Abstract
Dendritic cells (DCs) play a central role in instructing antiviral immune responses. DCs, however, can become targeted by different viruses themselves. We recently demonstrated that human DCs can be productively infected with echoviruses (EVs), but not coxsackie B viruses (CVBs), both of which are RNA viruses belonging to the Enterovirus genus of the Picornaviridae family. We now show that phagocytosis of CVB-infected, type I interferon-deficient cells induces an antiviral state in human DCs. Uptake of infected cells increased the expression of the cytoplasmic RNA helicases retinoic acid-inducible gene I and melanoma differentiation-associated gene 5 as well as other interferon-stimulated genes and protected DCs against subsequent infection with EV9. These effects depended on recognition of viral RNA and could be mimicked by exposure to the synthetic double-stranded RNA analogue poly(I:C) but not other Toll-like receptor (TLR) ligands. Blocking endosomal acidification abrogated protection, suggesting a role for TLRs in the acquisition of an antiviral state in DCs. In conclusion, recognition of viral RNA rapidly induces an antiviral state in human DCs. This might provide a mechanism by which DCs protect themselves against viruses when attracted to an environment with ongoing infection.
Dendritic cells (DCs) are crucial for the induction of antiviral immunity via instruction of both the innate and adaptive immune system (3, 4, 13). The hallmark of antiviral responses is production of type I interferons (IFNs). Although classically plasmacytoid DCs were thought to be the major producers of type I IFNs (6, 17), it has been shown that conventional DCs can produce similarly high type I IFN levels upon viral infection (11). In fact, all nucleated cells are capable of type I IFN production, resulting in the establishment of an antiviral state in neighboring cells via upregulation of so-called IFN-stimulated genes (ISGs).
DCs express a vast array of pattern recognition receptors, such as Toll-like receptors (TLRs), which enable them to recognize viral pathogen-associated molecular patterns (PAMPs) like double-stranded RNA (dsRNA) (2) and single-stranded RNA (10, 15, 24). TLRs responding to viral nucleic acids are localized within the endosomal compartment (26, 29) and are triggered upon endocytosis of viral particles or virus-infected cells but presumably do not sense cytoplasmic virus replication upon infection of the DC itself. Instead, recent studies have identified two structurally related RNA helicases, retinoic acid-inducible gene I (RIG-I) (39) and melanoma differentiation-associated gene 5 (MDA5) (18), both ISGs, as critical mediators in the response to infection with different RNA viruses in mice (14, 20, 38). Triggering of MDA5 or RIG-I causes recruitment of the shared adaptor protein IFN-β promoter stimulator 1 (IPS-1), also known as mitochondrial antiviral signaling protein (MAVS), virus-induced signaling adaptor (VISA), and CARD adaptor inducing IFN-β (Cardif) (21, 27, 33, 36), leading to induction of type I IFNs. RIG-I-deficient cells display greatly diminished type I IFN responses to various RNA viruses and in vitro-transcribed dsRNA (19, 20). Interestingly, MDA5-deficient cells are selectively unresponsive to certain picornaviruses, such as encephalomyocarditis virus and Theiler's encephalomyelitis virus as well as the synthetic dsRNA analogue poly(I:C) (14, 20). This specificity might be related to the presence or absence of 5′-triphosphate groups, which was recently reported to be a critical structure for RIG-I activation (16, 30). The essential contribution of these RNA helicases to antiviral immunity becomes evident in RIG-I- or MDA5-knockout mice that readily succumb upon infection with Japanese encephalitis virus or encephalomyocarditis virus, respectively (14, 20).
To date, most data regarding RIG-I and MDA5 are derived from mouse studies, while still little is known regarding the role of these viral sensors in human cells. We recently showed that infection of human monocyte-derived DCs with echovirus (EV), but not coxsackie B virus (CVB), results in rapid inhibition of TLR-mediated responses and massive cell death (22). As it is difficult to reconcile these in vitro effects with the generally mild clinical outcome of EV infections, we set out to investigate the conditions that could potentially alter DC susceptibility. In the present work, we demonstrate that phagocytosis of virus-infected cells results in enhanced expression of RIG-I, MDA5, and other ISGs and protects DCs against EV infection. These effects require intact endosomal acidification and depend on the presence of RNA in infected cells. Thus, DCs engage a state of antiviral resistance following recognition of viral RNA.
MATERIALS AND METHODS
Virus stocks and purification.
Reference strains echovirus 1 Farouk (EV1 Farouk), EV7 Wallace, EV8 Bryson, EV9 Hill, and EV11 Gregory were obtained from the National Institute for Public Health and the Environment (RIVM, Bilthoven, The Netherlands). Coxsackievirus B3 (CVB3) Nancy was kindly provided by R. Kandolf (University of Tübingen, Tübingen, Germany). Production of virus stocks and virus titrations were performed on buffalo green monkey cells as described previously (22). Serial 10-fold dilutions were tested in 96-well microtiter plates, and 50% tissue culture infective doses were calculated as described before (35).
Plasmids.
The EV9 Hill infectious cDNA clone (40) was generously provided by B. Nelsen-Salz (Virology Institute, University of Cologne, Cologne, Germany).
Stimulation of monocyte-derived DCs.
