Abstract
We have analyzed the intracellular transport of the spike (S) protein of infectious bronchitis virus (IBV), an avian coronavirus. Surface expression was analyzed by immunofluorescence microscopy, by surface biotinylation, and by syncytium formation by S-expressing cells. By applying these methods, the S protein was found to be retained intracellularly. Tyr1143 in the cytoplasmic tail was shown to be a crucial component of the retention signal. Deletion of a dilysine motif that has previously been suggested to function as a retrieval signal did not abolish intracellular retention. Treatment of the S proteins with endoglycosidases did not reveal any differences between the parental and the mutant proteins. Furthermore, all S proteins analyzed were posttranslationally cleaved into the subunits S1 and S2. In coexpression experiments, the S protein was found to colocalize with a Golgi marker. Taken together, these results indicate that the S protein of IBV is retained at a late Golgi compartment. Therefore, this viral surface protein differs from the S proteins of transmissible gastroenteritis virus and severe acute respiratory syndrome coronavirus, which are retained at a pre-Golgi compartment or transported to the cell surface, respectively. The implications of these differences are discussed.
Coronaviruses are enveloped viruses that obtain their membranes during the budding process at intracellular compartments. The virions are assembled at the endoplasmic reticulum (ER)-Golgi intermediate compartment (ERGIC) (12, 14). Therefore, the four structural proteins S, M, E, and N have to be located at the ERGIC membranes to allow coordinated budding. The spike (S) protein of coronaviruses is the major inducer of neutralizing antibodies and is responsible for virus binding to target cells. Recently we have demonstrated that sialic acid serves as a receptor determinant for IBV (15). The IBV S protein also has fusion activity and is cleaved into the subunits S1 and S2 at a multibasic cleavage site that is sensitive to furin or furin-like proteases (4).
It has been reported that the coronavirus M and E proteins are retained intracellularly when they are expressed in eukaryotic cells (5, 7). Intracellular retention has also been demonstrated for the S protein of a porcine coronavirus, transmissible gastroenteritis virus (TGEV). A YxxI motif in the cytoplasmic tail is responsible for the retention of the S protein of TGEV at a pre-Golgi compartment (13). Similar tyrosine-containing tetrapeptides are present in the cytoplasmic tails of many coronavirus S proteins. The infectious bronchitis virus (IBV) S protein also contains such a motif. However, Lontok and coworkers reported that a dilysine motif is responsible for the intracellular retention of this viral surface protein (8).
The aim of this study was to analyze the trafficking of the IBV S protein in more detail. We demonstrate that Tyr1143 is crucial for the intracellular retention of the S protein. By contrast, deletion of the dilysine did not result in surface expression of the S protein.
MATERIALS AND METHODS
Cells.
BHK-21 cells (German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany) were incubated in Eagle minimal essential medium supplemented with 3% fetal calf serum and 1% nonessential amino acids. Primary chicken fibroblasts were prepared from 10-day-old specific-pathogen-free chickens (Lohmann, Cuxhaven, Germany) and were incubated in medium 199 supplemented with 3% fetal calf serum.
Transient transfection of eukaryotic cells.
BHK-21 cells and primary chicken fibroblasts were transfected 24 h after seeding. If not otherwise indicated, 1 μg DNA/well was used in a 24-well plate and 4 μg DNA/well was used in a 6-well plate. For the colocalization experiments, 1 μg IBV wild-type S [S(WT)] DNA together with 0.5 μg marker DNA were cotransfected. Transfections were performed with the Lipofectamine 2000 transfection reagent (Invitrogen, Karlsruhe, Germany) following the manufacturer's instructions.
Plasmids.
