Abstract
Avian H5N1 influenza virus causes a remarkably severe disease in humans, with an overall case fatality rate of greater than 50%. Human influenza A viruses induce apoptosis in infected cells, which can lead to organ dysfunction. To verify the role of H5N1-encoded NS1 in inducing apoptosis, the NS1 gene was cloned and expressed in human airway epithelial cells (NCI-H292 cells). The apoptotic events posttransfection were examined by a terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick-end-labeling assay, flow cytometric measurement of propidium iodide, annexin V staining, and Western blot analyses with antibodies specific for proapoptotic and antiapoptotic proteins. We demonstrated that the expression of H5N1 NS1 protein in NCI-H292 cells was sufficient to induce apoptotic cell death. Western blot analyses also showed that there was prominent cleavage of poly(ADP-ribose) polymerase and activation of caspase-3, caspase-7, and caspase-8 during the NS1-induced apoptosis. The results of caspase inhibitor assays further confirmed the involvement of caspase-dependent pathways in the NS1-induced apoptosis. Interestingly, the ability of H5N1 NS1 protein to induce apoptosis was much enhanced in cells pretreated with Fas ligand (the time posttransfection required to reach >30% apoptosis was reduced from 24 to 6 h). Furthermore, 24 h posttransfection, an increase in Fas ligand mRNA expression of about 5.6-fold was detected in cells transfected with H5N1 NS1. In conclusion, we demonstrated that the NS1 protein encoded by avian influenza A virus H5N1 induced apoptosis in human lung epithelial cells, mainly via the caspase-dependent pathway, which encourages further investigation into the potential for the NS1 protein to be a novel therapeutic target.
The first documented instance of human infection with avian influenza A virus H5N1 occurred in Hong Kong in 1997. This avian influenza virus caused a remarkably severe disease in humans, with an overall case fatality rate of 33% (4, 57). Since the reemergence of this virus in 2003, H5N1 infections have reached the level of endemicity among poultry in multiple regions of the world, particularly the Southeast Asian countries. As of June 2007, more than 315 human infections had been reported to the World Health Organization (WHO), with a mortality rate greater than 50% (10, 54). While lymphopenia, hypercytokinemia, and hemophagocytosis have been the most notable clinicopathological findings, the biological basis accounting for the severity of H5N1 infection in humans remains unknown (10, 51). In a recent study, apoptosis among the alveolar epithelial cells of two patients who died of H5N1 infection was observed (53), suggesting that apoptosis may play a role in H5N1 pathogenesis in humans.
Apoptosis, or programmed cell death, is a series of defined cellular events that culminates in the efficient removal of the cell and its contents. Apoptosis consists of three distinct phases: an initiation phase, triggered by a variety of physiological agents, which involves the activation of heterogeneous intracellular signaling pathways; a commitment phase, during which the cells become irreversibly committed to die; and an execution phase, during which the characteristic morphological changes (membrane blebbing, cell shrinkage, and the condensation of the chromatin) become obvious. It has been postulated previously that the induction of apoptosis is a host defense mechanism, stopping the replication and spread of viruses (19). Many viral infections result in the apoptosis of host cells, whereas several viruses have evolved mechanisms to inhibit apoptosis (45, 50), allowing continued viral replication in infected host cells.
Influenza viruses are reported to induce apoptosis in numerous cell types, both in vivo (28, 37, 39) and in vitro (12, 17, 26, 29, 41, 42, 44, 48, 49). The virus induces apoptosis in infected cells as part of the mechanisms contributing to cellular and organ dysfunction (38, 52). Apoptosis induction is multifactorial and highly regulated. It has been shown previously that influenza virus strains differ in their abilities to induce apoptosis, and this phenomenon is also cell type specific. This specificity may be due to the fact that different viral proteins from different strains may vary in their abilities to modulate the apoptotic response (3). Several viral proteins (neuraminidase, M1, NS1, and PB1-F2) from different strains of human influenza viruses have been shown to induce or inhibit apoptosis in human cells (5, 6, 29, 46, 58, 59).
To date, whether and to what extent apoptosis contributes to the highly virulent property of influenza (H5N1) viruses are not clear. Moreover, the upstream signaling events and what initiates the apoptotic cascade are not known. There are reports showing that a poor-apoptosis-inducer strain can be converted into a strong-inducer strain by the substitution of the NS1 gene from a strong-inducer strain by a reverse-genetics technique, and vice versa (31). It has also been shown previously that NS1 proteins encoded by different strains may have different abilities to induce apoptosis (3, 30). Furthermore, there are reports showing that the H5N1 NS1 gene can circumvent the host antiviral cytokine responses and contribute to the virulence of H5N1 avian influenza viruses (25, 43).
In this study, we demonstrated the ability of the H5N1-encoded NS1 protein to induce apoptosis in human bronchiolar epithelial cells (NCI-H292 cells).
MATERIALS AND METHODS
Virus growth and cell culture.
Cells of the human bronchial epithelial cell line NCI-H292 (ATCC CCL-1848) were grown as monolayers in RPMI 1640 medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Invitrogen) at 37°C in a 5% CO2 incubator (16). The cells were infected with an H5N1 virus isolated from a patient in Hong Kong in 1997, A/HK/483/97(H5N1), at a multiplicity of infection of 1.
RNA extraction and construction of plasmids.
Total RNA was extracted from cell lysate using the QIAamp viral RNA mini kit (Qiagen, Hilden, Germany). The full-length NS1 gene from A/HK/483/97(H5N1) was amplified using the SuperScript III one-step reverse transcription-PCR (RT-PCR) system with Platinum Taq high-fidelity polymerase (Invitrogen) and ligated into the linearized pcDNA4/HisMaxTOPO vector according to the protocol of the vector manufacturer (Invitrogen) to produce the recombinant His-tagged construct pcDNA4-NS1. This vector contains the cytomegalovirus promoter for the expression of an NS1 protein with a six-histidine tag. Clones were screened for proper orientation by PCR and sequence analysis using the version 3.1 BigDye Terminator ready reaction cycle sequencing kit (Applied Biosystems, Foster City, CA). Competent Escherichia coli TOP10 cells (Invitrogen) were transformed with the plasmids, and the plasmids were amplified and purified using a high-purity plasmid purification kit (Invitrogen).
Transient transfection and protein expression system.
