Abstract
Inoculation with the neurotropic JHM strain of mouse hepatitis virus (JHMV) into the central nervous system (CNS) of mice results in an acute encephalitis associated with an immune-mediated demyelinating disease. During acute disease, infiltrating CD8+ T cells secrete gamma interferon (IFN-γ) that controls replication in oligodendrocytes, while infected astrocytes and microglia are susceptible to perforin-mediated lysis. The present study was undertaken to reveal the functional contributions of the activating NKG2D receptor in host defense and disease following JHMV infection. NKG2D ligands RAE-1, MULT1, and H60 were expressed within the CNS following JHMV infection. The immunophenotyping of infiltrating cells revealed that NKG2D was expressed on ∼90% of infiltrating CD8+ T cells during acute and chronic disease. Blocking NKG2D following JHMV infection resulted in increased mortality that correlated with increased viral titers within the CNS. Anti-NKG2D treatment did not alter T-cell infiltration into the CNS or the generation of virus-specific CD8+ T cells, and the expression of IFN-γ was not affected. However, cytotoxic T-lymphocyte (CTL) activity was dependent on NKG2D expression, because anti-NKG2D treatment resulted in a dramatic reduction in lytic activity by virus-specific CD8+ T cells. Blocking NKG2D during chronic disease did not affect either T-cell or macrophage infiltration or the severity of demyelination, indicating that NKG2D does not contribute to virus-induced demyelination. These findings demonstrate a functional role for NKG2D in host defense during acute viral encephalitis by selectively enhancing CTL activity by infiltrating virus-specific CD8+ T cells.
Viral infection of the central nervous system (CNS) presents unique challenges to the immune system with regard to controlling and eliminating the invading pathogen. These obstacles include the presence of a blood-brain barrier that provides a physical and physiological barrier that is difficult for cells and molecules to cross, the absence of classic lymphatic drainage that may impair the generation of an adaptive immune response, and the relative absence of major histocompatibility complex (MHC) class I or II expression on resident cells (3, 26, 39, 73). In addition, the CNS is composed of a variety of highly specialized cells, many of which have limited renewal capacity, that represent potential targets of infection by numerous different viruses (34, 81). Therefore, a significant hurdle encountered by infiltrating antigen-specific lymphocytes is the elimination of virus from infected cells while limiting the damage that may have long-term detrimental consequences to the host. Critical to this is the cellular tropism of the virus, as this is important in dictating the effector response by infiltrating lymphocytes. For example, neuronotropic viruses often are eliminated by noncytolytic mechanisms via cytokine-mediated control and/or neutralizing antibodies, whereas the infection of other cell populations can evoke cytolytic mechanisms for control (17, 33, 45, 53, 75, 78, 87, 91). Therefore, characterizing the mechanisms involved in regulating the interaction between immune cells and virus-infected targets is critical for understanding how viral infection of the CNS is controlled and eventually cleared.
The inoculation of mouse hepatitis virus (MHV; a positive-strand RNA virus that is a member of the Coronaviridae family), neurotropic strain JHM (JHMV), into the CNS of susceptible strains of mice provides an excellent model in which to examine host response mechanisms responsible for the control of viral replication within distinct cell lineages present in the brain (6). JHMV infection results in an acute encephalomyelitis characterized by wide-spread replication in astrocytes, microglia, and oligodendrocytes, with relatively few neurons being infected (10, 44, 69, 71). In addition, infection is associated with an orchestrated release of cytokines and chemokines that results in the inflammation and recruitment of inflammatory cells (47, 55, 68-70, 85, 90, 93). The control of viral replication during acute disease is primarily due to virus-specific CD8+ T cells, which function by two different effector mechanisms within the CNS: gamma interferon (IFN-γ) secretion is responsible for controlling viral replication in oligodendrocytes, whereas a perforin-dependent mechanism promotes viral clearance from astrocytes and microglia (53, 69). Infiltrating CD4+ T cells provide a supporting role for the maintenance and expansion of cytotoxic T-lymphocytes (CTLs) within the CNS as well as enhancing effector responses (80). Although a robust cell-mediated immune response occurs during acute disease, sterilizing immunity is not achieved, resulting in viral persistence (82). Factors contributing to this include oligodendrocytes becoming a predominant viral reservoir as well as substantial variation in viral RNA sequences (1, 29, 44). Virus-specific CD8+ T cells are retained within the CNS of persistently infected mice, and although lytic activity is muted, these cells retain the capacity to secrete IFN-γ, which affects viral replication in oligodendrocytes (5, 32, 61, 69). Ultimately, virus-specific antibody is critical in suppressing viral recrudescence and leads to the eventual clearance of virus from the CNS (52). Histological features associated with viral persistence include the development of an immune-mediated demyelinating disease that is similar to the human demyelinating disease multiple sclerosis, with both T cells and macrophages being important in amplifying disease severity by contributing to myelin damage (16, 71).
Although elegant work previously has been performed with regard to evaluating the functional role of CD8+ T cells and viral replication within the CNS of JHMV-infected mice (7, 62, 80, 83), it is likely that additional signaling molecules associated with CD8+ T cells also contribute to the control of viral replication. NKG2D is a type II transmembrane glycoprotein that is expressed as a disulfide-linked homodimer on the surface of NK cells, CD8+ T cells, and γδ T cells in mice (20, 41). In some situations, NKG2D functions as a costimulatory receptor for CD8+ T cells following an encounter with cells expressing NKG2D ligands, resulting in enhanced effector functions and cell proliferation (35, 41, 60, 64, 74). For example, antiviral effector responses by some human cytomegalovirus antigen-specific CD8+ T clones characterized by cytokine bursts are amplified when cultured with viral peptide-pulsed cells expressing the human NKG2D ligand MICA (35). Treatment of lymphocytic choriomeningitis virus-specific CD8+ T cells with an NKG2D-activating antibody augmented proliferation compared to that of cells treated with control antibody, indicating that NKG2D receptor signaling on CD8+ T cells offers protection against virus-induced disease (41). Ex vivo anti-NKG2D treatment of Mycobacterium tuberculosis antigen-specific CD8+ T cells resulted in decreased IFN-γ secretion and CTL activity when cultured with bacterial peptide-stimulated macrophages compared to treatment with an isotype-matched control antibody, suggesting that NKG2D receptor signaling on CD8+ T cells affects the outcome of disease (74). Finally, blocking NKG2D receptor signaling in mice persistently infected with JHMV results in diminished immune-mediated pathology, suggesting that IFN-γ production from γδ T cells is impaired (18). These studies highlight the potential protective role of NKG2D in host defense following microbial infection by evoking a range of responses in a variety of immune cells.
In this study, the functional role of NKG2D receptor signaling on T cells and NK cells during both acute and chronic virus-induced neurological disease was examined. Following JHMV infection of susceptible mice, mRNA transcripts specific for the NKG2D ligands H60, MULT1, and RAE-1 were expressed within the CNS, and the majority of infiltrating NK cells and CD8+ T cells expressed the NKG2D receptor. Blocking NKG2D receptor signaling in JHMV-infected SCID mice (lacking T and B cells, but functional NK cells are intact) did not affect survival or the viral burden within the brain, indicating that NKG2D does not enhance antiviral effector responses by infiltrating NK cells. However, anti-NKG2D treatment of JHMV-infected BALB/c mice resulted in increased mortality and increased viral burden within the brain. Indeed, treatment with NKG2D neutralizing antibody resulted in diminished CTL activity by virus-specific CD8+ T cells, yet IFN-γ expression was not affected, indicating that blocking NKG2D selectively affects antiviral effector function in response to acute viral infection of the CNS. The administration of anti-NKG2D antibody to persistently infected mice did not result in viral recrudescence, modulate IFN-γ levels, or influence the severity of demyelination, indicating that NKG2D receptor signaling does not influence chronic disease. Therefore, the findings presented highlight an important role for NKG2D in CD8+ T-cell antiviral immunity during acute infection of the CNS by enhancing cytolytic activity.
MATERIALS AND METHODS
Mice and reagents.
BALB/c mice and SCID mice on the BALB/c background (H-2d) were purchased from the National Cancer Institute. Mice were anesthetized by intraperitoneal (i.p.) injection of a 120- to 150-μl mixture of ketamine hydrochloride (Ketaject; 100 μg/ml; Phoenix, St. Joseph, MO) and xylazine hydrochloride (1.1 mg/ml; ICN Biomedical, Aurora, OH) in Hanks' balanced salt solution (HBSS). Anesthetized mice were injected intracranially (i.c.) with 15,000 PFU of JHMV (strain V2.2-1) suspended in 30 μl of sterile HBSS. Sham-infected animals were injected with 30 μl of sterile HBSS alone. Mice were sacrificed at defined time points, and brains were removed for the determination of virus titers on a susceptible astrocytoma cell line (DBT) (40, 48). The animal protocols used for these studies were reviewed and approved by the institutional animal care review board. The immortalized mouse macrophage cell line J774A.1 (H-2d haplotype) was purchased from the ATCC. The production of CX5 (rat anti-mouse NKG2D) monoclonal antibody (MAb) was performed as previously described (65). Saturated ammonium sulfate precipitation was performed to purify immunoglobulin G (IgG) from the ascites fluid. Small-molecular-weight proteins were removed by dialysis, and the antibody concentration was determined by using a rat IgG antibody-specific enzyme-linked immunosorbent assay (ELISA).
Anti-NKG2D treatment of mice.
JHMV-infected BALB/c immunocompetent and SCID mice were treated i.p. with 100 μg of anti-NKG2D MAb CX5 or rat IgG control antibody (Sigma-Aldrich, St. Louis, MO) suspended in 300 μl of 1× HBSS (Mediatech Inc., Herndon, VA) 2 days before infection and 5 and 10 days postinfection (p.i.) for innate and acute studies. Antibody for chronic studies was administered i.p. on days 19, 24, and 29 p.i. at the same concentration as that used for the innate and acute studies. Following a single injection of anti-NKG2D CX5 MAb, antibody can be detected up to 2 weeks postinjection, with reduced NKG2D receptor expression on NK cells lasting for at least 7 days (42, 57, 63, 64, and L. L. Lanier, unpublished observations).
Quantitative real-time PCR.