The cDNA of the IBV S protein, derived from an apathogenic IBV strain (Beaudette CK, accession number AJ311317), was kindly provided by Dave Cavanagh. It was amplified by PCR and cloned into the pCG1 vector using the BamH1 restriction site. For easier mutagenesis, an AatII restriction site was inserted at nucleotide 3000 by changing the triplets GAT and GTA into GAC and CTC using primers 4 and 5 (Table 1). Deletion mutants were generated and point mutations were introduced by using the primers indicated in Fig. 1. All constructs were sequenced to exclude any undesired sequence changes. For colocalization studies, cells were transfected with cDNA of green fluorescent protein (GFP)-tagged marker proteins. The pER-EGFP DNA (marker for the ER) was kindly provided by Frank van Kuppefeld, Nijmegen, The Netherlands; pEGFP-Golgi DNA was kindly provided by Eric Snijder, Leiden, The Netherlands; and pERGIC-GFP was constructed by inserting the GFP open reading frame behind the signal peptide of the ERGIC p53 DNA (ERGIC p53 was kindly provided by H.-P. Hauri, Basel, Switzerland).
TABLE 1.
Primers used for plasmid construction
| Primer no.a | Sequence (5′→3′) |
|---|---|
| 1 | CGC CGC GGA TCC ATG TTG GTA ACA CCT CTT TTA CTA GTG ACT |
| 2 | TTT GGA TCC TCA AAC AGA CTT TTT AGG TCT GTA TTG TTC |
| 3 | TTT GGA TCC TCA TTC AGT TAC CAC ATC GTT ATC AAA AGT CGT |
| 4 | CTG CAG GAG ACG TCG TTA CGC TTA CT |
| 5 | TAA GCG TAA CGA CGT CTC CTG CAG TA |
| 6 | GCC CGC TAA TGG TAG GGG TA |
| 7 | TTT GGA TCC TCA ACC ACA CTT ACT CAT TAG AGG CAT AAT GCC |
| 8 | GAA ATC TTC TGC TGC CAC GAC TTT TGA |
| 9 | AAA AGT CGT GGC AGC AGA AGA TTT CTT |
| 10 | GAA ATC TTC TGC TTA CAC GAC TTT T |
| 11 | AGT CGT GTA AGC AGA AGA TTT CTT |
| 12 | ATC TTC TTA TGC CAC GAC TTT TGA T |
| 13 | AAA AGT CGT GGC ATA AGA AGA TTT |
The primer numbers correspond to the numbers shown in Fig. 1.
FIG. 1.
Schematic drawing of mutant IBV S proteins. Numbers above arrows indicate the primers used for mutant construction (Table 1).
Immunofluorescence analysis.
Cells grown on coverslips were transfected with the indicated DNA. At 12 h posttransfection, the cells were fixed with 3% paraformaldehyde for 20 min at room temperature. After an incubation with 0.1 M glycine for 5 min, cells were permeabilized by treatment with 0.2% Triton X-100 for 5 min to allow intracellular staining. For detection of viral antigen, the samples were incubated with the monoclonal antibody Ch/IBV 26.1 directed against the S2 protein of IBV (ID Lelystad, The Netherlands) and an appropriate dye-labeled second antibody. If not otherwise indicated, antibodies were incubated with the cells for 1 h at room temperature, followed by three washing steps with phosphate-buffered saline. Nuclei were stained by incubating cells with DAPI (4′,6′-diamidino-2-phenylindole) stain. Fluorescence microscopy was performed with a Leica 2 inverted confocal microscope.
Surface biotinylation and immunoprecipitation.
Cells grown on coverslips were transfected with the different IBV S constructs. After 24 h, the surface proteins were biotinylated by incubation with the N-hydroxy-succinimide ester of biotin (0.5 mg/ml phosphate-buffered saline; Pierce), and cells were lysed with NP-40 lysis buffer. The lysates were immunoprecipitated with monoclonal anti-S antibody Ch/IBV 26.1 overnight as described by Zimmer et al. (17). The precipitated surface proteins were separated by electrophoresis on 8% sodium dodecyl sulfate (SDS)-polyacrylamide gels and blotted the onto nitrocellulose. The proteins were visualized by incubating the membrane with streptavidin-peroxidase.
Glycosidase digestion.
