Abstract
Poxvirus vectors have proven to be highly effective for boosting immune responses in diverse vaccine settings. Recent reports reveal marked differences in the gene expression of human dendritic cells infected with two leading poxvirus-based human immunodeficiency virus (HIV) vaccine candidates, New York vaccinia virus (NYVAC) and modified vaccinia virus Ankara (MVA). To understand how complex genomic changes in these two vaccine vectors translate into antigen-specific systemic immune responses, we undertook a head-to-head vaccine immunogenicity and efficacy study in the pathogenic HIV type 1 (HIV-1) model of AIDS in Indian rhesus macaques. Differences in the immune responses in outbred animals were not distinguished by enzyme-linked immunospot assays, but differences were distinguished by multiparameter fluorescence-activated cell sorter analysis, revealing a difference between the number of animals with both CD4+ and CD8+ T-cell responses to vaccine inserts (MVA) and those that elicit a dominant CD4+ T-cell response (NYVAC). Remarkably, vector-induced differences in CD4+/CD8+ T-cell immune responses persisted for more than a year after challenge and even accompanied antigenic modulation throughout the control of chronic infection. Importantly, strong preexposure HIV-1/simian immunodeficiency virus-specific CD4+ T-cell responses did not prove deleterious with respect to accelerated disease progression. In contrast, in this setting, animals with strong vaccine-induced polyfunctional CD4+ T-cell responses showed efficacies similar to those with stronger CD8+ T-cell responses.
The global spread of human immunodeficiency virus (HIV) has reached pandemic proportions (http://www.unaids.org). Despite more than two decades of research since the discovery of HIV as the etiologic agent of AIDS, the development of an effective HIV type 1 (HIV-1) vaccine remains an unfulfilled priority. While it is generally accepted that ultimately a prophylactic HIV-1 vaccine should induce both humoral and cell-mediated immune responses to a number of different HIV antigens (40, 63), envelope-based immunogens capable of inducing broadly neutralizing responses currently are not available (13, 79, 98). Recent approaches have focused on vaccines capable of inducing potent CD8+ T-cell responses to control the virus load, to reduce transmission, and to slow disease development (26, 53). Evidence from both humans and nonhuman primates for the role of T-cell responses in the control of HIV includes the correlation between HIV-specific CD8+ T cells and the control of plasma viremia (51, 52, 99); the association of certain restricting major histocompatibility complex (MHC) class I alleles, conserved T-cell epitopes, and slow disease progression (14, 27, 28, 48, 55, 59, 61, 64, 70, 72, 90, 100); and the rapid increase in viral load after experimental CD8+ lymphocyte depletion in simian immunodeficiency virus (SIV)- or simian-human immunodeficiency virus (SHIV)-infected rhesus macaques (2, 54, 86), providing a strong rationale for the development of T-cell-based vaccines. Recently the quality of the HIV-specific CD8+ T cells associated with the control of HIV-1 virus loads in human long-term nonprogessors has been described, revealing characteristics of a polyfunctional profile simultaneously capable of degranulation and of producing gamma interferon (IFN-γ), interleukin-2 (IL-2), tumor necrosis factor alpha (TNF-α), and macrophage inflammatory protein 1-β (9). In contrast, while anti-HIV CD8+ and CD4+ T-cell responses have been demonstrated to have a positive effect on controlling virus loads, HIV-1-specific CD4+ T-cell responses also have been implicated as being deleterious. Indeed, the finding that HIV-1 preferentially infects HIV-specific CD4+ T cells has suggested a possible contraindication for the prophylactic induction of strong HIV-1-specific CD4+ T-cell responses (12, 23).
Two of the leading poxvirus-based vaccine vector candidates for the delivery of HIV antigens for the induction of T-cell-mediated immune responses include modified vaccinia virus Ankara (MVA) and New York vaccinia virus (NYVAC) vectors. Following the successful global eradication of smallpox in the 1970s, attenuated vaccinia vectors now have the advantage of the relative absence of preexisting immunity to poxvirus in the large young human population at risk for HIV-1 infection. In animal models, MVA as a vaccine vector has been found to induce very immunogenic responses to its inserts when administered by systemic and mucosal routes as well as providing protection against various infectious agents, including immunodeficiency viruses (for reviews, see references 18, 30, and 92). The NYVAC vector is derived from the vaccinia virus strain Copenhagen (94), is able to express multiple antigens from a wide range of species (93), and has been evaluated in several preclinical and clinical trials (7, 29, 45, 66, 87). The complements of genes that have been altered, modified, or lost are very different between these two vectors, as has recently been revealed by gene profiling (36). In studies on human monocyte-derived dendritic cells (MDDC) infected with either MVA or NYVAC vector (37), type 1 IFN, IL-6, and toll-like receptor pathways were selectively induced by MVA at the mRNA level (37). Although IL-12β, IFN-β, and TNF-α were upregulated by both vectors, they were increased to higher levels by MVA than by NYVAC. In mice, a comparison of the immune responses revealed a greater magnitude of T-cell responses to HIV-1 inserts expressed by MVA than those expressed by NYVAC (33). However, differences in polyfunctional T-cell subsets have not been explored in either human or nonhuman primates.
Here, we have undertaken a detailed head-to-head comparison between MVA and NYVAC vectors expressing identical SIV/HIV-1 gene inserts. The study design utilized the DNA prime/poxvirus boost strategy (4, 15, 22, 34, 38, 41-43, 49, 58, 65, 68, 80). The immunization protocol was based on the same EuroVacc clinical trial design as that recently completed with human volunteers (39). Vaccine constructs contained identical SIV/HIV-1 antigen inserts to allow a proper immunologic comparison of MVA- and NYVAC-based vectors. As HIV-1 Env was one of the dominant immunogenic components, efficacy subsequently was evaluated in the SHIV model of AIDS in rhesus macaques (Macaca mulatta of Indian origin).
MATERIALS AND METHODS
Vaccines. (i) DNA immunogens HIV-1 Env and SIV Gag-Pol-Nef.
SIVmac239 pcDNA-gag-pol-nef and SHIV89.6P pcDNA-env (KB9 molecular clone; 47) constructs were made and provided by R. Wagner (University of Regensburg, Germany). The techniques used for the construction of synthetic polygenes were similar to those that were used for preparing the pcDNA-gag-pol-nef of HIV-1IIIB (20) and pcDNA-env of Chinese clade C HIV-1 strain CN54 (88), as described elsewhere (33).
RNA- and codon-optimized gag-pol-nef and env gene vector inserts were designed by Geneart AG using the GeneOptimizer software package. This procedure allows several parameters to be optimized in parallel, including the adaptation of the coding sequence to human codon usage (96) and the elimination of cryptic splice sites, AU-rich sequence clusters, palindromic sequences, direct repeats, and unfavorable restriction sites, and the introduction of favorable sequence elements, e.g., the 5′ Kozak sequence. SIVmac239 gag-pol-nef and SHIV89.6P env (gp120) sequences were used. The codon-optimized SHIV89.6P env construct comprises 1,500 nucleotides (nt) encoding an artificial signal peptide (MDRAKLLLLLLLLLLPQAQ) followed by gp120 SHIV89.6P. The 5′ part of the 4,254-nt SIVmac239 gag-pol-nef polygene construct encodes the group-specific antigen with a G2A modification that renders this polyprotein myristylation deficient. The gag sequence is followed by a 952-bp fragment containing the 5′ part of pol, including a D577N mutation leading to the inactivation of the viral protease. A 618-bp fragment containing a scrambled nef variant (the 5′ end of nt 8170 to 8469 linked to the 3′ end of nt 8470 to 8787) was fused to the 3′ end of pol coding sequence, replacing the active site of the reverse transcriptase. The 3′ pol reading frame (nt 2527 to 3591) lacking the integrase gene was extended by the 3′ end of the scrambled nef gene. The sequence stretch (nt 2407 to 2514) encoding the active site of the reverse transcriptase (amino acids [aa] 1382 to 1417 in Gag-Pol-Nef) was translocated to the 3′ end of the polygene construct, resulting in an open reading frame encoding the budding-defective ∼160-kDa nonglycosylated artificial Gag-Pol-Nef polyprotein. A schematic representation of the Gag-Pol-Nef polyprotein is given in Fig. 1. The synthetic SIVmac239 gag-pol-nef gene was cloned into the unique KpnI restriction site of the pCR-Script Amp(+) cloning vector (SrfI to KpnI in the 3′ region of the synthetic gene; Stratagene, La Jolla, CA), resulting in the vector pCR-Script-C-syn-gag-pol-nef. The synthetic SHIV89.6P env-gp120 gene was cloned into the SrfI and XhoI restriction sites of the pCR-Script Amp(+) cloning vector, resulting in the vector pCR-Script-C-syn-env-gp120. The SrfI restriction site thereby was destroyed. The pCR-Script-based vectors were used to generate the DNA vaccine candidate plasmids. Both plasmids lack any antibiotic resistance gene and instead include several copies of a lacI repressor binding site. Upscaling was performed by Cobra Biomanufacturing Plc., Keele, Staffordshire, United Kingdom.
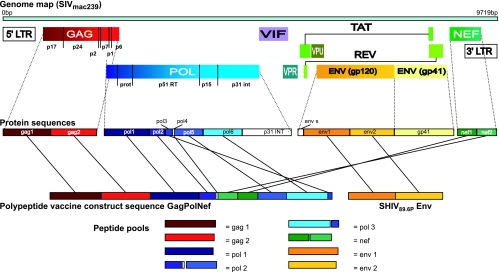
FIG. 1.
Schematic representation of the Gag-Pol-Nef polyprotein and Env protein in relation to the SIVmac239 virus genome and the peptide pools used for the analysis of immune responses. Different genes are indicated by different colors (gag in red, pol in blue, nef in green, and env in yellow). A detailed description of the construction can be found in Materials and Methods. LTR, long terminal repeat; GAG, group specific antigen; POL, polymerase; VIF, virion infectivity factor; VPR, viral protein regulator; VPU, viral protein unknown; TAT, transactivator of viral transcription; REV, regulator of viral protein expression; NEF, negative factor; PROT, protease; RT, reverse transcriptase; INT, integrase; ENV, envelope.
(ii) Generation of vaccine vectors.
Both the NYVAC (94) and MVA vectors expressing SIVmac239 gag-pol-nef and SHIV89.6P env were constructed as described elsewhere (32, 33) by using the DNA plasmids described above that were provided by R. Wagner and the pLZAW1 cloning vector that was provided by Sanofi-Pasteur. The resulting plasmids direct the insertion of the foreign genes into the thymidine kinase locus of the MVA and NYVAC genomes, allowing the construction of recombinant viruses lacking a selectable marker. The resulting MVA-89.6P-SIVGPN and NYVAC-89.6P-SIVGPN vaccine vectors contain two early/late promoters in a back-to-back orientation individually driving SHIV89.6P env and SIVmac239 gag-pol-nef genes. The vaccine vectors were purified through two 45% (wt/vol) sucrose cushions and titrated by being immunostained in chicken embryo fibroblasts. The genetic homogeneity and purity of the MVA-89.6P-SIVGPN and NYVAC-89.6P-SIVGPN vectors were confirmed by PCR analyses. The expression of 89.6P-gp120 and SIVGPN proteins by the vaccine vectors was confirmed by Western blotting.
Study design.
The study comprised three groups of seven animals each plus two naïve, infection control animals (outbred rhesus macaques [Macaca mulatta] of Indian origin). At weeks 0 and 4, two groups of seven animals were immunized (primed) with DNA. Prior to administration, the DNA constructs pcDNA-gag-pol-nef-SIVmac239 and pcDNA-env-SHIV89.6P were mixed together. A total of 4 mg of DNA was administered intramuscularly (i.m.) in the upper leg. One group of seven control animals received empty DNA vectors. At weeks 20 and 24, one group of seven animals was immunized (boosted) with MVA-89.6P-SIVGPN (5 × 108 PFU/500 μl i.m. in the right upper arm), while another group of seven animals was boosted with NYVAC-89.6P-SIVGPN (5 × 108 PFU/500 μl i.m). Seven control animals were boosted with empty NYVAC vectors (5 × 108 PFU/500 μl i.m. in the right upper arm).
