Abstract
The Wnt/β-catenin pathway is involved in cell functions governing development and disease. In modeling postentry restriction of human immunodeficiency virus (HIV) replication in astrocytes, we reported that part of this natural resistance to productive replication of HIV in astrocytes involved expression of proteins of the Wnt/β-catenin signaling pathway. We determined here whether induction of β-catenin signaling in peripheral blood mononuclear cells (PBMCs) can modulate HIV replication. Given that lithium is an inducer of β-catenin signaling, we used it as a tool to determine the impact of β-catenin signaling on HIV replication in PBMCs. We demonstrated that lithium inhibited the replication of T-tropic and primary isolates of HIV by >90% and did so in noncytotoxic/noncytostatic concentrations and in a β-catenin-dependent manner. Specifically, inhibiting β-catenin signaling by transfection of dominant-negative mutant constructs to either T-cell factor 4, the downstream effector of Wnt signaling, or β-catenin, the central mediator of this pathway, abrogated the ability of lithium to inhibit HIV replication. Moreover, when Wnt/β-catenin signaling was inhibited, the level of HIV replication was enhanced by fourfold. To confirm the in vivo relevance of the β-catenin pathway in repressing HIV replication, we evaluated HIV-positive antiretroviral therapy-naive patients who were on lithium therapy. These patients demonstrated a reduction in viral load, which increased as the dose of lithium was reduced. Collectively, these data indicate that β-catenin signaling is an intrinsic molecular pathway restricting HIV replication in PBMCs.
Although human immunodeficiency virus (HIV) predominately infects CD4+ T cells, it can enter a number of cells types that restrict its replication postentry (1). Understanding virus-host interaction that leads to limited HIV replication in these cell types can be a powerful tool to identify molecular mechanisms of innate/intrinsic restriction of HIV replication. We previously evaluated the mechanism by which astrocytes restrict postentry HIV replication in the brain. These studies indicated that active Wnt/β-catenin signaling in astrocytes is associated with limited HIV replication in these target cells (2).
Wnts are a family of 19 soluble secreted glycoproteins that are involved in signal transduction pathways that regulate the transcriptional activity of hundreds of genes that impact cell differentiation, communication, apoptosis/survival, and proliferation (21, 22). Wnt signaling is initiated by binding of the Wnt protein to the seven-transmembrane Frizzled family of receptors. After binding of Wnt to its receptor, Frizzled-dependent pathways are activated, specifically the canonical or Wnt/β-catenin pathway, the Wnt/Ca+2 pathway, or the planar cell polarity pathway. Binding of Wnt to Frizzled in the presence of the Wnt coreceptor low-density lipoprotein receptor-related protein 5/6 leads to activation of the β-catenin pathway. Wnt/β-catenin signaling leads to the inhibition of GSK-3, which thereby stabilizes β-catenin, leading to its accumulation in cells. β-Catenin then binds to the lymphoid enhancer binding factor (LEF)/T-cell factor (TCF) family of transcription factors (LEF1, TCF-1, TCF-3, and TCF-4), resulting in regulation of gene transcription.
Given that Wnt/β-catenin signaling is associated with repression of HIV replication in astrocytes, we evaluated the role of β-catenin signaling on HIV replication in permissive targets (peripheral blood mononuclear cells [PBMCs]). Because Wnt signaling is prominent in hematopoiesis and especially in thymopoiesis but its activity is diminished in mature lymphocytes (31), we used lithium as a tool to up-regulate β-catenin signaling in PBMCs. Lithium inhibits the activity of both the α and β isoforms of glycogen synthase kinase 3 (GSK-3) (10, 15), resulting in the activation of β-catenin signaling (10, 15, 33). We demonstrate here that activation of β-catenin signaling inhibits while inhibition of β-catenin signaling enhances HIV replication. Taken together, these data demonstrate that the β-catenin signaling pathway is a repressor of HIV replication in PBMCs and highlight this pathway for future therapeutic interventions that will exploit the interaction between β-catenin signaling and HIV.
MATERIALS AND METHODS
Cell culture.
PBMCs were isolated by Ficoll-Hypaque density gradient centrifugation from venous blood collected from healthy laboratory workers. PBMCs were suspended in RPMI 1640 medium (Biowhittaker; Walkersville, MD) supplemented with 10% heat-inactivated fetal bovine serum (Sigma, St Louis, MO), 1% penicillin-streptomycin (Gibco-BRL, Grand Island, NY), 2 mM l-glutamine (Gibco-BRL, Grand Island, NY), and human recombinant interleukin 2 (IL-2) (20 U/ml; AIDS Research Reagent and Reference Program, Germantown, MD). The cells were subsequently stimulated with 1 μg/ml soluble anti-CD3 and anti-CD28 antibodies (PharMingen, San Diego, CA), as indicated. J1.1 cells were cultured in complete medium as indicated above but without IL-2. To induce HIV from the latently infected J1.1 cell line, cells were stimulated with 100 U/ml of tumor necrosis factor alpha (TNF-α) (R&D Systems, Minneapolis, MN) for 24 h. Lithium was purchased from Sigma (St. Louis, MO) and filter sterilized.
HIV infection.
