Abstract
Group A rotaviruses are classified into serotypes, based on the reactivity pattern of neutralizing antibodies to VP4 and VP7, as well as into subgroups (SGs), based on non-neutralizing antibodies directed against VP6. The inner capsid protein (VP2) has also been described as a SG antigen; however, little is known regarding the molecular determinants of VP2 SG specificity. In this study, we characterize VP2 SGs by correlating genetic markers with the immunoreactivity of the SG-specific monoclonal antibody (YO-60). Our results show that VP2 proteins similar in sequence to that of the prototypic human strain Wa are recognized by YO-60, classifying them as VP2 SG-II. In contrast, proteins not bound by YO-60 are similar to those of human strains DS-1 or AU-1 and represent VP2 SG-I. Using a mutagenesis approach, we identified residues that determine recognition by either YO-60 or the group A-specific VP2 monoclonal antibody (6E8). We found that YO-60 binds to a conformationally dependent epitope that includes Wa VP2 residue M328. The epitope for 6E8 is also contingent upon VP2 conformation and resides within a single region of the protein (Wa VP2 residues A440 to T530). Using a high-resolution structure of bovine rotavirus double-layered particles, we predicted these epitopes to be spatially distinct from each other and located on opposite surfaces of VP2. This study reveals the extent of genetic variation among group A rotavirus VP2 proteins and illuminates the molecular basis for a previously described SG specificity associated with the rotavirus inner capsid protein.
Rotaviruses are nonenveloped, 11-segmented, double-stranded RNA (dsRNA) viruses and a leading cause of virus-induced acute gastroenteritis in young children and infants (22). The infectious virion is organized as three concentric protein shells, each comprised of unique viral capsid constituents (25). The structural proteins within each shell vary slightly among rotavirus strains, leading to antigenic differences that can be detected by using immunological assays (4, 14). As such, the reactivity pattern of antibodies against certain rotavirus capsid proteins is the primary method by which viruses in this family are classified (3, 4, 14). Specifically, the sero groups described for rotaviruses (A to G) are based on the binding of non-neutralizing monoclonal antibodies to the intermediate shell protein (VP6) (4, 14). Because group A rotaviruses are a predominant cause of human disease, they are further classified into serotypes and subgroups (SGs) (22). Serotypes are based on the neutralizing antibody responses generated against the outer capsid proteins (VP7 [G-types] and VP4 [P-types]) and, along with the more recently described genotypes, remain the most common method of classifying group A rotaviruses in epidemiological studies (1-3, 11, 33). SGs have been based predominantly on the immunoreactivity pattern of non-neutralizing monoclonal antibodies against VP6 and are used to further characterize group A rotavirus isolates (5, 7, 12, 13, 16, 20, 35). In addition to VP6, the rotavirus inner capsid protein (VP2) has been described as an SG antigen, but the classification of virus strains into VP2 SGs is limited (31, 34).
Group A rotaviruses can be described as VP6 SG-I, SG-II, SG-I/II, or non-SG-I/II based on their differential recognition by monoclonal antibodies (255/60 [SG-I] and 631/9 [SG-II]) (7, 10, 30, 35). These VP6 SG-specific antibodies each bind to a distinct conformational epitope present on the trimeric, but not the monomeric, form of the intermediate capsid protein (8, 18, 32). Although a small percentage of rotaviruses bear both or neither VP6 epitopes (SG-I/II or non-SG-I/II, respectively), most human strains are VP6 SG-I or SG-II (3, 14). In contrast to VP6, very little is known about an additional SG specificity that was based on the immunoreactivity of a monoclonal antibody (YO-60) directed against VP2. YO-60 was generated after immunization of mice using the human strain YO, and it is known to immunoreact with VP2 proteins from several human and porcine rotavirus strains (designated VP2 SG-II) (31, 34, 35). However, this antibody does not bind VP2 proteins from other human and animal strains (designated VP2 SG-I) (31). The differential binding of YO-60 suggests that VP2 SG-II proteins contain a divergent, but as-yet-unidentified, epitope that is absent in VP2 SG-I proteins. While the majority of rotaviruses shown to be VP2 SG-I are also VP6 SG-I, and likewise for SG-II, these antigens are capable of independently reassorting in nature (31). The observation that VP2 SGs (defined by YO-60) do not invariably correlate with VP6 SGs hampered the widespread use of the VP2 SG nomenclature when characterizing rotaviruses.
Comprising the innermost layer of a rotavirus virion, VP2 serves a number of important structural and functional roles. During particle assembly, 120 copies of VP2 form a pseudo T=1 icosahedral core scaffold, allowing for the packaging of VP1/VP3/RNA complexes and the addition of the outer capsid proteins (VP4, VP6, and VP7) (4). Moreover, VP2 plays a critical role in viral RNA synthesis and is required for triggering the RNA-dependent RNA polymerase activity of VP1 (23, 24, 36). The VP2 capsid layer is visualized in reconstructed cryo-electron microscopic images as a smooth, thin, contiguous shell, with a small portion of the protein extending further inward at the fivefold axis to form a pentagonal structure (17, 27). Biochemical studies indicate that this inwardly protruding piece is likely the amino terminus of the protein, which is thought to directly engage VP1/VP3/RNA and possibly trigger VP1 to catalyze RNA synthesis (15, 24, 37). The previous lack of sequence information has hindered progress in defining VP2 residues that are important for these described functions. Furthermore, without sequence information it remains unclear how VP2 SG-I or SG-II proteins are genetically defined or whether they exhibit structural or functional differences. Recent work by our lab and others has elucidated the genome sequences of several group A rotaviruses, affording the opportunity to resolve the basis of VP2 SG specificity and to identify potential functional domains within the inner capsid (19; E. Heiman et al., unpublished data).
In the present study, we analyzed the newly available sequence information to reveal the extent of variation among group A rotavirus VP2 proteins, and we correlated this variation with YO-60 immunoreactivity. Our results show that VP2 proteins similar to that of the prototypic human strain Wa were recognized by YO-60, defining them as SG-II. In contrast, YO-60 did not bind to VP2 proteins similar to strains AU-1 or DS-1, suggesting that they represent SG-I. Using a mutagenesis approach, we identified specific residues within Wa VP2 that mediate binding by either YO-60 or the group A-specific VP2 antibody (6E8). These antibody-binding sites are predicted to be spatially distinct from each other and located on opposite surfaces of the structured rotavirus core. The results of the present study greatly enhance our understanding of the molecular basis for a SG specificity associated with the rotavirus inner capsid. Equally, these results identify conserved residues that may be critical for the various functions of VP2 during rotavirus replication.
MATERIALS AND METHODS
Mammalian cells, rotaviruses, and antibodies.
Fetal rhesus monkey kidney (MA104) cells were used for rotavirus infections and cultured at 37°C and in 5% CO2 in M199 medium supplemented to contain 10% fetal bovine serum (FBS), 1% penicillin-streptomycin (CellGro), and 0.1% amphotericin B (CellGro). Rotavirus strains SA11-5N, DS-1, 69M, Wa, WI61, and P were propagated and titered in MA104 cells and used for infections after pretreatment with 10 μg of trypsin/ml as described previously (6). Infected cells were incubated in serum-free M199 medium for the indicated times. Human embryonic kidney (HEK 293T) cells were used for transfection experiments and were propagated at 37°C and in 5% CO2 in Dulbecco modified Eagle medium (DMEM) supplemented to contain 10% FBS, 1% nonessential amino acids (Cambrex), 1% penicillin-streptomycin (CellGro), and 0.1% amphotericin B (CellGro).
Polyclonal guinea pig VP1 antiserum (α-VP1; 59752) was prepared using recombinant protein, which was expressed and purified as described previously (36). Polyclonal guinea pig VP2 antiserum (pGP; 59750) and the mouse monoclonal immunoglobulin G antibodies (6E8 and YO-60) were generated previously (29, 31, 34).
Phylogenetic analyses and amino acid alignments.
Phylogenetic analyses and amino acid alignments were constructed by using MacVector 8.1.2. (Accelrys). Amino acid dendrograms were generated by using the neighbor-joining method (1,000 bootstrap repetitions) and the Poisson correction parameter. The GenBank accession numbers for the gene 2 sequences used in the present study are given parenthetically as follows: Wa (X14942), SA11-5N (DQ838631), DS-1 (EF583026), PO-13 (AB009630), Bristol (AJ303139), S2 (DQ870486), YO (DQ870498), KU (AB022766), Beijing (DQ862063), AU-1 (DQ490536), T152 (DQ146700), UK (X52589), RF (X14057), TB-Chen (AY787652), D (EF583022), P (EF583038), ST3 (EF583046), IAL28 (EF583030), SE584 (EF583042), 69M (EF583014), WI61 (EF583050), A64 (EF583018), and L26 (EF583034).
Structural analyses.
An atomic resolution crystal structure of bovine rotavirus double-layered particles (DLPs), which includes VP2-A residues P100 to L880 and VP2-B residues E81 to L880, was generously provided by Stephen Harrison, Harvard University (B. McClain, E. Settembre, R. Bellamy, and S. C. Harrison, unpublished data). The VP2 sequence of the crystallized DLP is 98% identical to that of bovine rotavirus UK. Image analyses were performed by using the UCSF Chimera-Molecular Modeling System (26).
