Abstract
While many studies show that the APOBEC3 family of cytidine deaminases can inhibit human immunodeficiency virus type 1 (HIV-1) replication, the clinical significance of this host defense mechanism is unclear. Elite suppressors are HIV-1-infected individuals who maintain viral loads below 50 copies/ml without antiretroviral therapy. To determine the role of APOBEC3G/F proteins in the control of viremia in these patients, we used a novel assay to measure the frequency of hypermutated proviral genomes. In most elite suppressors, the frequency was not significantly different than that observed in patients on highly active antiretroviral therapy. Thus, enhanced APOBEC3 activity alone cannot explain the ability of elite suppressors to control viremia.
The apolipoprotein B mRNA-editing enzyme catalytic polypeptide-like editing complex 3, or APOBEC3, proteins are a recently discovered family of cytidine deaminases that catalyze G→A hypermutation in retroviral genomes (14-16, 22, 23, 25, 30, 32, 38, 40, 42). Although human immunodeficiency virus type 1 (HIV-1) hypermutation had been documented previously (6, 36), the discovery that APOBEC3G was responsible for rendering cells nonpermissive to vif-deficient viruses led to an appreciation of the role of hypermutation in innate immunity to HIV-1 (9, 14, 15, 22, 25, 30, 42).
The activity of the APOBEC3 proteins is restricted by the HIV-1 protein Vif (12, 26, 30, 31, 41), which prevents the incorporation of these deaminases into budding virions. The extent to which APOBEC3 proteins affect replication is a matter of great interest (6, 17, 19, 36). Although hypermutated viral genomes are not present in plasma, they can be detected in proviral DNA from resting CD4+ T cells (20). Studies using primers based on sequences in the pol gene have shown that an average of 9% of integrated proviruses are hypermutated in patients on highly active antiretroviral therapy (HAART) (20).
In a subset of patients, APOBEC3 proteins may play a more significant role. Long-term nonprogressors (LTNPs) are HIV-1-infected individuals who remain asymptomatic and maintain normal CD4+ counts. An inverse correlation between APBOEC3G mRNA levels and disease progression has been observed in LTNPs (18) but not in progressors (11). A finding of defective Vif in one LTNP further suggests that APOBEC3 proteins may control viral replication in vivo (13). This hypothesis is further corroborated by the finding of minimal HIV-1 evolution and characteristic APOBEC3G-induced hypermutation in one LTNP (37). In another study of three LTNPs, the frequency of defective vif clones amplified from PBMC was similar to that found in patients with progressive disease (33). We recently noted that proviral gag in elite suppressors (ESs) had substantial levels of hypermutation (2). These patients, like LTNPs, maintain normal CD4+ counts but are further distinguished by undetectable viral loads (<50 copies/ml) in the absence of therapy. Recent studies have shown that some ESs are infected with replication-competent viruses, which they control through host defense mechanisms (5). Because these ESs are able to control viremia, it is important to understand the role of APOBEC3 proteins.
In this study, we examined the role of APOBEC3-induced hypermutation in ESs by directly sequencing the proviral DNA from ESs and from a control set of patients with progressive disease who had undetectable viral loads on HAART. Hypermutated sequences can be readily detected in proviral DNA from resting CD4+ T cells of all infected individuals (20). This population of cells constitutes the major stable viral reservoir (7), thus providing a record of APOBEC3 activity. While it remains possible that the APOBEC3G and -F proteins are uniquely active in another compartment, this has not yet been elucidated, and the significance is unknown. Therefore, resting CD4+ cells were purified from eight ESs and nine patients on HAART as previously described (1). Genomic DNA was isolated using a Puregene kit (Qiagen, Valencia, CA) (1). Limiting-dilution nested PCR (24) was then performed on the genomic DNA to amplify a region spanning the 3′ end of env and the 5′ start of nef (Fig. 1). This region was selected for its high rate of hypermutation (40, 35). Primers were designed to avoid APOBEC3G target sites. To minimize PCR error, positive reaction products were directly sequenced. Nonclonal sequences were discarded. Because amplifications were carried out at a limiting dilution, all reported sequences represent independent templates present in vivo (24). The extent of hypermutation was determined using the Hypermut 2.0 tool (www.hiv.lanl.gov/content/sequence/HYPERMUT/hypermut.html), which can distinguish plus-strand APOBEC3G/F hypermutation from background levels of mutation. Previous studies have established a dinucleotide sequence specificity of 5′-GG-3′ and 5′-GA-3′ for APOBEC3G and APOBEC3F, respectively, where the underlined plus-strand dG is complementary to the targeted minus-strand dC. APOBEC3G-mediated hypermutation does not occur when dC is in a downstream +2 position on the plus strand (3, 20, 21, 40). Thus, target sites were defined as either 5′-GG-3′ or 5′-GA-3′, depending on the enzyme being studied, and the control sequences were defined as 5′-GYN-3′ or 5′-GRC-3′. A sequence was considered hypermutated if it registered a P value of <0.05 on the Fisher exact test that compared the number of G→A changes in APOBEC3 versus control contexts.
