Abstract
Innate immune responses against viral infection, especially the induction of type I interferon, are critical for limiting the replication of the virus. Although it has been shown that DNA can induce type I interferon, to date no natural DNA ligand of a virus that induces type I interferon has been described. Here we screened the genome of murine gammaherpesvirus 68 with mutations at various genomic locations to map the region of DNA that induces type I interferon. A repetitive region termed the 100-base-pair repeat region is a ligand that is both necessary and sufficient for the viral genomic DNA to induce type I interferon. A region colinear with this ligand in the genome of Kaposi's sarcoma-associated herpesvirus also induces type I interferon. We have thus defined a repetitive region of the genomes of gammaherpesviruses as the first natural DNA virus ligand that induces type I interferon.
The innate immune system is a highly efficient defense system of multicellular organisms that is critical in protection against microorganisms. With its families of evolutionarily conserved receptors, the innate immune system recognizes and responds to ligands containing molecular patterns that are indicative of a particular microorganism type. These pathogen-associated molecular patterns (PAMPs) are unique to the type of microorganism that is being encountered, and the innate immune system responds to these PAMPs with the appropriate induction of signals (15, 28). For example, when the innate immune system encounters a viral PAMP, the quintessential innate response is to induce the release of type I interferon (IFN) (23).
IFNs were first described in the 1950s, when it was found that cells exposed to inactivated viruses secrete a chemical that interferes with the replication of subsequent viral infections (12, 13, 30). These secreted chemicals were proteins that were named “interferons” for their ability to “interfere” with viral infection. IFNs are recognized by the type I interferon receptor and signal in both autocrine and paracrine manners to induce the transcription of a number of specific genes (7, 33, 36). The IFN-induced gene products work in concert to produce an antiviral state making the cell refractory to further viral infection, to potentiate the cell to undergo apoptosis upon the stress of a viral infection, or to activate the adaptive arm of the immune response (20, 22, 32, 45, 50). Induction of IFN is key to mounting a proper and robust immune response to a viral infection (46).
For most of the years since the discovery of IFN, the mechanisms by which viral PAMPs are recognized and induce the production of IFN remained unknown. New virions are created through viral parasitization of the cellular transcription and translation machinery; consequently, most protein components of viruses are effectively host derived and not hypothesized to be effective PAMPs. On the other hand, viral genomic RNA and DNA are often highly structured and represent nucleic acid species not typically found in a host cell or in particular subcellular locations (1, 3, 39, 48, 49). Thus, viral nucleic acids are outstanding in the molecular environment of the cell and can serve as effective PAMPs. Several of these unique nucleic acid species are recognized by different innate immune receptors, such as Toll-like receptors (TLRs). For example, TLR3 recognizes extracellular double-stranded RNA, while TLR9 recognizes extracellular or endocytosed CpG DNA (1, 9, 21, 26, 39). Similarly, the recently described cytoplasmic RNA helicases, such as RIG-I, recognize cytoplasmic uncapped 5′ phosphates of viral RNA (10, 11, 18, 19). These nucleic acid receptors induce signal transduction cascades that converge on the activation of IFN regulatory factors (IRFs), which bind to IFN-stimulated response elements (ISREs) upstream of IFN-inducible genes, including those that encode the IFN proteins themselves (2, 31, 35, 44).
Although the mechanistic framework for the induction of IFN by RNA virus infection has recently been well studied, much still remains to be understood with regards to how DNA viruses induce IFN. CpG DNA, which is found in both bacterial DNA and some viral DNA, is recognized by TLR9 and, as such, was the main candidate for a DNA virus ligand/receptor pair. Other work, however, points to the importance of TLR9-independent induction of IFN (14, 16, 34, 37, 41, 43). When synthetic DNA or bacterial DNA is transfected into cells, IFN is induced. Other forms of DNA, including virally derived DNA, seem to be able to elicit an induction of IFN, but how these large segments of viral DNA function as a ligand in the context of the much larger chromosomal DNA remains a key question. Additionally, the diversity of viral pathogens may necessitate multiple systems of DNA virus detection. Here we focus on the induction of IFN by the genomic DNA from herpesvirus infection. We define the nature of the first IFN-inducing viral DNA ligand, a portion of genomic DNA from gammaherpesviruses.
MATERIALS AND METHODS
Cell culture, transfections, and infections.
Mouse embryonic fibroblasts (MEFs) and human embryonic kidney (HEK) 293T cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and antibiotics (penicillin and streptomycin). Transfection of viral DNA, reporter plasmids, or DNA carrier plasmids was done using Fugene 6 (Roche) according to the manufacturer's protocol. Transfection of excised DNA or PCR fragments was done using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. Viruses were diluted in Dulbecco's modified Eagle's medium to a multiplicity of infection of 1.0 and added to cells for a period of 48 h to allow accumulation of IFN.
Luciferase plasmids and reagents.
For luciferase assays, we used ISRE (from ISG15), 5× κB site, IFN-α4, recombination binding protein Jκ (RBP-Jκ), and hTERT plasmids in a pGL2 background. The RBP-Jκ and hTERT luciferase plasmids were gifts from Y. Liang and H. Ly, Emory University School of Medicine (24). We controlled for transfection efficiency by transfecting 1/10 the total DNA mass with pSV40-encoded Renilla luciferase. Cells were lysed in passive lysis buffer and assayed for firefly and Renilla luciferase levels by using a dual-luciferase assay kit (from Promega or Biotinium, as both worked comparably throughout in all scenarios).
Viral DNA experiments.
Viral DNA was extracted as described previously (25). Briefly, cell-associated viral DNA was prepared after infecting cells for 24 to 36 h at a high multiplicity of infection. When cells appeared to be undergoing cytopathic effects, the medium was removed, and the remaining cells were lysed in 10 mM Tris-HCl, 10 mM EDTA, and 0.6% sodium dodecyl sulfate. Cellular genomic DNA was precipitated overnight after the addition of 5 M NaCl to a final concentration of 0.85 M NaCl. Lysates were cleared by centrifugation at maximum speed in a microcentrifuge. The viral DNA was harvested using phenol-chloroform extraction followed by ethanol precipitation. The DNA was treated with RNase A at 25°C for 30 min and then with proteinase K at 50°C for 30 min. The viral DNA was again extracted with phenol-chloroform, followed by ethanol precipitation, and finally was resuspended in water.
