Abstract
The G protein-coupled m1 and m3 muscarinic acetylcholine receptors increase tyrosine phosphorylation of several proteins, including the focal adhesion-associated proteins paxillin and focal adhesion kinase (FAK), but the mechanism is not understood. Activation of integrins during adhesion of cells to extracellular matrix, or stimulation of quiescent cell monolayers with G protein-coupled receptor ligands including bradykinin, bombesin, endothelin, vasopressin, and lysophosphatidic acid, also induces tyrosine phosphorylation of paxillin and FAK and formation of focal adhesions. These effects are generally independent of protein kinase C but are inhibited by agents that prevent cytoskeletal assembly or block activation of the small molecular weight G protein Rho. This report demonstrates that tyrosine phosphorylation of paxillin and FAK elicited by stimulation of muscarinic m3 receptors with the acetylcholine analog carbachol is inhibited by soluble peptides containing the arginine–glycine–aspartate motif (the recognition site for integrins found in adhesion proteins such as fibronectin) but is unaffected by peptides containing the inactive sequence arginine–glycine–glutamate. Tyrosine phosphorylation elicited by carbachol, but not by cell adhesion to fibronectin, is reduced by the protein kinase C inhibitor GF 109203X. The response to carbachol is dependent on the presence of fibronectin. Moreover, immunofluorescence studies show that carbachol treatment induces formation of stress fibers and focal adhesions. These results suggest that muscarinic receptor stimulation activates integrins via a protein kinase C-dependent mechanism. The activated integrins transmit a signal into the cell’s interior leading to tyrosine phosphorylation of paxillin and FAK. This represents a novel mechanism for regulation of tyrosine phosphorylation by muscarinic receptors.
Muscarinic receptors belong to a class of receptors whose members possess seven transmembrane domains and transmit signals by coupling to heterotrimeric GTP binding (G) proteins. Five muscarinic receptor isoforms have been cloned (1–3), three (m1, m3, and m5) that stimulate phosphatidylinositol 4,5-bisphosphate (PIP2) breakdown and two (m2 and m4) that inhibit adenylyl cyclase (4, 5). The classic signaling cascade initiated by activation of PIP2-coupled receptors entails hydrolysis of PIP2 to form diacylglycerol and inositol 1,4,5-trisphosphate with subsequent activation of protein kinase C (PKC). It is now apparent that muscarinic receptors also activate other kinases, including mitogen-activated protein kinase (6–9). Moreover, PIP2-coupled muscarinic receptors induce tyrosine phosphorylation of a number of proteins including PKCδ, phospholipase Cγ, the delayed rectifier potassium channel Kv1.2, and the G protein Gαq/11 subunits (10–14). Two additional substrates for muscarinic receptor-induced tyrosine phosphorylation have also been identified; they are the focal adhesion-associated proteins paxillin and focal adhesion kinase (FAK) (15–17).
Focal adhesions are specialized sites of attachment found in cultured cells at locations where the extracellular domains of cell-surface integrins bind to immobilized extracellular matrix (ECM) proteins such as fibronectin (18). This interaction results in clustering of the integrins and the association of their intracellular domains with cytoskeletal proteins that anchor bundles of polymerized actin filaments (stress fibers) to these sites. A number of signaling proteins are recruited to focal adhesions, including the adapter protein paxillin and the tyrosine kinase FAK (19, 20). Thus, focal adhesions have both structural and signaling functions.
The formation of focal adhesions may be experimentally induced by allowing cells in suspension to attach to immobilized ECM proteins (20) or by the addition of growth factors or G protein-coupled receptor ligands to quiescent cell monolayers (21, 22). In both experimental paradigms, the stimulus results in transient tyrosine phosphorylation of a similar set of proteins, including tensin, p130cas, paxillin, and FAK (20, 23). Interdependence of integrin and G protein-coupled receptor signaling has been demonstrated in platelets, in which tyrosine phosphorylation of FAK requires costimulation of the platelet by epinephrine, and by the integrin αIIbβ3 ligand fibrinogen (24). Similarly, full activation of the tyrosine kinase Syk in platelets requires both the agonist thrombin and integrin engagement by fibrinogen (25). In fibroblasts, G protein-coupled receptor-mediated tyrosine phosphorylation and focal adhesion formation have been shown to depend on cytoskeletal integrity, on activation of the small molecular G-protein Rho, and on actomyosin contractility (21, 23, 26–29).
Evidence described in this report shows that integrin activation is required for signaling events elicited by muscarinic receptor stimulation. Tyrosine phosphorylation of paxillin and FAK in human embryonic kidney (HEK) cells stably expressing muscarinic m3 receptors increased in a time- and concentration-dependent manner in response to the muscarinic agonist carbachol and was inhibited by antagonists of integrin binding and by an inhibitor of PKC. Integrin dependence was restricted to a subset of the signaling pathways activated by carbachol. The results suggest that integrins represent novel downstream effectors of muscarinic receptors and may constitute a mechanism for the reported adhesion dependence of other receptor-initiated signaling pathways (30, 31).