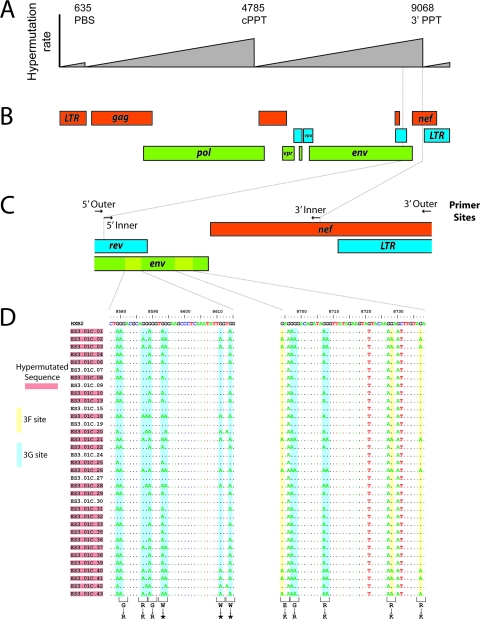
FIG. 1.
Detection of hypermutated proviral sequences in ESs. (A) Gradients of APOBEC3-mediated hypermutation as defined by Yu et al. (40) and Suspene et al. (35). The gradients peak just 5′ of the primer binding site (PBS), the central polypurine tract (cPPT), and the 3′ polypurine tract (3′ PPT). (B) Map of the HIV-1 genome indicating the region selected for analysis. This region is at the peak of the a gradient of hypermutation frequency. (C) Enlargement of the region amplified showing outer and inner PCR primers. Primers were chosen at sites lacking APOBEC3G consensus hypermutation sites. Sequences from two regions in the env gene are shown in detail in panel D. Plus-strand sequences for the indicated regions of env from 35 independent proviral sequences from ES3 are shown. APOBEC3F and APOBEC3G hypermutation sites are in yellow and blue boxes, respectively. Amino acid changes resulting from hypermutation are indicated at the bottom of the figure. *, stop codon.
As shown in Fig. 1D, hypermutated proviral sequences are readily detected by this method. A total of 978 independent proviral sequences were obtained by limiting dilution PCR, for an average of 57.5 per patient (range 35 to 99). The frequency of hypermutation is indicated in Fig. 2. Except for one outlier (ES3), ESs had levels of hypermutated proviral sequences that were comparable to or slightly lower than those of patients on HAART. As shown in Table 1, the average fraction of hypermutated clones for all ESs was 18.6% ± 25.5%, compared to 20.9% ± 14.6% for control patients. Sequences obtained from three ESs and four patients on HAART at a second time point confirmed that the rate of hypermutation is invariant with time, demonstrating the stability of the reservoir (average of 30.6 clones analyzed per patient; rate of hypermutation was not significantly different [data not shown]).
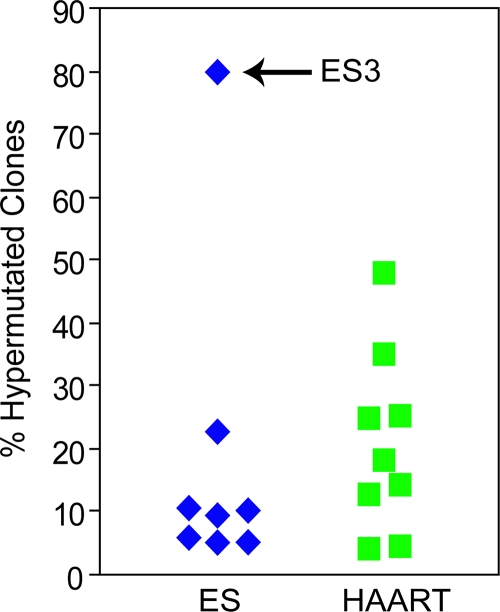
FIG. 2.
