Abstract
Respiratory syncytial virus (RSV) is a major cause of severe lower respiratory tract disease in infancy and early childhood. Despite its importance as a pathogen, there is no licensed vaccine against RSV. The G glycoprotein of RSV, a major attachment protein, is a potentially important target for protective antiviral immune responses. Here, a recombinant replication-deficient adenovirus-based vaccine, rAd/3xG, expressing the soluble core domain of G glycoprotein (amino acids 130 to 230) engineered by codon optimization and tandem repetition for higher-level expression, was constructed and evaluated for its potential as an RSV vaccine in a murine model. A single intranasal immunization with rAd/3xG provided potent protection against RSV challenge which lasted for more than 10 weeks. Strong mucosal immunoglobulin A responses were also induced by a single intranasal immunization but not by intramuscular or oral administration of rAd/3xG. Interestingly, neither gamma interferon- nor interleukin-4-producing CD4 T cells directed to I-Ed-restricted epitope were detected in the lungs of rAd/3xG-immune mice upon challenge, whereas priming with vaccinia virus expressing RSV G (vvG) elicited strong Th1/Th2 mixed CD4 T-cell responses. Lung eosinophilia and vaccine-induced weight loss were significantly lower in the rAd/3xG-immune group than in the vvG-primed group. Together, our data demonstrate that a single intranasal administration of rAd/3xG elicits beneficial protective immunity and represents a promising vaccine regimen against RSV infection.
Respiratory syncytial virus (RSV) is the most important viral pathogen causing serious respiratory tract disease in infants and young children worldwide. RSV is also receiving increasing recognition as an important cause of lower respiratory tract illness in immunocompromised patients and the elderly (13, 15, 16). Despite the importance of RSV as a respiratory pathogen, there is no licensed vaccine currently available against RSV infection. Thus, developing an effective and safe RSV vaccine remains a worldwide priority.
The RSV G glycoprotein was identified as the major RSV attachment protein (24) and is thought to be important for protection against RSV infection (39). G protein lacks any major histocompatibility complex class I-restricted epitope (8, 26, 36) and has not yet been demonstrated to elicit a cytotoxic T-lymphocyte response in either humans or mice (19, 29). It has a single immunodominant I-Ed epitope spanning amino acids 183 to 198 and largely induces a specific subset of CD4 T cells restricted to Vβ14 expression in the T-cell receptor (40, 42). Numerous studies have suggested that immunization with RSV G is associated with the induction of polarized Th2-type responses, which leads to pulmonary eosinophilia upon RSV challenge of G-immunized mice (17, 20, 30, 35, 40). In contrast, it was recently suggested that G-specific immune responses are not solely the basis for vaccine-enhanced illness and should not be excluded from potential vaccine strategies (21, 22). In addition, intramuscular (i.m.) injection of plasmid DNA encoding membrane G or secreted G induced balanced Th1/Th2 immunity without an atypical pulmonary inflammation after RSV challenge in mice and cotton rats (3, 25), suggesting that G protein may provide protection but not induce enhanced lung pathology, depending on the vehicle and/or method of delivery.
In the present study, we have targeted the RSV G protein fragment between residues 130 and 230 and engineered the sequence by codon optimization for optimal expression in animal cells and by the addition of a secretory signal sequence derived from tissue plasminogen activator (t-PA). Moreover, this sequence was engineered to be multiple-copy tandem repeats in the same open reading frame for higher immunogenicity (23, 31, 47). Replication-defective recombinant adenovirus (rAd) vaccines (rAd/1xG and rAd/3xG) were then generated and evaluated for their potential as vaccines. We show here that a single intranasal (i.n.) immunization of rAd/3xG induces a strong serum immunoglobulin G (IgG) response, a mucosal IgA response, and long-term protection following RSV challenge without vaccine-enhanced illness.
MATERIALS AND METHODS
Preparation of RSV stock.
RSV strain A2 was propagated in HEp-2 cells (ATCC, Manassas, VA) in Dulbecco's modified Eagle's medium (Life Technologies, Gaithersburg, MD) supplemented with 3% heat-inactivated fetal calf serum, 2 mM glutamine, 20 mM HEPES, nonessential amino acid, penicillin, and streptomycin and titrated for infectivity by plaque assay.
Construction of replication-defective rAds.
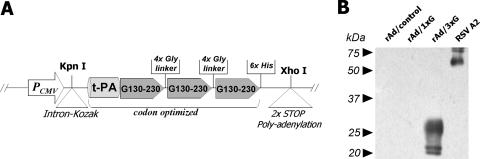
A coding sequence of RSV G protein spanning amino acid residues 130 to 230 (RSV strain A2) was synthesized, in which codon substitutions were made for minimized usage of rare codons (Bioneer Corp., Daejeon, Korea). Codons for a six-histidine stretch as a tag and two consecutive stop codons were attached at 3′ terminus. This synthetic DNA was sequenced and then subcloned into pGEM-t-EASY vector (Promega, Madison, WI). The RSV G protein region was again amplified with various oligonucleotide primers containing appropriate restriction enzyme sites and juxtaposed three times with four glycine residues as a linker between each repeated region (Fig. 1). The original RSV G sequences were also cloned into the same sites of the vector. A start codon and the signal sequence of human t-PA were then inserted in the N termini of the RSV G fragments. Finally, the entire open reading frame was excised by KpnI/XhoI digestion and inserted into the same sites of pShuttle-CMV vector. Replication-defective Ads (serotype 5) were generated by insertion of foreign sequences by homologous recombination and subsequent purification of recombinant progeny as described previously (18). Briefly, the shuttle vector plasmid was electroporated into electrocompetent BJ5183 cells carrying the pAdEasy-1 Ad genomic DNA. Recombinant Ad DNA was isolated and transfected into HEK293 cells to generate rAd/1xG and rAd/3xG viruses. Control Ad (rAd/control) was generated by the same method using the empty pShuttle-CMV vector. The recombinant viruses were amplified, purified on CsCl2 gradients, and titrated according to the protocols provided by the manufacturer. The correct expression and secretion of RSV G protein fragments by rAd/3xG-infected HEp-2 cells were verified by Western blotting using G-specific monoclonal antibody (clone 131-2G; Chemicon International, Inc., Temecula, CA) and horseradish peroxidase-conjugated streptavidin (Zymed Laboratories, San Francisco, CA).
