Abstract
The avian sarcoma and leukosis virus (ASLV) family of retroviruses contains five highly related envelope subgroups (A to E) thought to have evolved from a common viral ancestor in the chicken population. Three genetic loci in chickens determine the susceptibility or resistance of cells to infection by the subgroup A to E ASLVs. Some inbred lines of chickens display phenotypes that are somewhere in between either efficiently susceptible or resistant to infection by specific subgroups of ASLV. The tvb gene encodes the receptor for subgroups B, D, and E ASLVs. The wild-type TvbS1 receptor confers susceptibility to subgroups B, D, and E ASLVs. In this study, the genetic defect that accounts for the altered susceptibility of an inbred chicken line, line M, to infection by ASLV(B), ASLV(D), and ASLV(E) was identified. The tvb gene in line M, tvbr2, encodes a mutant TvbS1 receptor protein with a substitution of a serine for a cysteine at position 125 (C125S). Here, we show that the C125S substitution in TvbS1 significantly reduces the susceptibility of line M cells to infection by ASLV(B) and ASLV(D) and virtually eliminates susceptibility to ASLV(E) infection both in cultured cells and in the incidence and growth of avian sarcoma virus-induced sarcomas in chickens. The C125S substitution significantly reduces the binding affinity of the TvbS1 receptor for the subgroup B, D, and E ASLV envelope glycoproteins. These are the first results that demonstrate a possible role of the cysteine-rich domain 3 in the function of the Tvb receptors.
Retroviruses cause serious diseases in animals and humans. The disease process begins with the virus infecting a cell(s), a process mediated by the interaction of the retroviral envelope glycoproteins with specific cell surface proteins that act as receptors (14, 24). A proper viral-glycoprotein-receptor interaction initiates conformational changes in the viral glycoproteins that ultimately result in the fusion of the viral and cellular membranes and entry of viral components (9). Despite the complexity and specificity of the viral-glycoprotein-receptor interaction required for virus entry, families of closely related retroviruses have evolved their glycoproteins to use different cellular proteins as receptors. Presumably, the presence of multiple viral subgroups that utilize different receptors is an advantage for viruses in overcoming host resistance. Resistance to retroviral infection occurs when the specific receptor protein is not available. Genetic alteration(s) can account for resistance, resulting in the complete lack of receptor protein expression or the expression of an aberrant protein not suitable as a viral receptor. In addition, receptors can be saturated with viral glycoproteins expressed by the cell, physically blocking receptor accessibility, a phenomenon known as receptor interference (14, 24).
The avian sarcoma and leukosis virus (ASLV) family of retroviruses contains five highly related envelope subgroups (A to E) thought to have evolved in the chicken population from a common viral ancestor (4, 5, 24). Three genetic loci in chickens determine the susceptibility or resistance of cells to infection by the subgroup A to E ASLVs. Susceptibility to subgroup A ASLVs is determined by the tva locus; susceptibility to subgroup C ASLVs by the tvc locus; and susceptibility to the subgroup B, D, and E ASLVs by the tvb locus. The Tva proteins are related to the low-density lipoprotein receptor family (6, 25). The Tvb proteins are related to the tumor necrosis factor receptor (TNFR) family (2, 3, 7). The Tvc proteins are most closely related to mammalian butyrophilins of the immunoglobulin superfamily (11). The normal functions and ligands of the Tva, Tvb, and Tvc proteins in birds are unknown.
The genetic defects that account for resistance to infection by specific ASLVs, tvar, tvbr, and tvcr alleles, have been identified in some lines of inbred chickens (10, 11, 16). The mutations found in the resistant alleles result in premature termination codons, or frameshifts, leading to severely truncated proteins or no receptor expression. In addition, single-amino-acid substitutions, often changing a cysteine residue, that result in a dramatic decrease in the affinity of the ASLV envelope glycoprotein interaction for the mutant receptor protein have been identified. An understanding of the variation in receptor proteins that can be encountered by ASLVs in chickens, including related receptor orthologs in other birds, provides valuable information on the evolutionary pressures on these viruses and enables the identification of domains of the receptor and envelope glycoprotein critical for an efficient virus-receptor interaction and subsequent virus entry.
The structures of TNFR-related proteins have shown that homologous proteins (e.g., the Tvb proteins) likely contain three cysteine-rich domains (CRDs) in the extracellular domain (Fig. 1A) (15). Two naturally occurring tvb susceptibility alleles have been identified in chickens; the tvbs1 allele confers susceptibility to subgroups B, D, and E ASLVs, while the tvbs3 allele confers susceptibility to only subgroup B and D ASLVs (3, 7). The substitution of a serine for a cysteine at residue 62 in the CRD2 domain of TvbS3 distinguishes the TvbS1 and TvbS3 proteins. Presumably, this mutation alters the structure of CRD2, resulting in the loss of ASLV(E) binding and entry. The major determinants of the Tvb receptor important for entry of ASLV(B), ASLV(D), and ASLV(E) were identified previously using a variety of mutational approaches (1, 17, 18). A 15-amino-acid peptide in the Tvb CRD1 domain appears to be sufficient for ASLV(B) and ASLV(D) binding and entry. In contrast, the main determinants of Tvb important for ASLV(E) binding and entry reside in the CRD2 domain. These studies did not demonstrate any significant role of the CRD3 domain in the binding and entry of ASLV(B), ASLV(D), or ASLV(E). A tvb allele, tvbr, which contains a premature stop codon at residue 57 explaining the resistance to infection by all three subgroups (16), was identified and characterized from inbred line 72 chickens. Finally, a tvb homolog, tvbt, that confers susceptibility only to ASLV(E) (2), has been identified in turkeys.
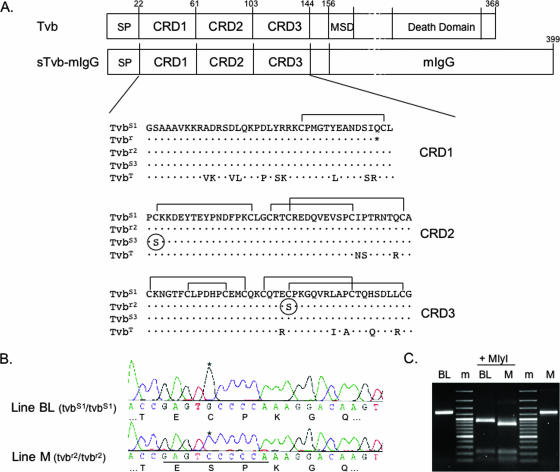
FIG. 1.