PBMCs were infected with either the laboratory-adapted CXCR4-utilizing HIV IIIB isolate or a CCR5-utilizing primary isolate, 302151 (93US151). Chemokine coreceptor usage was determined using the P4-R5 MAGI system as needed (AIDS Research and Reference Reagent Program, Germantown, MD). HIV infection of PBMCs was performed by incubating PBMCs with HIV at 10 ng p24/1 × 106 cells for 2 h at 37°C. Subsequently, unbound virus was removed by washing the cells at least twice. The cells were then cultured in complete medium supplemented with IL-2. In some experiments, lithium chloride (Sigma) was added in various amounts (0 to 1 mM) postinfection and cells were cultured at 37°C in 5% CO2. HIV replication was monitored 7 days postinfection by harvesting the supernatants, lysing the virions with 10% triton X-100 for 1 h at 37°C, and measuring HIV p24 by conventional enzyme-linked immunosorbent assay (ELISA) (AIDS vaccine program, Fredrick, MD).
Cell viability, apoptosis, and cell turnover assay.
Cell viability was monitored using the trypan blue exclusion assay. The level of apoptosis was evaluated by an annexin V/propidium iodide (PI) flow-based assay. This assay was performed according to the manufacturer's instructions (BD Biosciences, Franklin Lakes, NJ). Cell turnover was evaluated using the carboxyfluorescein succinimidyl ester (CFSE) dye tracking assay. Briefly, PBMCs were stained with CFSE according to the manufacturer's instructions (BD Biosciences), stimulated with anti-CD3/CD28, or left unstimulated, and LiCl (0 to 25 mM) was added if appropriate. Dilution of the CSFE dye was monitored at time zero and 48, 72, and 96 h of treatment. All flow-based assay analyses were performed using a FACSCalibur flow cytometer utilizing CELLQuest software (BD Biosciences, Franklin Lakes, NJ).
DNA constructs.
TOPflash and FOPflash constructs are widely used to evaluate β-catenin-dependent signaling events that drive the expression of TCF (13, 17). TOPflash is a TCF reporter plasmid containing two sets of three copies of wild-type TCF binding sites driven by the thymidine kinase minimal prompter and upstream of a luciferase reporter gene. FOPflash contains mutated TCF binding sites driven by the same thymidine kinase promoter and also upstream of the same luciferase open reading frame as TOPflash. FOPflash is used as a negative control for TOPflash activity. Both TOPflash and FOPflash were purchased from Upstate Cell Signaling Solutions (Billerica, MA). The Renilla luciferase internal control vector is driven by the cytomegalovirus (CMV) immediate-early promoter region and was purchased from Promega (Madison, WI). The vector encoding green fluorescent protein (GFP) is also driven by the CMV immediate-early promoter region and was purchased from Amaxa (Gaithersburg, MD). The TCF-4 and β-catenin dominant-negative (DN) mutant constructs were a gift from James O'Kelly (University of California, Los Angeles) and Jane B. Trepel (Center for Cancer Research, National institutes of Health, Bethesda, MD), respectively. Both constructs were described previously (3, 35). Briefly, The TCF-4 DN construct has the DNA binding domain but lacks the N terminus, which is required for β-catenin binding. The β-catenin DN construct lacks the N- and C-terminal domains necessary for β-catenin to function as a transcription coactivator (30). Both DN constructs block β-catenin signaling (3, 16, 35).
Transfection.
PBMCs were transfected using the human T-cell nucleofector kit, as recommended by the manufacturer (Amaxa; Gaithersburg, MD). To measure basal Wnt activity, 5 × 106 PBMCs were transfected with 3 μg of either TOPflash, FOPflash, or GFP along with 0.03 μg Renilla CMV construct. Luciferase reporter activity was measured by the dual-luciferase assay according to the manufacturer's protocol (Promega, Madison WI), using a Monolight 2010 luminometer (BD Biosciences) and normalizing to Renilla luciferase relative light unit values. Loss-of-function studies were performed by inhibiting endogenous TCF or β-catenin activity by transfecting 3 × 106 to 5 × 106 PBMCs with their respective DN construct at 5 to 10 μg of DNA per experimental condition. The total DNA amount was consistent within each experiment. Twenty-four hours posttransfection, PBMCs were infected with HIV, and HIV levels were measured by conventional ELISA on day 7 postinfection.
Western blot.
β-Catenin expression was evaluated by conventional Western blotting. Cell lysates containing 10 μg of protein were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (Hy-bond C Super; Amersham). The membrane was incubated with mouse anti-human active β-catenin (US Biological) at 0.5 μg/ml for 2 h and then with a 1:5,000 dilution of horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G antibody (Imgenex; San Diego, CA). The signals were revealed by enhanced chemiluminescence Western blotting (Amersham) and visualized by autoradiography. Subsequently the membranes were stripped, blocked, and blotted with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody as a control for equal amounts of proteins in all samples tested. Signal intensities of bands were measured using a conventional densitometer and analyzed using the Scion image software from NIH. The background band intensity was subtracted from all values.
Extraction of patient data.
A retrospective study was conduced at The Ruth M. Rothstein CORE Center (Chicago, IL) with approval from the Institutional Review Board of the John H. Stroger, Jr. Hospital of Cook County. The CORE Center is a freestanding inner-city outpatient comprehensive care clinic that provides care to 6,000 HIV-infected patients. The CORE center provides primary care, laboratory testing, specialty consultations, and mental health care at a single site. We identified HIV patients who are concurrently being treated with lithium for psychiatric disorders. Of the 22 HIV-positive patients on lithium, 16 patients were on antiretroviral therapy (ART) and 6 were ART naive. Two ART-naive patients were excluded for lack of complete records, and one ART-naive patient was excluded because lithium was initiated at the last visit with no additional evaluations. From their medical records, the following information was extracted: age, gender, race, time of HIV diagnosis, viral load, CD4 counts, lithium dose, ART regimen, and any other drug usage.
