Abstract
The genome of the influenza A virus is composed of eight different segments of negative-sense RNA. These eight segments are incorporated into budding virions in an equimolar ratio through a mechanism that is not fully understood. Two different models have been proposed for packaging the viral ribonucleoproteins into newly assembling virus particles: the random-incorporation model and the selective-incorporation model. In the last few years, increasing evidence from many different laboratories that supports the selective-incorporation model has been accumulated. In particular, different groups have shown that some large viral RNA regions within the coding sequences at both the 5′ and 3′ ends of almost every segment are sufficient for packaging foreign RNA sequences. If the packaging regions are crucial for the viability of the virus, we would expect them to be conserved. Using large-scale analysis of influenza A virus sequences, we developed a method of identifying conserved RNA regions whose conservation cannot be explained by population structure or amino acid conservation. Interestingly, the conserved sequences are located within the regions identified as important for efficient packaging. By utilizing influenza virus reverse genetics, we have rescued mutant viruses containing synonymous mutations within these highly conserved regions. Packaging of viral RNAs in these viruses was analyzed by reverse transcription using a universal primer and quantitative PCR for individual segments. Employing this approach, we have identified regions in the polymerase gene segments that, if mutated, result in reductions of more than 90% in the packaging of that particular polymerase viral RNA. Reductions in the level of packaging of a polymerase viral RNA frequently resulted in reductions of other viral RNAs as well, and the results form a pattern of hierarchy of segment interactions. This work provides further evidence for a selective packaging mechanism for influenza A viruses, demonstrating that these highly conserved regions are important for efficient packaging.
The influenza A virus genome consists of eight negative-sense RNA segments (19, 24), encoding up to 11 viral proteins (2, 20). During the infection of target cells, influenza viral RNA (vRNA) replication and transcription occurs in the nucleus. In order to be packaged into progeny virions, the vRNA is transported from the nucleus as a ribonucleoprotein complex composed of the three influenza virus polymerase proteins, the nucleoprotein (NP), and the vRNA, in association with the influenza virus matrix 1 (M1) protein and nuclear export protein (3). The process by which the vRNA is packaged is not well understood. However, it is known that vRNA is specifically packaged in preference to other cellular RNAs and that the different vRNAs are present in an equimolar ratio within a population of virus particles (18). For a virus particle to be fully infectious, it must contain a full complement of the eight vRNA segments.
Two simple models have been hypothesized for the packaging of influenza vRNA: random incorporation and selective incorporation (20). The random-incorporation model assumes that a common structural feature which enables them to be randomly incorporated into budding virions is present on all vRNAs. Assuming equal concentrations of vRNA segments in the cytoplasm, the probability that eight different segments are packed in one virion is very small (P value = 8!/88 = 0.0024), indicating that, if this is the case, only a few virus particles will be viable. If instead of eight segments we consider that more than eight are incorporated, the probability that eight of them are different goes to more-reasonable numbers (1, 7). If 12 vRNAs were packaged randomly, the mathematical models suggest that infectivity increases to approximately 10%, which is comparable with the percentage for the experimental data (4). As only 1 to 2% of the weight of the influenza virus particle is vRNA, it is difficult to accurately quantify the exact number of vRNA segments packaged.
The selective-incorporation model suggests that each vRNA segment contains a unique “packaging signal” allowing it to act independently and be selectively packaged. The use of green fluorescent protein (GFP) packaging construct incorporation signals has been described for all eight segments (9, 10, 12, 15, 17, 25). However, it is still not understood how these signals direct the packaging of vRNA into budding virions. Liang et al. (12) demonstrated that both PB1 and PA vRNA needed at least 40 5′ and 66 3′ coding nucleotides in order to package efficiently and that PB2 vRNA required only 80 5′ coding nucleotides, with packaging being independent of coding nucleotides at the 3′ end of the vRNA. It was also demonstrated that the packaging signals from individual vRNA segments work in conjunction. Pairing of a 3′ signal from one segment and a 5′ signal from a different segment did not direct the packaging of a GFP-based vRNA into budding virions. The result for the PB2 vRNA is consistent with previously reported data for PB2 defective interfering vRNA (6). Dos Santos Afonso et al. (5) also demonstrated the importance of the 5′ end of the PB2 vRNA and the necessity of it being adjacent to the 5′ untranslated region. This was demonstrated by the insertion of a flag epitope tag fused to the PB2 open reading frame. Placement of the tag at the 5′ end of the vRNA required duplication of the last 109 coding nucleotides of PB2, whereas the tag sequence could be inserted before the start codon at the 3′ end of the vRNA with no effect on vRNA packaging.
Using an approach similar to that of Liang et al. (12) with GFP packaging constructs, Muramoto et al. (15) also analyzed the requirements of the 3′ and 5′ coding regions as packaging signals for the polymerase vRNAs. Although their results differ slightly from those of the previous study, this was explained by the different approaches taken in the two studies, with the study by Liang et al. competing the packaging of GFP constructs with the packaging of the parental vRNA. In contrast to that of Liang et al. (12), the approach of Muramoto et al. (15) required 120 nucleotides at the 3′ end of the PB2 vRNA for efficient packaging, which was inconsistent with previous data suggesting that this end is not important for packaging. The Muramoto et al. study also suggested a hierarchy of vRNA incorporation, with data showing that the loss of incorporation of one segment leads to reduced incorporation of other segments. This effect was most prominent when the PB2 vRNA was omitted (15). This hierarchy and the intersegment interactions are supported by thin-section electron micrographs showing viral ribonucleoproteins organized in a distinct pattern within virions (16).
To summarize, increasing evidence from many different laboratories that supports the selective-incorporation model has been accumulated. First, there are vRNA regions within the coding sequence at both the 5′ and 3′ ends of almost every segment that are sufficient for packaging foreign RNA sequences. Second, loss of incorporation of one segment alters the incorporation of the others, and finally, electron micrographs have shown that there is a clear segmental pattern within a virion.
If the packaging regions are crucial for the viability of the virus, we expect them to be conserved. Factorizing out population structure and amino acid conservation, we have been able to find some conserved regions at the ends of each of the different segments. Possible explanations for the presence of these conserved regions in the vRNA have to do with RNA structure, RNA-RNA interactions, and RNA-protein interactions. We are able to show that these regions of highly conserved sequence are important for packaging of vRNA. Mutations in key regions of these highly conserved sequences resulted in 5- to 20-fold reductions in the levels of packaging of the mutated vRNAs compared with the level of wild-type (wt) vRNA packaging. We are also able to confirm the results of Muramoto et al. (15) demonstrating that the reduction in packaging of one vRNA results in reductions in other vRNAs.
While this study was being completed, Gog et al. (11) reported a result for the PB2 segment similar to ours. In that study, synonymous changes were introduced into a GFP packaging construct and the effect on packaging of this construct was analyzed. Changes in individual conserved residues resulted in a 20- to 50-fold reduction in packaging. In contrast to the experimental results reported herein, the packaging of the GFP construct was in competition with that of the parental PB2 vRNA, which was provided by infection with wt virus.