Immunofluorescence assays.
MA104 cells, grown to near confluence on glass coverslips, were either mock infected or infected at a multiplicity of infection of 5. At 8 h postinfection, cells were washed with phosphate-buffered saline and then fixed using 4% paraformaldehyde and permeabilized with 1% Triton X-100. Indirect immunostaining was performed as described previously (29). Primary antibodies and secondary antibodies conjugated to fluorophores (Molecular Probes) were used at a 1:1,000 dilution. Stained coverslips were mounted onto glass slides using ProLong Gold containing DAPI (4′,6′-diamidino-2-phenylindole; Invitrogen) and incubated at room temperature for 12 h prior to imaging. Fluorescence was detected using a Leica DM-IRE2 epifluorescence microscope with a 40× oil immersion objective lens. Image analyses and merging was performed by using Adobe Photoshop 9.0 (Adobe).
Cloning SA11, DS-1, and Wa gene 2 into pCI vectors.
The VP2-coding sequence (gene 2) from rotavirus strains SA11, DS-1, and Wa were cloned into the pCI vector (Promega) using reverse transcription-PCR. RNA was extracted from infected cells using TRIzol-LS as described in the manufacturer's protocol (Invitrogen). Reverse transcription was performed using SuperScript II reverse transcriptase (Invitrogen), denatured RNA, and an antisense primer complementary to the SA11-5N gene 2 5′ untranslated region. PCR (HF Platinum Taq; Invitrogen) was used to amplify gene 2 cDNAs, and the resulting amplicons were ligated into either pCR-XL-TOPO (Invitrogen) or pGEM-T Easy (Promega) and sequenced to ensure the fidelity of PCR. The cloned VP2 amino acid sequences exactly match what is reported in GenBank for the following virus strains (GenBank number): SA11-5N (DQ838631), DS-1 (EF583026), and Wa (X14942). The SA11, DS-1, or Wa VP2 coding regions were then subcloned into pCI using primer-generated restriction sites (5′EcoRI and 3′NotI).
Truncation mutagenesis of Wa gene 2 in pCI.
To generate truncations of the Wa VP2 amino or carboxy termini, PCR was performed using HF Platinum Taq (Invitrogen) and pCI-Wa-g2 as a template. For making carboxy-terminal truncations, the sense primer was 5′-GATGGAATTCATGGCGTACAGGAAA-3′, while the antisense primers were 5′-GAATAGCGGCCGCTTAGTTTAGTCTATTAGC-3′ (ΔC1), 5′-GAATAGCGGCCGCTTAAGTATTTATATGGC-3′ (ΔC2), and 5′-GAATAGCGGCCGCTTATCTATCAGCCTGAAC-3′ (ΔC3). For making amino-terminal truncations, the antisense primer was 5′-GAATAGCGGCCGCTTACAGTTCGTT-3′, while the sense primers were 5′-GATGGAATTCATGCAATTATCTGATAAA-3′ (ΔN1), 5′-GATGGAATTCATGGTTCAGGCTGATAGA-3′ (ΔN2), and 5′-GATGGAATTCATGGCCATAATAAATACT-3′ (ΔN3). The PCR products were directly cloned into pCI using restriction sites (5′EcoRI and 3′NotI) and sequenced to ensure the fidelity of PCR.
Generating pCI gene 2 constructs for 6E8 epitope mapping.
To generate pCI-Wa-g2-440-642, PCR was performed using Accuprime Pfx (Invitrogen), pCI-Wa-g2 as the template, and the primers ΔN3 (sense) and ΔC1 (antisense), which are described above. To generate pCI-Wa-g2-440-570 and pCI-Wa-g2-440-530, PCR was performed by using the sense primer for ΔN3 (above) and the antisense primers 5′-GATAGCGGCCGCTTAACAAGCCATTAG-3′ (amino acids 440 to 570) and 5′-GATAGCGGCCGCTTAAGTCGGAAATTG-3′ (amino acids 440 to 530).
The PCR amplicons were directly cloned into pCI using restriction sites (5′EcoRI and 3′NotI) and sequenced to ensure the fidelity of PCR.
Generating pCI gene 2 chimeras for YO-60 epitope mapping.
To generate pCI vectors to express chimeric (Chi) proteins, Chi-1 and Chi-2, restriction enzyme digests were performed using EcoRI and AflIII sites. Chi-1 gene 2 was made by replacing the pCI-Wa-g2 nucleotides between the EcoRI and AflII sites (Wa residues M1 to 578V) with those from DS-1 (DS-1 residues M1 to V567). Chi-2 gene 2 was made by replacing the pCI-DS-1-g2 nucleotides between EcoRI and AflII (DS-1 residues M1 to V567) with those from Wa (Wa residues M1to 578V). To generate a pCI vector to express Chi-3, DS-1 nucleotides 691 to 1284 (DS-1 residues V231 to L428) were replaced with Wa nucleotides 718 to 1317 (Wa residues V240 to L439) using PCR and blunt-ended ligation. The sense primer used for outward PCR of pCI-DS-1-g2 was 5′-GCTATAGTAAATACAATAA-3′, and the antisense primer was 5′-ACGCTGTCTCATCTCTGC-3′. To amplify Wa nucleotides 718 to 1317 (Wa residues V240 to L439), the 5′ phosphorylated sense primer was 5′-GTTCAGGCTGATAGAAAAT-3′, and the 5′ phosphorylated antisense primer was 5′-CAATTCGCATGCGACTAA-3′. The parental vectors pCI-DS-1-g2 and pCI-Wa-g2 were used as PCR templates, Accuprime Pfx was used as the polymerase, and the final ligated products were sequenced across the VP2-coding region.
Generating pCI gene 2 point mutants for YO-60 epitope mapping.
To generate single point mutations in the pCI-Wa-g2 or pCI-DS-1-g2 templates, outward PCR was performed by using 5′ phosphorylated primers and Accuprime Pfx. The PCRs were treated with DpnI for 1 h at 37°C prior to gel purification and ligation. The final ligated products were sequenced across the VP2-coding region to ensure incorporation of the engineered mutation and the absence of other changes. The primers for outward PCR were as follows: 5′-GTCGTCAATTATCCATCAATTTT-3′ (Wa I246V sense), 5′-ATTTCTATCAGCCTGAACCTG-3′ (Wa I246V antisense), 5′-CAGCATCAATTAGTCGAACCA-3′ (Wa N266Q sense), 5′-TAGAAAATATTCATTAAATGC-3′ (Wa N266Q antisense), 5′-GATATCATTTTTAATTATATACC-3′ (Wa E276D sense), 5′-ATTATTTAGTGGTTCGACTAA-3′ (Wa E276D antisense), 5′-CGTAATTTGCCATCAACAGCA-3′ (Wa M328 sense), 5′-ATCCATATTCAAAATATAGTT-3′ (Wa M328 antisense), 5′-AAAGAATTAGTTTCAACTGAA-3′ (Wa ΔKE sense), 5′-CAAATCAGGCACAACTGATC-3′ (Wa ΔKE antisense), 5′-GAGATAATATTTAATTATATACC-3′ (DS-1 D267E sense), 5′-ATTATTCAATGGTTCAACTAA-3′ (DS-1 D267E antisense), 5′-ATGAATCTACCATCAACTGCC-3′ (DS-1 R289M sense), and 5′-ATCCATATTGAGAATGTAATT-3′ (DS-1 R289M antisense).
Expression and radiolabeling proteins in cultured cells.
To label virus-expressed proteins, 2 × 106 MA104 cells in six-well plates were either infected at a multiplicity of infection of 5 or mock infected using M199 medium. Proteins were radiolabeled from 3 to 8 h postinfection using 100 μCi of [35S]EasyTag express protein labeling mix (Perkin-Elmer)/ml in 80% DMEM lacking methionine and cysteine and 20% complete medium. After the labeling period, cells were washed using 500 μl of phosphate-buffered saline and then lysed in 300 μl of lysis buffer (150 mM NaCl, 1% Nonidet P-40, 0.5% deoxycholate, 50 mM Tris-HCl [pH 8.0], protease inhibitor cocktail mix [Roche], and 1 μg each of leupeptin and aprotinin/ml). Lysates were subjected to centrifugation at 3,500 × g to remove cell nuclei, and cytoplasmic supernatants were used for immunoprecipitations.
To label plasmid-expressed VP2 proteins, HEK 293T cell monolayers in six-well plates were transfected with pCI plasmids according to the manufacturer's protocol. Briefly, approximately 106 cells were grown 24 h in complete DMEM at 37°C and in 5% CO2. Prior to transfection, the medium was replaced with DMEM lacking antibiotics but supplemented to contain 5% FBS and 1% nonessential amino acids. Each well was transfected with 4 μg of plasmid DNA using 10 μl of Lipofectamine 2000 in 500 μl of Opti-MEM-I. At 18 h posttransfection, the medium was replaced with 80% DMEM medium lacking methionine and cysteine and 20% complete medium. Proteins were radiolabeled from 24 to 48 h posttransfection using 100 μCi of [35S]EasyTag express protein labeling mix/ml, and cytoplasmic lysates were generated for immunoprecipitations as described above for infected cells.