Cells were transfected with 100 ng or the indicated amount of viral DNA along with reporter plasmids, and after 24 or 36 h, cells were lysed and assayed for both firefly and Renilla luciferase levels. Viral DNA was also treated with DNase for 30 min at 37°C and then phenol chloroform extracted and ethanol precipitated. A sample was also mock treated with DNase to account for the extraction process. UV treatment of viral DNA occurred by placing 100 ng of viral DNA in a microcentrifuge tube and exposing it to 120 mJ of UV via a Stratagene UV Stratalinker machine.
Cloning of repeat regions.
The 100-bp repeat region of murine gammaherpesvirus 68 (MHV-68) between nucleotides (nt) 93,951 and 103,288 was cloned into pUC-19, and the BglII-StyI fragment was gel purified. The 40-bp repeat region between nt 24,699 and 32,881 was cloned into pSMART-VC, and the AvrII-NheI fragment was extracted and used in this study. For the terminal repeats, NotI-excised MHV-68 genomic DNA was used in experiments. The plasmid containing the kaposin locus was a gift from C. McCormick (Dalhousie University) (27).
Construction of viral BAC and derivative mutants.
We generated an independent bacterial artificial chromosome (BAC) clone of MHV-68 (pBAC/MHV-68) (T. T. Wu, unpublished data). The BAC (pBeloBAC plasmid; Camr) was inserted at the left end of the viral genome (nt 1892). pEntranceposon (Kanr) containing Mu binding sites was used to clone a signature-tagged transposon, using XbaI and StuI sites. Twelve tags were designed for real-time PCR detection. The sequences of the signature tags and primers were as previously described (40).
pEntranceposons tagged with 12 unique sequences were cut with BglII to release the STM transposons. In vitro transposition was performed for 10 to 15 min with pBAC/MHV-68 and purified signature tag transposons (Finzyme, Finland). The reaction mixtures were transformed into bacteria (Escherichia coli DH10b), and colonies were recovered from LB plates containing kanamycin and chloramphenicol. BAC DNAs with signature tag mutants were subjected to sequencing analysis using the Seq1 primer (5′-GTCGCTTACTAGGATCCG-3′; Macrogen, Republic of Korea) to determine the location and orientation of inserted transposons. EcoRI digestion followed by Southern analysis was performed to confirm the correct genomic arrangement of signature tag mutants.
BAC DNAs were transfected into BHK21 cells in 96-well plates, using Lipofectamine Plus (Invitrogen). At 6 to 8 days posttransfection, supernatant was transferred at a 1:10 dilution to freshly seeded BHK21 cells for two rounds, and the cytopathic effects of each mutant were scored to determine the essentialness of the disrupted region for in vitro growth of the virus. Plaque assays and real-time PCR with ORF56-specific primers (nt 75598 to 75783) (M56F [5′-GTAACTCGAGACTGAAACCTCGCAGAGGTCC-3′] and M56R [5′-CCGAAGCTTGCACGGTGCAATGTGTCACAG-3′]) were used to measure the titers of replication-competent viruses.
DNA structure modeling.
DNA sequences were modeled for secondary structure by using the online bending and curvature plot in B-DNA (BANANA) program at http://bioweb.pasteur.fr/seqanal/interfaces/banana.html (8, 38). Sequences were submitted and bend and curvature values were obtained for each nucleotide location. The numbers were imported into Microsoft Excel, and line graphs were obtained and overlapped to gain the full representation of the genome. Individual sequences were done in a similar manner.
RESULTS
Viral genomic DNAs from different herpesviruses are ligands for IFN induction.
Induction of IFN occurs through various signal transduction cascades that initiate from the recognition of a PAMP by an innate immune receptor, converging on the induction of IFN gene transcription. We began by comparing the induction of IFN upon viral infection with members of two distinct herpesviruses families, i.e., herpes simplex virus type 1 (HSV-1), a representative member of the alphaherpesvirus subfamily, and MHV-68, a member of the gammaherpesvirus subfamily.
Upon infection of MEFs with either virus, IFN-α was released into the supernatant (Fig. 1A). Although the mechanism of induction of IFN by DNA viruses is not precisely defined, the idea that DNA could be an important PAMP in the induction of this IFN-α release led us to focus on the importance of the viral genomic DNA. We extracted viral genomic DNA from MEF cells infected with either HSV-1 or MHV-68. Viral genomic DNA from either HSV-1 infection or MHV-68 infection could induce ISRE activation (Fig. 1B).
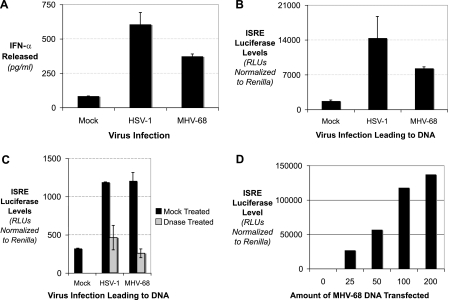
FIG. 1.
Herpesviral DNA induces type I IFN. (A) MEFs were mock infected or infected with either HSV-1 or MHV-68. Cell supernatants were collected, and the levels of IFN-α were determined by enzyme-linked immunosorbent assay. (B) MEFs were mock transfected or transfected with viral DNA derived from HSV-1 or MHV-68 infections, along with an ISRE luciferase reporter and a Renilla luciferase reporter transfection control. The levels of ISRE luciferase relative to Renilla luciferase were quantitated. (C) MEFs were mock transfected or transfected with viral DNA derived from HSV-1 or MHV-68 infections, along with an ISRE luciferase reporter and a Renilla luciferase transfection control. The viral DNA was either mock treated (black bars) or DNase treated (gray bars). (D) MEFs were mock transfected or transfected with increasing amounts of viral DNA derived from MHV-68 infections, along with an ISRE luciferase reporter and a Renilla luciferase transfection control.
To confirm that the viral genomic DNA was the ligand for induction of IFN, we treated the viral genomic DNA preparations with DNase. Neither the HSV-1 nor the MHV-68 viral genomic DNA could induce IFN after DNase treatment (Fig. 1C). In addition, titration of the MHV-68 viral genomic DNA into wild-type MEFs induced the ISRE reporter in a dose-dependent manner (Fig. 1D). Thus, herpesviral genomic DNA is a distinct ligand for induction of IFN. To define the nature of how the genomic DNA functions as a viral PAMP, we focused on the MHV-68 genome.
Viral genomic DNA in a BAC induces type I IFN.
The viral genomic DNA of MHV-68 that was used for the data shown in Fig. 1 was composed of approximately 120 kilobases of unique sequence, with various lengths of terminal repeats. However, this viral genomic DNA was derived from infected cells and thus may represent a mixture of nucleic acid species. In addition, this viral genomic DNA preparation may have had chemical or other modifications that made the DNA able to induce IFN. To negate these potential complications in understanding the nature of the viral genomic DNA as a ligand, we began using viral genomic DNA ligated into a BAC. The viral BAC contains the entire MHV-68 genomic DNA in a plasmid that contains the necessary genetic information to maintain and expand the large genomic DNA in bacteria and, as such, is also able to be manipulated genetically.