Eight weeks after the final immunization, all animals were challenged with 50 to 100 50% monkey infectious doses (MID50) of the pathogenic virus strain SHIV89.6P (17, 47, 77, 78), generously provided by N. Letvin (Beth Israel Deaconess Medical Center, Boston, MA), by the intravenous route (1 ml of a 1:1,000 dilution of the virus stock, containing 3.2 × 104 50% tissue culture infectious doses/ml [1 × 108 RNA equivalents/ml] and approximately 5 × 104 MID50/ml). The study protocol and experimental procedures were approved by the institute's animal ethical care and use committee and were performed in accordance with Dutch law and international ethical and scientific standards and guidelines.
Enumeration of peptide-specific T-cell responses by IFN-γ, IL-2, or IL-4 ELISpot assays.
The quantification of antigen-specific cytokine-secreting cells was performed on freshly isolated peripheral blood mononuclear cells (PBMC) by IFN-γ, IL-2, and IL-4 enzyme-linked immunospot (ELISpot) assays according to the manufacturer's instructions (U-Cytech, Utrecht, The Netherlands). The positive control was 1 μg/ml staphylococcal enterotoxin B (SEB), while the negative control was medium alone. Antigen-specific responses were measured against the following peptide pools (5 μg/ml each): 15-mers with an 11-aa overlap spanning the entire Gag-Pol-Nef polyprotein (provided by EuroVacc consortium; Synpep Corporation, Dublin, CA), and 20-mers overlapping by 10 aa and spanning the Env gp120 of SHIV89.6P (NIH AIDS Research and Reference Reagent Program, Rockville, MD). Peptides were allocated into pools as follows: Gag1, 54 peptides (SIVg1 to SIVg213); Gag2, 54 peptides (SIVg217 to SIVgp429); Pol1, 60 peptides (SIVgp433 to SIVp669); Pol2, 60 peptides (SIVp673 to SIVpn689 and SIVnp941 to SIVp1157); Pol3, 59 peptides (SIVp1161 to SIVp1393); Nef, 62 peptides (SIVpn693 to SIVnp937); and Env, 49 peptides (4702 to 4749). Results are expressed as the mean number of spot-forming cells (SFC) per 106 PBMC from triplicate assays minus background values (mean numbers of SFC plus twice the standard deviations of triplicate assays with medium alone). Mean SFC for each antigen (responses to both Gag pools were combined, as were those from the three Pol pools) were reported for individual macaques. Responses were considered positive if more than 50 SFC/106 PBMC (after background values were subtracted) were detected.
Phenotyping of T-cell responses by intracytoplasmic staining of cytokines.
The phenotype of responding T cells was analyzed by intracytoplasmic staining of cytokines and fluorescence-activated cell sorter (FACS) analysis as described elsewhere (6), with minor modifications. PBMC were incubated overnight in the presence of the costimulatory molecules CD28 and CD49d (2 μg/ml each), with medium alone (negative control), with 1 μg/ml SEB (positive control), or with 1 μg/ml of the same peptide pools as those used in the ELISpot assay described above. Cells were stained with directly conjugated antibodies (Becton Dickinson [BD] Biosciences) to CD3 (peridinin-chlorophyll protein [PerCP] labeled), CD4 (phycoerythrin-CY7 [PE-CY7] labeled), CD8 (allophycocyanin-CY7 [APC-CY7] labeled), and CD14 and CD20 (energy-coupled dye [ECD] labeled; Beckman Coulter, Marseille, France) to identify monocytes and B cells. The phenotyping of memory cells was possible by staining them with antibodies to CCR7 (fluorescein isothiocyanate [FITC] labeled; R&D Systems, Minneapolis, MN) and CD45RA (custom biotin labeled [BD Biosciences], followed by streptavidin labeling [Pacific Orange; Molecular Probes, Leiden, The Netherlands]). The cells were fixed and permeabilized with Cytofix/Cytoperm buffer (BD Biosciences) and stained intracellularly with antibodies to IFN-γ (APC labeled) and IL-2 (PE labeled) in perm/wash solution (BD Biosciences). A total of 100,000 to 200,000 events were acquired on the FACS Aria (BD Biosciences), and FACS Diva software was used for analysis. The two-proportion z test (two tailed) with a significance level of 0.05 was used to calculate whether antigen-specific responses were significantly greater than negative control responses. If yes, then the number of antigen-specific cytokine-producing T cells per 106 lymphocytes was calculated and is shown in the figures.
Antigen-specific proliferation.
The proliferative capacity upon antigen stimulation of PBMC was investigated using the 5-(and 6-)carboxyfluorescein diacetate, succinimidyl ester (CFSE) proliferation assay as described elsewhere (57, 75). Cells were stimulated with medium alone, the Gag peptide pool or the Env peptide pool (both at 4 μg/peptide/ml), or concanavalin A (5 μg/ml). Cells were incubated for 6 days at 37°C and 5% CO2. Cells were stained for viability with the Live/Dead fixable violet dead cell dye (L34955; Molecular Probes). Subsequently, cells were exposed to antibodies from BD Biosciences (CD3APC [custom made], CD4PerCPCy5.5, CD8APCCy7) CD14ECD, and from Beckman Coulter (CD20ECD). Cells were fixed overnight in 2% paraformaldehyde at 4°C, and data were acquired on a FACS Aria (BD Biosciences). Responses that were greater than twice the background values were considered to be positive.
Gene array analysis.
Human MDDC were infected with MVA or NYVAC and incubated for 6 h. Cells were collected and processed for RNA extraction. Chips carrying oligonucleotides from 19,256 genes were used to profile MDDC gene expression and hybridized cDNA samples from infected and uninfected (mock-infected) cells at 6 h postinfection as well as NYVAC-infected samples versus MVA-infected samples at 6 h postinfection. In each analysis, genes with an interreplicative mean signal of less than 100 or an interreplicative standard deviation of greater than 1 were filtered out. The microarray labeling, slide treatment, and gene expression analysis were described previously (37). The values in Table 1 are changes and were obtained from expression ratios. Ratio values of <1 were transformed into their negative reciprocals, i.e., the more negative a value is, the greater the downregulation it represents (a change of −2-fold is equivalent to a ratio of 0.5). The data for MVA versus NYVAC come from an independent hybridization, so variability with respect to the theoretical values calculated from the other two hybridizations is expected.
TABLE 1.
Differences in mRNA levels of immunomodulators possibly involved in CD4+ and CD8+ T-cell activation after MVA and NYVAC infection of human MDDCa
| Description | GenBank accession no. | Gene designation | Fold change in transcription level for:
|
||
|---|---|---|---|---|---|
| MVA vs mock infection | NYVAC vs mock infection | MVA vs NYVAC | |||
| IFN-induced protein with tetratricopeptide repeats 4 | NM_001549 | IFIT4 | 52.45 | 3.37 | 11.44 |
| TNF (TNF superfamily, member 2) | NM_000594 | TNF | 52.21 | 6.79 | 10.38 |
| IFN-induced protein with tetratricopeptide repeats 1 | NM_001548 | IFIT1 | 47.8 | 4.65 | 7.38 |
| Mitogen-activated protein kinase kinase 5 | NM_002757 | MAP2K5 | 15.59 | 2.07 | 6.54 |
| RNA helicase | NM_014314 | RIG-I | 21.81 | 5.72 | |
| Small inducible cytokine A4 | NM_002984 | SCYA4 | 3.74 | −1.14 | 5.61 |
| IFN-induced, hepatitis C-associated microtubular aggregate protein (44 kDa) | NM_006417 | MTAP44 | 20.95 | 2.63 | 5.52 |
| IL-12B (natural killer cell stimulatory factor 2, cytotoxic lymphocyte maturation factor 2) | NM_002187 | IL12B | 9.76 | 5.44 | |
| IFN-β1, fibroblast | NM_002176 | IFNB1 | 137.82 | 5.06 | |
| Melanoma differentiation-associated protein-5 | NM_022168 | MDA5 | 7.77 | 1.67 | 5.05 |
| Cyclin B1 | NM_031966 | CCNB1 | 7.87 | −1.06 | 3.64 |
| Small inducible cytokine A5 (RANTES) | NM_002985 | SCYA5 | 4.17 | −1.17 | 3.06 |
| IL-6 signal transducer (gp130, oncostatin M receptor) | NM_002184 | IL6ST | 2.15 | −1.19 | 2.91 |
| V-fos FBJ murine osteosarcoma viral oncogene homolog | NM_005252 | FOS | 3.38 | 1.00 | 2.86 |
| GRO2 oncogene (SCYB2) | NM_002089 | GRO2 | 6.58 | 2.22 | 2.78 |
| TNF (ligand) superfamily, member 13b | NM_006573 | TNFSF13B | 1.84 | −1.21 | 2.67 |
| Small inducible cytokine subfamily B (Cys-X-Cys), member 10 | NM_001565 | SCYB10 | 8.33 | 2.35 | |
| IRF-7 | NM_004031 | IRF7 | 2.52 | 1.3 | 2.31 |
| Menage a trois 1 (CAK assembly factor) | NM_002431 | MNAT1 | 5.85 | 1.72 | 2.27 |
| Small inducible cytokine A3 | NM_002983 | SCYA3 | 4.22 | 1.12 | |
| Colony-stimulating factor 2 (granulocyte-macrophage) | NM_000758 | CSF2 | 3.13 | −1.08 | 1.94 |
| Growth arrest and DNA damage-inducible, β type | NM_015675 | GADD45B | 2.22 | 1.56 | 1.31 |
| Nuclear factor of activated T cells 5, tonicity responsive | AF346509 | NFAT5 | 2.81 | 1.24 | |
| IL-10 receptor, α | NM_001558 | IL10RA | −1.39 | −2.12 | 1.21 |
| Major histocompatibility complex, class II, DQβ1 | NM_002123 | HLA-DQB1 | 2.18 | 2.49 | 1.02 |
| Proteasome (prosome, macropain) subunit, β type, 10 | NM_002801 | PSMB10 | −3.04 | −1.20 | −1.03 |
| IFN consensus sequence binding protein 1, IRF-8 | NM_002163 | ICSBP1 | −1.19 | 3.32 | −1.07 |
| TNF (ligand) superfamily, member 9 (4-1BB-L) | NM_003811 | TNFSF9 | −1.59 | −2.10 | −1.07 |
| Inhibitor of DNA binding 2B, dominant-negative helix-loop-helix protein | M96843 | ID2B | −2.16 | −1.78 | −1.07 |
| CD86 antigen (CD28 antigen ligand 2, B7-2 antigen) | NM_006889 | CD86 | 1.44 | 1.12 | −1.13 |
| Transforming growth factor β1 | NM_000660 | TGFB1 | −2.43 | −2.09 | −1.16 |
| CD80 antigen (CD28 antigen ligand 1, B7-1 antigen) | NM_005191 | CD80 | −1.53 | −1.14 | −1.20 |
| V-jun sarcoma virus 17 oncogene homolog (avian) | NM_002228 | JUN | 1.91 | 2.93 | −1.34 |
| IL-10 receptor, β | Z17227 | IL10RB | −2.59 | −1.21 | −1.38 |
| MHC class II, DRβ5 | NM_002125 | HLA-DRB5 | −2.92 | −5.59 | −1.65 |
| IL-8 | NM_000584 | IL-8 | −2.90 | −1.39 | −1.89 |
| Ras homolog gene family, member B | NM_004040 | ARHB | −1.50 | 2.08 | −1.97 |
| IFN-γ receptor 2 (IFN-γ transducer 1) | NM_005534 | IFNGR2 | −2.52 | −1.18 | −2.05 |
| Secreted phosphoprotein 1 (osteopontin, bone sialoprotein I, early T-lymphocyte activation 1) | NM_000582 | SPP1 | −1.60 | −2.64 | −2.13 |
| Jun D proto-oncogene | NM_005354 | JUND | 1.41 | 2.78 | −2.42 |
| IL-1α | NM_000575 | IL1A | −1.43 | 2.01 | −2.42 |
| Mal, T-cell differentiation protein 2 | NM_052886 | MAL2 | −2.49 | −1.09 | −2.54 |
| IRF-5 | NM_002200 | IRF5 | −2.31 | 1.06 | −2.65 |
| IL-1β | NM_000576 | IL1B | −2.25 | 1.39 | −3.14 |
| Prostate differentiation factor | NM_004864 | PLAB | −1.77 | 3.32 | −3.94 |
| FOS-like antigen 2 | AK055579 | FOSL2 | 1.36 | 4.13 | −4.09 |
| Deoxythymidylate kinase (thymidylate kinase) | NM_012145 | DTYMK | 1.54 | 12.02 | −8.18 |
| Cyclin-dependent kinase inhibitor 2C (p18, inhibits CDK4) | NM_001262 | CDKN2C | −3.10 | 1.18 | |
The description, accession number, gene designation, and change in transcription levels are indicated for each gene in three different hybridizations. Change values were obtained from the ratio of intensities. The change is shown in boldface for genes that are significantly upregulated (>2-fold) or downregulated (<−2-fold) in MVA-infected versus mock-infected MDDC, NYVAC-infected versus mock-infected MDDC, or MVA-infected versus NYVAC-infected MDDC. Upregulation or downregulation of <2-fold is considered not significant. FBJ, Finkel-Biskis-Jinkins; GRO2, growth-related gene; MAL, myelin and lymphocyte protein.