Monocyte-derived DCs were generated as described previously (22). Mature DCs were obtained by stimulating cells with poly(I:C) (20 μg/ml), lipopolysaccharide (LPS) (100 ng/ml), R848 (4 μg/ml), and PAM3Cys-SKKKK (PAM, 2 μg/ml) for a period of 24 h, unless indicated otherwise. To block the actions of type I IFN, cells were stimulated in the presence or absence of neutralizing anti-human type I IFN antibodies (1:100; Iivari, Kaaleppi, or bovine serum; courtesy of I. Julkunen, National Public Health Institute, Helsinki, Finland) (28). To block endosomal acidification, DCs were cultured with chloroquine (CQ, 10 μM) starting 1 h prior to poly(I:C) stimulation. For infection, immature or mature DCs were harvested using cold phosphate-buffered saline (PBS), washed, and infected at a multiplicity of infection (MOI) of 1 with indicated viruses in serum-free RPMI medium. After 60 min of incubation at 37°C, cells were washed three times in an excess volume of PBS, after which viral titers were determined at different time points postinfection (p.i.) as described above.
WB.
Equal amounts of protein were separated by 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electroblotted onto nitrocellulose membranes (Bio-Rad), followed by probing with the indicated antibodies. Anti-RIG-I and anti-protein kinase R (anti-PKR) antibodies were purchased from ProSci Incorporated and Becton Dickinson Transduction Laboratories, respectively. Production of rabbit polyclonal anti-MDA5 was described previously (23). RIG-I, PKR, and MDA5 antibodies were used in 1:1,000; 1:500, and 1:10,000 dilutions, respectively. After washes, membranes were incubated with IRDye anti-mouse or anti-rabbit immunoglobulin G (IgG) (1:15,000) (Li-Cor Biosciences). Imaging was done using the Odyssey System. Western blot (WB) analysis of viral protein 3A was done as described before (22).
Transfection of viral RNA.
pEV9Hill was linearized by digestion with NotI and transcribed in vitro with T7 RNA polymerase (Promega). DCs were harvested, washed with PBS, and resuspended in phenol-red free Optimem (Invitrogen Life Technologies). RNA electroporation was performed as described before (22).
RNA isolation.
Total RNA was isolated from DC cultures using TRIzol reagent (Invitrogen Life Technologies) according to the manufacturer's instructions, with minor modifications. RNA integrity was determined by analyzing the ribosomal 28S and 18S bands on a 1% agarose gel. The reverse transcription reaction was performed using Moloney murine leukemia virus reverse transcriptase (Invitrogen) according to the manufacturer's instructions. For each sample a “−RT” control was included in which the reverse transcriptase was replaced by diethyl pyrocarbonate-treated Milli-Q. The cDNA was stored at −20°C until further use.
qPCR.
Quantitative analysis of gene expression in DCs was done using Sybr green-based quantitative PCR (qPCR). The qPCRs were performed in a 25-μl volume containing 12.5 μl Sybr green mix (Applied Biosystems), 1.5 μl forward/reverse primer (300 nM final concentration), 4.5 μl Milli-Q, and 5 μl cDNA dilution. Reactions were performed on an ABI 7900HT Sequence Detection System (Applied Biosystems). Analysis was done using sequence detection system software (SDS, version 2.0; Applied Biosystems). Primer sequences are available upon request and were designed using the freely accessible Primer Bank program (34).
Uptake of Vero cells.
Vero cells were labeled using PKH26 (Sigma-Aldrich) according to the manufacturer's instructions and infected with CVB3 at an MOI of 10. Cells were harvested and washed 6 to 8 h p.i. and resuspended in fresh medium at a density of 5 × 106 cells/ml prior to being held at −20°C until further use. Vero cell preparations were added to DC cultures at a ratio of 1:1 (in some experiments DCs had been pretreated with CQ as described above). Alternatively, Vero cell preparations were exposed to a mixture of RNase A (Roche) and RNase V-I (Ambion) or an equal volume of PBS for a period of 15 min at 37°C prior to addition to DCs. Uptake of Vero cells by DCs was analyzed using flow cytometry and confocal microscopy.
Confocal microscopy.
DCs were harvested, washed, and allowed to adhere to poly-l-lysine-coated coverslips in serum-free medium for 1 h at 37°C. Cells were fixed with 1% paraformaldehyde and blocked in PBS with 3% bovine serum albumin, 10 mM glycine, and 2% human serum (blocking buffer [BB]). For cell surface staining, cells were incubated using mouse anti-human DC-SIGN (Beckman Coulter) or mouse-anti human HLA-DR/DP (ascites) in BB, washed, and incubated with isotype-specific Alexa-labeled goat anti-mouse IgGs (Alexa 568/Alexa 647; Molecular Probes). For intracellular staining, cells were fixed using 1% paraformaldehyde, permeabilized using 0.1% Triton X-100 in PBS, and incubated with rabbit polyclonal anti-MDA5 followed by incubation with goat anti-rabbit IgG-Alexa 488 (Molecular Probes) in BB. After final washes, cells were sealed using Mowiol (Merck) and analyzed using confocal microscopy (Bio-Rad MRC 1024).
Statistical analysis.
Statistical analysis was performed using Student's t test (two-tailed distribution). A P value < 0.05 was considered a significant difference.
RESULTS
Poly(I:C) induces resistance against viral infection in human DCs.