The tvbr2 allele in chicken line M contains a C125S substitution in CRD3. (A) Schematic representations of the Tvb proteins and a soluble form of Tvb fused to an mIgG are shown (not drawn to scale). The numbering of the Tvb proteins includes the signal peptide (SP) for historical reasons. MSD, membrane-spanning domain. The deduced amino acid sequences of the CRDs of the known Tvb receptors, including the turkey ortholog, TvbT, are compared, with residues different from the TvbS1 receptor indicated. The C125S substitution in TvbR2 and the C62S substitution in TvbS3 are circled. The putative disulfide bonds in the Tvb CRDs are indicated based on comparisons with the related TNFR proteins and the X-ray structure of the human DR5 TRAIL receptor protein. (B) Chromatograms of partial nucleotide and deduced amino acid sequences of CRD3s from chicken line M and outbred chicken line BL cDNAs. The G-to-C transversion is highlighted with an asterisk; the new MlyI site in line M is underlined. (C) A 1,259-bp tvb fragment was produced by reverse transcription-PCR using RNA from line BL and line M. After MlyI digestion, a 920-bp fragment was produced from the wild-type tvbs1 cDNA from line BL, while the additional MlyI site in the line M tvbr2 cDNA produced an 820-bp fragment. m, DNA marker 100-bp ladder.
Some inbred lines of chickens display phenotypes that are somewhere in between completely susceptible and resistant to infection by specific subgroups of ASLV. This altered susceptibility phenotype could be the result of mutations in the receptor that alter but do not eliminate the ability of ASLV to use the protein as a receptor. We hypothesized that such mutations, which reduce but do not eliminate the binding affinity of the mutant receptor for the viral glycoproteins, could explain this phenotype. In this study, the genetic defect that accounts for the altered susceptibility of an inbred chicken line, line M, to infection by ASLV(B), ASLV(D), and ASLV(E) was identified. The tvb gene in line M, tvbr2, encodes a mutant TvbS1 receptor protein with a substitution of a serine for a cysteine at position 125 (C125S). These are the first results that demonstrate a possible role of CRD3 in the efficient functioning of the Tvb receptors.
MATERIALS AND METHODS
Chicken lines.
The inbred chicken lines M (Black Minorca), L15, CC.R1, and WA and the outbred chicken population Brown Leghorn (BL) have been maintained at the Institute of Molecular Genetics, Prague (20). Hens and cockerels were kept in individual cages (4,200 cm2) under standard conditions. Feed and water were provided ad libitum, and a light-dark photoperiod of 12 h-12 h was applied. Fertilized eggs were incubated at 38°C and 60% relative humidity in a forced-air incubator with a tilting motion every 2 h through a 90° angle. All experiments were performed in accordance with Czech legal requirements for animal handling.
Cell culture and virus propagation.
Chicken embryo fibroblasts (CEFs) were prepared from 10-day-old embryos from line M, WA, and BL chickens as described previously (12). The DF-1 chicken fibroblast cell line (12a), the Rous sarcoma virus (RSV)-transformed 16Q quail cell line (19), and CEFs were grown in a mixture of two parts Dulbecco's modified Eagle's medium and one part F-12 medium supplemented with 5% calf serum, 5% fetal calf serum, 1% chicken serum, and penicillin/streptomycin (100 mg/ml each) in a 5% CO2 atmosphere at 37°C. RCASBP(A)GFP, RCASBP(B)GFP, RCASBP(C)GFP, and RCASBP(D)GFP viruses (12, 13) were propagated by transfection of plasmid DNA containing the reporter vector into DF-1 cells, which are free of closely related endogenous retrovirus loci. The RCASBP(E)GFP virus was propagated in line L15 CEFs. Transfection was performed by applying a mixture of 10 μg plasmid DNA and 100 μl linear polyethylenimine (Polysciences; molecular weight, 25,000; 1 mg/ml; pH 7.5) in 12 ml serum-free culture medium on subconfluent DF-1 cells grown in 100 mm plates. Virus spread was observed as an increasing proportion of green fluorescent protein (GFP)-positive cells, and viral stocks were established from cell supernatants at day 4 or 5 after transfection. Viral stocks were cleared of debris by centrifugation at 2,000 × g for 10 min at 10°C and stored at −80°C.
The transforming subgroup A, B, C, D, and E ASLV specificities for in vivo sarcoma induction were produced by rescuing the replication-defective Bryan high-titer-RSV present in the 16Q cell line. DF-1 cells were infected with RCASBP(A)GFP, RCASBP(B)GFP, RCASBP(C)GFP, RCASBP(D)GFP, or RCASBP(E)GFP, and virus spread was monitored by fluorescence. After 4 days, the infected GFP-positive DF-1 cells were mixed with 16Q cells (19) and cultivated for a further 5 days. Viral stocks containing GFP reporter viruses, as well as transforming viruses of the same subgroup, were centrifuged at 2,000 × g for 10 min at 10°C and stored at −80°C. The titers of the transforming viruses were quantitated by an src focus assay on BL CEFs and reached titers of 102 to 105 focus-forming unit (FFU) per ml.
Reverse transcription-PCR of the tvbr allele from chicken line M.
We prepared total RNA from blood collected from line M chickens using the RNeasy total RNA isolation system (Qiagen Inc.). Reverse transcription was carried out with 1 μg total RNA, Moloney murine leukemia virus reverse transcriptase (Promega), and oligo(dT)15 primers (Promega). The cDNA resulting from this reaction was PCR amplified with primers TVB3 (5′-CAGACCTCCAGAAGCCAGAC-3′) and TVB6 (5′-CGAGAGCACTGTCACAGAGAT-3′) with Taq polymerase (TaKaRa). The conditions for the amplification were as follows: 2 min at 94°C; 34 cycles of 15 s at 94°C, annealing for 40 s at 57°C, and 3 min at 72°C; and final extension for 10 min at 72°C. The final PCR product was treated with ExoSAP-IT (USB) and then sequenced using the BigDye Terminator v3.1 cycle-sequencing kit (PE Applied Biosystems). A 1,259-bp fragment of tvb was amplified from the cDNA using primers TVB5 (5′-TCCTAACTCGGTCCGAATCC-3′) and TVB6 and the Expand Long Template Polymerase (Roche). The conditions for amplification were 2 min at 94°C and 35 cycles of 15 s at 94°C, annealing for 40 s at 56°C, and 90 s at 68°C; final extension was for 7 min at 68°C. The product was digested with MlyI, and the products were separated by agarose electrophoresis.