Virus neutralization assay.
The neutralizing activity of the sera from immunized and infected rhesus macaques was assayed against the challenge virus strain SHIV89.6P. Virus stocks were prepared on human PBMC. For the neutralization assay, 50 tissue culture infectious doses/ml of SHIV89.6P were incubated with serum (final dilution, 1/15) from each animal in duplicate for 1 h at 37°C in a total volume of 150 μl Dulbecco's modified Eagle's medium with 10% fetal calf serum in 96-well flat-bottomed culture plates. Freshly trypsinized TZM-bl cells (10,000 cells in 100 μl medium containing 37.5 μg/ml DEAE-dextran) were added to each well. Control wells received cells plus virus (virus control) or cells only (background control) (97). After 48 h of culture, supernatant was removed and 150 μl lysis buffer (phosphate-buffered saline, 1% Triton, 1 mM CaCl2, and 1 mM MgCl2) was added and incubated for 45 min at 4°C. One hundred microliters of cell lysate was transferred to 96-well black/white solid plates for the measurement of luminescence using a Victor 3 light luminometer. One hundred microliters of Britelite reagent (Perkin Elmer) was added. The luminescence from samples before immunization (groups 1 and 2) or before challenge (group 3) was taken as equivalent to 100% of infected cells or alternatively as 0% neutralization.
Determination of plasma virus loads.
The plasma virus load was determined with quantitative competitive RNA reverse transcription-PCR using plasma from EDTA-treated blood samples (95). The lower detection limit was 40 RNA equivalents/ml.
T-lymphocyte subset analysis.
Quantitative changes in PBMC subsets after challenge were monitored by FACS analysis as previously described (62). The following monoclonal antibody combinations were used: (i) CD20FITC, CD16PE, HLA-DRPerCP, CD3APC, CD4PE−CY7, and CD8APC-CY7 and (ii) CCR7FITC, CD62LPE, and CD45RABiotin followed by streptavidinPerCP, CD3APC, CD4PE-CY7, and CD8APC-CY7. Monoclonal antibodies were obtained from BD Biosciences (CD20FITC, clone L27; CD16PE, clone B7.3.1; HLA-DRPerCP, clone L243; CD3APC, clone SP34; CD4PE-CY7, clone SK3; CD8APC-CY7, clone SK1; and CD45RABiotin, clone 5H9), R&D Systems (CCR7FITC, clone FAB197F), or AbD Serotec (Dusseldorf, Germany) (CD62LPE, clone FMC46). Polystyrene fluorospheres (Beckman Coulter) were used to calculate the absolute lymphocyte counts. Flow cytometry was performed on a FACS Aria (BD Biosciences). A total of 20,000 events in the lymphocyte gate were analyzed per monoclonal antibody mix.
Statistical analysis.
Statistical analysis was performed by the Wilcoxon's rank sum test or unpaired t test (with Welch correction) depending on the normal distribution of the data. A P value <0.05 was considered significant.
Nucleotide sequence accession number.
The DNA sequence of MVA-89.6p-SIVGPN in the thymidine kinase viral locus has been deposited in GenBank under accession number EU359568 (see Table S1 in the supplemental material).
RESULTS
Antigen-specific IFN-γ, IL-2, and IL-4 ELISpot responses.
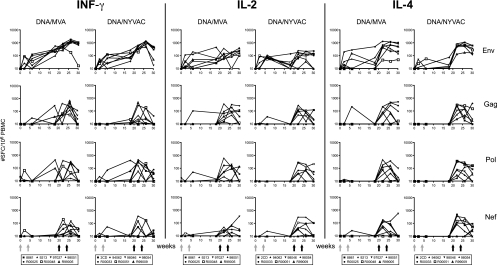
To investigate possible differences in the modulation of antigen-specific cytokine responses by the genetically distinct MVA and NYVAC vectors, we performed IFN-γ, IL-2, and IL-4 ELISpot assays to detect peptide-specific responses to each of the four vaccine-encoded antigens Env, Gag, Pol, and Nef. Priming with DNA induced low-level Env-specific cytokine responses (Fig. 2, top). As anticipated, antigen-specific responses were further increased after boosts with either type of poxvirus vector, increasing IFN-γ, IL-2, and IL-4 responses to all vaccine antigens (Fig. 2). The second poxvirus immunization resulted in an additional increase in the Env-specific IFN-γ responses (for MVA, from 560 to 1,171 mean SFC/106 PBMC [P < 0.035]; for NYVAC, from 446 to 1,074 mean SFC/106 PBMC [P < 0.004]) and Gag-specific IFN-γ responses (from 101 to 331 mean SFC/106 PBMC [P < 0.045] for MVA; Wilcoxon rank sum test). The highest observed response was to Env, which consistently scored positive in ELISpot assays during follow-up. This observation was entirely consistent with findings of the human clinical trial (39).
FIG. 2.
T-cell ELISpot responses elicited over time. Shown are IFN-γ (left panels), IL-2 (middle panels), and IL-4 (right panels) ELISpots from individual animals immunized with SHIV immunogens delivered by DNA/MVA or DNA/NYVAC to HIV-189.6P Env and SIVmac239 Gag, Pol, and Nef peptides. Background responses (mean numbers of SFC plus twice the standard deviations of triplicate assays with medium alone) were subtracted. Responses are presented as the number of SFC per 106 PBMC. Arrows indicate immunization time points at weeks 0, 4 (DNA), 20, and 24 (poxvirus).
Qualitative differences between poxvirus vector vaccine candidates reveal preferential CD8+ versus CD4+ T-cell responses.
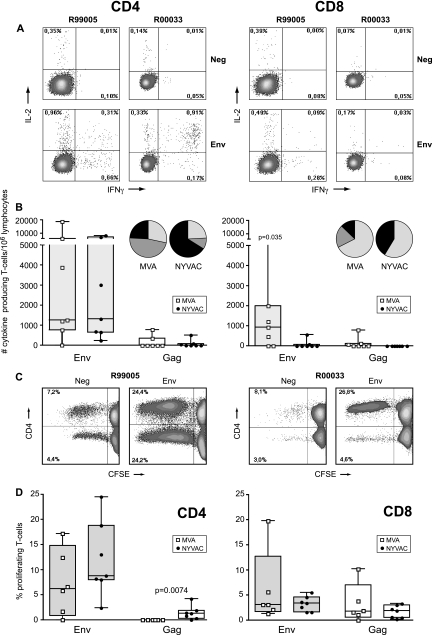
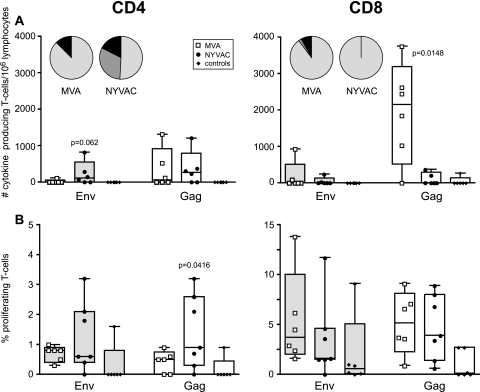
Multiparameter intracellular FACS analysis was employed to determine the phenotypic characteristics of the cytokine-producing T-cell populations (Fig. 3A). Following poxvirus vector immunizations, Env-specific cytokine-producing T cells were dominant in animals of both MVA and NYVAC groups (Fig. 3A and B). This confirmed the ELISpot data but revealed that responses were mainly from CD4+ T cells (Fig. 3A and B) of the central memory (CD45RA−/CCR7+) and effector memory (CD45RA−/CCR7−) phenotypes (data not shown) in both groups. Additionally, five out of seven MVA-boosted animals also developed Env-specific cytokine responses mediated by CD8+ T cells (P = 0.035) (Fig. 3B, right) and had an effector phenotype (CD8+/CD45RA+/CCR7−) (data not shown). Further analysis of the antigen-specific T cells revealed that the CD4+ and CD8+ T cells of the majority of the immunized animals produced either IFN-γ alone or IFN-γ plus IL-2 (Fig. 3B). The frequency of IFN-γ-producing CD8+ T cells was higher in MVA-boosted animals than in NYVAC-boosted animals (P = 0.0187).
FIG. 3.
Vaccine-induced antigen-specific CD4+ and CD8+ T-cell responses 2 weeks prior to challenge. (A) Representative flow cytometry profiles of vaccine-induced CD4+ (left) and CD8+ (right) T-cell responses directed against Env in monkeys R99005 and R00033 immunized with SHIV immunogens delivered by DNA/MVA or DNA/NYVAC, respectively. CD4 and CD8 T-cell responses were defined using polychromatic flow cytometry. Blood mononuclear cells were stimulated with the relevant peptide pools and stained with CD4, CD8, IFN-γ, and IL-2 antibodies. (B) Cytokine production by CD4+ (left) and CD8+ (right) T cells of all individual animals immunized with SHIV immunogens delivered by DNA/MVA (open squares) or DNA/NYVAC (black dots) to HIV-189.6p Env (gray boxes) and SIVmac239 Gag peptides (clear boxes) as measured by ICS assays 2 weeks before challenge. Box-whisker plots indicate the interquartile ranges and the medians (horizontal lines) of the groups. Responses are presented as the numbers of cytokine-producing CD3+ T cells per 106 lymphocytes. Pie charts represent the average response for all animals of each group of the CD4+ and CD8+ HIV-189.6p Env- and SIVmac239 Gag-specific T-cell responses grouped by function (expressing either one cytokine, IFN-γ [light gray] or IL-2 [dark gray], or the two simultaneously [black]) relative to the total antigen-specific response. (C) Representative flow cytometry profiles of CFSE-labeled PBMC of monkeys R99005 and R00033 immunized with SHIV immunogens delivered by DNA/MVA or DNA/NYVAC, respectively, unstimulated (Neg) or stimulated with Env peptides, cultured for 6 days, and stained with CD3, CD4, and CD8 antibodies. (D) Percentage of antigen-specific proliferating CD4+ and CD8+ T cells of all individual animals (dots) immunized with SHIV immunogens delivered by DNA/MVA (open squares) or DNA/NYVAC (black dots) to HIV-189.6p Env (gray boxes) and SIVmac239 Gag peptides (clear boxes) 2 weeks before challenge. Responses are presented as percentages of proliferating (CFSE low) CD3+/CD4+ and CD3+/CD8+ T cells. Background responses (medium alone) were subtracted.