Total RNA was extracted from homogenized brain and spinal cord samples by using TRIzol reagent (Invitrogen, Carlsbad, CA), DNase-treated, and purified by phenol-chloroform extraction. RNA was reverse transcribed by using a Moloney murine leukemia virus kit (Invitrogen, Carlsbad, CA) and random hexamers (Promega, Madison, WI). Quantitative (real-time) PCR was performed by using a Bio-Rad (Hercules, CA) iCycler instrument according to the manufacturer's instructions. Real-time PCR analysis for IFN-γ was performed by using previously described primers for IFN-γ and hypoxanthine phosphoribosyltransferase (65, 66). The cycling conditions used for quantitative PCR were the following: 95°C for 4 min, followed by 40 cycles at 95°C for 30 s, 58°C for 30 s, and 72°C for 30 s. Primers were tested for specificity and efficiency. Real-time PCR analysis for NKG2D ligand transcripts was performed by using previously described primers and probes (37, 64, 65). Probes were purchased from Integrated DNA Technologies (Coralville, IA), and primers were purchased from Invitrogen (Carlsbad, CA). Bio-Rad (Hercules, CA) iQ Supermix was used for the reactions. The cycling conditions used for the determination of NKG2D ligand mRNA expression by quantitative PCR were the following: 95°C for 4 min, followed by 40 cycles at 95°C for 30 s and at 58°C for 30 s. Data were analyzed by using the Bio-Rad (Hercules, CA) iCycler iQ version 3.0a software. Samples were normalized to hypoxanthine phosphoribosyltransferase mRNA expression. Data were quantified by using the relative expression software tool, version 2 (72).
Mononuclear cell isolation and flow cytometry.
The immunophenotyping of the cellular infiltrate present within brains of infected mice was accomplished by homogenizing isolated tissue and generating a single-cell suspension for analysis by flow cytometry, as previously described (48). Fc receptors were blocked by using a concentration of 2.5 μg/ml of anti-mouse CD16/CD32 MAb 2.4G2 (BD Pharmingen, San Diego, CA) in staining buffer (1× phosphate-buffered saline [PBS], 0.1 g/liter bovine serum albumin). The following antibodies were used for immunophenotyping: fluorescein isothiocyanate (FITC)-conjugated rat anti-B220 (BD Pharmingen, San Diego, CA) for B cells; FITC-conjugated rat anti-7/4 (AbD Serotec, Raleigh, NC) for granulocytes; FITC-, phycoerythrin (PE)-, or allophycocyanin (APC)-conjugated rat anti-mouse CD8α for CD8+ T cells (BD Pharmingen, San Diego, CA); FITC- or APC-conjugated rat anti-mouse CD4 (BD Pharmingen, San Diego, CA) for CD4+ T cells; APC-conjugated rat anti-mouse CD49b MAb DX5 (Miltenyi Biotec, Bergisch Gladbach, Germany); PE- or FITC-conjugated hamster anti-mouse CD3ɛ (BD Pharmingen, San Diego, CA) for NK cells (DX5+, CD3ɛ−); and APC-conjugated rat anti-mouse CD45 (eBioscience, San Diego, CA) and FITC-conjugated rat anti-F480 (AbD Serotec, Raleigh, NC) for macrophages and microglia. PE- or APC-conjugated rat anti-mouse NKG2D (CX5; eBioscience, San Diego, CA) was used to analyze NKG2D expression on different cell types. PE-conjugated rat anti-mouse I-A/I-E (BD Pharmingen, San Diego, CA) was used in conjunction with APC-conjugated rat anti-mouse CD45 (eBioscience, San Diego, CA) to determine the MHC class II expression on microglia. Virus-specific CD8+ T-cell infiltration within brain was determined by flow cytometry by using H2-Ld-N318 tetramers comprising the dominant viral nucleocapsid protein spanning amino acids 318 to 326 for mice on the BALB/c background (4, 61). For NKG2D ligand expression on J774A.1 cells, PE-conjugated rat anti-mouse H60 and panspecific RAE-1 (R&D Systems, Minneapolis, MN) were used. As controls, isotype-matched APC- and PE-conjugated MAbs were used. Cells were incubated with MAbs for 30 min at 4°C and washed. NKG2D receptor surface expression was determined by incubating cells with a biotin-conjugated NKG2D-specific antibody, MI-6 (41) (which recognizes a different epitope than the CX5 anti-NKG2D MAb used for the in vivo modulation of the receptor; eBioscience, San Diego, CA), at 2.5 μg/ml for 30 min at 4°C. Cells were washed and incubated with PE-conjugated streptavidin (eBioscience, San Diego, CA) at 2 μg/ml for 30 min at 4°C. Samples were analyzed on a FACStar (BD Biosciences, Mountain View, CA). Data are presented as the percentage and total number of positive cells within the gated population.
Intracellular cytokine staining.
Single-cell suspensions were obtained from the brains of infected mice at day 7 p.i. Intracellular staining for IFN-γ was performed by stimulating cells for 6 h in medium supplemented with GolgiStop (BD Pharmingen, San Diego, CA) with the CD8+ T-cell immunodominant peptide N318-326 at a concentration of 5 nM (4). Intracellular IFN-γ was detected by using PE-conjugated rat anti-mouse IFN-γ (XMG1.2; BD Pharmingen, San Diego, CA) for 30 min at 4°C (31).
CD8+ T-cell cytotoxicity assay.
Brains from mice infected with JHMV and treated with either rat IgG or anti-NKG2D were removed and homogenized, and infiltrating cells were purified by using Percoll gradients as described previously (48, 56, 90). In order to obtain a sufficient number of antigen-specific CD8+ T cells for the assay, brains from each treatment group were pooled. J774A.1 cells were pulsed with either the immunodominant CD8+ T-cell epitope for mice on the BALB/c background (H-2d), N318-326 (4), or a nonantigenic ovalbumin (OVA) peptide at a concentration of 5 nM for 1.5 to 2 h at 37°C in 5% CO2. Peptide-pulsed J774A.1 cells were washed and then plated at a concentration of 10,000 cells per well. Lymphocytes from total brain isolates from both groups were cocultured with the peptide-pulsed J774A.1 cells in the presence of 10 μg/ml of rat IgG or anti-NKG2D. The final volume of well cocultures was 100 μl of RPMI 1640 supplemented with 10% fetal bovine serum (FBS). Cocultures were incubated for 4 h at 37°C in 5% CO2. The amounts of lactate dehydrogenase released from lysed cells were determined by using a CytoTox 96 nonradioactive cytotoxicity assay (Promega, Madison, WI). The percentage of lysis was determined as specified by the manufacturer's protocols. The percentage of lysis of OVA peptide-pulsed cocultures was subtracted from the percentage of lysis of N318-326-pulsed cocultures to eliminate background CTL activity.
IFN-γ secretion assay.
Total brain isolates from mice infected with JHMV and treated with either rat IgG or anti-NKG2D were cultured in 200 μl RPMI-1640 supplemented with 10% FBS, l-glutamine, penicillin-streptomycin, and 10 μg/ml of the corresponding antibody. Viral peptide N318-326 or control OVA peptide was added to the cultures to a final concentration of 5 nM and incubated at 37°C for 48 h in 5% CO2. Supernatants were removed and clarified by centrifugation.
ELISA.
For whole-tissue ELISA, brains were removed and homogenized in PBS supplemented with Complete Mini, EDTA-free protease inhibitor cocktail (two tablets per 14 ml of PBS) (Roche, Mannheim, Germany). Supernatants were clarified by centrifugation. Sample supernatants from the cytokine secretion assay were clarified by centrifugation and used for ELISA. ELISAs were performed by using the Duoset mouse IFN-γ ELISA kit (R&D Systems, Minneapolis, MN), as specified by the manufacturer, to determine the levels of IFN-γ.
CNS pathology.
Spinal cords were removed at days 27 and 32 p.i., and sections (8 μm) were stained with luxol fast blue (LFB) and analyzed by light microscopy. Demyelination was scored as follows: 0, no demyelination; 1, mild inflammation accompanied by the loss of myelin integrity; 2, moderate inflammation with increasing myelin damage; 3, numerous inflammatory lesions accompanied by a significant increase in myelin stripping; and 4, intense areas of inflammation accompanied by numerous phagocytic cells engulfing myelin debris (48). Slides containing stained spinal cord sections were blinded and scored by three investigators, and scores were averaged and are presented as averages ± standard errors of the means (SEM). The distribution of viral antigen was determined by immunoperoxidase staining (Vectastain-ABC kit; Vector Laboratories, Burlingame, CA) using the anti-JHMV MAb J.3.3 specific for the carboxyl terminus of the viral nucleocapsid protein as a primary antibody and horse anti-mouse IgG as the secondary antibody (Vector Laboratories, Burlingame, CA) (7, 28, 90). The data presented represent a minimum of six spinal cord sections per mouse with no fewer than three mice per time point.
Statistical analysis.
Statistically significant differences between groups of mice were determined by t tests using Sigma-Stat 2.0 software (Jandel, Chicago, IL), and P values of <0.05 were considered significant. The statistical analysis for survival studies was performed by using the log-rank test, in which a P value of 0.05 or less was considered significant (2, 49).
RESULTS
NKG2D receptor and ligands are expressed in response to JHMV infection of the CNS.