Lysates of S-expressing cells were incubated overnight with 100 μl concanavalin A-agarose (Calbiochem) to pull down the S protein. The pelleted proteins were digested with 2 μl endoglycosidase H for 1 h at 37°C with buffers provided by the manufacturer (New England Biolabs) and then separated by 8% SDS-polyacrylamide gel electrophoresis. Following transfer to nitrocellulose membranes, the S protein was stained with polyclonal anti-IBV serum raised in specific-pathogen-free rabbits.
Antibody uptake assay.
BHK-21 cells grown on coverslips were transfected with the indicated DNA. After 24 h, the cells were incubated on ice at 4°C with anti-Beaudette serum for 1 h. Half of the coverslips were then incubated with prewarmed medium at 37°C for 1 h to allow endocytosis of protein-antibody complexes. The incubation with anti-rabbit Cy3-linked antibody was performed with all coverslips on ice for 1 h. This was followed by fixation of the cells with methanol-acetone for 1 min and staining with fluorescein isothiocyanate-labeled donkey anti-rabbit antibody for 1 h. Cells were analyzed with a Leica 2 inverted confocal microscope.
RESULTS
A tyrosine-based signal is responsible for intracellular localization of the IBV S protein.
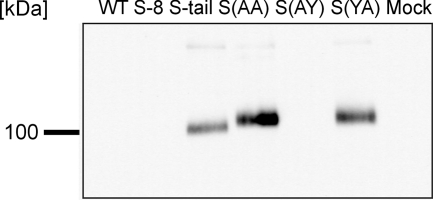
To analyze the trafficking of the S protein of IBV in more detail, we generated five mutants of the S protein which differed from the parental protein by deletions or point mutations in the cytoplasmic tail (Fig. 1). Mutant S-8 lacks the eight carboxy-terminal amino acids, including the dilysine motif that has been suggested to serve as a retrieval signal. Mutant S-tail lacks the 15 carboxy-terminal amino acids, including a dityrosine motif that may be part of a potential tyrosine-based retention signal similar to that described for the S protein of TGEV (13). In addition, we constructed mutant proteins where either of the two tyrosine residues or both were replaced by an alanine: S(YA), S(AY), and S(AA). Lontok and coworkers (8) reported that the IBV S protein is retained intracellularly when it is expressed in HeLa cells. They proposed that the dilysine motif in the cytoplasmic tail may be responsible for the retention. We used BHK-21 cells for our analysis and confirmed by immunofluorescence microscopy that the S protein is not detectable on the surface; rather, it accumulated inside the cells (Fig. 2A). Similar staining characteristics were found with the mutant protein lacking the eight carboxy-terminal amino acids. Though the dilysine motif had been deleted in this mutant, the S protein was not transported to the plasma membrane. From this result we conclude that the dilysine is not a major determinant for the intracellular retention of the S protein. By contrast, the mutant lacking the 15 carboxy-terminal amino acids (S-tail) is transported to the cell surface. Surface expression of this mutant protein was evident not only from immunostaining (Fig. 2A) but also from the extensive syncytium formation observed upon expression of the S-tail protein (Fig. 3). The generation of the S-tail mutant involved the deletion of a dityrosine motif (Fig. 1). As a tyrosine is a crucial element of the retention signal in the cytoplasmic tail of the S protein of TGEV (13), we generated point mutations in the S protein where either of the tyrosines or both were replaced by alanines. The double mutant S(AA) was readily detected on the cell surface and induced extensive syncytium formation, indicating that the S protein of IBV contains a tyrosine-based retention signal (Fig. 2A and 3). When the mutants with single amino acid exchanges were analyzed, surface expression was observed with the S(YA) but not with the S(AY) mutant. Similar behavior of the mutant proteins was observed when the proteins were expressed in primary chicken embryo fibroblasts. These cells are derived from the natural host of IBV and are susceptible to infection by IBV Beaudette (Fig. 2B). The surface transport of S-tail, S(AA), and S(YA) was confirmed when the proteins were analyzed by surface biotinylation of BHK cells (Fig. 4). These results indicate that within the dityrosine motif only the carboxy-terminal residue (Tyr1143) is important for intracellular retention of the S protein.