Virus entry analyzed by FACS.
Line BL or M CEFs were seeded at 5 × 105 per 60-mm plate and infected with RCASBP viruses the next day: 105 infectious units were applied in 0.5 ml medium, and after 1 h, the volume of the medium was increased to 4 ml. The percentage of GFP-positive cells was quantitated by fluorescence-activated cell sorting (FACS) using an LSR II analyzer (Becton Dickinson) on days 1, 2, 3, and 6 postinfection. Each day, one half of the cell culture was used for FACS and the other half was passaged on a new dish. For FACS analysis, trypsinized cells were first washed in phosphate-buffered saline (PBS) and then analyzed.
DNA constructs.
A gene encoding a soluble form of the chicken TvbS1C125S receptor (stvbs1C125S-mIgG) was constructed as described previously for soluble forms of the chicken TvbS3 receptor (stvbs3-mIgG) and TvbS1 receptor (stvbs1-mIgG) (11). These genes encode the extracellular domain of the ASLV receptor fused to the constant region of a mouse immunoglobulin G (mIgG) heavy chain and are in the CLA12NCO adaptor plasmid (12). The stvbs1C125S-mIgG, stvbs1-mIgG, and stvbS3-mIgG gene cassettes were isolated as ClaI fragments and subcloned into the ClaI site of the RCASBP(A) vector. DF-1 cells were infected with each virus, and infected cell supernatants that contained either the TvbS1C125S-mIgG, TvbS3-mIgG, or TvbS1-mIgG receptor proteins were collected. The supernatants were cleared by centrifugation at 2,000 × g for 10 min at 4°C and stored in aliquots at −80°C.
The recombinant, replication-competent ASLV vectors RCASBP(A)GFP, RCASBP(B)GFP, RCASBP(C)GFP, RCASBP(D)GFP, and RCASBP(E)GFP viruses containing the GFP gene have been described previously (12). The receptor subgroup of the viral envelope glycoprotein is denoted in parentheses [e.g., subgroup B by (B)].
Immunoprecipitations and Western immunoblot analysis.
The TvbS1C125S-mIgG, TvbS3-mIgG, and TvbS1-mIgG receptor proteins were immunoprecipitated separately from cell culture supernatants with anti-mIgG-agarose beads (Sigma) and analyzed by Western immunoblotting as previously described (11).
Binding affinity was analyzed by FACS.
Line L15 CEFs and line L15 CEFs infected with either RCASBP(B), RCASBP(D), or RCASBP(E) were removed from culture with trypsin de Larco (Quality Biological, Inc.) and washed with PBS. The cells were fixed with 4% paraformaldehyde in PBS at room temperature for 15 min and then washed with PBS. Approximately 1 × 106 cells in PBS supplemented with 1% calf serum (PBS-CS) were incubated with supernatant containing one of the receptor-mIgG fusion proteins on ice for 30 min. The DF-1 cells were then washed with PBS-CS and incubated with either 5 μl of goat anti-mIgG (heavy plus light chains) linked to phycoerythrin or 5 μl of goat anti-rabbit IgG (rIgG) (heavy plus light chains) linked to phycoerythrin (Kirkegaard & Perry Laboratories, Gaithersburg, MD) in PBS-CS (1 ml total volume) on ice for 30 min. The cell-soluble receptor-IgG-Ig-phycoerythrin complexes were washed with PBS-CS, resuspended in 0.5 ml PBS-CS, and analyzed with a Becton Dickinson FACSCalibur using CELLQuest 3.1 software.
KD calculations.
The maximum possible fluorescence and apparent dissociation constant (KD) value for each data set obtained from the FACS binding assays were estimated by fitting the data via nonlinear least squares to a log logistic growth curve function: 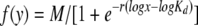 , where y is the mean fluorescence, M is the maximum fluorescence, r is the rate, x is the concentration of the receptor-mIgG or SU-rIgG fusion protein, and Kd is the dissociation constant, defined as the concentration of the receptor-mIgG or SU-rIgG fusion protein at half-maximal binding.
, where y is the mean fluorescence, M is the maximum fluorescence, r is the rate, x is the concentration of the receptor-mIgG or SU-rIgG fusion protein, and Kd is the dissociation constant, defined as the concentration of the receptor-mIgG or SU-rIgG fusion protein at half-maximal binding.
In vivo sarcoma induction and monitoring.
Ten-day-old chickens from lines M, CC.R1, and L15 were inoculated with 102 or 103 FFU of transforming virus rescued from 16Q cells in 0.1 ml of Iscove's Dulbecco's modified Eagle's medium subcutaneously into the pectoral muscle. The growth of sarcomas at the site of inoculation was monitored by calculating the area of prominent tumor. Transparent foil was placed on the tumor, the contours of the tumor were traced, and the area of the tumor was calculated in mm2 (21). Birds bearing vast nonregressing tumors were sacrificed before they reached the terminal stage.
RESULTS
The tvb allele from the inbred chicken line M (tvbr2) encodes a mutant TvbS1 receptor with the C125S substitution in CRD3.
cDNAs were synthesized from mRNAs isolated from inbred chicken line M and line WA CEFs and used to PCR amplify tvb sequences and to clone the tvb cDNAs. We determined the nucleotide and deduced amino acid sequences of the coding regions of tvb receptor cDNA clones and compared the nucleotide sequences with the sequences of tvbs1 and tvbs3 genes (3, 7). The tvbs1 allele of line WA encodes the same single-nucleotide mutation in codon 58 (CAG to UAG) that results in a premature termination codon described previously for the chicken line 72 tvbr allele (16). However, the tvb gene in line M contains a different single-nucleotide mutation that changes the cysteine at position 125 to serine (UGC to UCC) in the TvbS1 receptor protein (Fig. 1A) and is designated tvbr2. This single-nucleotide change creates an additional MlyI recognition site in tvbr2 compared to tvbs1 (Fig. 1B). The presence of this polymorphic marker was further demonstrated by digestion of a 1,259-bp fragment of tvb cDNA amplified from line M RNA. The diagnostic 920-bp MlyI fragment was obtained using the control tvbs1 cDNA from line BL; a shortened 820-bp fragment was obtained after MlyI cleavage of tvbr2 cDNA (Fig. 1C).