MATERIALS AND METHODS
Materials.
Antibodies and other reagents were obtained from the following sources. Anti-paxillin, anti-FAK, anti-phosphotyrosine (monoclonal clone PY20), anti-phosphotyrosine (recombinant peroxidase-linked RC20), and goat peroxidase-linked anti-mouse IgG were from Transduction Laboratories (Lexington, KY), and anti-vinculin antibodies were from Sigma. Protein G-agarose was purchased from Oncogene Science. Reagents and equipment and minigels for electrophoresis were supplied by Bio-Rad. GRGDS and GRGES peptides were purchased from American Peptide Company (Santa Clara, CA). Other reagents, including the RGDS peptide, were obtained from Sigma or Fisher Scientific.
Cell Culture.
HEK cells stably transfected with muscarinic m3 receptors were maintained in DMEM/F12 supplemented with 10% fetal bovine serum (16). These cells were shown previously to express approximately 200,000 m3 receptors per cell (5). For experiments using confluent monolayers, cells were grown in 60-mm culture dishes (Falcon, Beckton Dickinson Labware) coated with poly-d-lysine, for 3–4 days prior to an experiment. Cultures were incubated overnight in serum-free DMEM prior to an experiment. All subsequent pharmacological treatments were carried out in serum-free DMEM. To study adhesion to fibronectin, cells were grown on untreated tissue culture dishes, incubated overnight in serum-free DMEM, and detached by incubating for 5 min at 37°C in nonenzymatic cell dissociation solution (Sigma). The cell suspensions were triturated, gently pelleted in a clinical centrifuge, and resuspended in DMEM alone or in the presence of 0.75 mM GRGDS or GRGES peptides or 2.5 μM GF 109203X. After gentle mixing for 15 min, the cells were pelleted, resuspended in DMEM, transferred to untreated culture dishes or to dishes coated with fibronectin (Becton Dickinson Labware) or poly-d-lysine, and then allowed to adhere. The medium was removed, and lysis buffer was added without further washing of the cells. This was done to reduce cell loss in experiments in which adhesion was inhibited with RGD-containing peptides.
Immunoprecipitation and Determination of Tyrosine-Phosphorylated Proteins.
Cells were rinsed in PBS containing 1 mM sodium orthovanadate and collected in 1 ml of lysis buffer A containing 1% Nonidet P-40, 50 mM Tris, pH 7.5, 150 mM NaCl, 1 mM sodium orthovanadate, 25 mM NaF, 2 mM 4-(2-aminoethyl)benzenesulfonyl fluoride (AEBSF), 1 μg/ml leupeptin, and 2 μg/ml aprotinin. Lysates were centrifuged, normalized for protein content, incubated overnight with immunoprecipitating antibodies, usually at concentrations of 4 to 5 μg/500 μg of protein, with protein G-agarose (Oncogene Science) (3 mg per sample), centrifuged, and washed three times (in a washing buffer containing 0.1% Triton X-100/25 mM Tris, pH 7.5/250 mM NaCl/1 mM sodium orthovanadate). Immunoprecipitates were size-fractionated by SDS/PAGE, the proteins were transferred to poly(vinylidene difluoride) membranes, and the membranes were blocked with 3% gelatin or 5% milk in Tris-buffered saline containing 0.15% Tween-20. Tyrosine-phosphorylated proteins were immunoprecipitated with anti-phosphotyrosine antibodies (clone PY20). Immunoblots were probed with recombinant peroxidase-linked anti-phosphotyrosine (RC20) antibodies or antibodies to FAK or paxillin. Approximate molecular weights were estimated using calibrated prestained standards (Bio-Rad).
Measurement of Secreted Derivatives of the Amyloid Precursor Protein.
Medium was collected, desalted, dried, and analyzed by immunoblotting with an antibody to sAPP (6E10; Senetek, Maryland Heights, MO), as previously described (16).
Protein Analysis.
Protein contents were measured using the bicinchoninic acid reagent (Sigma).
Immunofluorescence Microscopy.
Cells were grown overnight in glass slide chambers coated with fibronectin in serum-containing DMEM/F12 and then preincubated for 4 h in serum-free medium. Serum-starved, quiescent cells were treated with carbachol-containing (100 μM) or control medium for 10 min, fixed in 3.7% paraformaldehyde in PBS for 5 min, permeabilized in 0.1% Triton X-100 in PBS for 5 min, and blocked for 30 min in 1% BSA in PBS. Cells were incubated for 30 min at 37°C with monoclonal antibodies to vinculin (Sigma), at a 1:400 dilution, followed by a fluorescent-conjugated secondary antibody (Alexa 488 goat anti-mouse IgG; Molecular Probes, Eugene OR), at a 1:200 dilution, for 30 min at 37°C. F-actin was stained with a fluorescent phalloidin conjugate (Alexa 488 phalloidin, Molecular Probes) at a final concentration of 5 U/ml. The preparations were examined by confocal microscopy (with a 60× water-immersion objective lens) using an argon laser with an excitation wavelength of 488 nm and an emission filter at 530 nm. Images were converted to TIFF format and processed using the NIH Image program.