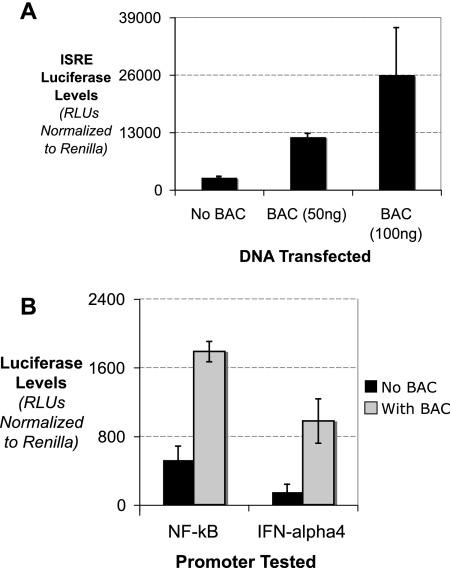
In MEFs, BACs containing the viral genome induced ISRE luciferase (Fig. 2A). Using different amounts of viral BAC, we saw that the induction was titratable and was very potent in inducing ISREs, potentially owing to the pure nature of the viral BAC population that was transfected. Although ISREs are key indicators of IFN induction, other promoters, such as the natural IFN promoters, may be affected by the viral BAC DNA in different ways (29). We tested the IFN-α4 promoter and NF-κB binding sites for the ability to be induced by the viral BAC DNA. As shown in Fig. 2B, viral BACs were able to induce NF-κB and IFN-α4 promoters. Viral BACs did not induce the unrelated hTERT promoter or the RBP-Jκ binding site (data not shown).
FIG. 2.
BACs containing MHV-68 genomic DNA induce type I IFN. (A) MEFs were transfected with plasmid DNA or BAC DNA containing the MHV-68 genome at two concentrations, along with an ISRE luciferase reporter and a Renilla luciferase transfection control. The levels of ISRE luciferase relative to Renilla luciferase were quantitated. (B) MEFs were mock transfected or transfected with BAC DNA containing the MHV-68 genome, along with either the NF-κB or IFN-α4 luciferase reporter plasmid and a Renilla luciferase transfection control.
A distinct, repetitive region of the MHV-68 genome is required for IFN induction.
The work in Fig. 1 and 2, along with previous studies, directly shows that viral DNA can be used to induce IFN (14, 41). Cells induce IFN through recognition of PAMPs by distinct cellular pattern recognition receptors. A central question was how the herpesviral genomes constitute a true, distinguishable PAMP. The PAMP could be based on specific viral DNA sequences that could be distinguished from cellular sequences. Furthermore, was there an intrinsic property of viral DNA that made it a potent PAMP or was it a particular region within the viral DNA that governed its ability to function as a PAMP?
To begin shedding light on these questions, we screened a library of mutant viral BACs. The viral BACs were mutated by random transposon mutagenesis throughout the genome. These mutants were characterized regarding the exact sequence of the mutation and their replication capability. A set of mutant viral BACs were individually transfected into cells along with the ISRE luciferase plasmid.
All of the mutants shown in Fig. 3A, except for one, were able to induce IFN to a level comparable to that induced by the wild-type viral BAC. These results show that the ability of the viral genomic DNA to induce IFN is highly resistant to random mutations throughout the genome. The fact that one mutant was unable to induce IFN was particularly intriguing to us. Interestingly, the region of that mutation was in a repetitive region of the viral genome that has been designated the 100-bp repeat region because the region is made up of approximately 20 repeats of a unit that is 100 base pairs in length (47).
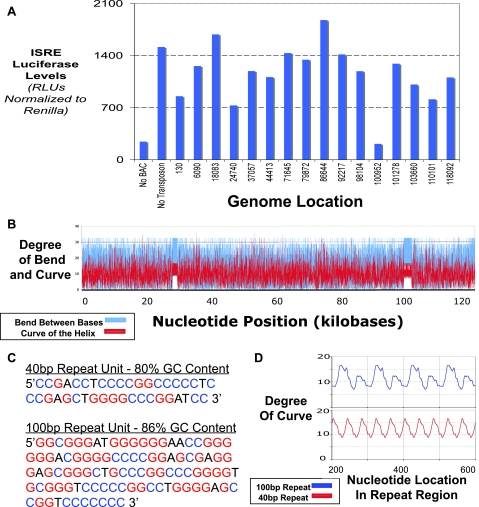
FIG. 3.
Screening of the MHV-68 genome identifies a region in the 100-bp repeat region as being critical for the genomic DNA to induce type I IFN. (A) MEFs were transfected with a panel of BAC DNAs containing the MHV-68 genome, mutagenized with transposons at various locations throughout the genome, along with an ISRE luciferase reporter and a Renilla luciferase reporter transfection control. (B) The sequence for the MHV-68 genome was analyzed by a structure modeling program that predicts the degree of bending between local bases and the overall curvature of the double helix. The values of the bends (blue line) and curves (red line) obtained were plotted as a line graph, as a function of nucleotide base position. As shown, most of the genome is irregular, except for the two ordered regions of the 40-bp repeat region and the 100-bp repeat region. (C) Sequences of the units of the 40-bp repeat unit and the 100-bp repeat unit. The GC content for each is listed, and the G's and C's are denoted in red and blue, respectively. (D) The curvature predictions for the 40-bp repeat region and the 100-bp repeat region are diagrammed. For each, the overall region was modeled and the denoted internal region was plotted for comparison.
To further determine how repetitive regions could be distinct in the viral genome, beyond sequence, we looked at predicted structures of the genomic DNA. Using DNA structure prediction algorithms, we analyzed the entire genome of MHV-68 to see how the DNA helix bends and curves (Fig. 3B). Bending is denoted in blue and represents how much successive base pairs tend not to be parallel to the previous base pair. Curvature of the helix, on the other hand (denoted in red), indicates where the axis of the DNA helix tends to be nonlinear. As shown in Fig. 3B, predicted structure modeling of the entire MHV-68 genome shows a random DNA structure, except for the 100-bp repeat region and one other repetitive region, termed the 40-bp repeat region (47). These two repetitive regions, noted in Fig. 3B, show higher degrees of bending between bases and overall curvature of the double helix, yet they are also distinct in their overall lengths and structure predictions. As shown in Fig. 3C, the sequences of the repeat units are distinct and the overall GC contents are different, with the 100-bp repeat unit having a surprisingly high level of GC nucleotides (86%). We focused on the curvature of the DNA helix within these two repeat units and compared the two regions directly. As shown in Fig. 3D, when graphed together, the degrees of curvature of the helix are distinctly different, with the period of peaks of the curvature being almost three times as long for the 100-bp repeat region as for the 40-bp repeat region. The period of the curvature peaks is exactly the length of the unit for each repeat and thus may hint at how the formation of distinct DNA structures may be governed. Thus, an intact 100-bp repeat region is necessary for the MHV-68 viral genomic DNA to induce IFN.