MATERIALS AND METHODS
Cell lines.
293T and MDCK cells were obtained from the American Type Culture Collection (Manassas, VA) and were maintained in Dulbecco's modified Eagle's medium and minimal essential medium, respectively (Gibco, Carlsbad, CA), supplemented with 10% fetal calf serum (HyClone, Logan, UT) and penicillin-streptomycin (Gibco).
Analysis of influenza virus sequences.
To analyze the genomes of influenza A viruses for conserved regions within the polymerase vRNA, approximately 600 avian sequences for each segment were aligned (667, 591, and 621 sequences for segments 1, 2 and 3, respectively). Sequences were obtained from the Influenza Virus Resource, National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/genomes/FLU/). Avian sequences were chosen because there is more sequence variation within the published sequences and to avoid the bias within the human sequences in the public database. The bias arises in part from the fact that a large percentage of sequences in the database have been isolated in New York and New Zealand within the past 10 years and from the fact that the human viruses present a more severe bottleneck structure than the avian viruses.
Constructs and cloning.
The plasmids used for the rescue of recombinant influenza A/WSN/33 (WSN) virus have been described previously (8). To generate recombinant WSN viruses with mutated PB1, PB2, or PA vRNA segments, pPOLI plasmids encoding the WSN gene of interest were subjected to site-directed mutagenesis using a Stratagene QuikChange XL site-directed mutagenesis kit (Stratagene, La Jolla, CA). For the generation of recombinant A/Puerto Rico/8/34 (PR/8) viruses with mutated PA vRNA segments, we utilized a Stratagene QuikChange XL site-directed mutagenesis kit (Stratagene) for mutagenesis of a pGEM plasmid containing the PA gene of PR/8. Sequences of each mutated construct were confirmed by automated sequencing. Correctly mutated PR/8 PA genes were then subcloned into pDZ (21). All primer sequences and vector maps are available upon request.
Reverse genetics for recombinant viruses.
The reverse-genetics systems for the generation of recombinant influenza WSN (8) and PR/8 (23) viruses have been described previously. For the generation of recombinant WSN viruses, 293T and MDCK cells were cotransfected with eight pPOLI vectors expressing negative-sense vRNA and four pCAGGS protein expression vectors encoding the viral polymerase subunits and NP. After 48 h of incubation, recombinant viruses were passaged to fresh MDCK cells for amplification. For the generation of recombinant PR/8 viruses, 293T cells were transfected with eight pDZ plasmids, which utilize bidirectional transcription to both encode the viral genomic RNA and express viral protein. Cells were collected 24 h posttransfection and inoculated into the allantoic cavities of 10-day-old embryonated chicken eggs. Rescue of recombinant viruses was assessed by hemagglutination activity. Each of the newly generated viruses was further plaque purified, and mutations were confirmed by sequencing of mutated genes.
Viral growth kinetics.
The growth of mutant recombinant WSN viruses was evaluated by inoculation of MDCK cells at a multiplicity of infection (MOI) of 0.001. Cells were incubated at 37°C for 48 h, at which time the virus titer in the supernatant was determined by plaque assay of MDCK cells. Growth kinetics of PR/8 viruses was determined by inoculation of 10-day-old eggs with 100 PFU of virus. At 48 h postinoculation, the virus titer in the allantoic fluid was determined by titration of plaques on MDCK cells.
Isolation of packaged vRNAs.
Packaged vRNA for WSN recombinants was analyzed by purification of virus from the supernatants of three 150-mm dishes of MDCK cells. Cells were inoculated at an MOI of 0.001. Cells were incubated for 36 to 48 h until maximal cytopathic effect was visible. Supernatant was clarified by low-speed centrifugation and then further clarified by centrifugation at 10,000 rpm using a Beckman SW20 rotor (Beckman Coulter, Fullerton, CA). Clarified supernatant was then layered on a 30% sucrose cushion and further centrifuged at 25,000 rpm for 2.5 h. Pelleted virus was resuspended in TMK (10 mM Tris-HCl, pH 7.5, 1.5 mM MgCl2, 10 mM KCl) and vRNA extracted using TRIzol LS reagent (Invitrogen). Precipitated vRNA was resuspended in a final volume of 15 μl of 10 mM Tris-HCl (pH 8.0) and stored at −80°C. To analyze packaged vRNA for PR/8 mutant viruses, 10-day-old eggs were inoculated with approximately 1,000 PFU and incubated for 2 days. Allantoic fluid was harvested and processed as described above.
qPCR analysis of packaged vRNAs.
Extracted vRNAs (approximately 200 ng) were reverse transcribed using a universal 3′ primer (5′-AGGGCTCTTCGGCCAGCRAAAGCAGG) and Superscript II reverse transcriptase (RT) (Invitrogen). The RT product was then diluted 10,000-fold and used as a template for quantitative PCR (qPCR). Separate PCRs were then carried out as previously reported (14) with segment-specific primers utilizing a LightCyler 480 apparatus (Roche, Nutley, NJ). The 10-μl reaction mixture contained 1 μl of diluted RT product, SYBR green I (Molecular Probes), a 0.5 μM concentration of each primer, a 200 μM concentration of each of the deoxynucleoside triphosphates, 3 mM MgCl2, 2 μl of 10× PCR buffer II, and 1 U of AmpliTaq Gold enzyme (Applied Biosystems, Foster City, CA). Relative concentrations of vRNA were determined on the basis of an analysis of cycle threshold values (14), normalization for the total vRNA amount by equalization of the level of PB2 vRNA, and then calculation of the percentages of incorporation relative to the levels of wt vRNA packaging. Results are presented as the average levels of incorporation of vRNA ± standard deviations, with results derived from two independent virus purifications, with vRNA levels quantified in triplicate.
RESULTS
Identification of highly conserved regions of the influenza A virus genome.
We performed an analysis of the conservation within individual segments, using approximately 600 vRNA sequences per segment. The initial data set used for analysis contained only avian isolates due to the enormous bias in the database of sequences from human influenza viruses (most of the sequences from human viruses have been collected in the last few years in a few places in the world, in particular, New York and New Zealand) and the greater diversity within avian isolates. At the end of the analysis, we checked that the conserved regions found in this data set also extend to other sequences, e.g., human viruses.
To determine whether sequence conservation was at the RNA level, we have to factor out amino acid conservation and the population structure. Viral sequences are highly related to each other due to the evolution of the virus and the sampling bias. This correlation in the sequences could produce false positives if a few sequences are overweighed due to the population of the virus or a sampling bias.