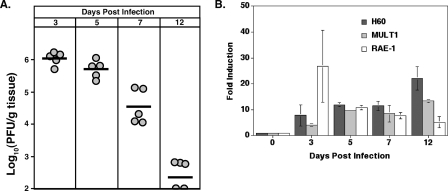
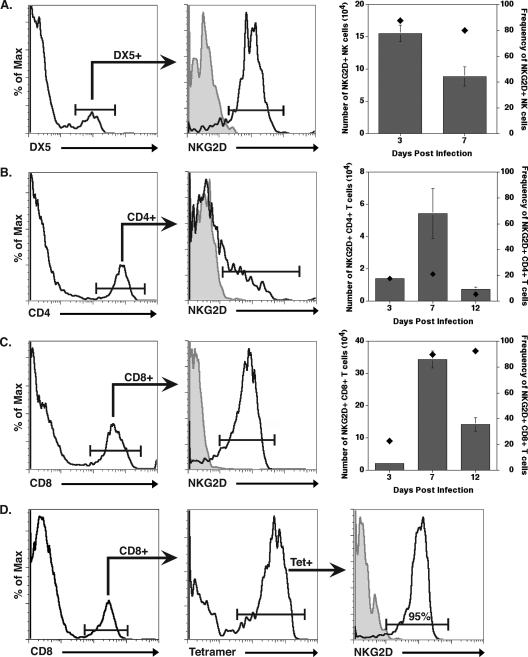
BALB/c mice were infected i.c. with JHMV, and viral titers within the brains were determined at days 3, 5, 7, and 12 p.i. As shown in Fig. 1A, the highest viral titers from the time points measured occurred at day 3 p.i., but they ultimately declined by day 12 p.i. as a result of the infiltration of virus-specific T cells. Transcripts specific for the NKG2D ligands H60, MULT1, and RAE-1 were up-regulated in the brains of infected mice compared to levels in sham-infected mice at days 3, 5, 7, and 12 p.i., as determined by quantitative real-time PCR (Fig. 1B). The immunophenotyping of cells for the NKG2D receptor expression determined that 90% of infiltrating NK cells (DX5+, CD3− cells) were NKG2D positive at day 3 p.i. (Fig. 2A). While the overall numbers of NK cells within the brain decreased by day 7 p.i., ∼80% of NK cells remained NKG2D positive (Fig. 2A). Very few NK cells were present within the CNS of mice at day 12 p.i., and NKG2D expression was not determined at this time point. Fewer than 20% of CD4+ T cells present within the CNS expressed NKG2D at any time point examined (Fig. 2B). At day 3 p.i., only ∼20% of infiltrating CD8+ T cells were NKG2D positive (Fig. 2C), but by day 7 p.i., the number of CD8+ T cells within the CNS of infected mice increased dramatically, with >90% expressing the NKG2D receptor (Fig. 2C). The numbers of CD8+ T cells within the CNS declined by day 12 p.i., and this correlated with the reduction in viral burden (Fig. 1A); however, the frequency of NKG2D-positive CD8+ T cells remained high, with ∼95% of N318-326 MHC class I tetramer-reactive CD8+ T cells expressing NKG2D (Fig. 2C and D). Taken together, these data demonstrate that NKG2D ligands are expressed in the brain following JHMV infection, and infiltrating NK cells and CD8+ T cells represent the predominant NKG2D-positive populations of immune cells examined that infiltrate into the CNS, suggesting a role for NKG2D in host defense following JHMV infection.
FIG. 1.
NKG2D ligands and the receptor are expressed following JHMV infection. (A) Viral titers within the brains of BALB/c mice infected i.c. with JHMV. Titers within the brains of individual mice are represented by circles, and the average titer for each time point is indicated by the dark line. Data presented are representative of two separate experiments. The sensitivity of the titer assay is ∼100 PFU/g tissue. (B) Transcripts specific for NKG2D ligands were up-regulated compared to levels for sham-infected mice at all time points assayed, as determined by quantitative real-time PCR. Data presented are representative of two separate experiments with a minimum of three mice for each time point.
FIG. 2.
NKG2D expression on cells infiltrating into the CNS of JHMV-infected mice. BALB/c mice were infected i.c. with JHMV, and NKG2D expression on infiltrating cells was determined at select times p.i. NK cells (A), CD4+ T cells (B), and CD8+ T cells (C) were found to express various levels of NKG2D at different times p.i. with virus. Representative histograms (left graphs in panels A to C) are from individual mice at day 7 p.i. For histograms in panels A to C, the shaded area represents isotype-matched control antibody staining and clear areas represent staining with the indicated antibody. In addition, both the overall numbers (gray bars; left y axis) and frequency (black diamonds; right y axis) of NKG2D-positive cells at defined times p.i. are provided (right graphs, panels A to C). (D) NKG2D expression on N318-326 epitope virus-specific CD8+ T cells (determined by tetramer staining) also was defined, with ∼95% of cells at day 12 p.i. expressing NKG2D. Flow cytometric data shown are from a representative experiment of two independent experiments with no fewer than three mice per time point. Data are presented as averages ± SEM.
NKG2D receptor signaling does not affect survival in JHMV-infected SCID mice.
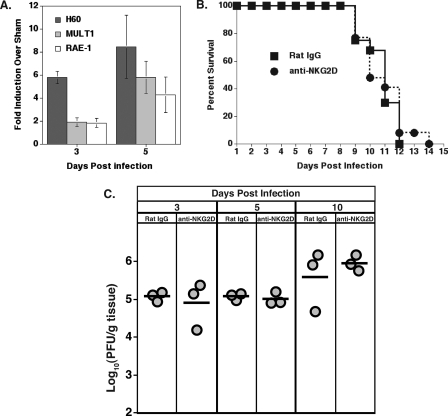
We next determined if NKG2D receptor signaling on NK cells was important in controlling viral infection within the CNS. JHMV infection of SCID mice, mice that are deficient in functional T and B cells while maintaining functional NK cells, resulted in the expression of H60, MULT1, and RAE-1 transcripts in the CNS on days 3 and 5 p.i. (Fig. 3A). Treatment with an anti-NKG2D neutralizing antibody did not affect survival or viral titers within the CNS compared to the survival and viral titers of infected mice treated with a control antibody (Fig. 3B and C). These data support earlier studies indicating that NKG2D receptor signaling on NK cells does not enhance innate immune responses following JHMV infection of the CNS (90a).
FIG. 3.
NKG2D neutralization in JHMV-infected SCID mice does not affect survival. (A) Quantitative real-time PCR of brain mRNA demonstrated that levels of transcripts for NKG2D ligands are increased in SCID mice compared to those of sham-infected mice at days 3 and 5 p.i. (B) Treatment of SCID mice with anti-NKG2D did not affect host survival compared to that of mice treated with control antibody. Data are presented as averages from two independent experiments with no fewer than six mice per group. (C) Levels of viral recovery from the brains of mice treated with anti-NKG2D or rat IgG were equivalent at all time points assayed. The viral burden is presented from a representative experiment of two independent experiments with three mice per group per time point. Circles represent individual mice, while bars represent the averages of the treatment group.
Blocking NKG2D increases mortality following JHMV infection of BALB/c mice.
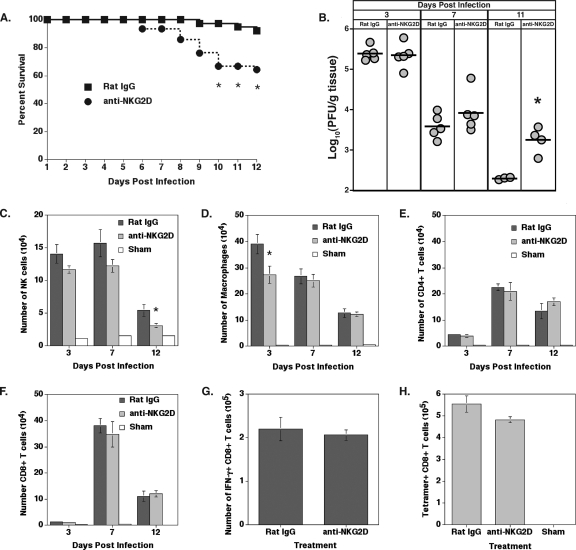
CD8+ T cells are the main effector cells responsible for controlling the replication and spread of JHMV following the infection of the CNS (83, 92). Infiltrating virus-specific CD8+ T cells present within the brains of JHMV-infected mice expressed NKG2D, suggesting a potential role in antiviral responses. Infected BALB/c mice were treated with anti-NKG2D or a control antibody to determine if NKG2D receptor signaling on CD8+ T cells has a functional role in host defense. NKG2D neutralization resulted in a significant decrease (P ≤ 0.05) in survival between days 10 and 12 p.i. compared to the levels of survival after control rat IgG treatment (Fig. 4A). An analysis of the viral burden within the brain at days 3 and 7 p.i. revealed that anti-NKG2D treatment did not alter the amount of infectious virus detected within the CNS compared to that of mice treated with rat IgG (Fig. 4B). However, by day 11 p.i., mice treated with anti-NKG2D neutralizing antibody exhibited a diminished (P ≤ 0.005) ability to reduce viral titers within the brain, indicating that antiviral T-cell responses might be impaired (Fig. 4B). The immunophenotyping of cell infiltrates in the brain following infection was performed to determine if NKG2D neutralization altered immune cell infiltration. NK cell infiltration was not affected by anti-NKG2D treatment at days 3 and 7 p.i. (Fig. 4C). However, a small but significant decrease (P ≤ 0.05) in the numbers of NK cells was observed at 12 days p.i. in the CNS of anti-NKG2D-treated mice compared to that of rat IgG-treated mice (Fig. 4C). Macrophage infiltration was diminished within the CNS of anti-NKG2D-treated mice at day 3 p.i., yet there were no differences between treatment groups between days 7 and 12 p.i. (Fig. 4D). Similarly, there were no detectable differences in CD4+ and CD8+ T-cell infiltration levels between treatment groups at any time point assayed (Fig. 4E and F). Finally, NKG2D neutralization did not affect the infiltration of virus-specific CD8+ T cells at day 7 p.i. as determined by intracellular IFN-γ staining (Fig. 4G) and N318-326 MHC class I tetramer staining (Fig. 4H). Rigorous analysis previously confirmed that CX5 treatment does not deplete NKG2D-bearing NK cells or T cells in vivo (42, 43, 63, 64). Therefore, these findings establish that NKG2D is important for protection from JHMV-induced CNS disease and is independent of immune cell infiltration, suggesting that antiviral T-cell responses are muted.
FIG. 4.
Anti-NKG2D treatment in immunocompetent BALB/c mice reduces host survival and antiviral activity. (A) NKG2D neutralization significantly (*, P ≤ 0.05) decreased survival, with only ∼65% of mice surviving at day 12 p.i. In contrast, ∼95% of mice treated with control antibody survived to day 12 p.i. Data are presented as averages from seven independent experiments with no fewer than four mice per group. (B) Analysis of viral titers from the brains of infected mice treated with either rat IgG or anti-NKG2D revealed no significant differences at days 3 and 7 p.i. However, blocking NKG2D resulted in greater (*, P ≤ 0.005) viral burden within the brain at day 11 p.i. than that of control-treated mice. The viral burden is presented from a representative experiment of two independent experiments with no fewer than three mice per group per time point. Circles represent individual mice; bars represent group averages. (C) Immunophenotyping of cellular infiltrates revealed that NK cell infiltration was unaffected by NKG2D neutralization at days 3 and 7 p.i. compared to that of rat IgG treatment. At day 12 p.i., there was a significant decrease (*, P ≤ 0.05) in the number of NK cells present within the brain. (D) Macrophage infiltration was significantly reduced (*, P ≤ 0.05) in anti-NKG2D-treated mice at day 3 p.i. but was similar to that of control-treated mice at days 7 and 12 p.i. Analysis of T-cell infiltration determined that numbers of CD4+ (E) and CD8+ (F) T cells are equivalent in mice treated with either rat IgG or anti-NKG2D. (G) Intracellular IFN-γ staining in response to N318-326 peptide revealed that the infiltration of virus-specific CD8+ T cells was unaffected by NKG2D neutralization compared to that of control-treated mice. (H) N318-326 MHC class I tetramer staining at day 7 p.i. also demonstrated that anti-NKG2D treatment does not affect virus-specific CD8+ T-cell infiltration into the CNS. Cell infiltration data shown in panels C to H are representative of two independent experiments with a minimum of five mice per group. Data are presented as averages ± SEM.