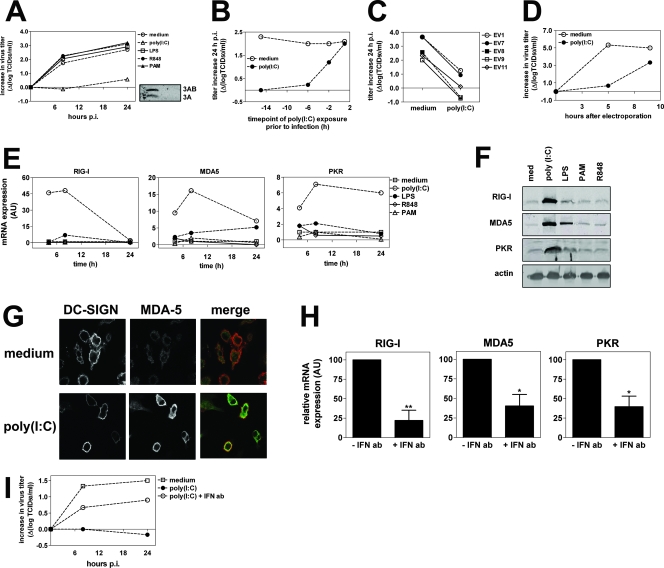
We previously reported that human DCs display a striking difference in susceptibility to infection with distinct types of Enterovirus, a genus of the Picornaviridae family. While DCs are productively infected with EV, they are resistant to infection with the closely related CVB (22). Here, we investigated the effect of stimulation with different TLR ligands on susceptibility for EV infection. Untreated DCs and DCs stimulated with LPS (TLR4), PAM3Cys (TLR1/2), or R848 (TLR7 or TLR8) were all highly susceptible to EV9 infection, leading to significant increases in virus titers and viral proteins (Fig. 1A). In contrast, stimulation with the synthetic viral dsRNA analogue poly(I:C) dramatically reduced EV9 replication (Fig. 1A), in a concentration-dependent manner (data not shown). Time course analysis showed that resistance to EV9 infection was accrued within 6 h after poly(I:C) exposure (Fig. 1B). Additionally, infection of DCs with EV1, EV7, EV8, and EV11 could also be inhibited by poly(I:C) treatment, showing that the protective effect applied to a broad range of EV strains (Fig. 1C). Poly(I:C)-stimulated DCs also displayed reduced virus replication upon delivery of in vitro-transcribed EV9 RNA directly into the DC cytoplasm, suggesting the induction of an active antiviral state, rather than simple downregulation of the (as-yet-unidentified) EV9 receptor on the cell surface (Fig. 1D).
FIG. 1.
Poly(I:C) stimulation protects human DCs against EV infection and increases expression of RIG-I, MDA5, and PKR. (A) DCs were left untreated or stimulated with poly(I:C) (20 μg/ml), LPS (100 ng/ml), R848 (4 μg/ml), or PAM3Cys-SKKKK (PAM, 2 μg/ml) for 24 h and infected with EV9 at an MOI of 1, after which viral titers were determined at several time points p.i. The inset shows the expression of viral protein 3A and its precursor 3AB in unstimulated DCs (left lane) or poly(I:C)-stimulated DCs (right lane). (B) DCs were stimulated with poly(I:C) (20 μg/ml) at different time points prior to infection with EV9 at an MOI of 1. The increase in viral titers was determined 24 h p.i. (C) DCs were left untreated or stimulated with poly(I:C) (20 μg/ml) for a period of 24 h and subsequently infected with EV1, EV7, EV8, EV9, or EV11 at an MOI of 1. Shown is the increase in viral titers at 24 h p.i. (D) DCs were stimulated with poly(I:C) (20 μg/ml) for 24 h and subsequently electroporated using 20 μg in vitro-transcribed RNA from the full-length cDNA clone of EV9. The increase in viral titers was determined at several time points after electroporation. (E) DCs were stimulated using LPS, R848, PAM, or poly(I:C) as described for panel A, and mRNA levels of RIG-I, MDA5, and PKR were determined using qPCR at several time points after stimulation. (F) Protein expression of RIG-I, MDA5, and PKR was analyzed by WB assay 24 h after stimulation of DCs as described for panel A. (G) DCs were left untreated or stimulated with poly(I:C) (20 μg/ml) for 24 h, after which DCs were harvested, stained using DC-SIGN and MDA5-specific antibodies, and analyzed using confocal microscopy as described in Materials and Methods. (H) DCs were stimulated with poly(I:C) in the presence or absence of type I IFN neutralizing antibodies (Iivari, Kaaleppi, and bovine anti-IFN-α; see Materials and Methods). After 8 h, mRNA expression levels of RIG-I, MDA5, and PKR were determined using qPCR. Shown are mean expression levels ± standard deviations of three independent experiments using different donors (*, P ≤ 0.05; **, P ≤ 0.01). (I) DCs were stimulated with poly(I:C) (20 μg/ml) in the presence or absence of type I IFN neutralizing antibodies for 24 h, washed, and subsequently infected with EV9 at an MOI of 1, after which virus titers were determined at several time points p.i. Data shown are representative of more than five (A and E), three (C, D, F, G, H, and I), or two (B) independent experiments using different donors.
To evaluate the effects of TLR triggering on expression of genes that are crucially involved in the innate antiviral response, we determined mRNA levels of the viral sensors RIG-I and MDA5 and the effector molecule PKR. Stimulation with PAM, R848, or lipoteichoic acid did not affect ISG expression, and only a modest increase was observed in some experiments using LPS. However, stimulation with poly(I:C) consistently induced a strong upregulation of RIG-I, MDA5, and PKR (Fig. 1E and data not shown). WB analysis corroborated our findings by qPCR and demonstrated elevated protein levels following exposure to poly(I:C) (Fig. 1F). Confocal analysis showed an increased expression of endogenous MDA5 in the cytoplasm of poly(I:C)-stimulated human DCs (Fig. 1G). Furthermore, neutralizing antibodies against type I IFN inhibited the effect of poly(I:C) on both expression of ISGs (Fig. 1H) and viral infection (Fig. 1I), implying that poly(I:C) exerts its effect at least in part via autocrine type I IFN stimulation.