Approximately 106 NCI-H292 cells were transfected with 4 μg of pcDNA4-NS1 or control plasmids in a six-well plate using Lipofectamine 2000 reagent according to the protocol of the manufacturer (Invitrogen). Cells were then collected at 0, 6, 12, 18, 24, and 30 h posttransfection, washed with phosphate-buffered saline (PBS), and trypsinized. The cell pellet was then resuspended in 100 μl of lysis buffer (2% sodium dodecyl sulfate, 10% glycerol, 0.0625 M Tris-HCl [pH 8.0]), and the suspension was incubated on ice for 30 min. After boiling for 10 min and centrifugation at 12,000 × g for 20 min, the supernatant was collected for Western blot analysis.
TUNEL assay.
At the late stage of apoptosis, the DNA of apoptotic cells is cleaved into a population of multimers of 180- to 200-bp fragments through the action of endogenous endonucleases, which then cause morphological changes in the nuclei of apoptotic cells. The terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick-end-labeling (TUNEL) system could label this fragmented DNA in situ. TUNEL staining for apoptotic nuclei was performed using the DeadEnd colorimetric TUNEL system according to the instructions of the manufacturer (Promega, Madison, WI). Briefly, cells were fixed in 4% paraformaldehyde for 25 min and then treated with permeabilization solution (0.2% Triton X-100 solution in PBS) for 5 min at room temperature. Labeling reactions were performed with 100 μl of reaction buffer for 60 min at 37°C in a humidified chamber. Color development was accomplished with diaminobenzidine for 8 min. Positively stained apoptotic nuclei were observed microscopically. Apoptosis was evaluated as the average number of positively stained cells per field at high-power magnification (×400).
PI staining and DNA content analysis by flow cytometry.
The propidium iodide (PI) flow cytometric assay is based on the principle that apoptotic cells are characterized by DNA fragmentation and the consequent loss of nuclear DNA content at the late phase of apoptosis. Briefly, cells (106) were washed with PBS and fixed with 70% ethanol overnight at 4°C. The fixed cells were then stained with 50 μg of PI (Sigma, St. Louis, MO)/ml with 1 μg of RNase A/ml at 4°C for 1 h. PI binds to DNA by intercalating between the bases, with no sequence preference. The DNA contents of the cells were then analyzed with a flow cytometer (XL-MCL; Beckman Coulter, Fullerton, CA). Cells at the late stage of apoptosis, i.e., sub-G1 (hypodiploid) cells, will have DNA contents lower than those of G1 cells. The proportions of these apoptotic cells, i.e., sub-G1 cells, at different time points posttransfection were determined.
Annexin V-FITC staining of apoptotic cells and analysis by flow cytometry.
Cells were collected, washed with PBS, and stained with fluorescein isothiocyanate (FITC)-conjugated annexin V (BD Biosciences, Franklin Lakes, NJ) and PI for 20 min at room temperature in the dark. The stained cells were then analyzed by a flow cytometer (Beckman Coulter). FITC-conjugated annexin V binds to surface phosphatidylserine translocated from the intra- to the extracellular plasma membrane early in apoptosis. Cells were simultaneously stained with PI to discriminate membrane-permeable necrotic cells from FITC-labeled apoptotic cells. Apoptotic cells were identified as those with annexin V-FITC staining only, and the results were expressed as the proportion of these cells among the total number of cells analyzed.
Western blot analysis.
Monolayers of cells transfected with DNA or untransfected cells were lysed, and the total protein concentration was determined by the bicinchoninic acid assay (Sigma). Proteins with equivalent concentrations were heated for 5 min at 100°C in sample buffer containing β-mercaptoethanol and were then resolved by 12% or 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The resolved proteins were transferred onto a polyvinylidene difluoride membrane (Bio-Rad, Richmond, CA) and blocked with 1% powdered milk in Tris-buffered saline with 0.1% Tween 20 (Amersham Pharmacia, Uppsala, Sweden) for 1 h at room temperature. Mouse or rabbit antibodies were then used to probe for His-tagged NS1 (Invitrogen) and poly(ADP-ribose) polymerase (PARP), caspase-3, caspase-7, caspase-8, caspase-9, Bid, Bad, Bim, and Bcl-xL (all from Cell Signaling Technology, Beverly, MA), with overnight incubation at 4°C. The membrane was subsequently incubated for 1 h at room temperature with 1:1,000 anti-rabbit or anti-mouse immunoglobulin G horseradish peroxidase-linked whole secondary antibody (Amersham Pharmacia, Uppsala, Sweden). The membrane was also probed for glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Chemicon, Temecula, CA) as a loading control.
Caspase inhibition assays.
The cell monolayers were treated with 50 μM caspase-8 inhibitor (Ac-IETD-CHO), caspase-3 inhibitor (Ac-DEVD-CHO), or general caspase inhibitor (Z-VAD-FMK) (all from BD Biosciences) 30 min before the transfection and protein expression.
Effects of Fas ligands on NS1 induction of apoptosis.
The effect of Fas ligands (FasL) on the induction of the H5N1 NS1 death receptor pathway was also studied. NCI-H292 monolayers were incubated in the absence and presence of human FasL (2 ng/ml; Upstate Biotechnology, Lake Placid, NY) for 1 h before transfection experiments were carried out as described above. Cells were also examined by phase microscopy to evaluate the morphological characteristics of apoptosis.
Quantification of mRNA by quantitative real-time RT-PCR.
To study the involvement of the Fas/CD95 cell surface death receptor in the induction of apoptosis in cells transfected with H5N1 NS1, quantitative real-time RT-PCR was used to compare the profiles of Fas and FasL mRNA expression following transfection. DNase-treated total RNA was isolated by using the TRIzol total RNA extraction kit according to the protocol of the manufacturer (Invitrogen). The cDNA was synthesized from mRNA with poly(dT) primers and Superscript III reverse transcriptase (Invitrogen) and quantified by real-time PCR analysis with an ABI PRISM 7700 sequence detection system (Applied Biosystems). The sense and antisense primers used for Fas were TCGGAGGATTGCTCAACAACC and AAGAAGAAGACAAAGCCACCCC, respectively, which target a 564-bp fragment (33). The sense and antisense primers used for FasL were AGGCAAGTCCAACTCAAGGTCC and CATCTTCCCCTCCATCATCACC, respectively, which target a 238-bp fragment (33). The PCR conditions were 95°C for 10 min, followed by 40 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s. PCRs were performed in triplicate using the SYBR green PCR master mix (Applied Biosystems). The expression of the beta-actin gene was also quantified in a similar way for normalization of the results obtained for Fas and FasL. Sense and antisense primers for the beta-actin gene were GCACGGCATCGTCACCAACT and CATCTTCTCGCGGTGGCCT, respectively. Comparative threshold cycle methods were used to analyze the results using beta-actin as the endogenous control, where expression levels of Fas and FasL in untreated and untransfected cells were set at 1.
RESULTS
The expression of H5N1 NS1 protein induced apoptosis in H292 cells.