IFN-γ expression during acute disease is not dependent on NKG2D.
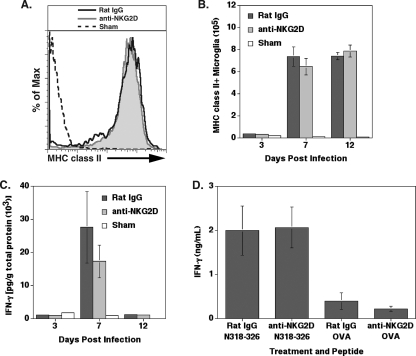
Previous studies have shown that blocking NKG2D receptor signaling diminishes IFN-γ secretion by T cells (9, 69, 74). Given that IFN-γ is important in the control of JHMV replication within the CNS and in host survival, it is plausible that anti-NKG2D treatment of JHMV-infected mice affects IFN-γ secretion by infiltrating T cells (69). MHC class II expression on microglia following MHV infection requires IFN-γ expression; therefore, the amount of MHC class II expressed by microglia can be used to determine the amounts of IFN-γ secretion within the CNS (7). Microglia from anti-NKG2D- and control-treated mice at day 7 p.i. revealed similar fluorescence intensities of MHC class II staining (Fig. 5A). In addition, the number of MHC class II+ microglia was similar between treatment groups on days 3, 7, and 12 p.i. (Fig. 5B). In parallel, there was no significant difference in IFN-γ protein levels within the brains at days 3, 7, and 12 p.i. in mice treated with rat IgG or anti-NKG2D (Fig. 5C). Total cells were isolated and enriched from the brains of rat IgG- or anti-NKG2D-treated mice at day 7 p.i. and cultured in the presence of the immunodominant CD8+ T-cell epitope N318-326 or a nonspecific OVA peptide as a control, and IFN-γ production was determined after 48 h. As shown in Fig. 5D, blocking NKG2D receptor signaling did not affect the secretion of IFN-γ by virus-specific CD8+ T cells compared to that of cells treated with control antibody. OVA peptide stimulation resulted in low-level IFN-γ production by both treatment groups compared to that of cultures containing the N318-326 peptide. Therefore, these findings indicate that NKG2D receptor signaling does not influence IFN-γ secretion within the CNS by virus-specific CD8+ T cells in response to JHMV infection.
FIG. 5.
NKG2D receptor signaling does not affect IFN-γ secretion in immunocompetent BALB/c mice. IFN-γ secretion within the brain was determined by MHC class II expression on microglia. (A) The fluorescence intensity of MHC class II on the surface of microglia was similar between rat IgG- and anti-NKG2D-treated mice at day 7 p.i., as demonstrated by a representative histogram. (B) Quantification of the mean fluorescence intensity (MFI) revealed that the level of MHC class II on microglia was similar at days 3, 7, and 12 p.i. in mice treated with either anti-NKG2D or control antibody. MHC class II expression data are representative of two independent experiments with no fewer than five mice per group. (C) IFN-γ protein amounts were reduced in the brains of anti-NKG2D-treated mice compared to those of rat IgG-treated mice at day 7 p.i.; however, the difference was not significant. There was no difference in the amounts of IFN-γ protein present in the brain at days 3 and 12 p.i. between treatment groups. Data presented are representative of two separate experiments, with each experiment using a minimum of four mice per time point. Data are presented as averages ± SEM. (D) Anti-NKG2D treatment of total brain isolates obtained at day 7 p.i. and pulsed with CD8+ T-cell epitope N318-326 resulted in increased IFN-γ secretion, but the difference was not significant compared to that of mice treated with rat IgG. Cells incubated with nonspecific OVA peptide are included to illustrate background levels of IFN-γ expression under the experimental conditions used. Data presented are representative of two separate experiments with three mice per experimental condition, and results are presented as averages ± SEM.
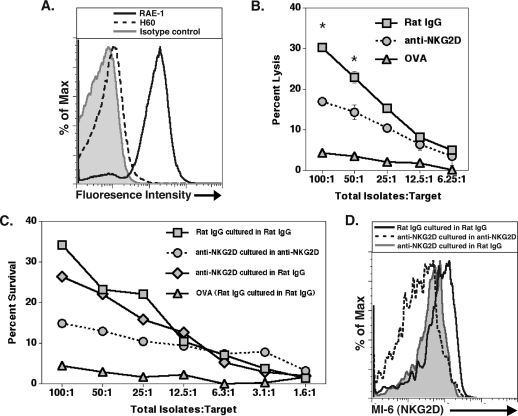
NKG2D neutralization reduces CD8+ CTL activity.
We next determined if blocking NKG2D affected CD8+ T-cell lytic activity. The target cells selected were of the J774A.1 cell line that expresses the NKG2D ligand RAE-1 (Fig. 6A) but low levels of H60. BALB/c mice were infected i.c. with JHMV and treated with either anti-NKG2D or control antibody. Infiltrating cells were isolated and enriched from the CNS at day 7 p.i. and incubated with J774A.1 cells pulsed with either the CD8+ T-cell epitope N318-326 or an OVA peptide as a control in the presence of either anti-NKG2D or rat IgG. CTL activity was significantly reduced (P ≤ 0.05) in cells from mice treated with anti-NKG2D and cultured in anti-NKG2D compared to that of cells from mice treated with control antibody and cultured in control antibody (Fig. 6B). The analysis of OVA peptide-pulsed target cells resulted in only limited lytic activity at all effector:target ratios tested compared to that of target cells pulsed with the CD8+ T-cell immunodominant MHV peptide. Binding of the anti-NKG2D antibody to NKG2D results in receptor internalization, suggesting that the expression of NKG2D on activated virus-specific T cells is required for optimal CTL activity (57, 64, 65). Therefore, to determine if the reemergence of NKG2D results in the recovery of CTL function, immune cells isolated from the CNS from mice treated with either anti-NKG2D or control IgG were cultured with N318-326-pulsed J774A.1 cells in the presence of either antibody. As shown in Fig. 6C, cells isolated from the brains of anti-NKG2D-treated mice and incubated with anti-NKG2D resulted in muted CTL activity compared to that of cells isolated from mice treated with rat IgG and incubated with rat IgG. However, CTL activity was partially restored in cells isolated from anti-NKG2D-treated mice that were cultured with rat IgG (Fig. 6C), and this correlated with the return in expression of NKG2D on the surface of CD8+ T cells (Fig. 6D). Lysis of OVA peptide-pulsed target cells was included to demonstrate background CTL activity (Fig. 6C). These data indicate that the expression of NKG2D is required for optimal lytic activity by virus-specific CD8+ T cells.
FIG. 6.
NKG2D neutralization diminishes CD8+ T-cell CTL activity. (A) J774A.1 target cells pulsed with N318-326 peptide express RAE-1 on the cell surface. (B) JHMV-infected mice treated with rat IgG or anti-NKG2D were sacrificed at day 7 p.i., and the CTL activity of CD8+ T cells was determined by using N318-326 peptide-pulsed J774A.1 cells as targets. Cocultures of total cell isolates and target cells were incubated in the presence of rat IgG or anti-NKG2D. Anti-NKG2D treatment (circles) resulted in reduced (*, P ≤ 0.05) CD8+ T-cell CTL activity compared to that of rat IgG-treated mice (squares). (C) Removal of anti-NKG2D (diamonds) partially restored CTL activity after 4 h, and this correlated with (D) the return of NKG2D expression on CD8+ T cells as demonstrated by using an antibody specific for NKG2D but recognizing a different epitope (MI-6) than that recognized by the antibody (CX5) used for neutralization. The percentage of lysis of OVA peptide-pulsed target cells (triangles in panels B and C) is included to demonstrate the background levels of CTL activity. Data presented are the averages from three independent experiments using a minimum of seven mice from each experimental group for analysis.
Blocking NKG2D does not affect the severity of demyelination.
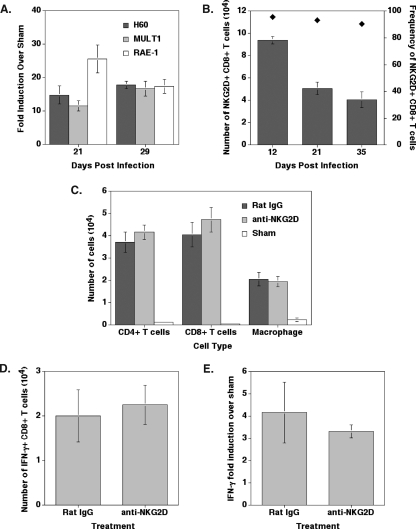
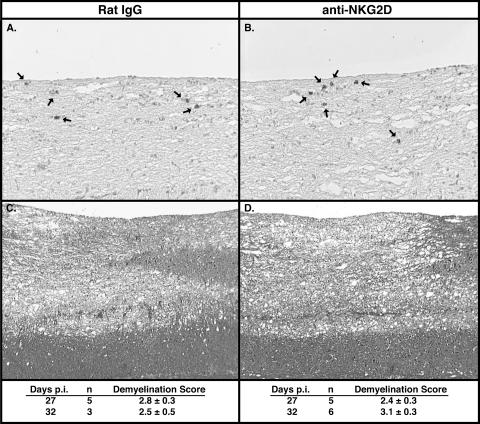
Transcripts for the NKG2D ligands H60, MULT1, and RAE-1 were detected within the spinal cords of mice persistently infected with JHMV (Fig. 7A). The numbers of NKG2D-positive CD8+ T cells within the spinal cords of JHMV-infected mice gradually declined from day 12 through day 35 p.i., although >90% of CD8+ T cells remained NKG2D positive (Fig. 7B). The presence of the NKG2D receptor on lymphocytes and NKG2D ligands in the CNS during MHV-induced chronic demyelinating disease raises the question of whether these molecules contribute to the pathogenesis of chronic disease. In order to test this, mice were infected with JHMV, and NKG2D-blocking antibodies were administered beginning at day 19, which represents a time at which demyelinating lesions are present within persistently infected mice (56). Treatment with anti-NKG2D did not modulate the infiltration of either CD4+ or CD8+ T cells or macrophages (Fig. 7C), and it did not affect the infiltration of virus-specific CD8+ T cells as determined by intracellular IFN-γ staining (Fig. 7D). Anti-NKG2D treatment did not affect IFN-γ mRNA expression within the spinal cord compared to that of control-treated mice at day 29 p.i. (Fig. 7E). Furthermore, there were no differences with either viral antigen (Fig. 8A and B) or in the severity of demyelination (Fig. 8C and D) between mice treated with anti-NKG2D and those treated with control antibody. These findings indicate that NKG2D receptor signaling does not contribute to chronic JHMV-induced demyelinating disease in immunocompetent mice.