Statistical analysis.
The data were analyzed using GraphPad Instat 3 software (San Diego, CA). Based on data distribution, either paired or unpaired testes were used. P values of <0.05 were considered significant.
RESULTS
Induction of β-catenin signaling in PBMCs.
We evaluated basal and inducible levels of β-catenin signaling in PBMCs by measuring protein expression of the central mediator of this pathway (β-catenin) and by measuring the transcriptional activity of a β-catenin-responsive luciferase reporter. To evaluate β-catenin expression, PBMCs were isolated from healthy individuals and treated with LiCl at its therapeutic plasma level (1 mM) (7) and at a higher dose (5 mM). Lithium chloride is a well-established inducer of β-catenin (10, 15, 27, 33). Also, because HIV replication is dependent on lymphocyte activation, lymphocytes were stimulated with anti-CD3/CD28 at 1 μg/ml each or left unstimulated as a control. Expression of active β-catenin (the dephosphorylated form) was evaluated by Western blotting at 24 h. A431 cells constitutively express β-catenin, and their lysate was used as a positive control. The GAPDH housekeeping gene was used as a loading control. LiCl induced active β-catenin in both unstimulated and stimulated PBMCs (Fig. 1A and B). Interestingly, anti-CD3/CD28 stimulation also induced β-catenin expression but to a lower magnitude than that with lithium stimulation (Fig. 1A and B). These data demonstrate that the basal level of β-catenin in PBMCs is low in resting cells but that it can be up-regulated in response to T-cell activation (anti-CD3/CD28 costimulation) and more potently in response to lithium treatment. These data confirm that lithium induces Wnt/β-catenin signaling and demonstrate that it can also occur in PBMCs.
FIG. 1.
Induction of β-catenin signaling in PBMCs. (A) PBMCs from healthy donors were left unstimulated or stimulated with anti-CD3/CD28 with or without LiCl at 1 mM or 5 mM. Western blotting was performed at 24 h for dephosphorylated (active) β-catenin or GAPDH expression. The positive control for active β-catenin is the A431 extract, which constitutively expresses β-catenin (US Biological, Swampscott, MA). Data are representative of at least three independent experiments. (B) Band intensities from the Western blots were measured using a conventional densitometer and analyzed using Scion software. Background intensity was subtracted from all measurements. Asterisk denote P values of <0.005 between unstimulated and stimulated cultures under the indicated lithium dose, as evaluated by the unpaired t test. (C) PBMCs were stimulated with anti-CD3/CD28 for 48 h, treated with LiCl (1 mM) for 24 h, and transfected with either GFP, TOPflash, or FOPflash along with a Renilla construct. At 24 h posttransfection, luciferase activity was measured using the dual-luciferase system. Data are presented as n-fold increases in firefly luciferase (LUC) over Renilla LUC activity. Data are representative of two independent experiments performed in triplicate. The asterisk denotes a P value of <0.01 in a comparison between untreated and lithium-treated TOPflash-transfected cells, as determined by the Mann-Whitney test.
To determine whether the observed increase in β-catenin levels led to activation of β-catenin-dependent transcription, PBMCs from healthy donors were transfected with a luciferase construct containing four native TCF/LEF binding sites (TOPflash) or its negative-control counterpart (FOPflash) containing four mutated LEF/TCF binding sites. A construct of GFP was used as the negative control. PBMCs were also cotransfected with an internal plasmid control (Renilla). Transfected PBMCs were left untreated or treated with LiCl at 1 mM and 5 mM. Luciferase activity was evaluated 24 h posttransfection and normalized to Renilla. Basal expression of TCF/LEF was low in PBMCs, as measured by TCF/LEF transcriptional activity, which was similar to that for the GFP control (Fig. 1C). Lithium treatment up-regulated β-catenin signaling by fourfold in TOPflash-transfected cultures but not in FOPflash-transfected cultures (Fig. 1C).
Induction of β-catenin signaling inhibits HIV replication.
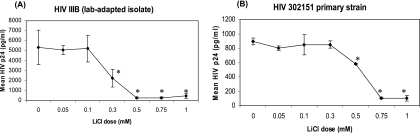
Given that lithium activates β-catenin signaling in PBMCs, we assessed whether lithium can modulate HIV replication in these cells. PBMCs were stimulated with anti-CD3/CD28, treated with LiCl at various doses (0 to 1 mM), and then infected with either a CXCR4 lab-adapted isolate of HIV (HIV IIIB) (26) or a CCR5-utilizing primary isolate of HIV 302151 (also known as HIV 93US151) (NIH AIDS Research and Reference Reagent Program, Germantown, MD). HIV replication was evaluated by measuring the HIV p24 core protein at day 7 postinfection using conventional ELISA. LiCl inhibited HIV IIIB by 50% at 0.3 mM, with >90% inhibition by 0.5 mM (Fig. 2A). Inhibition of the primary isolate by LiCl was also observed, but it required a higher dose of LiCl (0.75 mM) to reach >90% inhibition (Fig. 2B). These data indicate that treating PBMCs with LiCl leads to substantial inhibition of HIV replication of both primary and laboratory isolates of HIV.
FIG. 2.