FIG. 2.
Immunofluorescence analysis of BHK-21 cells (A) and primary chicken embryo fibroblasts (B) transfected with S-protein mutant-containing plasmids. Virus antigen was visualized with monoclonal anti-S antibody and fluorescein isothiocyanate-labeled second antibody. Images were taken with a Leica 2 inverted confocal microscope.
FIG. 3.
Syncytium formation by mutants of the S protein. BHK-21 cells were transfected with expression plasmids of the different constructs. At 24 h posttransfection, cells were analyzed by phase-contrast microscopy.
FIG. 4.
Surface biotinylation of transfected BHK-21 cells. Lysates of biotinylated BHK-21 cells were immunoprecipitated with monoclonal anti-S antibody, and the membrane was stained with streptavidin peroxidase.
The S protein is not endocytosed.
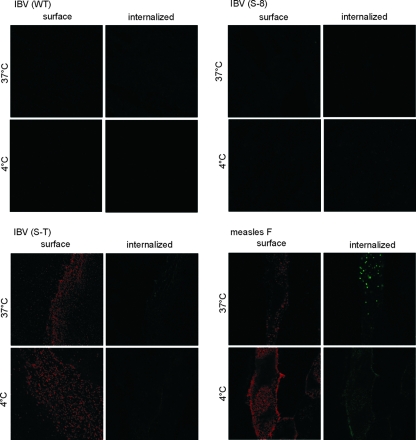
For several proteins it has been shown that YXXΦ (Φ indicates an amino acid with a bulky hydrophobic side chain) motifs can function as an endocytosis signal. Lontok and coworkers reported that in a chimeric protein that contains the cytoplasmic tail of the IBV S protein, a YXXΦ motif functions as an endocytosis signal (8). We performed an antibody uptake assay to analyze the S protein for endocytotic internalization. For this purpose we transfected BHK-21 cells with the parental protein and the two deletion mutants. We chose the measles virus F protein as a positive control, because this protein is strongly endocytosed after expression on the cell surface (11). At 24 h posttransfection, cells were stained with anti-Beaudette serum and incubated for 1 h at 37°C to allow endocytosis of the S-antibody complexes. Neither the parental protein nor the two deletion mutants, S-8 and S-tail, were internalized (Fig. 5). The S-8 mutant contains the YYXXΦ sequence but lacks the two lysine residues, and in the S-tail mutant both motifs are missing. This result shows that the YTTF motif does not function as an endocytosis signal in the IBV S, whether or not the two lysines are present.
FIG. 5.
BHK cells were transfected for expression of parental or mutant S proteins. The F protein of measles virus served as a control protein that is internalized by endocytosis. Prior to staining, cells were kept at 4°C or 37°C to prevent or allow endocytosis. Surface proteins are visualized by red fluorescence and internalized proteins by green fluorescence. Internalization was observed only with the measles virus F protein.
The S protein is located along the secretory pathway.
Having shown that the S protein is not transported to the cell surface, we were interested to localize the IBV glycoprotein at intracellular compartments. Using transfected BHK-21 cells, we analyzed whether the S protein colocalizes with marker proteins for the ER, the ERGIC, and the Golgi apparatus. As shown in Fig. 6, the parental protein colocalized with both the ER and the Golgi markers. This finding suggests that the S protein of IBV is not retained at a pre-Golgi compartment but is transported from the ER to the Golgi apparatus. To confirm these data, we analyzed the S protein from transfected cells by Western blotting. As shown in Fig. 7, both the uncleaved S0 and the proteolytic cleavage products S1 and S2 were detected in all samples. S0 was present as two bands, representing proteins with carbohydrate chains of the complex type (upper band) and proteins with high-mannose oligosaccharides (lower band). The S1 and S2 subunits have similar electrophoretic mobilities and are the major band visible. The proteolytic activation of the S protein is accomplished by furin or furin-like enzymes in a late Golgi compartment. The results from Fig. 7 indicate that all S proteins analyzed are transported to the Golgi apparatus. This was expected for the S-tail, S(YA), and S(AA) proteins that were detected on the cell surface. The finding that the parental S as well as the S-8 and S(AY) mutants are proteolytically cleaved is consistent with the fluorescence analysis in Fig. 6 and indicates that intracellular retention of the IBV S protein occurs at a late Golgi compartment.