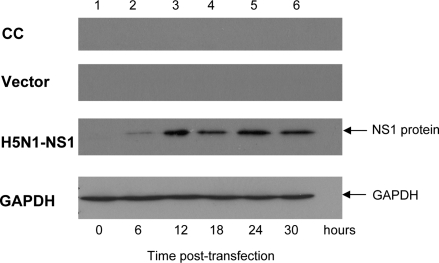
To assess the expression of NS1 protein in NCI-H292 cells after transfection with the pcDNA4-NS1 plasmid, the cells were collected at 0, 6, 12, 18, 24, and 30 h posttransfection. Total proteins from the cell lysates were subjected to Western blot analysis. The anti-His6 antibody specifically recognized a protein of about 26 kDa, corresponding to the predicted size of the H5N1 NS1 protein. The protein expression was detectable as early as 6 h and plateaued by 12 h posttransfection (Fig. 1).
FIG. 1.
Time course of H5N1-NS1 protein expression in H292 cells. Anti-His6 antibodies were used to detect the NS1 protein as a single protein band with a size of approximately 26 kDa in the Western blot analysis of the protein lysate of NCI-H292 cells transfected with pcDNA4-NS1. Approximately 25 μg of total cell lysate was loaded into each lane. CC, whole-cell lysates prepared from untransfected control cells; vector, whole-cell lysates prepared from cells transfected with pcDNA4 empty vector; H5N1-NS1, whole-cell lysates prepared from cells transfected with pcDNA4-NS1. GAPDH was detected as a loading control. The results shown are representative of three independent experiments.
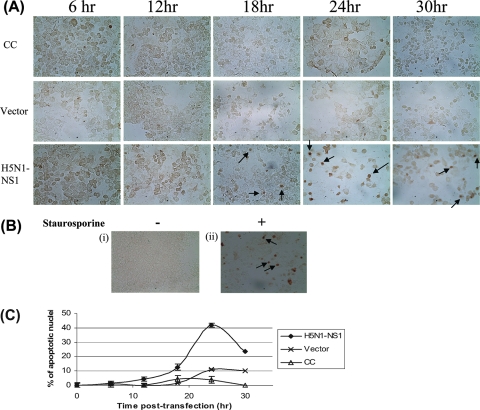
To examine whether apoptosis was initiated in the cells transfected with pcDNA4-NS1, the cells were fixed and examined by TUNEL, an assay that detects DNA strand breakage, which is a hallmark of apoptosis. The results showed that the cells transfected with pcDNA4-NS1 exhibited TUNEL staining in a time-dependent manner (Fig. 2A). The maximal TUNEL staining occurred at 24 h posttransfection (Fig. 2B). In contrast, the untransfected cells and the cells that were transfected with an empty vector were negative for TUNEL staining. As expected, cells treated with the apoptosis-inducing agent staurosporine showed positive TUNEL staining (Fig. 2A and B).
FIG. 2.
Apoptosis detected by the TUNEL assay. (A) Representative photographs showing the control and transfected cells stained for the detection of apoptotic nuclei by the TUNEL assay. The cells were harvested at 0, 6, 12, 18, 24, and 30 h posttransfection. Examples of apoptotic cells as indicated by positively stained nuclei are marked with arrows. Apoptosis was evaluated by counting the number of positively stained cells per field at a magnification of ×400. CC, untransfected control cells; vector, cells transfected with pcDNA4 empty vector; H5N1-NS1, cells transfected with pcDNA4-NS1. (B) Representative photographs showing the untransfected control cells not treated (i) or treated with the apoptosis-inducing agent staurosporine (0.5 μM) for 24 h (ii) and stained for the detection of apoptotic nuclei by the TUNEL assay. Examples of positively stained apoptotic cells are indicated by arrows. −, not treated; +, treated. (C) Percentage of apoptotic cells detected at various time points posttransfection using the TUNEL assay. Results are expressed as means ± standard deviations (SD) of results from three independent experiments.
To further document the occurrence of apoptosis among NS1 protein-expressing cells, PI staining and flow cytometry were used to analyze cellular DNA content. The results revealed the presence of a population of sub-G1 cells among the NS1 protein-expressing cells, but not among control cells, from 6 to 30 h posttransfection (Fig. 3A). Apoptosis among staurosporine-treated cells was observed, as expected (Fig. 3B). These results suggested that the cells expressing NS1 protein had an apoptotic loss of DNA content (Fig. 3C).
FIG. 3.
Apoptosis detected by cellular DNA analysis using flow cytometry. (A) Representative DNA histograms showing the proportions of apoptotic hypodiploid nuclei detected by flow cytometry. The proportion of apoptotic cells among the cells transfected with pcDNA4-NS1 increased in a time-dependent manner, but those among untransfected cells and cells transfected with empty vector did not. The percentage of apoptotic cells detected at each time point is shown. CC, untransfected control cells; vector, cells transfected with pcDNA4 empty vector; H5N1-NS1, cells transfected with pcDNA4-NS1. (B) NCI-H292 cells treated (+) or not treated (−) with the apoptosis-inducing agent staurosporine (0.5 μM) for 24 h were included as a positive control for the experiment. (C) Proportions of apoptotic cells among the untreated control cells, cells transfected with empty vector, and cells transfected with pcDNA4-NS1. Results are expressed as means ± SD of results from three independent experiments.
To complement our results, the cells expressing NS1 protein were stained with annexin V-FITC and PI and were then analyzed by flow cytometry (Fig. 4A and B). The results showed that approximately 21% of the NS1 protein-expressing cells were stained by annexin V-FITC, but not penetrated by PI, at 24 h posttransfection, compared to less than 4% of the control cells (Fig. 4C). Collectively, all these data indicated that apoptosis was initiated in the NCI-H292 cells overexpressing NS1 protein.
FIG. 4.
Apoptosis detected by annexin V and PI staining. (A) Representative dual-labeled quadrants of bivariant fluorescence dot plots showing the induction of apoptosis in the NCI-H292 cells transfected with pcDNA4-NS1. Apoptotic cells that stained positive for annexin V but not PI were identified in the right lower quadrant. Percentages shown are proportions of apoptotic cells detected at different time points, as indicated. CC, untransfected control cells; vector, cells transfected with pcDNA4 empty vector; H5N1-NS1, cells transfected with pcDNA4-NS1. (B) NCI-H292 cells treated (+) or not treated (−) with the apoptosis-inducing agent staurosporine (0.5 μM) for 24 h were included as a positive control. (C) Bar chart showing the proportions of apoptotic cells at different time points posttransfection. Results are expressed as means ± SD of results from three independent experiments.