Induction of Wnt signaling inhibits HIV replication. PBMCs were stimulated with anti-CD3/CD28, infected with 10 ng of HIV IIIB (A) or 302151 (B), and treated with 0 to 1 mM LiCl. HIV replication was determined by measuring p24 by ELISA at day 7. Data are representative of three experiments performed in triplicate. Asterisks denotes P values of <0.01, as determined by the Dunnett multiple-comparisons test between no lithium and respective lithium doses.
To exclude that the ability of lithium to inhibit HIV may be mediated by cytotoxic or cytostatic effects, we evaluated the impact of lithium on cell viability and proliferation. PBMCs were stimulated with anti-CD3/CD28 and treated with LiCl at 0 to 25 mM. Cell viability was evaluated at 24, 48, and 72 h by the trypan blue exclusion assay and costaining for annexin V and PI. At all of the doses tested, LiCl did not impact cell viability as determined by the trypan blue exclusion assay and staining for necrotic (PI-positive) and apoptotic (annexin V-positive) cells (Fig. 3A and B), even at doses that far exceeded the 0.5 to 0.75 mM doses demonstrated to inhibit HIV.
FIG. 3.
Lithium inhibition of HIV replication occurs at a noncytotoxic/noncytostatic dose. To determine the impact of lithium on cell viability and proliferation, PBMCs were stimulated with anti-CD3/CD28 for 24 h and then treated with LiCl at the dose indicated. Cell viability was measured by trypan blue exclusion assay (A) or annexin V/PI staining (B). In panel C, PBMCs were isolated and left unstimulated or stimulated with anti-CD3/CD28 for 48 h and then loaded with CFSE tracking dye and treated with 0, 1, 5, or 25 mM LiCl. Dilution of CFSE was evaluated by flow cytometry 96 h after lithium treatment. Data represent triplicate experiments.
To evaluate the impact of lithium on cell proliferation, PBMCs were loaded with CFSE, stimulated with anti-CD3/CD28, and then treated with 1, 5, or 25 mM LiCl or left without lithium treatment. Dilution of CFSE was measured by flow cytometry at 96 h. At lower doses (1 mM and 5 mM), lithium had no effect on cell turnover, but at a higher dose (25 mM), lithium reduced cell division (Fig. 3C). These data demonstrate that lithium inhibits HIV replication by >90% at 0.5 to 0.75 mM, depending on the HIV isolate used, which is a dose that is within the recommended therapeutic plasma level of lithium (0.6 to 1.2 mM) (7) and is neither cytotoxic nor cytostatic.
Lithium inhibits HIV replication in PBMCs through activation of β-catenin signaling.
To determine whether lithium inhibition of HIV is mediated through its activation of the β-catenin pathway, PBMCs from healthy donors were stimulated with anti-CD3/CD28 for 48 h and then transfected with a DN construct of TCF-4, β-catenin, or GFP and then infected with HIV IIIB or primary strain 302151 (93US151). After 24 h, lithium at 1 mM was added to the cultures, and HIV p24 ELISA was performed on day 7 postinfection. As seen in Fig. 4, inhibiting either the down-stream effector of the Wnt signaling pathway (TCF-4) or the central mediator of this pathway (β-catenin) by using their respective DN mutant constructs abrogated the ability of lithium to inhibit the replication of HIV strain IIIB and primary HIV strain 302151. These data indicate that lithium inhibits HIV through inducing β-catenin signaling.
FIG. 4.
Lithium inhibits HIV through the Wnt/β-catenin pathway. PBMCs from healthy donors were stimulated with anti-CD3/CD28 for 48 h, transfected with a DN construct of TCF-4, β-catenin, or GFP, and then infected with HIV IIIB (A) or primary strain 30215 (B). After 24 h, lithium at 1 mM was added to the cultures. HIV p24 ELISA was performed on day 7 postinfection. Asterisks denotes P values of <0.01, as determined by the Dunnett multiple-comparisons test between cultures without the DN mutants and those with the DN mutants, whether using GFP or Li as the comparative arm.
Inhibition of endogenous β-catenin signaling induces HIV replication.
We also observed that the level of HIV replication was enhanced with inhibition of the β-catenin pathway, by approximately fourfold (Fig. 4), suggesting that inhibiting the endogenous/basal level of β-catenin in PBMCs enhances HIV replication. We evaluated this phenomenon by exploring the role of endogenous β-catenin signaling in regulation of HIV replication. Specifically, stimulated PBMCs were transfected with either DN-TCF-4, DN-β-catenin, or GFP or treated with a cell-permeating N-acetyl-Leu-Leu-norleucinal (ALLN) peptide, which inhibits proteasome-mediated proteolysis and induces the accumulation of cellular β-catenin(24, 32). Twenty-four hours post-transfection or ALLN treatment, the cells were infected with HIV IIIB or strain 302151, and HIV replication was measured by p24 ELISA on day 7. Efficiency of transfection at day 2 posttransfection was approximately 59% as measured by GFP expression (data not shown). Inhibiting the activity of endogenous TCF-4 or β-catenin induced HIV replication by approximately fourfold in comparison to results for mock- or GFP-transfected cultures, independent of the HIV isolate used (Fig. 5A and B). Further, treating HIV-infected PBMCs with the ALLN peptide at either 5 or 25 μM also induced HIV IIIB and 30215 replication by three- and fivefold, respectively, in comparison to results for none-ALLN treated cultures (Fig. 5C and D). These data demonstrate that endogenous Wnt/β-catenin activity is an intrinsic host mechanism that represses HIV replication, removing this repression enhances HIV replication in PBMCs, and up-regulating this pathway by lithium treatment reduces HIV replication.