How can we factor out amino acid conservation and population structure? The first step we followed was to consider the conservation in third-codon positions, eliminating most of the conservation due to the protein structure. If the conservation is due to population structure (null hypothesis), we would expect the conserved positions to be independent from each other, i.e., for them to be randomly distributed along the genome of the virus and not clustered together. Fixing a conservation cutoff, c (in our analysis, we used a conservation cutoff of 98%; i.e., in at least 98% of the particular nucleotides, the position is conserved), we computed the probability that we would get by chance a position that is more conserved than the cutoff value. This probability is just the number of positions that are more conserved than the cutoff value, n(c), over the total number of positions, n(0). The quotient of these two quantities [Pc = n(c)/n(0)] defines the probability of finding a particular well-conserved position, i.e., of finding a nucleotide that is more conserved than the particular cutoff value. Now, the probability that there are N conserved third-codon positions together, assuming the accuracy of the null hypothesis (these are independent events), is PcN. After correcting for multiple hypotheses (Bonferroni correction, with a P of <0.01), we found the lengths, N, of nearby third-codon positions. In particular, for 98% conservation, we hold that a string of highly conserved third-codon positions with a length of >6 cannot be explained by the stratification of the data. Table 1 summarizes the conserved sequences found within the polymerase vRNAs. The results are very robust with any change in the conservation cutoff from 95 to 99%.
TABLE 1.
Conserved sequences identified within the three polymerase subunit vRNAs for avian and human influenza A virusesa
| Segment | Positions of conserved sequence | Length (nt) |
|---|---|---|
| PB2 | 2209-2304 | 96 |
| PB1 | 2256-2279 | 24 |
| PA | 691-731 | 41 |
| PA | 742-767 | 26 |
| PA | 2094-2156 | 63 |
Numbering of nucleotides (nt) is based on the positive-sense RNA sequence.
A data set of human sequences was then analyzed to determine whether sequence conservation is retained in human isolates. All conserved avian sequences were conserved in human viruses. These conserved regions can be explained only by RNA-RNA interactions, the RNA structure, or RNA-protein interactions. Interestingly, nearly all these conserved sequences were located toward the ends of the vRNAs, within the regions identified by Liang et al. (12) and Muramoto et al. (15) as being important for the incorporation of vRNA into progeny virions.
Mutational analysis of the conserved residues of the PB2 gene and their role in genome packaging.
The highly conserved region of the PB2 gene, nucleotides 2209 to 2304 (numbering corresponds to that of the positive-sense strand), is contained within the 5′ packaging region of the PB2 gene identified by Muramoto et al. (15), which includes 120 nucleotides at the 5′ end of the PB2 gene and is slightly longer than the 80 nucleotides that Liang et al. (12) reported to be essential for packaging. In order to determine the role of this highly conserved region in packaging, sequential regions of 15 to 20 nucleotides were mutated (Fig. 1).
FIG. 1.
Mutational analysis of the highly conserved region of the WSN PB2 vRNA. (Left) Schematic representations of the regions of synonymous mutations (white boxes) introduced into the WSN PB2 vRNA. nt, nucleotide; ORF, open reading frame. (Right) Synonymous nucleotide changes introduced for each construct. The upper line is the parental WSN virus PB2 sequence, and nucleotide changes are presented in bold on the lower line. The numbering of nucleotides is based on that of the positive-sense RNA sequence.
Each of these mutated PB2 constructs were then tested in a transfection-based assay for the ability of the vRNA to be replicated by the influenza virus polymerase. This was to ensure that any loss of packaging of the vRNA was not due to lower replication and therefore a lower abundance of the vRNA in infected cells. The vRNA replication was assessed by transfection of 293T cells with the different mutant pPOLI-WSN PB2 constructs along with plasmids expressing the influenza virus polymerases and NP. Replication of the vRNA expressed from the pPOLI constructs was assessed by qPCR and normalized to the replication of the NA vRNA from pPOLI-WSN NA as a control for transfection. The mutations introduced into the PB2 vRNAs had no effect on the ability of the influenza virus polymerase complex to recognize and replicate them. All mutant vRNAs were replicated to levels similar to those of wt WSN PB2 vRNA (data not shown).
Recombinant viruses were then rescued using each of the mutated PB2 constructs. The replication of each of these recombinant viruses was assessed by infecting MDCK cells at an MOI of 0.001 and incubating them at 37°C for 48 h. Virus titers from the supernatants at 48 h are shown in Table 2, with PB2-51 growing to a level similar to that of wt WSN. Mutant viruses PB2-52, PB2-53, PB2-54, and PB2-55 all showed an approximately 2-log reduction in titer compared with wt WSN, suggesting a role for this highly conserved region in the replication cycle.
TABLE 2.
Effect on replication in MDCK cells of recombinant WSN viruses with the introduction of synonymous changes within the highly conserved region of the PB2 vRNA
| Virus | Plaque titer |
|---|---|
| WSN wt | 6.1 × 107 |
| PB2-51 | 4.3 × 107 |
| PB2-52 | 5 × 105 |
| PB2-53 | 4 × 105 |
| PB2-54 | 6.5 × 105 |
| PB2-55 | 4 × 105 |
MDCK cells were infected at 0.001 MOI and incubated for 48 h. Virus produced was titrated by plaque assay in MDCK cells. Results represent the average titers from two independent infections titrated in duplicate (n = 4).
Viruses were amplified and purified through a sucrose cushion, and the vRNA was extracted. The packaging of individual vRNAs was then analyzed by qPCR (Table 3). The levels of packaging of the PB2 vRNA in progeny viruses were significantly reduced in all mutant viruses except for PB2-51, which corresponds to the replication data for these viruses. These data suggest that the highly conserved regions play a role in the packaging of the PB2 vRNA into progeny virions, and when packaging is compromised, virus replication is also attenuated, as expected.
TABLE 3.
Effect of synonymous changes within the highly conserved region of PB2 vRNA on the packaging of individual vRNAs into progeny WSN virions
| Virus | % of vRNA packaging vs that of parental WSN virus
|
|||||||
|---|---|---|---|---|---|---|---|---|
| PB2 | PB1 | PA | HA | NP | NA | M | NS | |
| WSN | 100.0 ± 3.8 | 100.0 ± 6.1 | 100.0 ± 2.1 | 100.0 ± 2.2 | 100.0 ± 5.9 | 100.0 ± 3.2 | 100.0 ± 3.3 | 100.0 ± 1.4 |
| PB2-51 | 65.5 ± 11.5 | 95.2 ± 17.0 | 73.4 ± 13.1 | 101.2 ± 9.4 | 87.9 ± 4.5 | 100.0 ± 11.3 | 66.4 ± 6.9 | 78.5 ± 8.2 |
| PB2-52 | 18.7 ± 12.0 | 86.0 ± 4.7 | 48.2 ± 1.9 | 115.5 ± 2.1 | 46.1 ± 3.3 | 100.0 ± 2.3 | 39.6 ± 1.3 | 40.1 ± 1.1 |
| PB2-53 | 14.5 ± 0.5 | 19.4 ± 3.1 | 19.3 ± 1.5 | 56.4 ± 3.5 | 25.1 ± 1.7 | 100.0 ± 3.5 | 14.4 ± 0.5 | 13.9 ± 0.5 |
| PB2-54 | 8.9 ± 1.6 | 18.2 ± 2.1 | 14.0 ± 3.2 | 90.9 ± 13.6 | 12.1 ± 1.6 | 100.0 ± 11.3 | 10.7 ± 7.1 | 8.7 ± 5.8 |
| PB2-55 | 6.7 ± 0.5 | 14.1 ± 1.4 | 11.5 ± 1.2 | 98.0 ± 3.1 | 11.5 ± 1.4 | 100.0 ± 4.7 | 9.4 ± 0.6 | 7.6 ± 0.4 |
Values given are means ± standard deviations. Results are from two independent experiments, with the assay performed in triplicate (n = 6).