FIG. 1.
Construction and characterization of rAd/3xG vaccine. (A) A shuttle vector was designed as shown in the diagram and constructed by several cloning steps as described in Materials and Methods. The vector was used to generate the replication-deficient rAd/3xG by homologous recombination with the Ad genomic DNA. (B) The expression of the recombinant RSV G protein fragment by rAd/3xG was confirmed by Western blotting. Culture supernatants were prepared from HEp-2 cells infected with rAd/1xG, rAd/3xG, or rAd/control and subjected to Western blotting with an anti-RSV glycoprotein monoclonal antibody. RSV A2-infected culture supernatant was used as a positive control.
Immunization and challenge.
Female BALB/c mice were purchased from Charles River Laboratories Inc. (Yokohama, Japan) and kept under specific-pathogen-free conditions. For immunization, 6- to 8-week-old mice were inoculated with various doses of replication-defective Ad via the i.n., i.m., or oral route. For i.n. immunizations, mice were lightly anesthetized by ether/chloroform inhalation, and 5 × 107 PFU vaccine or control virus in a volume of 50 μl was applied to the left nostril. i.m. immunization was performed by injection of 5 × 107 PFU of vaccine in 100 μl into mouse hind limbs. For oral immunization, mice were deprived of water and food a few hours before injection and immunized with 5 × 107 PFU of virus in 200 μl of phosphate-buffered saline (PBS) by proximal esophageal intubation with a mouse feeding needle. Male and female pups were immunized with 3 × 107 PFU of rAd in volumes of 20 to 30 μl at 7 to 14 days of age. Vaccinia virus expressing RSV G (vvG) (5 × 106 PFU) was inoculated at the base of the tail by scarification (36). Three to four weeks later the mice were challenged i.n. with 3 × 106 to 1 × 107 PFU of live RSV A2 if necessary. All animal studies were performed according to the guidelines of our Institutional Animal Care and Use Committee.
ELISA.
Blood was obtained from the retro-orbital plexus with a heparinized capillary tube, collected in an Eppendorf tube, and centrifuged, and serum was stored at −20°C. RSV G protein-specific antibody titers in immunized mice were measured by a direct enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well plates were coated overnight with 100 μl/well of 10-μg/ml RSV A2 antigen (U.S. Biological, Swampscott, MA) or 2 × 104 PFU of purified RSV A2 diluted in PBS and then blocked with PBS containing 2% bovine serum albumin and 0.05% Tween 20 for 2 h. Sera or lavage fluids were then added in serial dilutions and incubated for 2 h. The plates were washed five times with PBS containing 0.05% Tween 20 and incubated for 1 h with various dilutions of horseradish peroxidase-conjugated affinity-purified rabbit anti-mouse total IgG, IgG1, IgG2a, or IgA secondary antibody (Zymed Laboratories, San Francisco, CA). The plates were washed three times and developed with 3,3′,5,5′-tetramethylbenzidine, and the reaction was stopped with 1 M H3PO4 and analyzed at 450 nm with a Thermo ELISA plate reader.
RSV titer in lung.
Four days after RSV challenge, subsets of mice were euthanized and the lungs were removed into Eagle's modified essential medium. The tissues were then processed through a steel screen to obtain a single-cell suspension, and particulate matter was removed by passage through a 70-μm cell strainer (BD Labware, Franklin Lakes, NJ). The supernatants were collected, and RSV titers in the supernatants were measured by plaque assay on subconfluent HEp-2 monolayers. The data are expressed as the PFU per gram of lung tissue.
BAL.
At 5 days postchallenge, a subset of mice from each group were sacrificed and tracheotomy was performed. The lung airways were washed with 0.8 ml of PBS containing 1% fetal bovine serum (FBS). The collected bronchoalveolar lavage (BAL) fluid cells and supernatants were used for counting eosinophils by hematoxylin-eosin staining and measuring secretory IgA titers, respectively.
Preparation of lung lymphocytes and flow cytometric analysis.
The lungs were perfused with 5 ml of PBS containing 10 U/ml heparin (Sigma-Aldrich, St. Louis, MO) through the right ventricle using a syringe fitted with 25-gauge needle. The lungs were then removed and placed in RPMI medium supplemented with glutamine, gentamicin, penicillin G, and 10% FBS (HyClone, Logan, UT). The tissue was then processed through a steel screen to obtain a single-cell suspension, and particulate matter was removed by passage through a 70-μm Falcon cell strainer (BD Labware, Franklin Lakes, NJ). Freshly explanted BAL fluid or lung cells were purified by density gradient centrifugation and stained in PBS-3% FBS-0.09% NaN3 using fluorochrome-conjugated antibodies. The antibodies used were anti-CD3e (clone 145-2C11), anti-CD4 (clone RM4-5), anti-CD44 (clone IM7), anti-CD49d (clone R1-2), and anti-Vβ14 TCR (clone 14-2). All antibodies were purchased from BD PharMingen (San Diego, CA). After staining, cells were fixed in PBS-2% (wt/vol) paraformaldehyde, and events were acquired using a FACSCalibur flow cytometer (BD Biosciences, San Diego, CA). To enumerate the number of cytokine-producing cells, intracellular cytokine staining was performed. In brief, 2 × 106 freshly explanted lung lymphocytes were cultured in a culture tube. Cells were left untreated or stimulated with 10 μM G(183-195) peptide (WAICKRIPNKKPG) and then incubated for 5 h at 37°C in 5% CO2. Brefeldin A (5 μg/ml) (Sigma-Aldrich) was added for the duration of the culture period to facilitate intracellular cytokine accumulation. Cells were then stained for surface markers, washed, fixed, permeabilized with fluorescence-activated cell sorter buffer containing 0.5% saponin (Sigma- Aldrich, Seoul, Korea), and stained for gamma interferon (IFN-γ). The antibodies used were anti-IFN-γ (clone XMG1.2) or its control isotype antibody (rat IgG1). Dead cells were excluded on the basis of forward and side light scatter patterns. Data were collected using CELLQuest software (BD Biosciences) and analyzed with CELLQuest and WinMDI version 2.9 software (Scripps Research Institute, La Jolla, CA).