The C125S substitution in the TvbS1 receptor reduces the susceptibility of line M cells to subgroup B, D, and E ASLV infection.
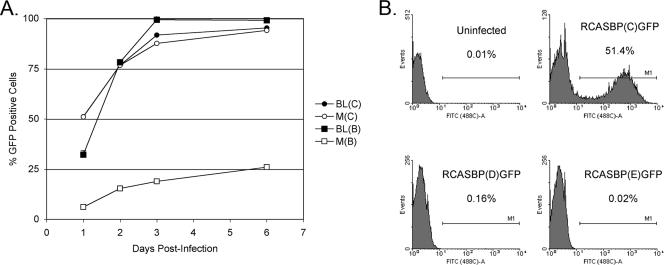
To determine the effect of the C125S substitution in the chicken TvbS1 receptor on ASLV susceptibility, line M and BL CEFs were infected at a multiplicity of infection (MOI) of 0.1 with RCASBPGFP reporter virus, a replication-competent ASLV vector encoding GFP, and the time course of infection was followed by quantitating the percentage of green fluorescent cells by flow cytometry. Line BL CEFs are susceptible to subgroup A, B, C, and D ASLVs and were used as a positive control. As expected, both line M and line BL CEFs were efficiently infected by RCASBP(C)GFP, as shown in Fig. 2A, with almost one-half of the cells infected at 1 day postinfection and virtually all cells infected by day 3. A very different result was obtained when the CEFs were infected with RCASBP(B)-GFP. As expected, line BL CEFs were efficiently infected with RCASBP(B)GFP at a rate similar to that of RCASBP(C)GFP infection (Fig. 2A). However, RCASBP(B)-GFP infected line M CEFs much less efficiently, with only 6.6% of the cells infected at day 1 (5- to 10-fold lower than line BL), and virus spread through the cells very slowly, with only ∼25% of the cell population infected by day 6. In a separate experiment, line M CEFs were infected with RCASBP(C)GFP, RCASBP(D)GFP, or RCASBP(E)GFP at 0.1 MOI, and the infected cells were quantitated the next day (Fig. 2B). As in the first experiment, ∼50% of the line M CEFs were infected with the control RCASBP(C)GFP virus, but only 0.16% of the line M CEFs were infected with RCASBP(D)GFP and virtually no line M CEFs were infected with RCASBP(E)GFP. These data clearly demonstrate, at least in cultured cells, that the C125S substitution in the TvbS1 receptor resulted in lower susceptibility of line M CEFs to ASLV(B) infection, including a significant decrease in the rate of virus spread, perhaps an even lower level of susceptibility to ASLV(D) infection and virus spread, and almost complete resistance to infection by ASLV(E).
FIG. 2.
The time course of infection of BL and line M (M) CEFs with ASLVs. CEFs were infected with replication-competent ASLVs encoding the GFP reporter proteins RCASBP(B)GFP, RCASBP(C)GFP, RCASBP(D)GFP, and RCASBP(E)GFP. (A) The proportion of GFP-positive cells was determined by FACS on the indicated day postinfection; the percentage of GFP-positive cells is indicated as a mean of three parallel dishes. (B) Line M CEFs were infected at an MOI of 0.1, and the percentage of GFP-positive cells was quantitated by FACS 1 day postinfection. In the histograms, the relative GFP fluorescence is plotted against the cell count and the percentage of GFP-positive cells is indicated as a mean of two parallel dishes.
The C125S substitution in TvbS1 lowers the binding affinity for the ASLV envelope glycoproteins.
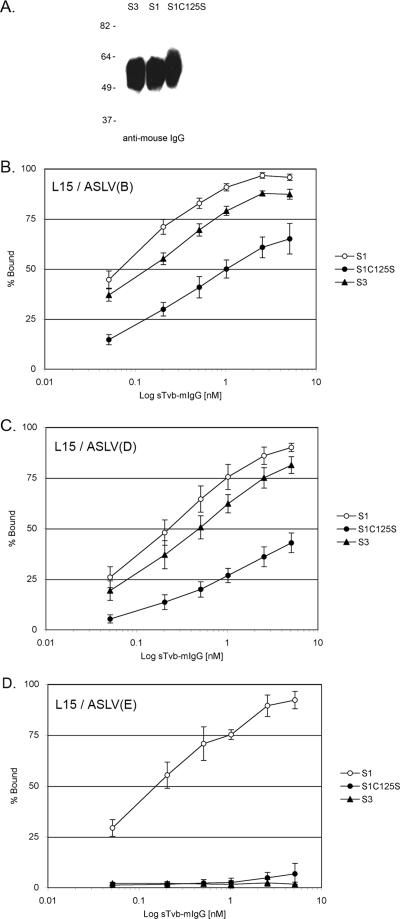
Soluble forms of the Tvb receptors fused to an mIgG domain (Fig. 1A) were used to estimate by FACS the binding affinities of the TvbS1, TvbS1C125S, and TvbS3 receptors for ASLV envelope glycoproteins expressed on the surfaces of infected line L15 CEFs, as described previously (11). The integrity of the soluble Tvb (sTvb)-mIgG proteins was verified by immunoprecipitation and Western immunoblot analysis (Fig. 3A). The concentration of each protein stock was determined by an enzyme-linked immunosorbent assay for mIgG as described previously (10). The sTvbS1-mIgG protein bound the ASLV(B)-infected cells (Fig. 3B), the ASLV(D)-infected cells (Fig. 3C), and the ASLV(E)-infected cells (Fig. 3D) with subnanomolar affinities (Table 1). As expected, the sTvbS3-mIgG protein bound subgroup B and D glycoproteins with affinities similar to that of sTvbS1-mIgG but did not bind subgroup E glycoproteins at a detectable level. Binding of the sTvbS1C125S-mIgG protein to all three ASLV glycoproteins could be detected, but with significantly lower affinities than for sTvbS1-mIgG: ASLV(B) at 10- to 25-fold lower affinity, ASLV(D) at 25- to 50-fold lower affinity, and ASLV(E) at barely detectable levels. The reduction in the binding affinity of sTvbS1C125S-mIgG correlates with the reduced infectivity of subgroups B, D, and E ASLVs in line M CEFs (Fig. 2).