Collectively, these data indicate that exposure to synthetic dsRNA causes upregulation of both viral sensors and effector molecules and protects DCs against EV infection.
Phagocytosis of infected cells by DCs leads to upregulation of ISGs.
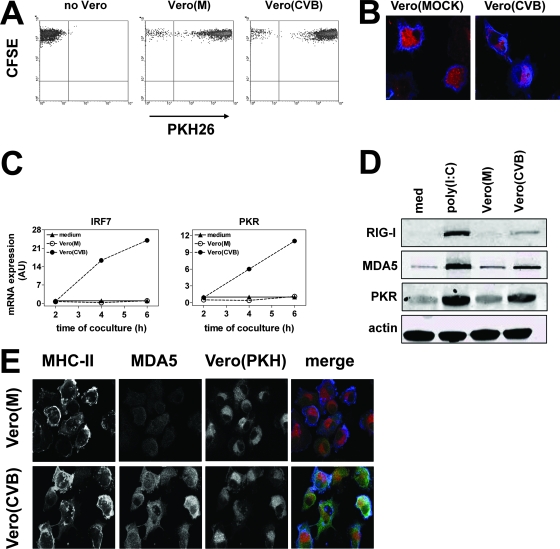
To study the DC-virus interaction in a more physiological model, we explored whether phagocytosis of CVB3-infected cells by DCs would induce effects similar to those of stimulation with poly(I:C). CVB3 was used because this virus, if released from infected (dying) cells, does not cause infection of DCs (22) or upregulation of RIG-I, MDA5, or PKR (data not shown). Since secretion of type I IFNs by infected cells could influence susceptibility for infection and ISG expression in DCs, all experiments were performed with Vero cells, which carry a genetic defect in type I IFN synthesis (9, 12). CVB3-infected, but not uninfected, Vero cells were efficiently taken up by DCs (data not shown), likely reflecting the effect of infection on Vero cell viability. For the remainder of our experiments we used freeze-thawed cell preparations that were taken up with equal efficiency, irrespective of the infection status of the cells (Fig. 2A). This enabled us to analyze the effect of uptake of uninfected or virus-infected cells on ISG expression and susceptibility in DCs. Confocal analysis confirmed the flow cytometry data by showing the presence of PKH26+ compartments within DCs (Fig. 2B). As the primers for human RIG-I and MDA5 cross-react with their Vero cell (Cercopithecus aethiops, African green monkey) homologues, we focused on mRNA expression of the ISGs IRF7 and PKR. Although uptake of mock-infected Vero cells had no effect, phagocytosis of CVB3-infected cells caused a rapid and profound upregulation of IRF7 and PKR in DCs (Fig. 2C). WB analysis showed that uptake of CVB-infected, but not of mock-infected, Vero cells resulted in increased protein levels of RIG-I, MDA5, and PKR (Fig. 2D). No ISG protein expression was detected in CVB3-infected Vero cell preparations as such (data not shown). Confocal analysis further confirmed increased MDA5 levels in DCs that had taken up CVB3-infected Vero cells compared to mock-infected cells (Fig. 2E). Thus, phagocytosis of CVB3-infected cells by DCs leads to rapid upregulation of molecules involved in the innate antiviral response and can occur independently of type I IFN released by infected cells.
FIG. 2.
Phagocytosis of virus-infected cells increases the expression of RIG-I, MDA5, and PKR. (A) PKH26-labeled Vero cell preparations (mock or CVB3 infected) were added to carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled DC cultures in a ratio of 1:1. After 24 h, the number of CFSE+ PKH26+ DCs was analyzed using flow cytometry. (B) Presence of PKH26+ Vero cell material within DCs after a 24-h coculture as analyzed using confocal microscopy. DCs were stained using anti-major histocompatibility complex class II. (C) DCs were cocultured with mock- or CVB3-infected Vero cell preparations, and the mRNA expression of PKR and IRF7 was determined using qPCR at different time points after the start of coculture. (D) DCs were cocultured with Vero cell preparations as described for panel A, and protein expression of RIG-I, MDA5, and PKR was determined using WB assay 24 h after the start of coculture. (E) DCs were cocultured with Vero cell preparations as described for panel A, and 24 h later cells were stained with major histocompatibility complex class II- and MDA5-specific antibodies and analyzed using confocal microscopy. Data shown are representative of more than six (A), four (B), or three (C to E) independent experiments using different donors.
Phagocytosis of CVB-infected cells protects DCs against EV infection.
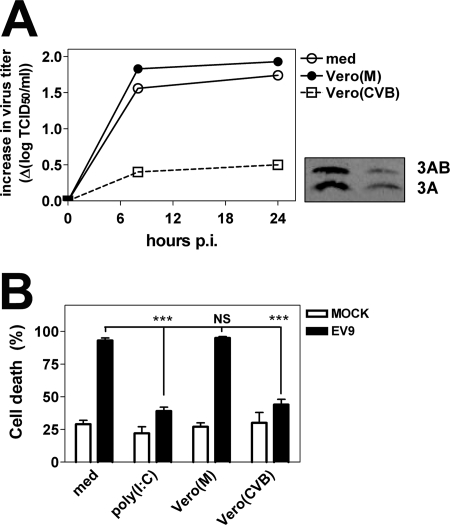
To determine whether upregulation of ISGs following uptake of infected cell preparations by DCs resulted in functional protection, DCs were subsequently infected with EV9. Vero cell preparations themselves did not support replication of EV9 (data not shown). Phagocytosis of infected preparations resulted in a markedly decreased EV replication in DC cultures, while uptake of uninfected cells had no effect (Fig. 3A). WB analysis showed notably lower levels of the viral protein 3A and its precursor 3AB in DCs that had taken up CVB-infected Vero cells, which confirmed inhibited EV growth (Fig. 3A).