Expression and radiolabeling proteins in rabbit reticulocyte lysate.
Radiolabeled proteins for epitope mapping were expressed from pCI plasmids using rabbit reticulocyte lysate (TNT T7 Quick-Coupled Transcription/Translation kit; Promega) in the presence of 100 μCi of [35S]EasyTag l-methionine (Perkin-Elmer)/ml. Approximately 1 μg of DNA was added to each 50-μl reaction according to the manufacturer's instructions.
Immunoprecipitation analyses.
Immunoprecipitations were performed in a final volume of 300 μl using protein A-Sepharose beads (Sigma), either 50 μl of radiolabeled cytoplasmic lysate or 5 to 14 μl of radiolabeled reticulocyte lysate, and 1.5 μl of antibody in lysis buffer. Reactions were incubated at 4°C for 3 h with rotation. Protein-bead conjugates were washed three times in lysis buffer supplemented to contain 0.1% sodium dodecyl sulfate (SDS), and proteins were eluted from beads by incubation at > 95°C for 5 min in 2× SDS-protein loading buffer (Invitrogen). Proteins were resolved by SDS-polyacrylamide gel electrophoresis (PAGE) in polyacrylamide Tris-glycine gels (Novex; Invitrogen) and analyzed by fluorography (Amplify; Amersham). The SeeBlue Plus-2 protein ladder (Invitrogen) was used as a molecular weight standard. Images were prepared by using Adobe Photoshop 9.0 (Adobe).
RESULTS
Genetic variation among group A rotavirus VP2 proteins.
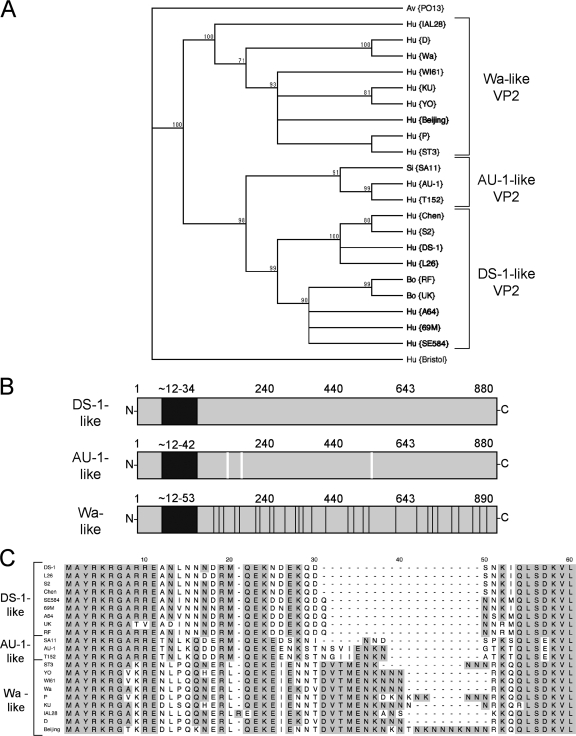
The VP2-coding segment (gene 2) has recently been sequenced from several human and animal group A strains as part of a comprehensive rotavirus genomics project (19; Heiman et al., unpublished). Upon analyzing this sequence information, we found that most group A rotavirus VP2 proteins can be placed into one of three distinct categories due to their phylogenetic similarities to prototypical human strains: AU-1, DS-1, or Wa (Fig. 1A). Overall, the three types of VP2 proteins are highly conserved (>84% amino acid similarity). However, they show a surprising amount of variation in their amino termini (approximately residues 1 to 100), a domain of the protein thought to extend into the core and engage VP1/VP3/RNA (Fig. 1B and C). In particular, a stretch of amino acids directly following residue E11 is completely unique for each category of VP2 (<10% amino acid similarity) (Fig. 1C). For the Wa-like VP2 proteins, this region (Wa residues N12 to Q42) contains an asparagine-lysine-rich amino acid insertion that varies in length (Fig. 1C). In contrast to the amino termini, the residues of VP2 thought to make up the pseudo T=1 core shell (approximately residues 100 to 890) are quite similar among the three categories; still, several specific amino acid changes are seen. Residues comprising the shells of AU-1-like proteins show three amino acid changes (SA11 VP2 residues R154, A208, and V504) compared to DS-1-like VP2 (Fig. 1B). The shells of Wa-like VP2 proteins are more divergent and show 29 amino acid changes downstream of Wa residue K41, including an additional 2-amino-acid insertion (K345 and E346 of Wa VP2) (Fig. 1B). The functional significance of this observed variation is unknown, but it might reflect differences in protein and RNA interactions during rotavirus replication. It was presumed that one or more variable residues in the rotavirus inner capsid comprise the binding epitope of the VP2 SG-specific antibody YO-60.
FIG. 1.
Analysis of group A VP2 amino acid sequences. (A) Phylogenetic dendrogram of group A VP2 proteins. The dendrogram was constructed using the neighbor-joining method (1,000 bootstrap repetitions) and the Poisson correction parameter. The virus strain is shown in parenthesis following the species of isolation: Hu (human), Si (simian), Bo (bovine), and Av (avian). The group C rotavirus VP2 sequence (strain Bristol) was used as an outgroup. Brackets indicate branches that segregate with VP2 from the prototypical human strains (DS-1, AU-1, and Wa), which are used as references in the present study. All accession numbers are listed in Materials and Methods. (B) Schematic diagram of the three categories of group A VP2 proteins: DS-1-like, AU-1-like, and Wa-like. The black box represents the hypervariable region of the amino termini. Approximate amino acids are listed above each protein, and amino acid changes that are unique to Wa-like VP2 are shown as black lines. Three residues in the shell region of AU-1-like proteins that are not conserved for DS-1-like proteins are shown as white lines. (C) Alignment of the group A VP2 amino termini. The VP2 amino acid sequence of several representative virus strains is shown. Dashes indicate gaps in the protein sequence. Shading indicates the conservation of amino acid identity. Brackets indicate the VP2 category based on phylogenetics.
Three-dimensional locations of residues unique to Wa-like VP2 proteins.
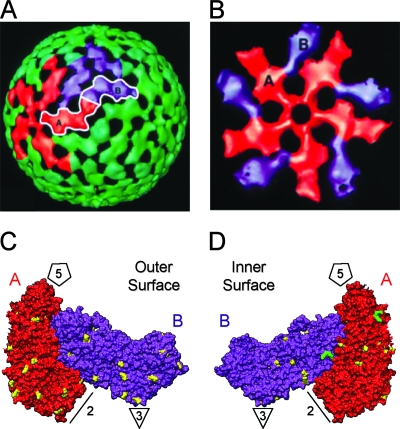
The inner capsid is an icosahedron composed of 120 subunits of VP2 arranged as 60 asymmetric dimers (Fig. 2A). Individual monomers within a dimer unit each adopt slightly different conformations (VP2-A and VP2-B), which allows them to interact and close the surface of the core (Fig. 2A). Around each of the 12 icosahedral fivefold axes, VP2-A and VP2-B are arranged into decamers similar to the petals of a cupped, inverted flower (Fig. 2B). Five copies of VP2-A cluster directly around the fivefold vertex, while five copies of VP2-B are situated farther away, interdigitated between the VP2-A subunits (Fig. 2B). A high-resolution crystal structure of bovine rotavirus DLPs shows that the overall fold of VP2 (VP2-A residues P100 to L880 and VP2-B residues E81 to L880) is very similar to what has been described for the inner capsid proteins of reovirus and bluetongue virus (λ1 and VP3, respectively) (9, 28; McClain et al., unpublished) (Fig. 2C and D). Like λ1 and VP3, the amino-terminal residues of bovine rotavirus VP2 (VP2-A residues M1 to E99 and VP2-B residues M1 to E80) are disordered and not visualized after icosahedral averaging. Nonetheless, we used this structure to predict the three-dimensional locations of residues unique to Wa-like VP2 proteins. Of the 29 changes downstream of Wa residue K41, 24 of them could be mapped onto the bovine VP2 dimer, while 5 reside in the disordered region of the protein (Fig. 2C and D). We found that these 24 residues are distributed throughout the structure, but most are located on the outer or inner surfaces of the protein. The location of the two-amino-acid insertion (K345 and E346 of Wa VP2) was predicted to be on the inner surface of the core, near the outer edge of each monomer (Fig. 2D).
FIG. 2.