FIG. 7.
NKG2D ligands and receptor are expressed in the spinal cords of mice persistently infected with JHMV. (A) Real-time PCR analysis revealed that levels of mRNA transcripts for NKG2D ligands were increased compared to those of sham-infected mice within the spinal cords of mice chronically infected at days 21 and 29 p.i. A minimum of three mice per time point was used for analysis. (B) Greater than 90% of CD8+ T cells (black diamonds; right y axis) retained in the spinal cords of infected mice expressed NKG2D on days 12, 21, and 35 p.i. The number of NKG2D+ CD8+ T cells (gray bars; left y axis) was greatest at day 12 p.i. and decreased at days 21 and 35 p.i. (C) Treatment with anti-NKG2D did not alter the infiltration of T cells or macrophages on day 29 p.i. (D) Anti-NKG2D treatment did not reduce the total number of virus-specific CD8+ T cells on day 32 p.i. as determined by intracellular IFN-γ staining following pulsing with N318-326 peptide, and (E) there were no differences in the overall IFN-γ transcript levels as determined by quantitative real-time PCR analysis on day 29 p.i. Data are presented as the averages ± SEM. Data are representative of two independent experiments with a minimum of three mice per time point.
FIG. 8.
Anti-NKG2D treatment does not affect the severity of demyelination in JHMV-infected mice. Representative spinal cord tissue sections from JHMV-infected mice at day 27 p.i. revealed no differences in either the number or the distribution of viral antigen in spinal cords of mice treated with either rat IgG (A) (magnification, ×100) or anti-NKG2D (B) (magnification, ×100). Viral antigen was detected by immunoperoxidase staining by using anti-JHMV MAb J.3.3, and antigen-positive cells were demonstrated by the presence of chromogen (arrows) (28, 90). Representative LFB-stained spinal cord sections from JHMV-infected cells at day 32 p.i. from mice treated with rat IgG (C) (magnification, ×40) or anti-NKG2D (D) (magnification, ×40) revealed no differences in the severity of white-matter damage. Demyelination scores were determined by the analysis of LFB-stained tissues and are provided for treatment groups below each representative image.
DISCUSSION
The present study demonstrates a role for NKG2D in host defense following viral infection of the CNS. Transcripts for NKG2D ligands are expressed within the brains of JHMV-infected mice, and infiltrating inflammatory cells, specifically CD8+ T cells and NK cells, express the NKG2D receptor. NKG2D neutralization in infected SCID mice resulted in levels of survival and viral burden similar to those of control-treated mice, demonstrating that NKG2D receptor signaling on NK cells does not affect the outcome of disease in the absence of an adaptive immune response. In contrast, NKG2D neutralization during acute disease in immunocompetent BALB/c mice resulted in enhanced mortality and an increased viral burden that was independent of immune cell infiltration into the CNS or IFN-γ secretion. Specifically, our findings suggest that anti-NKG2D treatment selectively diminished CD8+ T-cell CTL activity, indicating that NKG2D receptor signaling specifically amplifies this arm of the effector response and is important for protection during acute disease following JHMV infection of the CNS.
The inoculation of the CNS of immunodeficient mice with JHMV resulted in elevated levels of transcripts specific for NKG2D ligands as early as day 3 p.i. Similarly, NKG2D transcripts were present within the brains of JHMV-infected BALB/c mice at comparatively higher levels than those of infected SCID mice. These findings suggest that resident glial cells are capable of expressing ligands and that infiltrating leukocytes also express ligands. We know neither the cellular sources for transcripts within JHMV-infected SCID or BALB/c mice nor the molecular mechanisms associated with the induction of transcript expression. Previous studies have highlighted the importance of Toll-like receptor (TLR) signaling in NKG2D ligand induction (24, 35, 95). JHMV infection of the CNS does result in elevated levels of transcripts for several TLRs, including TLR-3 and TLR-6 (T. E. Lane, unpublished observations); therefore, it is possible that TLR signaling enhances NKG2D ligand expression within the CNS in response to JHMV infection. Moreover, recent reports indicate that viral infection of both cultured cells and mice results in the increased expression of NKG2D ligands within infected cells, indicating that viral infection is sufficient to trigger ligand expression (24, 27). In addition, it is possible that infiltrating leukocytes express NKG2D ligands and/or that cytokines released from activated cells enhance ligand expression on resident glial cells.
The importance of NKG2D receptor signaling on NK cells has been demonstrated in many tumor and pathogen models (11, 13, 15, 20, 21, 38, 46, 50, 57, 58, 84). The interaction between the NKG2D receptor and specific ligands results in enhanced effector functions by NK cells, including the secretion of IFN-γ and cytotoxic activity that augments the killing of NKG2D ligand-bearing target cells (14, 15, 20, 21, 57). Moreover, a previously unappreciated role for NKG2D and its ligands in nonclassical NK T-cell-mediated immune responses has been revealed by using a transgenic mouse model of human hepatitis B infection (89). In addition, NKG2D-mediated interactions between NK cells and professional antigen-presenting cells have been shown to lead to stronger priming of antigen-specific CD8+ T-cell responses by increasing interleukin-12 (IL-12) production from dendritic cells containing an antigen from Toxoplasma gondii lysate (36). Treatment of T. gondii-infected mice with anti-NKG2D antibody impaired the development of antigen-specific CD8+ T cells, which led to a reduced ability to clear parasites from infected tissues. We do not believe that NKG2D is functioning in a similar manner in our model system of virus-induced neurological disease. We have recently determined that in vivo blocking of IL-12 neither impairs the generation of virus-specific T cells nor mutes antiviral effector function leading to increased viral burden within the brain (K. S. Held, W. G. Glass, Y. I. Orlovsky, K. A. Shamberger, T. D. Petly, P. J. Branigan, M. R. Cunningham, J. M. Benson, and T. E. Lane, unpublished data). In addition, recent studies have emphasized that NK cells do not play a prominent role in defense against JHMV infection of the CNS (86, 96). Finally, we observed no differences in the frequencies or overall numbers of virus-specific CD8+ T cells within the brains of infected mice treated with either anti-NKG2D or control antibody, indicating that blocking NKG2D does not limit the development of an effective CD8+ T-cell response. Therefore, we conclude that NKG2D-mediated protection from virus-induced CNS disease is not dependent upon an NK cell-mediated pathway.
More important in the control of JHMV replication within the CNS is the infiltration of CD8+ T cells (6). Upon infection, virus-specific CD8+ T cells are primed within the draining cervical lymph nodes, and migration back into the CNS is aided by using specific chemokine receptors responding to chemokine ligands secreted from within the CNS (54, 55, 62, 85). During priming, naïve CD8+ T cells require T-cell receptor (TCR) engagement to MHC molecules presenting antigenic peptide and secondary costimulatory signals, such as CD28 ligation by members of the B7 family, in order to expand and differentiate into CTLs (88). Importantly, the costimulation of antigen-primed CTLs is not required for the release of effector molecules, but it can enhance effector functions (25, 51, 60). T cells express numerous receptors that act in controlling cellular responses through recognition and binding to specific ligands (88). Under the majority of circumstances, professional antigen-presenting cells express many of the ligands for these costimulatory molecules; however, inflammation also can induce the expression of additional ligands that enhance cytokine secretion and lytic activity following recognition by antigen-specific CD8+ T cells (8, 12, 23, 30, 67, 77). Numerous reports have suggested that NKG2D acts as a costimulatory molecule on both mouse and human CD8+ T cells (22, 35, 41, 59, 60). Indeed, studies examining the costimulation of TCR transgenic CTLs through either NKG2D-RAE-1 or CD28-B7 interaction revealed that responses occur regardless of the signaling pathway utilized (60). The engagement of NKG2D resulted in enhanced CTL expansion, IFN-γ secretion, and CTL activity, suggesting that NKG2D is a costimulatory molecule sharing functional similarities with CD28 (60). These findings differ from those of Ehrlich et al. (25), in which a rigorous analysis indicated that NKG2D expression on mouse CD8+ T cells acts as a costimulatory molecule only under restricted conditions or requires additional cofactors. The experimental conditions and model systems employed may explain the differences between these results.