FIG. 3.
Phagocytosis of infected cells protects DCs against EV infection. (A) DCs were left untreated or cultured with mock or CVB-infected Vero cell preparations for a period of 24 h, after which DCs were harvested, washed, and subsequently infected with EV9 at an MOI of 1. Viral titers were determined at different time points p.i. The inset shows the expression of viral protein 3A and its precursor 3AB following uptake of mock-infected (left lane) or CVB3-infected (right lane) Vero cells, 8 h after infection of DCs with EV9. (B) DCs were treated as described for panel A, and the percentage of dead cells was determined using a trypan blue exclusion assay 48 h p.i. The graph shows means + standard deviations of quadruplicates per condition (***, P ≤ 0.001). Data shown are representative of at least three independent experiments using different donors.
We next assessed whether phagocytosis of these preparations could protect DCs against EV9-induced cell death. As shown in Fig. 3B, EV9 caused massive cell death in both untreated DCs and DCs that had taken up uninfected cell preparations. In contrast, uptake of CVB3-infected cell preparations strongly enhanced cell survival. Also exposure to poly(I:C) decreased cell death following EV infection. Taken together, these data indicate that phagocytosis of virus-infected cells can effectively protect human DCs against the lethal effects of EV infection.
ISG upregulation in DCs following phagocytosis of infected cells requires intact endosomal acidification and is mediated via recognition of viral RNA.
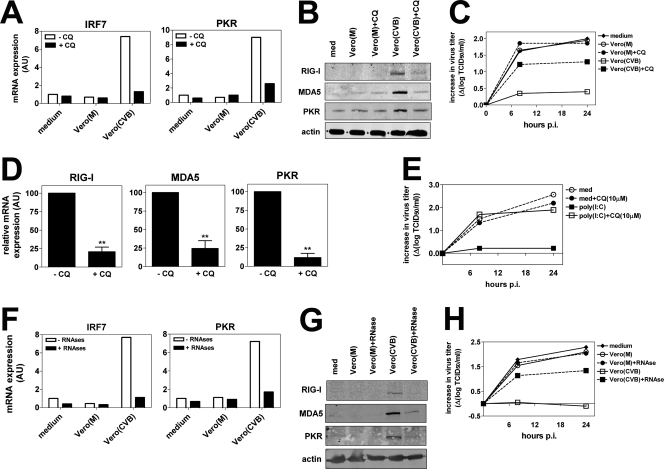
Infected, dying cells taken up by DCs are localized to so-called phagosomes, which subsequently “mature” via fusion with the endosomal/lysosomal compartments, resulting in a progressive decrease in pH (5). Interestingly, intracellular TLRs are also recruited to these compartments, enabling interaction with potentially released PAMPs, like dsRNA, which occurs in a pH-dependent fashion (8). To determine if endosomal acidification is required for the induction of viral resistance following uptake of infected cell preparations, we pretreated DCs with CQ, a chemical that blocks acidification of these compartments. Pretreatment of DCs with CQ markedly decreased mRNA expression of IRF7 and PKR (Fig. 4A) as well as RIG-I, MDA5, and PKR protein levels (Fig. 4B). Importantly, the CQ-mediated reduction in ISG levels was accompanied by reduced protection against EV infection (Fig. 4C). These effects were not related to impaired phagocytosis, since CQ had no effect on uptake of Vero cell preparations (data not shown). CQ also reduced the poly(I:C)-mediated increase in ISG expression (Fig. 4D) and completely abrogated the inhibitory effect of poly(I:C) on infection (Fig. 4E).
FIG. 4.
Induction of an antiviral state requires intact endosomal acidification and is mediated via recognition of viral RNA. (A) DCs were left untreated or preincubated with CQ (10 μM) for 1 h prior to addition of Vero cell preparations. After an 8-h coculture, cells were harvested and mRNA expression of IRF7 and PKR was analyzed using qPCR. (B) DCs were stimulated as for panel A, and protein expression of RIG-I, MDA5, and PKR was determined using WB assay 16 h after coculture. (C) DCs were stimulated as for panel A and infected with EV9 at an MOI of 1 16 h after coculture. Viral titers were determined at several time points p.i. (D) DCs were left untreated or preincubated with CQ (10 μM) prior to addition of poly(I:C) (20 μg/ml). After 8 h of stimulation, the expression of RIG-I, MDA5, PKR, and IRF7 was analyzed using qPCR. Shown are mean expression levels ± standard deviations of three independent experiments using different donors (**, P ≤ 0.01). (E) DCs were treated as described for panel A, and after 8 h of stimulation, cells were harvested, washed extensively, and infected with EV9 at an MOI of 1, after which viral titers were determined at several time points p.i. (F) Vero cell preparations were left untreated or exposed to a mixture of RNase A and RNase V-I prior to addition to DC cultures as described in Materials and Methods. Expression of PKR and IRF7 in DCs was analyzed using qPCR 8 h after addition of Vero cell preparations. (G) DCs were cocultured with Vero cell preparations as described for panel D, and protein expression of RIG-I, MDA5, and PKR was analyzed by WB assay 16 h after the start of coculture. (H) DCs were treated as described for panel D, and 16 h after the start of coculture, cells were harvested, washed, and infected with EV9 at an MOI of 1. Data are representative of three independent experiments using different donors.