Predicted three-dimensional locations of residues unique to Wa-like VP2. (A) Cryo-electron microscopic image reconstruction of the rotavirus inner capsid layer. The image was modified with permission from B. V. V. Prasad (Baylor University) (17) and shows the surface topology of the VP2 capsid. VP2-A monomers are shown in red and VP2-B monomers are shown in purple. (B) Arrangement of VP2-A and VP2-B around the fivefold axis. (C and D) Bovine rotavirus VP2-A-B dimer. A high-resolution structure of the VP2 component of bovine rotavirus DLPs was generously provided by Stephen Harrison (Harvard University). Locations of the five-, three-, and twofold axes are indicated and the VP2-A and VP2-B monomers are labeled. The image shown in panel C is of the outer surface of the dimer. The image shown in panel D was rotated 180° to view of the inner surface of the dimer. Residues of bovine VP2 corresponding to changes in Wa-like VP2 proteins are shown in yellow. The predicted location of the two-amino acid KE insertion is shown in green.
Correlation of VP2 sequence variation with YO-60 immunoreactivity.
The YO-60 monoclonal antibody was generated after immunization of mice using the human strain YO, which encodes a Wa-like VP2 (Fig. 1A). Of the few cell-culture-adapted rotavirus strains previously tested, those that were recognized by YO-60 represent strains that also encode Wa-like VP2 proteins, including the immunogen strain YO (31, 34). These data led us to hypothesize that Wa-like proteins represent VP2 SG-II and that one or more of the residues within Wa VP2 not conserved in AU-1-like and DS-1-like proteins makes up the YO-60 epitope. To test this hypothesis, VP2 proteins from various cell-culture-adapted rotavirus strains were assayed for the capacity to be recognized by YO-60 (Fig. 3). The viruses used in these experiments express VP2 proteins representing each of the three categories: AU-1-like (strain SA11), DS-1-like (strains DS-1 and 69M), and Wa-like (strains W161, P, and Wa). We found that neither YO-60 nor the group A-specific VP2 antibody 6E8 were able to bind VP2 in Western blots (data not shown). This result suggested that the epitopes for these antibodies require proper folding of the protein and, therefore, immunoprecipitation and immunofluorescence assays were used for subsequent experiments.
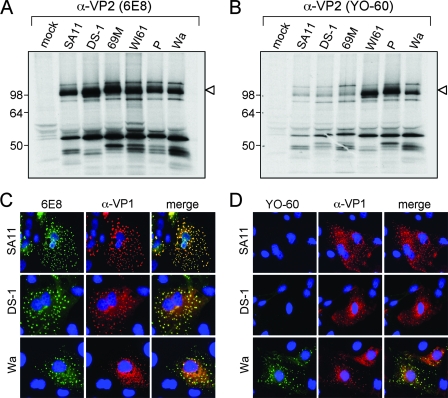
FIG. 3.
Immunoreactivity of 6E8 and YO-60 using rotavirus-infected cells. Mock-infected or rotavirus-infected MA104 cells were radiolabeled using [35S]methionine-cysteine from 3 to 8 h postinfection, and cytoplasmic extracts were immunoprecipitated using 6E8 (A) or YO-60 (B). Proteins were analyzed after SDS-PAGE and fluorography. The images were made from a 12-h exposure of the gels to film. Molecular mass markers (in kilodaltons) are shown on the left of the gels, and the location of VP2 is indicated by an arrowhead. Rotavirus-infected MA104 cells on glass coverslips were fixed at 8 h postinfection and subjected to indirect immunofluorescence using α-VP1 (red) and either 6E8 (C) or YO-60 (D) (green). Fluorescence was detected by using a Leica DM-IRE2 epifluorescence microscope with a 40× oil immersion objective lens. Colocalization of red and green pixels is shown as yellow pixels in the merged image. Cell nuclei are shown in blue. The virus strain used for infection is shown to the left of the image.
To determine whether YO-60 recognizes AU-1-like, DS-1-like, or Wa-like VP2 proteins, MA104 cells were either mock infected or infected with the different rotavirus strains, radiolabeled from 3 to 8 h postinfection using [35S]methionine-cysteine, and cytoplasmic extracts were subjected to immunoprecipitation (Fig. 3A and B). Using the group A-specific VP2 antibody 6E8, a 102-kDa protein (VP2) was immunoprecipitated from all infected cytoplasmic extracts (Fig. 3A). In contrast, VP2 was only strongly immunoprecipitated from cells infected with rotavirus strains WI61, P, and Wa using YO-60 (Fig. 3B). In extracts from cells infected with SA11, DS-1, and 69M a faint 102-kDa band was seen after YO-60 immunoprecipitation (Fig. 3B). However, this band was also detected after incubation of cytoplasmic extracts in the absence of antibody, but in the presence of protein A-Sepharose beads (data not shown). Several smaller-molecular-mass proteins were also immunoprecipitated by both antibodies and are predicted to be VP2-interacting viral proteins (such as VP6 and NSP2). Despite the presence of other bands, these results suggested that YO-60 strongly immunoreacts with Wa-like VP2 proteins. To verify these results, rotavirus-infected MA104 cells on glass coverslips were subjected to dual-label indirect immunofluorescence assays using 6E8 or YO-60 and guinea pig polyclonal VP1 antiserum (α-VP1) (Fig. 3C and D). Specific, punctate, fluorescence staining that colocalized with VP1 was detected in cells infected with SA11, DS-1, or Wa after incubation with 6E8 (Fig. 3C), suggesting that this antibody immunoreacts with VP2 from all of the viruses. In contrast, the YO-60 antibody only showed specific fluorescence staining that colocalized with VP1 in cells infected with the rotavirus strain Wa (Fig. 3D). The level of YO-60 fluorescence in cells infected with strains SA11 and DS-1 was indistinguishable from mock-infected cells (data not shown). This immunofluorescence result corroborates the results using immunoprecipitation and supports the hypothesis that Wa-like proteins represent VP2 SG-II, whereas AU-1-like and DS-1-like proteins comprise VP2 SG-I.
Truncation mutagenesis identifies regions of Wa VP2 required for 6E8 and YO-60 binding.
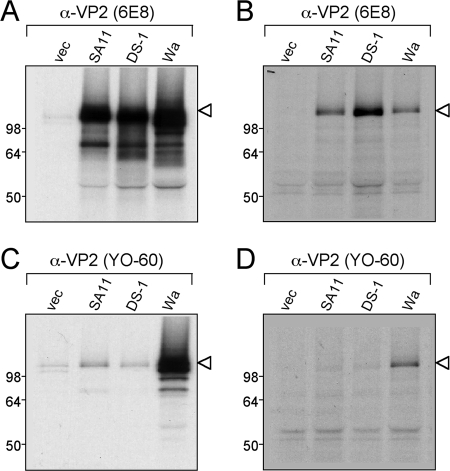
Having correlated VP2 SG specificity with sequence variation, we next sought to map the epitopes recognized by 6E8 and YO-60. Sequences encoding VP2 from rotavirus strains SA11 (VP2 SG-I), DS-1 (VP2 SG-I), and Wa (VP2 SG-II) were cloned into the pCI plasmid downstream from both a T7 promoter and a cytomegalovirus immediate-early promoter. This plasmid allows for the expression of recombinant proteins in coupled transcription-translation reactions in vitro and in mammalian cell culture. We first tested the capacity of the recombinant VP2 proteins to be recognized by the monoclonal antibodies (Fig. 4). Radiolabeled SA11, DS-1, and Wa VP2 proteins were expressed in either rabbit reticulocyte lysate or after transfection of HEK 293T cells and then subjected to immunoprecipitation using 6E8 or YO-60. The 6E8 antibody was capable of binding all three recombinant VP2 proteins (SA11, DS-1, and Wa) following expression in vitro or in cell culture (Fig. 4A and B). In contrast, using the YO-60 antibody, only Wa VP2 was immunoprecipitated from the reticulocyte or 293T cell lysates (Fig. 4C and D). Several smaller-molecular-weight proteins were immunoprecipitated from the reticulocyte lysate to various degrees using the VP2 antibodies. These bands most likely represent abortive VP2 translation products and are commonly seen using this system. Nonetheless, this result further confirmed that YO-60 specifically recognizes Wa-like VP2 proteins. Moreover, the fact that the recombinant VP2 proteins were still recognized as antigens demonstrated that they could be utilized for epitope-mapping studies. Due to the ease of protein expression using the reticulocyte lysate, this system was used for all subsequent experiments.
FIG. 4.
Expression and immunoprecipitation of recombinant VP2 proteins. Radiolabeled VP2 proteins were expressed from pCI vectors using rabbit reticulocyte lysate (A and C) or after transfection of HEK 293T cells (B and D). The lysates were subjected to immunoprecipitation using 6E8 (A and B) or YO-60 (C and D). Proteins were analyzed after SDS-PAGE and fluorography. The images were made from a 12-h exposure of the gels to film. Molecular mass markers (in kilodaltons) are shown on the left of the gels, and the location of recombinant VP2 is indicated with an arrowhead. The pCI vector with no insert (vec) was used as a control.