FIG. 3.
Binding affinities of ASLV envelope glycoproteins for Tvb receptors. (A) Western immunoblot analysis of the soluble forms of the Tvb proteins, sTvbS3-mIgG (S3), sTvbS1-mIgG (S1), and sTvbS1C125S (S1C125S), immunoprecipitated with anti-mIgG agarose beads, denatured and separated by sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis, and transferred to nitrocellulose. The mIgG-tagged proteins were probed with anti-mIgG-conjugated to horseradish peroxidase and visualized by chemiluminescence. Molecular masses (in kilodaltons) are given on the left. (B to D) Line L15 CEFs chronically infected with ASLV(B) (B), ASLV(D) (C), or ASLV(E) (D) were fixed with paraformaldehyde and incubated with different amounts of soluble receptor. The viral glycoprotein-soluble-receptor complexes were bound to goat-anti-mIgG linked to phycoerythrin. The amount of phycoerythrin bound to the cells was quantitated by FACS, and the maximum fluorescence was estimated (see Materials and Methods). The data were plotted as percent maximum fluorescence. The values shown are the averages and standard deviations from four experiments.
TABLE 1.
Estimated binding affinities of soluble forms of the Tvb receptors for ASLV envelope glycoproteins expressed on the surfaces of L15 CEFs
| Cells | Apparent KD (nM)a
|
||
|---|---|---|---|
| sTvbS1-mIgG | sTvbS1C125S-mIgG | sTvbS3-mIgG | |
| L15/ASLV(B) | 0.07 ± 0.01 | 1.27 ± 0.65 | 0.15 ± 0.03 |
| L15/ASLV(D) | 0.23 ± 0.08 | 8.77 ± 3.17 | 0.50 ± 0.14 |
| L15/ASLV(E) | 0.17 ± 0.05 | ≫1,000b | NDBc |
Apparent KD values were estimated by fitting data via nonlinear least squares to log logistic growth curve function as described in Materials and Methods. Each result is the average and standard deviation from three experiments.
Binding of the sTvbS1C125S-mIgG receptor was detectable but not sufficient to calculate a reliable apparent KD (Fig. 3).
NDB, no detectable binding.
Compared to chickens with a wild-type TvbS1 receptor, the incidence and growth of sarcomas induced by subgroups B, D, and E ASVs were significantly altered in line M chickens.
In order to see the in vivo effects of the C125S substitution in the TvbS1 receptor, line M chickens were infected with transforming viruses and the formation and growth rates of the induced sarcomas were measured. ASLVs containing the src oncogene (avian sarcoma virus [ASV]) were produced by the rescue of env-defective Bryan high-titer-RSV from 16Q cells (19) by infection with the appropriate envelope subgroup RCASBPGFP virus. The titer of the rescued transforming virus stocks was quantitated by in vitro focus assay. Ten-day-old line M chicks and age-matched controls of line CC.R1 and line L15 were challenged with 1,000 FFU or 100 FFU of the ASV stock injected into the pectoral muscle. Line CC.R1 is susceptible to subgroup B, C, and D ASLV infection. Line L15 is susceptible to subgroup A, B, D, and E ASLVs—a rare inbred chicken line susceptible to subgroup E viruses (8). The incidence and growth rates of sarcomas induced at the site of virus inoculation were quantitated.
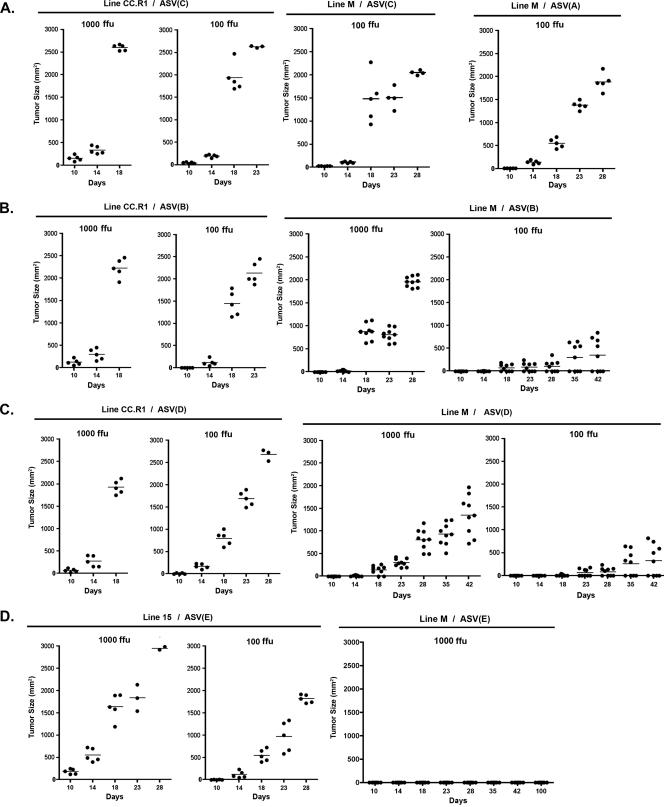
As expected, line CC.R1 was susceptible to sarcoma formation induced by ASV(C), ASV(B), and ASV(D) infection while line M was susceptible to sarcoma formation induced by ASV(A) and ASV(C) infection. In these cases, the tumor incidence was 100% in both lines, with similar tumor growth rates, depending on the initial dose of ASV (Fig. 4A, B, and C). Animals that received the 1,000-FFU dose were terminated due to tumor burden by day 18 and those that received the 100-FFU dose by days 23 to 28. Very different tumor incidence and progression were observed with ASV(B) and ASV(D) infections of line M chicks. The 1,000-FFU ASV(B) dose induced sarcomas in 100% of the infected line M animals, but the tumors grew at significantly lower rates than in line CC.R1, with all animals requiring termination by day 28 (Fig. 4B). The 100-FFU ASV(B) dose induced sarcomas in only 45% of the infected line M animals (four of nine chicks), and the tumors that did form grew very slowly. A similar pattern was observed with ASV(D) infection of line M. The 1,000-FFU ASV(D) dose induced tumors in 100% of the infected animals, but the tumors grew at an even lower rate (Fig. 4C) than ASV(B)-induced tumors (Fig. 4B). The 100-FFU ASV(D) dose induced sarcomas in only 45% of the infected animals (four of nine chicks), and the tumors that did form grew even more slowly. Finally, ASV(E) induced sarcomas in 100% of the infected line L15 animals, and the tumor growth rate was dependent on the virus dose (Fig. 4D). However, even at the 1,000-FFU ASV(E) dose, no infected line M animals (zero of eight chicks) produced detectable tumors even after 100 days postinfection. Assuming that the continual cell-to-cell spread of a transforming virus by reinfection is the major factor facilitating the progression of ASV-induced sarcomas (22), we conclude that the C125S substitution in the TvbS1 receptor is responsible for the reduced susceptibility of line M to ASLV(B) and ASLV(D) infection and the complete reisistance to ASLV(E) infection in vivo.