We next investigated the potential contribution of viral RNA present in the infected Vero cell preparations to both upregulation of ISGs and the induction of a resistant state. To this aim, CVB3-infected and uninfected cell preparations were incubated with a mixture of RNases prior to addition to DCs. RNase treatment did not affect uptake of preparations by DCs (data not shown). Degradation of RNA resulted in a strongly reduced upregulation of IRF7 and PKR mRNA levels (Fig. 4F) and RIG-I, MDA5, and PKR protein expression in DCs (Fig. 4G). Furthermore, RNase treatment abrogated the protective effect on EV infection of DCs (Fig. 4H), demonstrating the essential role of recognition of viral RNA in the induction of an antiviral state.
DISCUSSION
Phagocytosis of infected cells represents one of the defense mechanisms against viral infection and is executed by different immune cells. Here we report that uptake of CVB3-infected cells by human DCs resulted in rapid increase in both mRNA and protein levels of viral sensors including the RNA helicases RIG-I and MDA5 and effector molecules like PKR. Upregulation of these ISGs required intact endosomal acidification, was dependent on the presence of viral RNA, and could be mimicked by exposure to the synthetic dsRNA analogue poly(I:C). Moreover, DCs that had taken up CVB-infected cells were protected against lethal infection with EV.
Recent murine studies have highlighted the role of various RIG-like helicases in recognition of viral RNA (14, 19, 20). These findings have challenged our view of the relative importance of TLRs and non-TLRs in antiviral immunity. For instance, recognition of the synthetic dsRNA mimetic poly(I:C) was classically thought to be mediated via TLR3 (2), while more recent data implicate MDA5 as a crucial component for type I IFN production upon poly(I:C) stimulation or viral infection in mice (14, 20). Very limited human data are available regarding these novel RNA sensors in the human setting, but one study shows that poly(I:C) responses in human cell lines are RIG-I dependent (7), suggesting the existence of species-specific or cell-type-specific differences, analogues to the species-specific difference in recognition of single-stranded RNAs by TLR7 and TLR8 (15).
We previously demonstrated the dramatic consequences of EV infection for the function and viability of human DCs (22). We presently document that distinct TLR ligands displayed a remarkably different effect on the induction of viral resistance in DCs. While poly(I:C) rapidly increased the level of different ISGs, including RIG-I and MDA5, and protected DCs against infection, other ligands such as PAM, lipoteichoic acid, and R848 did not. LPS caused a modest increase in ISG expression. This might imply that ISG upregulation is mediated in a TRIF-dependent fashion, as both poly(I:C) and LPS have been reported to signal via this adaptor protein (37).
To study the effects of viral RNA recognition under conditions that more closely resemble the physiological situation, we used a model system in which DCs acquired virus-infected cells via phagocytosis. Uptake of CVB3-infected cells resulted in ISG upregulation and protection against EV, both of which could be blocked by CQ. As CQ is known to increase endosomal pH and it has been shown that the interaction between poly(I:C)/TLR3 (8) and CpG/TLR9 (32) is pH dependent, this would suggest a role for TLR3 in our experiments. Alternatively, CQ could hamper transport of viral structures, such as dsRNA, to the cytoplasm where recognition by other PPRs like RIG-I or MDA5 could take place. However, a recent study has shown that CQ treatment of human DCs rather increases export of soluble antigen from early endosomes into the cytosol, thereby promoting antigen cross-presentation (1). Identification of the exact routing of viral PAMPs following uptake by DCs can possibly aid in dissecting the relative contribution of TLRs and RNA helicases to different facets of the antiviral immune response.
It has been shown that, besides viral nucleic acids, viral proteins can also alter the activation status of DCs (25, 31), which could affect DC susceptibility following phagocytosis of infected cells. Our experiments identified RNA within infected Vero cell preparations as the crucial component for both ISG upregulation and resistance against infection. Since uptake of noninfected cells had no effect, we favor a role for viral RNA in these processes. However, we cannot exclude the possibility that infection leads to modification of host RNA structures that could be subsequently recognized by different RNA sensors. The use of Vero cells in our experiments showed that phagocytosis of infected cells by DCs can induce protection independently of type I IFN released by infected cells. Thus, recognition of RNA could preserve the ability of DCs to engage an antiviral state, even when type I IFN responses in the infected cells are blocked as a consequence of viral immune evasion strategies.
In conclusion, recognition of viral RNA rapidly induces an antiviral state in human DCs. This might reveal a mechanism by which DCs protect themselves against viruses when attracted to an environment with ongoing infection, thereby facilitating adequate instruction of virus-specific T cells to clear viral infection.
Acknowledgments
We thank Bianca Blom (Amsterdam Medical Center/University of Amsterdam, Amsterdam, The Netherlands) for help with the type I IFN blocking experiments.
This work was supported by grants from The Netherlands Diabetes Foundation (DFN 2001.00.047) to M.K. and The Netherlands Organization for Scientific Research to F.J.M.V.K. (NWO-VIDI-917.46.305) and G.J.A. (NWO-912-02-034). P.M.B. and P.B.F. were supported by a National Institutes of Health General Medical Sciences grant (1R01 GM068448).
Footnotes
Published ahead of print on 9 January 2008.