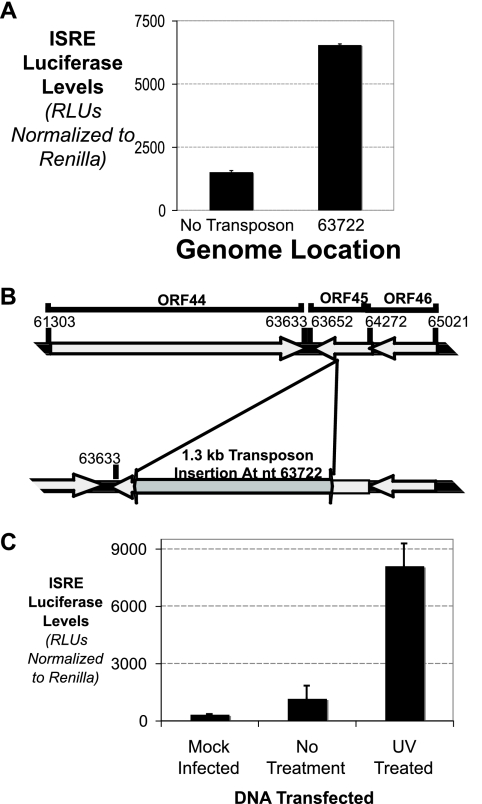
One other mutant (Fig. 4A), with a mutation at nucleotide 63722, is also interesting because it was able to induce a much higher level of ISRE induction upon transfection. This region is diagrammed in Fig. 4B. Mutation at nucleotide 63722 disrupts the ORF45 gene, whose gene product in Kaposi's sarcoma-associated herpesvirus (KSHV) has been shown to block IRF7-mediated IFN induction by KSHV (51). Thus, an intact ORF45 gene is required for dampening of the IFN induction caused by viral genomic DNA. In addition, this result is recapitulated from UV treatment of virally derived genomic DNA (Fig. 4C). UV treatment is assumed to disrupt most open reading frames (ORFs), including ORF45, allowing the cell to respond to viral DNA by producing IFN at an uninhibited level.
FIG. 4.
MHV-68 genomic DNA directly opposes DNA induction of IFN. (A) MEF cells were transfected with wild-type or ORF45 mutant viral BACs, along with ISRE luciferase and Renilla luciferase. The levels of ISRE luciferase relative to Renilla luciferase were quantitated. (B) Schematic of the coding region around ORF45 and how transposon mutagenesis at nt 63722 disrupts the ORF. (C) Viral genomic DNA was extracted from MHV-68-infected cells. The genomic DNA was either mock treated or UV treated. The viral genomic DNA was transfected into MEF cells, along with ISRE luciferase and Renilla luciferase. The levels of ISRE luciferase relative to Renilla luciferase were quantitated.
The 100-bp repeat region of MHV-68 is sufficient to induce type I IFN.
Our mutational mapping of the MHV-68 viral genomic DNA shows that an intact 100-bp repeat region within the viral genome is critical for the ability of the genome to induce IFN. In addition, analysis of the overall bending and curvature of the genomic DNA points out that this region has intrinsic, unique structural properties that may allow it to be recognized by the cellular antiviral machinery and to induce IFN. An important additional question was how other repetitive regions lead to or influence IFN induction.
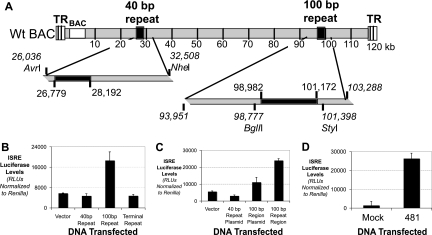
The MHV-68 genome contains three main repetitive regions, including the 100-bp repeat region, the 40-bp repeat region, and the terminal repeat region (Fig. 5A). We isolated and purified the repetitive regions of the MHV-68 viral genome and transfected these individual fragments into cells. The 100-bp repeat region alone was sufficient to induce IFN (Fig. 5B). Neither the 40-bp repeat region nor the terminal repeats were able to induce the ISRE reporter. Placing the 100-bp repeat region into a plasmid slightly decreased its ability to induce the ISRE reporter but was still able to create a plasmid that induced IFN (Fig. 5C). Thus, even outside circular or larger pieces of DNA, the 100-bp repeat region is sufficient to induce IFN.
FIG. 5.
The 100-bp repeat region is sufficient to induce type I IFN. (A) Schematic of the MHV-68 genome and locations of the repeat regions along with cloning sites. (B) Induction of ISRE luciferase by the 100-bp repeat region (BglII/StyI fragment), the 40-bp repeat region (AvrI/NheI fragment), and the excised terminal repeats (NotI fragment). The levels of ISRE luciferase relative to Renilla luciferase were quantitated. Only the 100-bp repeat region induced ISRE. (C) Induction of the ISRE reporter upon transfection with the 100-bp repeat region in a plasmid and after excision from the carrier plasmid, using the sites indicated in panel A. The levels of ISRE luciferase relative to Renilla luciferase were quantitated. A plasmid with the 40-bp repeat region was included as a negative control. (D) Induction of the ISRE reporter upon transfection with a PCR product containing the last 481 nt of the 100-bp repeat region. The levels of ISRE luciferase relative to Renilla luciferase were quantitated.
All of the DNAs with the repeat regions in Fig. 5A to C were from bacterial species; thus, bacterial modification of DNA could be involved in the ability to induce IFN. The 100-bp repeat region is approximately 2 kb in length and is composed of 100-bp repeat units. Repeated attempts to recreate the structure from 100-nt-long oligonucleotides, either single stranded or duplexed, were not successful in creating a nucleic acid species that could induce IFN (data not shown). We were able to clone a 481-bp region at the 3′ end of the 100-bp repeat region. This region was PCR amplified and purified to remove any contaminating bacterial DNA. This region, but not other, unrelated PCR products of comparable size, was able to induce IFN (Fig. 5D). This does not discount the rest of the 100-bp repeat region, as this 481-bp region contains the most 3′ region of the repeat region and is still structurally comparable to the repetitive structure of the rest of the region.