Data analysis.
Comparison of differences was conducted by using an unpaired, two-tailed Student t test. The difference was considered statistically significant when the P value was ≤0.05.
RESULTS
Construction of rAd expressing an RSV G protein fragment.
The strategy that we employed to enhance the vaccine potential the of RSV G protein fragment (amino acid residues 130 to 230) was to increase expression by codon optimization and secretion of the recombinant protein by the addition of a signal sequence from t-PA. In addition, three copies of this RSV G fragment were tandemly repeated for higher immunogenicity (23, 31, 47). Given the natural tropism of Ad, a replication-defective rAd vector might be an ideal candidate for delivery of an RSV vaccine. Thus, the engineered coding sequence was placed into Ad DNA under the control of a cytomegalovirus enhancer and promoter by homologous recombination, resulting in the generation of the replication-defective rAd rAd/3xG (Fig. 1A). For comparison, rAd/1xG, containing a single copy of the RSV G fragment, was used. Using a G-specific monoclonal antibody and immunoblotting, multiple bands of approximately 20 kDa to 30 kDa were detected only in the culture supernatant from HEp-2 cells infected with rAd/3xG and not in supernatant from either rAd/control- or rAd/1xG-infected HEp-2 cells (Fig. 1B). However, we have observed a weak positive signal in an ELISA with an rAd/1xG-infected sample, indicating a much lower level of G fragment expression by rAd/1xG (data not shown). These results indicate that rAd/3xG successfully expresses the specific RSV G protein fragment and that the level of expression was significantly enhanced by use of our strategy.
Humoral immune response to rAd/3xG.
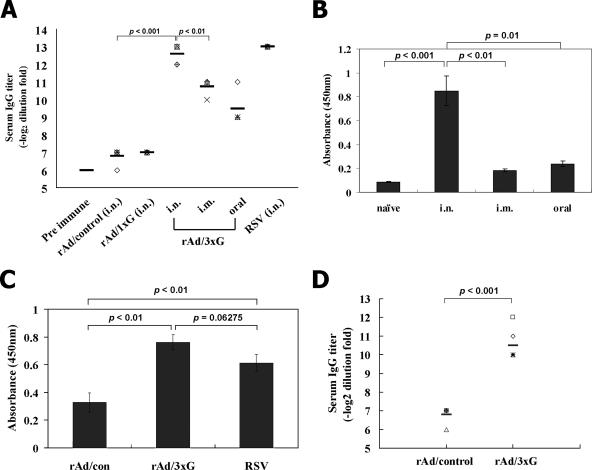
We next examined whether rAd/3xG vaccine was capable of eliciting antigen-specific immune responses in vivo and, if so, what were the characteristics of such immunity and which route was the best one. BALB/c mice were inoculated once via the i.n., i.m., or oral route with the same doses of rAd/1xG, rAd/3xG, or control Ad. As a comparison, live RSV A2 was given once by i.n. instillation, and at 2 weeks after immunization total anti-RSV IgG titers were determined by ELISA. Both i.n. and i.m. immunization with rAd/3xG induced strong total IgG responses in serum even after a single injection, as demonstrated in Fig. 2A. Oral administration led to a relatively weaker RSV-specific serum IgG response than i.n. or i.m. application. However, i.n. immunization with the same dose of rAd/1xG failed to elicit any detectable IgG response above the control value (Fig. 2A). Notably, the levels of serum IgG responses in rAd/3xG-immunized mice (i.n.) were comparable to those induced by live RSV inoculation (i.n.) (Fig. 2A), indicating potent immunogenicity of the rAd/3xG vaccine in vivo.
FIG. 2.
Characterization of humoral immune responses induced by rAd/3xG. (A) BALB/c mice were immunized once with rAd (rAd/1xG, rAd/3xG, or rAd/control) or with live RSV A2 via the indicated routes, and systemic anti-RSV IgG antibody titers were measured by serum ELISA 3 weeks after immunization. The results represent log2 end point values from four or five individual mice. (B) Average IgA titers in the BAL fluid were measured 3 weeks after rAd/3xG immunization. Error bars indicate the standard deviation. (C) Total IgA titers in the supernatants of lung homogenates were determined for each group 4 days after RSV challenge. (D) Fourteen-day-old pups were immunized with 3 × 107 PFU of rAd/3xG or rAd/control in a volume of 20 μl. Anti-RSV IgG antibody titers were determined 2 weeks later.
Secretory IgA is the first line of host defense against aerial pathogens and appears to protect against infection of upper respiratory tract (6). Thus, an effective RSV vaccine should induce virus-specific secretory IgA preferably in the respiratory mucosal area. To examine whether rAd/3xG vaccination elicits an IgA response in the respiratory tract, BAL was performed after immunization and levels of IgA were determined by RSV-specific ELISA. As shown in Fig. 2B, the level of mucosal IgA was much higher in the i.n. immunized group than in the other groups when BAL fluid was tested at 21 days after immunization. Each group of mice immunized i.m. or orally with rAd/3xG exhibited basal levels of IgA response. We also checked the level of anti-RSV IgA in the lungs at 4 days after RSV challenge, and rAd/3xG-immune and RSV-immune mice showed significantly higher levels of IgA than control mice (P < 0.01) (Fig. 2C). The difference between the rAd/3xG-immune and RSV-immune mice was not statistically significant, although the level was slightly higher in rAd/3xG group.
Next, we investigated whether immunization with rAd/3xG also induces strong humoral responses in young pups or neonates. The 14-day-old pups immunized with rAd/3xG showed anti-RSV IgG responses (Fig. 2D), although the mean titer was lower than that in the adult group (Fig. 2A). However, we failed to detect an anti-RSV humoral response in 7- and 10-day-old pups or neonates (data not shown), possibly due to immaturity of the immune system (1).