Statistical Analysis.
Comparisons based on immunoblotting data were derived from samples processed on the same blot. The statistical significance of differences was estimated by analysis of variance followed by the Tukey test. Differences were taken to be significant at P ≤ 0.05.
RESULTS AND DISCUSSION
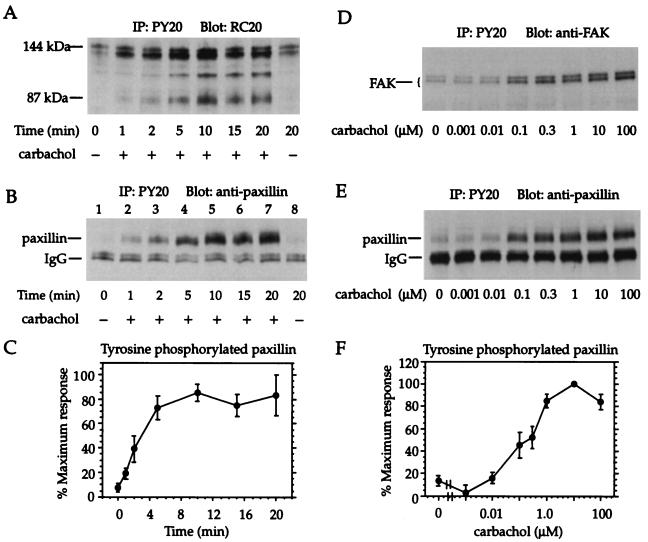
HEK cells stably expressing muscarinic m3 receptors were treated with the muscarinic agonist carbachol. In accordance with previous studies in cells expressing m1 or m3 receptors (15–17, 32, 33), carbachol treatment resulted in the appearance of a number of tyrosine-phosphorylated protein species with apparent molecular masses ranging from approximately 70 to 150 kDa (Fig. 1A). A prominent, diffuse band of approximately 70 kDa was identified as the focal adhesion-associated protein paxillin; a protein of the same size was detected when antiphosphotyrosine immunoprecipitates from carbachol-treated cells were immunoblotted with antibodies to paxillin (Fig. 1B). The tyrosine phosphorylation of paxillin induced by carbachol was maximal within 5 min and remained elevated for at least 20 or 30 min (Fig. 1C). In view of earlier reports identifying FAK as an additional substrate for muscarinic receptor-induced tyrosine phosphorylation (15, 17), antiphosphotyrosine immunoprecipitates from cells treated with varying concentrations of carbachol were analyzed by immunoblotting with antibodies to FAK. A doublet was observed in the 120–140 molecular weight range that increased in intensity as a function of increasing carbachol concentration (Fig. 1D). A parallel, concentration-dependent increase in tyrosine-phosphorylated paxillin was observed in these cells (Fig. 1 E and F). Tyrosine phosphorylation was also assessed by the reverse procedure, in which lysates from treated cells were subjected to immunoprecipitation with antibodies to paxillin or FAK, and the immunoprecipitated proteins were analyzed by immunoblotting with anti-phosphotyrosine antibodies; again, the results showed that carbachol increased the phosphotyrosine content of paxillin (16) and FAK (not shown); in both cases, FAK appeared as a doublet. Moreover, the effect of carbachol was reduced by the tyrosine kinase inhibitors tyrphostin A25 and genistein (16). Thus, tyrosine phosphorylation of paxillin and FAK are increased in a time- and concentration-dependent manner by activation of muscarinic m3 receptors.
Figure 1.
Activation of muscarinic m3 receptors increases tyrosine phosphorylation of paxillin and FAK. (A–C) Confluent monolayers of HEK cells stably transfected with m3 receptors were treated with 100 μM carbachol or vehicle for various periods of time. (D–F) Cell monolayers were incubated for 10 min in serum-free medium containing various concentrations of carbachol. Cells were lysed, and antiphosphotyrosine immunoprecipitates were analyzed by immunoblotting with antiphosphotyrosine antibodies (A), antibodies to paxillin (B, E), or antibodies to FAK (D). (C, F) Paxillin content of antiphosphotyrosine immunoprecipitates was quantitated by densitometry. Data are expressed as a percentage of the maximal response. Means ± SEM from three to four experiments are shown.