REFERENCES
- 1.Accapezzato, D., V. Visco, V. Francavilla, C. Molette, T. Donato, M. Paroli, M. U. Mondelli, M. Doria, M. R. Torrisi, and V. Barnaba. 2005. Chloroquine enhances human CD8+ T cell responses against soluble antigens in vivo. J. Exp. Med. 202817-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413732-738. [DOI] [PubMed] [Google Scholar]
- 3.Andoniou, C. E., S. L. van Dommelen, V. Voigt, D. M. Andrews, G. Brizard, C. Asselin-Paturel, T. Delale, K. J. Stacey, G. Trinchieri, and M. A. Degli-Esposti. 2005. Interaction between conventional dendritic cells and natural killer cells is integral to the activation of effective antiviral immunity. Nat. Immunol. 61011-1019. [DOI] [PubMed] [Google Scholar]
- 4.Barth, H., A. Ulsenheimer, G. R. Pape, H. M. Diepolder, M. Hoffmann, C. Neumann-Haefelin, R. Thimme, P. Henneke, R. Klein, G. Paranhos-Baccala, E. Depla, T. J. Liang, H. E. Blum, and T. F. Baumert. 2005. Uptake and presentation of hepatitis C virus-like particles by human dendritic cells. Blood 1053605-3614. [DOI] [PubMed] [Google Scholar]
- 5.Blander, J. M., and R. Medzhitov. 2006. On regulation of phagosome maturation and antigen presentation. Nat. Immunol. 71029-1035. [DOI] [PubMed] [Google Scholar]
- 6.Cella, M., F. Facchetti, A. Lanzavecchia, and M. Colonna. 2000. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat. Immunol. 1305-310. [DOI] [PubMed] [Google Scholar]
- 7.Cheng, G., J. Zhong, J. Chung, and F. V. Chisari. 2007. Double-stranded DNA and double-stranded RNA induce a common antiviral signaling pathway in human cells. Proc. Natl. Acad. Sci. USA 1049035-9040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Bouteiller, O., E. Merck, U. A. Hasan, S. Hubac, B. Benguigui, G. Trinchieri, E. E. Bates, and C. Caux. 2005. Recognition of double-stranded RNA by human toll-like receptor 3 and downstream receptor signaling requires multimerization and an acidic pH. J. Biol. Chem. 28038133-38145. [DOI] [PubMed] [Google Scholar]
- 9.Desmyter, J., J. L. Melnick, and W. E. Rawls. 1968. Defectiveness of interferon production and of rubella virus interference in a line of African green monkey kidney cells (Vero). J. Virol. 2955-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diebold, S. S., T. Kaisho, H. Hemmi, S. Akira, and C. Reis e Sousa. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 3031529-1531. [DOI] [PubMed] [Google Scholar]
- 11.Diebold, S. S., M. Montoya, H. Unger, L. Alexopoulou, P. Roy, L. E. Haswell, A. Al-Shamkhani, R. Flavell, P. Borrow, and C. Reis e Sousa. 2003. Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature 424324-328. [DOI] [PubMed] [Google Scholar]
- 12.Emeny, J. M., and M. J. Morgan. 1979. Regulation of the interferon system: evidence that Vero cells have a genetic defect in interferon production. J. Gen. Virol. 43247-252. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez, N. C., A. Lozier, C. Flament, P. Ricciardi-Castagnoli, D. Bellet, M. Suter, M. Perricaudet, T. Tursz, E. Maraskovsky, and L. Zitvogel. 1999. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat. Med. 5405-411. [DOI] [PubMed] [Google Scholar]
- 14.Gitlin, L., W. Barchet, S. Gilfillan, M. Cella, B. Beutler, R. A. Flavell, M. S. Diamond, and M. Colonna. 2006. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc. Natl. Acad. Sci. USA 1038459-8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heil, F., H. Hemmi, H. Hochrein, F. Ampenberger, C. Kirschning, S. Akira, G. Lipford, H. Wagner, and S. Bauer. 2004. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 3031526-1529. [DOI] [PubMed] [Google Scholar]
- 16.Hornung, V., J. Ellegast, S. Kim, K. Brzozka, A. Jung, H. Kato, H. Poeck, S. Akira, K. K. Conzelmann, M. Schlee, S. Endres, and G. Hartmann. 2006. 5′-Triphosphate RNA is the ligand for RIG-I. Science 314994-997. [DOI] [PubMed] [Google Scholar]
- 17.Jarrossay, D., G. Napolitani, M. Colonna, F. Sallusto, and A. Lanzavecchia. 2001. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur. J. Immunol. 313388-3393. [DOI] [PubMed] [Google Scholar]
- 18.Kang, D. C., R. V. Gopalkrishnan, Q. Wu, E. Jankowsky, A. M. Pyle, and P. B. Fisher. 2002. mda-5: an interferon-inducible putative RNA helicase with double-stranded RNA-dependent ATPase activity and melanoma growth-suppressive properties. Proc. Natl. Acad. Sci. USA 99637-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kato, H., S. Sato, M. Yoneyama, M. Yamamoto, S. Uematsu, K. Matsui, T. Tsujimura, K. Takeda, T. Fujita, O. Takeuchi, and S. Akira. 2005. Cell type-specific involvement of RIG-I in antiviral response. Immunity 2319-28. [DOI] [PubMed] [Google Scholar]
- 20.Kato, H., O. Takeuchi, S. Sato, M. Yoneyama, M. Yamamoto, K. Matsui, S. Uematsu, A. Jung, T. Kawai, K. J. Ishii, O. Yamaguchi, K. Otsu, T. Tsujimura, C. S. Koh, C. Reis e Sousa, Y. Matsuura, T. Fujita, and S. Akira. 2006. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441101-105. [DOI] [PubMed] [Google Scholar]