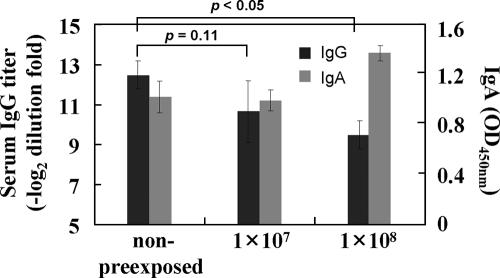
We next examined the effect of preexisting immunity against the Ad vector. The mice were preexposed to rAd/control, rested for 3 weeks, and then immunized with the rAd/3xG vaccine. There was no significant difference in the anti-RSV antibody response between the nonpreexposed group and the group preexposed to 1 × 107 PFU (P = 0.11), although the mean titer in the group preexposed to 1 × 108 PFU was decreased (P < 0.05) (Fig. 3). More interestingly, the levels of secreted anti-RSV IgA in BAL fluid were not significantly changed among all groups of mice (Fig. 3), and all groups of mice showed no detectable RSV replication in the lungs when challenged with 2 × 106 PFU of RSV at 4 weeks after immunization (data not shown). Taken together, our results suggest that a single immunization with rAd/3xG vaccine via the i.n. route is more efficient than that by other administration routes in inducing both serum IgG and respiratory IgA and is sufficiently immunogenic even in the presence of preexisting anti-Ad immunity.
FIG. 3.
Immunogenicity of rAd/3xG in the presence of existing vector immunity. The mice were first inoculated i.n. with various doses of rAd/control as indicated and then after 3 weeks were immunized i.n. with 5 × 107 PFU of rAd/3xG. Anti-RSV IgG antibody titers were measured by serum ELISA 3 weeks later. Mucosal IgA titers in the BAL fluid were also measured 3 weeks after immunization. OD, optical density. Error bars indicate the standard deviation.
T-cell response to rAd/3xG.
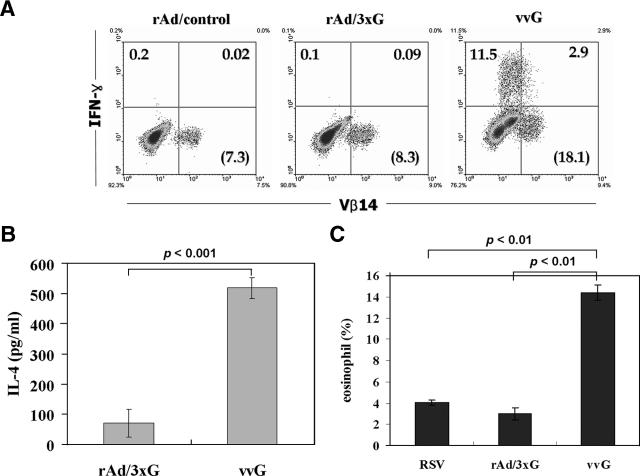
It has been well demonstrated that sensitization of mice with vvG primes G-specific CD4 T-cell responses and results in pulmonary eosinophilia following RSV challenge (5, 17, 20, 30, 36). Since the G protein fragment expressed by the rAd/3xG vaccine contains an I-Ed-restricted CD4 T-cell epitope (43), we examined whether rAd/3xG immunization primes G-specific CD4 T cells. To this end, rAd/3xG- or vvG-immune mice were challenged with RSV, and 4 days after challenge lung lymphocytes were prepared, stimulated with I-Ed-restricted G(183-195) epitope peptide, and stained for IFN-γ production. As shown in Fig. 4A, a G-specific CD4 T-cell response was barely detected in the lungs of the rAd/3xG-immunized group (≤0.2% of lung CD4 T cells), while a strong response (≥10% of lung CD4 T cells) was observed in the vvG-immunized group by intracellular IFN-γ staining. We also checked the level of interleukin-4 (IL-4) cytokine after in vitro stimulation of lung mononuclear cells from both groups. As shown in Fig. 4B, in vitro-stimulated cells from vvG-immune mice produced much more IL-4 than those from rAd/3xG-immune mice. Taken together, these results clearly demonstrate that rAd/3xG immunization does not prime a significant G-specific Th1/Th2 response, in contrast to vvG scarification.
FIG. 4.
G-specific CD4 T-cell response and lung eosinophilia in rAd/3xG-immune mice. (A) Mice were immunized with rAd i.n. or with vvG by scarification and challenged with RSV 3 weeks after immunization. Lung mononuclear cells were prepared from the lungs of the same groups of mice (n = 4) 4 days after challenge and stimulated with G(183-195) peptide in the presence of brefeldin A. Cells were stained for CD4, IFN-γ, and T-cell receptor Vβ14 and analyzed by flow cytometry. Cells gated for CD4 expression are shown in each dot plot, and the percentages represent the frequency of G-specific IFN-γ-positive cells. The numbers in parentheses indicate the percentage of total Vβ14+ cells among CD4+ cells. The results are representative of two independent experiments. (B) Lung mononuclear cells were stimulated in vitro with plate-coated anti-CD3 for 48 h, and the levels of IL-4 in the culture supernatant were determined by sandwich ELISA. One representative from two separate experiments is shown. (C) BAL was performed 5 days after challenge, cell pellets were differentially stained, and the number of eosinophils was counted. Data represent means ± standard deviations (n = 4). The results are representative of three independent experiments.