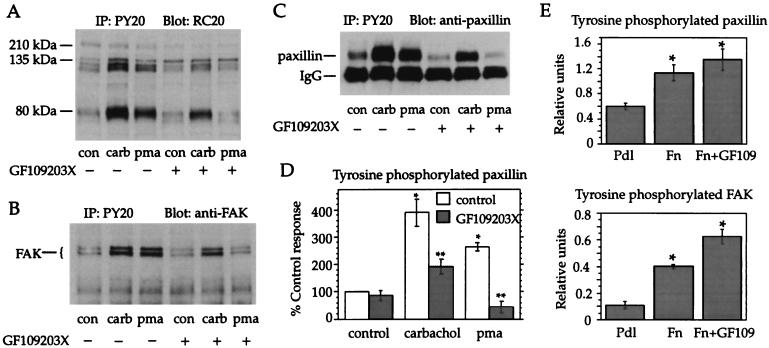
Muscarinic m3 receptor stimulation activates PKC (34, 35), a serine–threonine kinase that has been implicated in protein tyrosine phosphorylation evoked by the G protein-coupled receptor ligand bradykinin (36, 37), an effect presumably mediated via activation of a tyrosine kinase or inhibition of a tyrosine phosphatase. Therefore, the effect of the selective PKC inhibitor GF 109203X (38) on carbachol-mediated tyrosine phosphorylation of paxillin and FAK was determined. Parallel cultures were treated with the PKC activator phorbol 12-myristate 13-acetate (PMA), which also stimulates tyrosine phosphorylation, though often to a lesser extent than many receptor agonists (39, 40). The inhibitor GF 109203X essentially abolished tyrosine phosphorylation of paxillin and FAK elicited by PMA and caused a partial reduction in carbachol-induced tyrosine phosphorylation of these proteins (Fig. 2 A–C). Quantitation of the inhibitory effect on paxillin phosphorylation revealed that the response to carbachol was reduced by 64% in the presence of GF 109203X (Fig. 2D). A comparable reduction in carbachol-induced tyrosine phosphorylation of FAK was also observed (data not shown). In contrast to these results, others have reported that the stimulatory effects on FAK and paxillin tyrosine phosphorylation of the G protein-coupled receptor ligands bombesin, vasopressin, endothelin, and lysophosphatidic acid were not inhibited by GF 109203X or by down-regulation of protein kinase C (22, 29, 40, 41).
Figure 2.
PKC dependence of muscarinic receptor-mediated tyrosine phosphorylation. Cell monolayers were pretreated for 15 min with medium containing 2.5 μM GF 109203X or dimethyl sulfoxide (the vehicle in which the inhibitor was dissolved). The medium was replaced with fresh medium containing dimethyl sulfoxide or GF 109203X alone, or with carbachol (100 μM) or PMA (1 μM). Anti-phosphotyrosine immunoprecipitates were analyzed by immunoblotting with anti-phosphotyrosine (RC20) antibodies (A), antibodies to FAK (B), or antibodies to paxillin (C). (D) Paxillin content of anti-phosphotyrosine immunoprecipitates was quantitated by densitometry. Data are expressed as a percentage of the control response. Means ± SEM from at least three experiments are shown. ∗, Significant difference from control; ∗∗, significant difference from carbachol or PMA (P < 0.05). (E) Cells in suspension were treated with 2.5 μM GF 109203X (GF109) or dimethyl sulfoxide for 15 min and then allowed to attach for 30 min to culture dishes coated with poly-d-lysine (Pdl) or fibronectin (Fn). Anti-phosphotyrosine immunoprecipitates from cell lysates were analyzed by immunoblotting with antibodies to paxillin (Upper) or FAK (Lower). Data are means ± SEM from three experiments. ∗, significant difference from control (P < 0.05).
Paxillin and FAK are recruited to focal adhesions, and transiently phosphorylated on tyrosine residues, during integrin-mediated cell adhesion to ECM molecules such as fibronectin (42). Consistent with these reports, when HEK cells were placed in suspension and then plated onto fibronectin-coated dishes for 30 min, the cells showed increased tyrosine phosphorylation of paxillin and FAK relative to cells plated onto poly-d-lysine (Fig. 2E). However, although muscarinic receptor-mediated tyrosine phosphorylation was PKC-dependent, tyrosine phosphorylation induced by adhesion of these cells to fibronectin was not inhibited by GF 109203X (Fig. 2E).