FIG. 6.
Colocalization of the S(WT) protein with marker proteins of the ER, ERGIC, and Golgi apparatus in BHK-21 cells. The S protein was stained with the monoclonal anti-S antibody and Cy3-labeled second antibody. The marker proteins were GFP tagged. Nuclei were stained with DAPI.
FIG. 7.
Lysates of transfected BHK-21 cells. S proteins were detected with polyclonal anti-IBV serum. All mutants give the same bands as S(WT). All proteins are cleaved into the subunits S1 and S2, and they show a double band at about 220 kDa representing proteins with mannose-rich and complex oligosaccharides.
To obtain further data on the intracellular transport of the S protein, an endoglycosidase H digestion of the parental S and the mutant proteins was performed. Incubation with endoglycosidase H resulted in an increased electrophoretic mobility of both the uncleaved S0 and the cleavage products S1/S2 (Fig. 8). The upper band of the uncleaved protein shows only a moderate downshift, indicating that the majority of oligosaccharides are complex glycans. The lower S0 band is highly sensitive to endoglycosidase H, resulting in a dramatic increase of the electrophoretic mobility. The respective bands of all samples are at similar positions, indicating that there is no major difference between the intracellularly retained S proteins and the surface-expressed mutants in the processing of the oligosaccharide side chains. Following removal of high-mannose oligosaccharides, the S1/S2 proteins have an estimated molecular mass of about 100 kDa. Removal of both complex and high-mannose oligosaccharides by treatment with endoglycosidase F results in S1/S2 subunits with a molecular mass of 60 kDa (not shown). Therefore, the majority of the oligosaccharides are complex glycans. As there is no difference in the electrophoretic mobility between the surface-expressed and the intracellularly retained S proteins, all proteins have passed the site where the conversion of high-mannose to complex oligosaccharides occurs. This result excludes the possibility that the S protein is retained in a pre-Golgi compartment and is, therefore, consistent with the conclusion drawn from the Fig. 6 and 7 that the S protein of IBV is retained intracellularly at a late Golgi compartment.
FIG. 8.
Endoglycosidase H digest of S mutants. Lysates of transfected BHK-21 cells were precipitated with concanavalin A-agarose. The proteins pulled down were exposed to endoglycosidase H treatment and then separated by SDS-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. S proteins were visualized with anti-Beaudette serum. Only a small portion of the S0 protein is endoglycosidase H sensitive (black arrows), whereas the majority of the glycans are endoglycosidase H resistant (open arrows).