FIG. 4.
The growth of sarcomas induced after infection with 1,000 FFU or 100 FFU of ASV in line CC.R1, line L15, or line M 10-day-old chicks. The sizes of tumors induced in chicks infected with ASV(A) or ASV(C) (A), ASV(B) (B), ASV(D) (C), or ASV(E) (D) were measured on the days indicated. The tumor size in each bird is indicated by an individual dot, with the average tumor size for the group shown with a horizontal bar.
DISCUSSION
In this study, we describe the identification of the molecular defect in the tvb gene of inbred line M chickens, the C125S substitution in the TvbS1 receptor, that significantly decreases the sensitivity of these birds to infection by ASLV(B) and ASLV(D) and completely abrogates their sensitivity to ASLV(E) infection. This single-amino-acid substitution in the TvbR2 receptor in the inbred line M chickens significantly reduces the binding affinity of the TvbS1 receptor for the ASLV glycoproteins and explains the decreased susceptibility to the ASLV(B) and ASLV(D) infection and resistance to ASLV(E) infection. This is the first reported example of a mutation in the ASLV receptors that results in a quantitative effect on ASLV susceptibility and pathogenesis. Various levels of resistance to human immunodeficiency virus type 1 infection have been reported that are explained by polymorphisms in the CD4 receptor and/or the viral coreceptors that result in a significant reduction in the efficiency of the human immunodeficiency virus type 1 glycoprotein interaction with the mutant receptor (23). Therefore, one mechanism whereby this initial interaction between the receptor and virus required for entry can cause selective pressure on the virus population is the interaction of the viral glycoproteins with variant receptors, resulting in quantitative differences in host sensitivity. The altered susceptibility of line M for infection by ASLV(B), ASLV(D), and ASLV(E) was observed both in cultured CEFs and in chicks challenged with ASV.
Originally, the major determinants of the interaction of the ASLV(B), ASLV(D), and ASLV(E) glycoproteins with the Tvb receptors were thought to be independent of CRD3. Indeed, mutant receptors with CRD3 completely deleted were constructed in previous studies and appeared to bind the viral glycoproteins and to function as receptors for these three ASLV subgroups as efficiently as wild-type Tvb (Fig. 5). A 15-amino-acid domain in Tvb CRD1, residues 32 to 46, has been reported to contain the interaction determinants for subgroup B and D ASLVs: viral entry can be mediated using only this domain as a synthesized peptide (18). Residues L36, Q37, L41, and Y42 in this domain are critical for ASLV(B) and ASLV(D) binding and entry (18). The disulfide bond pattern of CRD1 did not appear to be important for Tvb to function as a receptor for ASLV(B) and ASLV(D) in these studies. From the sequence alignment of the Tvb receptor with several TNFR family members, and based on the X-ray structure of the DR5 TRAIL receptor, the Tvb32-46 domain is predicted to be in the nonstructured N terminus of the CRD1 domain, perhaps explaining the retention of receptor function without a particular pattern of disulfide bonds in CRD1.
FIG. 5.
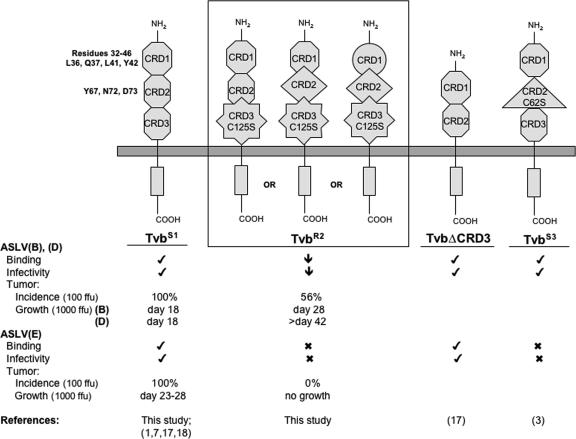
Schematic representations of membrane-bound forms of the known and functional chicken Tvb proteins. Hypothetical models are shown for TvbS1, TvbR2 (A, B, and C), the published TvbS1 with CRD3 deleted (TvbΔCDR3), and TvbS3. TvbS1 depicts the wild-type structure of the CRDs (hexagons). The different shapes of the CRDs denote altered structures induced by either the C125S substitution in TvbR2 or the C62S substitution in TvbS3. The abilities of the proteins to function as ASLV(B), ASLV(D), and ASLV(E) receptors are indicated. Check mark, wild-type activity; ↓, significantly reduced activity; X, little or no detectable activity. Tumor incidence (the percentages of chickens infected with 100 FFU of ASVs that formed tumors) and growth (the day when all chickens were sacrificed due to tumor burden from infection with 1,000 FFU ASVs) were summarized from Fig. 4.