Similar to what was reported by Muramoto et al. (15), reductions in the packaging of the PB2 vRNA resulted in reduced packaging of other vRNAs. There is a strong correlation between the level of PB2 packaged and the levels of PB1, PA, NP, M, and NS vRNAs packaged, suggesting some cooperation between these vRNAs in packaging. No significant reduction was seen in the packaging of the hemagglutinin (HA) or NA vRNAs except with PB2-53, suggesting that these vRNA segments are packaged independently of the PB2 vRNA. In the case of PB2-53, the reduction in HA vRNA packaging is more modest than that of the other genes.
In an attempt to select for mutations restoring the packaging of the PB2 vRNA, PB2-52, PB2-53, PB2-54, and PB2-55 viruses were sequentially passaged at a low MOI 8 to 10 times in MDCK cells. Even after the final passage in MDCK cells, no restoration of virus replication to wt levels was achieved. Additionally, no changes in the reduced packaging of the PB2 vRNA were observed. This suggests that more than one nucleotide is important for packaging and that multiple changes are necessary in order to restore packaging to the correct balance.
Mutational analysis of the single conserved residues of the PB2 gene and their role in vRNA packaging.
While this work was being completed, Gog et al. (11) reported a similar study identifying and examining the same highly conserved sequence of the PB2 vRNA. In that study, Gog et al. created a packaging construct with a GFP gene flanked by 159 coding nucleotides of the 3′ end and 166 coding nucleotides of the 5′ end of the PB2 coding nucleotides, along with the untranslated regions. When this construct was transfected into cells, followed by infection at a high MOI with influenza A virus WSN, they were able to passage the GFP construct onto fresh MDCK cells. By making changes to single conserved amino acid codons, they were able to reduce the transduction of this GFP construct from 20- to 50-fold. To examine the effects of these nucleotide changes in the context of a complete PB2 vRNA, we engineered viruses with the same changes (Fig. 2).
FIG. 2.
Mutation of single highly conserved codons of the WSN PB2 vRNA. Synonymous nucleotide changes were introduced for each mutated codon. The upper line is the parental WSN virus PB2 sequence, and nucleotide changes are presented in bold on the lower line. Numbering of residues and changes introduced are based on the report by Gog et al. (11). mut, mutation.
Analysis of the vRNA packaged in progeny virions demonstrated that the mutation of single codons was sufficient to see a reduction in vRNA packaging of up to 10-fold (Table 4). Residues 744 (nucleotides 2218 to 2220), 745 (nucleotides 2221 to 2224), and 757 (nucleotides 2296 to 2298) were shown to be the most critical for efficient packaging of the PB2 vRNA. Although the reduction in packaging seen in this experiment (5- to 10-fold) is not a great as that seen in the study by Gog et al. (11) (20- to 50-fold), this can be explained by the fact that in the experiment by Gog et al. (11) the GFP construct was competing with a wt PB2 vRNA for packaging. Even a small reduction in the ability of the GFP construct to be packaged could result in the wt vRNA out-competing the mutant for incorporation into the virion.
TABLE 4.
Effect of single residue synonymous changes in WSN PB2 mutants on the packaging of individual vRNAs into progeny virions
| Virus | PB2 | PB1 | PA | HA | NP | NA | M | NS |
|---|---|---|---|---|---|---|---|---|
| WSN | 100.0 ± 4.0 | 100.0 ± 1.4 | 100.0 ± 2.2 | 100.0 ± 2.4 | 100.0 ± 3.6 | 100.0 ± 3.7 | 100.0 ± 1.3 | 100.0 ± 2.0 |
| PB2m731 | 102.0 ± 2.3 | 115.3 ± 8.4 | 130.9 ± 3.4 | 100.0 ± 1.5 | 88.1 ± 2.4 | 177.8 ± 4.9 | 100.7 ± 3.5 | 83.9 ± 3.8 |
| PB2m737 | 129.2 ± 2.2 | 116.8 ± 3.6 | 171.9 ± 2.7 | 100.0 ± 1.6 | 64.7 ± 2.3 | 115.5 ± 3.3 | 73.6 ± 1.0 | 99.6 ± 1.5 |
| PB2m744b | 18.9 ± 2.7 | 115.5 ± 4.5 | 41.5 ± 1.4 | 100.0 ± 4.8 | 59.4 ± 1.3 | 89.8 ± 3.6 | 110.8 ± 4.5 | 154.8 ± 5.9 |
| PB2m745 | 11.6 ± 2.2 | 16.4 ± 2.9 | 51.1 ± 4.4 | 100.0 ± 3.4 | 14.9 ± 1.2 | 57.7 ± 2.9 | 37.8 ± 0.6 | 54.7 ± 3.1 |
| PB2m748 | 85.0 ± 1.2 | 82.3 ± 4.0 | 80.4 ± 0.2 | 100.0 ± 1.4 | 63.0 ± 0.8 | 87.7 ± 1.2 | 48.0 ± 0.5 | 104.9 ± 0.9 |
| PB2m751 | 56.3 ± 0.5 | 70.6 ± 1.3 | 56.6 ± 1.3 | 100.0 ± 1.1 | 40.6 ± 0.4 | 86.6 ± 0.9 | 32.4 ± 0.5 | 61.4 ± 0.8 |
| PB2m757 | 8.2 ± 0.2 | 143.0 ± 5.2 | 81.0 ± 2.2 | 100.0 ± 1.2 | 53.8 ± 1.3 | 74.4 ± 0.9 | 90.5 ± 0.7 | 92.0 ± 2.7 |
Values given are percentages of packaging of vRNA in comparison to those of parental WSN virus ± standard deviation. Results are from two independent experiments, with assay performed in triplicate (n = 6).
Mutational analysis of the conserved residues of the PB1 gene and their role in vRNA packaging.