Rates of hypermutation in ESs versus patients on HAART. The percentage of hypermutated genomes amplified from resting CD4+ T cells from ESs and patients on HAART is shown. Patient ES3 had an unusually high frequency of hypermutated proviral sequences.
TABLE 1.
Interpatient variability in extent of hypermutation
| Clinical status and patient ID | No. of clones
|
Fraction (%) of clones
|
Fraction (%) of sites with G→A
|
||||
|---|---|---|---|---|---|---|---|
| Sequenced | Hyper mutated | Hypermutated | With 3G patterna | With 3F patternb | 3G | 3F | |
| HAARTc | |||||||
| 99 | 57 | 8 | 14 | 100 | 25 | 44 | 27 |
| 108 | 50 | 9 | 18 | 100 | 67 | 64 | 30 |
| 134 | 55 | 7 | 13 | 100 | 57 | 54 | 27 |
| 136 | 57 | 21 | 37 | 90 | 81 | 70 | 34 |
| 139 | 70 | 3 | 4 | 100 | 33 | 33 | 30 |
| 140 | 46 | 22 | 48 | 95 | 55 | 64 | 35 |
| 148 | 48 | 2 | 4 | 100 | 100 | 70 | 48 |
| 154 | 56 | 14 | 25 | 86 | 93 | 72 | 34 |
| 147 | 61 | 15 | 25 | 93 | 67 | 61 | 28 |
| Mean ± SD | 55.6 ± 7.3 | 11.2 ± 7.2 | 20.9 ± 14.6 | 96.0 ± 5.3 | 64.2 ± 25.1 | 59.1 ± 13.0 | 32.7 ± 6.5 |
| ESd | |||||||
| ES8 | 62 | 14 | 23 | 100 | 43 | 47 | 24 |
| ES1 | 76 | 7 | 9 | 100 | 57 | 45 | 45 |
| ES3 | 35 | 28 | 80 | 100 | 39 | 54 | 28 |
| ES5 | 80 | 4 | 5 | 100 | 100 | 73 | 37 |
| ES11 | 47 | 5 | 11 | 100 | 100 | 16 | 65 |
| ES6 | 99 | 6 | 6 | 100 | 100 | 65 | 27 |
| ES7 | 40 | 4 | 10 | 50 | 75 | 62 | 40 |
| ES4 | 39 | 2 | 5 | 100 | 50 | 49 | 64 |
| Mean ± SD | 59.8 ± 23.3 | 8.8 ± 8.6 | 18.6 ± 25.5 | 93.8 ± 17.7 | 70.5 ± 26.7 | 51.3 ± 17.3 | 41.2 ± 15.9 |
The observed level of hypermutation is higher than previous estimates (20) because of the use of unbiased primers targeting the most heavily hypermutated region of the genome. It is also important to note that the levels of hypermutation observed in sequencing of proviruses may represent an underestimate of the actual fraction of viral cDNAs that are inactivated by APOBEC3 proteins because of both nonmutagenic viral control by APOBEC3 (4, 8, 32) and the fact that hypermutated genomes destroyed through UNG-dependent mechanisms (29) are not detected.
Within both populations of patients, however, there was significant patient-to-patient variation in the levels of hypermutation (Fig. 2; Table 1). In patients on HAART, the percentage of hypermutated proviruses ranged from 4% to 48%. In ESs, the range was broader due to a single outlier (ES3, 80%). Moreover, the patients also varied in the type of hypermutation they exhibited (Table 1; Fig. 1D). Overall, APOBEC3G patterns of hypermutation dominated, being present in 97% of pooled hypermutated sequences from all ESs and 94% in patients on HAART. APOBEC3F patterns, on the other hand, were present in 56% of pooled hypermutated sequences from ESs and 66% in patients on HAART. Interestingly, 95% of all clones with APOBEC3F patterns of hypermutation also had evidence of APOBEC3G hypermutation, possibly indicating some degree of cooperation between the two enzymes.