FIG. 5.
Inhibiting basal Wnt activity enhances replication of HIV IIIB or primary strain 302151. PBMCs were stimulated with anti-CD3/CD28 for 48 h and then transfected with GFP, DN-TCF-4, or DN-β-catenin prior to infection with HIV IIIB (A) or 302151 (B). (C and D) PBMCs were stimulated with anti-CD3/CD28, infected with HIV IIIB (C) or 302151 (D) for 2 h, washed extensively, and treated with either 5 or 25 μM ALLN peptide or left untreated. HIV p24 levels were measured 7 days post-infection or transfection. Asterisks denote P values of <0.01 in comparison to no lithium treatment with TNF-α, as determined by the Dunnett multiple-comparisons test.
Lithium inhibits reactivation of latent HIV.
To evaluate whether lithium can also inhibit reactivation of latent HIV, we used the J1.1 latent-infection cell model. J1.1 is a latently infected Jurkat cell line whereby HIV is reactivated with TNF-α or mitogen stimulation (25). J1.1 cells were treated with TNF-α (100 U/ml) with or without lithium (0.25 to 1,000 μM) and HIV replication evaluated by p24 ELISA 3 days poststimulation. Lithium inhibited TNF-α-mediated induction of HIV replication by >80% starting at 2 μM (Fig. 6).
FIG. 6.
Lithium inhibits TNF-α-mediated induction of HIV. J1.1 cells were treated with TNF-α (100 U/ml) with or without LiCl (0 to 1,000 μM) for 3 days and HIV replication monitored by p24 ELISA 3 days posttreatment. The basal level of HIV replication in J1.1 (without TNF-α treatment) was <50 pg/ml. Data represent a minimum of three experiments. Asterisks denotes P values of <0.01 in comparison to no lithium treatment with TNF-α, as determined by the Dunnett multiple-comparisons test.
HIV-positive ART-naive patients on lithium demonstrate lower HIV viral load.
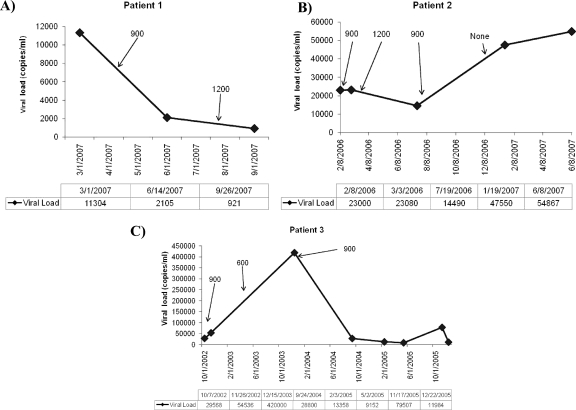
To confirm the in vivo relevance of the β-catenin pathway as a repressor of HIV replication, we performed a medical-chart review of patients identified as HIV positive and concurrently on lithium treatment for bipolar disorder at The Ruth M. Rothstein CORE Center (Chicago, IL). Of the 22 HIV-positive patients on lithium at the CORE center, three patients were ART naive and were further evaluated for longitudinal changes in viral load with lithium treatment (Table 1). These HIV-positive ART-naive patients on lithium demonstrated a remarkable reduction in their viral load with lithium treatment, which increased as the lithium dose was reduced or lithium treatment was stopped. Specifically, patient 1 demonstrated a 12-fold reduction in viral load with continued lithium treatment, while patient 2 showed a fourfold increase in viral load with termination of lithium treatment (Fig. 7A and B). Interestingly, patient 3 demonstrated both of these observations with a sevenfold increase in viral load upon a decrease of lithium treatment from 900 mg/day to 600 mg/day, which was completely abrogated (35-fold viral load decrease) with a return to 900 mg lithium per day (Fig. 7C). While there is a significant effect of lithium treatment on the viral load, we did not observe a correlation between lithium treatment and the CD4 count. We also observed a significant decrease in viral load among HIV patients who had detectable viremia on stable ART when lithium was started, with some patients achieving a plasma HIV-1 RNA level of <75 copies/ml after the initiation of lithium (data not shown). However, for these ART-experienced patients, it is difficult to attribute the reduction in viral load solely to lithium usage, since these patients may also have become more compliant with ART as a consequence of lithium treatment stabilizing their psychiatric disorder.
TABLE 1.
Profiles of ART-naive, HIV-positive patients on lithium
| Donor | Age (yr) | Sexa | Ethnicity | Yr of HIV dxb | ART | Low CD4 count (cells/mm3) | High CD4 count (cells/mm3) |
|---|---|---|---|---|---|---|---|
| Patient 1 | 44 | M | White | 2004 | None | 514 | 708 |
| Patient 2 | 42 | F | Non-Hispanic black | 2004 | None | 322 | 674 |
| Patient 3 | 34 | F | White | 2000 | None | 222 | 560 |
M, male; F, female.
dx, diagnosis.
FIG. 7.
Lithium treatment affects viral load in HIV-positive, ART-negative patients. Viral loads from HIV-positive patients who are ART naive and are concurrently being treated with lithium for bipolar disorders are shown. Arrows identify known lithium treatment doses at specified times.
DISCUSSION
A number of endogenous host factors have been found to restrict HIV replication in nonpermissive cells, including APOPEC 3G and TRIM5a. We demonstrate here that the β-catenin signaling pathway is also an endogenous inhibitor of HIV replication in PBMCs. The mechanism by which active β-catenin signaling inhibits HIV replication is still not clearly delineated.