Our findings suggest that blocking NKG2D during acute disease in JHMV-infected mice emphasizes a role for this molecule in host defense. What is interesting is that anti-NKG2D treatment selectively compromised CTL effector functions, while IFN-γ secretion remained intact. It is unclear whether one mechanism contributing to this differential response of CTL-mediated killing relates to the restricted expression of NKG2D ligands on CTL-sensitive populations. For example, NKG2D ligands may be expressed by astrocytes and microglia that are sensitive to CTL-mediated lysis, whereas NKG2D ligand expression may be absent on oligodendrocytes, thus rendering these cells resistant to CTL-mediated killing (32, 69). The proliferation of antigen-specific CD8+ T cells was not affected following anti-NKG2D treatment, because there were no differences in the total numbers of T cells or virus-specific CD8+ T cells within the CNS following viral infection. Other reports indicate that blocking NKG2D on mouse CTLs negatively affects numerous effector functions, including proliferation, the production of IFN-γ, and lytic activity (41, 60, 64, 74). There are several possible explanations for the results obtained from our experiments. First, treatment with anti-NKG2D antibody may have selectively blocked cytolytic activity in a subpopulation of mouse CD8+ T cells previously characterized as developing in a thymic-independent manner and phenotypically as CD8+ CD44high and NKG2D positive (19). These cells exhibited preferential cytolytic killing of syngeneic tumor target cells that was enhanced by the NKG2D engagement of RAE-1. We do not feel that this is a reasonable explanation, as these cells are speculated to preferentially express TCRs with a strong bias toward self antigen as a result of extrathymic development and likely function in a surveillance manner to protect against cellular transformation (19). Second, it is possible that JHMV infection results in the expansion of a subpopulation of virus-specific CD8+ CD28− T cells that selectively function in the lysis of virus-infected cells. This possibility is supported by the finding that blocking NKG2D inhibited MHC-restricted lysis of cytomegalovirus-infected targets by human CD8+ CD28− T-cell clones (35). However, data from our studies suggest this is unlikely, as CD28 was detected on all NKG2D+ CD8+ T cells examined, indicating that JHMV-specific CTLs are not functioning in a CD28-independent manner (data not shown). Alternatively, the ligation of NKG2D may initiate intracellular signaling events that allow for specific effector functions to evolve, e.g., IFN-γ secretion and the release of cytotoxic granules. Again, we do not endorse this possibility, as numerous studies examining intracellular signaling associated with NKG2D have not revealed divergent pathways controlling defined T-cell responses. Finally, NKG2D has been shown to be involved in immunological synapse (IS) formation in mouse and human CTLs (60, 79). The ability to form IS by CTLs has been suggested to potentially alert the cell for possible TCR engagement and subsequent induction of effector responses (79). Therefore, one explanation for our results is that treatment with anti-NKG2D antibody inhibited mature IS formation, and this resulted in a downstream effect on CTL-mediated killing by not allowing for the focused delivery of cytotoxic effector molecules to target cells. Our findings that the removal of anti-NKG2D from the CTL culture system results in the return of surface expression of NKG2D and the restoration of lytic activity support this possibility. Related to this matter is the duration of efficacy of anti-NKG2D MAb following the treatment of mice. Previous studies have indicated that antibody can be detected for up to 2 weeks in mice following a single treatment, with a sustained reduction of NKG2D for up to 7 days posttreatment (42, 57, 63, 64, and Lane, unpublished).
Although our in vitro results suggest that NKG2D expression can be detected relatively quickly in the absence of blocking antibody, we believe that in vivo treatment results in sustained activity throughout the experimental conditions employed. Additionally, animals received multiple injections of anti-NKG2D antibody, which further supports a long-lasting blocking effect in vivo.
Although NKG2D ligands are expressed within the spinal cord in mice persistently infected with JHMV, and although ∼95% of CD8+ T cells present were NKG2D positive, NKG2D neutralization had no effect on the severity of demyelination. This is in contrast to a previous study, in which JHMV infection of TCR β−/− mice (lacking T cells with a functional αβ TCR) resulted in demyelination, possibly by modulating IFN-γ secretion by γδ T cells, which was partially mediated by NKG2D receptor signaling (18). However, demyelination in immunocompetent mice infected with JHMV is amplified by IFN-γ-secreting CD4+ T cells as well as macrophage infiltration (31, 48, 56, 94). These findings highlight potential differences between T-cell subsets (e.g., αβ T cells and γδ T cells) with regard to IFN-γ secretion and suggest that, in the absence of the traditional TCR, γδ T cells depend more on alternative pathways such as NKG2D for IFN-γ to be produced. The adoptive transfer of CD8+ T cells into JHMV-infected Rag1−/− recipients does result in demyelinating lesions, demonstrating that CD8+ T cells can contribute to white-matter damage in JHMV-infected mice; our findings indicate that CD8+ T-cell contributions to demyelination are NKG2D independent. This is not entirely surprising, given the demonstration that NKG2D enhances CTL activity by virus-specific T cells during acute disease. This is relevant in light of studies by Bergmann and colleagues (5) that demonstrated that CD8+ T cells that are retained within the CNS during JHMV persistence exhibit impaired cytolytic function. Therefore, blocking NKG2D may not have any substantial effect on CD8+ T cells during the chronic phase of disease due to the inherent deficiencies in these cells to lyse virus-infected targets. Interestingly, a recent study determined that NKG2D ligands are expressed by cultured human oligodendrocytes as well as by oligodendrocytes located within white-matter sections obtained from multiple sclerosis lesions (76). Moreover, inhibiting the NKG2D-NKG2D ligand interactions in culture resulted in a marked inhibition of the killing of oligodendrocytes by NK cells, γδ T cells, and CD8+ T cells, highlighting the possible contributions of NKG2D receptor signaling in cytotoxic responses within the CNS (76). Additional information derived from other relevant animal models of demyelination will be necessary to further clarify the importance of NKG2D receptor signaling in chronic neurodegenerative diseases.
Acknowledgments
We are indebted to Wenqiang Wei (University of Southern California, Los Angeles, CA) for tissue staining and immunostaining for viral antigen.
National Institutes of Health grants NS41249 to T.E.L. and AI066897 to L.L.L. supported this work. A Pilot Research Award from the National Multiple Sclerosis Society to T.E.L. also supported this work. L.L.L. is an American Cancer Society Research Professor.
L.L.L. and the University of California (San Francisco, CA) have licensed intellectual property rights relative to NKG2D for commercial applications.
Footnotes
Published ahead of print on 26 December 2007.
REFERENCES
- 1.Adami, C., J. Pooley, J. Glomb, E. Stecker, F. Fazal, J. O. Fleming, and S. C. Baker. 1995. Evolution of mouse hepatitis virus (MHV) during chronic infection: quasispecies nature of the persisting MHV RNA. Virology 209337-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agresti, A. 1992. A survey of exact interference for contingency tables. Stat. Sci. 7131-177. [Google Scholar]
- 3.Aloisi, F., F. Ria, and L. Adorini. 2000. Regulation of T-cell responses by CNS antigen-presenting cells: different roles for microglia and astrocytes. Immunol. Today 21141-147. [DOI] [PubMed] [Google Scholar]
- 4.Bergmann, C., M. McMillan, and S. Stohlman. 1993. Characterization of the Ld-restricted cytotoxic T-lymphocyte epitope in the mouse hepatitis virus nucleocapsid protein. J. Virol. 677041-7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergmann, C. C., J. D. Altman, D. Hinton, and S. A. Stohlman. 1999. Inverted immunodominance and impaired cytolytic function of CD8+ T cells during viral persistence in the central nervous system. J. Immunol. 1633379-3387. [PubMed] [Google Scholar]
- 6.Bergmann, C. C., T. E. Lane, and S. A. Stohlman. 2006. Coronavirus infection of the central nervous system: host-virus stand-off. Nat. Rev. Microbiol. 4121-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergmann, C. C., B. Parra, D. R. Hinton, R. Chandran, M. Morrison, and S. A. Stohlman. 2003. Perforin-mediated effector function within the central nervous system requires IFN-gamma-mediated MHC up-regulation. J. Immunol. 1703204-3213. [DOI] [PubMed] [Google Scholar]
- 8.Bertram, E. M., W. Dawicki, and T. H. Watts. 2004. Role of T cell costimulation in anti-viral immunity. Semin. Immunol. 16185-196. [DOI] [PubMed] [Google Scholar]
- 9.Borchers, M. T., N. L. Harris, S. C. Wesselkamper, S. Zhang, Y. Chen, L. Young, and G. W. Lau. 2006. The NKG2D-activating receptor mediates pulmonary clearance of Pseudomonas aeruginosa. Infect. Immun. 742578-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchmeier, M. J., and T. E. Lane. 1999. Viral-induced neurodegenerative disease. Curr. Opin. Microbiol. 2398-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell, J. A., D. S. Trossman, W. M. Yokoyama, and L. N. Carayannopoulos. 2007. Zoonotic orthopoxviruses encode a high-affinity antagonist of NKG2D. J. Exp. Med. 2041311-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carreno, B. M., and M. Collins. 2002. The B7 family of ligands and its receptors: new pathways for costimulation and inhibition of immune responses. Annu. Rev. Immunol. 2029-53. [DOI] [PubMed] [Google Scholar]