Differential activation of caspases in NS1-expressing cells.
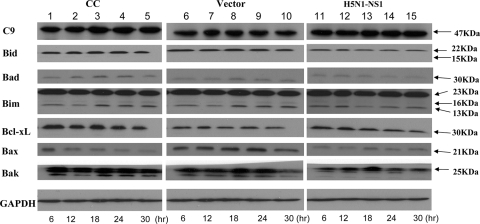
To delineate the apoptotic pathway, we first measured the cleavage of caspase-activated PARP at 6, 12, 18, 24, and 30 h posttransfection using Western blot analysis. There were increases of about 1.6- and 2-fold in the levels of the cleaved PARP fragment in NS1-expressing cells at 24 and 30 h postinfection, respectively, compared with the levels in cells transfected with the empty vector or mock transfection controls (Fig. 5A). PARP helps cells to maintain their viability; the cleavage of PARP facilitates cellular disassembly and serves as a marker of cells undergoing apoptosis. PARP is also one of the cleavage targets of caspase-3 and caspase-7 in vivo. Therefore, we further investigated the activities of caspase-3 and caspase-7. Our results showed that the cleaved fragments of caspase-3 and caspase-7 were detectable in NS1 protein-expressing cells at 24 h posttransfection (Fig. 5B and C).
FIG. 5.
Caspase pathway activation in NCI-H292 cells induced by the H5N1 NS1 protein. The total protein from cell lysates was harvested at different time points posttransfection (lanes 1 to 5: 6, 12, 18, 24, and 30 h, respectively). Western blot analysis was performed to detect the cleavage of proapoptotic proteins in untransfected control cells (CC), cells transfected with pcDNA4 empty vector (vector), and cells transfected with pcDNA4-NS1 (H5N1-NS1). Total proteins from NCI-H292 cells treated (+) and not treated (−) with the apoptosis-inducing agent staurosporine (0.5 μM) for 24 h were included as a positive control. (A) The cleavage of PARP in cells transfected with pcDNA4-NS1 was detected by using anti-PARP antibody. (B) Procaspase-3 (C3) activation in cells transfected with pcDNA4-NS1 was detected by using anti-caspase-3 antibody. (C) Procaspase-7 (C7) activation in cells transfected with pcDNA4-NS1 was detected by using anti-caspase-7 antibody. (D) Procaspase-8 (C8) activation in cells transfected with pcDNA4-NS1 was detected by using anti-procaspase-8 antibody. GAPDH was used as the loading control for each membrane preparation from the untransfected control cells, the cells transfected with pcDNA4 empty vector, and the cells transfected with pcDNA4-NS1. The results shown are representative of three separate experiments. The GAPDH results shown correspond to those for cells transfected with pcDNA4-NS1. The level of induction (n-fold) was calculated from density values for protein bands relative to the GAPDH results corresponding to the untransfected control cells, the cells transfected with pcDNA4 empty vector, or the cells transfected with pcDNA4-NS1.
To further delineate the caspase cascade pathway by which NS1 protein might be involved in the induction of apoptosis, we examined another important apoptotic marker, caspase-8. Consistent with the caspase-3 findings, NS1 protein also induced the degradation of procaspase-8 (1.3-fold reduction) beginning at 24 h posttransfection (Fig. 5D).
Apoptosis can be activated through either the mitochondrion-mediated (14) or the receptor-mediated (1) pathway. Communication between the two pathways can occur through the caspase-8-mediated cleavage of Bid, a member of the Bcl-2 family, which induces the release of cytochrome c. Therefore, we also studied the effect of NS1 protein on mitochondrion-mediated apoptotic pathway-related protein expression, such as that of Bid, Bim, Bad, Bcl-xL, and caspase-9. Our results showed that NS1 protein expression did not affect the levels of the Bcl-2 family proteins and caspase-9 (Fig. 6).
FIG. 6.
Expression of pro- and antiapoptotic proteins in NCI-H292 cells after transfection with H5N1 NS1. Cell lysates harvested at different time points (6, 12, 18, 24, and 30 h) posttransfection were used for Western blot analysis to detect the expression of procaspase-9, Bid, Bad, Bim, Bcl-xL, Bax, and Bak. CC, untransfected control cells; vector, cells transfected with pcDNA4 empty vector; H5N1-NS1, cells transfected with pcDNA4-NS1. The results shown are representatives of triplicate experiments. The level of induction (n-fold) was calculated from density values for protein bands relative to the corresponding GAPDH results.
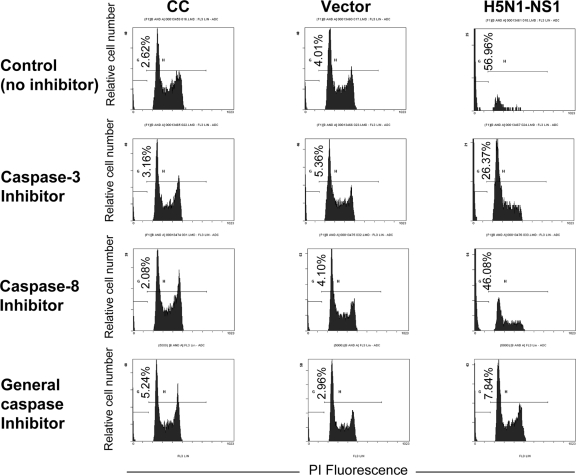
We further examined the pathway involved in NS1 protein-induced apoptosis by treating the NCI-H292 cells with inhibitors specific for caspase-3 and caspase-8 and a general inhibitor of all caspases before transfection. Cells were collected for analysis at 24 h posttransfection (Fig. 7). This time point was selected based on the observation that NS1 protein was highly expressed at 24 h posttransfection, and at this time point, both the early and late apoptotic events were detected at high levels.
FIG. 7.
Effects of caspase inhibitors on NS1 protein-induced apoptosis in NCI-H292 cells. NCI-H292 cells (106/ml) were pretreated with a caspase-3 inhibitor, a caspase-8 inhibitor, or a general caspase inhibitor before transfection. Cells were harvested at 24 h posttransfection and stained with PI at 4°C for 1 h. Apoptotic hypodiploid nuclei were detected by fluorescence-activated flow cytometry analysis. The values in the lower left corners indicate average percentages of apoptotic cells. The results shown are representatives of triplicate experiments. CC, untransfected control cells; vector, cells transfected with pcDNA4 empty vector; H5N1-NS1, cells transfected with pcDNA4-NS1.
Following the inhibition by the caspase-3 inhibitor, the caspase-8 inhibitor, and the general caspase inhibitor, apoptosis inhibition levels of 31%, 11%, and 49% were detected by PI staining (Table 1). These results further suggested the induction of apoptosis by NS1 protein via the caspase-dependent death receptor pathway.