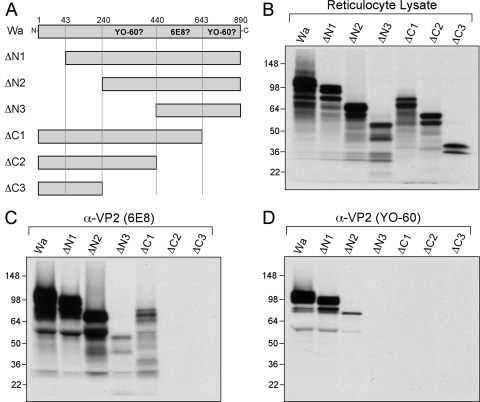
To define the region(s) of Wa VP2 required for interactions with either 6E8 or YO-60, pCI vectors were generated that encode amino- and carboxy-terminal truncation mutants (Fig. 5A). The Wa VP2 truncation mutants were made in vitro by using reticulocyte lysate and tested for the capacity to be recognized by 6E8 or YO-60 by using immunoprecipitation assays (Fig. 5B to D). All of the mutant proteins showed the correct molecular mass as determined by gel electrophoresis: full-length VP2 (102 kDa), ΔN1 (98 kDa), ΔN2 (75 kDa), ΔN3 (52 kDa), ΔC1 (75 kDa), ΔC2 (52 kDa), and ΔC3 (29 kDa) (Fig. 5B). After immunoprecipitation of the truncated proteins by the 6E8 antibody, ΔN1, ΔN2, ΔN3, and ΔC1 were detected, suggesting that residues A440 to N642 might contain the 6E8 epitope (Fig. 5C). YO-60 was only capable of immunoprecipitating ΔN1 and ΔN2 proteins (Fig. 5D). The result that ΔN1 was sufficiently recognized by YO-60 strongly indicates that the amino-terminal hypervariable residues do not make up the antibody epitope. In contrast, these results suggested that at least two distinct regions of Wa VP2 might be required for YO-60 recognition: (i) residues V240 to L439 and (ii) residues L643 to L890.
FIG. 5.
Wa VP2 truncation mutagenesis and immunoprecipitations. (A) Schematic diagram of Wa VP2 truncation mutants. The engineered deletions within Wa VP2 are illustrated with amino acid numbers listed above the full-length protein. Regions of Wa VP2 predicted to be required for 6E8 or YO-60 binding are indicated. (B) Expression of mutant proteins. The Wa VP2 truncation mutants were expressed using rabbit reticulocyte lysate in the presence of [35S]methionine. Proteins were analyzed after SDS-PAGE and fluorography. The images were made from a 12-h exposure of the gel to film. Molecular mass markers (in kilodaltons) are shown on the left of the gels. (C and D) Immunoprecipitations. Truncated Wa VP2 proteins were immunoprecipitated using either 6E8 (C) or YO-60 (D). Proteins were analyzed as described for panel B.
Wa VP2 residues A440 to T530 are sufficient for recognition by the 6E8 antibody.
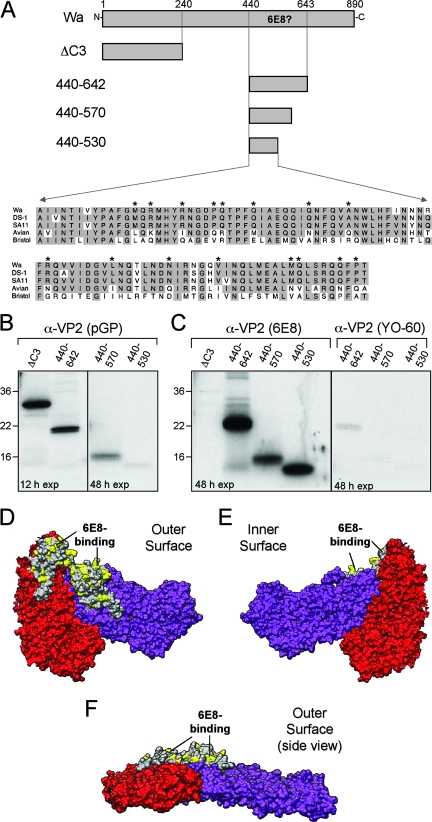
The 6E8 monoclonal antibody was isolated from a mouse immunized with the rotavirus strain Wa (31). 6E8 immunoreacts with most group A VP2 proteins, avian strains being the only known exception (31). The immunoprecipitation results with truncated forms of Wa VP2 indicated that 6E8 might bind to a region within Wa VP2 residues A440 to N642 (Fig. 5). To determine whether this region of the protein is sufficient for recognition by 6E8, it was expressed alone as a 23-kDa protein (protein 440-642) in reticulocyte lysate and assayed for immunoprecipitation (Fig. 6A to C). Also, to define a more minimal domain within Wa VP2 residues A440 to N642 that might encompass the 6E8 epitope, we generated two carboxy-terminal truncation mutants (Fig. 6A). As with 440-642, these two small-molecular-mass proteins, 440-570 (15 kDa) and 440-530 (10 kDa), were expressed in vitro and assayed by immunoprecipitation (Fig. 6A to C). The 29-kDa Wa VP2 mutant ΔC3, which lacks residues A440 to N642, served as a negative control for 6E8 specificity. Because the small-molecular-mass proteins contained few radiolabeled methionines, they were difficult to detect following direct analysis of the programmed reticulocyte lysate. Nonetheless, a guinea pig polyclonal VP2 antiserum (pGP) was capable of immunoprecipitating each of these proteins, showing that they were appropriately expressed in the lysates (Fig. 6B). As expected, 6E8 did not recognize ΔC3; however, this antibody did immunoprecipitate proteins 440-642, 440-570, and 440-530 (Fig. 6C). The recognition of these small-molecular-mass proteins by 6E8 was specific, since YO-60 did not bind 440-642, 440-570, or 440-530 (Fig. 6C). The result that 6E8 immunoprecipitated the 10-kDa fragment (i.e., 440-530) indicates that the epitope for this antibody resides entirely within Wa VP2 residues A440 to T530.
FIG. 6.
Identification of the 6E8-binding region within Wa VP2. (A) Schematic of Wa VP2 mutants and alignment of the 6E8-binding region. Amino acid numbers are listed above the Wa VP2 schematic, and the region predicted to be required for 6E8 binding is indicated. The amino acid sequences corresponding to Wa VP2 residues A440 to T530 are shown for several representative virus strains. Shaded residues are conserved among VP2 proteins. Asterisks represent residues that differ between VP2 proteins recognized by 6E8 (Wa, DS-1, and SA11) and those that are not recognized (Avian and Bristol). (B and C) Immunoprecipitations. Wa VP2 truncation mutants were expressed using rabbit reticulocyte lysate in the presence of [35S]methionine. Proteins were immunoprecipitated using either pGP (B), 6E8 (C), or YO-60 (C) and analyzed after SDS-PAGE and fluorography. The exposure time (in hours) of the gels to film is indicated. Molecular mass markers (in kilodaltons) are shown on the left of the gels. (D to F) Three-dimensional location of the 6E8-binding site. The residues of bovine VP2 corresponding to Wa VP2 residues A440 to T530 are shown in gray. Amino acids shown in yellow, which correspond to the asterisks in panel A, are possible sites of 6E8 interactions. The images show the outer surface (D), the inner surface (E), and a side view looking directly at the VP2-A twofold axis (F).
Having defined a specific region of Wa VP2 sufficient for recognition by 6E8, we next sought to determine the three-dimensional location of this epitope using the bovine VP2 structure (Fig. 6D to F). The 6E8-binding region (Wa VP2 residues A440 to T530) corresponds to bovine VP2 residues A430 to T520. This region forms a small, seemingly independent, domain that is proximal to the fivefold axis and protrudes away from the outer surface of VP2 (Fig. 6D to F). To identify residues within this domain that 6E8 might directly engage, we generated an amino acid sequence alignment corresponding to Wa VP2 residues A440 to T530 (Fig. 6A). The alignment shows 15 single-amino-acid changes between those VP2 proteins recognized by 6E8 (most group A strains) versus those from group A avian or group C rotavirus strains (Fig. 6A). Each of the nonconserved residues are surface exposed on the structured protein and thus available for antibody binding (Fig. 6D to F). Future mutagenesis studies individually targeting these 15 residues should reveal the 6E8-binding footprint.
Identification of a domain necessary for recognition by the YO-60 antibody.