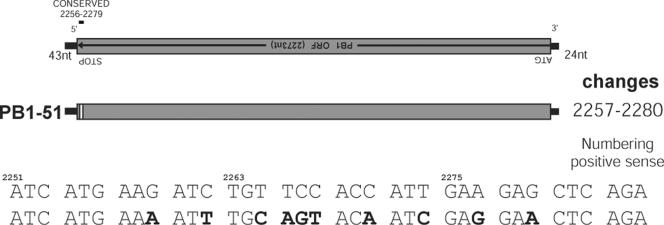
Analysis of the PB1 vRNA sequence identified a short region (nucleotides 2256 to 2279) (Fig. 3) within that reported by both Liang et al. (12) and Muramoto et al. (15) as essential for the packaging of a GFP construct. In a study similar to the one carried out previously with the PB2 gene, synonymous mutations were engineered within a plasmid (Fig. 3), and an analysis of the ability of the influenza virus polymerase to drive the replication of the encoded vRNA demonstrated no difference from what occurred with the wt sequence (data not shown). A recombinant virus was then rescued and the replication of this virus assessed in MDCK cells. At a low MOI (0.001), after 48 h, this virus showed a significant reduction (3 log units) in replication compared with the replication level of wt WSN. Analysis of the packaged vRNA showed a significant reduction in the packaging of PB1 vRNA (Table 5). Unlike that seen with the PB2 mutants, the reduction in packaging of other vRNAs was not reduced to the same extent in the absence of the PB1 vRNA. Without a further understanding of the packaging process, it is not possible to understand this difference. Clearly then, the PB1 and PB2 segments interact differently with other influenza vRNA segments.
FIG. 3.
Mutational analysis of the short highly conserved region of the WSN PB1 vRNA. (Top) Schematic representations of the region of synonymous mutations (white boxes) introduced into the WSN PB1 vRNA. nt, nucleotide; ORF, open reading frame. (Bottom) Synonymous nucleotide changes introduced. The upper line is the parental WSN virus PB1 sequence, and nucleotide changes are presented in bold on the lower line. The numbering of nucleotides is based on that of the positive-sense RNA sequence.
TABLE 5.
Effect of synonymous changes within the highly conserved region of WSN PB1 on the packaging of individual vRNAs into progeny virions
| Virus | PB2 | PB1 | PA | HA | NP | NA | M | NS |
|---|---|---|---|---|---|---|---|---|
| WSN | 100.0 ± 4.4 | 100.0 ± 6.9 | 100.0 ± 2.6 | 100.0 ± 2.3 | 100.0 ± 2.4 | 100.0 ± 3.0 | 100.0 ± 1.6 | 100.0 ± 1.7 |
| PB1-51 | 58.7 ± 1.4 | 9.5 ± 0.3 | 97.8 ± 4.9 | 131.6 ± 3.8 | 77.0 ± 2.7 | 136.3 ± 2.2 | 51.4 ± 0.8 | 100.0 ± 2.0 |
Values given are percentages of packaging of vRNA in comparison to those of parental WSN virus ± standard deviation. Results are from two independent experiments, with assay performed in triplicate (n = 6).
Mutational analysis of the conserved residues of the WSN PA gene and their role in vRNA packaging.
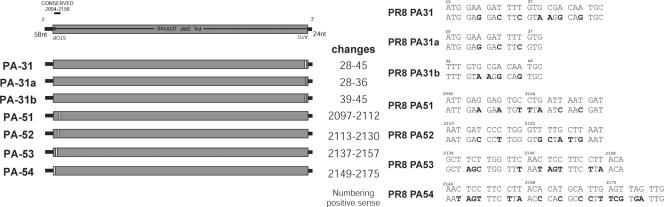
Analysis of the PA sequence for influenza A viruses identified a highly conserved region (nucleotides 2094 to 2156) that was adjacent to the 21 nucleotides essential for packaging identified by Muramoto et al. (15). However, Liang et al. (12) reported that 40 nucleotides from the 5′ end were necessary for the efficient incorporation of their GFP construct, which partially overlaps this conserved region. The other two highly conserved regions within the PA gene were not analyzed in this study, as they have been shown by others as not being essential for efficient packaging. In an approach similar to that described above, four constructs containing blocks of synonymous changes across the conserved region were prepared (Fig. 4). In addition to mutations across the conserved region, there were mutations in the packaging regions identified by Muramoto et al. (12) (PA31 and PA54) (Fig. 4). Analysis of influenza virus polymerase-driven replication did not change in comparison to that of wt WSN PA.
FIG. 4.
Mutational analysis of the highly conserved region of the WSN PA vRNA. (Left) Schematic representation of the regions of synonymous mutations (white boxes) introduced into the WSN PA vRNA. nt, nucleotide; ORF, open reading frame. (Right) Synonymous nucleotide changes introduced for each construct. The upper line is the parental WSN virus PA sequence, and nucleotide changes are presented in bold on the lower line. The numbering of nucleotides is based on that of the positive-sense RNA sequence.
Viruses with mutations within the highly conserved region (PA51-53) and the 5′ packaging region (PA54) were rescued easily; however, the virus containing the PA31 mutations was not able to be rescued, even after multiple rescue attempts. This suggests that the sequence conservation of this region is essential for virus replication. The growth of the viruses with 5′ mutations after the infection of MDCK cells at a low MOI (0.001) (Table 6) showed an attenuation of growth for all viruses. The level of replication was 2 to 3 logs lower than that of wt WSN.
TABLE 6.
Effect on replication in MDCK cells of recombinant WSN viruses with the introduction of synonymous changes within the highly conserved region of the PA vRNA
| Virus | Plaque titer |
|---|---|
| WSN wt | 6.1 × 107 |
| WSN PA51 | 3.3 × 105 |
| WSN PA52 | 1.6 × 106 |
| WSN PA53 | 4.3 × 104 |
| WSN PA54 | 9.0 × 104 |
MDCK cells were infected at 0.001 MOI and incubated for 48 h. Virus produced was titrated by plaque assay in MDCK cells. Results represent the average titer from two independent infections titrated in duplicate (n = 4).
An analysis of packaged vRNAs for the four WSN PA mutant viruses showed reductions in the levels of packaging of PA vRNAs (Table 7). The biggest reduction in the packaging of PA vRNA was seen with the PA53 virus, which showed a >5-fold reduction in the packaging of the PA vRNA. The region mutated in virus PA53 is within the extra region identified in the study by Liang et al. (12) but not that by Muramoto et al. (15). This suggests, as concluded in the work of Liang et al., that this region is actually involved in packaging. A consequence of the reduction in packaging of PA vRNA was a reduction in the packaging of PB2 and NP, suggesting cooperation between these vRNAs for packaging. Mutant PA52 viruses, although showing no reduction in PA vRNA packaging, demonstrated reductions in the packaging of other segments, further indicating interactions. Further work is necessary to elucidate any interactions between these vRNAs sequences.
TABLE 7.