To further corroborate polyfunctionality by using FACS-based assays, the proliferative capacity of both CD4+ and CD8+ T-cell populations was studied in each animal from both immunization groups (Fig. 3C and D). Proliferation was mainly Env specific, consistent with ELISpot and intracellular cytokine staining (ICS) data. In MVA-boosted animals, Env-specific proliferation was mediated by both CD4+ and CD8+ T cells, while in NYVAC-boosted animals Env-specific proliferation was preferentially mediated by CD4+ T cells (Fig. 3C and D). Furthermore, Gag-specific proliferating CD4+ T cells were detected in NYVAC-immunized animals (P = 0.0074), while in MVA-boosted animals the Gag-specific proliferating T cells were CD8+ (Fig. 3D). The calculation of the relative contribution of CD4+ and CD8+ T cells to the total response revealed a significantly larger proportion of CD8+ T-cell-mediated antigen-specific responses induced by MVA compared to those induced by NYVAC immunization (25% versus 7.3% of total cytokine responses [P = 0.042] and 59.8% versus 31.4% of total proliferative responses [P = 0.0227]).
Gene array analysis supports differential poxvirus vector-induced CD4+/CD8+ T-cell responses.
The reanalysis of gene profiling data from human dendritic cells (DC) activated by MVA or NYVAC infection (Table 1) revealed a highly marked upregulation in MVA-infected cells at the mRNA level of IL-12, IFN-α, and IFN-β as well as IFN regulatory factor 7 (IRF-7), which is implicated in the type I IFN production (MDA5, RIG). Consequently, many IFN-stimulated genes (ISGs) also were differentially upregulated in MVA-infected DC (i.e., IFIT1 [ISG56], IFIT4 [ISG60], and SCYB10 [a CXCR3 ligand chemokine chiefly active on effector Th1 cells] genes). Moreover, the differentiation program initiated by IL-12 and IFN-α/β regulates numerous genes involved in several functions. Of these, genes relevant for effector cell regulation of gene expression, such as the GADD45B (56) and the transcription factor NFAT5 (91) genes, genes involved in signal transduction (MAP2K5 gene) and cell cycle regulation (cyclin B1 gene) (60), and members of the TNF family (5) are consistently upregulated in MVA infection. Interestingly, genes encoding proinflammatory cytokines such as TNF and IL-6 and CC chemokines such as SCYA3, SCYA4, and SCYA5 (RANTES), which are involved in the modulation of the immune response, are differentially expressed by MVA and NYVAC vectors. These profiling data support the preferential stimulation of CD8+ T cells by MVA. In the case of NYVAC, it is significant that the expression of all of the above-described CD8+ T-cell-stimulatory genes is markedly reduced after virus infection of DC, further supporting a differential CD8 versus CD4 behavior between the two vaccine vector strains in vivo.
Vaccine efficacy against pathogenic challenge.
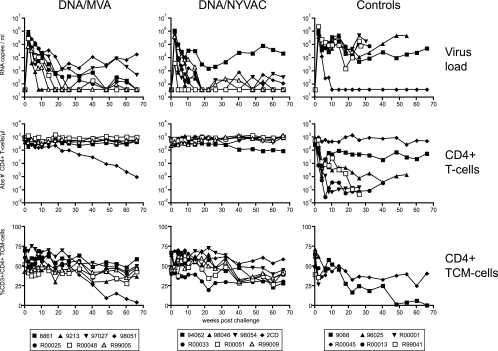
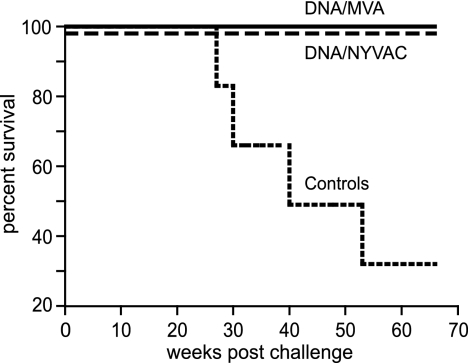
After challenge, all animals became infected (Fig. 4). Peak virus loads at 2 weeks after challenge ranged from 4.1 × 103 to 2.5 × 106 RNA copies/ml and were similar in all three groups (Fig. 4, top). Subsequently, virus loads in five control animals remained high and persisted in four of these five animals at above 105 RNA copies/ml, followed by ensuing AIDS-like disease. Upon necropsy, histology confirmed the diagnosis of AIDS, hallmarked by lymphoid depletion in the peripheral and mesenteric lymph nodes, spleen, gut-associated lymphoid tissue, and tonsils. In contrast to controls, six out of seven immunized animals from each group were able to reduce the virus load to below 104 RNA copies/ml (Fig. 4, top left). Histological examination of lymphoid tissues upon necropsy revealed mild lymphoid depletion of gut-associated lymphoid tissue in only one animal of the MVA group (98051). All immunized animals remained healthy during the study period and showed prolonged survival compared to that of the control animals (Fig. 5).
FIG. 4.
Vaccine efficacy. The viral RNA load (top), absolute number of circulating CD4+ T cells (middle), and percentage of CD4+ Tcm cells (bottom) after the challenge of each individual animal are presented. The left column represents results from animals immunized with SHIV immunogens delivered by DNA/MVA, the middle column shows results from animals immunized with SHIV immunogens delivered by DNA/NYVAC, and the right column presents results from control animals.
FIG. 5.
Survival. The percentages of animals that remained disease free after SHIV89.6P challenge are shown. The animals were immunized with SHIV immunogens delivered by DNA/MVA (solid line) or DNA/NYVAC (dashed line) or were control animals (declining line).
Absolute CD4+ T-cell loss inversely correlated with virus load after challenge (Fig. 4, middle row). In four of five control animals with high virus loads, a dramatic progressive loss of CD4+ T cells occurred, while in another control animal a 10-fold reduction of absolute CD4+ T-cell counts was observed. In both immunized groups (n = 14), only the two animals that were unable to control virus load below 104 RNA copies/ml developed evidence of CD4+ T-cell loss (Fig. 4).
A more sensitive indicator of changes within the CD4+ T-cell population is the loss of the CD4+ central memory T-cell subset (Tcm; CD4+/CCR7+/CD45RA−) (71). In control animals with high virus loads, a decline of the CD4+ Tcm was observed after challenge. In the immunized animals from both groups, the CD4+ Tcm population remained relatively stable until 30 weeks after infection. Subsequently, a slow decline of the CD4+ Tcm population also could be observed in these animals (Fig. 4, bottom).
Changes in antigen-specific T-cell responses following infection.
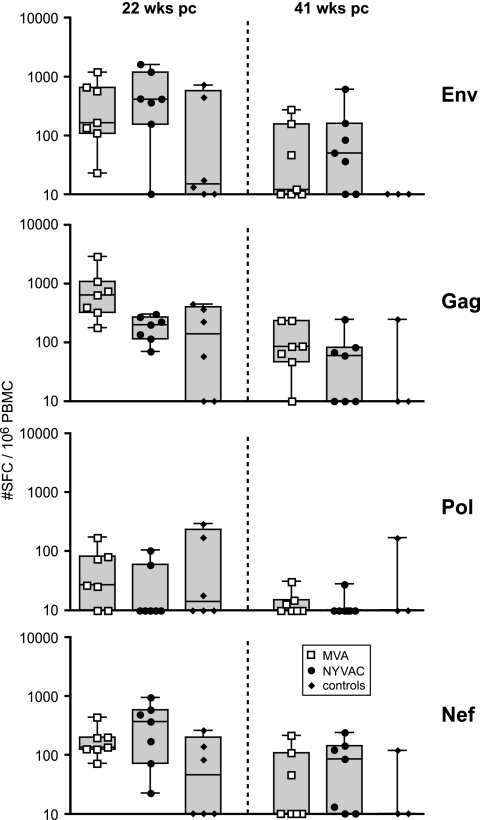
To further elucidate immune responses associated with protection from disease in the immunized groups, antigen-specific cellular immune responses were studied after challenge. Surprisingly, anamnestic T-cell responses were not observed immediately following challenge. This may have been due to an initial decline of antigen-specific IFN-γ, IL-2, and IL-4 responses in both vaccine groups (data not shown). In contrast to the control animals, HIV-1 Env-, SIV Gag-, and SIV Nef-specific IFN-γ responses in vaccinated animals at 22 weeks after challenge were similar to those observed before challenge (Fig. 6, left). These responses subsequently persisted until 41 weeks after challenge (Fig. 6, right).
FIG. 6.
T-cell ELISpot responses after challenge. IFN-γ production by PBMC of individual control animals (black diamonds) or animals immunized with SHIV immunogens delivered by DNA/MVA (open squares) or DNA/NYVAC (black dots) to HIV-189.6P Env and SIVmac239 Gag, Pol, and Nef peptides at 22 weeks after challenge (pc) (left panels) and at 41 weeks after challenge (right panels). Box-whisker plots indicate the interquartile ranges and the medians (horizontal lines) of the groups. Background responses (mean numbers of SFC plus twice the standard deviations of triplicate assays with medium alone) were subtracted. Responses are presented as the number of SFC per 106 PBMC.
At the time of euthanasia (66 weeks after challenge for immunized animals and earlier for symptomatic control animals), antigen-specific cytokine induction was monitored intracellularly by polychromatic FACS analysis (ICS). In contrast to the prechallenge ICS data, postinfection responses were directed primarily against SIV Gag and, to a lesser extent, against HIV-1 Env (Fig. 7A). The Gag-specific CD4+ T cells were of the central memory (CD45RA−/CCR7+) and effector memory (CD45RA−/CCR7−) phenotypes, while the CD8+ T cells were of the effector phenotype (CD45RA+/CCR7−) (data not shown). Similarly to the situation prior to challenge, CD8-mediated cytokine responses were higher in the MVA-boosted animals (P = 0.0148 for SIV Gag) than in the NYVAC-boosted animals, and a relatively higher proportion of the total cytokine response was mediated by CD8+ T cells in this group (74% for MVA versus 20% for NYVAC; P = 0.0072). The predominant cytokine produced was IFN-γ, with a small contribution from IFN-γ and IL-2 double-positive cells (Fig. 7A). In contrast to controls, detectable levels of Env- and Gag-specific proliferating CD4+ and CD8+ T cells were observed in immunized animals at the time of euthanasia (Fig. 7B). Although low, the frequency of Gag-specific CD4+ proliferating T cells was significantly higher in NYVAC-boosted animals than in MVA-boosted animals (P = 0.0416).
FIG. 7.
Antigen-specific CD4+ and CD8+ T-cell responses at the time of euthanasia. (A) Cytokine production by CD4+ (left) and CD8+ (right) T cells of control animals (black diamonds) or individual animals (dots) immunized with SHIV immunogens delivered by DNA/MVA (open squares) or DNA/NYVAC (black dots) to HIV-189.6p Env (gray boxes) and SIVmac239 Gag peptides (clear boxes) as measured by ICS assays at the time of euthanasia. Box-whisker plots indicate the interquartile ranges and the medians (horizontal lines) of the groups. Responses are presented as the number of cytokine-producing CD3+ T cells per 106 lymphocytes. Pie charts represent the average response for all animals of each group of the CD4+ and CD8+ HIV-189.6p Env- and SIVmac239 Gag-specific T-cell responses grouped by function (expressing either one cytokine, IFN-γ [light gray] or IL-2 [dark gray], or the two simultaneously [black]) relative to the total antigen-specific response. (B) Percentage of antigen-specific proliferating CD4+ (left) and CD8+ (right) T cells of control animals (black diamonds) or individual animals (dots) immunized with SHIV immunogens delivered by DNA/MVA (open squares) or DNA/NYVAC (black dots) to HIV-189.6p Env (gray boxes) and SIVmac239 Gag peptides (clear boxes). Responses are presented as the percentage of proliferating (CFSE low) CD3+/CD4+ and CD3+/CD8+ T cells. Background responses (medium alone) were subtracted. Statistically significant differences between immunization groups are given by showing the P values (Mann-Whitney test).