- 21.Kawai, T., K. Takahashi, S. Sato, C. Coban, H. Kumar, H. Kato, K. J. Ishii, O. Takeuchi, and S. Akira. 2005. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol. 6981-988. [DOI] [PubMed] [Google Scholar]
- 22.Kramer, M., B. M. Schulte, L. W. Toonen, M. A. de Bruijni, J. M. Galama, G. J. Adema, and F. J. van Kuppeveld. 2007. Echovirus infection causes rapid loss-of-function and cell death in human dendritic cells. Cell. Microbiol. 91507-1518. [DOI] [PubMed] [Google Scholar]
- 23.Lin, L., Z. Su, I. V. Lebedeva, P. Gupta, H. Boukerche, T. Rai, G. N. Barber, P. Dent, D. Sarkar, and P. B. Fisher. 2006. Activation of Ras/Raf protects cells from melanoma differentiation-associated gene-5-induced apoptosis. Cell Death Differ. 131982-1993. [DOI] [PubMed] [Google Scholar]
- 24.Lund, J. M., L. Alexopoulou, A. Sato, M. Karow, N. C. Adams, N. W. Gale, A. Iwasaki, and R. A. Flavell. 2004. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. USA 1015598-5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinelli, E., C. Cicala, D. Van Ryk, D. J. Goode, K. Macleod, J. Arthos, and A. S. Fauci. 2007. HIV-1 gp120 inhibits TLR9-mediated activation and IFN-{alpha} secretion in plasmacytoid dendritic cells. Proc. Natl. Acad. Sci. USA 1043396-3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsumoto, M., K. Funami, M. Tanabe, H. Oshiumi, M. Shingai, Y. Seto, A. Yamamoto, and T. Seya. 2003. Subcellular localization of Toll-like receptor 3 in human dendritic cells. J. Immunol. 1713154-3162. [DOI] [PubMed] [Google Scholar]
- 27.Meylan, E., J. Curran, K. Hofmann, D. Moradpour, M. Binder, R. Bartenschlager, and J. Tschopp. 2005. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 4371167-1172. [DOI] [PubMed] [Google Scholar]
- 28.Mogensen, K. E., L. Pyhala, and K. Cantell. 1975. Raising antibodies to human leukocyte interferon. Acta Pathol. Microbiol. Scand. B 83443-450. [DOI] [PubMed] [Google Scholar]
- 29.Nishiya, T., E. Kajita, S. Miwa, and A. L. Defranco. 2005. TLR3 and TLR7 are targeted to the same intracellular compartments by distinct regulatory elements. J. Biol. Chem. 28037107-37117. [DOI] [PubMed] [Google Scholar]
- 30.Pichlmair, A., O. Schulz, C. P. Tan, T. I. Naslund, P. Liljestrom, F. Weber, and C. Reis e Sousa. 2006. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science 314997-1001. [DOI] [PubMed] [Google Scholar]
- 31.Rolland, A., E. Jouvin-Marche, C. Viret, M. Faure, H. Perron, and P. N. Marche. 2006. The envelope protein of a human endogenous retrovirus-W family activates innate immunity through CD14/TLR4 and promotes Th1-like responses. J. Immunol. 1767636-7644. [DOI] [PubMed] [Google Scholar]
- 32.Rutz, M., J. Metzger, T. Gellert, P. Luppa, G. B. Lipford, H. Wagner, and S. Bauer. 2004. Toll-like receptor 9 binds single-stranded CpG-DNA in a sequence- and pH-dependent manner. Eur. J. Immunol. 342541-2550. [DOI] [PubMed] [Google Scholar]
- 33.Seth, R. B., L. Sun, C. K. Ea, and Z. J. Chen. 2005. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell 122669-682. [DOI] [PubMed] [Google Scholar]
- 34.Wang, X., and B. Seed. 2003. A PCR primer bank for quantitative gene expression analysis. Nucleic Acids Res. 31e154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wessels, E., D. Duijsings, R. A. Notebaart, W. J. Melchers, and F. J. van Kuppeveld. 2005. A proline-rich region in the coxsackievirus 3A protein is required for the protein to inhibit endoplasmic reticulum-to-Golgi transport. J. Virol. 795163-5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu, L. G., Y. Y. Wang, K. J. Han, L. Y. Li, Z. Zhai, and H. B. Shu. 2005. VISA is an adapter protein required for virus-triggered IFN-beta signaling. Mol. Cell 19727-740. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto, M., S. Sato, H. Hemmi, K. Hoshino, T. Kaisho, H. Sanjo, O. Takeuchi, M. Sugiyama, M. Okabe, K. Takeda, and S. Akira. 2003. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 301640-643. [DOI] [PubMed] [Google Scholar]
- 38.Yoneyama, M., M. Kikuchi, K. Matsumoto, T. Imaizumi, M. Miyagishi, K. Taira, E. Foy, Y. M. Loo, M. Gale, Jr., S. Akira, S. Yonehara, A. Kato, and T. Fujita. 2005. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J. Immunol. 1752851-2858. [DOI] [PubMed] [Google Scholar]
- 39.Yoneyama, M., M. Kikuchi, T. Natsukawa, N. Shinobu, T. Imaizumi, M. Miyagishi, K. Taira, S. Akira, and T. Fujita. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5730-737. [DOI] [PubMed] [Google Scholar]
- 40.Zimmermann, H., H. J. Eggers, A. Zimmermann, W. Kraus, and B. Nelsen-Salz. 1995. Complete nucleotide sequence and biological properties of an infectious clone of prototype echovirus 9. Virus Res. 39311-319. [DOI] [PubMed] [Google Scholar]