Effect of synonymous changes within the highly conserved region of PA on the packaging of individual vRNAs into progeny WSN virions
| Virus | PB2 | PB1 | PA | HA | NP | NA | M | NS |
|---|---|---|---|---|---|---|---|---|
| WSN | 100.0 ± 4.0 | 100.0 ± 1.4 | 100.0 ± 2.2 | 100.0 ± 2.4 | 100.0 ± 3.6 | 100.0 ± 3.7 | 100.0 ± 1.3 | 100.0 ± 2.0 |
| WSN PA51 | 19.1 ± 2.5 | 103.3 ± 2.7 | 37.3 ± 1.7 | 112.7 ± 3.3 | 21.5 ± 0.2 | 100.0 ± 4.5 | 151.9 ± 0.4 | 63.6 ± 1.2 |
| WSN PA52 | 22.0 ± 0.3 | 35.6 ± 0.0 | 91.6 ± 1.4 | 92.3 ± 1.4 | 43.6 ± 0.4 | 100.0 ± 0.0 | 42.4 ± 0.0 | 69.2 ± 0.1 |
| WSN PA53 | 36.3 ± 1.4 | 112.4 ± 3.7 | 18.9 ± 0.5 | 106.8 ± 1.2 | 14.9 ± 1.3 | 100.0 ± 1.7 | 73.5 ± 1.0 | 154.8 ± 5.6 |
| WSN PA54 | 9.5 ± 1.2 | 154.3 ± 9.1 | 44.3 ± 7.7 | 117.5 ± 8.3 | 23.5 ± 3.6 | 100.0 ± 3.1 | 151.9 ± 2.0 | 76.0 ± 1.5 |
Values given are percentages of packaging of vRNA in comparison to those of parental WSN virus ± standard deviation. Results are from two independent experiments, with assay performed in triplicate (n = 6).
Mutational analysis of the conserved residues of the PR/8 PA gene and their role in vRNA packaging.
In order to see if this reduction in packaging of the PA gene is common to other viruses or unique to WSN, we made similar mutations in the PR/8 background, a related virus. As we were unable to rescue the WSN virus with a mutation in the 3′ packaging region, two extra mutants were created within this region. The PA31 mutant still covered the entire region identified as essential for packaging (15); in mutants PA31a and PA31b, this region was divided in half to see if the entire region was important (Fig. 5). All viruses were successfully rescued except for the PA31 virus (this correlated with our inability to rescue the WSN PA31 virus). Although it was not possible to rescue the PA31 virus, both PA31a and PA31b were able to be rescued, suggesting that although this region is highly important, conservation of the full region is not required.
FIG. 5.
Mutational analysis of the conserved region of the PR/8 PA vRNA. (Left) Schematic representations of the regions of synonymous mutations (white boxes) introduced into the PR/8 PA vRNA. (Right) Synonymous nucleotide changes introduced at the 3′ and 5′ ends of the vRNA for each construct. The upper line is the parental PR/8 virus PA sequence, and nucleotide changes are presented in bold on the lower line. The numbering of nucleotides is based on that of the positive-sense RNA sequence.
The growth of recombinant viruses was assessed by inoculation of 10-day-old eggs with 100 PFU of allantoic fluid 48 h postinoculation, and results are shown in Table 8. In a PR/8 background, only the PA53 virus showed a reduction in replication, with the peak virus titer approximately 1 log less than those of wt PR/8 and the other mutants.
TABLE 8.
Effect on replication in embryonated eggs of recombinant PR/8 viruses with the introduction of synonymous changes within the highly conserved region of the PA vRNA
| Virus | Plaque titer |
|---|---|
| PR/8 wt | 3.2 × 109 |
| PR/8 PA31a | 2.7 × 109 |
| PR/8 PA31a | 4.0 × 109 |
| PR/8 PA51 | 1.9 × 109 |
| PR/8 PA52 | 1.4 × 109 |
| PR/8 PA53 | 1.2 × 108 |
| PR/8 PA54 | 2.1 × 109 |
10 day-old embryonated eggs were inoculated with 100 PFU of virus. 48 h post inoculation, virus titer in the allantoic fluid was assessed by plaque assay on MDCK cells. Results represent the average titer from two independent infections titrated in duplicate (n = 4).
Analysis of PA vRNA packaging for the PR/8 mutants showed a reduction of nearly 20-fold in PA53 virus (Table 9). Unlike the results seen with WSN mutants PA51 and PA54, no reduction in the level of packaging of the PA vRNA was seen for the corresponding mutants in the PR/8 background. No significant reduction was seen in the packaging of the PA vRNA with the PA31a and PA31b viruses, suggesting that sequence conservation of the entire 3′ packaging region identified by Muramoto et al. (15) is not required for correct packaging of the PA vRNA It should be noted that this region at the start of the coding sequence of the PA gene is not highly conserved, with variability particularly in codon usage. Reductions in PA vRNA packaging in PR/8 virus mutants resulted in reductions in NP vRNA, similar to that seen with the corresponding WSN virus mutants.
TABLE 9.
Effect of synonymous changes within the highly conserved region of PA on the packaging of individual vRNAs into progeny PR/8 virions
| Virus | PB2 | PB1 | PA | HA | NP | NA | M | NS |
|---|---|---|---|---|---|---|---|---|
| PR/8 | 100.0 ± 3.0 | 100.0 ± 3.7 | 100.0 ± 2.6 | 100.0 ± 4.7 | 100.0 ± 5.4 | 100.0 ± 6.6 | 100.0 ± 2.7 | 100.0 ± 5.3 |
| PR/8 PA31a | 91.6 ± 6.9 | 74.1 ± 9.2 | 73.2 ± 2.8 | 92.5 ± 7.1 | 88.7 ± 9.5 | 96.0 ± 4.1 | 100.0 ± 4.5 | 86.6 ± 4.1 |
| PR/8 PA31b | 81.4 ± 4.5 | 64.9 ± 1.4 | 78.1 ± 3.7 | 87.2 ± 5.9 | 87.6 ± 7.3 | 93.1 ± 1.8 | 100.0 ± 4.8 | 89.4 ± 1.3 |
| PR/8 PA51 | 70.3 ± 1.9 | 67.2 ± 2.9 | 43.7 ± 3.4 | 84.4 ± 4.0 | 60.2 ± 3.8 | 80.8 ± 9.3 | 100.0 ± 1.9 | 94.2 ± 6.3 |
| PR/8 PA52 | 61.1 ± 5.6 | 53.9 ± 5.1 | 62.9 ± 2.4 | 84.3 ± 1.9 | 74.7 ± 7.2 | 85.5 ± 8.9 | 100.0 ± 8.2 | 97.8 ± 5.4 |
| PR/8 PA53 | 76.7 ± 1.9 | 43.8 ± 2.1 | 5.9 ± 0.7 | 66.2 ± 3.8 | 15.8 ± 1.1 | 73.6 ± 1.3 | 100.0 ± 6.2 | 61.4 ± 5.7 |
| PR/8 PA54 | 58.9 ± 4.8 | 48.3 ± 3.0 | 68.9 ± 2.0 | 81.2 ± 5.3 | 75.4 ± 3.0 | 77.6 ± 3.2 | 100.0 ± 5.7 | 86.4 ± 6.4 |
Values given are percentages of packaging of vRNA in comparison to those of parental PR/8 virus ± standard deviation. Results are from two independent experiments, with assay performed in triplicate (n = 6).