Neutralizing antibodies.
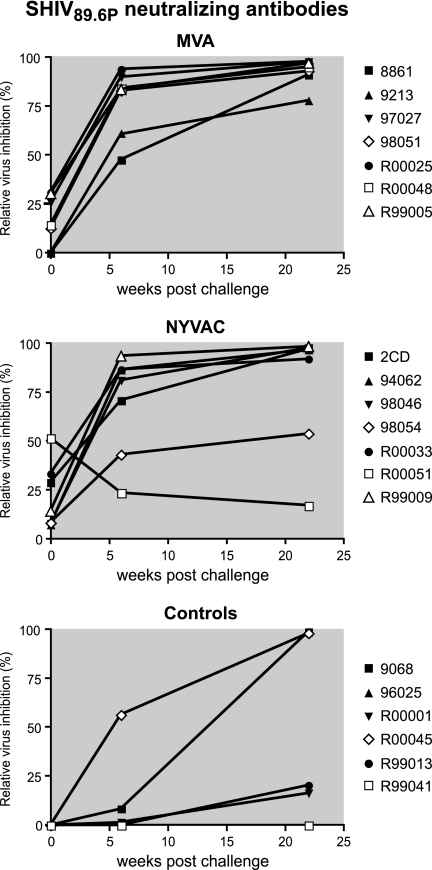
As expected with this T-cell-based vaccine approach, prior to challenge there were insufficient neutralizing antibody responses to protect animals from infection (Fig. 8). After challenge, neutralizing antibodies developed quickly in immunized animals (at similar rates in MVA- and NYVAC-immunized animals) and were significantly higher at 6 weeks after challenge compared to antibody titers in controls (P = 0.0012 for MVA versus controls and P = 0.0023 for NYVAC versus controls).
FIG. 8.
Neutralizing antibody titers. Relative inhibition of virus infection of indicator cells with the challenge virus SHIV89.6p is shown. The infection of indicator cells with virus incubated with individual sera from before immunization or before challenge (controls) was set at 0% inhibition.
DISCUSSION
This head-to-head study evaluated the immunogenicity and efficacy of two DNA prime/poxvirus boost HIV-1 vaccine candidates in the Indian rhesus macaque model of AIDS. Our results demonstrated that both poxvirus boosts were highly immunogenic, significantly boosting DNA-primed cellular immune responses to the HIV-1 and SIV inserts. This observation was consistent with earlier findings in which a broadening of the DNA-primed immune response to other (subdominant) epitopes by poxvirus boosting was reported (42, 83). The Env-specific vaccine responses prior to challenge were dominant and mediated mainly by CD4+ T cells. This may be attributable to the vaccine construct, which was designed such that the Env protein would be secreted, likely favoring CD4+ T-cell responses (84). The cause of the relative dominance of Env remains speculative and may be related to the level of antigen expression at the cellular level. However, Western blot analysis of MVA- and NYVAC-infected chicken embryo fibroblasts showed similar levels of expression of both Env protein and the polyprotein Gag-Pol-Nef. When the immunogenicity of these vaccine vectors was evaluated in HLA-A2 class I transgenic mice, a similar Env immunodominant response was observed (33). Factors such as MHC binding affinity, the efficiency of epitope processing, and competition between T cells for access to antigen-presenting cells could contribute to the observed immunodominant response (32, 73, 81).
Phenotypic analyses by ICS and CFSE proliferation assays revealed that the MVA vaccine vector induced CD8+ T-cell responses in addition to CD4 responses, while the NYVAC vector boosted CD4+ T-cell-mediated responses to a higher level than MVA. These differences were likely influenced by the different immune-modulatory effects that these poxvirus vectors have on their host cells. Moreover, in a recent study of mice that evaluated the biodistribution of MVA and NYVAC recombinant viruses, a more sustained infection was induced by NYVAC than by MVA (31), which possibly influences the T-cell response. In contrast to CD8+ T cells, for which a relatively short antigen pulse seems sufficient for antigen-presenting cells to drive clonal expansion and differentiation, antigen persistence is required for CD4+ T cells throughout their expansion phase (69). A human gene profiling analysis of MVA-infected HeLa cells has revealed an upregulation of immunomodulatory genes such as those encoding IL-1a, IL-6, IL-7, IL-8, and IL-15 (35), which could create a proinflammatory microenvironment allowing the expansion of CD8+ T-cell populations. The maintenance of specific CD8+ memory T cells could contribute to MVA-induced expression of cytokines such as IL-15 (85). Conversely, NYVAC induced much more cell apoptosis than MVA (36, 67), resulting in vaccine antigens being processed through the endocytosis pathway, facilitating presentation by MHC class II molecules and, thus, favoring CD4+ T-cell-mediated immune responses (44). Alternatively, cross-presentation and stimulation of CD8+ T-cell responses cannot be ruled out (1, 10), and both phenomena likely contribute to the cumulative antigen-specific T-cell response. The preferential induction of HIV-specific CD4+ T cells by the NYVAC vector did not result in a greater propensity for accelerated disease progression due to preferential infection and the loss of HIV antigen-specific CD4+ T cells (23), since both poxvirus vaccines induced similar levels of protection from disease progression and prolonged survival. Importantly, antigen-specific T cells induced by both NYVAC and MVA vectors were polyfunctional in that they were able to express both IFN-γ and IL-2 simultaneously, showed proliferative capacity, and were of central memory (CD45RA−/CCR7+) and effector memory (CD45RA−/CCR7−) phenotypes before challenge and of memory (CD4+/CD45RA−/CCR7+/−) and effector (CD8+/CD45RA+/CCR7−) phenotypes after challenge. It has been proposed that these phenotypes define effector CD8 T cells at an advanced stage of differentiation (16). It remains to be determined whether these cells also actually exhibit cytotoxic potential. The induction of polyfunctional T cells by vaccinia virus immunization confirms earlier findings by others (76), but we have not investigated whether these were of the unusual phenotype (CD45RO− CD27intermediate). The presence of polyfunctional phenotypes has been shown to correlate with a long-term nonprogressor status in rhesus macaques (82) and HIV-infected individuals (9, 11, 74) and to correlate with a beneficial control of virus load (2, 46, 101). However, vaccine-induced T-cell and humoral immune responses were not sufficient to protect animals from SHIV89.6p infection, but immunized animals that became infected were protected from disease progression for more than a year (66 weeks), while the majority of control animals developed evidence of AIDS-like disease. Others also have previously shown vaccine-induced control of the SHIV89.6P load and protection from disease progression (3, 8, 19, 21, 24). It is possible that protection is relatively easy to induce in the challenge model used (25). It remains to be determined whether the observed equivalent protection mediated by differential CD4 and CD8 responses is restricted to the SHIV89.6P challenge model or if similar results would have been obtained following challenge with the more pathogenic SIVmac239.
The majority of immunized animals in our study controlled virus loads and maintained CD4+ T-cell numbers, including the CD4+ Tcm population, at preinfection levels. The potential contributions of the various antigen-specific CD4+ or CD8+ T-cell-mediated mechanisms to the improved survival in the immunized animals need further investigation. The preservation of CD4+ T cells in immunized and in control animals without disease allowed the development of neutralizing antibodies by B cells after challenge, which also may ultimately have facilitated long-term control.
The Env-dominant response observed prior to infection gradually shifted toward a Gag-dominant response following infection. The vaccine-induced Env-specific responses were mediated mainly by CD4+ T cells, which are the targets for SHIV infection. Since all vaccinated animals became infected, the function and the number of these Env-specific CD4+ T cells might have been transiently affected by the virus infection (50, 89), even though no clear CD4+ T-cell decline was observed. The postchallenge induction of Gag as well as Nef responses represents an anamnestic response preferentially observed in immunized animals. This underscores the conclusion that, despite the relative immunodominance of Env, immunization against the other vaccine antigens has been beneficial.
Although immunization did not protect against SHIV infection, viral loads were reduced after the acute phase, CD4+ Tcm cells were maintained, and prolonged survival was induced. However, the relative loss of Env-specific responses, reduced CD4 responses, reduced IL-2 production (according to ELISpot and ICS assays), and reduced proliferative capacity after challenge would indicate an insidious loss of T-cell function, which may eventually have an effect on long-term survival. While both vaccine candidates induced similar levels of efficacy, this efficacy apparently was exerted through different mechanisms, which may have implications for the long-term management of the infection and future refinements in HIV vaccine design.
Supplementary Material
Acknowledgments
We are indebted to T. de Koning and H. van Westbroek for documentation. We thank D. Mortier, S. Hofman, Z. Fagrouch, and W. Koornstra for technical support. We are grateful to Jose Manuel Gonzalez for bioinformatic assistance in gene array analysis.
This study was supported by funding from the EuroVacc (European Vaccine Effort Against HIV/AIDS) project QLK2-CT-1999-01321.