TABLE 1.
Percentages of apoptotic cells with respect to the caspase inhibitors addeda
| Cell population | % Of apoptotic cells among cells pretreated with:
|
|||
|---|---|---|---|---|
| No inhibitor | Caspase-3 inhibitor | Caspase-8 inhibitor | General caspase inhibitor | |
| Untransfected control cells | 2.62 | 3.16 | 2.08 | 5.24 |
| Cells transfected with pcDNA4 empty vector | 4.01 | 5.36 | 4.10 | 2.96 |
| Cells transfected with pcDNA4-NS1 | 56.96 | 26.37 | 46.08 | 7.84 |
| % Inhibition of apoptosis relative to level of apoptosis with no inhibitor | 30.59 | 10.88 | 49.12 | |
Apoptotic cells were counted by PI staining at 24 h posttransfection.
Fas ligand (FasL) levels and effects of Fas ligands on NS1 induction of apoptosis.
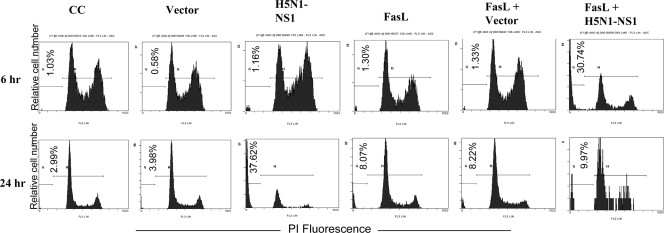
Interestingly, the ability of H5N1 NS1 protein to induce apoptosis in FasL-pretreated cells was much enhanced compared to that in nonpretreated cells. Among cells transfected with H5N1 NS1, the proportion of apoptotic cells detected at 24 h posttransfection was 37.6%. However, among FasL-pretreated cells, the proportion of apoptotic cells achieved as early as 6 h posttransfection was 30.7% (Fig. 8). These results showed that bronchial epithelial NCI-H292 cells could respond to the FasL apoptotic stimulus and that this stimulus also enhanced the apoptotic induction by the H5N1 NS1 protein.
FIG. 8.
Effect of Fas ligand (FasL) pretreatment on NS1 protein-induced apoptosis in NCI-H292 cells. NCI-H292 cells (106/ml) were pretreated or not treated with FasL (2 ng/ml) for 1 h before transfection. Cells were harvested at 6 and 24 h posttransfection and stained with PI at 4°C for 1 h. Apoptotic hypodiploid nuclei were detected by fluorescence-activated flow cytometry. The values in the lower left corners indicate average percentages of apoptotic cells. The results shown are a representative of triplicate experiments. CC, untransfected control cells; vector, cells transfected with pcDNA4 empty vector; H5N1-NS1, cells transfected with pcDNA4-NS1.
At 6 h posttransfection, no significant difference in the levels of Fas and FasL mRNAs in the FasL-pretreated, transfected cells compared to those in the corresponding controls was observed (Fig. 9A). However, at 24 h posttransfection, there was a 5.6-fold increase in FasL expression in cells transfected with H5N1 NS1 but not pretreated with FasL; in the cells transfected with H5N1 NS1 and pretreated with FasL, there was a 44.9-fold increase in FasL expression (Fig. 9B).
FIG. 9.
Expression of Fas and Fas ligand (FasL) genes in NCI-H292 cells upon FasL pretreatment and H5N1 NS1 protein expression. NCI-H292 cells were not treated or were pretreated with FasL (2 ng/ml) for 1 h before being transfected with H5N1 NS1 or pcDNA4 empty vector. Six hours (A) and 24 h (B) posttransfection, the levels of Fas and FasL mRNAs were measured. FL, cells pretreated with FasL only; FL-H5N1(NS1), FasL-pretreated cells transfected with H5N1 NS1; FL-vector, FasL-pretreated cells transfected with pcDNA4 empty vector; H5N1-NS1, cells transfected with pcDNA4-NS1 without FasL pretreatment; vector only, cells transfected with pcDNA4 empty vector without FasL pretreatment; control, untreated and untransfected control cells. Comparative threshold cycle methods were used to analyze the results using beta-actin mRNA as the endogenous control. Results are expressed as means ± SD of results from three independent experiments.
DISCUSSION
Most of the in vitro studies of influenza virus-induced apoptosis have been carried out with monocyte/macrophage cell lines (3, 27). However, it should be noted that respiratory epithelial cells rather than these immune cells are the primary target of virus replication. Furthermore, these immune cells will not be present in large numbers until they have been recruited into the area at a later stage of infection. In fact, by using human biopsy or brush samples, it has been shown previously that in the normal healthy respiratory epithelium, immune cells typically make up less than 1% (bronchial epithelium) or 2% (nasal epithelium) of the cell population (9). Since apoptosis was observed mainly in the alveolar epithelial cells of autopsy specimens taken from patients who died of H5N1 infection (52), it is important to use respiratory epithelial cells as a model to study H5N1-induced apoptosis.
The reason why influenza virus induces apoptosis has been hotly debated (31). It was thought previously that apoptosis is primarily a host defense mechanism limiting virus replication and that influenza virus overcomes this mechanism by rapid multiplication before apoptosis occurs (23). However, there is now evidence to show that the induction of apoptosis is essential for influenza virus mRNA synthesis, as well as for virus propagation (46, 47, 54, 55). Furthermore, H5N1 viral RNA was detected previously in the lungs and tracheas of infected subjects, where apoptosis and inflammation were both prominent. In contrast, only viral RNA was detected in the livers, but apoptosis and inflammation were not observed. These findings may suggest that apoptosis plays an important role in mediating inflammation and the subsequent tissue damage in severe H5N1 infections (52).
NS1 is the only influenza virus protein that has been shown to be both pro- and antiapoptotic in infected cells (18). It has been shown previously that the H5N9 NS1 protein induces apoptosis in MDCK and HeLa cells (41). However, another study has reported that NS1 from H1N1 or H3N2 recombinant viruses down-regulates apoptosis in MDCK and Vero cells (31, 58). Furthermore, NS1 from H5N1 has been shown previously to reduce lymphoid depletion in an in vivo mouse model (18). Recently, there has been a report showing that NS1 from H1N1 mediates antiapoptotic signaling responses (11). At present, it is still not certain whether the NS1 protein is proapoptotic or antiapoptotic. The diverse observations may also be a result of differences in influenza virus subtypes and strains, as well as the host cell system being used in the experiments. Therefore, a detail characterization of the protein and the mechanisms involved in the induction of apoptosis is essential for understanding the pathogenesis of influenza viruses, particularly H5N1 (22).