In contrast to ASLV(B) and ASLV(D), both CRD1 and CRD2 of Tvb are required for efficient ASLV(E) binding and entry (17). The structural integrity of the protein, including the disulfide bonds and residues Y67, N72, and D73 in CRD2, are critical for proper receptor function. Modeling Tvb on the X-ray structure of DR5 TRAIL receptor predicts that these critical residues reside in a highly structured region of the protein. The TvbS3 protein contains the C62S substitution, which presumably alters the structure of the critical CRD2, eliminating binding of the ASLV(E) glycoproteins and virus entry but having no detectable effect on ASLV(B) and ASLV(D) binding and entry (Fig. 5). It has also been suggested that the TvbS1 receptor protein may exist in at least two forms that result from alternate patterns of protein folding and disulfide bonds: type 1 confers susceptibility to subgroup B, D, and E ASLVs; type 2 confers susceptibility to only subgroup B and D ASLVs (1).
The C125S substitution likely alters the folding and final structure of at least CRD3 in the TvbR2 protein, since the C125 residue in Tvb is predicted to exist as a disulfide bond with C143 in CRD3 (Fig. 5, TvbR2 A). Published studies have demonstrated that Tvb receptors with the CRD3 domain deleted (TvbΔCRD3) appear to function as efficiently as a wild-type TvbS1 receptor (17). Therefore, a simple model requiring the interaction of the viral glycoproteins with two domains for efficient virus binding and entry, ASLV(B) and ASLV(D) with the Tvb32-46 domain and CRD3 and ASLV(E) with CRD2 and CRD3, does not seem likely. The Tvb32-46 domain expressed in a heterologous protein can also act as an ASLV(B) and ASLV(D) receptor (18). Invariably, experiments that study the functions of different domains and/or mutations in receptor function express the proteins at very high levels compared to the levels found naturally in chicken cells. In an earlier study, we reported that the tvar allele found in line C chickens contains a cysteine-to-tryptophan substitution that significantly reduces the binding affinity of ASLV(A) glycoproteins for the Tva receptor (10). However, the ectopic expression of the TvaR receptor at high levels in resistant cells conferred susceptibility to ASLV(A) infection at levels similar to wild-type Tva. The true resistant phenotype of TvaR became apparent experimentally only when very low levels of the receptors were expressed: wild-type Tva still conferred a high level of susceptibility, while the TvaR protein was >1,000-fold less efficient as an ASLV(A) receptor. It may be that when the Tvb proteins are expressed at wild-type levels, mutant proteins with only the Tvb32-46 domain or just CRD1 and CRD2 may still function but at a lower efficiency than receptors that also contain CRD3. The Tvb receptors expressed at levels found on chicken cells may require the interaction of the ASLV glycoproteins with the known domains and CRD3 on the receptor for optimal function.
Alternatively, since the Tvb proteins may naturally exist in several forms, the C125S mutation may result in structural alterations in other regions of the protein. Given the existence of two types of TvbS1 receptors with altered disulfide bonds and the TvbS3 receptor, both with putative alterations in the CRD2 domain that eliminate ASLV(E) binding and entry, it seems obvious to propose that the C125S substitution may also cause alterations in the CRD2 domain structure. Another possible model of the TvbR2 protein to explain the altered ASLV susceptibility phenotype would have an altered CRD3 structure interfering with and/or altering the structure of CRD2, thereby blocking the use of the protein as an ASLV(E) receptor. However, in contrast to the phenotype of the TvbS3 receptor, the binding and entry of ASLV(B) and ASLV(D) are also affected in the TvbR2 receptor. Therefore, if C125S alters both CRD3 and CRD2, the structure of CRD2 in TvbR2 would likely be different than TvbS3 and might inhibit the interaction of ASLV(B) and ASLV(D) with CRD1 (Fig. 5, TvbR2 B). Finally, the C125S substitution could result in an alteration in the structures of all three CRDs (Fig. 5, TvbR2 C), eliminating the use of the protein as an ASLV(E) receptor and significantly reducing, but not eliminating, the binding and entry of ASLV(B) and ASLV(D). Other combinations of these and other models could account for the phenotype of the TvbR2 receptor, including the altered structure of CRD3 resulting from the C125S substitution altering the presentation of intact CRD1 and CRD2 on the membrane so that the virus is sterically blocked from effective interactions.
The normal functions of the Tvb proteins in chickens are unknown and can be estimated only from their similarity to the TNFR family of proteins. Until we identify these functions, the effect of the C125S substitution on the normal activity of this protein cannot be evaluated. The ASLV glycoproteins may interact with regions of the receptor that do not compete with the interaction of normal ligand(s) of the protein, providing possible scenarios in which the protein can still act as a receptor but loses normal function, and vice versa. Subgroup E ASLVs are endogenous to the germ lines of almost all chickens. To control the spread and accumulation of endogenous ASLV proviruses and possible pathogenesis, polymorphisms in the ASLV receptors that altered the binding of the ASLV glycoproteins while retaining normal function might have offered a positive selective advantage in the chicken population. Selection of mutant Tvb proteins that have lost the ability to function as an ASLV(E) receptor while possibly retaining the “normal” Tvb function may have provided the greatest selective advantage, resulting in fixing of the tvbs3 and tvbr2 loci in the germ lines of certain lines of inbred chickens.
Acknowledgments
We thank Vìěra Hoserová, Helena Burešová, and Lenka Mikušová for technical assistance with molecular cloning and cell cultures.
This work was supported by the Grant Agency of the Czech Republic (grants no. 523/07/1171 to J.H. and 523/07/1282 to J.G.), by the Academy of Sciences of the Czech Republic (grant no. AV0Z50529514), and by National Institutes of Health grant AI48682 (M.J.F.).
Footnotes
Published ahead of print on 19 December 2007.