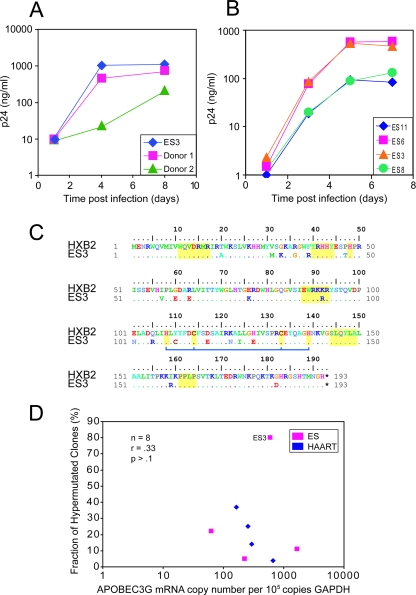
ES3 exhibited a statistically significant elevated level of hypermutated sequences (80%, P < 0.01) compared to other ESs (Z test, z = 2.38) and thus warranted further investigation (Table 1; Fig. 3). To test whether this patient's CD4+ cells were permissive for viral replication, CD4+ T cells from ES3 were activated with phytohemagglutinin and infected with the Ba-L isolate of HIV-1 as previously described (5). At various times after infection, p24 levels in the supernatant were measured. As shown in Fig. 3A and B, there was no difference in the kinetics of Ba-L replication between CD4+ T cells from ES3 and those from three other ESs who had lower levels of proviral hypermutation. In addition, the virus replicated as well in cells from ES3 as it did in cells from two seronegative donors. To determine whether vif was defective in this patient, we amplified this gene from the low level of free virus in the patient's plasma (viral load, <50 copies/ml). Figure 3C shows that were no stop codons, insertions, deletions, or novel mutations in this gene. In addition, there were no substitutions at amino acid positions known to be important for Vif function (13, 28, 33), although we were unable to definitively rule this out with a phenotypic analysis of the protein. Furthermore, no polymorphisms were identified in the APBOEC3G gene amplified from mRNA from this patient (data not shown).
FIG. 3.
Characterization of ES3. Susceptibility of CD4+ T cells from ES3 to spreading HIV-1 infection. CD4+ T lymphoblasts from ES3 and from two normal controls (donors 1 and 2) (A) and three ESs (ES5, ES8, and ES11) (B) were isolated and infected with Ba-L. Virus growth was measured by quantitating HIV-1 p24 antigen in the culture supernatant. (C) Sequence of the vif gene from ES3. No novel substitutions, insertions, or deletions were found. There were no significant changes at sites that are known to be important in Vif functionality (regions indicated in yellow). The blue bar denotes amino acids that constitute a zinc binding motif important for APOBEC3G degradation (28, 33, 39). (D) Expression of APOBEC3G mRNA was measured using a real-time PCR assay in four ESs (ES3, ES5, ES8, and ES11) and four HAART-treated patients (patients 99, 136, 147, and 148). mRNA levels were calculated as copies of APOBEC3G per 100,000 copies of GAPDH. No correlation between the fraction of hypermutated clones and expression of APOBEC3G was seen.
To investigate the levels of APOBEC3G mRNA expression in the rate of hypermutation, we performed real-time PCR analysis on four ESs and four patients on HAART from our study. We found no correlation between APOBEC3G expression and the rate of hypermutation (Fig. 3D). Because APOBEC3G and -F are coordinately regulated (11), no association would be expected for APOBEC3F either. Additionally, no relationship was seen between disease status and APOBEC3G mRNA levels, contrary to previous findings (18).
While these findings suggest that innate host mechanisms involving APOBEC3 proteins are not solely responsible for the control of viremia in ES3, further investigation is warranted. Interestingly, Greene and colleagues have demonstrated that in activated T cells, APOBEC3G is in high-molecular-mass complexes with limited enzymatic activity (10, 34). We have shown that ES3, like other ESs, has a low level of free virus in the plasma that can be detected with extremely sensitive RT-PCR techniques. Nonhypermutated gag (2), pol (2), env (1), and nef sequences have been amplified from the patient's plasma. Furthermore, the plasma gag clones show evidence of viral evolution in response to selective pressure from CD8+ T cells (2). These findings strongly suggest that APOBEC3-mediated hypermutation does not completely inhibit viral replication in this ES. This patient also is positive for the HLA-B*57 allele (2), which is overrepresented in ESs, and thus, it is likely that multiple factors contribute to the control of viral replication.