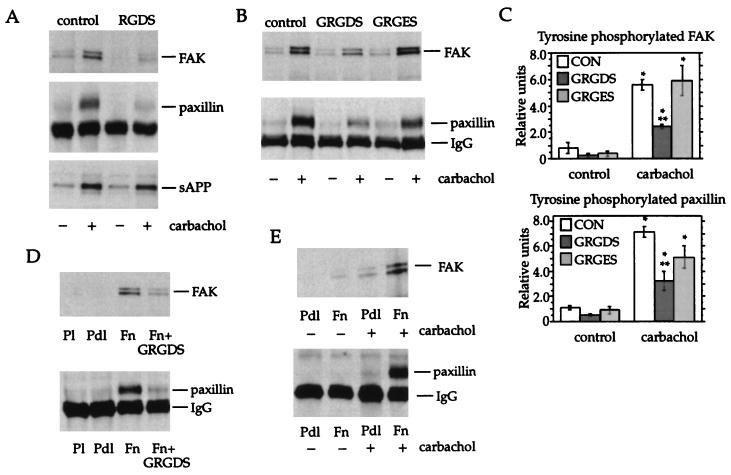
In view of recent reports that stimulation of G protein-coupled receptors leads to phosphorylation and activation of the epidermal growth factor receptor, which mediates some of the downstream signaling events initiated by the G protein receptor agonist (17, 37, 43), the possibility arose that integrins might be similarly activated by muscarinic receptor stimulation, resulting in tyrosine phosphorylation of paxillin and FAK. To test this hypothesis, cell monolayers were incubated with carbachol in the presence of the peptide arginine-glycine-aspartate-serine (RGDS), which mimics the integrin recognition site on the ECM molecules fibronectin and vitronectin and other adhesion proteins (44) and has been shown to inhibit integrin-mediated cell adhesion (45). This peptide blocked tyrosine phosphorylation of paxillin and FAK by carbachol (Fig. 3A Upper and Middle). In contrast, it did not affect carbachol-mediated release of soluble derivatives of the amyloid precursor protein (Fig. 3A Lower), a response previously shown to be elicited by muscarinic receptors coupled to PIP2 turnover and dependent on PKC and tyrosine phosphorylation (16, 33, 46).
Figure 3.
Effect of RGD-containing peptides on muscarinic receptor- and fibronectin-mediated tyrosine phosphorylation. (A) Confluent cell monolayers were incubated for 20 min in control medium, or in medium containing carbachol (100 μM), in the presence or absence of RGDS peptide (1 mM). Cell lysates were assayed for tyrosine phosphorylation of FAK (Upper) or paxillin (Middle). The medium was assayed for soluble derivatives of the amyloid precursor protein (sAPP) (Lower). (B) Cell monolayers were treated for 20 min with or without carbachol (100 μM) in the presence or absence of GRGDS or GRGES peptides (0.75 mM) and analyzed for tyrosine phosphorylation of FAK (Upper) and paxillin (Lower). (C) Paxillin and FAK content of anti-phosphotyrosine immunoprecipitates was quantitated by densitometry. Data are expressed as relative units. Means ± SEM from three or four experiments are shown. ∗, Significant difference from corresponding control group; ∗∗, significant difference from carbachol (P < 0.05). (D) The GRGDS peptide blocked tyrosine phosphorylation stimulated by adhesion of cells for 30 min to fibronectin (Fn). No increase in tyrosine phosphorylation was observed in cells adherent to plastic (Pl) or poly-d-lysine (Pdl). (E) Cells in suspension were allowed to adhere to culture dishes coated with Pdl or Fn. After 2.5 h, cells were incubated for 20 min in fresh medium in the presence or absence of 100 μM carbachol and analyzed for tyrosine phosphorylation of FAK (Upper) and paxillin (Lower). This experiment was repeated three times with similar results.
These observations indicate that integrin activation mediates a subset of muscarinic receptor-mediated signaling pathways. To test the specificity of the blocking peptide, the effects of a related peptide with the sequence GRGDS and a control peptide, GRGES, on the responses to carbachol were compared. Whereas the GRGDS peptide caused a marked reduction in the phosphorylation of paxillin and FAK induced by carbachol, the inactive analogue GRGES at the same concentration did not significantly reduce this response, further supporting the contention that the observed inhibition was due to specific interference with integrin activation (Fig. 3 B and C). In accordance with previous reports (42, 47–49), when cells in suspension were allowed to attach to fibronectin-coated culture dishes, similar increases in tyrosine phosphorylation of paxillin and FAK were observed within 30 min. These responses were inhibited by the GRGDS peptide (Fig. 3D).
To further test the importance of integrin activation on carbachol-induced tyrosine phosphorylation, cells were stimulated with carbachol in the presence and absence of fibronectin. Serum-starved cells were suspended in serum-free DMEM and then plated onto dishes coated with poly-d-lysine or fibronectin. After 2.5 h, an interval sufficient for the initial tyrosine phosphorylation response to fibronectin to return to baseline levels, cells were incubated for an additional 20 min in control or carbachol-containing medium. Tyrosine phosphorylation of paxillin and FAK in response to carbachol was markedly attenuated in cells plated on poly-d-lysine, indicating that integrin binding to fibronectin is a necessary component of the response (Fig. 3E).
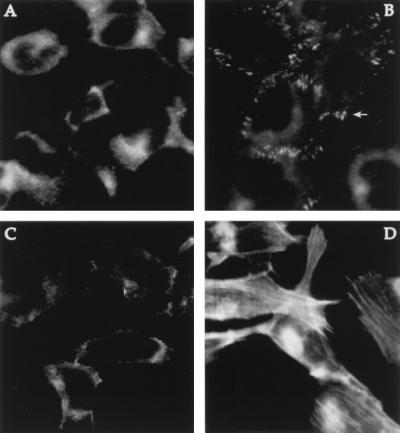
Finally, the ability of carbachol to activate integrins was tested using an immunofluorescence approach. Treatment of serum-starved cells with carbachol resulted in the formation of stress fibers, visualized with a fluorescent phalloidin conjugate (Fig. 4D), and the accumulation of vinculin, a major component of focal adhesions, into discrete patches at the cell periphery (Fig. 4B).