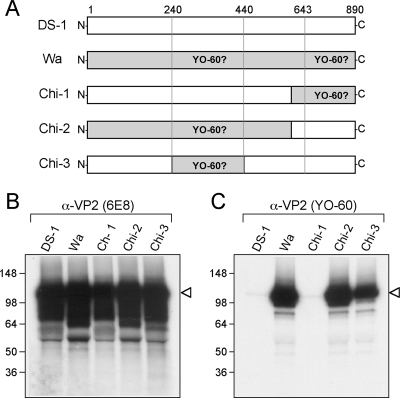
The results using truncated forms of Wa VP2 indicated that YO-60 might bind to two distinct regions of the protein: (i) residues V240 to L439 and (ii) residues L643 to L890. Alternatively, it is possible that deletion of either of these regions abrogates the YO-60 epitope by causing the protein to misfold. Therefore, to identify the region(s) of the properly folded protein bound by YO-60, we constructed full-length chimeric proteins between DS-1 VP2 (SG-I) and Wa VP2 (SG-II) (Fig. 7A). Chimeras 1 and 2 (Chi-1 and Chi-2, respectively) were designed to resolve which of the two regions identified by truncation mutagenesis are required for YO-60 recognition (Fig. 7A). As a control, the 6E8 antibody was used and shown to immunoprecipitate the wild-type (DS-1 and Wa) and chimeric VP2 proteins (Fig. 7B). However, the YO-60 antibody was only capable of immunoprecipitating Chi-2, but not Chi-1 (Fig. 7C). This result demonstrated that the carboxy-terminal residues L643 to L890 were not directly bound by YO-60 but instead were likely required for proper protein folding. Moreover, the result that Chi-2 was immunoprecipitated by YO-60 suggested that Wa VP2 residues V240 to L439 might indeed encompass the antibody recognition site. To test this prediction, chimera 3 (Chi-3) was constructed as a DS-1 VP2 that contains Wa residues V240 to L439 in place of DS-1 residues V231 to L428 (Fig. 7A). The results show that Chi-3 was immunoprecipitated by the control antibody 6E8, as well as by YO-60, confirming that this region of Wa VP2 contains residues specific to SG-II proteins (Fig. 7B and C).
FIG. 7.
Identification of the YO-60-binding region within Wa VP2. (A) Schematic diagram of Wa VP2 chimeric mutants. The diagram illustrates the engineered DS-1/Wa chimeric VP2 proteins. Amino acid numbers (corresponding to Wa VP2) are listed above the proteins. Regions of the protein predicted to be required YO-60 binding are labeled. The chimeric mutants were expressed using rabbit reticulocyte lysate in the presence of [35S]methionine and immunoprecipitated using either 6E8 (B) or YO-60 (C). Proteins were analyzed after SDS-PAGE and fluorography. The images were made from a 12-h exposure of the gels to film. Molecular mass markers (in kilodaltons) are shown on the left of the gels, and the location of VP2 is indicated with an arrowhead.
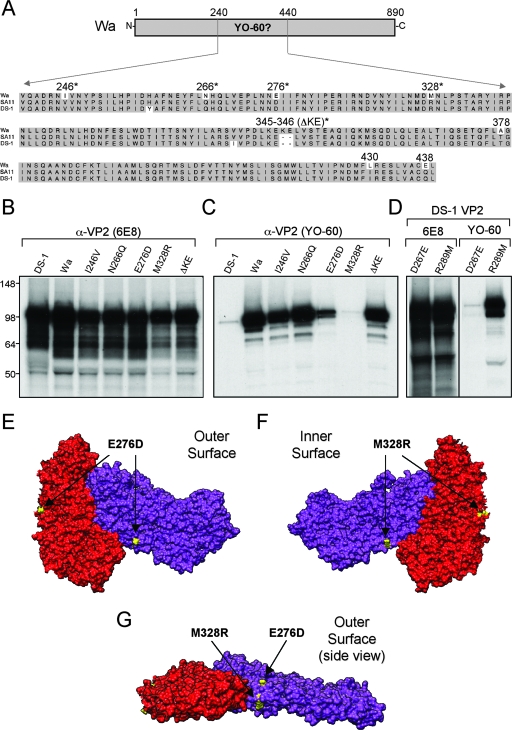
The YO-60 epitope contains Wa VP2 residue M328.
To identify the precise residues necessary for YO-60-binding, we generated an amino acid sequence alignment of the region corresponding to Wa VP2 residues V240 to L439 using several group A VP2 sequences (Fig. 8A). The alignment showed seven single-amino-acid changes between VP2 SG-I and SG-II proteins (Wa residues I246, N266, E276, M328, A378, L430, and E438), as well as a two-amino-acid insertion seen in VP2 SG-II (Wa residues K345 and E346) (Fig. 8A). Of these changes, several were predicted to be surface-exposed residues (I246, N266, E276, M328, K345, and E346) and available for antibody binding (Fig. 8A). To delineate which of these amino acids contributed to YO-60 immunoreactivity, each exposed residue was singly changed in Wa VP2 to match the corresponding residue in DS-1 VP2. To assay the influence of the K345-E346 insertion, both of these residues were deleted in a single mutant (ΔKE). We predicted that mutation of any residue in the VP2 SG-II-specific YO-60 epitope would result in the abolishment of antibody binding. The mutant proteins were expressed in vitro using reticulocyte lysate and assayed for antibody recognition by immunoprecipitation (Fig. 8B and C). As expected, the control 6E8 antibody immunoprecipitated the wild-type VP2 proteins (DS-1 and Wa), as well as each of the Wa VP2 point mutants (Fig. 8B). However, YO-60 was only capable of immunoprecipitating wild-type Wa VP2, the point mutants I246V, N266Q, and ΔKE and, to a lesser extent, mutant E276D (Fig. 8C). The lack of immunoreactivity against M328R indicated that this residue is a component of the YO-60 epitope, whereas the observation that YO-60 bound less efficiently to E276D suggested that this residue influences the recognition of M328. To determine whether either the identified glutamic acid or methionine residue is sufficient for YO-60 binding in the context of a VP2 SG-I protein, we generated two DS-1 VP2 point mutants (DS-1 mutants D267E and R289M) and assayed the in vitro-translated proteins by immunoprecipitation (Fig. 8D). The results show that YO-60 gained the capacity to recognize DS-1 VP2 following the R289M mutation but not following the D267E mutation (Fig. 8D). These results demonstrate that the methionine residue at position 328 in Wa VP2, which is an arginine in all VP2 SG-I proteins, is a critical molecular determinant of VP2 SG-II specificity.
FIG. 8.
Point mutagenesis of Wa VP2 and YO-60 immunoreactivity. (A) Alignment of the YO-60-binding region from group A VP2 proteins. The amino acid sequences of the region corresponding to Wa VP2 residues V240 to L439 are shown for several representative virus strains. Dashes indicate gaps in the protein sequence. Shaded residues are conserved between SG-I and SG-II VP2 proteins. The numbers correspond to Wa VP2 residues that are not conserved in SG-I. Asterisks indicate residues that are predicted to be surface exposed. (B and C) Expression and immunoprecipitation of Wa VP2 point mutants. Proteins were expressed using rabbit reticulocyte lysate in the presence of [35S]methionine and immunoprecipitated using either 6E8 (B) or YO-60 (C). Proteins were analyzed after SDS-PAGE and fluorography. The images were made from a 12-h exposure of the gels to film. Molecular mass markers (in kilodaltons) are shown on the left of the gels. (D) Expression and immunoprecipitation of DS-1 VP2 point mutants. Proteins were analyzed as described for panel B and C. (E to G) Three-dimensional location of the YO-60-binding site. The residues of bovine VP2 corresponding to Wa VP2 residues E276 and M328 are shown in yellow. The images show the outer surface (E), the inner surface (F), and a side view looking directly at the VP2-A twofold axis (G).
The three-dimensional location of Wa VP2 residues E276 and M328 (bovine VP2 residues D268 and R290, respectively) were predicted by using the known structure (Fig. 8E to G). Both residues are exposed on the VP2 dimer; however, they are located on opposite faces of the protein. Residue E276 localizes to the outer surface of VP2, near the left edge of each monomer (Fig. 8E). In contrast, M328 localizes to the inward-facing surface near the two- and threefold axes (Fig. 8F). Despite residing on alternate faces of VP2, E276 and M328 are predicted to be very close together on the side of each monomer (Fig. 8G).
DISCUSSION
In this study, we correlated group A rotavirus VP2 sequence variation with the immunoreactivity pattern of two non-neutralizing monoclonal antibodies. We found that the 6E8 monoclonal antibody is capable of binding all tested group A VP2 proteins. In contrast, the YO-60 monoclonal antibody only binds to proteins similar in sequence to Wa VP2 but not to other group A VP2 proteins (DS-1-like or AU-1-like). To investigate the residues within Wa VP2 required for interactions with 6E8 or YO-60, a mutagenesis approach was used. We show that a 90-amino-acid region (Wa residues A440 to T530), which is predicted to protrude from the surface of VP2 near the fivefold axis, is sufficient for 6E8 binding. The YO-60 epitope contains Wa VP2 residue M328 and is thought to be located on the inner surface of the structured protein, spatially distinct from the 6E8-binding site. Together, these results clarify the molecular basis for the previously described VP2 SGs (31, 34). Moreover, the present study demonstrates the genetic and antigenic variation that exists among group A rotavirus VP2 proteins and raises several important questions regarding rotavirus replication and classification.
Does sequence variation reflect differences in VP2 interactions?