In summary, we found that hypermutation occurs in ∼20% of proviral sequences with significant patient-to-patient variability. As previously noted, APOBEC3G patterns of mutation dominate, being present in 95% of hypermutated sequences. Our sequencing data indicate that, despite previous reports of elevated levels of APOBEC3G in LTNP, hypermutation cannot explain the control of viremia in the majority of ESs. Suppression in these patients is probably maintained via a combination of other viral and host factors, including CTL responses. While APOBEC3 proteins do not appear to be solely responsible for control of viral replication in ESs, our study does not rule out a contribution of these cytidine deaminases in ES status.
Nucleotide sequence accession numbers.
The sequences determined in this study have been deposited in GenBank under accession numbers EU383053 to EU384245.
Acknowledgments
This work was supported by the NIH (grants K08 AI51191 and R56 AI73185-01A1) and HHMI.
We thank Megan Wind-Rotolo and Amanda Chase for their input.
Footnotes
Published ahead of print on 12 December 2007.
REFERENCES
- 1.Bailey, J. R., K. G. Lassen, H. Yang, T. C. Quinn, S. C. Ray, J. N. Blankson, and R. F. Siliciano. 2006. Neutralizing antibodies do not mediate suppression of human immunodeficiency virus type 1 in elite suppressors or selection of plasma virus variants in patients on highly active antiretroviral therapy. J. Virol. 804758-4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey, J. R., T. M. Williams, R. F. Siliciano, and J. N. Blankson. 2006. Maintenance of viral suppression in HIV-1-infected HLA-B*57+ elite suppressors despite CTL escape mutations. J. Exp. Med. 2031357-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beale, R. C. L., S. K. Petersen-Mahrt, I. N. Watt, R. S. Harris, C. Rada, and M. S. Neuberger. 2004. Comparison of the differential context-dependence of DNA deamination by APOBEC enzymes: correlation with mutation spectra in vivo. J. Mol. Biol. 337585-596. [DOI] [PubMed] [Google Scholar]
- 4.Bishop, K. N., R. K. Holmes, and M. H. Malim. 2006. Antiviral potency of APOBEC proteins does not correlate with cytidine deamination. J. Virol. 808450-8458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blankson, J. N., J. R. Bailey, S. Thayil, H. Yang, K. Lassen, J. Lai, S. K. Gandhi, J. D. Siliciano, T. M. Williams, and R. F. Siliciano. 2007. Isolation and characterization of replication-competent human immunodeficiency virus type 1 from a subset of elite suppressors. J. Virol. 812508-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borman, A. M., C. Quillent, P. Charneau, K. M. Kean, and F. Clavel. 1995. A highly defective HIV-1 group O provirus: evidence for the role of local sequence determinants in G → A hypermutation during negative-strand viral DNA synthesis. Virology 208601-609. [DOI] [PubMed] [Google Scholar]
- 7.Brenchley, J., B. Hill, D. Ambrozak, D. Price, F. Guenaga, J. Casazza, J. Kuruppu, J. Yazdani, S. Migueles, M. Connors, M. Roederer, D. Douek, and R. Koup. 2004. T-cell subsets that harbor human immunodeficiency virus (HIV) in vivo: implications for HIV pathogenesis. J. Virol. 781160-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiu, Y., and W. C. Greene. 2006. Multifaceted antiviral actions of APOBEC3 cytidine deaminases. Trends Immunol. 27291-297. [DOI] [PubMed] [Google Scholar]
- 9.Chiu, Y., H. E. Witkowska, S. C. Hall, M. Santiago, V. B. Soros, C. Esnault, T. Heidmann, and W. C. Greene. 2006. High-molecular-mass APOBEC3G complexes restrict Alu retrotransposition. Proc. Natl. Acad. Sci. USA 10315588-15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiu, Y., V. B. Soros, J. F. Kreisberg, K. Stopak, W. Yonemoto, and W. C. Greene. 2005. Cellular APOBEC3G restricts HIV-1 infection in resting CD4+ T cells. Nature 435108-114. [DOI] [PubMed] [Google Scholar]