Footnotes
Published ahead of print on 9 January 2008.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Albert, M. L. 2004. Death-defying immunity: do apoptotic cells influence antigen processing and presentation? Nat. Rev. Immunol. 4223-231. [DOI] [PubMed] [Google Scholar]
- 2.Amara, R. R., C. Ibegbu, F. Villinger, D. C. Montefiori, S. Sharma, P. Nigam, Y. Xu, H. M. McClure, and H. L. Robinson. 2005. Studies using a viral challenge and CD8 T cell depletions on the roles of cellular and humoral immunity in the control of an SHIV-89.6P challenge in DNA/MVA-vaccinated macaques. Virology 343246-255. [DOI] [PubMed] [Google Scholar]
- 3.Amara, R. R., K. Patel, G. Niedziela, P. Nigam, S. Sharma, S. I. Staprans, D. C. Montefiori, L. Chenareddi, J. G. Herndon, H. L. Robinson, H. M. McClure, and F. J. Novembre. 2005. A combination DNA and attenuated simian immunodeficiency virus vaccine strategy provides enhanced protection from simian/human immunodeficiency virus-induced disease. J. Virol. 7915356-15367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 29269-74. [DOI] [PubMed] [Google Scholar]
- 5.Anel, A., A. Bosque, J. Naval, A. Pineiro, L. Larrad, M. A. Alava, and M. J. Martinez-Lorenzo. 2007. Apo2L/TRAIL and immune regulation. Front. Biosci. 122074-2084. [DOI] [PubMed] [Google Scholar]
- 6.Balla-Jhagjhoorsingh, S. S., G. Koopman, P. Mooij, W. Koornstra, S. McCormack, J. Weber, G. Pantaleo, and J. L. Heeney. 2004. Long-term persistence of HIV-1 vaccine-induced CD4+CD45RA−CD62L−CCR7− memory T-helper cells. AIDS 18837-848. [DOI] [PubMed] [Google Scholar]
- 7.Benson, J., C. Chougnet, M. Robert-Guroff, D. Montefiori, P. Markham, G. Shearer, R. C. Gallo, M. Cranage, E. Paoletti, K. Limbach, D. Venzon, J. Tartaglia, and G. Franchini. 1998. Recombinant vaccine-induced protection against the highly pathogenic simian immunodeficiency virus SIVmac251: dependence on route of challenge exposure. J. Virol. 724170-4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertley, F. M., P. A. Kozlowski, S. W. Wang, J. Chappelle, J. Patel, O. Sonuyi, G. Mazzara, D. Montefiori, A. Carville, K. G. Mansfield, and A. Aldovini. 2004. Control of simian/human immunodeficiency virus viremia and disease progression after IL-2-augmented DNA-modified vaccinia virus Ankara nasal vaccination in nonhuman primates. J. Immunol. 1723745-3757. [DOI] [PubMed] [Google Scholar]
- 9.Betts, M. R., M. C. Nason, S. M. West, S. C. De Rosa, S. A. Migueles, J. Abraham, M. M. Lederman, J. M. Benito, P. A. Goepfert, M. Connors, M. Roederer, and R. A. Koup. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 1074781-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blachère, N. E., R. B. Darnell, and M. L. Albert. 2005. Apoptotic cells deliver processed antigen to dendritic cells for cross-presentation. PLoS Biol. 3e185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boaz, M. J., A. Waters, S. Murad, P. J. Easterbrook, and A. Vyakarnam. 2002. Presence of HIV-1 Gag-specific IFN-gamma+ IL-2+ and CD28+ IL-2+ CD4 T cell responses is associated with nonprogression in HIV-1 infection. J. Immunol. 1696376-6385. [DOI] [PubMed] [Google Scholar]
- 12.Brenchley, J. M., L. E. Ruff, J. P. Casazza, R. A. Koup, D. A. Price, and D. C. Douek. 2006. Preferential infection shortens the life span of human immunodeficiency virus-specific CD4+ T cells in vivo. J. Virol. 806801-6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burton, D. R., R. C. Desrosiers, R. W. Doms, W. C. Koff, P. D. Kwong, J. P. Moore, G. J. Nabel, J. Sodroski, I. A. Wilson, and R. T. Wyatt. 2004. HIV vaccine design and the neutralizing antibody problem. Nat. Immunol. 5233-236. [DOI] [PubMed] [Google Scholar]
- 14.Carrington, M., and S. J. O'Brien. 2003. The influence of HLA genotype on AIDS. Annu. Rev. Med. 54535-551. [DOI] [PubMed] [Google Scholar]
- 15.Cebere, I., L. Dorrell, H. McShane, A. Simmons, S. McCormack, C. Schmidt, C. Smith, M. Brooks, J. E. Roberts, S. C. Darwin, P. E. Fast, C. Conlon, S. Rowland-Jones, A. J. McMichael, and T. Hanke. 2006. Phase I clinical trial safety of DNA- and modified virus Ankara-vectored human immunodeficiency virus type 1 (HIV-1) vaccines administered alone and in a prime-boost regime to healthy HIV-1-uninfected volunteers. Vaccine 24417-425. [DOI] [PubMed] [Google Scholar]
- 16.Champagne, P., G. S. Ogg, A. S. King, C. Knabenhans, K. Ellefsen, M. Nobile, V. Appay, G. P. Rizzardi, S. Fleury, M. Lipp, R. Forster, S. Rowland-Jones, R. P. Sekaly, A. J. McMichael, and G. Pantaleo. 2001. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature 410106-111. [DOI] [PubMed] [Google Scholar]
- 17.Collman, R., J. W. Balliet, S. A. Gregory, H. Friedman, D. L. Kolson, N. Nathanson, and A. Srinivasan. 1992. An infectious molecular clone of an unusual macrophage-tropic and highly cytopathic strain of human immunodeficiency virus type 1. J. Virol. 667517-7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corbett, M., W. M. Bogers, J. L. Heeney, S. Gerber, C. Genin, A. Didierlaurent, H. Oostermeijer, R. Dubbes, G. Braskamp, S. Lerondel, C. E. Gomez, M. Esteban, I. Kondova, P. Mooij, S. S. Balla-Jhagjhoorsingh, N. Beenhakker, G. Koopman, S. Van der Burg, J. P. Kraehenbuhl, and A. Le Pape. Aerosol immunization with NYVAC and MVA vectored vaccines: simple, safe and immunogenic. Proc. Natl. Acad. Sci. USA, in press.
- 19.Demberg, T., R. H. Florese, M. J. Heath, K. Larsen, I. Kalisz, V. S. Kalyanaraman, E. M. Lee, R. Pal, D. Venzon, R. Grant, L. J. Patterson, B. Korioth-Schmitz, A. Buzby, D. Dombagoda, D. C. Montefiori, N. L. Letvin, A. Cafaro, B. Ensoli, and M. Robert-Guroff. 2007. A replication-competent adenovirus-human immunodeficiency virus (Ad-HIV) tat and Ad-HIV env priming/Tat and envelope protein boosting regimen elicits enhanced protective efficacy against simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques. J. Virol. 813414-3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Didierlaurent, A., J. C. Ramirez, M. Gherardi, S. C. Zimmerli, M. Graf, H. A. Orbea, G. Pantaleo, R. Wagner, M. Esteban, J. P. Kraehenbuhl, and J. C. Sirard. 2004. Attenuated poxviruses expressing a synthetic HIV protein stimulate HLA-A2-restricted cytotoxic T-cell responses. Vaccine 223395-3403. [DOI] [PubMed] [Google Scholar]
- 21.Doria-Rose, N. A., C. Ohlen, P. Polacino, C. C. Pierce, M. T. Hensel, L. Kuller, T. Mulvania, D. Anderson, P. D. Greenberg, S. L. Hu, and N. L. Haigwood. 2003. Multigene DNA priming-boosting vaccines protect macaques from acute CD4+ T-cell depletion after simian-human immunodeficiency virus SHIV89.6P mucosal challenge. J. Virol. 7711563-11577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dorrell, L., H. Yang, B. Ondondo, T. Dong, K. di Gleria, A. Suttill, C. Conlon, D. Brown, P. Williams, P. Bowness, N. Goonetilleke, T. Rostron, S. Rowland-Jones, T. Hanke, and A. McMichael. 2006. Expansion and diversification of virus-specific T cells following immunization of human immunodeficiency virus type 1 (HIV-1)-infected individuals with a recombinant modified vaccinia virus Ankara/HIV-1 Gag vaccine. J. Virol. 804705-4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Douek, D. C., J. M. Brenchley, M. R. Betts, D. R. Ambrozak, B. J. Hill, Y. Okamoto, J. P. Casazza, J. Kuruppu, K. Kunstman, S. Wolinsky, Z. Grossman, M. Dybul, A. Oxenius, D. A. Price, M. Connors, and R. A. Koup. 2002. HIV preferentially infects HIV-specific CD4+ T cells. Nature 41795-98. [DOI] [PubMed] [Google Scholar]
- 24.Egan, M. A., S. Y. Chong, S. Megati, D. C. Montefiori, N. F. Rose, J. D. Boyer, M. K. Sidhu, J. Quiroz, M. Rosati, E. B. Schadeck, G. N. Pavlakis, D. B. Weiner, J. K. Rose, Z. R. Israel, S. A. Udem, and J. H. Eldridge. 2005. Priming with plasmid DNAs expressing interleukin-12 and simian immunodeficiency virus gag enhances the immunogenicity and efficacy of an experimental AIDS vaccine based on recombinant vesicular stomatitis virus. AIDS Res. Hum. Retrovir. 21629-643. [DOI] [PubMed] [Google Scholar]
- 25.Feinberg, M. B., and J. P. Moore. 2002. AIDS vaccine models: challenging challenge viruses. Nat. Med. 8207-210. [DOI] [PubMed] [Google Scholar]
- 26.Fischer, W., S. Perkins, J. Theiler, T. Bhattacharya, K. Yusim, R. Funkhouser, C. Kuiken, B. Haynes, N. L. Letvin, B. D. Walker, B. H. Hahn, and B. T. Korber. 2007. Polyvalent vaccines for optimal coverage of potential T-cell epitopes in global HIV-1 variants. Nat. Med. 13100-106. [DOI] [PubMed] [Google Scholar]
- 27.Frahm, N., S. Adams, P. Kiepiela, C. H. Linde, H. S. Hewitt, M. Lichterfeld, K. Sango, N. V. Brown, E. Pae, A. G. Wurcel, M. Altfeld, M. E. Feeney, T. M. Allen, T. Roach, M. A. St. John, E. S. Daar, E. Rosenberg, B. Korber, F. Marincola, B. D. Walker, P. J. Goulder, and C. Brander. 2005. HLA-B63 presents HLA-B57/B58-restricted cytotoxic T-lymphocyte epitopes and is associated with low human immunodeficiency virus load. J. Virol. 7910218-10225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frahm, N., P. Kiepiela, S. Adams, C. H. Linde, H. S. Hewitt, K. Sango, M. E. Feeney, M. M. Addo, M. Lichterfeld, M. P. Lahaie, E. Pae, A. G. Wurcel, T. Roach, M. A. St. John, M. Altfeld, F. M. Marincola, C. Moore, S. Mallal, M. Carrington, D. Heckerman, T. M. Allen, J. I. Mullins, B. T. Korber, P. J. Goulder, B. D. Walker, and C. Brander. 2006. Control of human immunodeficiency virus replication by cytotoxic T lymphocytes targeting subdominant epitopes. Nat. Immunol. 7173-178. [DOI] [PubMed] [Google Scholar]
- 29.Franchini, G., S. Gurunathan, L. Baglyos, S. Plotkin, and J. Tartaglia. 2004. Poxvirus-based vaccine candidates for HIV: two decades of experience with special emphasis on canarypox vectors. Expert Rev. Vaccines 3S75-S88. [DOI] [PubMed] [Google Scholar]
- 30.Gherardi, M. M., and M. Esteban. 2005. Recombinant poxviruses as mucosal vaccine vectors. J. Gen. Virol. 862925-2936. [DOI] [PubMed] [Google Scholar]
- 31.Gómez, C. E., J. L. Najera, E. Domingo-Gil, L. Ochoa-Callejero, G. Gonzalez-Aseguinolaza, and M. Esteban. 2007. Virus distribution of the attenuated MVA and NYVAC poxvirus strains in mice. J. Gen. Virol. 882473-2478. [DOI] [PubMed] [Google Scholar]
- 32.Gómez, C. E., J. L. Najera, E. P. Jimenez, V. Jimenez, R. Wagner, M. Graf, M. J. Frachette, P. Liljestrom, G. Pantaleo, and M. Esteban. 2007. Head-to-head comparison on the immunogenicity of two HIV/AIDS vaccine candidates based on the attenuated poxvirus strains MVA and NYVAC coexpressing in a single locus the HIV-1(BX08) gp120 and HIV-1(IIIB) Gag-Pol-Nef proteins of clade B. Vaccine 252863-2885. [DOI] [PubMed] [Google Scholar]
- 33.Gómez, C. E., J. L. Najera, V. Jimenez, K. Bieler, J. Wild, L. Kostic, S. Heidari, M. Chen, M. J. Frachette, G. Pantaleo, H. Wolf, P. Liljestrom, R. Wagner, and M. Esteban. 2007. Generation and immunogenicity of novel HIV/AIDS vaccine candidates targeting HIV-1 Env/Gag-Pol-Nef antigens of clade C. Vaccine 251969-1992. [DOI] [PubMed] [Google Scholar]