- 13.Cerboni, C., F. Neri, N. Casartelli, A. Zingoni, D. Cosman, P. Rossi, A. Santoni, and M. Doria. 2007. Human immunodeficiency virus 1 Nef protein downmodulates the ligands of the activating receptor NKG2D and inhibits natural killer cell-mediated cytotoxicity. J. Gen. Virol. 88242-250. [DOI] [PubMed] [Google Scholar]
- 14.Cerwenka, A., A. B. Bakker, T. McClanahan, J. Wagner, J. Wu, J. H. Phillips, and L. L. Lanier. 2000. Retinoic acid early inducible genes define a ligand family for the activating NKG2D receptor in mice. Immunity 12721-727. [DOI] [PubMed] [Google Scholar]
- 15.Cerwenka, A., J. L. Baron, and L. L. Lanier. 2001. Ectopic expression of retinoic acid early inducible-1 gene (RAE-1) permits natural killer cell-mediated rejection of a MHC class I-bearing tumor in vivo. Proc. Natl. Acad. Sci. USA 9811521-11526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheever, F. S., J. B. Daniels, A. M. Pappenheimer, and O. T. Bailey. 1949. A murine virus (JHM) causing disseminated encephalomyelitis with extensive destruction of myelin. J. Exp. Med. 90181-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen, Z., K. Li, R. R. Rowland, and P. G. Plagemann. 1999. Selective antibody neutralization prevents neuropathogenic lactate dehydrogenase-elevating virus from causing paralytic disease in immunocompetent mice. J. Neurovirol. 5200-208. [DOI] [PubMed] [Google Scholar]
- 18.Dandekar, A. A., K. O'Malley, and S. Perlman. 2005. Important roles for gamma interferon and NKG2D in γδ T-cell-induced demyelination in T-cell receptor β-deficient mice infected with a coronavirus. J. Virol. 799388-9396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhanji, S., and H. S. Teh. 2003. IL-2-activated CD8+CD44high cells express both adaptive and innate immune system receptors and demonstrate specificity for syngeneic tumor cells. J. Immunol. 1713442-3450. [DOI] [PubMed] [Google Scholar]
- 20.Diefenbach, A., A. M. Jamieson, S. D. Liu, N. Shastri, and D. H. Raulet. 2000. Ligands for the murine NKG2D receptor: expression by tumor cells and activation of NK cells and macrophages. Nat. Immunol. 1119-126. [DOI] [PubMed] [Google Scholar]
- 21.Diefenbach, A., E. R. Jensen, A. M. Jamieson, and D. H. Raulet. 2001. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature 413165-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diefenbach, A., E. Tomasello, M. Lucas, A. M. Jamieson, J. K. Hsia, E. Vivier, and D. H. Raulet. 2002. Selective associations with signaling proteins determine stimulatory versus costimulatory activity of NKG2D. Nat. Immunol. 31142-1149. [DOI] [PubMed] [Google Scholar]
- 23.Dustin, M. L., R. Rothlein, A. K. Bhan, C. A. Dinarello, and T. A. Springer. 1986. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J. Immunol. 137245-254. [PubMed] [Google Scholar]
- 24.Ebihara, T., H. Masuda, T. Akazawa, M. Shingai, H. Kikuta, T. Ariga, M. Matsumoto, and T. Seya. 2007. Induction of NKG2D ligands on human dendritic cells by TLR ligand stimulation and RNA virus infection. Int. Immunol. 191145-1155. [DOI] [PubMed] [Google Scholar]
- 25.Ehrlich, L. I., K. Ogasawara, J. A. Hamerman, R. Takaki, A. Zingoni, J. P. Allison, and L. L. Lanier. 2005. Engagement of NKG2D by cognate ligand or antibody alone is insufficient to mediate costimulation of human and mouse CD8+ T cells. J. Immunol. 1741922-1931. [DOI] [PubMed] [Google Scholar]
- 26.Fabry, Z., C. S. Raine, and M. N. Hart. 1994. Nervous tissue as an immune compartment: the dialect of the immune response in the CNS. Immunol. Today 15218-224. [DOI] [PubMed] [Google Scholar]
- 27.Fang, M., L. L. Lanier, and L. J. Sigal. A role for NKG2D in NK cell-mediated resistance to poxvirus disease. PLoS Pathog., in press. [DOI] [PMC free article] [PubMed]
- 28.Fleming, J. O., S. A. Stohlman, R. C. Harmon, M. M. Lai, J. A. Frelinger, and L. P. Weiner. 1983. Antigenic relationships of murine coronaviruses: analysis using monoclonal antibodies to JHM (MHV-4) virus. Virology 131296-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fleury, H. J., R. D. Sheppard, M. B. Bornstein, and C. S. Raine. 1980. Further ultrastructural observations of virus morphogenesis and myelin pathology in JHM virus encephalomyelitis. Neuropathol. Appl. Neurobiol. 6165-179. [DOI] [PubMed] [Google Scholar]
- 30.Frauwirth, K. A., and C. B. Thompson. 2002. Activation and inhibition of lymphocytes by costimulation. J. Clin. Investig. 109295-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glass, W. G., M. J. Hickey, J. L. Hardison, M. T. Liu, J. E. Manning, and T. E. Lane. 2004. Antibody targeting of the CC chemokine ligand 5 results in diminished leukocyte infiltration into the central nervous system and reduced neurologic disease in a viral model of multiple sclerosis. J. Immunol. 1724018-4025. [DOI] [PubMed] [Google Scholar]
- 32.González, J. M., C. C. Bergmann, C. Ramakrishna, D. R. Hinton, R. Atkinson, J. Hoskin, W. B. Macklin, and S. A. Stohlman. 2006. Inhibition of interferon-gamma signaling in oligodendroglia delays coronavirus clearance without altering demyelination. Am. J. Pathol. 168796-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Griffin, D., B. Levine, W. Tyor, S. Ubol, and P. Despres. 1997. The role of antibody in recovery from alphavirus encephalitis. Immunol. Rev. 159155-161. [DOI] [PubMed] [Google Scholar]
- 34.Griffin, D. E. 2003. Immune responses to RNA-virus infections of the CNS. Nat. Rev. Immunol. 3493-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Groh, V., R. Rhinehart, J. Randolph-Habecker, M. S. Topp, S. R. Riddell, and T. Spies. 2001. Costimulation of CD8αβ T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat. Immunol. 2255-260. [DOI] [PubMed] [Google Scholar]
- 36.Guan, H., M. Moretto, D. J. Bzik, J. Gigley, and I. A. Khan. 2007. NK cells enhance dendritic cell response against parasite antigens via NKG2D pathway. J. Immunol. 179590-596. [DOI] [PubMed] [Google Scholar]
- 37.Hamerman, J. A., K. Ogasawara, and L. L. Lanier. 2004. Cutting edge: Toll-like receptor signaling in macrophages induces ligands for the NKG2D receptor. J. Immunol. 1722001-2005. [DOI] [PubMed] [Google Scholar]
- 38.Hayakawa, Y., J. M. Kelly, J. A. Westwood, P. K. Darcy, A. Diefenbach, D. Raulet, and M. J. Smyth. 2002. Cutting edge: tumor rejection mediated by NKG2D receptor-ligand interaction is dependent upon perforin. J. Immunol. 1695377-5381. [DOI] [PubMed] [Google Scholar]
- 39.Hickey, W. F. 2001. Basic principles of immunological surveillance of the normal central nervous system. Glia 36118-124. [DOI] [PubMed] [Google Scholar]
- 40.Hirano, N., T. Murakami, K. Fujiwara, and M. Matsumoto. 1978. Utility of mouse cell line DBT for propagation and assay of mouse hepatitis virus. Jpn. J. Exp. Med. 4871-75. [PubMed] [Google Scholar]
- 41.Jamieson, A. M., A. Diefenbach, C. W. McMahon, N. Xiong, J. R. Carlyle, and D. H. Raulet. 2002. The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity 1719-29. [DOI] [PubMed] [Google Scholar]
- 42.Kim, J., C. K. Chang, T. Hayden, F. Liu, J. A. H. Benjamin, L. L. Lanier, and S. Kang. 2007. The activating immunoreceptor NKG2D and its ligands are involved in allograft transplant rejection. J. Immunol. 1796416-6420. [DOI] [PubMed] [Google Scholar]
- 43.Kjellev, S., C. Haase, D. Lundsgaard, B. Urso, D. Tornehave, and H. Markholst. 2007. Inhibition of NKG2D receptor function by antibody therapy attenuates transfer-induced colitis in SCID mice. Eur. J. Immunol. 371397-1406. [DOI] [PubMed] [Google Scholar]
- 44.Knobler, R. L., M. Dubois-Dalcq, M. V. Haspel, A. P. Claysmith, P. W. Lampert, and M. B. Oldstone. 1981. Selective localization of wild type and mutant mouse hepatitis virus (JHM strain) antigens in CNS tissue by fluorescence, light and electron microscopy. J. Neuroimmunol. 181-92. [DOI] [PubMed] [Google Scholar]
- 45.Komatsu, T., Z. Bi, and C. S. Reiss. 1996. Interferon-gamma induced type I nitric oxide synthase activity inhibits viral replication in neurons. J. Neuroimmunol. 68101-108. [DOI] [PubMed] [Google Scholar]
- 46.Krmpotic, A., M. Hasan, A. Loewendorf, T. Saulig, A. Halenius, T. Lenac, B. Polic, I. Bubic, A. Kriegeskorte, E. Pernjak-Pugel, M. Messerle, H. Hengel, D. H. Busch, U. H. Koszinowski, and S. Jonjic. 2005. NK cell activation through the NKG2D ligand MULT-1 is selectively prevented by the glycoprotein encoded by mouse cytomegalovirus gene m145. J. Exp. Med. 201211-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lane, T. E., V. C. Asensio, N. Yu, A. D. Paoletti, I. L. Campbell, and M. J. Buchmeier. 1998. Dynamic regulation of alpha- and beta-chemokine expression in the central nervous system during mouse hepatitis virus-induced demyelinating disease. J. Immunol. 160970-978. [PubMed] [Google Scholar]
- 48.Lane, T. E., M. T. Liu, B. P. Chen, V. C. Asensio, R. M. Samawi, A. D. Paoletti, I. L. Campbell, S. L. Kunkel, H. S. Fox, and M. J. Buchmeier. 2000. A central role for CD4+ T cells and RANTES in virus-induced central nervous system inflammation and demyelination. J. Virol. 741415-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Langsrud, Ø., K. Jørgensen, R. Ragni Ofstad, and T. Næs. Analyzing designed experiments with multiple responses. J. Appl. Stat., in press.