Studies with astrocytes, which restrict HIV replication pre- and postentry (8), have identified the downstream effector of the Wnt/β-catenin pathway, TCF-4, as a repressor of HIV transcription (2, 19, 28, 34). TCF-4 represses basal and Tat-mediated transactivation of the HIV long terminal repeat (LTR), but the details of this interaction are also not clearly defined (34). It is presumed, albeit for astrocytes, that the TCF-4-mediated inhibition of HIV LTR activity occurs in a β-catenin-independent manner (34). While our data are consistent with the observation that TCF-4 is a repressor of HIV replication, we provide evidence to indicate that in PBMCs this repression is β-catenin dependent, as demonstrated by abrogation of the inhibitory effect when β-catenin activity was inhibited though its respective DN mutant.
It is likely that TCF-4 forms a multiprotein complex on the HIV LTR that leads to repression of HIV transcription. We previously demonstrated that TCF-4 is part of a transcriptional complex that is immunoprecipitated with the HIV LTR between +1 to +160 of the transcription initiation site when HIV replication is repressed but not in association with productive HIV replication (2). Others have reported that TCF-4 also binds to Tat, forming a stable complex detected in the cytoplasm and nucleus (9). TCF-4 was also reported to interact with Sp-1 and inhibit its ability to transactivate the HIV LTR (28). Therefore, TCF-4 may bind to the LTR alone or in association with other proteins, leading to transcriptional repression. A multiprotein complex was recently identified that inhibits HIV transcription, whereby c-Myc, Sp-1, and histone deacetylase all tether on the HIV LTR and induce its repression, promoting a state of latency (14). Findings that TCF-4 binds Sp-1 and that c-Myc is under the gene regulation of the β-catenin signaling pathway suggest that TCF-4 may also be a component of this multiprotein HIV repression complex. Understanding the details of how these proteins tether on the HIV LTR is helpful in understanding signals that induce HIV latency, which may be exploited for novel HIV therapy or even in strategies to purge the latent HIV reservoir.
Our findings also identify lithium as having anti-HIV properties. Lithium is a commonly used drug for treatment of bipolar disorder. It has been in clinical use for over 50 years. At therapeutic plasma levels, lithium inhibits the activities of both the α and β isoforms of GSK-3 (10, 15), resulting in the activation of β-catenin signaling (10, 15, 27, 33). However, at higher concentrations (4 to 5 mM), lithium inhibits inositol monophosphatase, inositol trisphosphate, and inositol polyphosphatase, leading to a decreased response. The dose of lithium (0.3 to 0.75 mM) determined previously to inhibit HIV and subsequently used here was ideal for Wnt/β-catenin activation and not inositol trisphosphate effects. We further determined that inhibiting β-catenin activity abrogates the ability of lithium to suppress HIV replication. The lithium-mediated inhibition occurred at a dose that was neither cytotoxic nor cytostatic for PBMCs. Lithium, however, inhibited cell turnover at a higher dose (25 mM). This observation is consistent with a number of reports, albeit not for PBMCs, demonstrating that at doses exceeding 10 mM, lithium causes cell cycle arrest (4, 9, 29). Finally, our observation that lithium inhibited TNF-α-mediated induction of HIV in J1.1 cells (an NF-κB-dependent mechanism) at a much lower dose (2 μM) than de novo infection (1 mM) suggests that lithium may also be inhibiting NF-κB. GSK-3β induces NF-κB function (12). Therefore, since lithium inhibits GSK-3β, this may lead to NF-κB inhibition as well. In J1.1 cells, lithium may be activating a repressor (Wnt/β-catenin) and inhibiting an activator (NF-κB) of HIV, leading to potent suppression at a lower dose. Alternatively, lithium is known to induce soluble TNF-α receptor, which may absorb the exogenous TNF-α, thereby reducing its effects on reactivation of latent HIV (11).
Although antiretroviral drugs have successfully targeted components of the viral life cycle, such as entry and fusion, reverse transcription, integration, and viral protein processing, viral mutations against these targets continue to diminish the efficacy of ART, necessitating new approaches. The fact that lithium is inexpensive and is easily administered suggests that lithium treatment may be of benefit in developing countries with limited access to current ART. Side effects of lithium are well known, with weight gain, hypothyroidism, and tremor being most common. Toxicities and most side effects can be managed by initial and periodic monitoring of lithium serum levels and monitoring of thyroid and renal functions. Several animal and human studies further suggest a benefit of lithium administration in neuroprotection in HIV (5, 6, 18, 20) and in Alzheimer's disease (23). Specifically, lithium prevents gp120- and Tat-induced HIV neurodegeneration in vitro (5, 20) and increases soluble TNF-α receptor, which absorbs the neurotoxic TNF-α cytokine (11). Lithium therapy significantly improved HIV-associated neurocognitive impairment and was well tolerated among HIV-positive patients at doses of 600 to 1,200 mg/day, with no grade 3 or 4 adverse effects (18).
Our data indicate that the Wnt/β-catenin pathway, which regulates TCF-4 activity, is an intracellular innate pathway that restricts HIV replication in PBMCs. Understanding the interaction between HIV and the Wnt/β-catenin pathway will allow for a better understanding of host-HIV interactions and more importantly for translating this relationship between Wnt/β-catenin and HIV into the development of either commercially available reagents or new drugs to activate and/or inactivate this pathway to regulate the extent of HIV replication in target cells.