- 11.Cho, S., H. Drechsler, R. C. Burke, M. Q. Arens, W. Powderly, and N. O. Davidson. 2006. APOBEC3F and APOBEC3G mRNA levels do not correlate with human immunodeficiency virus type 1 plasma viremia or CD4+ T-cell count. J. Virol. 802069-2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conticello, S. G., R. S. Harris, and M. S. Neuberger. 2003. The Vif protein of HIV triggers degradation of the human antiretroviral DNA deaminase APOBEC3G. Curr. Biol. 132009-2013. [DOI] [PubMed] [Google Scholar]
- 13.Farrow, M. A., M. Somasundaran, C. Zhang, D. Gabuzda, J. L. Sullivan, and T. C. Greenough. 2005. Nuclear localization of HIV type 1 Vif isolated from a long-term asymptomatic individual and potential role in virus attenuation. AIDS Res. Hum. Retroviruses 21565-574. [DOI] [PubMed] [Google Scholar]
- 14.Goff, S. P. 2003. Death by deamination: a novel host restriction system for HIV-1. Cell 114281-283. [DOI] [PubMed] [Google Scholar]
- 15.Harris, R. S., K. N. Bishop, A. M. Sheehy, H. M. Craig, S. K. Petersen-Mahrt, I. N. Watt, M. S. Neuberger, and M. H. Malim. 2003. DNA deamination mediates innate immunity to retroviral infection. Cell 113803-809. [DOI] [PubMed] [Google Scholar]
- 16.Harris, R. S., and M. T. Liddament. 2004. Retroviral restriction by APOBEC proteins. Nat. Rev. Immunol. 4868-877. [DOI] [PubMed] [Google Scholar]
- 17.Janini, M., M. Rogers, D. R. Birx, and F. E. McCutchan. 2001. Human immunodeficiency virus type 1 DNA sequences genetically damaged by hypermutation are often abundant in patient peripheral blood mononuclear cells and may be generated during near-simultaneous infection and activation of CD4+ T cells. J. Virol. 757973-7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin, X., A. Brooks, H. Chen, R. Bennett, R. Reichman, and H. Smith. 2005. APOBEC3G/CEM15 (hA3G) mRNA levels associate inversely with human immunodeficiency virus viremia. J. Virol. 7911513-11516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin, X., H. Wu, and H. Smith. 2007. APOBEC3G levels predict rates of progression to AIDS. Retrovirology 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kieffer, T. L., P. Kwon, R. E. Nettles, Y. Han, S. C. Ray, and R. F. Siliciano. 2005. G→A hypermutation in protease and reverse transcriptase regions of human immunodeficiency virus type 1 residing in resting CD4+ T cells in vivo. J. Virol. 791975-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langlois, M., R. C. L. Beale, S. G. Conticello, and M. S. Neuberger. 2005. Mutational comparison of the single-domained APOBEC3C and double-domained APOBEC3F/G anti-retroviral cytidine deaminases provides insight into their DNA target site specificities. Nucleic Acids Res. 331913-1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lecossier, D., F. Bouchonnet, F. Clavel, and A. J. Hance. 2003. Hypermutation of HIV-1 DNA in the absence of the Vif protein. Science 3001112. [DOI] [PubMed] [Google Scholar]
- 23.Liddament, M. T., W. L. Brown, A. J. Schumacher, and R. S. Harris. 2004. APOBEC3F properties and hypermutation preferences indicate activity against HIV-1 in vivo. Curr. Biol. 141385-1391. [DOI] [PubMed] [Google Scholar]
- 24.Liu, S. L., A. G. Rodrigo, R. Shankarappa, G. H. Learn, L. Hsu, O. Davidov, L. P. Zhao, and J. I. Mullins. 1996. HIV quasispecies and resampling. Science 273415-416. [DOI] [PubMed] [Google Scholar]
- 25.Mangeat, B., P. Turelli, G. Caron, M. Friedli, L. Perrin, and D. Trono. 2003. Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature 42499-103. [DOI] [PubMed] [Google Scholar]
- 26.Marin, M., K. M. Rose, S. L. Kozak, and D. Kabat. 2003. HIV-1 Vif protein binds the editing enzyme APOBEC3G and induces its degradation. Nat. Med. 91398-1403. [DOI] [PubMed] [Google Scholar]
