predict that the α(2,8)-linked sialic acid motifs on gangliosides are important for binding and infection. This could not be confirmed due to the lack of commercially available α(2,8)-ST, but future studies determining the role of the α(2,8) linkage are in progress. Another possibility is that both JCV and BKV transiently bind sialic acid moieties on gangliosides, and this less stable binding was not observed in our study due to the rigorous washing after the binding and infection steps. Gangliosides are associated with and enriched within the lipid raft portions of cell membranes (8, 19, 27). Adding exogenous gangliosides to cells, as was done in other studies, could increase the number of endocytic events or signaling events that might reorganize the surface membrane and induce viral entry (8, 19, 27). This would explain why others have shown that the addition of gangliosides to cell culture media can make nonpermissive cells susceptible to infection (8, 19).
A previous study reported that JCV binds terminal α(2,6)-linked sialic acid attached to an N-linked glycoprotein, while another study suggested a role for α(2,3)-sialic acid and gangliosides (15, 18). Lui et al. suggested an exclusive role for α(2,6)-linked sialic acid in JCV binding, because α(2,3)-specific NA had no effect on JCV binding, hemagglutination, or infection (18). Our data are not inconsistent with prior data, although our interpretation is different. We believe that JCV can bind α(2,3) and α(2,6) linkages and that the binding of JCV to α(2,3)-linked sialic acid is independent of its binding to α(2,6)-linked sialic acid. Therefore, a reduction in only one linkage might not necessarily result in a decrease in binding or infection as long as cells are challenged with virus at a subsaturating concentration where the number of available receptors exceeds the number of viral particles.
In conclusion, our data indicate that the human polyomavirus receptors involved in binding correlate with those required for infection. This interaction depends on the three terminal sugars of an N-linked glycoprotein. Additionally, we have elucidated a novel role for the α(2,3)-linked sialic acid of an N-linked glycoprotein in the JCV life cycle.
Acknowledgments
We thank all members of the Atwood lab for critical discussions during the course of this work. We also thank Tammy Glass, Wendy Virgadamo, and Heather Forand for administrative assistance.
Work in our laboratory was supported by a grant from the National Cancer Institute (R01CA71878) and by a grant from the National Institute of Neurological Disorders and Strokes (R01NS43097) to W.J.A. M.L.G. is supported by a Ruth L. Kirschstein National Research Service Award (F31 NS053340). A.S.D. is supported by a GAANN training grant from the Department of Education (P200A030100) and by the Frederic Poole Gorham Biological Fellowship.
We declare that we have no competing financial interests.
Footnotes
Published ahead of print on 19 December 2007.
REFERENCES
- 1.Arnberg, N., K. Edlund, A. H. Kidd, and G. Wadell. 2000. Adenovirus type 37 uses sialic acid as a cellular receptor. J. Virol. 7442-48. [PMC free article] [PubMed] [Google Scholar]
- 2.Atwood, W. J., and L. C. Norkin. 1989. Class I major histocompatibility proteins as cell surface receptors for simian virus 40. J. Virol. 634474-4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauer, P. H., C. Cui, T. Stehle, S. C. Harrison, J. A. DeCaprio, and T. L. Benjamin. 1999. Discrimination between sialic acid-containing receptors and pseudoreceptors regulates polyomavirus spread in the mouse. J. Virol. 735826-5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger, J. R., and E. O. Major. 1999. Progressive multifocal leukoencephalopathy. Semin. Neurol. 19193-200. [DOI] [PubMed] [Google Scholar]
- 5.Brockhausen, I., G. Moller, A. Pollex-Kruger, V. Rutz, H. Paulsen, and K. L. Matta. 1992. Control of O-glycan synthesis: specificity and inhibition of O-glycan core 1 UDP-galactose: N-acetylgalactosamine-alpha-R beta 3-galactosyltransferase from rat liver. Biochem. Cell Biol. 7099-108. [DOI] [PubMed] [Google Scholar]
- 6.Chen, M. H., and T. Benjamin. 1997. Roles of N-glycans with alpha2,6 as well as alpha2,3 linked sialic acid in infection by polyoma virus. Virology 233440-442. [DOI] [PubMed] [Google Scholar]
- 7.Dugan, A. S., S. Eash, and W. J. Atwood. 2005. An N-linked glycoprotein with α(2,3)-linked sialic acid is a receptor for BK virus. J. Virol. 7914442-14445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilbert, J., J. Dahl, C. Riney, J. You, C. Cui, R. Holmes, W. Lencer, and T. Benjamin. 2005. Ganglioside GD1a restores infectibility to mouse cells lacking functional receptors for polyomavirus. J. Virol. 79615-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirsch, H. H., and J. Steiger. 2003. Polyomavirus BK. Lancet Infect. Dis. 3611-623. [DOI] [PubMed] [Google Scholar]
- 10.Hou, J., and E. O. Major. 2000. Progressive multifocal leukoencephalopathy: JC virus induced demyelination in the immune compromised host. J. Neurovirol. 6(Suppl. 2)S98-S100. [PubMed] [Google Scholar]
- 11.Isa, P., C. F. Arias, and S. Lopez. 2006. Role of sialic acids in rotavirus infection. Glycoconj. J. 2327-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knibbs, R. N., I. J. Goldstein, R. M. Ratcliffe, and N. Shibuya. 1991. Characterization of the carbohydrate binding specificity of the leukoagglutinating lectin from Maackia amurensis. Comparison with other sialic acid-specific lectins. J. Biol. Chem. 26683-88. [PubMed] [Google Scholar]
- 13.Knowles, W. A. 2001. The epidemiology of BK virus and the occurrence of antigenic and genomic subtypes, p. 527-560. In K. Khalili and G. L. Stoner (ed.), Human polyomaviruses: molecular and clinical perspectives. Wiley, New York, NY.