Figure 4.
Formation of focal contacts and stress fibers is induced by carbachol treatment. Immunofluorescence microscopy was used to visualize vinculin (A and B), a major focal adhesion-associated protein, and actin stress fibers (C and D). In serum-starved cells, vinculin staining was diffuse (A), but following exposure to 100 μM carbachol for 10 min, vinculin became concentrated in focal contacts, one of which is indicated by the arrow (B). Similarly, few stress fibers were observed in quiescent cells (C) but were a prominent feature in cells treated with carbachol (D). Actin filaments were detected with a fluorescent phalloidin conjugate. Focal adhesions were visualized with an antibody to vinculin.
The modification of integrin function by intracellular signals is a process known as “inside-out signaling” [for a review see Schwartz et al. (50)]. For example, binding of thrombin to its G protein-coupled receptor in platelets increases the affinity of the cell surface integrin αIIbβ3 for fibrinogen (51). Direct activation of PKC (e.g., by phorbol esters) also promotes cell spreading and adhesion (52–54). Interestingly, both thrombin-induced FAK phosphorylation (55) and PMA-induced actin assembly (54) in platelets were inhibited by RGD-containing peptides, which prevent fibrinogen binding to αIIbβ3 and platelet aggregation, suggesting that some of the downstream effects of these agonists were mediated by activation of integrins and subsequent “outside-in” signaling by these integrins. The PKC and integrin dependence of muscarinic receptor-mediated tyrosine phosphorylation observed in the present study is consistent with the data derived from platelets and suggests that PKC activation may be the mechanism by which muscarinic receptor stimulation activates integrins in HEK cells expressing m3 receptors. That PKC activation lies upstream of integrins in the muscarinic receptor-mediated signaling cascade is further supported by the observation that tyrosine phosphorylation induced by direct activation of integrins (via attachment to fibronectin) was unaffected by the PKC inhibitor GF 109203X. “Outside-in” signaling by integrins requires occupancy of the ligand binding site and clustering of the integrins (56, 57); both conditions are met when integrins bind to an immobilized ligand. These results suggest that integrins activated in response to stimulation of muscarinic receptors transmit a signal into the cell’s interior leading to tyrosine phosphorylation of paxillin and FAK. It is likely that the ECM proteins required for integrin activation are secreted by the HEK cells themselves. Many cells in culture, including a number of kidney cell lines, secrete fibronectin and assemble it into a matrix, and the α1β5 integrin, which is expressed by HEK cells (58), mediates adhesion of these cells to fibronectin (58). Indeed, the attenuation of the tyrosine phosphorylation response in cells stimulated with carbachol shortly after plating onto poly-d-lysine (that is, before they have time to secrete appreciable amounts of fibronectin), relative to that in cells plated on fibronectin, provides strong support for this hypothesis.
Several different lines of evidence have implicated integrin activation and focal adhesion assembly in signaling by G protein-coupled receptors. Thus, activation of these receptors, as well as integrin engagement by ECM proteins, stimulates focal adhesion formation concomitantly with tyrosine phosphorylation. Moreover, receptor-induced tyrosine phosphorylation is blocked by inhibitors of the small molecular weight G protein Rho, required for focal adhesion assembly (21, 26, 27, 59), by inhibition of actomyosin-based increases in contractility, hypothesized to supply the driving force for Rho-induced focal adhesion assembly (20, 23, 28), and by disruption of actin filaments, and thus cytoskeletal integrity, by cytochalasin D (22, 29, 40). However, it has also been reported that Rho-induced tyrosine phosphorylation can occur in the absence of stress fiber formation; thus, the involvement of integrin activation in this response is still an open question (60). The results presented here were derived using several different approaches and provide strong support for the participation of integrins in muscarinic receptor signaling.
In summary, the observations described in this report suggest that binding of carbachol to muscarinic m3 receptors leads to activation of integrins, apparently via an increase in PKC activity, and subsequent, tyrosine phosphorylation of paxillin and FAK that is dependent on the interaction of integrins with ECM proteins.
Acknowledgments
I thank E. Peralta for the generous gift of the transfected HEK cells, Susana Zanello, David Larson, and Ignacio Lopez for assistance with immunofluorescence microscopy, Michael Holick for the use of his confocal microscope, and Jan K. Blusztajn for helpful discussions. This work was supported by National Institutes of Health Grant NS30791.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: ECM, extracellular matrix; FAK, focal adhesion kinase; HEK, human embryonic kidney; PIP2, phosphatidylinositol 4,5-bisphosphate; PKC, protein kinase C; PMA, phorbol 12-myristate 13-acetate.