Acknowledgments
The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: HTLV-IIIB/H9 from Robert Gallo, HIV isolate 302151 (revised nomenclature, HIV-1 93US151) from Cecelia Hutto, J1.1 cell line from Thomas Folks, P4.R5 MAGI from Nathaniel Landau, and human rIL-2 from Maurice Gately at Hoffmann-La Roche, Ltd. We thank Toni Fredrick (University of California Keck School of Medicine, Los Angeles, CA) for statistical consultation.
This work was supported by a grant from the Department of Immunology/Microbiology at Rush University Medical Center (Chicago, IL). R.T.M. is an investigator of HHMI.
Footnotes
Published ahead of print on 16 January 2008.
REFERENCES
- 1.Al-Harthi, L., and A. Landay. 2001. Alternative targets of productive HIV infection: role of CD4 up-regulation on susceptibility of cells to HIV infection. AIDS Rev. 367-74. [Google Scholar]
- 2.Carroll-Anzinger, D., A. Kumar, V. Adarichev, F. Kashanchi, and L. Al-Harthi. 2007. human immunodeficiency virus-restricted replication in astrocytes and the ability of gamma interferon to modulate this restriction are regulated by a downstream effector of the Wnt signaling pathway. J. Virol. 815864-5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung, E. J., S. G. Hwang, P. Nguyen, S. Lee, J. S. Kim, J. W. Kim, P. A. Henkart, D. P. Bottaro, L. Soon, P. Bonvini, S. J. Lee, J. E. Karp, H. J. Oh, J. S. Rubin, and J. B. Trepel. 2002. Regulation of leukemic cell adhesion, proliferation, and survival by beta-catenin. Blood 100982-990. [DOI] [PubMed] [Google Scholar]
- 4.Erdal, E., N. Ozturk, T. Cagatay, E. Eksioglu-Demiralp, and M. Ozturk. 2005. Lithium-mediated downregulation of PKB/Akt and cyclin E with growth inhibition in hepatocellular carcinoma cells. Int. J. Cancer 115903-910. [DOI] [PubMed] [Google Scholar]
- 5.Everall, I. P., C. Bell, M. Mallory, D. Langford, A. Adame, E. Rockestein, and E. Masliah. 2002. Lithium ameliorates HIV-gp120-mediated neurotoxicity. Mol. Cell Neurosci. 21493-501. [DOI] [PubMed] [Google Scholar]
- 6.Gallicchio, V. S., M. L. Cibull, N. K. Hughes, and K. F. Tse. 1993. Effect of lithium in murine immunodeficiency virus infected animals. Pathobiology 61216-221. [DOI] [PubMed] [Google Scholar]
- 7.Georgotas, A., and S. Gershon. 1979. Lithium plasma levels. Psychopharmacol. Bull. 1535-37. [PubMed] [Google Scholar]
- 8.Gorry, P. R., C. Ong, J. Thorpe, S. Bannwarth, K. A. Thompson, A. Gatignol, S. L. Vesselingh, and D. F. Purcell. 2003. Astrocyte infection by HIV-1: mechanisms of restricted virus replication, and role in the pathogenesis of HIV-1-associated dementia. Curr. HIV Res. 1463-473. [DOI] [PubMed] [Google Scholar]
- 9.Hasgekar, N. N., P. P. Gokhale, M. K. Amin, R. Seshadri, and V. S. Lalitha. 1996. Lithium inhibits growth in a murine neural precursor cell line. Cell Biol. Int. 20781-786. [DOI] [PubMed] [Google Scholar]
- 10.Hedgepeth, C. M., L. J. Conrad, J. Zhang, H. C. Huang, V. M. Lee, and P. S. Klein. 1997. Activation of the Wnt signaling pathway: a molecular mechanism for lithium action. Dev. Biol. 18582-91. [DOI] [PubMed] [Google Scholar]
- 11.Himmerich, H., D. Koethe, A. Schuld, A. Yassouridis, and T. Pollmacher. 2005. Plasma levels of leptin and endogenous immune modulators during treatment with carbamazepine or lithium. Psychopharmacology (Berlin) 179447-451. [DOI] [PubMed] [Google Scholar]
- 12.Hoeflich, K. P., J. Luo, E. A. Rubie, M. S. Tsao, O. Jin, and J. R. Woodgett. 2000. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature 40686-90. [DOI] [PubMed] [Google Scholar]
- 13.Ishitani, T., J. Ninomiya-Tsuji, S. Nagai, M. Nishita, M. Meneghini, N. Barker, M. Waterman, B. Bowerman, H. Clevers, H. Shibuya, and K. Matsumoto. 1999. The TAK1-NLK-MAPK-related pathway antagonizes signalling between beta-catenin and transcription factor TCF. Nature 399798-802. [DOI] [PubMed] [Google Scholar]
- 14.Jiang, G., A. Espeseth, D. J. Hazuda, and D. M. Margolis. 2007. c-Myc and Sp1 contribute to proviral latency by recruiting histone deacetylase 1 to the human immunodeficiency virus type 1 promoter. J. Virol. 8110914-10923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein, P. S., and D. A. Melton. 1996. A molecular mechanism for the effect of lithium on development. Proc. Natl. Acad. Sci. USA 938455-8459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korinek, V., N. Barker, P. J. Morin, D. van Wichen, R. de Weger, K. W. Kinzler, B. Vogelstein, and H. Clevers. 1997. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science 2751784-1787. [DOI] [PubMed] [Google Scholar]