It was previously reported that the CD4 T-cell response directed to the I-Ed-restricted immunodominant epitope spanning amino acids 183 to 195 of RSV G is sufficient to induce enhanced disease after RSV challenge (40). Our results have indicated that rAd/3xG immunization barely primes G-specific CD4 T cells (Fig. 4A and B). Thus, it is quite unlikely that rAd/3xG immunization would elicit vaccine-enhanced eosinophilia after RSV challenge. To determine whether immunization with rAd/3xG potentiates vaccine-enhanced immunopathology, mice were challenged with RSV 3 weeks after immunization and the levels of eosinophilia in BAL were examined at 5 days after challenge. As previously reported (36, 40), eosinophilia was markedly enhanced in vvG-immunized animals (Fig. 4C) (15 to 25% of total BAL fluid cells). However, a weak infiltrate of eosinophils, ranging from 1 to 3% of the total BAL fluid cells, was observed in mice previously immunized with the rAd/3xG vaccine. This influx was similar in magnitude to that observed in mice previously infected with live RSV, suggesting that rAd/3xG hardly increases the risk of development of eosinophil-related lung pathology.
Protective efficacy of rAd/3xG against RSV challenge.
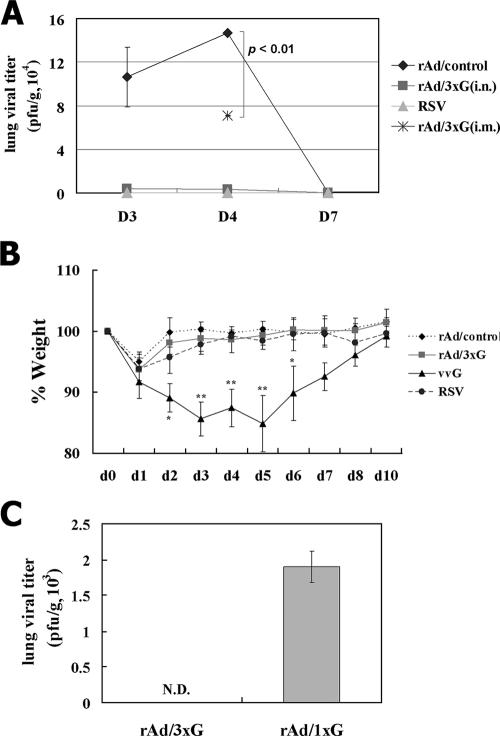
Having characterized the immune responses, we next wanted to examine the immune protection against pulmonary RSV challenge conferred by mucosal and parenteral rAd/3xG vaccination. To this end, immune mice were challenged with live RSV A2 at 4 weeks after immunization. While there was active RSV replication in the lungs of the control mice, i.n. immunization with rAd/3xG prevented any detectable RSV replication in the lungs during the course of infection (Fig. 5A). A group of mice previously infected with live RSV A2 also exhibited complete protection against the challenge. However, i.m. immunization with rAd/3xG resulted in partial protection at the peak of viral replication (day 4 after challenge) (Fig. 5A). In keeping with potent lung protection, there was no significant weight loss upon RSV challenge (Fig. 5B) and no significant disease score (data not shown) in rAd/3xG-immune mice compared to vvG-scarified mice. This protective immunity conferred by rAd/3xG immunization lasted for more than 10 weeks, since RSV challenge at 10 weeks after immunization resulted in no detectable virus replication in the lung (Fig. 5C). Together, these results suggest that a single mucosal rAd/3xG vaccination gives rise to long-term protective immunity without priming of pathological CD4 T cells and subsequent vaccine-enhanced disease.
FIG. 5.
Immune protection from respiratory RSV challenge and reduced weight loss by vaccination with rAd/3xG. (A) Each group of immune mice was challenged with 1 × 107 PFU RSV at 4 weeks after immunization, and the level of RSV replication in the lungs was determined by plaque assay during the postchallenge periods. Results are expressed as the means ± standard errors of the means from four or five mice for each time point per group. The limit of detection is 200 PFU/gram of lung. (B) The same groups of immune mice were challenged with RSV and then weighed each day. Results are expressed as the means ± standard errors of the means from five mice for each group. *, P < 0.01; **, P < 0.001. (C) Immune mice were challenged with 1 × 106 PFU RSV at 10 weeks after immunization, and the level of RSV replication was determined at day 4 after infection. N.D., not detected.
DISCUSSION
In this study, we targeted an RSV G protein fragment as an immunogen, expressed by replication-defective rAd, to evaluate its vaccine potential. We chose this G protein fragment for several reasons: (i) the G glycoprotein is one of the major immunogens among viral proteins in both humans and rodents (27), (ii) this region contains a conserved B-cell epitope of both subgroups A and B (2, 37, 41, 43), and (iii) a subunit vaccine containing this G protein fragment (9, 33) and a plasmid DNA vaccine expressing secreted G protein (3, 25) were shown to induced protective immunity in rodents without induction of lung pathology. The evidence for the exact role of G in vaccine-enhanced pathology is still contradictory (21, 28, 44). Thus, it is worthwhile to investigate the protective role of RSV G-specific immunity elicited by a novel Ad-based vaccine. rAd vectors have been used successfully and safely in many preclinical and clinical settings (reviewed in reference 38). Replication-defective Ad is an attractive vaccine delivery vehicle since it has intrinsic properties that activate both the innate and the adaptive arms of the immune system (11). It has also been shown to have very high transduction rate (48). Our results clearly show that immunization with the rAd/3xG vaccine induces strong serum and mucosal antibody responses after a single dose without priming of potently immunopathogenic CD4 T cells, which leads to long-term protection from experimental RSV infection without vaccine-enhanced illness. It is noteworthy that single-dose immunization with rAd/3xG is sufficient for the induction of protective immunity, while three immunizations with plasmid DNA encoding RSV G were required in the DNA vaccine regimen (25). This could be relative advantage of rAd/3xG over other vaccine candidates, considering the fact that infant vaccinees must be protected from infection as soon as possible after birth.
The exact mechanisms responsible for the lack of CD4 T-cell priming and induction of beneficial and protective immunity by rAd/3xG need further investigation. One possible explanation could be based on the intrinsic properties of defective rAd vaccines. A number of reports of studies using rAd-based vaccines describe similar findings that Ad vectors induce potent transgene product-specific antibody and/or CD8 T-cell responses but no overly potent CD4 T-cell responses in experimental animal systems (reviewed in reference 38). Second, it has been proposed that RSV G has an immunomodulatory effect on both innate and adaptive immunity (7, 32). For example, Bukreyev et al. showed that the secreted form of G and the cysteine-rich region have a positive modulatory effect on cytotoxic T-lymphocyte function, which is independent of virus titers and RSV-induced inflammation (7). Thus, it is possible that secreted G protein fragments expressed by rAd/3xG affect the priming environment in a way more favorable to beneficial protective immunity. Together, our results strongly suggest that RSV G protein could be successfully applied as a protective immunogen against RSV infection, if appropriate delivery vectors such as recombinant Ad are employed.