- 50.Lenac, T., M. Budt, J. Arapovic, M. Hasan, A. Zimmermann, H. Simic, A. Krmpotic, M. Messerle, Z. Ruzsics, U. H. Koszinowski, H. Hengel, and S. Jonjic. 2006. The herpesviral Fc receptor fcr-1 down-regulates the NKG2D ligands MULT-1 and H60. J. Exp. Med. 2031843-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liang, L., and W. C. Sha. 2002. The right place at the right time: novel B7 family members regulate effector T cell responses. Curr. Opin. Immunol. 14384-390. [DOI] [PubMed] [Google Scholar]
- 52.Lin, M. T., D. R. Hinton, N. W. Marten, C. C. Bergmann, and S. A. Stohlman. 1999. Antibody prevents virus reactivation within the central nervous system. J. Immunol. 1627358-7368. [PubMed] [Google Scholar]
- 53.Lin, M. T., S. A. Stohlman, and D. R. Hinton. 1997. Mouse hepatitis virus is cleared from the central nervous systems of mice lacking perforin-mediated cytolysis. J. Virol. 71383-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu, M. T., D. Armstrong, T. A. Hamilton, and T. E. Lane. 2001. Expression of Mig (monokine induced by interferon-gamma) is important in T lymphocyte recruitment and host defense following viral infection of the central nervous system. J. Immunol. 1661790-1795. [DOI] [PubMed] [Google Scholar]
- 55.Liu, M. T., B. P. Chen, P. Oertel, M. J. Buchmeier, D. Armstrong, T. A. Hamilton, and T. E. Lane. 2000. The T cell chemoattractant IFN-inducible protein 10 is essential in host defense against viral-induced neurologic disease. J. Immunol. 1652327-2330. [DOI] [PubMed] [Google Scholar]
- 56.Liu, M. T., H. S. Keirstead, and T. E. Lane. 2001. Neutralization of the chemokine CXCL10 reduces inflammatory cell invasion and demyelination and improves neurological function in a viral model of multiple sclerosis. J. Immunol. 1674091-4097. [DOI] [PubMed] [Google Scholar]
- 57.Lodoen, M., K. Ogasawara, J. A. Hamerman, H. Arase, J. P. Houchins, E. S. Mocarski, and L. L. Lanier. 2003. NKG2D-mediated natural killer cell protection against cytomegalovirus is impaired by viral gp40 modulation of retinoic acid early inducible 1 gene molecules. J. Exp. Med. 1971245-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lodoen, M. B., G. Abenes, S. Umamoto, J. P. Houchins, F. Liu, and L. L. Lanier. 2004. The cytomegalovirus m155 gene product subverts natural killer cell antiviral protection by disruption of H60-NKG2D interactions. J. Exp. Med. 2001075-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maasho, K., J. Opoku-Anane, A. I. Marusina, J. E. Coligan, and F. Borrego. 2005. NKG2D is a costimulatory receptor for human naive CD8+ T cells. J. Immunol. 1744480-4484. [DOI] [PubMed] [Google Scholar]
- 60.Markiewicz, M. A., L. N. Carayannopoulos, O. V. Naidenko, K. Matsui, W. R. Burack, E. L. Wise, D. H. Fremont, P. M. Allen, W. M. Yokoyama, M. Colonna, and A. S. Shaw. 2005. Costimulation through NKG2D enhances murine CD8+ CTL function: similarities and differences between NKG2D and CD28 costimulation. J. Immunol. 1752825-2833. [DOI] [PubMed] [Google Scholar]
- 61.Marten, N. W., S. A. Stohlman, R. D. Atkinson, D. R. Hinton, J. O. Fleming, and C. C. Bergmann. 2000. Contributions of CD8+ T cells and viral spread to demyelinating disease. J. Immunol. 1644080-4088. [DOI] [PubMed] [Google Scholar]
- 62.Marten, N. W., S. A. Stohlman, J. Zhou, and C. C. Bergmann. 2003. Kinetics of virus-specific CD8+ T-cell expansion and trafficking following central nervous system infection. J. Virol. 772775-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ogasawara, K., J. Benjamin, R. Takaki, J. H. Phillips, and L. L. Lanier. 2005. Function of NKG2D in natural killer cell-mediated rejection of mouse bone marrow grafts. Nat. Immunol. 6938-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ogasawara, K., J. A. Hamerman, L. R. Ehrlich, H. Bour-Jordan, P. Santamaria, J. A. Bluestone, and L. L. Lanier. 2004. NKG2D blockade prevents autoimmune diabetes in NOD mice. Immunity 20757-767. [DOI] [PubMed] [Google Scholar]
- 65.Ogasawara, K., J. A. Hamerman, H. Hsin, S. Chikuma, H. Bour-Jordan, T. Chen, T. Pertel, C. Carnaud, J. A. Bluestone, and L. L. Lanier. 2003. Impairment of NK cell function by NKG2D modulation in NOD mice. Immunity 1841-51. [DOI] [PubMed] [Google Scholar]
- 66.Overbergh, L., A. Giulietti, D. Valckx, R. Decallonne, R. Bouillon, and C. Mathieu. 2003. The use of real-time reverse transcriptase PCR for the quantification of cytokine gene expression. J. Biomol. Tech. 1433-43. [PMC free article] [PubMed] [Google Scholar]
- 67.Owens, T. 2002. Identification of new therapeutic targets for prevention of CNS inflammation. Expert Opin. Ther. Targets 6203-215. [DOI] [PubMed] [Google Scholar]
- 68.Parra, B., D. R. Hinton, M. T. Lin, D. J. Cua, and S. A. Stohlman. 1997. Kinetics of cytokine mRNA expression in the central nervous system following lethal and nonlethal coronavirus-induced acute encephalomyelitis. Virology 233260-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Parra, B., D. R. Hinton, N. W. Marten, C. C. Bergmann, M. T. Lin, C. S. Yang, and S. A. Stohlman. 1999. IFN-gamma is required for viral clearance from central nervous system oligodendroglia. J. Immunol. 1621641-1647. [PubMed] [Google Scholar]
- 70.Pearce, B. D., M. V. Hobbs, T. S. McGraw, and M. J. Buchmeier. 1994. Cytokine induction during T-cell-mediated clearance of mouse hepatitis virus from neurons in vivo. J. Virol. 685483-5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Perlman, S. R., T. E. Lane, and M. J. Buchmeier. 1999. Coronaviruses: hepatitis, peritonitis, and central nervous system disease, p. 331-348. In M. W. Cunningham and R. S. Fujinami (ed.), Effects of microbes on the immune system, vol. 1. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 72.Pfaffl, M. W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ransohoff, R. M., P. Kivisakk, and G. Kidd. 2003. Three or more routes for leukocyte migration into the central nervous system. Nat. Rev. Immunol. 3569-581. [DOI] [PubMed] [Google Scholar]
- 74.Rausch, A., M. Hessmann, A. Holscher, T. Schreiber, S. Bulfone-Paus, S. Ehlers, and C. Holscher. 2006. Interleukin-15 mediates protection against experimental tuberculosis: a role for NKG2D-dependent effector mechanisms of CD8+ T cells. Eur. J. Immunol. 361156-1167.16619285 [Google Scholar]
- 75.Rossi, C. P., A. McAllister, M. Tanguy, D. Kagi, and M. Brahic. 1998. Theiler's virus infection of perforin-deficient mice. J. Virol. 724515-4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saikali, P., J. P. Antel, J. Newcombe, Z. Chen, M. Freedman, M. Blain, R. Cayrol, A. Prat, J. A. Hall, and N. Arbour. 2007. NKG2D-mediated cytotoxicity toward oligodendrocytes suggests a mechanism for tissue injury in multiple sclerosis. J. Neurosci. 271220-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Seko, Y., N. Takahashi, H. Oshima, O. Shimozato, H. Akiba, K. Takeda, T. Kobata, H. Yagita, K. Okumura, M. Azuma, and R. Nagai. 2001. Expression of tumour necrosis factor (TNF) ligand superfamily costimulatory molecules CD30L, CD27L, OX40L, and 4-1BBL in murine hearts with acute myocarditis caused by coxsackievirus B3. J. Pathol. 195593-603. [DOI] [PubMed] [Google Scholar]
- 78.Shrestha, B., and M. S. Diamond. 2004. Role of CD8+ T cells in control of West Nile virus infection. J. Virol. 788312-8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Somersalo, K., N. Anikeeva, T. N. Sims, V. K. Thomas, R. K. Strong, T. Spies, T. Lebedeva, Y. Sykulev, and M. L. Dustin. 2004. Cytotoxic T lymphocytes form an antigen-independent ring junction. J. Clin. Investig. 11349-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stohlman, S. A., C. C. Bergmann, M. T. Lin, D. J. Cua, and D. R. Hinton. 1998. CTL effector function within the central nervous system requires CD4+ T cells. J. Immunol. 1602896-2904. [PubMed] [Google Scholar]
- 81.Stohlman, S. A., and D. R. Hinton. 2001. Viral induced demyelination. Brain Pathol. 1192-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stohlman, S. A., and L. P. Weiner. 1981. Chronic central nervous system demyelination in mice after JHM virus infection. Neurology 3138-44. [DOI] [PubMed] [Google Scholar]
- 83.Sussman, M. A., R. A. Shubin, S. Kyuwa, and S. A. Stohlman. 1989. T-cell-mediated clearance of mouse hepatitis virus strain JHM from the central nervous system. J. Virol. 633051-3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Takaki, R., Y. Hayakawa, A. Nelson, P. V. Sivakumar, S. Hughes, M. J. Smyth, and L. L. Lanier. 2005. IL-21 enhances tumor rejection through a NKG2D-dependent mechanism. J. Immunol. 1752167-2173. [DOI] [PubMed] [Google Scholar]
- 85.Trifilo, M. J., C. C. Bergmann, W. A. Kuziel, and T. E. Lane. 2003. CC chemokine ligand 3 (CCL3) regulates CD8+-T-cell effector function and migration following viral infection. J. Virol. 774004-4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Trifilo, M. J., C. Montalto-Morrison, L. N. Stiles, K. R. Hurst, J. L. Hardison, J. E. Manning, P. S. Masters, and T. E. Lane. 2004. CXC chemokine ligand 10 controls viral infection in the central nervous system: evidence for a role in innate immune response through recruitment and activation of natural killer cells. J. Virol. 78585-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Trottier, M. D., Jr., B. M. Palian, and C. Shoshkes Reiss. 2005. VSV replication in neurons is inhibited by type I IFN at multiple stages of infection. Virology 333215-225. [DOI] [PubMed] [Google Scholar]
- 88.Tseng, S. Y., and M. L. Dustin. 2002. T-cell activation: a multidimensional signaling network. Curr. Opin. Cell Biol. 14575-580. [DOI] [PubMed] [Google Scholar]
- 89.Vilarinho, S., K. Ogasawara, L. L. Lanier, S. Nishimura, and J. L. Baron. 2007. Blockade of NKG2D on NKT cells prevents hepatitis and the acute immune response to hepatitis B virus. Proc. Natl. Acad. Sci. USA 10418187-18192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Walsh, K. B., R. A. Edwards, K. M. Romero, M. V. Kotlajich, S. A. Stohlman, and T. E. Lane. 2007. Expression of CXC chemokine ligand 10 from the mouse hepatitis virus genome results in protection from viral-induced neurological and liver disease. J. Immunol. 1791155-1165. [DOI] [PubMed] [Google Scholar]
- 90a.Walsh, K. B., M. B. Lodoen, R. A. Edwards, L. L. Lanier, and T. E. Lane. 2008. Evidence for differential roles for NKG2D receptor signaling in innate host defense against coronavirus-induced neurological and liver disease. J. Virol. 823021-3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang, Y., M. Lobigs, E. Lee, and A. Mullbacher. 2003. CD8+ T cells mediate recovery and immunopathology in West Nile virus encephalitis. J. Virol. 7713323-13334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Williamson, J. S., and S. A. Stohlman. 1990. Effective clearance of mouse hepatitis virus from the central nervous system requires both CD4+ and CD8+ T cells. J. Virol. 644589-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Williamson, J. S., K. C. Sykes, and S. A. Stohlman. 1991. Characterization of brain-infiltrating mononuclear cells during infection with mouse hepatitis virus strain JHM. J. Neuroimmunol. 32199-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu, G. F., A. A. Dandekar, L. Pewe, and S. Perlman. 2000. CD4 and CD8 T cells have redundant but not identical roles in virus-induced demyelination. J. Immunol. 1652278-2286. [DOI] [PubMed] [Google Scholar]
- 95.Zhou, R., H. Wei, R. Sun, J. Zhang, and Z. Tian. 2007. NKG2D recognition mediates Toll-like receptor 3 signaling-induced breakdown of epithelial homeostasis in the small intestines of mice. Proc. Natl. Acad. Sci. USA 1047512-7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zuo, J., S. A. Stohlman, J. B. Hoskin, D. R. Hinton, R. Atkinson, and C. C. Bergmann. 2006. Mouse hepatitis virus pathogenesis in the central nervous system is independent of IL-15 and natural killer cells. Virology 350206-215. [DOI] [PMC free article] [PubMed] [Google Scholar]