In the present study, the ability of H5N1 NS1 protein to induce apoptosis in NCI-H292 cells was verified by several apoptosis detection assays. These assays target different stages of apoptosis. The annexin V staining assay detects the early phase of apoptosis, whereas the TUNEL assay and PI staining detect DNA fragmentation that occurs at a later stage of apoptosis. The annexin V staining results showed that the early stage of apoptosis was most readily detectable at 24 h posttransfection, whereas at 30 h posttransfection, DNA fragmentation was readily detected by PI staining and the TUNEL assay.
Our study of apoptotic protein activities and caspase inhibition effects confirmed that the apoptosis-inducing ability of H5N1 NS1 protein was mediated mainly via the death receptor signaling cascades (40). Our results also indicated that the pretreatment of NCI-H292 cells with FasL greatly increased the apoptosis-inducing property of the H5N1 NS1 protein. This phenomenon may be explained by the homology between H5N1 NS1 protein and the cytoplasmic domain of the proapoptotic Fas antigen (15, 17, 19). Apoptosis can occur through the ligation of cell surface death receptors such as Fas/CD95, a member of the tumor necrosis factor receptor family (20, 24, 56). This event leads to the recruitment and activation of an adapter protein, FADD (Fas-associated death domain-containing protein), resulting in the activation of caspases that exist as inactive zymogens in normal cells (8, 39). The apical target of FADD is caspase-8/FLICE (2, 32). Activated caspase-8 is able to cleave additional downstream caspases, including caspase-3, that mediate apoptosis.
A recent study based on the infection of primary blood macrophages with whole virus in vitro has also shown that H5N1 can induce a delayed onset of apoptosis compared to that induced by H1N1 (27). Similar to our findings, the caspase cascade was also found to be involved in H5N1-induced apoptosis (27). Taken together, these data indicate that NS1 is one of the key influenza virus-encoded proteins that trigger the caspase cascade, resulting in apoptosis. However, whether the NS1 proteins from different virus subtypes are associated with different patterns of onset of apoptosis and, thus, different implications for pathogenesis needs to be further investigated. In addition, the apoptotic effects of the H5N1 NS1 protein may also differ between human and nonhuman (e.g., avian and mammalian) hosts.
The apoptotic nature of NS1 may also contribute to the hypercytokinemic state of humans infected with H5N1. Evidence is accumulating to support the idea that apoptosis and inflammation are linked in influenza infection (3, 15, 35, 36, 51, 52). For example, it has been shown previously that a proinflammatory cytokine, interleukin-8, is secreted upon the induction of apoptosis in bronchiolar epithelial cells by Fas ligation (7, 13, 15, 21).
The present study provides evidence that the NS1 protein encoded by avian influenza A virus H5N1 can induce apoptosis in human respiratory epithelial cells via the death receptor caspase pathway. Since the apoptotic destruction of host cells may contribute to severe disease, any drug that can prevent this process may reduce disease severity and improve clinical outcomes (34). Hence, if either the NS1 protein or the Fas ligand plays a crucial role in inducing apoptosis, then blocking the production or the action of either of these may have a therapeutic benefit for humans with H5N1 influenza infections. For example, Fas and NS1 gene expression can be blocked by specific small interfering RNAs, and the action of the Fas ligand may be blocked using a Fas fusion protein. Therefore, further investigations as to whether NS1 and/or the Fas ligand may be worthwhile therapeutic targets for ameliorating the outcome of avian influenza infection in humans should be considered.
Acknowledgments
This study was supported by the Research Fund for the Control of Infectious Diseases from the Health, Welfare and Food Bureau of the Hong Kong Special Administrative Region government. Part of the work was performed at the Lo Kwee Cheong Research Laboratory.
Footnotes
Published ahead of print on 16 January 2008.
REFERENCES
- 1.Ashkenazi, A., and V. M. Dixit. 1998. Death receptors: signaling and modulation. Science 2811305-1308. [DOI] [PubMed] [Google Scholar]
- 2.Balachandran, S., P. C. Roberts, T. Kipperman, K. N. Bhalla, R. W. Compans, D. R. Archer, and G. N. Barber. 2000. Alpha/beta interferons potentiate virus-induced apoptosis through activation of the FADD/caspase-8 death signaling pathway. J. Virol. 741513-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brydon, E. W., S. J. Morris, and C. Sweet. 2005. Role of apoptosis and cytokines in influenza virus morbidity. FEMS Microbiol. Rev. 29837-850. [DOI] [PubMed] [Google Scholar]
- 4.Chan, P. K. 2002. Outbreak of avian influenza A(H5N1) virus infection in Hong Kong in 1997. Clin. Infect. Dis. 34(Suppl. 2)S58-S64. [DOI] [PubMed] [Google Scholar]
- 5.Chanturiya, A. N., G. Basanez, U. Schubert, P. Henklein, J. W. Yewdell, and J. Zimmerberg. 2004. PB1-F2, an influenza A virus-encoded proapoptotic mitochondrial protein, creates variably sized pores in planar lipid membranes. J. Virol. 786304-6312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, W., P. A. Calvo, D. Malide, J. Gibbs, U. Schubert, I. Bacik, S. Basta, R. O'Neill, J. Schickli, P. Palese, P. Henklein, J. R. Bennink, and J. W. Yewdell. 2001. A novel influenza A virus mitochondrial protein that induces cell death. Nat. Med. 71306-1312. [DOI] [PubMed] [Google Scholar]
- 7.Cheung, C. Y., L. L. Poon, A. S. Lau, W. Luk, Y. L. Lau, K. F. Shortridge, S. Gordon, Y. Guan, and J. S. Peiris. 2002. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet 3601831-1837. [DOI] [PubMed] [Google Scholar]
- 8.Chinnaiyan, A. M., K. O'Rourke, M. Tewari, and V. M. Dixit. 1995. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell 81505-512. [DOI] [PubMed] [Google Scholar]