Mutation of the 100-bp repeat region of MHV-68 genomic DNA ablates the ability of viral DNA to induce an antiviral state.
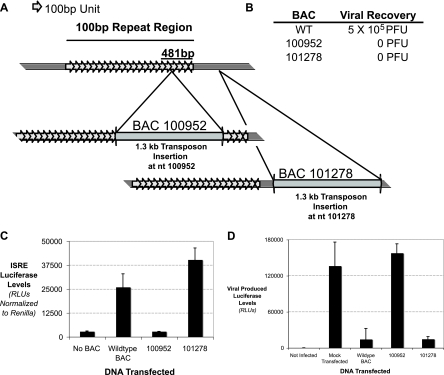
It is interesting that a transposon mutant (nt 100952) that did not induce ISRE luciferase had a mutation in the 481-nt region (Fig. 6C). When sequentially arrayed, the next transposon insertion in the screen was at nt 101278, which is immediately downstream of the 100-bp repeat region. A schematic of the location of each transposon relative to the 100-bp repeat region is shown in Fig. 6A. Upon transfection of these viral BACs, it was found that mutations at regions in or proximal to the 100-bp repeat region do not allow for viral growth, as monitored by observing cytopathic effects and virus titers (Fig. 6B) (40). Neither BAC 100952 nor BAC 101278 was able to lead to virus production. Thus, this region is critical for viral growth, and disruptions of the region can be detrimental to an overall viral infection if defective virions propagate or are taken up by cells. We next wanted to show the consequence of having DNA taken up by cells in terms of allowing virus propagation.
FIG. 6.
The 100-bp repeat region allows viral DNA to be recognized and leads to an antiviral state. (A) Schematic of the 100-bp repeat region and how transposon mutagenesis at nt 100952 and 101143 affects the region. Note that mutation at nt 100952 disrupts the 481-bp region shown in Fig. 5D, while mutation at nt 101143 does not affect any of the 100-bp repeat region. (B) Ability of mutant BACs to allow for viral growth compared to that of wild-type BAC. Viral growth was assessed by monitoring cytopathic effects as well as viral titers. (C) MEFs were transfected with either irrelevant DNA or a BAC mutant with mutation at nt 100952 or 101143, along with an ISRE luciferase reporter and a Renilla luciferase reporter transfection control. The levels of ISRE luciferase relative to Renilla luciferase were quantitated. (D) MEFs were transfected with either irrelevant DNA or a BAC mutant with mutation at nt 100952 or 101143. After 48 h, the cells were then infected with MHV-68 expressing a luciferase reporter gene, and after 12 h of infection, cell lysates were prepared and the levels of firefly luciferase were analyzed.
We purified new viral BACs at a high concentration and confirmed that they behaved as in the genomic screen. The viral BAC with a mutation in the 100-bp repeat region (nt 100952) was unable to induce the ISRE, while the viral BAC with a mutation immediately outside the 100-bp repeat region (nt 101278) was able to induce the ISRE (Fig. 6C). This confirms that viral genomic DNA that is completely wild type except for a disrupted 100-bp repeat region is not sufficient to induce IFN.
To monitor viral growth, we used MHV-68 encoding firefly luciferase, which can be monitored easily using standard luciferase assays (S. Hwang, unpublished data). Cells were transfected with viral BACs and, after 48 h, were infected with MHV-68 luciferase. Wild-type viral BACs induced an antiviral state that strongly inhibited viral infection (Fig. 6D), while viral BAC mutants with mutation outside the 100-bp repeat region (nt 101278) also induced a potent antiviral state. On the other hand, transfection of a viral BAC with a mutation in the 100-bp repeat region (nt 100952) did not induce an antiviral state and thus afforded no protection for the cells from subsequent viral infection. These cells allowed viral growth similar to that of mock-transfected cells. Thus, induction of IFN through recognition of the 100-bp repeat region leads to a meaningful induction of an antiviral state that limits the growth potential of a viral infection.
KSHV contains a repeat region, colinear with the 100-bp repeat region, that also induces type I IFN.
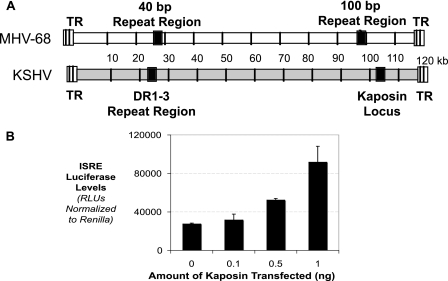
To confirm that our results are indicative of a true gammaherpesvirus PAMP, we extended our investigation to KSHV. KSHV is a human gammaherpesvirus commonly modeled by MHV-68, with a genomic organization similar to that of MHV-68 (Fig. 7A) (6, 42). When aligned, the 100-bp repeat region is colinear with a distinct repetitive region in KSHV, the kaposin locus. Alignment of the MHV-68 and KSHV genomes locates regions that can be aligned and thus termed colinear. As a repetitive region that is colinear with the 100-bp repeat region, the kaposin locus is a prime candidate for the IFN-inducing region of the KSHV genomic DNA.
FIG. 7.
Gammaherpesvirus DNA is recognized through colinear repeat regions. (A) Schematic alignment of MHV-68 and KSHV genomes showing the regions where repeat regions are colinear in gammaherpesviruses. The kaposin locus is colinear to the 100-bp repeat region. (B) Transfection of increasing amounts of a plasmid containing the kaposin locus, along with the ISRE reporter plasmid. The levels of ISRE luciferase relative to Renilla luciferase were quantitated.
To test the ability of the kaposin locus to induce IFN, we transfected plasmids containing the kaposin locus into HEK 293T cells, along with the ISRE luciferase reporter construct. As shown in Fig. 7B, titration of the kaposin locus into cells induced the ISRE reporter at high levels. This shows that in gammaherpesviruses, repetitive regions colinear with the 100-bp repeat region are indeed the regions of genomic DNA that are recognized by the cell and induce IFN.
DISCUSSION
A key issue in understanding the immune response to a viral infection is knowing how the cells of the body recognize the actual infectious agent. Viruses present a profound challenge to the host defense systems in that to create progeny virions they use the metabolic machinery and genetic architecture of the very cells they infect. For the cell to induce IFN, a pattern recognition receptor must recognize viral DNA or RNA, as viral nucleic acids are the best potential PAMP that a cell can recognize. Previous work focused on the ability of synthetic DNA and some infectious DNA to induce IFN, yet the actual nature of the DNA that induced IFN was never dissected (34, 41). A key question is the determination of the actual sequence or structure of a natural ligand of a DNA virus that can induce IFN. Here we took the genome of MHV-68 and, using a whole-genome approach, elucidated the 100-bp repeat region as the portion of viral DNA that serves as a PAMP to induce IFN.