DISCUSSION
To efficiently package the influenza virus' eight different vRNA segments into a virus particle during an infectious cycle, the virus must solve two significant problems: (i) it must discriminate between vRNA, cRNA, and cellular RNAs because virions have little or no cRNA or cellular RNA and (ii) it must assemble eight different vRNA molecules into a virus particle from a pool of vRNA segments that can undergo reassortments or mixing in an infected cell. This is likely accomplished by the presence of unique “packaging sequences” in each of the vRNA segments, and the specificity of virion packaging would then derive from virion protein-vRNA recognition, vRNA-RNA interactions, and the mechanism of replication and assembly of vRNAs into virions. The alternative, random packaging of any vRNA segment into a virion, would lead to an extremely low PFU-to-particle ratio (smaller than 8!/88) if there were only eight RNAs per particle. The hypothetical RNA packaging sequences in each RNA segment would need to be conserved between viruses, even avian and human viruses, because these vRNA segments can reassort into different progeny viruses. These sequences could well be in the coding region of the vRNA, which will also be conserved because of selection for amino acid sequences. However the third position of a codon is very degenerate and commonly drifts with viral replication but it would not be expected to drift if RNA sequence recognition was the essential function of these oligonucleotide sequences. For this reason, we identified long sequences of RNA (23 to 95 nucleotides) in the PB2, PB1, PA, NA, NP, and M segments (see Table 1 for the conserved regions in the polymerase genes) that were highly conserved in all avian influenza viruses in the database. These same RNA sequences were also highly conserved in all of the human viruses in the database, as was expected if human and avian vRNA segments can each be packaged by the same influenza virus. In addition, these sequences overlapped those identified by previous experiments that have attempted to map the influenza virus packaging sequences (9, 10, 12, 15, 17, 25).
To date there have been two different approaches to identify and map the cis-acting RNA packaging sequences in the eight segments of the influenza virus: (i) deletion of nonessential RNA sequences from a gene segment and replacement with a gene (GFP) that permits the detection of this segment when the gene is packaged into a virus particle and delivered to a new host cell (9, 10, 12, 15, 17, 25) and (ii) mutation of the essential RNA sequences for packaging the RNA followed by a quantitative measurement of the packaging of each of the eight RNA species in a virus preparation (11, 15). In the present study, we first introduced mutations (that did not change an amino acid) into the third position of a codon located in the conserved avian and human influenza virus RNA sequences of the PB1, PB2, and PA genes of the WSN and PR/8 viruses (Tables 2 to 9). These mutant viruses were replicated in cells and the virus progeny purified. Employing one RNA segment to normalize the RNA levels in the virions (the NA segment), we quantitated the levels of the other seven RNA segments in this virus preparation. Several interesting conclusions and patterns emerged from these studies. (i) Selected mutations in PB2 reduced the packaging of PB2 by up to 93% of the normalized level of NA. (ii) Mutations in the PB1 segment reduced the level of packaging of PB1 by up to 90% compared with that of a normalized NA control. (iii) Mutations in the PA segment reduced the packaging of the PA segment by up to 81% compared with that of the NA segment. Clearly, these RNA sequences play a role in segment packaging into influenza virus. However, it appears that not all of the conserved bases are involved in packaging, and the reason for this conservation is still unknown.
Perhaps more interesting were the observations that demonstrate that mutations in one segment of RNA also can have a dramatic impact upon the packaging of another segment into a virion, and this pattern is clearly nonrandom; i.e., changes in one segment affect the other segments in a nonuniform fashion. For example, selected mutations in the conserved sequence of PB2 (PB2-55) (Table 3) reduced the packaging of PB1 (86%), PA (89%), NP (88%), M (91%), and NS (92%) vRNAs. These same mutations failed to have any impact upon the levels of either HA or NA RNA segments in virions. In contrast, mutations in the conserved region of PB1 (PB2-51) that drastically reduced PB1 vRNA segment packaging (90%) had only a modest or no effect upon the reduction of packaging of PB2 (41%), PA (2%), NP (33%), or M (49%) vRNA and no impact upon HA or NA vRNA. Mutations in PA (PB2-53) reduced PA vRNA packaging by 81%, PB2 vRNA packaging by 64%, and NP vRNA packaging by 85% but had no impact upon the packaging of PB1, HA, NA, or M vRNA. These results both confirm and extend the work of Gog et al. (11), who focused upon PB2 and employed an approach different from that used in the studies reported here. The fact that different segments of the influenza virus RNA impact the packaging of other RNA segments suggests an ordered, nonrandom process of packaging where RNA-RNA and possibly RNA-protein interactions guide into the virion the eight different segments from a pool of mixed (able to undergo reassortment) RNA molecules. It is of some interest that none of the mutations in PA, PB1, or PB2 had any impact upon the packaging of the HA and NA segments and that no conserved RNA sequences that span both avian and human NA and HA sequences have been identified. This suggests that the packaging of these two segments (and therefore the reassortment of these two segments) may be accomplished by a mechanism different than the ones described here. It should be noted, however, that a virus lacking the HA RNA segment and grown in a complementing cell line expressing an HA protein also showed differential packaging of the other vRNAs (11). Thus, the HA RNA is also packaged by a mechanism which is dependent on or affects the incorporation of the other segments.
Just as the packaging of the eight RNA segments of the influenza virus appears to be nonrandom and has certain asymmetries, we have previously shown that the reassortment of the eight influenza virus gene segments does not fit a binomial distribution, which would be expected if all of the eight segments randomly reassorted into progeny viruses (13, 22). Lubeck et al. (13) first showed that a mixed infection of cells in culture by two different viruses gave rise to progeny viruses that did not fit a random segregation of all eight segments (a binomial distribution) and that would give rise to 256 different viruses. This experiment was carried out in cell culture so that no immunoselective forces acted upon the progeny obtained. The nonrandom nature of the progeny gave rise to patterns. (i) The most common reassorted virus had one new segment and seven segments from the other virus. Most often the new segment was the HA or NA segment. (ii) When the PB2 segment reassorted from one virus, it commonly brought along several other segments (PB1, PA, NP, M, and NS). These patterns are similar to those identified as important in the packaging process, and these data suggest that the packaging process guides the reassortment of segments into progeny virus in a nonrandom fashion (not a binomial distribution). A recent analysis (22) of two different human epidemics in New York and New Zealand has demonstrated a nonrandom pattern of reassortment of different vRNA segments in wild populations of viruses. In spite of the fact that both reassortment and immunoselection act upon this process, the results of that analysis in vivo (22) were similar to the results of the in vitro study of Lubeck et al. (13). Most commonly, when a single segment reassorts, it is usually the HA or NA segment. When several segments reassort, there is a complex pattern.
In conclusion, we have rescued and passaged recombinant influenza viruses with mutations within highly conserved regions located within previously identified packaging signals of the polymerase genes. These mutations resulted in a ≤10-fold reduction in the packaging level of that particular vRNA and also reductions in the levels of packaging of other vRNAs. This work complements other studies using GFP reporter packaging constructs to identify these regions as important for packaging, demonstrating in the context of a fully infectious eight-segmented virus the importance of these sequences. Further studies of the packaging regions in other RNA segments of a variety of subtype viruses will be necessary in order to better understand the precise molecular mechanism by which vRNAs are incorporated into influenza virions.