DISCUSSION
Our study confirms a previous report that the S protein of IBV, a group 3 coronavirus, is not transported to the cell surface but is retained at an intracellular compartment (8). The same observation has been made for coronaviruses of groups 1 and 2, TGEV and bovine coronavirus (6, 13). For TGEV it has been demonstrated that the sequence YEPI in the cytoplasmic tail is crucial for the intracellular retention (13). Replacement of the tyrosine and/or the isoleucine residues within this tetrapeptide by alanines abolished intracellular retention. By contrast, a dilysine motif in the cytoplasmic tail has been reported by others to be essential for retention of the IBV S protein (8). However, our results demonstrate that removal of the dilysine motif by deletion of the eight carboxy-terminal amino acids does not abolish intracellular retention. On the other hand, replacement of tyrosine1143 by an alanine resulted in a mutant that was transported to the plasma membrane. Expression of S on the cell surface was evident not only from surface immunofluorescence and surface biotinylation experiments but also from a strong syncytium formation that has not been observed with the parental protein. Therefore, the retention signal in the IBV S protein is tyrosine dependent, like the corresponding signal of the TGEV S protein. The discrepancy between our results and those reported by Lontok and coworkers may be related to the fact that those authors observed only an inefficient expression of the IBV S protein and, therefore, most studies were performed with chimeric proteins where only the 11 carboxy-terminal amino acids were derived from the IBV S protein. Using such chimeric proteins, a dibasic motif served as a retrieval signal when the carboxy-terminal amino acids of the severe acute respiratory syndrome coronavirus (SARS-CoV) were analyzed (8). However, in the context of the full-length S protein of SARS-CoV, the dibasic motif only slows down the intracellular transport but does not prevent the surface expression (10). The finding that intracellular retention is tyrosine dependent is consistent with the presence of the respective tyrosine in all IBV S proteins that have been sequenced so far. On the other hand, there are variants of IBV which lack dilysine motifs because of carboxy-terminal deletions of the S protein (1). In this context, it is interesting that we were able to demonstrate tyrosine-dependent retention of the S protein also in primary cells from the natural host of IBV, chicken embryo fibroblasts.
The S proteins of IBV and TGEV are both intracellularly retained by a tyrosine-dependent signal. Nevertheless, the site of retention is different for the two viral glycoproteins. The intracellularly retained S protein of TGEV contains only high-mannose oligosaccharides, whereas a mixture of complex and high-mannose oligosaccharides was detected on the surface-expressed mutant proteins (13). This finding shows that the parental S protein of TGEV is retained at a pre-Golgi compartment where oligosaccharides are still endoglycosidase H sensitive. By contrast, in the case of IBV S, no difference was found between the parental and the mutant proteins. This finding points to a site of the Golgi apparatus where the oligosaccharides have already been converted from the high-mannose to the complex type. This conclusion is consistent with the colocalization of the S protein and a Golgi marker. Further support is provided by our data on the proteolytic processing of the S protein. Cleavage of the precursor S0 into the subunits S1 and S2 was demonstrated for both the intracellularly retained and the surface-expressed forms of the S protein. Proteolytic activation of the S protein is mediated by furin or furin-like enzymes that are located at the trans-Golgi network. Thus, in contrast to the TGEV S protein, which is retained in a pre-Golgi compartment, the IBV-S protein is retained at a late Golgi compartment.
The difference between the S proteins of IBV and TGEV in the site of retention raises the question of how the tyrosine signals mediate the different sorting processes. Apart from coronaviruses, tyrosine-dependent intracellular retention has also been reported for the CD3ɛ chain of the T-cell receptor (9). For TGEV S and CD3ɛ it has been shown that the retention motif comprises the tetrapeptide YXXΦ, with Φ representing an amino acid with a bulky hydrophobic side chain. This consensus sequence appears to be valid also for IBV S, because the crucial tyrosine residue is contained in the sequence 1142YYTTF1146. The Tyr1143 together with the Phe1146 fit into the consensus sequence shown above. Only this tyrosine residue was found to be involved in intracellular retention of the S protein. Replacement of Tyr1142 did not abolish intracellular retention because of the presence of a threonine (i.e., no large aliphatic amino acid) at the +3 position. Signals of the YXXΦ type have been described for different sorting events: (i) rapid internalization from the cell surface, (ii) lysosomal targeting, (iii) localization to specialized organelles such as the antigen-processing compartment or the trans-Golgi network, and (iv) delivery to the basolateral plasma membrane of polarized epithelial cells (2). For any of these sorting events, the YXXΦ motif has to interact with a tetrameric adapter complex. One of the subunits, μ2, has pockets for both the Y and the Φ residue that mediate the interaction between the adapter complex and the target protein. Amino acid exchanges within or around the YXXΦ motif may determine the preferential affinity for either of the adapter complexes and thus explain the different sorting pathways. In this way, the intracellular retention of the coronavirus S proteins may also be explained. In the future, it has to be determined which adapter complexes interact with the glycoproteins of TGEV and IBV. For the CD3ɛ chain of the T-cell receptor, it has been shown that replacement of Arg183 abolishes the intracellular retention of CD3ɛ, resulting in a mutant protein that is internalized from the cell surface by endocytosis (3). We did not observe endocytosis of the IBV S protein and the two deletion mutants in the antibody uptake assay, but a modification of the adjacent amino acid sequence may identify amino acids which are essential for the function of the YTTF sequence as a retention signal. Replacement of such amino acids should result in mutants that are transported to the surface and from there endocytosed because of the YXXΦ motif. Such mutants would also explain why Lontok and coworkers found the YTTF signal to function as an endocytosis signal in a chimeric S protein (8). For the S proteins of IBV and TGEV, it remains to be shown which amino acids determine the sorting to a pre-Golgi or a late Golgi compartment, respectively.