This viral DNA segment is a novel, defined PAMP that is necessary and sufficient for the proper induction of IFN by recognition of viral genomic DNA. As a PAMP, the 100-bp repeat region has the intrinsic ability to direct the induction of an antiviral state. Viral genomes, especially those of the herpesvirus family, which have DNA genomes with sizes of >100 kilobases, must harbor all the information for a productive viral infection. With their large size, herpesvirus genomes have a large coding capacity but are also required to have proper nucleic acid structures to allow for efficient replication. This includes regions that allow for initiation of replication of the genome, proper packaging of the genome into a new virion, and interactions of the viral nucleic acids with host or viral protein. As such, the genomes of different viruses are structurally varied throughout, containing a topologic landscape unique to each virus.
It is in this very unique genomic landscape that the host cell has the best opportunity to recognize a signature of viral infection. Much of any viral genome is used by the virus to carry ORFs that are requisite for production of progeny virions as well as modulation of the host cell physiology. The ORF products are encoded like most other cellular proteins, and as such, the structure of these regions of DNA is not thought to be an efficient PAMP for recognition by the cell. This idea was confirmed through our screening of mutations throughout the MHV-68 genome, in which all of the transposon mutations that affected individual ORFs did not affect the ability of the viral DNA to induce IFN (Fig. 3A). In scanning the predicted bending and curvature of the genomic DNA of MHV-68, one quickly notices few structurally distinct regions that could be distinguishable to the cell as a PAMP (Fig. 3B). However, we hypothesized that those regions that were structurally distinct would be most distinguishable to the cell as a PAMP. This was dramatically confirmed in that disruption of the 100-bp repeat region, a region of approximately 2 kilobases that contains a repetitive, high-GC-content unit (Fig. 3C), ablated the ability of the viral DNA to induce IFN. The transposon mutant is an insertion, and thus the entire sequence of the 100-bp repeat region is kept in the genome, underscoring the fact that a full structure, not the sequence of the DNA, is recognized.
Not only was the 100-bp repeat region necessary for the viral DNA to induce IFN, but it was sufficient, and even outside the context of the viral genome, the 100-bp repeat region could induce IFN, either as a free piece of DNA or in a plasmid (Fig. 5B and C). This region is made of many repeats of 100 bp in length. The repeat as an individual unit is not sufficient to induce IFN, as transfection of the units as single-stranded or duplexed units did not induce ISRE reporters (data not shown). However, in Fig. 5D, we show that a region amplified via PCR from a fragment cloned from the 3′ end of the 100-bp repeat region is sufficient to induce the ISRE promoter, which supports the idea that beyond a sequence signature, cells recognize viral DNA based on a sequence-initiated structure. In addition, the kaposin locus of KSHV, which is colinear with the 100-bp repeat region, is an effective PAMP for IFN induction (Fig. 7), underscoring the ability of this region to be recognized by cells as a signature of gammaherpesvirus infection.
A particularly important point is that regions of a genome that are outstanding structurally are not necessarily PAMPs. The 40-bp repeat region stands out dramatically in the overall genome analysis (Fig. 3B), yet it does not induce IFN like the 100-bp repeat region does (Fig. 5B). The 40-bp repeat region is composed of a unit repeat that is different from that of the 100-bp repeat region (Fig. 3C) and is predicted to have an entirely different curvature geometry from that of the 100-bp repeat region (Fig. 3D). Thus, it is not sufficient to be structurally unique in the genomic DNA, but there is some property of the 100-bp repeat region that makes it a PAMP that can induce IFN. We are pursuing further work to determine what property of the 100-bp repeat region makes it structurally unique.
It is interesting that the viral BACs that were mutated around the 100-bp repeat region were themselves unable to produce progeny virions. Because the 100-bp repeat region is located near a viral origin of replication as well as other viral ORFs, there could be multiple reasons for why this region is critical for viral replication (5). This also explains why any virus would keep intact a region of genomic DNA that could induce IFN. Without the 100-bp repeat region, the virus is unable to actively replicate, regardless of the IFN-inducing properties of this region. Viruses have evolved to circumvent this problem by carrying various viral proteins that can block the induction or effects of IFN.
The ORF45 protein is a tegument protein of gammaherpesviruses and, as such, is immediately released into the cytoplasm upon viral entry. In addition, the KSHV homologue of ORF45 was shown to block IRF-7-mediated induction of IFN (4). RNA interference of MHV-68 ORF45 blocks viral replication, and BAC-MHV-68, with an ORF45 nonsense mutation, is unable to produce a productive infection (17). In our studies, a viral genomic BAC with an ORF45 null mutation induced ISRE activation at a severalfold higher level than that with the wild-type viral genomic BAC. This may represent one potential explanation of why the loss of ORF45 leads to disruption of viral replication: without ORF45, the induction of IFN by the viral genomic DNA is high enough to block viral replication.
It is notable that ORF45 does not ablate the induction of IFN completely. Every cell transfected with wild-type viral genomic BACs has the potential to express ORF45, and thus ORF45 is not 100% effective as an IFN block. This concept moves back to our developing an understanding of the interaction between viruses and the immune system. The idea of immune evasion, where the virus is completely able to hide from the immune system, was recently redefined to the idea of immune modulation. Immune modulation is where a certain amount of immune activation still occurs due to both the strength of the immune system and the less-than-perfect functioning of viral proteins that interact with the immune system. This allows for the virus to replicate at a controlled rate, permitting some level of survival of the host and also allowing the virus to pass on to new hosts. This view of the immune interaction with viral pathogens exemplifies the fact that viruses have coevolved with their hosts.
Taken together, these studies define the first viral DNA ligand for induction of IFN. In addition, we have shed new light on the complexity of the interplay of the induction of IFN upon DNA virus infection. The ability of a cell to distinguish signatures of viral infection from the host's own components is also central to the development of new antivirals and more effective vaccination strategies. Current use of therapies based on the innate immune system, such as exogenous IFN treatment, is sometimes effective but often has serious side effects. These findings are the next step in the development of the next generation of antiviral drugs, which will focus on creating an efficacious and safe immune response against an infection.
Acknowledgments
D.J.S. was supported by a UCLA Tumor Cell Biology training grant (5 T32 CA009056-29). D.M. was supported by a UCLA Clinical Immunology and Allergy training grant (5T32AI007126-30). A.S. was a Van Trees Scholar through the UCLA Undergraduate Research Scholars Program. This work was supported by NIH grant ROI-AI069120.