REFERENCES
- 1.Adkins, H. B., S. C. Blacklow, and J. A. T. Young. 2001. Two functionally distinct forms of a retroviral receptor explain the nonreciprical receptor interference among subgroups B, D, and E avian leukosis viruses. J. Virol. 753520-3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adkins, H. B., J. Brojatsch, J. Naughton, M. M. Rolls, J. M. Pesola, and J. A. T. Young. 1997. Identification of a cellular receptor for subgroup E avian leukosis virus. Proc. Natl. Acad. Sci. USA 9411617-11622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adkins, H. B., J. Brojatsch, and J. A. T. Young. 2000. Identification and characterization of a shared TNFR-related receptor for subgroup B, D and E avian leukosis viruses reveal cysteine residues required specifically for subgroup E viral entry. J. Virol. 743572-3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnard, R. J., D. Elleder, and J. A. T. Young. 2006. Avian sarcoma and leukosis virus-receptor interactions: from classical genetics to novel insights into virus-cell membrane fusion. Virology 34425-29. [DOI] [PubMed] [Google Scholar]
- 5.Barnard, R. J., and J. A. T. Young. 2003. Alpharetrovirus envelope-receptor interactions. Curr. Top. Microbiol. Immunol. 281107-136. [DOI] [PubMed] [Google Scholar]
- 6.Bates, P., J. A. T. Young, and H. E. Varmus. 1993. A receptor for subgroup A Rous sarcoma virus is related to the low density lipoprotein receptor. Cell 741043-1051. [DOI] [PubMed] [Google Scholar]
- 7.Brojatsch, J., J. Naughton, M. M. Rolls, K. Zingler, and J. A. T. Young. 1996. CAR1, a TNFR-related protein, is a cellular receptor for cytopathic avian leukosis-sarcoma viruses and mediates apoptosis. Cell 87845-855. [DOI] [PubMed] [Google Scholar]
- 8.Crittenden, L. B., S. McMahon, M. S. Halpern, and A. M. Fadley. 1987. Embryonic infection with the endogenous avian leukosis virus Rous-associated virus-0 alters resposes to exogenous avian leukosis virus infection. J. Virol. 61722-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Earp, L. J., S. E. Delos, H. E. Park, and J. M. White. 2005. The many mechanisms of viral membrane fusion proteins. Curr. Top. Microbiol. Immunol. 28525-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elleder, D., D. C. Melder, K. Trejbalová, J. Svoboda, and M. J. Federspiel. 2004. Two different molecular defects in the Tva receptor gene explain the resistance of two tvar lines of chickens to infection by subgroup A avian sarcoma and leukosis viruses. J. Virol. 7813489-13500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elleder, D., V. Stepanets, D. C. Melder, F. Šenigl, J. Geryk, P. Pajer, J. Plachý, J. Hejnar, J. Svoboda, and M. J. Federspiel. 2005. The receptor for the subgroup C avian sarcoma and leukosis viruses, Tvc, is related to mammalian butrophilins, members of the immunoglobulin superfamily. J. Virol. 7910408-10419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Federspiel, M. J., and S. H. Hughes. 1997. Retroviral gene delivery. Methods Cell Biol. 52179-214. [PubMed] [Google Scholar]
- 12a.Himly, M., D. N. Foster, I. Bottoli, J. S. Iacovoni, and P. K. Vogt. 1998. The DF-1 chicken fibroblast cell line: transformation induced by diverse oncogenes and cell death resulting from infection by avian leukosis viruses. Virology 248295-304. [DOI] [PubMed] [Google Scholar]
- 13.Hughes, S. H. 2004. The RCAS vector system. Folia Biol. 50107-119. [PubMed] [Google Scholar]
- 14.Hunter, E. 1997. Viral entry and receptors., p. 71-120. In J. M. Coffin, S. H. Hughes, and H. E. Varmus (ed.), Retroviruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [PubMed]
- 15.Hymowitz, S. G., H. W. Christinger, G. Fuh, M. Ultsch, M. O'Connell, R. F. Kelley, A. Ashkenazi, and A. de Vos. 1999. Triggering cell death: the crystal structure of Apo2L/TRAIL in a complex with death receptor 5. Mol. Cell 4563-571. [DOI] [PubMed] [Google Scholar]
- 16.Klucking, S., H. B. Adkins, and J. A. T. Young. 2002. Resistance of infection by subgroups B, D and E avian sarcoma and leukosis viruses is explained by a premature stop codon within a resistance allele of the tvb receptor gene. J. Virol. 767918-7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klucking, S., and J. A. T. Young. 2004. Amino acid residues Tyr-67, Asn-72, and Asp-73 of the Tvb receptor are important for subgroup E avian sarcoma and leukosis virus interaction. Virology 318371-380. [DOI] [PubMed] [Google Scholar]
- 18.Knauss, D. J., and J. A. T. Young. 2002. A Fifteen-amino-acid Tvb peptide serves as a minimal soluble receptor for subgroup B avian leukosis and sarcoma viruses. J. Virol. 765404-5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy, H. M. 1977. A new, replication-defective variant of the Bryan high-titer strain Rous sarcoma virus. Virology 77705-721. [DOI] [PubMed] [Google Scholar]
- 20.Plachý, J. 2000. The chicken—a laboratory animal of the class Aves. Folia Biol. 4617-24. [PubMed] [Google Scholar]
- 21.Plachý, J., J. Hejnar, K. Trtková, K. Trejbalová, J. Svoboda, and K. Hála. 2001. DNA vaccination against v-src oncogene-induced tumors in congenic chickens. Vaccine 194526-4535. [DOI] [PubMed] [Google Scholar]
- 22.Ponten, J. 1964. The in vivo growth mechanism of avian Rous sarcoma. Natl. Cancer Inst. Monogr. 17131-145. [Google Scholar]
- 23.Reiche, E. M., A. M. Bonametti, J. C. Voltarelli, H. K. Morimoto, and M. A. Watanabe. 2007. Genetic polymorphisms in the chemokine and chemokine receptors: impact on clinical course and therapy of the human immunodeficiency virus type 1 infection. Curr. Med. Chem. 141325-1334. [DOI] [PubMed] [Google Scholar]
- 24.Weiss, R. A. 1992. Cellular receptors and viral glycoproteins involved in retrovirus entry, p. 1-108. In J. A. Levy (ed.), The retroviruses, vol. 2. Plenum Press, New York, NY. [Google Scholar]
- 25.Young, J. A. T., P. Bates, and H. E. Varmus. 1993. Isolation of a chicken gene that confers susceptibility to infection by subgroup A avian leukosis and sarcoma viruses. J. Virol. 671811-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]