Our study has also shown that the i.n. route of injection is better than any other route tested with regard to protective immunity induced by rAd/3xG. The higher levels of virus-specific mucosal IgA and serum IgG raised by i.n. immunization of rAd/3xG seem to be correlated with better protection against RSV challenge (Fig. 2A and B and 5A). These results are consistent with previous reports that stimulation and maintenance of local mucosal antibody may be important in the generation of effective protection (12, 34). Since the infection route for RSV is via the respiratory tract, mucosal delivery might be critical for the development of protective immunity at the site of infection. In addition, mucosal immunization has been reported to play an important role in avoiding interference from maternal antibodies against the Ad vector (4, 10). One of the major concerns about Ad-vectored vaccines is that the induction of immune responses to this common human pathogen in neonates and young infants is impaired by maternally transferred antibodies (45). We have shown that i.n. immunization with rAd/3xG is capable of inducing strong serum IgG and mucosal IgA responses even in the presence of vector immunity (Fig. 3). Previously, it has also been shown that mucosally delivered Ad vectors expressing viral antigen were capable of inducing neutralizing antibodies and protective immunity in neonatal mice even in the presence of maternal antibodies against the vaccine carrier (46). These results suggest that when delivered through i.n. route, the rAd/3xG vaccine might be effective in human populations in which the majority have preexisting immunity to Ad serotype 5 as a result of natural exposure. Furthermore, injection of rAd has been shown to generally induce inflammatory reactions by releasing proinflammatory cytokines such as IL-6, tumor necrosis factor alpha, and IL-1 (14), which may compensate for the Th2 bias of the immature immune system in neonates. In this regard, delivery of the RSV G protein fragment by an Ad vector as a neutralizing antigen might have some advantages over the use of other vehicles, particularly as neonatal vaccines.
In summary, we have provided evidence that a replication-deficient rAd-based RSV vaccine represents a promising candidate for an RSV vaccine. One of the major advantages associated with this RSV vaccine might be its effectiveness in inducing long-term protective immunity with a single dose. It is also possible that the level and/or duration of protection induced by mucosal vaccination with our vaccine candidate could be further enhanced when given with appropriate adjuvant(s). We are currently investigating whether the use of various doses, mixtures with various adjuvants, and/or multiple administrations of the immunogen by prime-boost regimens could further improve the efficacy of our vaccine candidate.
Acknowledgments
We acknowledge the technical support from Sung-A Kim, Min-Ha Lee, and Ji-Hye Yoon in the completion of this work.
This work was supported by a Korea Research Foundation grant funded by the Korean government (MOEHRD) (KRF-2006-E00130), by grant R15-2006-020 from the NCRC program of MOST and KOSEF through the Center for Cell Signaling and Drug Discovery Research at Ewha Womans University, and by the Brain Korea 21 project.
Footnotes
Published ahead of print on 19 December 2007.
REFERENCES
- 1.Adkins, B., C. Leclerc, and S. Marshall-Clarke. 2004. Neonatal adaptive immunity comes of age. Nat. Rev. Immunol. 4553-564. [DOI] [PubMed] [Google Scholar]
- 2.Akerlind-Stopner, B., G. Utter, M. A. Mufson, C. Orvell, R. A. Lerner, and E. Norrby. 1990. A subgroup-specific antigenic site in the G protein of respiratory syncytial virus forms a disulfide-bonded loop. J. Virol. 645143-5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson, C., P. Liljestrom, S. Stahl, and U. F. Power. 2000. Protection against respiratory syncytial virus (RSV) elicited in mice by plasmid DNA immunisation encoding a secreted RSV G protein-derived antigen. FEMS Immunol. Med. Microbiol. 29247-253. [DOI] [PubMed] [Google Scholar]
- 4.Belyakov, I. M., B. Moss, W. Strober, and J. A. Berzofsky. 1999. Mucosal vaccination overcomes the barrier to recombinant vaccinia immunization caused by preexisting poxvirus immunity. Proc. Natl. Acad. Sci. USA 964512-4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bembridge, G. P., R. Garcia-Beato, J. A. Lopez, J. A. Melero, and G. Taylor. 1998. Subcellular site of expression and route of vaccination influence pulmonary eosinophilia following respiratory syncytial virus challenge in BALB/c mice sensitized to the attachment G protein. J. Immunol. 1612473-2480. [PubMed] [Google Scholar]
- 6.Brandtzaeg, P. 2003. Role of secretory antibodies in the defence against infections. Int. J. Med. Microbiol. 2933-15. [DOI] [PubMed] [Google Scholar]
- 7.Bukreyev, A., M. E. Serra, F. R. Laham, G. A. Melendi, S. R. Kleeberger, P. L. Collins, and F. P. Polack. 2006. The cysteine-rich region and secreted form of the attachment G glycoprotein of respiratory syncytial virus enhance the cytotoxic T-lymphocyte response despite lacking major histocompatibility complex class I-restricted epitopes. J. Virol. 805854-5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connors, M., A. B. Kulkarni, P. L. Collins, C. Y. Firestone, K. L. Holmes, H. C. Morse III, and B. R. Murphy. 1992. Resistance to respiratory syncytial virus (RSV) challenge induced by infection with a vaccinia virus recombinant expressing the RSV M2 protein (Vac-M2) is mediated by CD8+ T cells, while that induced by Vac-F or Vac-G recombinants is mediated by antibodies. J. Virol. 661277-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corvaia, N., P. Tournier, T. N. Nguyen, J. F. Haeuw, U. F. Power, H. Binz, and C. Andreoni. 1997. Challenge of BALB/c mice with respiratory syncytial virus does not enhance the Th2 pathway induced after immunization with a recombinant G fusion protein, BBG2NA, in aluminum hydroxide. J. Infect. Dis. 176560-569. [DOI] [PubMed] [Google Scholar]