- 9.Danel, C., S. C. Erzurum, N. G. McElvaney, and R. G. Crystal. 1996. Quantitative assessment of the epithelial and inflammatory cell populations in large airways of normals and individuals with cystic fibrosis. Am. J. Respir. Crit. Care Med. 153362-368. [DOI] [PubMed] [Google Scholar]
- 10.de Jong, M. D., and T. T. Hien. 2006. Avian influenza A (H5N1). J. Clin. Virol. 352-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehrhardt, C., T. Wolff, S. Pleschka, O. Planz, W. Beermann, J. G. Bode, M. Schmolke, and S. Ludwig. 2007. Influenza A virus NS1 protein activates the PI3K/Akt pathway to mediate antiapoptotic signaling responses. J. Virol. 813058-3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fesq, H., M. Bacher, M. Nain, and D. Gemsa. 1994. Programmed cell death (apoptosis) in human monocytes infected by influenza A virus. Immunobiology 190175-182. [DOI] [PubMed] [Google Scholar]
- 13.Fujimoto, I., T. Takizawa, Y. Ohba, and Y. Nakanishi. 1998. Co-expression of Fas and Fas-ligand on the surface of influenza virus-infected cells. Cell Death Differ. 5426-431. [DOI] [PubMed] [Google Scholar]
- 14.Gustafsson, A. B., and R. A. Gottlieb. 2007. Bcl-2 family members and apoptosis, taken to heart. Am. J. Physiol. Cell Physiol. 292C45-C51. [DOI] [PubMed] [Google Scholar]
- 15.Hagimoto, N., K. Kuwano, M. Kawasaki, M. Yoshimi, Y. Kaneko, R. Kunitake, T. Maeyama, T. Tanaka, and N. Hara. 1999. Induction of interleukin-8 secretion and apoptosis in bronchiolar epithelial cells by Fas ligation. Am. J. Respir. Cell Mol. Biol. 21436-445. [DOI] [PubMed] [Google Scholar]
- 16.Hierholzer, J. C., E. Castells, G. G. Banks, J. A. Bryan, and C. T. McEwen. 1993. Sensitivity of NCI-H292 human lung mucoepidermoid cells for respiratory and other human viruses. J. Clin. Microbiol. 311504-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hinshaw, V. S., C. W. Olsen, N. Dybdahl-Sissoko, and D. Evans. 1994. Apoptosis: a mechanism of cell killing by influenza A and B viruses. J. Virol. 683667-3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hyland, L., R. Webby, M. R. Sandbulte, B. Clarke, and S. Hou. 2006. Influenza virus NS1 protein protects against lymphohematopoietic pathogenesis in an in vivo mouse model. Virology 349156-163. [DOI] [PubMed] [Google Scholar]
- 19.Ito, T., Y. Kobayashi, T. Morita, T. Horimoto, and Y. Kawaoka. 2002. Virulent influenza A viruses induce apoptosis in chickens. Virus Res. 84 27-35. [DOI] [PubMed] [Google Scholar]
- 20.Itoh, N., S. Yonehara, A. Ishii, M. Yonehara, S. Mizushima, M. Sameshima, A. Hase, Y. Seto, and S. Nagata. 1991. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell 66233-243. [DOI] [PubMed] [Google Scholar]
- 21.Julkunen, I., K. Melen, M. Nyqvist, J. Pirhonen, T. Sareneva, and S. Matikainen. 2000. Inflammatory responses in influenza A virus infection. Vaccine 19(Suppl. 1)S32-S37. [DOI] [PubMed] [Google Scholar]
- 22.Krug, R. M., W. Yuan, D. L. Noah, and A. G. Latham. 2003. Intracellular warfare between human influenza viruses and human cells: the roles of the viral NS1 protein. Virology 309181-189. [DOI] [PubMed] [Google Scholar]
- 23.Kurokawa, M., A. H. Koyama, S. Yasuoka, and A. Adachi. 1999. Influenza virus overcomes apoptosis by rapid multiplication. Int. J. Mol. Med. 3527-530. [DOI] [PubMed] [Google Scholar]
- 24.Lehmann, C., H. Sprenger, M. Nain, M. Bacher, and D. Gemsa. 1996. Infection of macrophages by influenza A virus: characteristics of tumour necrosis factor-alpha (TNF alpha) gene expression. Res. Virol. 147123-130. [DOI] [PubMed] [Google Scholar]
- 25.Li, Z., Y. Jiang, P. Jiao, A. Wang, F. Zhao, G. Tian, X. Wang, K. Yu, Z. Bu, and H. Chen. 2006. The NS1 gene contributes to the virulence of H5N1 avian influenza viruses. J. Virol. 8011115-11123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lowy, R. J., and D. S. Dimitrov. 1997. Characterization of influenza virus-induced death of J774.1 macrophages. Exp. Cell Res. 234249-258. [DOI] [PubMed] [Google Scholar]
- 27.Mok, C. K., D. C. Lee, C. Y. Cheung, M. Peiris, and A. S. Lau. 2007. Differential onset of apoptosis in influenza A virus. J. Gen. Virol. 881275-1280. [DOI] [PubMed] [Google Scholar]
- 28.Mori, I., T. Komatsu, K. Takeuchi, K. Nakakuki, M. Sudo, and Y. Kimura. 1995. In vivo induction of apoptosis by influenza virus. J. Gen. Virol. 762869-2873. [DOI] [PubMed] [Google Scholar]
- 29.Morris, S. J., G. E. Price, J. M. Barnett, S. A. Hiscox, H. Smith, and C. Sweet. 1999. Role of neuraminidase in influenza virus-induced apoptosis. J. Gen. Virol. 80137-146. [DOI] [PubMed] [Google Scholar]
- 30.Morris, S. J., H. Smith, and C. Sweet. 2002. Exploitation of the herpes simplex virus translocating protein VP22 to carry influenza virus proteins into cells for studies of apoptosis: direct confirmation that neuraminidase induces apoptosis and indications that other proteins may have a role. Arch. Virol. 147961-979. [DOI] [PubMed] [Google Scholar]
- 31.Morris, S. J., K. Nightingale, H. Smith, and C. Sweet. 2005. Influenza A virus-induced apoptosis is a multifactorial process: exploiting reverse genetics to elucidate the role of influenza A virus proteins in virus-induced apoptosis. Virology 335198-211. [DOI] [PubMed] [Google Scholar]
- 32.Muzio, M., A. M. Chinnaiyan, F. C. Kischkel, K. O'Rourke, A. Shevchenko, J. Ni, C. Scaffidi, J. D. Bretz, M. Zhang, R. Gentz, M. Mann, P. H. Krammer, M. E. Peter, and V. M. Dixit. 1996. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell 85817-827. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura, M., G. Matute-Bello, W. C. Liles, S. Hayashi, O. Kajikawa, S. M. Lin, C. W. Frevert, and T. R. Martin. 2004. Differential response of human lung epithelial cells to Fas-induced apoptosis. Am. J. Pathol. 1641949-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parrino, J., R. S. Hotchkiss, and M. Bray. 2007. Prevention of immune cell apoptosis as potential therapeutic strategy for severe infections. Emerg. Infect. Dis. 13191-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pirhonen, J., T. Sareneva, M. Kurimoto, I. Julkunen, and S. Matikainen. 1999. Virus infection activates IL-1β and IL-18 production in human macrophages by a caspase-1-dependent pathway. J. Immunol. 1627322-7329. [PubMed] [Google Scholar]