- 17.Korinek, V., N. Barker, K. Willert, M. Molenaar, J. Roose, G. Wagenaar, M. Markman, W. Lamers, O. Destree, and H. Clevers. 1998. Two members of the Tcf family implicated in Wnt/beta-catenin signaling during embryogenesis in the mouse. Mol. Cell. Biol. 181248-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Letendre, S. L., S. P. Woods, R. J. Ellis, J. H. Atkinson, E. Masliah, G. van den Brande, J. Durelle, I. Grant, and I. Everall. 2006. Lithium improves HIV-associated neurocognitive impairment. AIDS 201885-1888. [DOI] [PubMed] [Google Scholar]
- 19.Lu, Y., D. Q. Sheng, Z. C. Mo, H. F. Li, N. H. Wu, and Y. F. Shen. 2005. A negative regulatory element-dependent inhibitory role of ITF2B on IL-2 receptor alpha gene. Biochem. Biophys. Res. Commun. 336142-149. [DOI] [PubMed] [Google Scholar]
- 20.Maggirwar, S. B., N. Tong, S. Ramirez, H. A. Gelbard, and S. Dewhurst. 1999. HIV-1 Tat-mediated activation of glycogen synthase kinase-3beta contributes to Tat-mediated neurotoxicity. J. Neurochem. 73578-586. [DOI] [PubMed] [Google Scholar]
- 21.Miller, J. R., and R. T. Moon. 1996. Signal transduction through beta-catenin and specification of cell fate during embryogenesis. Genes Dev. 102527-2539. [DOI] [PubMed] [Google Scholar]
- 22.Moon, R. T., J. D. Brown, and M. Torres. 1997. WNTs modulate cell fate and behavior during vertebrate development. Trends Genet. 13157-162. [DOI] [PubMed] [Google Scholar]
- 23.Nunes, P. V., O. V. Forlenza, and W. F. Gattaz. 2007. Lithium and risk for Alzheimer's disease in elderly patients with bipolar disorder. Br. J. Psychiatry 190359-360. [DOI] [PubMed] [Google Scholar]
- 24.Orford, K., C. Crockett, J. P. Jensen, A. M. Weissman, and S. W. Byers. 1997. Serine phosphorylation-regulated ubiquitination and degradation of beta-catenin. J. Biol. Chem. 27224735-24738. [DOI] [PubMed] [Google Scholar]
- 25.Perez, V. L., T. Rowe, J. S. Justement, S. T. Butera, C. H. June, and T. M. Folks. 1991. An HIV-1-infected T cell clone defective in IL-2 production and Ca2+ mobilization after CD3 stimulation. J. Immunol. 1473145-3148. [PubMed] [Google Scholar]
- 26.Popovic, M., M. G. Sarngadharan, E. Read, and R. C. Gallo. 1984. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science 224497-500. [DOI] [PubMed] [Google Scholar]
- 27.Rao, A. S., N. Kremenevskaja, J. Resch, and G. Brabant. 2005. Lithium stimulates proliferation in cultured thyrocytes by activating Wnt/beta-catenin signalling. Eur. J. Endocrinol. 153929-938. [DOI] [PubMed] [Google Scholar]
- 28.Rossi, A., R. Mukerjee, P. Ferrante, K. Khalili, S. Amini, and B. E. Sawaya. 2006. Human immunodeficiency virus type 1 Tat prevents dephosphorylation of Sp1 by TCF-4 in astrocytes. J. Gen. Virol. 871613-1623. [DOI] [PubMed] [Google Scholar]
- 29.Smits, V. A., M. A. Essers, D. S. Loomans, R. Klompmaker, G. Rijksen, and R. H. Medema. 1999. Inhibition of cell proliferation by lithium is associated with interference in cdc2 activation. FEBS Lett. 45723-27. [DOI] [PubMed] [Google Scholar]
- 30.Steinhusen, U., V. Badock, A. Bauer, J. Behrens, B. Wittman-Liebold, B. Dorken, and K. Bommert. 2000. Apoptosis-induced cleavage of beta-catenin by caspase-3 results in proteolytic fragments with reduced transactivation potential. J. Biol. Chem. 27516345-16353. [DOI] [PubMed] [Google Scholar]
- 31.Timm, A., and R. Grosschedl. 2005. Wnt signaling in lymphopoiesis. Curr. Top. Microbiol. Immunol. 290225-252. [DOI] [PubMed] [Google Scholar]
- 32.van Noort, M., M. van de Wetering, and H. Clevers. 2002. Wnt signaling controls the phosphorylation status of beta-catenin. J. Biol. Chem. 2771791-1795. [DOI] [PubMed] [Google Scholar]
- 33.Williams, R. S., and A. J. Harwood. 2000. Lithium therapy and signal transduction. Trends Pharmacol. Sci. 2161-64. [DOI] [PubMed] [Google Scholar]
- 34.Wortman, B., N. Darbinian, B. E. Sawaya, K. Khalili, and S. Amini. 2002. Evidence for regulation of long terminal repeat transcription by Wnt transcription factor TCF-4 in human astrocytic cells. J. Virol. 7611159-11165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie, D., D. Yin, X. Tong, J. O'Kelly, A. Mori, C. Miller, K. Black, D. Gui, J. W. Said, and H. P. Koeffler. 2004. Cyr61 is overexpressed in gliomas and involved in integrin-linked kinase-mediated Akt and beta-catenin-TCF/Lef signaling pathways. Cancer Res. 641987-1996. [DOI] [PubMed] [Google Scholar]