References
- 1.Bonner T I, Buckley N J, Young A C, Brann M R. Science. 1987;237:527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- 2.Peralta E G, Ashkenazi A, Winslow J W, Smith D H, Ramachandran J, Capon D J. EMBO J. 1987;6:3923–3929. doi: 10.1002/j.1460-2075.1987.tb02733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonner T I, Young A C, Brann M R, Buckley N J. Neuron. 1988;1:403–410. doi: 10.1016/0896-6273(88)90190-0. [DOI] [PubMed] [Google Scholar]
- 4.Peralta E G, Ashkenazi A, Winslow J W, Ramachandran J, Capon D J. Nature (London) 1988;334:434–437. doi: 10.1038/334434a0. [DOI] [PubMed] [Google Scholar]
- 5.Sandmann J, Peralta E G, Wurtman R J. J Biol Chem. 1991;266:6031–6034. [PubMed] [Google Scholar]
- 6.Crespo P, Xu N, Simonds W F, Gutkind J S. Nature (London) 1994;369:418–420. doi: 10.1038/369418a0. [DOI] [PubMed] [Google Scholar]
- 7.Hawes B E, Van Biesen T, Koch W J, Luttrell L M, Lefkowitz R J. J Biol Chem. 1995;270:17148–17153. doi: 10.1074/jbc.270.29.17148. [DOI] [PubMed] [Google Scholar]
- 8.Van Biesen T, Hawes B E, Raymond J R, Luttrell L M, Koch W J, Lefkowitz R J. J Biol Chem. 1996;271:1266–1269. doi: 10.1074/jbc.271.3.1266. [DOI] [PubMed] [Google Scholar]
- 9.Wan Y, Kurosaki T, Huang X Y. Nature (London) 1996;380:541–544. doi: 10.1038/380541a0. [DOI] [PubMed] [Google Scholar]
- 10.Offermanns S, Bombien E, Schultz G. Biochem J. 1993;294:545–550. doi: 10.1042/bj2940545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang X-Y, Morielli A D, Peralta E G. Cell. 1993;75:1145–1156. doi: 10.1016/0092-8674(93)90324-j. [DOI] [PubMed] [Google Scholar]
- 12.Gusovsky F, Lueders J E, Kohn E C, Felder C C. J Biol Chem. 1993;268:7768–7772. [PubMed] [Google Scholar]
- 13.Soltoff S P, Toker A. J Biol Chem. 1995;270:13490–13495. doi: 10.1074/jbc.270.22.13490. [DOI] [PubMed] [Google Scholar]
- 14.Umemori H, Inoue T, Kume S, Sekiyama N, Nagao M, Itoh H, Nakanishi S, Mikoshiba K, Yamamoto T. Science. 1997;276:1878–1881. doi: 10.1126/science.276.5320.1878. [DOI] [PubMed] [Google Scholar]
- 15.Gutkind J S, Robbins K C. Biochem Biophys Res Commun. 1992;188:155–161. doi: 10.1016/0006-291x(92)92363-3. [DOI] [PubMed] [Google Scholar]
- 16.Petryniak M A, Wurtman R J, Slack B E. Biochem J. 1996;320:957–963. doi: 10.1042/bj3200957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsai W, Morielli A D, Peralta E G. EMBO J. 1997;16:4597–4605. doi: 10.1093/emboj/16.15.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burridge K, Fath K, Kelly T, Nuckolls G, Turner C. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- 19.Clark E A, Brugge J S. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 20.Burridge K, Chrzanowska-Wodnicka M, Zhong C. Trends Cell Biol. 1997;7:342–347. doi: 10.1016/S0962-8924(97)01127-6. [DOI] [PubMed] [Google Scholar]
- 21.Ridley A J, Hall A. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 22.Seufferlein T, Rozengurt E. J Biol Chem. 1994;269:9345–9351. [PubMed] [Google Scholar]
- 23.Burridge K, Chrzanowska-Wodnicka M. Annu Rev Cell Dev Biol. 1996;12:463–518. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- 24.Shattil S J, Haimovich B, Cunningham M, Lipfert L, Parsons J T, Ginsberg M H, Brugge J S. J Biol Chem. 1994;269:14738–14745. [PubMed] [Google Scholar]
- 25.Clark E A, Shattil S J, Ginsberg M H, Bolen J, Brugge J S. J Biol Chem. 1994;269:28859–28864. [PubMed] [Google Scholar]
- 26.Kumagai N, Morii N, Fujisawa K, Nemoto Y, Narumiya S. J Biol Chem. 1993;268:24535–24538. [PubMed] [Google Scholar]
- 27.Rankin S, Morii N, Narumiya S, Rozengurt E. FEBS Lett. 1994;354:315–319. doi: 10.1016/0014-5793(94)01148-6. [DOI] [PubMed] [Google Scholar]
- 28.Chrzanowska-Wodnicka M, Burridge K. J Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sinnett-Smith J, Zachary I, Valverde A M, Rozengurt E. J Biol Chem. 1993;268:14261–14268. [PubMed] [Google Scholar]