The biological importance of the intracellular retention is not clear. It may appear obvious to connect the transport behavior of these proteins with the formation of virus particles which occurs by a budding process at a pre-Golgi compartment. The intracellular retention of the TGEV-S protein would be consistent with the explanation that the glycoprotein is retained at the site of virus maturation. However, the S protein of IBV is transported beyond the compartment where the budding process takes place. Similar data have been reported for the M protein. The M proteins of TGEV and IBV are retained at the cis-Golgi network, the counterpart from mouse hepatitis virus is transported to the trans-Golgi network. In this context it is interesting that the S protein of SARS-CoV, which is transported to the cell surface, is retained at the Golgi when coexpressed with the M protein of this virus (10). Thus, interaction with other viral proteins may affect the intracellular localization. In this way, interaction between the E, the M, and the S proteins may be the crucial determinant for coronavirus maturation at a pre-Golgi compartment.
The retention signals in the cytoplasmic tail of the S proteins may function to prevent the surface expression of those glycoproteins that are not incorporated into virions. As shown here for IBV, surface expression of a viral protein may have a dramatic effect on the infected cell. IBV S proteins that were transported to the plasma membrane induced the formation of large syncytia and thus could easily be distinguished from the intracellularly retained proteins. Intracellular retention of the S protein may delay this cytopathic effect and allow virus production to proceed for a longer time. Another reason for preventing the surface expression of a viral glycoprotein may be to make the infected cell less accessible to host defense mechanisms such as antibodies. To obtain experimental data on the importance of intracellular retention, it is necessary to generate mutant virus with an S protein that is transported to the cell surface. Attempts to generate recombinant IBV with a replacement of the Tyr1142 and Tyr1143 residues were not successful (16). Therefore, other mutations that abolish intracellular retention but do not prevent formation of infectious virus have to be analyzed.
Acknowledgments
We thank D. Cavanagh, F. van Kuppeveld, E. Snijder, and H.-P. Hauri for kindly providing plasmids containing the IBV S, pER-EGFP, pEGFP-Golgi, and ERGIC p53 DNAs, respectively. We thank Martina Kaps for technical assistance.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (GRK 745, NE 221/5-1, and SFB621).
Footnotes
Published ahead of print on 19 December 2007.