- 36.Pirhonen, J., T. Sareneva, I. Julkunen, and S. Matikainen. 2001. Virus infection induces proteolytic processing of IL-18 in human macrophages via caspase-1 and caspase-3 activation. Eur. J. Immunol. 31726-733. [DOI] [PubMed] [Google Scholar]
- 37.Price, G. E., H. Smith, and C. Sweet. 1997. Differential induction of cytotoxicity and apoptosis by influenza virus strains of differing virulence. J. Gen. Virol. 782821-2829. [DOI] [PubMed] [Google Scholar]
- 38.Roberts, N. J., Jr., and J. E. Nichols. 1989. Regulation of lymphocyte proliferation after influenza virus infection of human mononuclear leukocytes. J. Med. Virol. 27179-187. [DOI] [PubMed] [Google Scholar]
- 39.Roulston, A., R. C. Marcellus, and P. E. Branton. 1999. Viruses and apoptosis. Annu. Rev. Microbiol. 53577-628. [DOI] [PubMed] [Google Scholar]
- 40.Salvesen, G. S., and V. M. Dixit. 1997. Caspases: intracellular signaling by proteolysis. Cell 91443-446. [DOI] [PubMed] [Google Scholar]
- 41.Schultz-Cherry, S., and V. S. Hinshaw. 1996. Influenza virus neuraminidase activates latent transforming growth factor beta. J. Virol. 708624-8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schultz-Cherry, S., N. Dybdahl-Sissoko, G. Neumann, Y. Kawaoka, and V. S. Hinshaw. 2001. Influenza virus NS1 protein induces apoptosis in cultured cells. J. Virol. 757875-7881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seo, S. H., E. Hoffmann, and R. G. Webster. 2004. The NS1 gene of H5N1 influenza viruses circumvents the host anti-viral cytokine responses. Virus Res. 103107-113. [DOI] [PubMed] [Google Scholar]
- 44.Shan, B., and W. H. Lee. 1994. Deregulated expression of E2F-1 induces S-phase entry and leads to apoptosis. Mol. Cell. Biol. 148166-8173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shen, Y., and T. E. Shenk. 1995. Viruses and apoptosis. Curr. Opin. Genet. Dev. 5105-111. [DOI] [PubMed] [Google Scholar]
- 46.Stasakova, J., B. Ferko, C. Kittel, S. Sereinig, J. Romanova, H. Katinger, and A. Egorov. 2005. Influenza A mutant viruses with altered NS1 protein function provoke caspase-1 activation in primary human macrophages, resulting in fast apoptosis and release of high levels of interleukins 1beta and 18. J. Gen. Virol. 86185-195. [DOI] [PubMed] [Google Scholar]
- 47.Stray, S. J., and G. M. Air. 2001. Apoptosis by influenza viruses correlates with efficiency of viral mRNA synthesis. Virus Res. 773-17. [DOI] [PubMed] [Google Scholar]
- 48.Takizawa, T., S. Matsukawa, Y. Higuchi, S. Nakamura, Y. Nakanishi, and R. Fukuda. 1993. Induction of programmed cell death (apoptosis) by influenza virus infection in tissue culture cells. J. Gen. Virol. 742347-2355. [DOI] [PubMed] [Google Scholar]
- 49.Takizawa, T., C. Tatematsu, K. Ohashi, and Y. Nakanishi. 1999. Recruitment of apoptotic cysteine proteases (caspases) in influenza virus-induced cell death. Microbiol. Immunol. 43245-252. [DOI] [PubMed] [Google Scholar]
- 50.Teodoro, J. G., and P. E. Branton. 1997. Regulation of apoptosis by viral gene products. J. Virol. 711739-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.To, K. F., P. K. Chan, K. F. Chan, W. K. Lee, W. Y. Lam, K. F. Wong, N. L. Tang, D. N. Tsang, R. Y. Sung, T. A. Buckley, J. S. Tam, and A. F. Cheng. 2001. Pathology of fatal human infection associated with avian influenza A H5N1 virus. J. Med. Virol. 63242-246. [DOI] [PubMed] [Google Scholar]
- 52.Tumpey, T. M., X. Lu, T. Morken, S. R. Zaki, and J. M. Katz. 2000. Depletion of lymphocytes and diminished cytokine production in mice infected with a highly virulent influenza A (H5N1) virus isolated from humans. J. Virol. 746105-6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Uiprasertkul, M., R. Kitphati, P. Puthavathana, R. Kriwong, A. Kongchanagul, K. Ungchusak, S. Angkasekwinai, K. Chokephaibulkit, K. Srisook, N. Vanprapar, and P. Auewarakul. 2007. Apoptosis and pathogenesis of avian influenza A (H5N1) virus in humans. Emerg. Infect. Dis. 13708-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.World Health Organization. 25 June 2007, posting date. Cumulative number of confirmed human cases of avian influenza A/(H5N1) reported to WHO. World Health Organization, Geneva, Switzerland. http://www.who.int/csr/disease/avian_influenza/country/cases_table_2007_06_25/en/index.html.
- 55.Wurzer, W. J., O. Planz, C. Ehrhardt, M. Giner, T. Silberzahn, S. Pleschka, and S. Ludwig. 2003. Caspase 3 activation is essential for efficient influenza virus propagation. EMBO J. 222717-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wurzer, W. J., C. Ehrhardt, S. Pleschka, F. Berberich-Siebelt, T. Wolff, H. Walczak, O. Planz, and S. Ludwig. 2004. NF-kappaB-dependent induction of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and Fas/FasL is crucial for efficient influenza virus propagation. J. Biol. Chem. 27930931-30937. [DOI] [PubMed] [Google Scholar]
- 57.Yuen, K. Y., P. K. Chan, M. Peiris, D. N. Tsang, T. L. Que, K. F. Shortridge, P. T. Cheung, W. K. To, E. T. Ho, R. Sung, and A. F. Cheng. 1998. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet 351467-471. [DOI] [PubMed] [Google Scholar]
- 58.Zhirnov, O. P., A. L. Ksenofontov, S. G. Kuzmina, and H. D. Klenk. 2002. Interaction of influenza A virus M1 matrix protein with caspases. Biochemistry (Moscow) 67534-539. [DOI] [PubMed] [Google Scholar]
- 59.Zhirnov, O. P., T. E. Konakova, T. Wolff, and H. D. Klenk. 2002. NS1 protein of influenza A virus down-regulates apoptosis. J. Virol. 761617-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]