Group A VP2 proteins have a high level of overall sequence homology; however, proteins from even closely related strains show marked variation in their amino termini. The first ∼100 residues of VP2 are predicted to lie inside the core shell and to bind the viral enzyme-RNA complex (VP1/VP3/RNA) (17, 27). These interactions are thought to serve two functional roles during replication: (i) tethering the enzymes and RNA to the inner surface during particle assembly and (ii) triggering VP1 to catalyze viral RNA synthesis. The dramatic sequence variation within this important region of the protein might reflect differences in protein-protein or protein-RNA interactions and RNA synthesis for these viruses. In support of this idea, it has been reported that rotavirus strains UK and Wa exhibit different in vitro transcription levels (8). Because UK and Wa show sequence variations in other capsid proteins in addition to VP2, the reason for the disparity in mRNA synthesis is not fully understood. The results from ongoing and future studies in our lab using recombinant proteins will determine whether AU-1-like, DS-1-like, and Wa-like VP2 exhibit differences in amino-terminal protein or RNA interactions and/or in viral RNA synthesis.
In contrast to the amino termini, the residues of VP2 that are predicted to form the pseudo T=1 icosahedral core shell are more conserved. During virion assembly, trimers of VP6 would engage outer surface-exposed VP2 amino acids, encapsulating the core and forming a DLP. The structural location and organization of VP6 trimers around the fivefold axis is slightly different between VP6 SG-I and SG-II rotavirus strains (8). It is feasible that, like VP6, the structure of the core shell varies for VP2 SG-I and SG-II proteins. Moreover, the VP2 amino acids involved in interactions with VP6 have not yet been determined. It is possible that the changes seen between the shell regions of VP2 SG-I and SG-II proteins result in slight differences in VP6 interactions. If this is the case, one might expect the genes encoding these SG antigens to be evolutionarily linked and to segregate together in nature. However, rotavirus strains that exhibit unlinked VP2 and VP6 antigens have been described (31, 34). For example, the porcine rotavirus strain OSU is classified as VP2 SG-II and VP6 SG-I, demonstrating a lack of absolute cosegregation of these antigens (31). Nevertheless, most of the rotavirus strains classified as VP6 SG-I encode either DS-1-like or AU-1-like VP2 proteins (VP2 SG-I). In the same manner, the majority of strains that bear VP6 SG-II epitopes encode Wa-like VP2 proteins (VP2 SG-II). This observation is consistent with the hypothesis that variability among group A rotavirus VP2 proteins might be mirrored in viral proteins that interact with the core shell. The recent systematic analysis of several rotavirus genome sequences supports this hypothesis and suggests that genes encoding the components of the DLP (VP1, VP2, VP3, and VP6) and the virus replication machinery (NSP2, NSP4, and NSP5) tend to segregate together (19; Heiman et al., unpublished). This is in contrast to the genes encoding the outer capsid proteins (VP4 and VP7), which appear to exchange readily. Certainly, further investigation into the possible genetic linkage between sets of rotavirus genes is warranted.
How are the 6E8 and YO-60 epitopes presented in the context of the icosahedral core?
The results of the 6E8 and YO-60 epitope-mapping studies reveal insights into the possible secondary and higher-ordered structure of VP2. We show that 6E8 is capable of recognizing a 23-kDa fragment of Wa VP2 comprised of residues A440 to T530. This result suggests that the domain containing residues A440 to T530 folds independently from the rest of the protein and presents the 6E8 epitope in the correct context. The 6E8-binding region is hypothesized to protrude from the outer surface of each structured VP2 monomer, near the icosahedral fivefold axis. Because this domain does not seem to form contacts between neighboring VP2 subunits in an A-B dimer, the antibody most likely binds to a monovalent site within a single VP2 monomer. Therefore, it is expected that VP2 core assembly is not required for 6E8-binding, but that this antibody likely immunoreacts with all VP2 assembly intermediates.
Unlike the 6E8 epitope, the YO-60 epitope is putatively located on the inner surface of each structured VP2 monomer, very near the two- and threefold axes. This location was surprising and suggests that the antibody might not be capable of binding to a closed core shell. The original report describing the generation of YO-60 indicates that this antibody is capable of agglutinating DLPs (historically referred to as single-shelled particles) (34). Although it is possible that the methionine at position 328 is exposed in the higher-ordered structure of a Wa DLP, our data suggest that this residue is probably buried within the core particle. Moreover, the three-dimensional context by which M328 is presented seems very important for YO-60 recognition. Truncation mutagenesis of the protein outside of the YO-60-binding region (Wa VP2 residues L643 to L890) abolished antibody interaction. Most likely, the deletion caused the protein to misfold, abrogating the antibody-antigen interaction. Furthermore, mutation of Wa VP2 residue 276 from an aspartic acid to a glutamic acid weakened the immunoprecipitation by YO-60. Residue D276 is predicted to be located very close to M328 in structured VP2, suggesting that replacement with a larger glutamic acid might have sterically hindered YO-60 from accessing its epitope.
VP2 antibody-binding sites are similar to previously characterized VP6 SG epitopes, which are also are dependent upon proper protein conformation. The epitope for the VP6 SG-I antibody (255/60) includes residues A172, A305, and R296 to N299 and spans two monomers of a VP6 trimer unit (8, 18, 32). The epitope for the VP6 SG-II antibody (631/9) includes residues A305, A306, and G315, which are all present on a single chain of the trimerized protein. Like what is seen for YO-60, mutagenesis of VP6 residues surrounding the binding sites for 255/60 or 631/9 hinders the recognition by these antibodies. The fact that second-site changes can indirectly reduce antibody recognition supports the use of sequence analyses, in addition to antibody binding studies, when characterizing group A rotaviruses.
What is the distribution of rotaviruses encoding AU-1-like, DS-1-like, or Wa-like VP2 proteins?
The results from the present study corroborate previous reports that used YO-60 to classify rotavirus strains into SGs (31, 34). Together, the available data suggest that VP2 SG-II contains solely human and porcine strains, whereas other human and animal strains (such as simian, bovine, lapine, feline, and canine) can be classified as VP2 SG-I (31, 34). The phylogenetic dendrogram of VP2 shows branchings that segregate with rotavirus genogroups, a distinction that was originally based on RNA hybridization using prototypic virus strains (AU-1, DS-1, and Wa) (21). The relationship among VP2 proteins is consistent with them sharing common evolutionary lineages with the genogroup strains. Connections between VP2 sequence variation and rotavirus serotype are less clear; however, some overall trends can be seen. Specifically, the VP2 SG-II proteins classified thus far, either by sequence analysis or by YO-60 immunoreactivity, belong to serotypes G1, G3, G4, G5, G9, and G12. VP2 SG-I proteins belong to rotavirus serotypes G2, G3, G6, G8, G10, and G12. All G1 serotype viruses we have analyzed encode Wa-like VP2 proteins, whereas all G2 serotype viruses seem to encode DS-1-like VP2 proteins. Serotype G3 rotavirus strains are the most ubiquitous, encoding VP2 proteins that represent each of the three categories (AU-1-like, DS-1-like, and Wa-like). Given that G1, G2, G3, and G4 are the main serotypes currently circulating in nature, it is likely that rotaviruses encoding all three categories of VP2 proteins are prominent (3). However, with the exception of strains AU-1 and T152, all human group A rotaviruses of which sequences are available encode either DS-1-like or Wa-like VP2 proteins. More detailed molecular surveillance studies need to be performed to study the transmission dynamics of rotaviruses encoding different types of VP2 proteins. The results presented here are expected to create a foundation for such epidemiological studies. Equally, our findings set the stage for future experiments aimed at understanding the impact of VP2 variation on rotavirus replication.
Acknowledgments
We thank Stephen Harrison and Ethan Settembre for structural insights into the locations of the 6E8 and YO-60 epitopes and Koki Taniguchi for generously sharing the YO-60 antibody. We also thank Zenobia Taraporewala, Hongyan Yang, Al Kapikian, Taka Hoshino, Jelle Matthijnssens, and Phil Dormitzer for advice and critical reading of the manuscript.
This study was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases.
Footnotes
Published ahead of print on 23 January 2008.
REFERENCES
- 1.Birch, C. J., R. L. Heath, and I. D. Gust. 1988. Use of serotype-specific monoclonal antibodies to study the epidemiology of rotavirus infection. J. Med. Virol. 2445-53. [DOI] [PubMed] [Google Scholar]
- 2.Coulson, B. S., L. E. Unicomb, G. A. Pitson, and R. F. Bishop. 1987. Simple and specific enzyme immunoassay using monoclonal antibodies for serotyping human rotaviruses. J. Clin. Microbiol. 25509-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desselberger, U., M. Iturriza-Gomara, and J. J. Gray. Rotavirus epidemiology and surveillance. Novartis Found. Symp. 238125-152. [DOI] [PubMed]
- 4.Estes, M. K. 2001. Rotaviruses and their replication, p. 1747-1785. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott/The Williams & Wilkins Co., Philadelphia, PA.
- 5.Gerna, G., M. Torsellini, N. Passarani, M. Battaglia, E. Percivalle, A. Sarasini, D. Torre, and P. Ferrante. 1984. Subgrouping of human rotavirus strains by complement fixation, indirect double-antibody sandwich enzyme-linked immunosorbent assay, and solid-phase immune electron microscopy. Arch. Virol. 81193-203. [DOI] [PubMed] [Google Scholar]
- 6.Gray, J., and U. Desselberger. 2000. Rotaviruses: methods and protocols. Humana Press, Totowa, NJ.