- 30.Lin T H, Chen Q M, Howe A, Juliano R L. J Biol Chem. 1997;272:8849–8852. [PubMed] [Google Scholar]
- 31.Ruoslahti E, Öbrink B. Exp Cell Res. 1996;227:1–11. doi: 10.1006/excr.1996.0243. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt M, Hüwe S M, Fasselt B, Homann D, Rümenapp U, Sandmann J, Jakobs K H. Eur J Biochem. 1994;225:667–675. doi: 10.1111/j.1432-1033.1994.00667.x. [DOI] [PubMed] [Google Scholar]
- 33.Slack B E, Breu J, Petryniak M A, Srivastava K, Wurtman R J. J Biol Chem. 1995;270:8337–8344. doi: 10.1074/jbc.270.14.8337. [DOI] [PubMed] [Google Scholar]
- 34.Trilivas I, Brown J H. J Biol Chem. 1989;264:3102–3107. [PubMed] [Google Scholar]
- 35.Trilivas I, McDonough P M, Brown J H. J Biol Chem. 1991;266:8431–8438. [PubMed] [Google Scholar]
- 36.Leeb-Lundberg L M F, Song X-H, Mathis S A. J Biol Chem. 1994;269:24328–24334. [PubMed] [Google Scholar]
- 37.Coutant K D, Corvaia N, Ryder N S. Biochem Biophys Res Commun. 1995;210:774–780. doi: 10.1006/bbrc.1995.1726. [DOI] [PubMed] [Google Scholar]
- 38.Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, Duhamel L, Charon D, Kirilovsky J. J Biol Chem. 1991;266:15771–15781. [PubMed] [Google Scholar]
- 39.Zachary I, Sinnett-Smith J, Rozengurt E. J Biol Chem. 1991;266:24126–24133. [PubMed] [Google Scholar]
- 40.Zachary I, Sinnett-Smith J, Turner C E, Rozengurt E. J Biol Chem. 1993;268:22060–22065. [PubMed] [Google Scholar]
- 41.McLees A, Graham A, Malarkey K, Gould G W, Plevin R. Biochem J. 1995;307:743–748. doi: 10.1042/bj3070743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burridge K, Turner C E, Romer L H. J Cell Biol. 1992;119:893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Daub H, Weiss F U, Wallasch C, Ullrich A. Nature (London) 1996;379:557–560. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- 44.Hynes R O. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 45.Yamada K M. J Biol Chem. 1991;266:12809–12812. [PubMed] [Google Scholar]
- 46.Nitsch R M, Slack B E, Wurtman R J, Growdon J H. Science. 1992;258:304–307. doi: 10.1126/science.1411529. [DOI] [PubMed] [Google Scholar]
- 47.Schaller M D, Borgman C A, Cobb B C, Reynolds A B, Parsons J T. Proc Natl Acad Sci USA. 1992;89:5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hanks S K, Calalb M B, Harper M C, Patel S K. Proc Natl Acad Sci USA. 1992;89:8487–8491. doi: 10.1073/pnas.89.18.8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guan J-L, Shalloway D. Nature. 1992;358:690–692. doi: 10.1038/358690a0. [DOI] [PubMed] [Google Scholar]
- 50.Schwartz M A, Schaller M D, Ginsberg M H. Annu Rev Cell Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 51.Shattil S J, Brass L F. J Biol Chem. 1987;262:992–1000. [PubMed] [Google Scholar]
- 52.Vuori K, Ruoslahti E. J Biol Chem. 1993;268:21459–21462. [PubMed] [Google Scholar]
- 53.Lewis J M, Cheresh D A, Schwartz M A. J Cell Biol. 1996;134:1323–1332. doi: 10.1083/jcb.134.5.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hartwig J H, Kung S, Kovacsovics T, Janmey P A, Cantley L C, Stossel T P, Toker A. J Biol Chem. 1996;271:32986–32993. doi: 10.1074/jbc.271.51.32986. [DOI] [PubMed] [Google Scholar]
- 55.Law D A, Nannizzi-Alaimo L, Phillips D R. J Biol Chem. 1996;271:10811–10815. doi: 10.1074/jbc.271.18.10811. [DOI] [PubMed] [Google Scholar]
- 56.Miyamoto S, Teramoto H, Coso O A, Gutkind J S, Burbelo P D, Akiyama S K, Yamada K M. J Cell Biol. 1995;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miyamoto S, Akiyama S K, Yamada K M. Science. 1995;267:883–885. doi: 10.1126/science.7846531. [DOI] [PubMed] [Google Scholar]
- 58.Bodary S C, McLean J W. J Biol Chem. 1990;265:5938–5941. [PubMed] [Google Scholar]
- 59.Seckl M J, Morii N, Narumiya S, Rozengurt E. J Biol Chem. 1995;270:6984–6990. doi: 10.1074/jbc.270.12.6984. [DOI] [PubMed] [Google Scholar]
- 60.Flinn H M, Ridley A J. J Cell Sci. 1996;109:1133–1141. doi: 10.1242/jcs.109.5.1133. [DOI] [PubMed] [Google Scholar]