- 14.Knowles, W. A., P. Pipkin, N. Andrews, A. Vyse, P. Minor, D. W. Brown, and E. Miller. 2003. Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J. Med. Virol. 71115-123. [DOI] [PubMed] [Google Scholar]
- 15.Komagome, R., H. Sawa, T. Suzuki, Y. Suzuki, S. Tanaka, W. J. Atwood, and K. Nagashima. 2002. Oligosaccharides as receptors for JC virus. J. Virol. 7612992-13000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee, Y. C., N. Kojima, E. Wada, N. Kurosawa, T. Nakaoka, T. Hamamoto, and S. Tsuji. 1994. Cloning and expression of cDNA for a new type of Galβ1,3GalNAc α2,3-sialyltransferase. J. Biol. Chem. 26910028-10033. [PubMed] [Google Scholar]
- 17.Levery, S. B. 2005. Glycosphingolipid structural analysis and glycosphingolipidomics. Methods Enzymol. 405300-369. [DOI] [PubMed] [Google Scholar]
- 18.Liu, C. K., G. Wei, and W. J. Atwood. 1998. Infection of glial cells by the human polyomavirus JC is mediated by an N-linked glycoprotein containing terminal α(2-6)-linked sialic acids. J. Virol. 724643-4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Low, J. A., B. Magnuson, B. Tsai, and M. J. Imperiale. 2006. Identification of gangliosides GD1b and GT1b as receptors for BK virus. J. Virol. 801361-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Major, E. O., K. Amemiya, C. S. Tornatore, S. A. Houff, and J. R. Berger. 1992. Pathogenesis and molecular biology of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin. Microbiol. Rev. 549-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marth, J. D. 1996. Complexity in O-linked oligosaccharide biosynthesis engendered by multiple polypeptide N-acetylgalactosaminyltransferases. Glycobiology 6701-705. [DOI] [PubMed] [Google Scholar]
- 22.Padgett, B. L., and D. L. Walker. 1973. Prevalence of antibodies in human sera against JC virus, an isolate from a case of progressive multifocal leukoencephalopathy. J. Infect. Dis. 127467-470. [DOI] [PubMed] [Google Scholar]
- 23.Ponticelli, C. 2004. Renal transplantation 2004: where do we stand today? Nephrol. Dial. Transplant. 192937-2947. [DOI] [PubMed] [Google Scholar]
- 24.Seganti, L., P. Mastromarino, F. Superti, L. Sinibaldi, and N. Orsi. 1981. Receptors for BK virus on human erythrocytes. Acta Virol. 25177-181. [PubMed] [Google Scholar]
- 25.Shibuya, N., I. J. Goldstein, W. F. Broekaert, M. Nsimba-Lubaki, B. Peeters, and W. J. Peumans. 1987. The elderberry (Sambucus nigra L.) bark lectin recognizes the Neu5Ac(alpha 2-6)Gal/GalNAc sequence. J. Biol. Chem. 2621596-1601. [PubMed] [Google Scholar]
- 26.Shukla, A. K., and R. Schauer. 1986. Analysis of sialidase and N-acetylneuraminate pyruvate-lyase substrate specificity by high-performance liquid chromatography. Anal. Biochem. 158158-164. [DOI] [PubMed] [Google Scholar]
- 27.Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature 387569-572. [DOI] [PubMed] [Google Scholar]
- 28.Skehel, J. J., and D. C. Wiley. 2000. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu. Rev. Biochem. 69531-569. [DOI] [PubMed] [Google Scholar]
- 29.Smith, R. D., J. H. Galla, K. Skahan, P. Anderson, C. C. Linnemann, Jr., G. S. Ault, C. F. Ryschkewitsch, and G. L. Stoner. 1998. Tubulointerstitial nephritis due to a mutant polyomavirus BK virus strain, BKV(Cin), causing end-stage renal disease. J. Clin. Microbiol. 361660-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stehle, T., Y. Yan, T. L. Benjamin, and S. C. Harrison. 1994. Structure of murine polyomavirus complexed with an oligosaccharide receptor fragment. Nature 369160-163. [DOI] [PubMed] [Google Scholar]
- 31.Tsai, B., J. M. Gilbert, T. Stehle, W. Lencer, T. L. Benjamin, and T. A. Rapoport. 2003. Gangliosides are receptors for murine polyoma virus and SV40. EMBO J. 224346-4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vacante, D. A., R. Traub, and E. O. Major. 1989. Extension of JC virus host range to monkey cells by insertion of a simian virus 40 enhancer into the JC virus regulatory region. Virology 170353-361. [DOI] [PubMed] [Google Scholar]
- 33.White, J. S., and D. C. White. 1997. Source book of enzymes. CRC Press, Boca Raton, FL.
- 34.Williams, M. A., H. Kitagawa, A. K. Datta, J. C. Paulson, and J. C. Jamieson. 1995. Large-scale expression of recombinant sialyltransferases and comparison of their kinetic properties with native enzymes. Glycoconj. J. 12755-761. [DOI] [PubMed] [Google Scholar]