REFERENCES
- 1.Binns, M. M., M. E. Boursnell, F. M. Tomley, and D. K. Brown. 1986. Comparison of the spike precursor sequences of coronavirus IBV strains M41 and 6/82 with that of IBV Beaudette. J. Gen. Virol. 672825-2831. [DOI] [PubMed] [Google Scholar]
- 2.Bonifacino, J. S., and E. C. Dell'Angelica. 1999. Molecular bases for the recognition of tyrosine-based sorting signals. J. Cell Biol. 145923-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borroto, A., J. Lama, F. Niedergang, A. Dautry-Varsat, B. Alarcon, and A. Alcover. 1999. The CD3 epsilon subunit of the TCR contains endocytosis signals. J. Immunol. 16325-31. [PubMed] [Google Scholar]
- 4.Cavanagh, D., P. J. Davis, D. J. Pappin, M. M. Binns, M. E. Boursnell, and T. D. Brown. 1986. Coronavirus IBV: partial amino terminal sequencing of spike polypeptide S2 identifies the sequence Arg-Arg-Phe-Arg-Arg at the cleavage site of the spike precursor propolypeptide of IBV strains Beaudette and M41. Virus Res. 4133-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klumperman, J., J. K. Locker, A. Meijer, M. C. Horzinek, H. J. Geuze, and P. J. Rottier. 1994. Coronavirus M proteins accumulate in the Golgi complex beyond the site of virion budding. J. Virol. 686523-6534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kunkel, F., and G. Herrler. 1996. Structural and functional analysis of the S proteins of two human coronavirus OC43 strains adapted to growth in different cells. Arch. Virol. 1411123-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim, K. P., and D. X. Liu. 2001. The missing link in coronavirus assembly. Retention of the avian coronavirus infectious bronchitis virus envelope protein in the pre-Golgi compartments and physical interaction between the envelope and membrane proteins. J. Biol. Chem. 27617515-17523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lontok, E., E. Corse, and C. E. Machamer. 2004. Intracellular targeting signals contribute to localization of coronavirus spike proteins near the virus assembly site. J. Virol. 785913-5922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mallabiabarrena, A., M. A. Jimenez, M. Rico, and B. Alarcon. 1995. A tyrosine-containing motif mediates ER retention of CD3-epsilon and adopts a helix-turn structure. EMBO J. 142257-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McBride, C. E., J. Li, and C. E. Machamer. 2007. The cytoplasmic tail of the severe acute respiratory syndrome coronavirus spike protein contains a novel endoplasmic reticulum retrieval signal that binds COPI and promotes interaction with membrane protein. J. Virol. 812418-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moll, M., H. D. Klenk, G. Herrler, and A. Maisner. 2001. A single amino acid change in the cytoplasmic domains of measles virus glycoproteins H and F alters targeting, endocytosis, and cell fusion in polarized Madin-Darby canine kidney cells. J. Biol. Chem. 27617887-17894. [DOI] [PubMed] [Google Scholar]
- 12.Risco, C., I. M. Anton, M. Muntion, J. M. Gonzalez, J. L. Carrascosa, and L. Enjuanes. 1998. Structure and intracellular assembly of the transmissible gastroenteritis coronavirus. Adv. Exp. Med. Biol. 440341-346. [DOI] [PubMed] [Google Scholar]
- 13.Schwegmann-Wessels, C., M. Al Falah, D. Escors, Z. Wang, G. Zimmer, H. Deng, L. Enjuanes, H. Y. Naim, and G. Herrler. 2004. A novel sorting signal for intracellular localization is present in the S protein of a porcine coronavirus but absent from severe acute respiratory syndrome-associated coronavirus. J. Biol. Chem. 27943661-43666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tooze, J., S. Tooze, and G. Warren. 1984. Replication of coronavirus MHV-A59 in sac− cells: determination of the first site of budding of progeny virions. Eur. J. Cell Biol. 33281-293. [PubMed] [Google Scholar]
- 15.Winter, C., C. Schwegmann-Wessels, D. Cavanagh, U. Neumann, and G. Herrler. 2006. Sialic acid is a receptor determinant for infection of cells by avian infectious bronchitis virus. J. Gen. Virol. 871209-1216. [DOI] [PubMed] [Google Scholar]
- 16.Youn, S., E. W. Collisson, and C. E. Machamer. 2005. Contribution of trafficking signals in the cytoplasmic tail of the infectious bronchitis virus spike protein to virus infection. J. Virol. 7913209-13217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zimmer, G., L. Budz, and G. Herrler. 2001. Proteolytic activation of respiratory syncytial virus fusion protein. Cleavage at two furin consensus sequences. J. Biol. Chem. 27631642-31650. [DOI] [PubMed] [Google Scholar]