- 7.Greenberg, H., V. McAuliffe, J. Valdesuso, R. Wyatt, J. Flores, A. Kalica, Y. Hoshino, and N. Singh. 1983. Serological analysis of the subgroup protein of rotavirus, using monoclonal antibodies. Infect. Immun. 3991-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greig, S. L., J. A. Berriman, J. A. O'Brien, J. A. Taylor, A. R. Bellamy, M. J. Yeager, and A. K. Mitra. 2006. Structural determinants of rotavirus subgroup specificity mapped by cryo-electron microscopy. J. Mol. Biol. 356209-221. [DOI] [PubMed] [Google Scholar]
- 9.Grimes, J. M., J. N. Burroughs, P. Gouet, J. M. Diprose, R. Malby, S. Zientara, P. P. Mertens, and D. I. Stuart. 1998. The atomic structure of the bluetongue virus core. Nature 395470-478. [DOI] [PubMed] [Google Scholar]
- 10.Hoshino, Y., M. Gorziglia, J. Valdesuso, J. Askaa, R. I. Glass, and A. Z. Kapikian. 1987. An equine rotavirus (FI-14 strain) which bears both subgroup I and subgroup II specificities on its VP6. Virology 157488-496. [DOI] [PubMed] [Google Scholar]
- 11.Hoshino, Y., M. M. Sereno, K. Midthun, J. Flores, A. Z. Kapikian, and R. M. Chanock. 1985. Independent segregation of two antigenic specificities (VP3 and VP7) involved in neutralization of rotavirus infectivity. Proc. Natl. Acad. Sci. USA 828701-8704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalica, A. R., H. B. Greenberg, R. G. Wyatt, J. Flores, M. M. Sereno, A. Z. Kapikian, and R. M. Chanock. 1981. Genes of human (strain Wa) and bovine (strain UK) rotaviruses that code for neutralization and subgroup antigens. Virology 112385-390. [DOI] [PubMed] [Google Scholar]
- 13.Kapikian, A. Z., W. L. Cline, H. B. Greenberg, R. G. Wyatt, A. R. Kalica, C. E. Banks, H. D. James, Jr., J. Flores, and R. M. Chanock. 1981. Antigenic characterization of human and animal rotaviruses by immune adherence hemagglutination assay (IAHA): evidence for distinctness of IAHA and neutralization antigens. Infect. Immun. 33415-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kapikian, A. Z., Y. Hoshino, and R. M. Chanock. 2001. Rotaviruses, p. 1787-1833. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott/The Williams & Wilkins Co., Philadelphia, PA.
- 15.Labbe, M., P. Baudoux, A. Charpilienne, D. Poncet, and J. Cohen. 1994. Identification of the nucleic acid binding domain of the rotavirus VP2 protein. J. Gen. Virol. 75(Pt. 12)3423-3430. [DOI] [PubMed] [Google Scholar]
- 16.Lambert, J. P., P. Marbehant, D. Marissens, and G. Zissis. 1984. Monoclonal antibodies directed against different antigenic determinants of rotavirus. J. Virol. 5147-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lawton, J. A., C. Q. Zeng, S. K. Mukherjee, J. Cohen, M. K. Estes, and B. V. Prasad. 1997. Three-dimensional structural analysis of recombinant rotavirus-like particles with intact and amino-terminal-deleted VP2: implications for the architecture of the VP2 capsid layer. J. Virol. 717353-7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez, S., R. Espinosa, H. B. Greenberg, and C. F. Arias. 1994. Mapping the subgroup epitopes of rotavirus protein VP6. Virology 204153-162. [DOI] [PubMed] [Google Scholar]
- 19.Matthijnssens, J., M. Ciarlet, F. Heiman, I. Arijs, T. Delbeke, S. M. McDonald, E. A. Palombo, M. Iturriza-Gomara, P. Maes, J. T. Patton, M. Rahman, and M. Van Ranst. Full genome-based classification of rotaviruses reveals common origin between human Wa-like and porcine rotavirus strains and human DS-1-like and bovine rotavirus strains. J. Virol., in press. [DOI] [PMC free article] [PubMed]
- 20.Nakagomi, O., T. Kutsuzawa-Nakagomi, H. Oyamada, T. Suto, and A. Ochi. 1984. Enzyme immunoassay for subgrouping human rotaviruses using monoclonal antibodies. Tohoku J. Exp. Med. 144105-106. [DOI] [PubMed] [Google Scholar]
- 21.Nakagomi, O., T. Nakagomi, K. Akatani, and N. Ikegami. 1989. Identification of rotavirus genogroups by RNA-RNA hybridization. Mol. Cell. Probes 3251-261. [DOI] [PubMed] [Google Scholar]
- 22.Parashar, U. D., E. G. Hummelman, J. S. Bresee, M. A. Miller, and R. I. Glass. 2003. Global illness and deaths caused by rotavirus disease in children. Emerg. Infect. Dis. 9565-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patton, J. T. 1996. Rotavirus VP1 alone specifically binds to the 3′ end of viral mRNA, but the interaction is not sufficient to initiate minus-strand synthesis. J. Virol. 707940-7947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patton, J. T., M. T. Jones, A. N. Kalbach, Y. W. He, and J. Xiaobo. 1997. Rotavirus RNA polymerase requires the core shell protein to synthesize the double-stranded RNA genome. J. Virol. 719618-9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pesavento, J. B., S. E. Crawford, M. K. Estes, and B. V. Prasad. 2006. Rotavirus proteins: structure and assembly. Curr. Top. Microbiol. Immunol. 309189-219. [DOI] [PubMed] [Google Scholar]
- 26.Petterson, E. F., T. D. Goddard, C. C. Huang, G. S. Couch, D. M. Greenblatt, E. C. Meng, and T. E. Ferrin. 2004. UCSF Chimera: a visualization system for exploratory research and analysis. J. Comp. Chem. 251605-1612. [DOI] [PubMed] [Google Scholar]
- 27.Prasad, B. V., R. Rothnagel, C. Q. Zeng, J. Jakana, J. A. Lawton, W. Chiu, and M. K. Estes. 1996. Visualization of ordered genomic RNA and localization of transcriptional complexes in rotavirus. Nature 382471-473. [DOI] [PubMed] [Google Scholar]
- 28.Reinisch, K. M., M. L. Nibert, and S. C. Harrison. 2000. Structure of the reovirus core at 3.6 Å resolution. Nature 404960-967. [DOI] [PubMed] [Google Scholar]
- 29.Silvestri, L. S., Z. F. Taraporewala, and J. T. Patton. 2004. Rotavirus replication: plus-sense templates for double-stranded RNA synthesis are made in viroplasms. J. Virol. 787763-7774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Svensson, L., L. Grahnquist, C. A. Pettersson, M. Grandien, G. Stintzing, and H. B. Greenberg. 1988. Detection of human rotaviruses which do not react with subgroup I- and II-specific monoclonal antibodies. J. Clin. Microbiol. 261238-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Svensson, L., L. Padilla-Noriega, K. Taniguchi, and H. B. Greenberg. 1990. Lack of cosegregation of the subgroup II antigens on genes 2 and 6 in porcine rotaviruses. J. Virol. 64411-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang, B., J. M. Gilbert, S. M. Matsui, and H. B. Greenberg. 1997. Comparison of the rotavirus gene 6 from different species by sequence analysis and localization of subgroup-specific epitopes using site-directed mutagenesis. Virology 23789-96. [DOI] [PubMed] [Google Scholar]
- 33.Taniguchi, K., T. Urasawa, Y. Morita, H. B. Greenberg, and S. Urasawa. 1987. Direct serotyping of human rotavirus in stools by an enzyme-linked immunosorbent assay using serotype 1-, 2-, 3-, and 4-specific monoclonal antibodies to VP7. J. Infect. Dis. 1551159-1166. [DOI] [PubMed] [Google Scholar]
- 34.Taniguchi, K., T. Urasawa, and S. Urasawa. 1986. Reactivity patterns to human rotavirus strains of a monoclonal antibody against VP2, a component of the inner capsid of rotavirus. Arch. Virol. 87135-141. [DOI] [PubMed] [Google Scholar]
- 35.Taniguchi, K., T. Urasawa, S. Urasawa, and T. Yasuhara. 1984. Production of subgroup-specific monoclonal antibodies against human rotaviruses and their application to an enzyme-linked immunosorbent assay for subgroup determination. J. Med. Virol. 14115-125. [DOI] [PubMed] [Google Scholar]
- 36.Tortorici, M. A., T. J. Broering, M. L. Nibert, and J. T. Patton. 2003. Template recognition and formation of initiation complexes by the replicase of a segmented double-stranded RNA virus. J. Biol. Chem. 27832673-32682. [DOI] [PubMed] [Google Scholar]
- 37.Zeng, C. Q., M. K. Estes, A. Charpilienne, and J. Cohen. 1998. The N terminus of rotavirus VP2 is necessary for encapsidation of VP1 and VP3. J. Virol. 72201-208. [DOI] [PMC free article] [PubMed] [Google Scholar]