- 10.Crowe, J. E., Jr. 2001. Influence of maternal antibodies on neonatal immunization against respiratory viruses. Clin. Infect. Dis. 331720-1727. [DOI] [PubMed] [Google Scholar]
- 11.Ertl, H. C., and Z. Xiang. 1996. Novel vaccine approaches. J. Immunol. 1563579-3582. [PubMed] [Google Scholar]
- 12.Etchart, N., B. Baaten, S. R. Andersen, L. Hyland, S. Y. Wong, and S. Hou. 2006. Intranasal immunisation with inactivated RSV and bacterial adjuvants induces mucosal protection and abrogates eosinophilia upon challenge. Eur. J. Immunol. 361136-1144. [DOI] [PubMed] [Google Scholar]
- 13.Falsey, A. R. 1998. Respiratory syncytial virus infection in older persons. Vaccine 161775-1778. [DOI] [PubMed] [Google Scholar]
- 14.Ginsberg, H. S., L. L. Moldawer, P. B. Sehgal, M. Redington, P. L. Kilian, R. M. Chanock, and G. A. Prince. 1991. A mouse model for investigating the molecular pathogenesis of adenovirus pneumonia. Proc. Natl. Acad. Sci. USA 881651-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glezen, W. P., S. B. Greenberg, R. L. Atmar, P. A. Piedra, and R. B. Couch. 2000. Impact of respiratory virus infections on persons with chronic underlying conditions. JAMA 283499-505. [DOI] [PubMed] [Google Scholar]
- 16.Han, L. L., J. P. Alexander, and L. J. Anderson. 1999. Respiratory syncytial virus pneumonia among the elderly: an assessment of disease burden. J. Infect. Dis. 17925-30. [DOI] [PubMed] [Google Scholar]
- 17.Hancock, G. E., D. J. Speelman, K. Heers, E. Bortell, J. Smith, and C. Cosco. 1996. Generation of atypical pulmonary inflammatory responses in BALB/c mice after immunization with the native attachment (G) glycoprotein of respiratory syncytial virus. J. Virol. 707783-7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He, T. C., S. Zhou, L. T. da Costa, J. Yu, K. W. Kinzler, and B. Vogelstein. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 952509-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heidema, J., G. J. de Bree, P. M. De Graaff, W. W. van Maren, P. Hoogerhout, T. A. Out, J. L. Kimpen, and G. M. van Bleek. 2004. Human CD8(+) T cell responses against five newly identified respiratory syncytial virus-derived epitopes. J. Gen. Virol. 852365-2374. [DOI] [PubMed] [Google Scholar]
- 20.Johnson, T. R., J. E. Johnson, S. R. Roberts, G. W. Wertz, R. A. Parker, and B. S. Graham. 1998. Priming with secreted glycoprotein G of respiratory syncytial virus (RSV) augments interleukin-5 production and tissue eosinophilia after RSV challenge. J. Virol. 722871-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson, T. R., M. N. Teng, P. L. Collins, and B. S. Graham. 2004. Respiratory syncytial virus (RSV) G glycoprotein is not necessary for vaccine-enhanced disease induced by immunization with formalin-inactivated RSV. J. Virol. 786024-6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson, T. R., S. M. Varga, T. J. Braciale, and B. S. Graham. 2004. Vβ14+ T cells mediate the vaccine-enhanced disease induced by immunization with respiratory syncytial virus (RSV) G glycoprotein but not with formalin-inactivated RSV. J. Virol. 788753-8760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kjerrulf, M., B. Lowenadler, C. Svanholm, and N. Lycke. 1997. Tandem repeats of T helper epitopes enhance immunogenicity of fusion proteins by promoting processing and presentation. Mol. Immunol. 34599-608. [DOI] [PubMed] [Google Scholar]
- 24.Levine, S., R. Klaiber-Franco, and P. R. Paradiso. 1987. Demonstration that glycoprotein G is the attachment protein of respiratory syncytial virus. J. Gen. Virol. 682521-2524. [DOI] [PubMed] [Google Scholar]
- 25.Li, X., S. Sambhara, C. X. Li, L. Ettorre, I. Switzer, G. Cates, O. James, M. Parrington, R. Oomen, R. P. Du, and M. Klein. 2000. Plasmid DNA encoding the respiratory syncytial virus G protein is a promising vaccine candidate. Virology 26954-65. [DOI] [PubMed] [Google Scholar]
- 26.Martinez, I., J. Dopazo, and J. A. Melero. 1997. Antigenic structure of the human respiratory syncytial virus G glycoprotein and relevance of hypermutation events for the generation of antigenic variants. J. Gen. Virol. 782419-2429. [DOI] [PubMed] [Google Scholar]
- 27.Murphy, B. R., S. L. Hall, A. B. Kulkarni, J. E. Crowe, Jr., P. L. Collins, M. Connors, R. A. Karron, and R. M. Chanock. 1994. An update on approaches to the development of respiratory syncytial virus (RSV) and parainfluenza virus type 3 (PIV3) vaccines. Virus Res. 3213-36. [DOI] [PubMed] [Google Scholar]
- 28.Murphy, B. R., A. Sotnikov, P. R. Paradiso, S. W. Hildreth, A. B. Jenson, R. B. Baggs, L. Lawrence, J. J. Zubak, R. M. Chanock, J. A. Beeler, et al. 1989. Immunization of cotton rats with the fusion (F) and large (G) glycoproteins of respiratory syncytial virus (RSV) protects against RSV challenge without potentiating RSV disease. Vaccine 7533-540. [DOI] [PubMed] [Google Scholar]