- 34.Goonetilleke, N., S. Moore, L. Dally, N. Winstone, I. Cebere, A. Mahmoud, S. Pinheiro, G. Gillespie, D. Brown, V. Loach, J. Roberts, A. Guimaraes-Walker, P. Hayes, K. Loughran, C. Smith, J. De Bont, C. Verlinde, D. Vooijs, C. Schmidt, M. Boaz, J. Gilmour, P. Fast, L. Dorrell, T. Hanke, and A. J. McMichael. 2006. Induction of multifunctional human immunodeficiency virus type 1 (HIV-1)-specific T cells capable of proliferation in healthy subjects by using a prime-boost regimen of DNA- and modified vaccinia virus Ankara-vectored vaccines expressing HIV-1 Gag coupled to CD8+ T-cell epitopes. J. Virol. 804717-4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guerra, S., L. A. Lopez-Fernandez, R. Conde, A. Pascual-Montano, K. Harshman, and M. Esteban. 2004. Microarray analysis reveals characteristic changes of host cell gene expression in response to attenuated modified vaccinia virus Ankara infection of human HeLa cells. J. Virol. 785820-5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guerra, S., L. A. Lopez-Fernandez, A. Pascual-Montano, J. L. Najera, A. Zaballos, and M. Esteban. 2006. Host response to the attenuated poxvirus vector NYVAC: upregulation of apoptotic genes and NF-κB-responsive genes in infected HeLa cells. J. Virol. 80985-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guerra, S., J. L. Najera, J. M. Gonzalez, L. Lopez, N. Climent, J. M. Gatell, T. Gallart, and M. Esteban. 2007. Distinct gene expression profiling after infection of immature human monocyte-derived dendritic cells by the attenuated poxvirus vectors MVA and NYVAC. J. Virol. 818707-8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanke, T., R. V. Samuel, T. J. Blanchard, V. C. Neumann, T. M. Allen, J. E. Boyson, S. A. Sharpe, N. Cook, G. L. Smith, D. I. Watkins, M. P. Cranage, and A. J. McMichael. 1999. Effective induction of simian immunodeficiency virus-specific cytotoxic T lymphocytes in macaques by using a multiepitope gene and DNA prime-modified vaccinia virus Ankara boost vaccination regimen. J. Virol. 737524-7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harari, A., P. A. Bart, W. Stöhr, G. Tapia, M. Garcia, E. Medjitna-Rais, S. Burnet, O. Erlwein, T. Barber, C. Moog, P. Liljestrom, R. Wagner, H. Wolf, J. P. Kraehenbuhl, M. Esteban, J. L. Heeney, M. J. Frachette, J. Tartaglia, S. McCormack, A. Babiker, J. Weber, and G. Pantaleo. 2008. An HIV-1 clade C DNA prime, NYVAC boost vaccine regimen induces vigorous, broad, polyfunctional and long-lasting T-cell responses. J. Exp. Med. 20563-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heeney, J. L., and S. A. Plotkin. 2006. Immunological correlates of protection from HIV infection and disease. Nat. Immunol. 71281-1284. [DOI] [PubMed] [Google Scholar]
- 41.Hel, Z., J. Nacsa, E. Tryniszewska, W. P. Tsai, R. W. Parks, D. C. Montefiori, B. K. Felber, J. Tartaglia, G. N. Pavlakis, and G. Franchini. 2002. Containment of simian immunodeficiency virus infection in vaccinated macaques: correlation with the magnitude of virus-specific pre- and postchallenge CD4+ and CD8+ T cell responses. J. Immunol. 1694778-4787. [DOI] [PubMed] [Google Scholar]
- 42.Hel, Z., W. P. Tsai, A. Thornton, J. Nacsa, L. Giuliani, E. Tryniszewska, M. Poudyal, D. Venzon, X. Wang, J. Altman, D. I. Watkins, W. Lu, A. von Gegerfelt, B. K. Felber, J. Tartaglia, G. N. Pavlakis, and G. Franchini. 2001. Potentiation of simian immunodeficiency virus (SIV)-specific CD4(+) and CD8(+) T cell responses by a DNA-SIV and NYVAC-SIV prime/boost regimen. J. Immunol. 1677180-7191. [DOI] [PubMed] [Google Scholar]
- 43.Im, E. J., J. P. Nkolola, K. di Gleria, A. J. McMichael, and T. Hanke. 2006. Induction of long-lasting multi-specific CD8+ T cells by a four-component DNA-MVA/HIVA-RENTA candidate HIV-1 vaccine in rhesus macaques. Eur. J. Immunol. 362574-2584. [DOI] [PubMed] [Google Scholar]
- 44.Inaba, K., S. Turley, F. Yamaide, T. Iyoda, K. Mahnke, M. Inaba, M. Pack, M. Subklewe, B. Sauter, D. Sheff, M. Albert, N. Bhardwaj, I. Mellman, and R. M. Steinman. 1998. Efficient presentation of phagocytosed cellular fragments on the major histocompatibility complex class II products of dendritic cells. J. Exp. Med. 1882163-2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kanesa-Thasan, N., J. J. Smucny, C. H. Hoke, D. H. Marks, E. Konishi, I. Kurane, D. B. Tang, D. W. Vaughn, P. W. Mason, and R. E. Shope. 2000. Safety and immunogenicity of NYVAC-JEV and ALVAC-JEV attenuated recombinant Japanese encephalitis virus-poxvirus vaccines in vaccinia-nonimmune and vaccinia-immune humans. Vaccine 19483-491. [DOI] [PubMed] [Google Scholar]
- 46.Kannanganat, S., B. G. Kapogiannis, C. Ibegbu, L. Chennareddi, P. Goepfert, H. L. Robinson, J. Lennox, and R. R. Amara. 2007. Human immunodeficiency virus type 1 controllers but not noncontrollers maintain CD4 T cells coexpressing three cytokines. J. Virol. 8112071-12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karlsson, G. B., M. Halloran, J. Li, I. W. Park, R. Gomila, K. A. Reimann, M. K. Axthelm, S. A. Iliff, N. L. Letvin, and J. Sodroski. 1997. Characterization of molecularly cloned simian-human immunodeficiency viruses causing rapid CD4+ lymphocyte depletion in rhesus monkeys. J. Virol. 714218-4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaslow, R. A., M. Carrington, R. Apple, L. Park, A. Munoz, A. J. Saah, J. J. Goedert, C. Winkler, S. J. O'Brien, C. Rinaldo, R. Detels, W. Blattner, J. Phair, H. Erlich, and D. L. Mann. 1996. Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nat. Med. 2405-411. [DOI] [PubMed] [Google Scholar]
- 49.Kent, S. J., A. Zhao, S. J. Best, J. D. Chandler, D. B. Boyle, and I. A. Ramshaw. 1998. Enhanced T-cell immunogenicity and protective efficacy of a human immunodeficiency virus type 1 vaccine regimen consisting of consecutive priming with DNA and boosting with recombinant fowlpox virus. J. Virol. 7210180-10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koopman, G., H. Niphuis, W. Newman, T. K. Kishimoto, V. C. Maino, and J. L. Heeney. 2001. Decreased expression of IL-2 in central and effector CD4 memory cells during progression to AIDS in rhesus macaques. AIDS 152359-2369. [DOI] [PubMed] [Google Scholar]
- 51.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 684650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuroda, M. J., J. E. Schmitz, W. A. Charini, C. E. Nickerson, M. A. Lifton, C. I. Lord, M. A. Forman, and N. L. Letvin. 1999. Emergence of CTL coincides with clearance of virus during primary simian immunodeficiency virus infection in rhesus monkeys. J. Immunol. 1625127-5133. [PubMed] [Google Scholar]
- 53.Letvin, N. L. 2006. Progress and obstacles in the development of an AIDS vaccine. Nat. Rev. Immunol. 6930-939. [DOI] [PubMed] [Google Scholar]
- 54.Lifson, J. D., J. L. Rossio, M. Piatak, Jr., T. Parks, L. Li, R. Kiser, V. Coalter, B. Fisher, B. M. Flynn, S. Czajak, V. M. Hirsch, K. A. Reimann, J. E. Schmitz, J. Ghrayeb, N. Bischofberger, M. A. Nowak, R. C. Desrosiers, and D. Wodarz. 2001. Role of CD8+ lymphocytes in control of simian immunodeficiency virus infection and resistance to rechallenge after transient early antiretroviral treatment. J. Virol. 7510187-10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Loffredo, J. T., J. Maxwell, Y. Qi, C. E. Glidden, G. J. Borchardt, T. Soma, A. T. Bean, D. R. Beal, N. A. Wilson, W. M. Rehrauer, J. D. Lifson, M. Carrington, and D. I. Watkins. 2007. Mamu-B*08-positive macaques control simian immunodeficiency virus replication. J. Virol. 818827-8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu, B. 2006. The molecular mechanisms that control function and death of effector CD4+ T cells. Immunol. Res. 36275-282. [DOI] [PubMed] [Google Scholar]
- 57.Lyons, A. B., and C. R. Parish. 1994. Determination of lymphocyte division by flow cytometry. J. Immunol. Methods 171131-137. [DOI] [PubMed] [Google Scholar]
- 58.Mäkitalo, B., P. Lundholm, J. Hinkula, C. Nilsson, K. Karlen, A. Morner, G. Sutter, V. Erfle, J. L. Heeney, B. Wahren, G. Biberfeld, and R. Thorstensson. 2004. Enhanced cellular immunity and systemic control of SHIV infection by combined parenteral and mucosal administration of a DNA prime MVA boost vaccine regimen. J. Gen. Virol. 852407-2419. [DOI] [PubMed] [Google Scholar]
- 59.McNeil, A. J., P. L. Yap, S. M. Gore, R. P. Brettle, M. McColl, R. Wyld, S. Davidson, R. Weightman, A. M. Richardson, and J. R. Robertson. 1996. Association of HLA types A1-B8-DR3 and B27 with rapid and slow progression of HIV disease. QJM 89177-185. [DOI] [PubMed] [Google Scholar]
- 60.Mescher, M. F., J. M. Curtsinger, P. Agarwal, K. A. Casey, M. Gerner, C. D. Hammerbeck, F. Popescu, and Z. Xiao. 2006. Signals required for programming effector and memory development by CD8+ T cells. Immunol. Rev. 21181-92. [DOI] [PubMed] [Google Scholar]
- 61.Migueles, S. A., M. S. Sabbaghian, W. L. Shupert, M. P. Bettinotti, F. M. Marincola, L. Martino, C. W. Hallahan, S. M. Selig, D. Schwartz, J. Sullivan, and M. Connors. 2000. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc. Natl. Acad. Sci. USA 972709-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mooij, P., W. M. Bogers, H. Oostermeijer, W. Koornstra, P. J. Ten Haaft, B. E. Verstrepen, G. Van Der Auwera, and J. L. Heeney. 2000. Evidence for viral virulence as a predominant factor limiting human immunodeficiency virus vaccine efficacy. J. Virol. 744017-4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mooij, P., and J. L. Heeney. 2001. Rational development of prophylactic HIV vaccines based on structural and regulatory proteins. Vaccine 20304-321. [DOI] [PubMed] [Google Scholar]
- 64.Mothé, B. R., J. Weinfurter, C. Wang, W. Rehrauer, N. Wilson, T. M. Allen, D. B. Allison, and D. I. Watkins. 2003. Expression of the major histocompatibility complex class I molecule Mamu-A*01 is associated with control of simian immunodeficiency virus SIVmac239 replication. J. Virol. 772736-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mwau, M., I. Cebere, J. Sutton, P. Chikoti, N. Winstone, E. G. Wee, T. Beattie, Y. H. Chen, L. Dorrell, H. McShane, C. Schmidt, M. Brooks, S. Patel, J. Roberts, C. Conlon, S. L. Rowland-Jones, J. J. Bwayo, A. J. McMichael, and T. Hanke. 2004. A human immunodeficiency virus 1 (HIV-1) clade A vaccine in clinical trials: stimulation of HIV-specific T-cell responses by DNA and recombinant modified vaccinia virus Ankara (MVA) vaccines in humans. J. Gen. Virol. 85911-919. [DOI] [PubMed] [Google Scholar]
- 66.Myagkikh, M., S. Alipanah, P. D. Markham, J. Tartaglia, E. Paoletti, R. C. Gallo, G. Franchini, and M. Robert-Guroff. 1996. Multiple immunizations with attenuated poxvirus HIV type 2 recombinants and subunit boosts required for protection of rhesus macaques. AIDS Res. Hum. Retrovir. 12985-992. [DOI] [PubMed] [Google Scholar]