Acknowledgments
R.R. thanks Michael Krasnitz for very stimulating discussions about the subject. pDZ plasmids and an optimized protocol for rescue of recombinant influenza PR/8 viruses were provided by Elena Carnero. Real-time qPCR was performed in James Wetmur's laboratory at the Mount Sinai School of Medicine.
Partial support of this work was provided by NIH grants PO1AI058113, Northeast Biodefense Center grant U54 AI05718, and UO1AI070469 and Center for Research on Influenza Pathogenesis grant HHSN2662000700010C. The work of A.J.L. and R.R. was supported by the Simons Foundation, the Ambrose Monell Foundation, and the Leon Levy Foundation.
Footnotes
Published ahead of print on 19 December 2007.
REFERENCES
- 1.Bancroft, C. T., and T. G. Parslow. 2002. Evidence of segment-nonspecific packaging of the influenza virus A virus genome. J. Virol. 767133-7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen, W., P. A. Calvo, D. Malide, J. Gibbs, U. Schubert, I. Bacik, S. Basta, R. O'Neill, J. Schickli, P. Palese, P. Henklein, J. R. Bennink, and J. W. Yewdell. 2001. A novel influenza A virus mitochondrial protein that induces cell death. Nat. Med. 71306-1312. [DOI] [PubMed] [Google Scholar]
- 3.Cros, J. F., and P. Palese. 2003. Trafficking of viral genomic RNA into and out of the nucleus: influenza, Thogoto and Borna disease viruses. Virus Res. 953-12. [DOI] [PubMed] [Google Scholar]
- 4.Donald, H. B., and A. Isaacs. 1954. Counts of influenza virus particles. J. Gen. Microbiol. 10457-464. [DOI] [PubMed] [Google Scholar]
- 5.Dos Santos Afonso, E., N. Escriou, I. Leclercq, S. van der Werf, and N. Naffakh. 2005. The generation of recombinant influenza A viruses expressing a PB2 fusion protein requires the conservation of a packaging signal overlapping the coding and noncoding regions at the 5′ end of the PB2 segment. Virology 34134-46. [DOI] [PubMed] [Google Scholar]
- 6.Duhaut, S. D., and N. J. Dimmock. 2002. Defective segment 1 RNAs that interfere with production of infectious influenza A virus require at least 150 nucleotides of 5′ sequence: evidence from a plasmid-driven system. J. Gen. Virol. 83403-411. [DOI] [PubMed] [Google Scholar]
- 7.Enami, M., W. Luytjes, M. Krystal, and P. Palese. 1990. Introduction of site-specific mutations into the genome of influenza virus. Proc. Natl. Acad. Sci. USA 873802-3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fodor, E., L. Devenish, O. G. Engelhardt, P. Palese, G. G. Brownlee, and A. García-Sastre. 1999. Rescue of influenza A virus from recombinant DNA. J. Virol. 739679-9682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujii, K., Y. Fujii, T. Noda, Y. Muramoto, T. Watanabe, A. Takada, H. Goto, T. Horimoto, and Y. Kawaoka. 2005. Importance of both the coding and the segment-specific noncoding regions of the influenza A virus NS segment for its efficient incorporation into virions. J. Virol. 793766-3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujii, Y., H. Goto, T. Watanabe, T. Yoshida, and Y. Kawaoka. 2003. Selective incorporation of influenza virus RNA segments into virions. Proc. Natl. Acad. Sci. USA 1002002-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gog, J. R., E. Dos Santos Afonso, R. M. Dalton, I. Leclercq, L. Tiley, D. Elton, J. C. von Kirchbach, N. Naffakh, N. Escriou, and P. Digard. 2007. Codon conservation in the influenza A virus genome defines RNA packaging signals. Nucleic Acids Res. 351897-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang, Y., Y. Hong, and T. G. Parslow. 2005. cis-Acting packaging signals in the influenza virus PB1, PB2, and PA genomic RNA segments. J. Virol. 7910348-10355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lubeck, M. D., P. Palese, and J. L. Schulman. 1979. Nonrandom association of parental genes in influenza A virus recombinants. Virology 95269-274. [DOI] [PubMed] [Google Scholar]
- 14.Marsh, G. A., R. Hatami, and P. Palese. 2007. Specific residues of the influenza A virus hemagglutinin viral RNA are important for efficient packaging into budding virions. J. Virol. 819727-9736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muramoto, Y., A. Takada, K. Fujii, T. Noda, K. Iwatsuki-Horimoto, S. Watanabe, T. Horimoto, H. Kida, and Y. Kawaoka. 2006. Hierarchy among viral RNA (vRNA) segments in their role in vRNA incorporation into influenza A virions. J. Virol. 802318-2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noda, T., H. Sagara, A. Yen, A. Takada, H. Kida, R. H. Cheng, and Y. Kawaoka. 2006. Architecture of ribonucleoprotein complexes in influenza A virus particles. Nature 439490-492. [DOI] [PubMed] [Google Scholar]
- 17.Ozawa, M., K. Fujii, Y. Muramoto, S. Yamada, S. Yamayoshi, A. Takada, H. Goto, T. Horimoto, and Y. Kawaoka. 2007. Contributions of two nuclear localization signals of influenza A virus nucleoprotein to viral replication. J. Virol. 8130-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palese, P. 1977. The genes of influenza virus. Cell 101-10. [DOI] [PubMed] [Google Scholar]
- 19.Palese, P., and J. L. Schulman. 1976. Differences in RNA patterns of influenza A viruses. J. Virol. 17876-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palese, P., and M. L. Shaw. 2007. Orthomyxoviridae: the viruses and their replication, p. 1647-1689. In B. N. Fields et al. (ed.), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 21.Quinlivan, M., D. Zamarin, A. García-Sastre, A. Cullinane, T. Chambers, and P. Palese. 2005. Attenuation of equine influenza viruses through truncations of the NS1 protein. J. Virol. 798431-8439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rabadan, R., A. J. Levine, and M. Krasnitz. Non-random reassortment in human influenza A viruses. Influenza Other Respir. Viruses, in press. [DOI] [PMC free article] [PubMed]
- 23.Schickli, J. H., A. Flandorfer, T. Nakaya, L. Martinez-Sobrido, A. García-Sastre, and P. Palese. 2001. Plasmid-only rescue of influenza A virus vaccine candidates. Philos. Trans. R. Soc. Lond. B 3561965-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scholtissek, C., E. Harms, W. Rohde, M. Orlich, and R. Rott. 1976. Correlation between RNA fragments of fowl plague virus and their corresponding gene functions. Virology 74332-344. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe, T., S. Watanabe, T. Noda, Y. Fujii, and Y. Kawaoka. 2003. Exploitation of nucleic acid packaging signals to generate a novel influenza virus-based vector stably expressing two foreign genes. J. Virol. 7710575-10583. [DOI] [PMC free article] [PubMed] [Google Scholar]