We thank members of the Cheng lab for their helpful insight and discussions, especially J. Bontemps and T. Ha.
Footnotes
Published ahead of print on 12 December 2007.
REFERENCES
- 1.Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413732-738. [DOI] [PubMed] [Google Scholar]
- 2.Au, W. C., P. A. Moore, W. Lowther, Y. T. Juang, and P. M. Pitha. 1995. Identification of a member of the interferon regulatory factor family that binds to the interferon-stimulated response element and activates expression of interferon-induced genes. Proc. Natl. Acad. Sci. USA 9211657-11661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barton, G. M., J. C. Kagan, and R. Medzhitov. 2006. Intracellular localization of Toll-like receptor 9 prevents recognition of self DNA but facilitates access to viral DNA. Nat. Immunol. 749-56. [DOI] [PubMed] [Google Scholar]
- 4.Coscoy, L. 2007. Immune evasion by Kaposi's sarcoma-associated herpesvirus. Nat. Rev. Immunol. 7391-401. [DOI] [PubMed] [Google Scholar]
- 5.Deng, H., J. T. Chu, N. H. Park, and R. Sun. 2004. Identification of cis sequences required for lytic DNA replication and packaging of murine gammaherpesvirus 68. J. Virol. 789123-9131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doherty, P. C., J. P. Christensen, G. T. Belz, P. G. Stevenson, and M. Y. Sangster. 2001. Dissecting the host response to a gamma-herpesvirus. Philos. Trans. R. Soc. Lond. B 356581-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doyle, S., S. Vaidya, R. O'Connell, H. Dadgostar, P. Dempsey, T. Wu, G. Rao, R. Sun, M. Haberland, R. Modlin, and G. Cheng. 2002. IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity 17251-263. [DOI] [PubMed] [Google Scholar]
- 8.Goodsell, D. S., and R. E. Dickerson. 1994. Bending and curvature calculations in B-DNA. Nucleic Acids Res. 225497-5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408740-745. [DOI] [PubMed] [Google Scholar]
- 10.Honda, K., A. Takaoka, and T. Taniguchi. 2006. Type I interferon gene induction by the interferon regulatory factor family of transcription factors. Immunity 25349-360. [DOI] [PubMed] [Google Scholar]
- 11.Hornung, V., J. Ellegast, S. Kim, K. Brzozka, A. Jung, H. Kato, H. Poeck, S. Akira, K. K. Conzelmann, M. Schlee, S. Endres, and G. Hartmann. 2006. 5′-Triphosphate RNA is the ligand for RIG-I. Science 314994-997. [DOI] [PubMed] [Google Scholar]
- 12.Isaacs, A., and J. Lindenmann. 1957. Virus interference. I. The interferon. Proc. R. Soc. Lond. B 147258-267. [PubMed] [Google Scholar]
- 13.Isaacs, A., J. Lindenmann, and R. C. Valentine. 1957. Virus interference. II. Some properties of interferon. Proc. R. Soc. Lond. B 147268-273. [DOI] [PubMed] [Google Scholar]
- 14.Ishii, K. J., C. Coban, H. Kato, K. Takahashi, Y. Torii, F. Takeshita, H. Ludwig, G. Sutter, K. Suzuki, H. Hemmi, S. Sato, M. Yamamoto, S. Uematsu, T. Kawai, O. Takeuchi, and S. Akira. 2006. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat. Immunol. 740-48. [DOI] [PubMed] [Google Scholar]
- 15.Janeway, C. A., Jr. 1989. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 541-13. [DOI] [PubMed] [Google Scholar]
- 16.Jensen, K. E., A. L. Neal, R. E. Owens, and J. Warren. 1963. Interferon responses of chick embryo fibroblasts to nucleic acids and related compounds. Nature 200433-434. [DOI] [PubMed] [Google Scholar]
- 17.Jia, Q., V. Chernishof, E. Bortz, I. McHardy, T. T. Wu, H. I. Liao, and R. Sun. 2005. Murine gammaherpesvirus 68 open reading frame 45 plays an essential role during the immediate-early phase of viral replication. J. Virol. 795129-5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato, H., S. Sato, M. Yoneyama, M. Yamamoto, S. Uematsu, K. Matsui, T. Tsujimura, K. Takeda, T. Fujita, O. Takeuchi, and S. Akira. 2005. Cell type-specific involvement of RIG-I in antiviral response. Immunity 2319-28. [DOI] [PubMed] [Google Scholar]
- 19.Kato, H., O. Takeuchi, S. Sato, M. Yoneyama, M. Yamamoto, K. Matsui, S. Uematsu, A. Jung, T. Kawai, K. J. Ishii, O. Yamaguchi, K. Otsu, T. Tsujimura, C. S. Koh, C. Reis e Sousa, Y. Matsuura, T. Fujita, and S. Akira. 2006. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441101-105. [DOI] [PubMed] [Google Scholar]
- 20.Kolumam, G. A., S. Thomas, L. J. Thompson, J. Sprent, and K. Murali-Krishna. 2005. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J. Exp. Med. 202637-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krug, A., G. D. Luker, W. Barchet, D. A. Leib, S. Akira, and M. Colonna. 2004. Herpes simplex virus type 1 activates murine natural interferon-producing cells through Toll-like receptor 9. Blood 1031433-1437. [DOI] [PubMed] [Google Scholar]
- 22.Le Bon, A., N. Etchart, C. Rossmann, M. Ashton, S. Hou, D. Gewert, P. Borrow, and D. F. Tough. 2003. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat. Immunol. 41009-1015. [DOI] [PubMed] [Google Scholar]
- 23.Le Page, C., P. Genin, M. G. Baines, and J. Hiscott. 2000. Interferon activation and innate immunity. Rev. Immunogenet. 2374-386. [PubMed] [Google Scholar]
- 24.Liang, Y., J. Chang, S. J. Lynch, D. M. Lukac, and D. Ganem. 2002. The lytic switch protein of KSHV activates gene expression via functional interaction with RBP-Jkappa (CSL), the target of the Notch signaling pathway. Genes Dev. 161977-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin, C. L., H. Li, Y. Wang, F. X. Zhu, S. Kudchodkar, and Y. Yuan. 2003. Kaposi's sarcoma-associated herpesvirus lytic origin (ori-Lyt)-dependent DNA replication: identification of the ori-Lyt and association of K8 bZip protein with the origin. J. Virol. 775578-5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lund, J., A. Sato, S. Akira, R. Medzhitov, and A. Iwasaki. 2003. Toll-like receptor 9-mediated recognition of herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 198513-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCormick, C., and D. Ganem. 2005. The kaposin B protein of KSHV activates the p38/MK2 pathway and stabilizes cytokine mRNAs. Science 307739-741. [DOI] [PubMed] [Google Scholar]