- 27.Nettles, R. E., T. L. Kieffer, P. Kwon, D. Monie, Y. Han, T. Parsons, J. Cofrancesco, Jr., J. E. Gallant, T. C. Quinn, B. Jackson, C. Flexner, K. Carson, S. Ray, D. Persaud, and R. F. Siliciano. 2005. Intermittent HIV-1 viremia (blips) and drug resistance in patients receiving HAART. JAMA 293817-829. [DOI] [PubMed] [Google Scholar]
- 28.Russell, R. A., and V. K. Pathak. 2007. Identification of two distinct human immunodeficiency virus type 1 Vif determinants critical for interactions with human APOBEC3G and APOBEC3F. J. Virol. 818201-8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schrofelbauer, B., Q. Yu, S. G. Zeitlin, and N. R. Landau. 2005. Human immunodeficiency virus type 1 Vpr induces the degradation of the UNG and SMUG uracil-DNA glycosylases. J. Virol. 7910978-10987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheehy, A. M., N. C. Gaddis, J. D. Choi, and M. H. Malim. 2002. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature 418646-650. [DOI] [PubMed] [Google Scholar]
- 31.Sheehy, A. M., N. C. Gaddis, and M. H. Malim. 2003. The antiretroviral enzyme APOBEC3G is degraded by the proteasome in response to HIV-1 Vif. Nat. Med. 91404-1407. [DOI] [PubMed] [Google Scholar]
- 32.Shindo, K., A. Takaori-Kondo, M. Kobayashi, A. Abudu, K. Fukunaga, and T. Uchiyama. 2003. The enzymatic activity of CEM15/Apobec-3G is essential for the regulation of the infectivity of HIV-1 virion but not a sole determinant of its antiviral activity. J. Biol. Chem. 27844412-44416. [DOI] [PubMed] [Google Scholar]
- 33.Simon, V., V. Zennou, D. Murray, Y. Huang, D. D. Ho, and P. D. Bieniasz. 2005. Natural variation in Vif: differential impact on APOBEC3G/3F and a potential role in HIV-1 diversification. PLoS Pathogens 1e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stopak, K. S., Y. Chiu, J. Kropp, R. M. Grant, and W. C. Greene. 2007. Distinct patterns of cytokine regulation of APOBEC3G expression and activity in primary lymphocytes, macrophages, and dendritic cells. J. Biol. Chem. 2823539-3546. [DOI] [PubMed] [Google Scholar]
- 35.Suspene, R., C. Rusniok, J. Vartanian, and S. Wain-Hobson. 2006. Twin gradients in APOBEC3 edited HIV-1 DNA reflect the dynamics of lentiviral replication. Nucleic Acids Res. 344677-4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vartanian, J. P., A. Meyerhans, B. Asjo, and S. Wain-Hobson. 1991. Selection, recombination, and G→A hypermutation of human immunodeficiency virus type 1 genomes. J. Virol. 651779-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang, B., M. Mikhail, W. B. Dyer, J. J. Zaunders, A. D. Kelleher, and N. K. Saksena. 2003. First demonstration of a lack of viral sequence evolution in a nonprogressor, defining replication-incompetent HIV-1 infection. Virology 312135-150. [DOI] [PubMed] [Google Scholar]
- 38.Wiegand, H. L., B. P. Doehle, H. P. Bogerd, and B. R. Cullen. 2004. A second human antiretroviral factor, APOBEC3F, is suppressed by the HIV-1 and HIV-2 Vif proteins. EMBO J. 232451-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiao, Z., E. Ehrlich, K. Luo, Y. Xiong, and X. F. Yu. 2007. Zinc chelation inhibits HIV Vif activity and liberates antiviral function of the cytidine deaminase APOBEC3G. FASEB J. 21217-222. [DOI] [PubMed] [Google Scholar]
- 40.Yu, Q., R. Konig, S. Pillai, K. Chiles, M. Kearney, S. Palmer, D. Richman, J. M. Coffin, and N. R. Landau. 2004. Single-strand specificity of APOBEC3G accounts for minus-strand deamination of the HIV genome. Nat. Struct. Mol. Biol. 11435-442. [DOI] [PubMed] [Google Scholar]
- 41.Yu, X., Y. Yu, B. Liu, K. Luo, W. Kong, P. Mao, and X. Yu. 2003. Induction of APOBEC3G ubiquitination and degradation by an HIV-1 Vif-Cul5-SCF complex. Science 3021056-1060. [DOI] [PubMed] [Google Scholar]
- 42.Zhang, H., B. Yang, R. J. Pomerantz, C. Zhang, S. C. Arunachalam, and L. Gao. 2003. The cytidine deaminase CEM15 induces hypermutation in newly synthesized HIV-1 DNA. Nature 42494-98. [DOI] [PMC free article] [PubMed] [Google Scholar]