- 29.Nicholas, J. A., K. L. Rubino, M. E. Levely, E. G. Adams, and P. L. Collins. 1990. Cytolytic T-lymphocyte responses to respiratory syncytial virus: effector cell phenotype and target proteins. J. Virol. 644232-4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Openshaw, P. J., S. L. Clarke, and F. M. Record. 1992. Pulmonary eosinophilic response to respiratory syncytial virus infection in mice sensitized to the major surface glycoprotein G. Int Immunol. 4493-500. [DOI] [PubMed] [Google Scholar]
- 31.Oscherwitz, J., M. E. Zeigler, T. E. Gribbin, and K. B. Cease. 1999. A V3 loop haptenic peptide sequence, when tandemly repeated, enhances immunogenicity by facilitating helper T-cell responses to a covalently linked carrier protein. Vaccine 172392-2399. [DOI] [PubMed] [Google Scholar]
- 32.Polack, F. P., P. M. Irusta, S. J. Hoffman, M. P. Schiatti, G. A. Melendi, M. F. Delgado, F. R. Laham, B. Thumar, R. M. Hendry, J. A. Melero, R. A. Karron, P. L. Collins, and S. R. Kleeberger. 2005. The cysteine-rich region of respiratory syncytial virus attachment protein inhibits innate immunity elicited by the virus and endotoxin. Proc. Natl. Acad. Sci. USA 1028996-9001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Power, U. F., H. Plotnicky-Gilquin, T. Huss, A. Robert, M. Trudel, S. Stahl, M. Uhlen, T. N. Nguyen, and H. Binz. 1997. Induction of protective immunity in rodents by vaccination with a prokaryotically expressed recombinant fusion protein containing a respiratory syncytial virus G protein fragment. Virology 230155-166. [DOI] [PubMed] [Google Scholar]
- 34.Singleton, R., N. Etchart, S. Hou, and L. Hyland. 2003. Inability to evoke a long-lasting protective immune response to respiratory syncytial virus infection in mice correlates with ineffective nasal antibody responses. J. Virol. 7711303-11311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sparer, T. E., S. Matthews, T. Hussell, A. J. Rae, B. Garcia-Barreno, J. A. Melero, and P. J. Openshaw. 1998. Eliminating a region of respiratory syncytial virus attachment protein allows induction of protective immunity without vaccine-enhanced lung eosinophilia. J. Exp. Med. 1871921-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Srikiatkhachorn, A., and T. J. Braciale. 1997. Virus-specific CD8+ T lymphocytes downregulate T helper cell type 2 cytokine secretion and pulmonary eosinophilia during experimental murine respiratory syncytial virus infection. J. Exp. Med. 186421-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sullender, W. M., K. Anderson, and G. W. Wertz. 1990. The respiratory syncytial virus subgroup B attachment glycoprotein: analysis of sequence, expression from a recombinant vector, and evaluation as an immunogen against homologous and heterologous subgroup virus challenge. Virology 178195-203. [DOI] [PubMed] [Google Scholar]
- 38.Tatsis, N., and H. C. Ertl. 2004. Adenoviruses as vaccine vectors. Mol. Ther. 10616-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor, G., E. J. Stott, M. Bew, B. F. Fernie, P. J. Cote, A. P. Collins, M. Hughes, and J. Jebbett. 1984. Monoclonal antibodies protect against respiratory syncytial virus infection in mice. Immunology 52137-142. [PMC free article] [PubMed] [Google Scholar]
- 40.Tebbey, P. W., M. Hagen, and G. E. Hancock. 1998. Atypical pulmonary eosinophilia is mediated by a specific amino acid sequence of the attachment (G) protein of respiratory syncytial virus. J. Exp. Med. 1881967-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trudel, M., F. Nadon, C. Seguin, and H. Binz. 1991. Protection of BALB/c mice from respiratory syncytial virus infection by immunization with a synthetic peptide derived from the G glycoprotein. Virology 185749-757. [DOI] [PubMed] [Google Scholar]
- 42.Varga, S. M., X. Wang, R. M. Welsh, and T. J. Braciale. 2001. Immunopathology in RSV infection is mediated by a discrete oligoclonal subset of antigen-specific CD4(+) T cells. Immunity 15637-646. [DOI] [PubMed] [Google Scholar]
- 43.Varga, S. M., E. L. Wissinger, and T. J. Braciale. 2000. The attachment (G) glycoprotein of respiratory syncytial virus contains a single immunodominant epitope that elicits both Th1 and Th2 CD4+ T cell responses. J. Immunol. 1656487-6495. [DOI] [PubMed] [Google Scholar]
- 44.Vaux-Peretz, F., J. M. Chapsal, and B. Meignier. 1992. Comparison of the ability of formalin-inactivated respiratory syncytial virus, immunopurified F, G and N proteins and cell lysate to enhance pulmonary changes in Balb/c mice. Vaccine 10113-118. [DOI] [PubMed] [Google Scholar]
- 45.Wang, Y., Z. Xiang, S. Pasquini, and H. C. Ertl. 1997. Immune response to neonatal genetic immunization. Virology 228278-284. [DOI] [PubMed] [Google Scholar]
- 46.Xiang, Z., Y. Li, G. Gao, J. M. Wilson, and H. C. Ertl. 2003. Mucosally delivered E1-deleted adenoviral vaccine carriers induce transgene product-specific antibody responses in neonatal mice. J. Immunol. 1714287-4293. [DOI] [PubMed] [Google Scholar]
- 47.Yankai, Z., Y. Rong, H. Yi, L. Wentao, C. Rongyue, Y. Ming, L. Taiming, L. Jingjing, and W. Jie. 2006. Ten tandem repeats of beta-hCG 109-118 enhance immunogenicity and anti-tumor effects of beta-hCG C-terminal peptide carried by mycobacterial heat-shock protein HSP65. Biochem. Biophys. Res. Commun. 3451365-1371. [DOI] [PubMed] [Google Scholar]
- 48.Zhong, L., A. Granelli-Piperno, Y. Choi, and R. M. Steinman. 1999. Recombinant adenovirus is an efficient and non-perturbing genetic vector for human dendritic cells. Eur. J. Immunol. 29964-972. [DOI] [PubMed] [Google Scholar]