- 28.Medzhitov, R., and C. Janeway, Jr. 2000. Innate immune recognition: mechanisms and pathways. Immunol. Rev. 17389-97. [DOI] [PubMed] [Google Scholar]
- 29.Mesplede, T., S. Navarro, P. Genin, P. Morin, M. L. Island, E. Bonnefoy, and A. Civas. 2003. Positive and negative control of virus-induced interferon-A gene expression. Autoimmunity 36447-455. [DOI] [PubMed] [Google Scholar]
- 30.Nagano, Y., and Y. Kojima. 1958. Inhibition of vaccinia infection by a liquid factor in tissues infected by homologous virus. C. R. Sci. Soc. Biol. Fil. 1521627-1629. [PubMed] [Google Scholar]
- 31.Nguyen, H., J. Hiscott, and P. M. Pitha. 1997. The growing family of interferon regulatory factors. Cytokine Growth Factor Rev. 8293-312. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen, K. B., T. P. Salazar-Mather, M. Y. Dalod, J. B. Van Deusen, X. Q. Wei, F. Y. Liew, M. A. Caligiuri, J. E. Durbin, and C. A. Biron. 2002. Coordinated and distinct roles for IFN-alpha beta, IL-12, and IL-15 regulation of NK cell responses to viral infection. J. Immunol. 1694279-4287. [DOI] [PubMed] [Google Scholar]
- 33.Novick, D., B. Cohen, and M. Rubinstein. 1994. The human interferon alpha/beta receptor: characterization and molecular cloning. Cell 77391-400. [DOI] [PubMed] [Google Scholar]
- 34.Okabe, Y., K. Kawane, S. Akira, T. Taniguchi, and S. Nagata. 2005. Toll-like receptor-independent gene induction program activated by mammalian DNA escaped from apoptotic DNA degradation. J. Exp. Med. 2021333-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pitha, P. M., W. C. Au, W. Lowther, Y. T. Juang, S. L. Schafer, L. Burysek, J. Hiscott, and P. A. Moore. 1998. Role of the interferon regulatory factors (IRFs) in virus-mediated signaling and regulation of cell growth. Biochimie 80651-658. [DOI] [PubMed] [Google Scholar]
- 36.Platanias, L. C., S. Uddin, and O. R. Colamonici. 1994. Tyrosine phosphorylation of the alpha and beta subunits of the type I interferon receptor. Interferon-beta selectively induces tyrosine phosphorylation of an alpha subunit-associated protein. J. Biol. Chem. 26917761-17764. [PubMed] [Google Scholar]
- 37.Rotem, Z., R. A. Cox, and A. Isaacs. 1963. Inhibition of virus multiplication by foreign nucleic acid. Nature 197564-566. [DOI] [PubMed] [Google Scholar]
- 38.Satchwell, S. C., H. R. Drew, and A. A. Travers. 1986. Sequence periodicities in chicken nucleosome core DNA. J. Mol. Biol. 191659-675. [DOI] [PubMed] [Google Scholar]
- 39.Schulz, O., S. S. Diebold, M. Chen, T. I. Naslund, M. A. Nolte, L. Alexopoulou, Y. T. Azuma, R. A. Flavell, P. Liljestrom, and C. Reis e Sousa. 2005. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature 433887-892. [DOI] [PubMed] [Google Scholar]
- 40.Song, M. J., S. Hwang, W. H. Wong, T. T. Wu, S. Lee, H. I. Liao, and R. Sun. 2005. Identification of viral genes essential for replication of murine gamma-herpesvirus 68 using signature-tagged mutagenesis. Proc. Natl. Acad. Sci. USA 1023805-3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stetson, D. B., and R. Medzhitov. 2006. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity 2493-103. [DOI] [PubMed] [Google Scholar]
- 42.Stevenson, P. G., and S. Efstathiou. 2005. Immune mechanisms in murine gammaherpesvirus-68 infection. Viral Immunol. 18445-456. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki, K., A. Mori, K. J. Ishii, J. Saito, D. S. Singer, D. M. Klinman, P. R. Krause, and L. D. Kohn. 1999. Activation of target-tissue immune-recognition molecules by double-stranded polynucleotides. Proc. Natl. Acad. Sci. USA 962285-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taniguchi, T., K. Ogasawara, A. Takaoka, and N. Tanaka. 2001. IRF family of transcription factors as regulators of host defense. Annu. Rev. Immunol. 19623-655. [DOI] [PubMed] [Google Scholar]
- 45.van Boxel-Dezaire, A. H., M. R. Rani, and G. R. Stark. 2006. Complex modulation of cell type-specific signaling in response to type I interferons. Immunity 25361-372. [DOI] [PubMed] [Google Scholar]
- 46.van den Broek, M. F., U. Muller, S. Huang, M. Aguet, and R. M. Zinkernagel. 1995. Antiviral defense in mice lacking both alpha/beta and gamma interferon receptors. J. Virol. 694792-4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Virgin, H. W. T., P. Latreille, P. Wamsley, K. Hallsworth, K. E. Weck, A. J. Dal Canto, and S. H. Speck. 1997. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J. Virol. 715894-5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoneyama, M., M. Kikuchi, T. Natsukawa, N. Shinobu, T. Imaizumi, M. Miyagishi, K. Taira, S. Akira, and T. Fujita. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5730-737. [DOI] [PubMed] [Google Scholar]
- 49.Yoshida, H., Y. Okabe, K. Kawane, H. Fukuyama, and S. Nagata. 2005. Lethal anemia caused by interferon-beta produced in mouse embryos carrying undigested DNA. Nat. Immunol. 649-56. [DOI] [PubMed] [Google Scholar]
- 50.Zhang, X., S. Sun, I. Hwang, D. F. Tough, and J. Sprent. 1998. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity 8591-599. [DOI] [PubMed] [Google Scholar]
- 51.Zhu, F. X., S. M. King, E. J. Smith, D. E. Levy, and Y. Yuan. 2002. A Kaposi's sarcoma-associated herpesviral protein inhibits virus-mediated induction of type I interferon by blocking IRF-7 phosphorylation and nuclear accumulation. Proc. Natl. Acad. Sci. USA 995573-5578. [DOI] [PMC free article] [PubMed] [Google Scholar]