- 67.Nájera, J. L., C. E. Gomez, E. Domingo-Gil, M. M. Gherardi, and M. Esteban. 2006. Cellular and biochemical differences between two attenuated poxvirus vaccine candidates (MVA and NYVAC) and role of the C7L gene. J. Virol. 806033-6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nkolola, J. P., E. G. Wee, E. J. Im, C. P. Jewell, N. Chen, X. N. Xu, A. J. McMichael, and T. Hanke. 2004. Engineering RENTA, a DNA prime-MVA boost HIV vaccine tailored for eastern and central Africa. Gene Ther. 111068-1080. [DOI] [PubMed] [Google Scholar]
- 69.Obst, R., H. M. van Santen, D. Mathis, and C. Benoist. 2005. Antigen persistence is required throughout the expansion phase of a CD4(+) T cell response. J. Exp. Med. 2011555-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.O'Connor, D. H., B. R. Mothe, J. T. Weinfurter, S. Fuenger, W. M. Rehrauer, P. Jing, R. R. Rudersdorf, M. E. Liebl, K. Krebs, J. Vasquez, E. Dodds, J. Loffredo, S. Martin, A. B. McDermott, T. M. Allen, C. Wang, G. G. Doxiadis, D. C. Montefiori, A. Hughes, D. R. Burton, D. B. Allison, S. M. Wolinsky, R. Bontrop, L. J. Picker, and D. I. Watkins. 2003. Major histocompatibility complex class I alleles associated with slow simian immunodeficiency virus disease progression bind epitopes recognized by dominant acute-phase cytotoxic-T-lymphocyte responses. J. Virol. 779029-9040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Okoye, A., M. Meier-Schellersheim, J. M. Brenchley, S. I. Hagen, J. M. Walker, M. Rohankhedkar, R. Lum, J. B. Edgar, S. L. Planer, A. Legasse, A. W. Sylwester, M. Piatak, Jr., J. D. Lifson, V. C. Maino, D. L. Sodora, D. C. Douek, M. K. Axthelm, Z. Grossman, and L. J. Picker. 2007. Progressive CD4+ central memory T cell decline results in CD4+ effector memory insufficiency and overt disease in chronic SIV infection. J. Exp. Med. 2042171-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pal, R., D. Venzon, N. L. Letvin, S. Santra, D. C. Montefiori, N. R. Miller, E. Tryniszewska, M. G. Lewis, T. C. VanCott, V. Hirsch, R. Woodward, A. Gibson, M. Grace, E. Dobratz, P. D. Markham, Z. Hel, J. Nacsa, M. Klein, J. Tartaglia, and G. Franchini. 2002. ALVAC-SIV-gag-pol-env-based vaccination and macaque major histocompatibility complex class I (A*01) delay simian immunodeficiency virus SIVmac-induced immunodeficiency. J. Virol. 76292-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Palmowski, M. J., E. M. Choi, I. F. Hermans, S. C. Gilbert, J. L. Chen, U. Gileadi, M. Salio, A. Van Pel, S. Man, E. Bonin, P. Liljestrom, P. R. Dunbar, and V. Cerundolo. 2002. Competition between CTL narrows the immune response induced by prime-boost vaccination protocols. J. Immunol. 1684391-4398. [DOI] [PubMed] [Google Scholar]
- 74.Pantaleo, G., and A. Harari. 2006. Functional signatures in antiviral T-cell immunity for monitoring virus-associated diseases. Nat. Rev. Immunol. 6417-423. [DOI] [PubMed] [Google Scholar]
- 75.Parish, C. R. 1999. Fluorescent dyes for lymphocyte migration and proliferation studies. Immunol. Cell Biol. 77499-508. [DOI] [PubMed] [Google Scholar]
- 76.Precopio, M. L., M. R. Betts, J. Parrino, D. A. Price, E. Gostick, D. R. Ambrozak, T. E. Asher, D. C. Douek, A. Harari, G. Pantaleo, R. Bailer, B. S. Graham, M. Roederer, and R. A. Koup. 2007. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8+ T cell responses. J. Exp. Med. 2041405-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reimann, K. A., J. T. Li, R. Veazey, M. Halloran, I. W. Park, G. B. Karlsson, J. Sodroski, and N. L. Letvin. 1996. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J. Virol. 706922-6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reimann, K. A., J. T. Li, G. Voss, C. Lekutis, K. Tenner-Racz, P. Racz, W. Lin, D. C. Montefiori, D. E. Lee-Parritz, Y. Lu, R. G. Collman, J. Sodroski, and N. L. Letvin. 1996. An env gene derived from a primary human immunodeficiency virus type 1 isolate confers high in vivo replicative capacity to a chimeric simian/human immunodeficiency virus in rhesus monkeys. J. Virol. 703198-3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Richman, D. D., T. Wrin, S. J. Little, and C. J. Petropoulos. 2003. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Natl. Acad. Sci. USA 1004144-4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Robinson, H. L., D. C. Montefiori, R. P. Johnson, K. H. Manson, M. L. Kalish, J. D. Lifson, T. A. Rizvi, S. Lu, S. L. Hu, G. P. Mazzara, D. L. Panicali, J. G. Herndon, R. Glickman, M. A. Candido, S. L. Lydy, M. S. Wyand, and H. M. McClure. 1999. Neutralizing antibody-independent containment of immunodeficiency virus challenges by DNA priming and recombinant pox virus booster immunizations. Nat. Med. 5526-534. [DOI] [PubMed] [Google Scholar]
- 81.Rodriguez, F., S. Harkins, M. K. Slifka, and J. L. Whitton. 2002. Immunodominance in virus-induced CD8+ T-cell responses is dramatically modified by DNA immunization and is regulated by gamma interferon. J. Virol. 764251-4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sadagopal, S., R. R. Amara, D. C. Montefiori, L. S. Wyatt, S. I. Staprans, N. L. Kozyr, H. M. McClure, B. Moss, and H. L. Robinson. 2005. Signature for long-term vaccine-mediated control of a simian and human immunodeficiency virus 89.6P challenge: stable low-breadth and low-frequency T-cell response capable of coproducing gamma interferon and interleukin-2. J. Virol. 793243-3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Santra, S., D. H. Barouch, B. Korioth-Schmitz, C. I. Lord, G. R. Krivulka, F. Yu, M. H. Beddall, D. A. Gorgone, M. A. Lifton, A. Miura, V. Philippon, K. Manson, P. D. Markham, J. Parrish, M. J. Kuroda, J. E. Schmitz, R. S. Gelman, J. W. Shiver, D. C. Montefiori, D. Panicali, and N. L. Letvin. 2004. Recombinant poxvirus boosting of DNA-primed rhesus monkeys augments peak but not memory T lymphocyte responses. Proc. Natl. Acad. Sci. USA 10111088-11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Santra, S., Y. Sun, J. G. Parvani, V. Philippon, M. S. Wyand, K. Manson, A. Gomez-Yafal, G. Mazzara, D. Panicali, P. D. Markham, D. C. Montefiori, and N. L. Letvin. 2007. Heterologous prime/boost immunization of rhesus monkeys by using diverse poxvirus vectors. J. Virol. 818563-8570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schluns, K. S., K. Williams, A. Ma, X. X. Zheng, and L. Lefrancois. 2002. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J. Immunol. 1684827-4831. [DOI] [PubMed] [Google Scholar]
- 86.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283857-860. [DOI] [PubMed] [Google Scholar]
- 87.Siemens, D. R., S. Crist, J. C. Austin, J. Tartaglia, and T. L. Ratliff. 2003. Comparison of viral vectors: gene transfer efficiency and tissue specificity in a bladder cancer model. J. Urol. 170979-984. [DOI] [PubMed] [Google Scholar]
- 88.Su, L., M. Graf, Y. Zhang, H. von Briesen, H. Xing, J. Kostler, H. Melzl, H. Wolf, Y. Shao, and R. Wagner. 2000. Characterization of a virtually full-length human immunodeficiency virus type 1 genome of a prevalent intersubtype (C/B′) recombinant strain in China. J. Virol. 7411367-11376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sun, Y., S. R. Permar, A. P. Buzby, and N. L. Letvin. 2007. Memory CD4+ T-lymphocyte loss and dysfunction during primary simian immunodeficiency virus infection. J. Virol. 818009-8015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sun, Y., J. E. Schmitz, A. P. Buzby, B. R. Barker, S. S. Rao, L. Xu, Z. Y. Yang, J. R. Mascola, G. J. Nabel, and N. L. Letvin. 2006. Virus-specific cellular immune correlates of survival in vaccinated monkeys after simian immunodeficiency virus challenge. J. Virol. 8010950-10956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sundrud, M. S., and A. Rao. 2007. New twists of T cell fate: control of T cell activation and tolerance by TGF-beta and NFAT. Curr. Opin. Immunol. 19287-293. [DOI] [PubMed] [Google Scholar]
- 92.Sutter, G., and C. Staib. 2003. Vaccinia vectors as candidate vaccines: the development of modified vaccinia virus Ankara for antigen delivery. Curr. Drug Targets Infect. Disord. 3263-271. [DOI] [PubMed] [Google Scholar]
- 93.Tartaglia, J., W. I. Cox, S. Pincus, and E. Paoletti. 1994. Safety and immunogenicity of recombinants based on the genetically-engineered vaccinia strain, NYVAC. Dev. Biol. Stand. 82125-129. [PubMed] [Google Scholar]
- 94.Tartaglia, J., M. E. Perkus, J. Taylor, E. K. Norton, J. C. Audonnet, W. I. Cox, S. W. Davis, J. van der Hoeven, B. Meignier, M. Riviere, et al. 1992. NYVAC: a highly attenuated strain of vaccinia virus. Virology 188217-232. [DOI] [PubMed] [Google Scholar]
- 95.Ten Haaft, P., B. Verstrepen, K. Uberla, B. Rosenwirth, and J. Heeney. 1998. A pathogenic threshold of virus load defined in simian immunodeficiency virus- or simian-human immunodeficiency virus-infected macaques. J. Virol. 7210281-10285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wada, K., S. Aota, R. Tsuchiya, F. Ishibashi, T. Gojobori, and T. Ikemura. 1990. Codon usage tabulated from the GenBank genetic sequence data. Nucleic Acids Res. 18(Suppl.)2367-2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wei, X., J. M. Decker, H. Liu, Z. Zhang, R. B. Arani, J. M. Kilby, M. S. Saag, X. Wu, G. M. Shaw, and J. C. Kappes. 2002. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob. Agents Chemother. 461896-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wei, X., J. M. Decker, S. Wang, H. Hui, J. C. Kappes, X. Wu, J. F. Salazar-Gonzalez, M. G. Salazar, J. M. Kilby, M. S. Saag, N. L. Komarova, M. A. Nowak, B. H. Hahn, P. D. Kwong, and G. M. Shaw. 2003. Antibody neutralization and escape by HIV-1. Nature 422307-312. [DOI] [PubMed] [Google Scholar]
- 99.Wilson, N. A., J. Reed, G. S. Napoe, S. Piaskowski, A. Szymanski, J. Furlott, E. J. Gonzalez, L. J. Yant, N. J. Maness, G. E. May, T. Soma, M. R. Reynolds, E. Rakasz, R. Rudersdorf, A. B. McDermott, D. H. O'Connor, T. C. Friedrich, D. B. Allison, A. Patki, L. J. Picker, D. R. Burton, J. Lin, L. Huang, D. Patel, G. Heindecker, J. Fan, M. Citron, M. Horton, F. Wang, X. Liang, J. W. Shiver, D. R. Casimiro, and D. I. Watkins. 2006. Vaccine-induced cellular immune responses reduce plasma viral concentrations after repeated low-dose challenge with pathogenic simian immunodeficiency virus SIVmac239. J. Virol. 805875-5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yant, L. J., T. C. Friedrich, R. C. Johnson, G. E. May, N. J. Maness, A. M. Enz, J. D. Lifson, D. H. O'Connor, M. Carrington, and D. I. Watkins. 2006. The high-frequency major histocompatibility complex class I allele Mamu-B*17 is associated with control of simian immunodeficiency virus SIVmac239 replication. J. Virol. 805074-5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zimmerli, S. C., A. Harari, C. Cellerai, F. Vallelian, P. A. Bart, and G. Pantaleo. 2005. HIV-1-specific IFN-gamma/IL-2-secreting CD8 T cells support CD4-independent proliferation of HIV-1-specific CD8 T cells. Proc. Natl. Acad. Sci. USA 1027239-7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.